Abstract
Coxiella burnetii is the causative agent of Q fever. All C. burnetii isolates encode either an autonomously replicating plasmid (QpH1, QpDG, QpRS, or QpDV) or QpRS‐like chromosomally integrated plasmid sequences. The role of the ORFs present in these sequences is unknown. Here, the role of the ORFs encoded on QpH1 was investigated. Using a new C. burnetii shuttle vector (pB‐TyrB‐QpH1ori), we cured the C. burnetii Nine Mile Phase II strain of QpH1. The ΔQpH1 strain grew normally in axenic media but had a significant growth defect in Vero cells, indicating QpH1 was important for C. burnetii virulence. We developed an inducible CRISPR interference system to examine the role of individual QpH1 plasmid genes. CRISPRi of cbuA0027 resulted in significant growth defects in axenic media and THP‐1 cells. The cbuA0028/cbuA0027 operon encodes CBUA0028 (ToxP) and CBUA0027 (AntitoxP), which are homologous to the HigB2 toxin and HigA2 antitoxin, respectively, from Vibrio cholerae. Consistent with toxin–antitoxin systems, overexpression of toxP resulted in a severe intracellular growth defect that was rescued by co‐expression of antitoxP. ToxP inhibited protein translation. AntitoxP bound the toxP promoter (PtoxP) and ToxP, with the resulting complex binding also PtoxP. In summary, our data indicate that C. burnetii maintains an autonomously replicating plasmid because of a plasmid‐based toxin–antitoxin system.
Keywords: antitoxin, Coxiella, CRISPRi, toxin
Coxiella burnetii is an obligate intracellular bacterium and causative agent of Q fever. Here we report that the C. burnetii genome encodes 11 toxin–antitoxin systems, which is highly unusual for intracellular bacteria, and that one TA system is encoded on the large endogenous plasmid found in almost all C. burnetii isolates. This TA system has a “noncanonical” toxin first gene orientation and acts at the RNA polymerase to cleave incoming mRNAs to prevent protein translation.
1. INTRODUCTION
Coxiella burnetii is an obligate intracellular bacterial pathogen and the etiological agent of human Q fever. Other than New Zealand, this bacterium has a worldwide distribution (Maurin & Raoult, 1999). Domestic animals, such as sheep, goats, and cows, are the main reservoirs for human infection, and their contaminated by‐products provide the vehicle for release of the highly infectious and stable bacterium (Angelakis & Raoult, 2010). Human infections are often asymptomatic (~60%) but can present as an acute flu‐like illness or under rare instances manifest as a more serious chronic infection (Eldin et al., 2017). C. burnetii has a tropism for mononuclear phagocytes (Khavkin & Tabibzadeh, 1988; Stein et al., 2005) where the bacterium replicates within a large phagolysosomal‐like parasitophorous vacuole termed the Coxiella‐containing vacuole (CCV). During phagolysosomal maturation, C. burnetii converts from an environmentally stable small‐cell variant (SCV) into a metabolically active large‐cell variant (LCV) (Coleman et al., 2004). Replication within the CCV requires the Dot/Icm type IVB secretion system, which delivers essential effector proteins into the cytosol of infected cells. Secretion assays have identified greater than 100 C. burnetii Dot/Icm substrates (Larson et al., 2016).
The C. burnetii genome consists of a small chromosome (1.9–2.2 Mb) and either one of at least four different autonomously replicating plasmids (QpH1, QpDG, QpDV, or QpRS) or a chromosomally integrated QpRS‐like plasmid sequence (IPS). The C. burnetii plasmids range in size from 32.6 to 54.2 kb while the IPS consists of 17.5 kb (Beare et al., 2009). A comparison of the open reading frame (ORF) content of the autonomous plasmids is shown that 22 open reading frames (ORFs) are conserved (Beare et al., 2009) yet of these only five ORFs (cbuA0007a, cbuA0010, cbuA0013, cbuA0023, and cbuA0027) are strictly conserved (having an identical ORF size), whereas a further three ORFs (cbuA0006, cbuA0011, and cbuA0012) have slightly different sizes when the IPS region is included in the comparison (Beare et al., 2009). Of these eight ORFs, cbuA0006, cbuA0013, and cbuA0023 have been identified as encoding Dot/Icm substrates and were found to localize to small cytoplasmic puncta (CBUA0006), autophagosomes (CBUA0013) or displayed no specific localization (CBUA0023), respectively, when ectopically expressed in host cells (Voth et al., 2011). The conservation of these ORFs suggests that they are important for C. burnetii pathogenesis.
The recent creation of an axenic media (Omsland et al., 2009) (Sandoz et al., 2016), and its use in generating himar1 transposon libraries in C. burnetii have allowed for a random method for identifying ORFs essential to bacterial virulence (Martinez et al., 2014; Newton et al., 2014; Weber et al., 2013). Using this approach, Martinez et al. (2014) isolated 14 himar1 mutants located in eight different QpH1 ORFs (cbuA0001, cbuA0003, cbuA0008b, cbuA0023, cbuA0032, cbuA0036, cbuA0039, and cbuA0040). Of these 14 mutants, two ORFs (cbuA0036‐parB and cbuA0039‐repA), with predicted roles in plasmid replication, had both strong replication and internalization defects in infected Vero cells, while two different mutants in cbuA0023 had either a mild replication defect or no defect. The development of a method for creating targeted gene deletions has allowed further analysis of the role of ORFs in C. burnetii pathogenesis (Beare et al., 2012). Using this technique, Colonne et al. (2016) created gene deletions of cbuA0015 and cbuA0016 and showed these mutants had a small atypical CCV in infected THP‐1 macrophages (Colonne et al., 2016). Recently, a new shuttle vector (pQGK) was developed and was used to cure C. burnetii of the QpH1 plasmid (Luo et al., 2021). This QpH1‐deficient strain of C. burnetii had a significant growth defect in bone marrow‐derived macrophages (Luo et al., 2021). Together, these studies indicate that the ORF content of the C. burnetii plasmid is important for virulence.
In this current study, we used a C. burnetii shuttle vector containing a new tyrosine‐based nutritional selection marker and QpH1 replication machinery (QRM; cbuA0036‐cbuA0039a) to create a ΔQpH1 strain of Nine Mile RSA439 (NMII). The ΔQpH1 strain displayed a severe growth defect in Vero cells. To determine the roles of the ORFs on QpH1, we developed an inducible CRISPR interference (CRISRPi) system, containing a new proline‐based nutritional selection marker. CRISPRi was used to individually knock down the expression of all the ORFs on the QpH1 plasmid. Knockdown of cbuA0027 resulted in severe replication defects in axenic media and in THP‐1 macrophage cells. Sequence analysis of cbuA0027 indicated that it was part of an operon with cbuA0028 and that the products of these ORFs encoded an antitoxin and toxin, respectively. Overexpression analysis, pulldown assays, and electrophoretic mobility shift assays (EMSA) confirm CBUA0028 and CBUA0027 are a functional type II toxin–antitoxin systems present on the QpH1 plasmid.
2. RESULTS
2.1. Deletion of the QpH1 plasmid results in defective intracellular growth
Every C. burnetii strain sequenced to date has either an endogenous plasmid (QpH1, QpRS, QpDG, and QpDV) or a QpRS‐like chromosomal insertion. To examine the role of the plasmid in C. burnetii virulence, we constructed a plasmid‐less strain of Nine Mile phase II. To delete the endogenous QpH1 plasmid, we constructed a C. burnetii–Escherichia coli shuttle vector termed pB‐TyrB‐QpH1ori that contains the QpH1 replication machinery (QRM), an E. coli origin of replication (pMB1), a selectable marker (CAT) for E. coli, and a new C. burnetii nutritional selection marker. This marker is based on the complementation of tyrosine auxotrophy, whereby C. burnetii expressing tyrB and grown in the presence of 4‐hydroxyphenylpyruvate (4‐HPP) will make L‐tyrosine (Figure 1a,b). Introduction of pB‐TyrB‐QpH1ori into C. burnetii and selection in axenic media in the absence of tyrosine resulted in the curing of the endogenous QpH1 plasmid (ΔQpH1) (Figure 1c). Growth of ΔQpH1 was indistinguishable from that of wild‐type NMII in an axenic medium (Figure 2a), with both strains increasing in more than 2500‐fold genome equivalents (GE) over 7 days. Replication in Vero cells following a 6‐day infection was then examined (Figure 2b). The ΔQpH1 strain displayed a significant reduction in growth (21.9‐fold GE increase) compared to NMII (577‐fold GE increase) (Figure 2b). In infected Vero cells, ΔQpH1 was contained within a small compressed CD63‐positive vacuole (Figure 2c,d), which is like the phenotype seen for some Dot/Icm substrate mutants (Larson et al., 2015). These results are consistent with previous data showing transposon mutants in the parB (cbuA0036) and repA (cbuA0039) ORFs located on QpH1 that have strong replication defects in Vero cells (Martinez et al., 2014). We were unsuccessful in creating a shuttle vector containing the entire QpH1 plasmid, presumably due to the very large size of QpH1 (39.3 kb), and therefore were unable to complement ΔQpH1.
FIGURE 1.
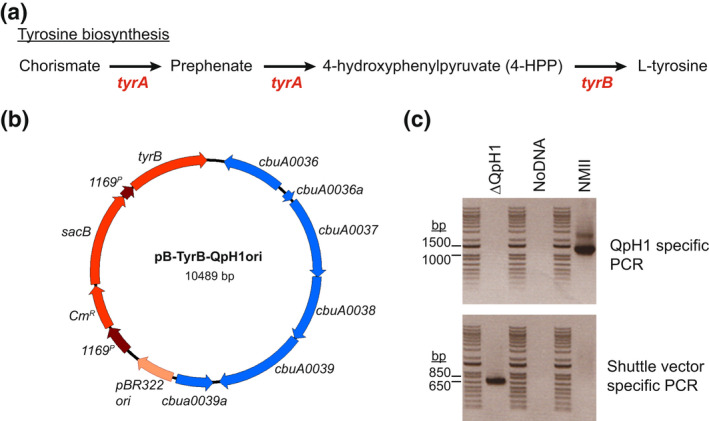
Curing Nine Mile RSA439 (NMII) of the QpH1 plasmid. (a) Schematic of the tyrosine biosynthesis pathway. Coxiella burnetii is auxotrophic for L‐tyrosine and missing both tyrA and tyrB genes required to convert chorismate into L‐tyrosine. (b) Plasmid map of the pB‐TyrB‐QpH1ori shuttle vector. (c) PCR detection of a QpH1‐specific region (PcbuA0023‐cbuA0023) and a shuttle‐specific gene (cm r ). Oligonucleotide primer pairs used for PCR detection are listed in Table S2. PCR was carried out using ΔQpH1 and NMII gDNA as a template DNA. A no DNA template control was also used. The 1 kb plus DNA ladder (Invitrogen) was used for size comparison (bands are sized at 15,000, 10,000, 8000, 7000, 6000, 5000, 4000, 3000, 2000, 1500, 1000, 850, 650, 500, 400, 300, 200, 100 bp). The QpH1 specific PCR band (1235 bp) was not detected in the ΔQpH1 strain but was present in NMII, while the shuttle vector specific PCR band (650 bp) was only seen in ΔQpH1. These data indicate that QpH1 has been cured from ΔQpH1
FIGURE 2.
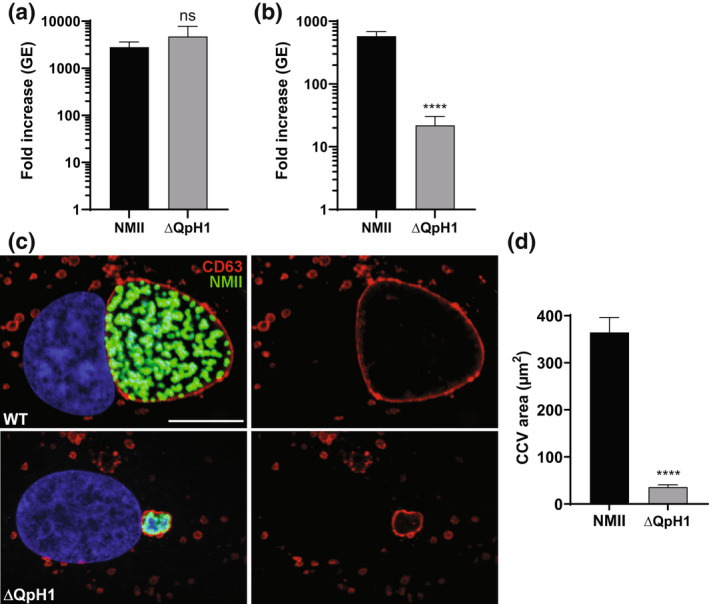
Coxiella burnetii ΔQpH1 has a severe intracellular growth defect. Replication of wild‐type NMII C. burnetii and the ΔQpH1 mutant in (a) ACCM‐D and (b) Vero cells. Replication is shown as fold increase in C. burnetii genome equivalents (GE) in axenic media (7 days) and Vero cells (6 days). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (Student's t test, ****p < .0001) compared with values for wild‐type C. burnetii. ns indicated no significant difference. (c) Confocal fluorescence micrographs of Vero cells infected for 3 days with NMII and ΔQpH1 mutant. CD63 (red) and C. burnetii (green) were stained by indirect immunofluorescence, and DNA (blue) was stained with Hoescht 33,342. Bar, 10 μm. (d) The histogram depicts the mean intensity of CCV area +/− SD of >50 cells for at least three independent experiments. Statistical difference was determined using the Student's t test (****p < .0001)
2.2. CRISPR interference of QpH1 ORF expression identifies a potential plasmid‐based toxin–antitoxin system
To investigate the requirement of the plasmid ORFs for virulence, we developed a miniTn7T‐based inducible CRISPR interference (CRISPRi) system for the knockdown of C. burnetii ORFs (Figure 3a). The C. burnetii CRISPRi system contains isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) inducible expression of a C‐terminal 3x‐myc‐tagged dCas9 and one or more targeting single guide RNA (sgRNA), while a selection of CRISPRi transformants was achieved using complementation of the proline auxotrophy in C. burnetii (Figure 3b). The CRISPRi system was tested for its ability to repress expression of single (Figure S1) and multiple (Figure S2) C. burnetii ORFs, showing knockdown of either icmD or icmD and scvA, respectively. Complementation of the icmD CRISPRi knockdown system was achieved using an anhydrotetracycline (aTc) inducible system expressing a codon‐optimized version of icmD, incorporating silent mutations into the codons targeted by the sgRNA (Figure S1). CRISPRi was used to target each of the plasmid ORFs contained in QpH1. Three targeting sequences were chosen for each ORF, except for cbuA0023a that only had two nGG PAM sequences and was cloned upstream of the sgRNA sequence. The effect of CRISPRi‐based QpH1 ORF knockdown on growth in axenic media (Figure S3) and in THP‐1 macrophages (Figure S4) was examined following 6‐ and 5‐day incubations, respectively. Induction of the CRISPRi system was analyzed by immunoblot analysis of dCas9 production (Figure S5). The Nine Mile QpH1 plasmid encodes 8 Dot/Icm type IVB secretion substrates (Maturana et al., 2013). Knockdown of the ORFs encoding these proteins resulted in no defect in either ACCM‐D growth (Figure 4a) or in THP‐1 macrophage growth (Figure 4b) suggesting that they are dispensable for growth under these conditions. Targeted knockdown was examined by qRT‐PCR for one target for each type 4B secretion substrate ORF (Figure S6) and showed knockdown of cbuA0006, cbuA0013, cbuA0014, cbuA0015, cbuA0023, and cbuA0025 but not cbuA0016 or cbuA0034. Although cbuA0016, the downstream part of an operon with cbuA0015, presumably would also be knocked down in the cbuA0015 CRISPRi strain. Only one ORF, cbuA0027, displayed significant growth defects in both axenic media (Figure 5a and Figure S3) and THP‐1 cells (Figure 5b and Figure S4) with all CRISPRi target sequences tested. The targeted knockdown of cbuA0027 was confirmed by qRT‐PCR (Figure S7). Complementation of cbuA0027 CRISPRi knockdown was achieved using an aTc‐inducible codon‐optimized version of cbuA0027 (Figure 6a) that contained a modified DNA sequence in the region targeted by the cbuA0027 CRISPRi constructs (Figure 6b). Complementation resulted in the restoration of intracellular growth in THP‐1 macrophages (Figure 6c). The cbuA0027 ORF is located downstream of cbuA0028 forming a two‐gene operon (Figure 7a) (Mao et al., 2009; Mao et al., 2014). A structural‐based I‐Tasser analysis of CBUA0028 and CBUA0027 indicated that they are homologous to the HigB2 toxin and HigA2 antitoxin from Vibrio cholerae, respectively (Figure 7b,c). CBUA0028 displays 26% identity with HigB2 and conservation of the five amino acids (K49, R51, R64, Y82, and K84) previously shown to comprise the HigB2 active site (Hadzi et al., 2017). CBUA0027 has a 30% identity with HigA2 and displays a conserved helix‐turn‐helix motif that is essential for DNA binding (Park et al., 2020). These data suggested CBUA0028 (ToxP) and CBUA0027 (AntitoxP) function as a toxin and antitoxin, respectively.
FIGURE 3.
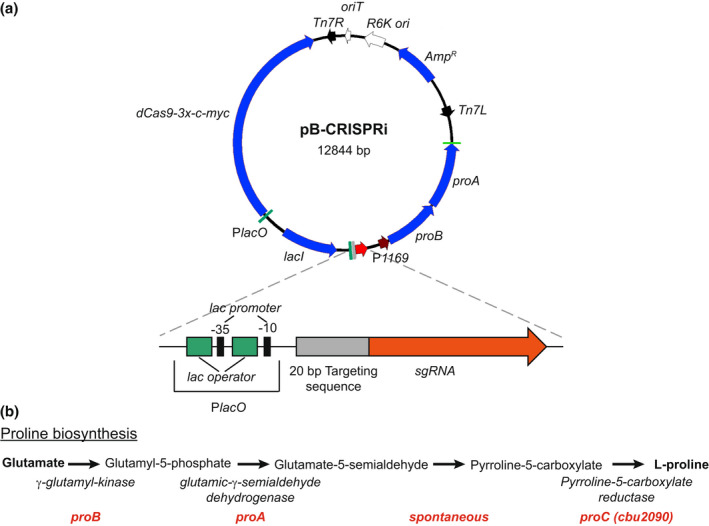
Components of the inducible Coxiella burnetii CRISPRi system. (a) Plasmid map of pB‐CRISPRi. Proline complementation was achieved by cloning the proBA operon from Legionella pneumophilia in front of the cbu1169 promoter (P1169). Inducible expression of dcas9 and sgRNA is controlled by the lac promoter (PlacO). The PlacO‐sgRNA region is enlarged and depicts two lac operators flanking the lac‐35 promoter sequence upstream of the 20 bp targeting sequence fused to sgRNA. (b) Schematic of the proline synthesis pathway. The genes required to convert glutamate to proline are depicted in red. C. burnetii encodes proC (cbu2090) but is missing proA and proB
FIGURE 4.
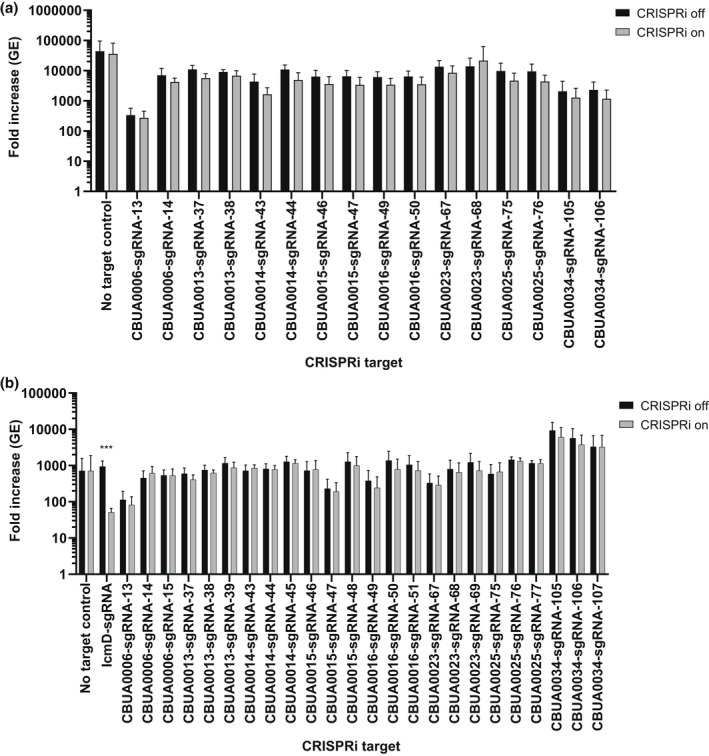
CRISPRi repression of QpH1 Dot/Icm subtrate expression does not affect axenic or THP‐1 macrophage growth. Replication is shown as a fold increase in Coxiella burnetii genome equivalents (GE) of Dot/Icm substrates CRISPRi strains after 6 days in (a) ACCM‐D or after a 5‐day infection of (b) THP‐1 macrophages in the absence (CRISPRi off) or the presence (CRISPRi on) of CRISPRi system inducer molecule IPTG. Replication of a no sgRNA target control was also examined as a negative control. CRISPRi repression of the essential icmD dot/Icm apparatus gene was used as a positive control in THP‐1 macrophages. Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (Student's t test, ***p < .001) compared with values for the CRISPRi off samples
FIGURE 5.
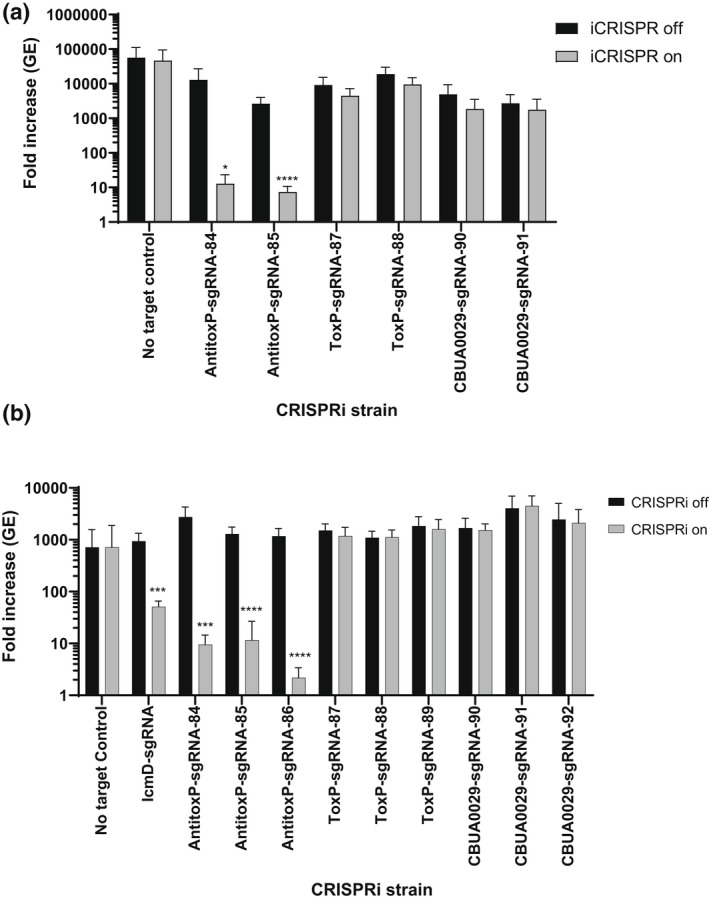
CRISRPi repression of antitoxP expression causes a severe growth defect in axenic media and THP‐1 macrophages. Replication is shown as a fold increase in Coxiella burnetii genome equivalents (GE) of CRISPRi strains targeting antitoxP, toxP, and cbuA0029 genes after 6 days in (a) ACCM‐D or after a 5‐day infection of (b) THP‐1 macrophages in the absence (CRISPRi off) or the presence (CRISPRi on) of CRISPRi system inducer molecule IPTG. Replication of a no sgRNA target control was also examined as a negative control. CRISPRi repression of the essential icmD Dot/Icm apparatus gene was used as a positive control in THP‐1 macrophages. Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (Student's t test, *p < .05, ***p < .001, and ****p < .0001) compared with values for the CRISPRi off samples
FIGURE 6.
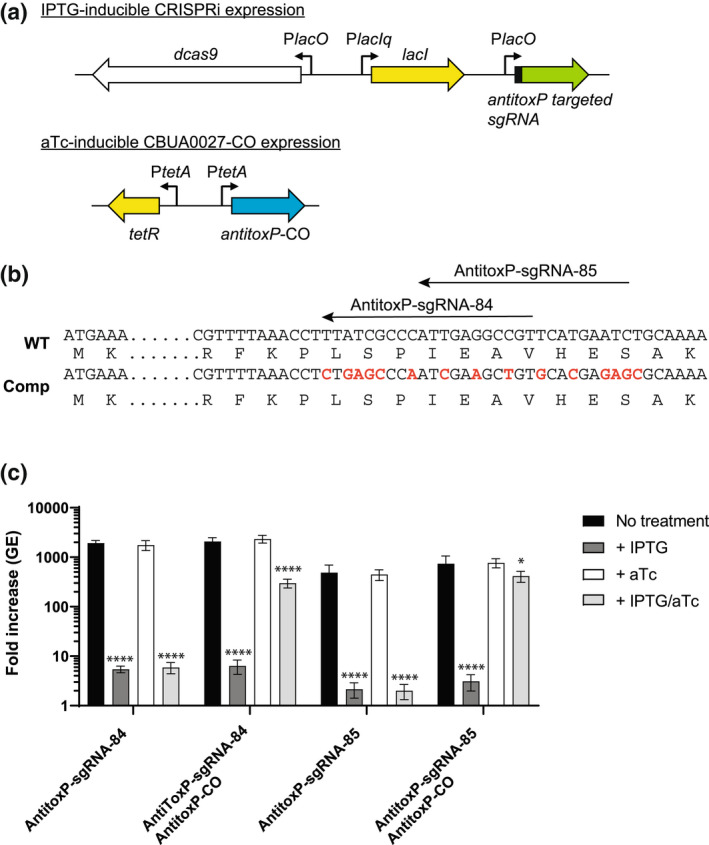
Complementation of antitoxP CRISPRi repression allows restoration of intracellular growth. (a) Schematics of the IPTG‐inducible antitoxP CRISPRi repression system and aTc‐inducible expression of the codon‐optimized antitoxP gene. In the presence of IPTG expression of dCas9‐3x‐c‐myc and antitoxP‐targeted sgRNA occurs from the Plac promoters. The presence of aTc expression of the codon‐optimized antitoxP gene occurs from the PtetA promoter. (b) Partial sequences of the wild‐type (WT) and codon‐optimized antitoxP gene are depicted. The location of the two antitoxP sgRNA targeting sequences is shown with arrows. Nucleotides that were changed are depicted in red. For toxP‐sgRNA‐84, 9 bp of the target sequence was changed and for toxP‐sgRNA‐85, 8 bp of the target sequence was changed. These changes did not affect the protein sequence. (c) Expression of antitoxP‐CO allows complementation of THP‐1 macrophage growth in antitoxP CRISPRi repression strains. Replication is shown as a fold increase in Coxiella burnetii genome equivalents (GE) of antitoxP CRISPRi strains with or without the antitoxP‐CO complement plasmid (pJB‐lysCA‐TetRA‐antitoxP‐CO) after a 5‐day infection of THP‐1 macrophages in the absence of any inducer (no treatment; CRISPRi off/complement off), presence of IPTG (CRISPRi on/complement off), presence of aTc (CRISPRi off/complement on), or presence of both IPTG and aTc (CRISPRi on/complement on). Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (one‐way ANOVA, *p < .05, ****p < .0001) compared with values for the no‐treatment samples
FIGURE 7.
The ToxP/AntitoxP TA modules retain essential regions of the Vibrio cholerae HigBA2 TA system. (a) Schematic of the toxP/antitoxP operon, showing their toxin–antitoxin gene orientation, and their corresponding DNA and amino acid sequences. The ToxP and AntitoxP amino acid sequences are depicted in red and blue. (b) I‐Tasser structural‐based alignment of ToxP and HigB2 toxin of V. cholerae. Secondary structure prediction is denoted in blue (C – coil, H – helix, S – strand). Identical residues are depicted with an asterisk (*), whereas similar residues are denoted as a single dot (.) or colon (:). The location of essential active site residues in HigB2 (K49, R51, R64, Y82, and K84) are boxed and conserved in ToxP. (c) I‐Tasser structural‐based alignment of AntitoxP and HigA2 antitoxin of V. cholerae. Secondary structure prediction is denoted in blue (C – coil, H – helix, S – strand). Identical residues are depicted with an asterisk (*), whereas similar residues are denoted as a single dot (.) or colon (:). The residues comprising the essential helix‐turn‐helix DNA‐binding motif in HigA2 are colored red. The predicted helix‐turn‐helix DNA‐binding motif in AntitoxP is highlighted by a red box and aligns with the same motif in HigA2
2.3. Overexpression of toxP inhibits the growth of C. burnetii
To ascertain whether ToxP functions as a toxin, toxP was cloned into the low‐copy miniTn7T transposon vector, whereby its expression was controlled by an aTc‐inducible promoter (Figure 8a; pMiniTn7T‐proBA‐TetRA‐cbuA0028). Expression of toxP using its own promoter was not possible in E. coli due to the toxic effects of ToxP, resulting in mutations to the cloned toxP ORF (data not shown). One mutation of note, resulted in a lysine to glutamic acid change at residue 79 in ToxP that had no toxic effect on E. coli growth (data not shown), presumably because K79 is predicted to be one of the active site residues in ToxP (Figure 7b). The pMiniTn7T‐proBA‐TetRA‐cbuA0028 expression vector was transformed into ΔQpH1, containing a clean background absent of the native toxP and antitoxP ORFs. The transformation was only possible using ΔQpH1 containing an IPTG‐inducible antitoxP construct (ΔQpH1/AntitoxP) (Figure 8b), with prior induction of antitoxP necessary for the survival of the pMiniTn7T‐proBA‐TetRA‐cbuA0028 transformants. The toxP ORF is conserved in all C. burnetii except genomic group V (Beare et al., 2006), the group containing the chromosomally integrated QpRS‐like sequences. Within this genomic group, the toxP ORF is N‐terminally truncated (Figure S8a) and the toxP promoter is missing while the complete antitoxP ORF is intact (Figure S8b). We decided to test if this truncated ORF (toxPG) could also function as a toxin (Figure 8c). The effect of toxP and toxPG overexpression was investigated by examining C. burnetii growth in THP‐1 macrophages in the presence and absence of the putative antitoxin AntitoxP (Figure 8d). Induction of antitoxP alone had no effect on C. burnetii growth. Overexpression of toxP resulted in a severe growth defect in THP‐1 macrophages, with an approximate two‐fold GE increase over a 5‐day infection (Figure 8d). This growth defect was alleviated by co‐expression of antitoxP. Overexpression of toxPG had no effect on C. burnetii growth in THP‐1 macrophages. In the absence of any treatment less growth was seen in the ΔQpH1/AntitoxP/ToxP strain suggesting that a background level of toxP expression was occurring (Figure 8d). This data show that toxP expression prevents C. burnetii growth in the absence of AntitoxP and suggests the N‐terminus of ToxP is essential to its function.
FIGURE 8.
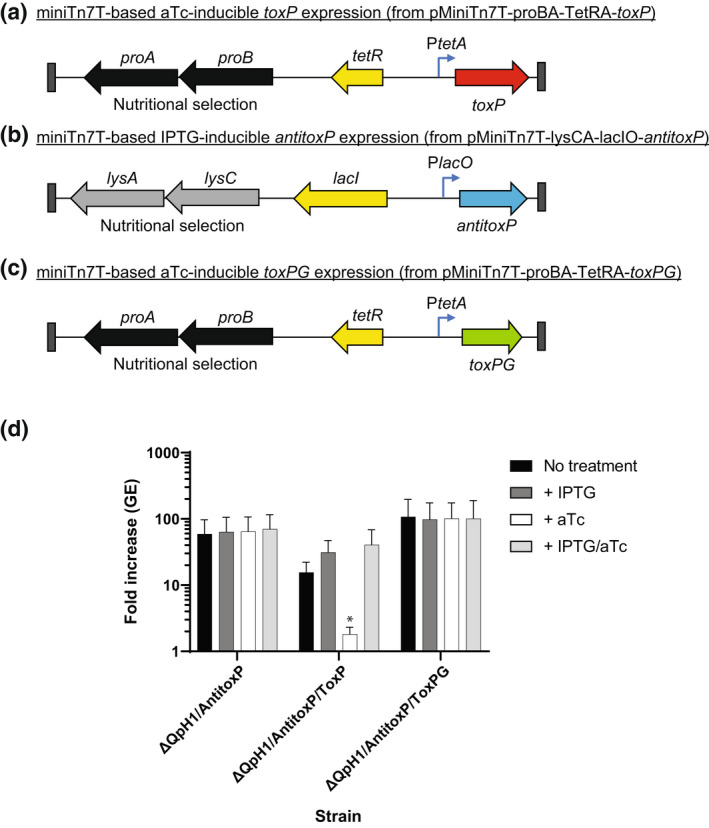
Expression of toxP in the absence of AntitoxP prevents Coxiella burnetii replication. Schematic of miniTn7T transposons designed to allow (a) aTc‐inducible expression of toxP (from pMiniTn7T‐proBA‐TetRA‐toxP) or (b) IPTG‐inducible expression of antitoxP (from pMiniTn7T‐proBA‐lacIO‐antitoxP) or (c) aTc‐inducible expression of the N‐terminally truncated version of toxP from G Q212 (toxPG) (from pMiniTn7T‐proBA‐TetRA‐toxPG). The ΔQpH1 mutant was first transformed with the pMiniTn7T‐lysCA‐lacIO‐antitoxP using the lysine‐based nutritional selection to integrate the IPTG‐inducible into the C. burnetii genome. The resulting strain ΔQpH1/AntitoxP was treated with IPTG to induce antitoxP expression and then transformed with miniTn7T transposons containing either aTc‐inducible toxP or toxPG expression using proline‐base nutritional selection to create ΔQpH1/AntitoxP/ToxP and ΔQpH1/AntitoxP/ToxPG, respectively. (d) Expression of toxP caused a severe growth defect in THP‐1 macrophages that could be alleviated by co‐expressing antitoxP. Replication is shown as a fold increase in C. burnetii genome equivalents (GE) of the ΔQpH1/AntitoxP strain with or without the ToxP or ToxPG miniTn7T transposons after a 5‐day infection of THP‐1 macrophages in the absence of any inducer (no treatment), presence of IPTG, presence of aTc, or presence of both IPTG and aTc. Results are expressed as the means of results from two biological replicates from three independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (one‐way ANOVA, *p < .05) compared with values for the no‐treatment samples
2.4. ToxP production prevents the translation of coproduced proteins
The HigB2 toxin has a RelE‐like active site and when bound to the ribosome A site cleaves translating mRNA molecules without a preference for specific sequences (Hadzi et al., 2017). Due to the conservation of the HigB2 active site residues in ToxP, we wanted to explore whether ToxP is functioning in the same manner. To investigate this phenomenon, a T7 polymerase‐based cell‐free expression system containing a control gene expression plasmid, expressing the type 4B secretion substrate cbu0665 (pEXP1‐cbu0665), and decreasing amounts of toxP expression plasmid (pET28a‐cbuA0028) as DNA templates was used. Production of N‐terminally XpressT‐tagged CBU0665 and C‐terminally V5‐tagged ToxP protein was then examined by immunoblot (Figure 9a,b). The results show a dose‐responsive increase in the level of CBU0665 when decreasing amounts of ToxP were present. Co‐production of AntitoxP rescued the effect of ToxP on CBU0665 production. These data confirm that ToxP prevents the production of an unrelated protein (CBU0665) and that AntitoxP can alleviate this effect, results that are consistent with ToxP and AntitoxP being a toxin and antitoxin, respectively.
FIGURE 9.
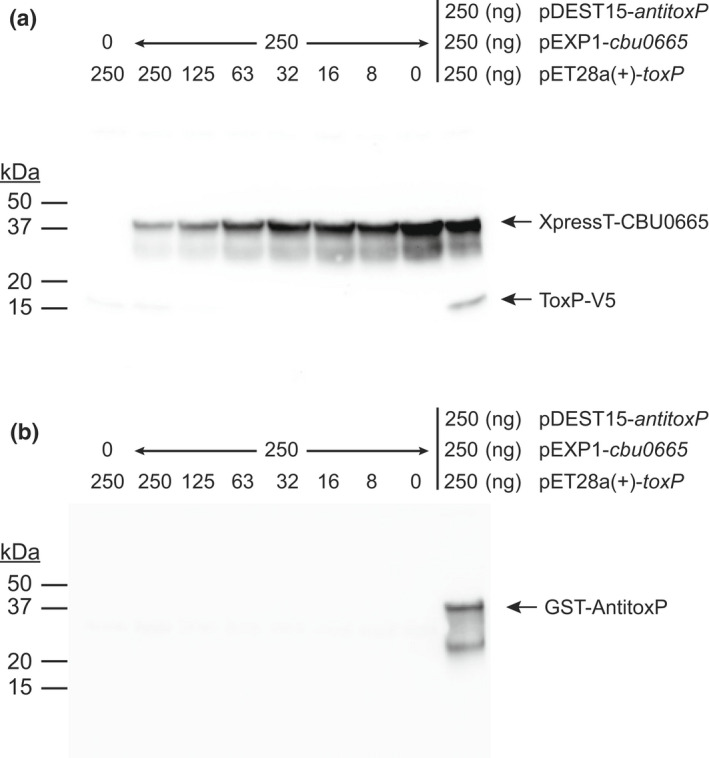
Production of ToxP inhibits protein translation. Cell‐free in vitro transcription and translation (IVTT) reactions were incubated with pEXP1‐cbu0665 (250 ng) and decreasing amounts of pET28a(+)‐toxP (250 ng – 8 ng) as a DNA template. Control IVTT reactions were conducted with either only pEXP1‐cbu0665 (250 ng) or pET28a(+)‐toxP (250 ng) plasmids or with all three plasmids together (250 ng of pEXP1‐cbu0665, pET28a(+)‐toxP, and pDEST15‐antitoxP) as a DNA template. (a) Production of CBU0665 (42.5 kDa) and ToxP (18 kDa) was detected by immunoblot of cell‐free IVTT lysates probed simultaneously with anti‐XpressT and anti‐V5 antibodies, respectively. (b) Production of AntitoxP (40.1 kDa) was detected by immunoblot of cell‐free lysates probed with anti‐GST antibody. A dose–response affect was seen on CBU0665 production when decreasing amounts of ToxP were present indicating ToxP inhibited translation of CBU0665. Translation inhibition of CBU0665 production by ToxP was alleviated when AntitoxP was present
2.5. The AntitoxP antitoxin directly binds the ToxP toxin
The HigB2 and HigA2 proteins are part of the RelE superfamily of type II TA systems (Christensen‐Dalsgaard & Gerdes, 2006). In type II TA systems, the antitoxin binds to the toxin to prevent the degradation of ribosome‐associated mRNA (Fraikin et al., 2020). To confirm that ToxP and AntitoxP are part of a type II TA system, pulldowns with N‐terminally tagged AntitoxP were investigated. Cell‐free expression was used to produce GST‐AntitoxP or simultaneously produce GST‐AntitoxP and ToxP‐V5 or XpressT‐CBU0665 (Figure S9). Cell‐free extracts were then incubated with GST‐binding beads to extract proteins bound to GST‐AntitoxP and these complexes were eluted from the beads with a reduced glutathione buffer. Immunoblots of the pulldowns indicated specific binding between AntitoxP and ToxP (Figure 10), while the control pulldown with CBU0665 displayed no binding with AntitoxP. No ToxP protein was seen in the GST pulldown elutions containing ToxP alone. Together, these data indicate that AntitoxP directly binds ToxP confirming its function as an antitoxin.
FIGURE 10.
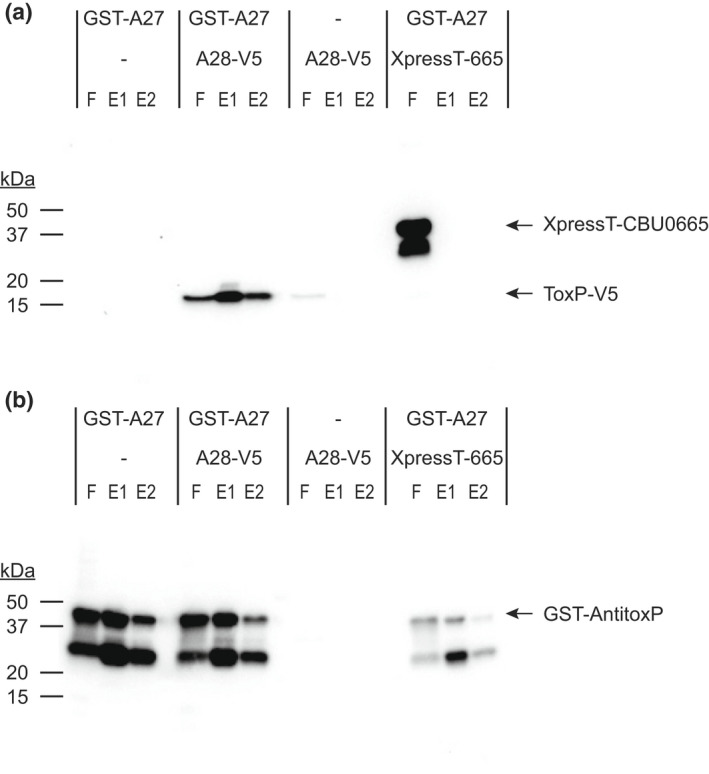
AntitoxP binds directly to ToxP. Cell‐free IVTT reactions containing GST‐tagged AntitoxP (GST‐A27), GST‐A27 and V5‐tagged ToxP (A28‐V5), A28‐V5 or GST‐A27, and XpressT‐tagged CBU00665 (XpressT‐665) (Figure S9) were incubated with GST‐affinity beads. After incubation beads were washed and bead‐bound proteins were eluted using a reduced glutathione buffer. Flow‐through (F), elution 1 (E1), and elution 2 (E2) fractions were analyzed by immunoblot probed simultaneously with (a) anti‐XpressT and anti‐V5 or (b) anti‐GST. GST‐AntitoxP pulldown of only ToxP‐V5 was observed
2.6. AntitoxP and the ToxP/AntitoxP complex bind the toxP promoter
RelE‐like antitoxins form a complex with their cognate toxin and this complex while preventing toxin binding to the ribosome and subsequent mRNA cleavage, also binds to the TA promoter to repress expression of the TA system (Jurenas et al., 2022; Overgaard et al., 2009). RelB alone has also been found to bind the relBE promoter (Overgaard et al., 2008). To investigate the binding of AntitoxP and AntitoxP/ToxP complex with the toxP promoter (PtoxP) electrophoretic mobility shift assays (EMSA) were performed. Elutions obtained from the AntitoxP and AntitoxP/ToxP pulldowns were incubated with labeled PtoxP or control groES promoter and these protein/DNA complexes were analyzed. AntitoxP and the AntitoxP/ToxP complex both resulted in a mobility shift when in the presence of PtoxP but not the control groES promoter (Figure 11 and Figure S10) indicating specific DNA binding with PtoxP.
FIGURE 11.
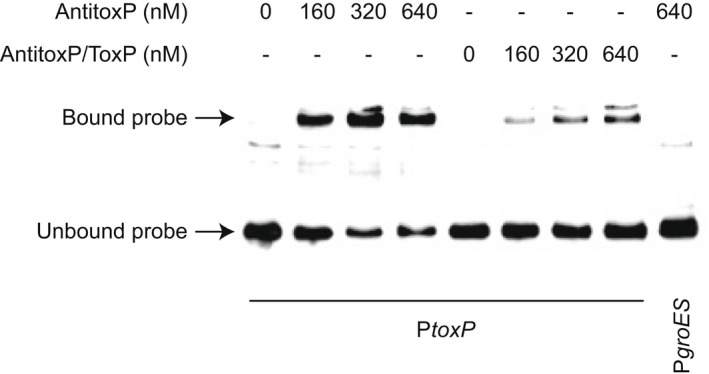
AntitoxP and AntitoxP/ToxP complex bind to the toxP promoter. EMSAs show interactions between biotin‐labeled toxP promoter (PtoxP) and increasing concentrations of purified AntitoxP or AntitoxP/ToxP complex. Biotin‐labeled PgroES with AntitoxP (640 nM) was included as a negative control. The location of bound and unbound probe is depicted by arrows
2.7. toxP expression is repressed in the presence of ToxP/AntitoxP
Canonical type II TA systems have a promoter‐toxin–antitoxin operonic arrangement, whereby the C‐terminal region of the antitoxin overlaps the toxin, which presumably allows for a molar excess of antitoxin via translational coupling. However, noncanonical type II systems, such as mqsRA, display a promoter‐toxin–antitoxin operonic organization, whereby the N‐terminus of the antitoxin overlaps the toxin, and in this configuration (Fraikin et al., 2019), one would expect that there would be a molar excess of toxin. TA systems like mqsRA alleviate this problem using promoters present within the toxin gene (Fraikin et al., 2019). The toxP/antitoxP TA system displays a noncanonical promoter‐toxin–antitoxin arrangement (Figure 7a). We predicted that an additional promoter(s) would be present within toxP that would allow PtoxP‐independent expression of antitoxP. The motif prediction tool in Geneious Prime was used to predict potential sigma 70 binding sites. Two potential antitoxP promoters (P2 and P3) were identified and compared with PtoxP (P1) (Figure 12a and Figure S11), with the P3 promoter also still present in the G Q212 toxP sequence (Figure S11). To test if these promoters are transcriptionally active in C. burnetii fragments containing either P1 (PA28‐F1), both P2 and P3 (PA27‐F1) or just the P3 region (PA27‐F2) were fused to the red fluorescent protein mScarlet‐i (Figure 12b) and a single copy inserted into wild‐type NMII and ΔQpH1 using the miniT7nT transposon system (Beare et al., 2011). Promoter activity was analyzed by measuring fluorescence on days 3, 5, 7, and 14 to compare promoter strength in LCVs and SCVs and between NMII and ΔQpH1. Expression from PtoxP was only detected in ΔQpH1 and was highest at Day 14, indicating in the absence of ToxP and AntitoxP the PtoxP promoter becomes dysregulated. No promoter activity was seen with the putative antitoxP promoters suggesting that some other mechanism regulates the levels of ToxP and AntitoxP in C. burnetii to maintain a balance between toxin and antitoxin.
FIGURE 12.
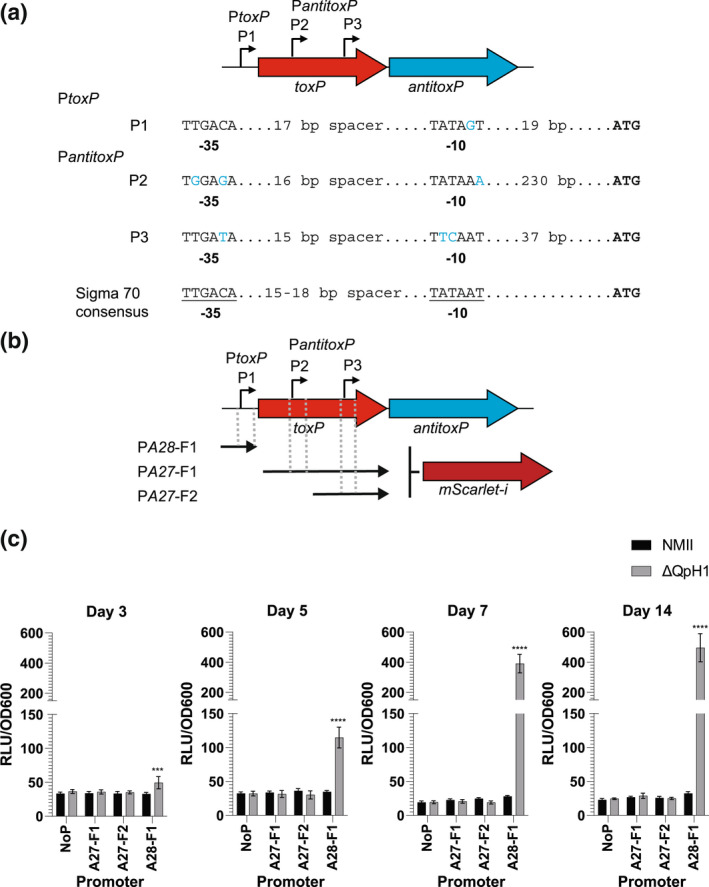
Identification of potential antitoxP promoters within the toxP coding sequence. (a) Schematic of the toxP/antitoxP operon and location of toxP and antitoxP promoters. The sequence of each predicted promoter (−35 and −10 regions) was compared with the consensus sigma 70 promoters, and deviations to this sequence are depicted in blue. (b) The DNA fragments used to create transcriptional fusions to mScarlet‐i and their location in relation to antitoxP and toxP are depicted. (c) The activity of the transcriptional fusions in NMII and ΔQpH1 strains. The axenic medium was inoculated with Coxiella burnetii and incubated for 3, 5, 7, or 14 days then fluorescence was measured. Absorbance at OD600 was used as a proxy for C. burnetii growth and used to normalize bacteria fluorescence. Results are expressed as the means of results from two biological replicates from four independent experiments. Error bars indicate the standard deviations from the means, and asterisks indicate a statistically significant difference (two‐way ANOVA, ***p < .001, ****p < .0001)
3. DISCUSSION
All C. burnetii strains contain an autonomously replicating plasmid (QpH1, QpDG, QpDV, or QpRS) or IPS (Beare et al., 2006) suggesting that ORFs encoded by these DNA sequences are necessary for C. burnetii pathogenesis. In this study, we investigated the role of the ORFs found on QpH1. To initiate this research, we first cured the native QpH1 plasmid from NMII using a shuttle vector containing the QRM. Incompatibility between the shuttle vector QRM and the QRM on QpH1 and the use of a new nutritional‐based selection for the shuttle vector in C. burnetii would cure NMII of QpH1 to generate ΔQpH1. Based on the known QpH1 ORF content, the resulting strain was predicted to grow normally in axenic media. Plasmid incompatibility was recently used as a method to cure NMII of the QpH1 plasmid (Luo et al., 2021). Growth analysis of the ΔQpH1 strain found no growth defect in axenic media but the presence of QpH1 was essential for growth in Vero cells. Consistent with this finding, transposon mutants located in the parB and repA ORFs of the C. burnetii QpH1 plasmid have strong replication defects in Vero cells (Martinez et al., 2014). A recent study also reported the curing of QpH1 from NMII and showed that QpH1‐deficient bacterium had a severe growth defect in murine bone marrow‐derived macrophages, although no growth defect was observed in Buffalo green kidney cells and growth defects in THP‐1 macrophages were inocula dependent (Luo et al., 2021). Due to the sequence conservation of the QRM ORFs within the four known C. burnetii plasmids, plasmid curing of QpDG, QpDV, and QpRS (Beare et al., 2009) is possible using our shuttle vector and may give insight into the role of these plasmids in the pathogenesis of different isolates, such as the K (Q154) and Dugway isolates that have unique sets of Dot/Icm substrates (Maturana et al., 2013).
Site‐specific gene deletion in C. burnetii remains a challenging and lengthy process and to date has only resulted in 24 mutants (Beare et al., 2014; Clemente et al., 2018; Colonne et al., 2016; Cunha et al., 2015; Friedrich et al., 2021; Larson et al., 2013, 2015, 2019; Pechstein et al., 2020; Sandoz et al., 2021; Schäfer et al., 2020; Vallejo Esquerra et al., 2017) (Long et al., 2021; Moormeier et al., 2019; Stead et al., 2018), two of which are located on QpH1 (Colonne et al., 2016). To overcome the difficulty in creating large numbers of individual gene deletions, we developed a miniTn7T‐based IPTG‐inducible CRISPRi system to knock down ORFs in C. burnetii. This system integrates as a single copy in the C. burnetii chromosome and selection for transformants is modulated using a new nutritional‐based marker for proline. The dcas9 we employed originated from Streptococcus pyogenes and recognizes the nGG protospacer adjacent motif (PAM) site (Peters et al., 2019). Previous attempts to use the dCas9 from Staphylococcus aureus and Streptococcus thermophilus were unsuccessful (data not shown). The number of S. pyogenes dCas9 PAM sites in the NMII genome is substantial, with 197,777 and 3073 nGG sites present in the chromosome and QpH1 plasmid, respectively. The CRISPRi system was first tested by knocking down the production of the host cell essential Dot/Icm protein IcmD (Figure S1) and then by simultaneously knocking down both IcmD and the small SCV‐specific protein ScvA production (39 amino acids) (Figure S2). This result indicated that the inducible CRISPRi system could target genes essential for intracellular infection and small SCV‐specific genes. Constitutive CRISPRi knockdown of the Dot/Icm substrate CirB in C. burnetii was recently described (Fu et al., 2022). This shuttle vector‐based system adopts an always‐on approach using the cbu1169 promoter to drive both sgRNA and dcas9 expression. While this system is functional, it is limited for use on nonessential genes for growth in axenic media. During testing of our CRISPRi system, we found that expression of large amounts of dCas9 affected growth of C. burnetii. Also, bacteria containing knocked down essential genes would eventually start to replicate due to mutation of dcas9, the inducible promoter or sgRNA sequences or because of secondary compensatory mutations elsewhere in the genome (data not shown), thus becoming inert to further CRISPRi induction. The benefit of our inducible CRISPRi system is the ability to knockdown genes at any time during the bacterial growth cycle, target essential genes, and multiple genes at once and to function in both axenic media and infected host cells.
Complementation of gene deletions is a marker of Koch's molecular postulates (Falkow, 1988). We sought to fulfill these postulates for CRISPRi repressed genes. Complementation of CRISPRi knockdowns in C. burnetii was achieved using aTc‐inducible expression of a codon‐optimized version of the targeted gene in trans (Figure 6c and Figure S1), whereby silent mutations were made in the 20 bp target region and/or PAM sequence. Complementation of CRISPRi knockdown has also been achieved in Chlamydia trachomatis, where the complementing incA gene was transcriptionally fused 3′ to the aTc‐inducible dcas9 (Ouellette et al., 2021). In this case, the CRISPRi target against incA was in the promoter region and so dCas9 would not bind to the plasmid‐based complement. A recent study by Brockett et al. (2021) reported that transcriptional fusion of a modified Chlamydial bacA gene, containing silent mutations to the CRISPRi target region, could complement CRISPRi knockdown of the chromosomal bacA gene. Together, these data indicate that CRISPRi repression can be complemented by simultaneously expressing a codon‐optimized version of the knocked down gene.
The Dot/Icm type IVb system is essential for C. burnetii virulence (Beare et al., 2011; Carey et al., 2011) and many of its substrates are required for bacteria growth and CCV biogenesis (Friedrich et al., 2021; Larson et al., 2013, 2015; Martinez et al., 2014; Newton et al., 2014; Weber et al., 2013). CRISPRi targeting of each individual plasmid ORFs found no effect of knockdown of the Dot/Icm substrates located on QpH1. The absolute conservation of cbuA0013 and cbuA0023 in C. burnetii suggests that these genes are important for pathogenesis. It is possible that the products of these genes are redundant and their loss in function is alleviated by other Dot/Icm effectors (Isaac & Isberg, 2014; Luo & Isberg, 2004). Incomplete knockdown of the genes may also result in partial production of the encoded product. This has been observed in our lab for other CRISPRi targeted genes and can be alleviated by co‐expressing multiple targets to the same gene (data not shown). CRISPRi silencing can also have unexpected results due to off‐targeting of the dCas9‐sgRNA complex to other regions of the genome (Zhang et al., 2021). In fact, knockdown of cbuA0021 resulted in off‐target effects on cbu0334 (thiDE) expression, which may have affected growth in axenic media but not growth in THP‐1 cells, and possibly cbu0055 (ubiA) which may have affected growth in THP‐1 cells (Figure S12). The thiDE gene products are required for thiamine biosynthesis (Liu et al., 2022). Mutants of thiDE have significant growth defects in media grown bacteria, which can be restored by adding exogenous thiamine (Liu et al., 2022). Hence, reduction in cbu0334 expression may prevent thiamine production in axenic media but in THP‐1 macrophages the bacteria can transport thiamine from the host. UbiA is part of a superfamily of intramembrane prenyltransferases that have roles in cellular respiration and antioxidation (Li, 2016). Therefore, it is possible that off‐targeting effects on cbu0055 may have a greater effect within the toxic environment of the THP‐1 macrophage lysosome than in axenic media.
Using the newly developed inducible CRISPRi system, we identified a TA system on QpH1 encoded by toxP and antitoxP. Historically TA systems were identified as a two‐gene module that was associated with the maintenance of conjugative plasmids (Gerdes et al., 1986; Ogura & Hiraga, 1983). TA systems are ubiquitous throughout free‐living prokaryotes and have been found on both chromosomal and extrachromosomal genetic elements (Schuster & Bertram, 2013). TA systems can be classified into eight different types, and in most instances, the antitoxin is 5′ in the toxin module (Jurenas et al., 2022; Song & Wood, 2020). Toxin modules have a range of cellular effects including impairing degrading RNAs and disrupting translation, disrupting cell wall structure, inducing metabolic stress and DNA replication (Jurenas et al., 2022). Antitoxins are generally either RNA or protein molecules that abrogate the effects of the toxin modules (Jurenas et al., 2022). Although TA systems are widespread in free‐living prokaryotes, they are generally not found in many host‐associated bacterium including Chlamydia species and Borrelia burgdorferi (Pandey & Gerdes, 2005). TA two‐gene modules have been identified in several Rickettsia species, while in other intracellular bacteria such as Orientia tsutsugamushi, Wolbachia species, Ehrlichia chaffeensis, and Anaplasma species only orphan toxin and antitoxin genes are present (Audoly et al., 2011; Botelho‐Nevers et al., 2012; Socolovschi et al., 2013).
The ToxP/AntitoxP TA system is the first toxin–antitoxin identified and characterized in C. burnetii. This TA module is both sequence and structurally homologous to V. cholerae HigBA2, a type II TA system and member of the RelE superfamily. Type II toxins are most often ribonucleases that either cleave‐free mRNA, such as HicA and MazF (Christensen et al., 2003; Jorgensen et al., 2009) or as with HigBA2 and the RelE superfamily, act by cleaving mRNAs in a ribosome‐dependent manner (Hurley & Woychik, 2009; Pedersen et al., 2003). The data presented here are consistent with other type II TA systems whereby the antitoxin directly binds its cognate toxin (Overgaard et al., 2009) (Figure 10), the toxin prevents growth in the absence of its cognate antitoxin (Heaton et al., 2012) (Figure 8), and toxin production inhibits protein synthesis, which can be restored in the presence of its antitoxin (Schureck et al., 2016) (Figure 9). The toxP/antitoxP TA module displays a noncanonical “reverse” gene orientation that is seen in other TA systems including higBA, mqsRA, hicAB, and rnlAB, where the toxin is upstream of the antitoxin (Deter et al., 2017). In the canonical gene orientation, bicistronic transcription of the TA module allows a molar excess of the antitoxin through translational coupling (Fraikin et al., 2020). Noncanonical TA systems require different mechanisms to achieve a molar excess of antitoxin including additional promoters inside the toxin gene coding sequence (Fraikin et al., 2019; Otsuka et al., 2010). Consistent with rnlB and mqsA antitoxin expression, internal promoter(s) were identified within the ToxP toxin coding sequence (Figure 12). Interestingly, transcriptional activity was not seen from these putative promoters in C. burnetii under the conditions tested (Figure 12). Previous data from both microarray (Sandoz et al., 2014) and RNAseq experiments (Beare et al., 2014) carried out in axenic media indicated that the level of toxP transcript is higher than antitoxP. However, mass spectrometry data from C. burnetii grown in axenic media showed that at day 4, more AntitoxP (normalized spectral count = 28.27) was detected relative to ToxP (normalized spectral count = 11.86) (Beare et al., 2014) and that the amount of AntitoxP increased as the bacteria entered stationary phase (Sandoz et al., 2014). Together, these data indicate a disconnect between antitoxP expression and AntitoxP protein production but show that an excess in AntitoxP is present within C. burnetii. Expression from PtoxP was detected but only in ΔQpH1 (Figure 12). This data indicated that deletion of the QpH1 plasmid alleviates the transcriptional control of the ToxP/AntitoxP complex on the PtoxP promoter, consistent with results observed with the mqsR promoter activity in a ΔmqsRA mutant (Fraikin et al., 2019). CRISPRi repression of antitoxP expression is in line with the toxin–antitoxin molar excess model, whereby repression of antitoxP expression will result in a molar excess of ToxP and toxic effects to the bacterial cell (Figures 5, 6, 8 and 9). Restoration of AntitoxP production by complementation alleviated the effects of CRISPRi repression (Figures 6, 8 and 9). Using the data presented here, we developed a model for the ToxP/AntitoxP toxin–antitoxin system (Figure 13).
FIGURE 13.
Putative model of ToxP/AntitoxP TA system. The toxP/antitoxP operon has a nonconical “reverse” TA gene organization. The first gene encodes the putative mRNA interferase and corepressor toxin ToxP (red) and the second gene encodes the AntitoxP antitoxin. The location of the toxP promoter (PtoxP) is depicted upstream of toxP. Consistent with the HigB2 toxin from V. cholerae, the ToxP toxin is predicted to interact with the ribosome A site, where it would cleave mRNA and prevent the translation from the corresponding mRNA. The AntitoxP antitoxin binds ToxP, thereby preventing translation inhibition, and this complex can then bind to the PtoxP promoter resulting in repression of transcription from PtoxP. AntitoxP also binds PtoxP in the absence of ToxP. Gray arrows represent interactions between components of the model. Black arrows represent the production of protein from toxP and antitoxP
CBU1490 is identified as an addiction molecule antidote protein and its crystal structure has been solved (Franklin et al., 2015). Recently, CBU1490 was found to be structurally homologous to the Mycobacterium tuberculosis HigA3 antitoxin (Park et al., 2020). Based on the finding of another potential antitoxin in C. burnetii, we mined the genome for other putative toxin and antitoxins. In addition to the plasmid‐based ToxP/AntitoxP TA system, ten additional Type II TA modules were found on the C. burnetii chromosome (Figure S13, Table 1). This analysis identified CBU1491 as the toxin module for CBU1490. Interestingly, over half of these TA systems adopted the noncanonical gene orientation, each containing potential sigma 70 promoters in their cognate toxin coding sequence. Six TA modules were conserved in all C. burnetii suggesting that they are important for C. burnetii pathogenesis. Both CBU0285 and AntitoxP antitoxins are conserved throughout all C. burnetii, indicating that while their cognate toxin modules are missing in some strains, these antitoxin modules may be necessary for other functions outside of their role as an antitoxin. Consistent with this hypothesis was the ability of AntitoxP to bind DNA in the absence of its cognate toxin (Figure 11) and reports showing antitoxins acting as transcription factors that control other regulons (Kim et al., 2010; Lin et al., 2013). Transposon mutants within toxin genes cbu0007a and cbu1992 or cbu0644 result in strong or mild replication defects in Vero cells suggesting that their loss or disruption of their cognate antitoxin expression is important for intracellular survival, while himar1 mutant of cbu1294 had no growth defects (Table 1) (Martinez et al., 2014; Newton et al., 2014). Furthermore, mutation of the cbu0007, cbu0285, or cbu2084 antitoxins also display a strong replication defect in Vero cells presumably because of their cognate toxins (Martinez et al., 2014). The large number of TA systems in C. burnetii is unusual for a host‐associated bacterium (Pandey & Gerdes, 2005) but fits the hypothesis that pathogenic bacteria have a larger number of TA systems (Georgiades & Raoult, 2011).
TABLE 1.
TA systems in Coxiella burnetii
| TA locus | TA family a | Toxin | Antitoxin | Conservation in C. burnetii b | himar1‐mutant phenotypes c | Expression in Vero cells d |
|---|---|---|---|---|---|---|
| cbu0007a‐cbu0007 e | BrnTA | BrnT | BrnA | Both deleted in GGIV‐a | cbu0007a ‐ SRD, MID:cbu0007 ‐ SRD | Highest late in infection |
| cbu0283‐cbu0284/cbu0285 e | RelE/ParE | RelE‐like | H‐T‐H transcriptional regulator | cbu0283 deleted/cbu0284 truncated in GGIV‐a and GGIV‐b | cbu0284 (stop) ‐ SRD:cbu0285 ‐ SRD, MID | No change |
| cbu0645/cbu0644 | RelE/ParE | RelE‐like | Phd/YefM | Yes | cbu0664 ‐ MRD | Highest late in infection |
| cbu1238/cbu1237 | VapBC | VapC‐like | CopG family | Yes | – | Highest early in infection |
| cbu1295/cbu1294 | RelE/ParE | VapC‐like | Phd/YefM | Yes | cbu1294 ‐ NRD | Highest late in infection |
| cbu1304/cbu1303 e | RatAB | RatA‐family | RnfH family | Yes | – | Highest early in infection |
| cbu1491/cbu1490 e | HigBA | RelE/ParE family | HigA | Yes | – | No change |
| cbu1692/cbu1691 e | HigBA | RelE/ParE family | HigA | Yes | – | No change |
| cbu1991/cbu1992 | RelE/ParE | RelE | RelE | cbu1991 deleted/cbu1992 truncated in GGII‐a | cbu1992 ‐ SRD | Highest early in infection |
| cbu2084/cbu2085 e | BrnTA | BrnT | BrnA | Both were deleted in GGII‐b | cbu2084 ‐ SRD | No change |
| cbuA0028/cbu0027 e | HigBA | HigBA2 | HigA2 | cbuA0028 truncated in GGV | – | Highest late in infection |
Closest homology to known TA systems.
Conservation across the 27 genome sequence in the NCBI blastn metablast search when using the standard database nr and Coxiella burnetii (Taxid:777) organism.
himar1‐mutant data from Martinez et al. (2014) and Newton et al. (2014), SRD ‐ strong replication defect, MRD ‐ mild replication defect, NRD ‐ no replication defect, MID ‐ mild internalization defect. (stop) indicated himar1 mutant is located in the genes stop codon.
Microarray data from Sandoz et al. (2016) comparing gene expression from days 5, 7, 14, and 21 infections in Vero cells to day 3.
TA systems with a non‐conical module orientation.
Type II TA systems have a range of functions including maintenance of plasmids, virulence, and pathogenicity and bacterial stress responses (Kamruzzaman et al., 2021). It is intriguing to speculate on their role in C. burnetii pathogenesis. ToxP/AntitoxP have an obvious role in maintenance of the endogenous plasmid found in most C. burnetii strains. However, the absolute conservation of antitoxP, including the conservation of its promoter internal to toxP, and its ability to bind DNA suggests that there are other roles for this TA system in C. burnetii survival. The toxP/antitoxP TA system is expressed highest in late stages of Vero cell infection (Table 1) (Sandoz et al., 2016) suggesting that it is important for late‐stage infection and possibly for transition from LCV to SCV, which requires a large redefinition of protein content, metabolism, and cellular structure of the bacterium (Coleman et al., 2004, 2007; Omsland et al., 2009; Sanchez et al., 2021). Similar roles for TA systems have been associated with reduced metabolism and persister cell formation upon stress conditions in other bacteria (Wang & Wood, 2011). Three other C. burnetii TA systems also have higher expression levels late in Vero cell infection (Table 1) (Sandoz et al., 2016) and may also aid in LCV to SCV transition or SCV persistence, while three TA systems have higher expression levels early in infection and may play a role in the SCV to LCV transition, including cbu1238/cbu1237 which appears to be regulated by the cbu0712 (gacA) regulator (data not shown, Wachter et al., 2022). Four additional C. burnetii TA systems were expressed throughout the infection cycle (Table 1) (Sandoz et al., 2016) suggesting that they may be important for responding to the harsh environmental conditions present in the C. burnetii CCV (Gutierrez & Colombo, 2005; Heinzen et al., 1999). Consistent with this hypothesis are findings that show TA systems also mediate several stress responses, including oxidative and nutritional stresses (Chan et al., 2018; Christensen et al., 2001, 2003).
In summary, this study utilized a newly developed inducible CRISPRi system and two new nutritional selection systems to examine the role of the QpH1 plasmid and its gene content in C. burnetii pathogenesis. The data presented here identified and characterized the first TA system in C. burnetii, encoded by the QpH1 plasmid, and explained why most C. burnetii strains still contain an autonomously replicating plasmid. The ToxP/AntitoxP TA system is one of the 11 TA systems discovered in the C. burnetii genome and suggests an important role for TA systems in Coxiella pathogenesis.
4. EXPERIMENTAL PROCEDURES
4.1. Bacterial strains and mammalian cell lines
Bacterial strains used in this study are listed in Table S1. The C. burnetii Nine Mile phase II (RSA439, clone 4, NMII) strain was used for this work. NMII C. burnetii and genetic transformants were axenically cultured in ACCM‐D as previously described (Sandoz et al., 2014), with the addition of 4‐hydroxyphenylpyruvic acid (4‐HPP) for tyrosine‐based nutritional selection. For storage, bacteria were pelleted following 7 days of growth, washed three times in phosphate‐buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4), and then suspended in a freezing medium (ACCM‐D containing 10% dimethyl sulfoxide) and frozen at −80°C. E. coli Stellar (Takara) and E. coli PIR2 (Invitrogen) cells were used for recombinant DNA procedures and cultivated in Luria‐Bertani (LB) broth or terrific broth. THP‐1 macrophages (TIB‐202; ATCC) and African green monkey kidney (Vero) cells (CCL‐81; ATCC) were maintained in an RPMI‐1640 medium containing 10% FBS at 37°C and 5% CO2. C. burnetii replication in host cells or in ACCM‐D was measured by quantitative PCR of genome equivalents (GE) as previously described (Howe et al., 2010; Omsland et al., 2009) using a probe specific to groEL.
4.2. DNA amplification and plasmid construction
Plasmids used in this study are listed in Table S1. Oligonucleotides were purchased from Integrated DNA Technologies and are listed in Table S2. PCRs were carried out using AccuPrime Pfx or Taq (Invitrogen). PCRs purified using the Nucleospin gel and PCR clean‐up kit were cloned into plasmid backbones digested with restriction enzymes (NEB) using the In‐Fusion HD cloning system (Takara). In‐Fusion reactions were transformed into E. coli Stellar cells (Takara) for non‐R6K origin of replication‐based vectors or PIR2 cells (Invitrogen) for R6K origin of replication plasmids. The sgRNA dsDNA fragments were cloned into plasmid backbones using the NEBridge Golden Gate Assembly kit (BsaI‐HF®v2) Construction of all the plasmids used in this study is detailed in File S1.
4.3. Transformation and selection of C. burnetii transformants
C. burnetii grown in ACCM‐D was transformed by electroporation as described previously (Omsland et al., 2011). For transformation with pMiniTn7T or pB‐CRISPRi vectors, bacteria were also co‐electroporated with pTnS2::1169 P ‐tnsABCD as previously described (Beare et al., 2011). Selection of tyrosine‐complemented strains was achieved using ACCM‐D minus tyrosine and additional 4‐HPP. Selection of arginine‐, lysine‐, and proline‐complemented strains was carried out using ACCM‐D minus arginine, lysine, or proline, respectively. For arginine selection, citrulline was also added.
4.4. Immunofluorescence microscopy
Vero cells were fixed with 4% paraformaldehyde in phosphate‐buffered saline (PBS) for 30 min at room temperature and simultaneously permeabilized and blocked for 30 min with 0.1% Triton X‐100 pLus 1% BSA. Antibodies (3–5 μg/ml) were diluted in Triton buffer and samples stained for 30–60 min. Cells were stained for indirect immunofluorescence as previously described (Howe et al., 2003). Rabbit anti‐C. burnetii serum and a mouse monoclonal antibody directed against the lysosomal marker CD63 (LAMP3) (clone H5C6, BD Biosciences) were used as primary antibodies. Alexa Fluor 488‐ and 594‐IgG (Invitrogen) were used as secondary antibodies. Nuclei were stained with Hoescht 33342 (ThermoFisher). Coverslips were mounted using ProLong Gold (ThermoFisher Scientific). Microscopy was conducted using a Zeiss LSM‐710 confocal fluorescence microscope (Carl Zeiss). The area of CCVs was measured using CD63 as a CCV membrane marker. Unless otherwise stated, a minimum of 50 cells for each condition from three independent experiments was used for analyses. Fiji (ImageJ, National Institutes of Health) was used for all image analysis.
4.5. Induction and complementation of the CRISPRi system
Induction of dCas9‐3x‐cMyc and targeted sgRNA in the CRISPRi system was carried out using IPTG. Induction in axenic media and in host cells was achieved using 0.1 mM and 2 mM IPTG, respectively. Induction of the CRISPRi complementation constructs was carried out using aTc. In axenic media and host cells, 5 ng/ml and 25 ng/ml of aTc was used to induce expression of the codon‐optimized complement constructs.
4.6. qRT‐PCR analysis of CRISPRi knockdown
Bacterial cell culture pellets were lysed in 1.0 ml of Trizol (ThermoFisher Scientific, Waltham, MA). RNA‐containing aqueous phase was collected according to manufacturer's recommended protocol except 1‐Bromo‐3‐chloropropane was used instead of chloroform (MilliporeSigma, St. Louis, MO). RNA‐containing aqueous phase was combined with equal volume of RLT lysis buffer (Qiagen, Valencia, CA) and extracted using Qiagen AllPrep DNA/RNA 96‐well system (Valencia, CA). The RNA quality was assessed using the Agilent 2100 Bioanalyzer using RNA 6000 Pico kit (Agilent Technologies, Santa Clara, CA). qRT‐PCR probe and primer sets (Table S2) were designed using Primer Express version 3.0 (Life Technologies, Carlsbad, CA). qRT‐PCR assay was performed using the AgPath‐ID One‐Step RT‐PCR Buffer and Enzyme Mix (Life Technologies, Carlsbad, CA). AgPath‐ID one‐step RT‐PCR reactions were carried out in 20 μl reactions with 1X RT‐PCR Buffer, 1X RT‐PCR Enzyme mix, 400 nM forward and reverse primers, and 120 nM of the fluorescent TaqMan probes. The groEL forward, reverse, and fluorescent oligo were combined with the oligos for each gene listed in the table. Oligos were purchased from Biosearch Technologies located in Novato, CA. The QPCR reactions were carried out at 50°C for 10 min, 95°C for 10 min, 55 cycles of 95°C for 15 s, and 60°C for 45 s. Data were analyzed using ABI 7900HT version 2.4 sequence detection system software (Life Technologies, Carlsbad, CA). Normalized gene expressions were determined by comparative CT method (ThermoFisher Scientific, Waltham, MA).
4.7. Immunoblotting
C. burnetii production of IcmD, IcmK, dCas9‐3x‐cMyc, GST‐AntitoxP, ToxP‐V5‐6xHis, and XpressT‐6xHis‐CBU0665 was examined by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and immunoblotting. PVDF membrane was incubated with the following antibodies: polyclonal rabbit anti‐IcmD antibody (1:5000), polyclonal rabbit anti‐IcmK antibody (1:2500; generously provided by Edward Shaw, PCOM South Georgia), mouse monoclonal anti‐c‐myc antibody (1:5000; clone 9E10 BD Biosciences), goat polyclonal anti‐GST antibody (1:5000, Sigma‐Aldrich), mouse monoclonal anti‐V5 antibody (1:5000; Invitrogen), and mouse monoclonal anti‐XpressT antibody (1:5000; Invitrogen). Following incubation of membranes with primary antibody, reacting proteins were detected using anti‐rabbit (IcmD and IcmK), antigoat (GST‐AntitoxP), or antimouse (dCas9‐3x‐c‐myc, ToxP‐V5‐6xHis, and XpressT‐6xHis‐CBU0665) IgG secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL) and chemiluminescence using ECL Pico or Femto reagent (Pierce). Chemiluminescence was detected using the UVP ChemStudio plus imager (Analytik Jena), and images were processed using the VisionWorks software (Version 9.1.21054.7804).
4.8. Cell‐free protein expression
T7‐polymerase‐based expression plasmids (pET28a(+)‐toxP, pDEST15‐antitoxP, and pEXP1‐cbu0665) were used as DNA templates in cell‐free in vitro transcription and translation (IVTT) reactions (100 μl) using the Expressway mini cell‐free expression kit (Invitrogen) to achieve cell‐free production of protein. The reactions were incubated at 30°C with shaking at 300 rpm for 30 min, followed by the addition of feeding buffer and further incubation at 30°C with shaking at 300 rpm for 5 h. Lysates were then used in pulldown assays (see next section) or processed for SDS‐PAGE analysis by resuspending in SDS‐PAGE loading buffer and boiling for 10 min. Proteins were separated by SDS‐PAGE on a 4–20% gradient gel and then immunoblotted as described above using antibodies directed against the GST tag (Sigma‐Aldrich) to detect AntitoxP, V5 tag to detect ToxP, or the Xpress tag (Invitrogen) to detect CBU0665.
4.9. Pulldown assays
IVTT reaction mixtures (100 μl) were incubated at 4°C for 1 h with a 50% slurry of PBS/Glutathione‐Sepharose 4B (GE Healthcare) with gentle mixing. Glutathione‐Sepharose was pelleted in Pierce spin cups (Thermo Fisher) by centrifugation at 1000 × g for 1 min and washed 8 times in Tris‐buffered saline (pH 7.2) containing 1% Triton X‐100. Proteins bound to the Glutathione‐Sepharose beads were eluted using a 10 mM glutathione buffer (50 mM Tris, 10 mM reduced glutathione, pH 8.0). The final eluted protein mixes were then used in EMSA's (see next section) or processed for SDS‐PAGE analysis by resuspending in SDS‐PAGE loading buffer and boiling for 10 min. Proteins were separated by SDS‐PAGE on a 4–20% gradient gel and then immunoblotted as described above using antibodies directed against the GST tag (EMD Millipore) to detect AntitoxP, V5 tag to detect ToxP or the Xpress tag (Invitrogen) to detect CBU0665.
4.10. Electrophoretic mobility shift assays
The upstream regions of the toxP, cbuA0029, and groEL genes were first selected for PCR amplification. PCR products (1 μg) of desired templates were 3′ end‐labeled using a Pierce biotin 3′ End DNA Labeling Kit (Thermo Scientific). The resulting probe reaction mixtures were electrophoresed on a 0.8% agarose gel for 30 min at 100 V and then gel purified with a NucleoSpin Gel and PCR Clean‐up kit (Takara Bio USA). The EMSA binding reaction, consisting of 2.5% glycerol, 5 mM MgCl2, 50 mM KCl, 1 nM biotin‐labeled DNA, and varying concentrations of either ToxP or AntitoxP/ToxP in 1X Binding Buffer (LightShift Chemiluminescence kit; Thermo Scientific), was assembled and incubated at room temperature for 30 min. A nondenaturing loading dye (0.25% bromophenol blue) was added, and the resulting RNA mixtures were resolved on a 10% polyacrylamide gel for 2 h at 100 V. DNA/protein complexes were transferred to a Hybond‐N+ positively charged nylon membrane (Amersham Pharmacia Biotech) using an electroblot transfer system (Bio‐Rad) and cross‐linked with short‐wave UV light in a GS gene linker UV chamber (Bio‐Rad). A North2South chemiluminescence hybridization and detection kit (Thermo Scientific) was used to detect resulting bands. The blot was imaged on a UVP ChemStudio PLUS Imager (Analytik Jena).
4.11. Promoter‐mScarlet‐i fluorescence reporter
Individual wells of a 12‐well tissue culture plate containing 2 ml of ACCM‐D minus lysine were inoculated with C. burnetii harboring promoter‐mScarlet‐i constructs at a cell density of 2 × 106 GE/ml. After 7 or 14 days of incubation, the growth medium was thoroughly mixed by pipetting and 200 μl of each culture was added to a black Cellstar 96‐well microplate (Greiner Bio‐One). Fluorescence was measured using a Cytation 5 plate reader (Agilent).
4.12. Computer analysis
Geneious Prime 2021.1.1 (https://www.geneious.com) was used to analyze C. burnetii genome sequences for TA systems and to conduct sigma 70 motif searches. DNA alignments were carried out using the Clustal Omega multiple sequence alignment software (https://www.ebi.ac.uk/Tools/msa/clustalo/). Structural‐based homology searches were carried out using the I‐Tasser program (https://zhanggroup.org/I‐TASSER/).
4.13. Statistical analysis
Statistical analyses were performed using a one‐way analysis of variance (ANOVA) or unpaired Student's t test and Prism software (Version 9.3.1; GraphPad Software, Inc., La Jolla, CA).
AUTHOR CONTRIBUTIONS
Conceptualization: Paul A. Beare. Methodology and investigation: Paul A. Beare, Shaun Wachter, Diane C. Cockrell, Heather E. Miller, Kimmo Virtaneva, Benjamin Darwitz, and Kishore Kanakabandi. Data analysis: Paul A. Beare and Shaun Wachter. Supervision: Robert A. Heinzen. Writing original draft: Paul A. Beare. Review and editing: all authors.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest regarding the publication of this research.
ETHICS STATEMENT
Genes conferring resistance to chloramphenicol, kanamycin or ampicillin and for complementing amino acid auxotrophies using Legionella pneumophilia and Escherchia coli genes are approved in C. burnetii genetic transformation studies by the Rocky Mountain Laboratories Institutional Biosafety Committee.
Supporting information
Supinfo S1
ACKNOWLEDGMENTS
We thank Rose Perry‐Gottschalk of the Research Technologies Branch, National Institute of Allergy and Infectious Diseases for graphical support. This work was supported by funding from the Intramural Research Program of the NIH, NIAID (AI00946‐18). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Wachter, S. , Cockrell, D. C. , Miller, H. E. , Virtaneva, K. , Kanakabandi, K. , Darwitz, B. , Heinzen, R. A. & Beare, P. A. (2022). The endogenous Coxiella burnetii plasmid encodes a functional toxin–antitoxin system. Molecular Microbiology, 118, 744–764. 10.1111/mmi.15001
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Angelakis, E. & Raoult, D. (2010) Q Fever. Veterinary Microbiology, 140, 297–309. [DOI] [PubMed] [Google Scholar]
- Audoly, G. , Vincentelli, R. , Edouard, S. , Georgiades, K. , Mediannikov, O. , Gimenez, G. et al. (2011) Effect of rickettsial toxin VapC on its eukaryotic host. PLoS One, 6, e26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P.A. , Gilk, S.D. , Larson, C.L. , Hill, J. , Stead, C.M. , Omsland, A. et al. (2011) Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. MBio, 2, e00175‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P.A. , Larson, C.L. , Gilk, S.D. & Heinzen, R.A. (2012) Two systems for targeted gene deletion in Coxiella burnetii . Applied and Environmental Microbiology, 78, 4580–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P.A. , Samuel, J.E. , Howe, D. , Virtaneva, K. , Porcella, S.F. & Heinzen, R.A. (2006) Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray‐based whole‐genome comparisons. Journal of Bacteriology, 188, 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P.A. , Sandoz, K.M. , Larson, C.L. , Howe, D. , Kronmiller, B. & Heinzen, R.A. (2014) Essential role for the response regulator PmrA in Coxiella burnetii type 4B secretion and colonization of mammalian host cells. Journal of Bacteriology, 196, 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, P.A. , Unsworth, N. , Andoh, M. , Voth, D.E. , Omsland, A. , Gilk, S.D. et al. (2009) Comparative genomics reveal extensive transposon‐mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infection and Immunity, 77, 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho‐Nevers, E. , Edouard, S. , Leroy, Q. & Raoult, D. (2012) Deleterious effect of ciprofloxacin on Rickettsia conorii‐infected cells is linked to toxin‐antitoxin module up‐regulation. The Journal of Antimicrobial Chemotherapy, 67, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Brockett, M.R. , Lee, J. , Cox, J.V. , Liechti, G.W. & Ouellette, S.P. (2021) A dynamic, ring‐forming Bactofilin critical for maintaining cell size in the obligate intracellular bacterium Chlamydia trachomatis . Infection and Immunity, 89, e0020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, K.L. , Newton, H.J. , Luhrmann, A. & Roy, C.R. (2011) The Coxiella burnetii dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathogens, 7, e1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, W.T. , Domenech, M. , Moreno‐Cordoba, I. , Navarro‐Martinez, V. , Nieto, C. , Moscoso, M. et al. (2018) The streptococcus pneumonia eyefM‐yoeB and relBE toxin‐antitoxin operons participate in oxidative stress and biofilm formation. Toxins (Basel), 10, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K. , Mikkelsen, M. , Pedersen, K. & Gerdes, K. (2001) RelE, a global inhibitor of translation, is activated during nutritional stress. Proceedings of the National Academy of Sciences of the United States of America, 98, 14328–14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K. , Pedersen, K. , Hansen, F.G. & Gerdes, K. (2003) Toxin‐antitoxin loci as stress‐response‐elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. Journal of Molecular Biology, 332, 809–819. [DOI] [PubMed] [Google Scholar]
- Christensen‐Dalsgaard, M. & Gerdes, K. (2006) Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids. Molecular Microbiology, 62, 397–411. [DOI] [PubMed] [Google Scholar]
- Clemente, T.M. , Mulye, M. , Justis, A.V. , Nallandhighal, S. , Tran, T.M. & Gilk, S.D. (2018) Coxiella burnetii blocks intracellular Interleukin‐17 signaling in macrophages. Infection and Immunity, 86, e00532‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, S.A. , Fischer, E.R. , Cockrell, D.C. , Voth, D.E. , Howe, D. , Mead, D.J. et al. (2007) Proteome and antigen profiling of Coxiella burnetii developmental forms. Infection and Immunity, 75, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, S.A. , Fischer, E.R. , Howe, D. , Mead, D.J. & Heinzen, R.A. (2004) Temporal analysis of Coxiella burnetii morphological differentiation. Journal of Bacteriology, 186, 7344–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonne, P.M. , Winchell, C.G. , Graham, J.G. , Onyilagha, F.I. , Macdonald, L.J. , Doeppler, H.R. et al. (2016) Vasodilator‐stimulated phosphoprotein activity is required for Coxiella burnetii growth in human macrophages. PLoS Pathogens, 12, e1005915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha, L.D. , Ribeiro, J.M. , Fernandes, T.D. , Massis, L.M. , Khoo, C.A. , Moffatt, J.H. et al. (2015) Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nature Communications, 6, 10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter, H.S. , Jensen, R.V. , Mather, W.H. & Butzin, N.C. (2017) Mechanisms for differential protein production in toxin‐antitoxin systems. Toxins (Basel), 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin, C. , Melenotte, C. , Mediannikov, O. , Ghigo, E. , Million, M. , Edouard, S. et al. (2017) From Q fever to Coxiella burnetii infection: a paradigm change. Clinical Microbiology Reviews, 30, 115–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow, S. (1988) Molecular Koch's postulates applied to microbial pathogenicity. Reviews of Infectious Diseases, 10(Suppl 2), S274–S276. [DOI] [PubMed] [Google Scholar]
- Fraikin, N. , Goormaghtigh, F. & Van Melderen, L. (2020) Type II toxin‐antitoxin systems: evolution and revolutions. Journal of Bacteriology, 202, e00763‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraikin, N. , Rousseau, C.J. , Goeders, N. & Van Melderen, L. (2019) Reassessing the role of the type II MqsRA toxin‐antitoxin system in stress response and biofilm formation: mqsA is transcriptionally uncoupled from mqsR. MBio, 10, e02678‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, M.C. , Cheung, J. , Rudolph, M.J. , Burshteyn, F. , Cassidy, M. , Gary, E. et al. (2015) Structural genomics for drug design against the pathogen Coxiella burnetii . Proteins, 83, 2124–2136. [DOI] [PubMed] [Google Scholar]
- Friedrich, A. , Beare, P.A. , Schulze‐Luehrmann, J. , Cordsmeier, A. , Pazen, T. , Sonnewald, S. et al. (2021) The Coxiella burnetii effector protein CaeB modulates endoplasmatic reticulum (ER) stress signalling and is required for efficient replication in Galleria mellonella . Cellular Microbiology, 23, e13305. [DOI] [PubMed] [Google Scholar]
- Fu, M. , Liu, Y. , Wang, G. , Wang, P. , Zhang, J. , Chen, C. et al. (2022) A protein‐protein interaction map reveals that the Coxiella burnetii effector CirB inhibits host proteasome activity. PLoS Pathogens, 18, e1010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades, K. & Raoult, D. (2011) Genomes of the most dangerous epidemic bacteria have a virulence repertoire characterized by fewer genes but more toxin‐antitoxin modules. PLoS One, 6, e17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, K. , Rasmussen, P.B. & Molin, S. (1986) Unique type of plasmid maintenance function: postsegregational killing of plasmid‐free cells. Proceedings of the National Academy of Sciences of the United States of America, 83, 3116–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, M.G. & Colombo, M.I. (2005) Autophagosomes: a fast‐food joint for unexpected guests. Autophagy, 1, 179–181. [DOI] [PubMed] [Google Scholar]
- Hadzi, S. , Garcia‐Pino, A. , Haesaerts, S. , Jurenas, D. , Gerdes, K. , Lah, J. et al. (2017) Ribosome‐dependent Vibrio cholerae mRNAse HigB2 is regulated by a beta‐strand sliding mechanism. Nucleic Acids Research, 45, 4972–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, B.E. , Herrou, J. , Blackwell, A.E. , Wysocki, V.H. & Crosson, S. (2012) Molecular structure and function of the novel BrnT/BrnA toxin‐antitoxin system of Brucella abortus . The Journal of Biological Chemistry, 287, 12098–12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen, R.A. , Hackstadt, T. & Samuel, J.E. (1999) Developmental biology of Coxiella burnettii . Trends in Microbiology, 7, 149–154. [DOI] [PubMed] [Google Scholar]
- Howe, D. , Melnicakova, J. , Barak, I. & Heinzen, R.A. (2003) Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cellular Microbiology, 5, 469–480. [DOI] [PubMed] [Google Scholar]
- Howe, D. , Shannon, J.G. , Winfree, S. , Dorward, D.W. & Heinzen, R.A. (2010) Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome‐like compartments of human macrophages. Infection and Immunity, 78, 3465–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley, J.M. & Woychik, N.A. (2009) Bacterial toxin HigB associates with ribosomes and mediates translation‐dependent mRNA cleavage at A‐rich sites. The Journal of Biological Chemistry, 284, 18605–18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac, D.T. & Isberg, R. (2014) Master manipulators: an update on Legionella pneumophila Icm/dot translocated substrates and their host targets. Future Microbiology, 9, 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, M.G. , Pandey, D.P. , Jaskolska, M. & Gerdes, K. (2009) HicA of Escherichia coli defines a novel family of translation‐independent mRNA interferases in bacteria and archaea. Journal of Bacteriology, 191, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenas, D. , Fraikin, N. , Goormaghtigh, F. & Van Melderen, L. (2022) Biology and evolution of bacterial toxin‐antitoxin systems. Nature Reviews. Microbiology, 20, 335–350. [DOI] [PubMed] [Google Scholar]
- Kamruzzaman, M. , Wu, A.Y. & Iredell, J.R. (2021) Biological functions of type II toxin‐antitoxin systems in bacteria. Microorganisms, 9, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavkin, T. & Tabibzadeh, S.S. (1988) Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii . Infection and Immunity, 56, 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Wang, X. , Zhang, X.S. , Grigoriu, S. , Page, R. , Peti, W. et al. (2010) Escherichia coli toxin/antitoxin pair MqsR/MqsA regulate toxin CspD. Environmental Microbiology, 12, 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C.L. , Beare, P.A. & Heinzen, R.A. (2019) Dependency of Coxiella burnetii type 4B secretion on the chaperone IcmS. Journal of Bacteriology, 201, e00431‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C.L. , Beare, P.A. , Howe, D. & Heinzen, R.A. (2013) Coxiella burnetii effector protein subverts clathrin‐mediated vesicular trafficking for pathogen vacuole biogenesis. Proceedings of the National Academy of Sciences of the United States of America, 110, E4770‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C.L. , Beare, P.A. , Voth, D.E. , Howe, D. , Cockrell, D.C. , Bastidas, R.J. et al. (2015) Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infection and Immunity, 83, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C.L. , Martinez, E. , Beare, P.A. , Jeffrey, B. , Heinzen, R.A. & Bonazzi, M. (2016) Right on Q: genetics begin to unravel Coxiella burnetii host cell interactions. Future Microbiology, 11, 919–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. (2016) Bringing bioactive compounds into membranes: the UbiA superfamily of intramembrane aromatic Prenyltransferases. Trends in Biochemical Sciences, 41, 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.Y. , Awano, N. , Masuda, H. , Park, J.H. & Inouye, M. (2013) Transcriptional repressor HipB regulates the multiple promoters in Escherichia coli . Journal of Molecular Microbiology and Biotechnology, 23, 440–447. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhang, X. , Deng, S. , Wang, H. & Zhao, Y. (2022) Thiamine is required for virulence and survival of Pseudomonas syringae pv. Tomato DC3000 on tomatoes. Frontiers in Microbiology, 13, 903258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C.M. , Beare, P.A. , Cockrell, D.C. , Fintzi, J. , Tesfamariam, M. , Shaia, C.I. et al. (2021) Contributions of lipopolysaccharide and the type IVB secretion system to Coxiella burnetii vaccine efficacy and reactogenicity. NPJ Vaccines, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, S. , Lu, S. , Fan, H. , Sun, Z. , Hu, Y. , Li, R. et al. (2021) The Coxiella burnetii QpH1 plasmid is a virulence factor for colonizing bone marrow‐derived murine macrophages. Journal of Bacteriology, 203, e00588‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Z.Q. & Isberg, R.R. (2004) Multiple substrates of the Legionella pneumophila dot/Icm system identified by interbacterial protein transfer. Proceedings of the National Academy of Sciences of the United States of America, 101, 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, F. , Dam, P. , Chou, J. , Olman, V. & Xu, Y. (2009) DOOR: a database for prokaryotic operons. Nucleic Acids Research, 37, D459–D463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X. , Ma, Q. , Zhou, C. , Chen, X. , Zhang, H. , Yang, J. et al. (2014) DOOR 2.0: presenting operons and their functions through dynamic and integrated views. Nucleic Acids Research, 42, D654–D659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, E. , Cantet, F. , Fava, L. , Norville, I. & Bonazzi, M. (2014) Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi‐phenotypic high‐content screening. PLoS Pathogens, 10, e1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana, P. , Graham, J.G. , Sharma, U.M. & Voth, D.E. (2013) Refining the plasmid‐encoded type IV secretion system substrate repertoire of Coxiella burnetii . Journal of Bacteriology, 195, 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin, M. & Raoult, D. (1999) Q fever. Clinical Microbiology Reviews, 12, 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormeier, D.E. , Sandoz, K.M. , Beare, P.A. , Sturdevant, D.E. , Nair, V. , Cockrell, D.C. et al. (2019) Coxiella burnetii RpoS regulates genes involved in morphological differentiation and intracellular growth. Journal of Bacteriology, 201, e00009‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, H.J. , Kohler, L.J. , Mcdonough, J.A. , Temoche‐Diaz, M. , Crabill, E. , Hartland, E.L. et al. (2014) A screen of Coxiella burnetii mutants reveals important roles for dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathogens, 10, e1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, T. & Hiraga, S. (1983) Partition mechanism of F plasmid: two plasmid gene‐encoded products and a cis‐acting region are involved in partition. Cell, 32, 351–360. [DOI] [PubMed] [Google Scholar]
- Omsland, A. , Beare, P.A. , Hill, J. , Cockrell, D.C. , Howe, D. , Hansen, B. et al. (2011) Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Applied and Environmental Microbiology, 77, 3720–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omsland, A. , Cockrell, D.C. , Howe, D. , Fischer, E.R. , Virtaneva, K. , Sturdevant, D.E. et al. (2009) Host cell‐free growth of the Q fever bacterium Coxiella burnetii . Proceedings of the National Academy of Sciences of the United States of America, 106, 4430–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka, Y. , Miki, K. , Koga, M. , Katayama, N. , Morimoto, W. , Takahashi, Y. et al. (2010) IscR regulates RNase LS activity by repressing rnlA transcription. Genetics, 185, 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette, S.P. , Blay, E.A. , Hatch, N.D. & Fisher‐Marvin, L.A. (2021) CRISPR interference to inducibly repress gene expression in Chlamydia trachomatis . Infection and Immunity, 89, e0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard, M. , Borch, J. & Gerdes, K. (2009) RelB and RelE of Escherichia coli form a tight complex that represses transcription via the ribbon‐helix‐helix motif in RelB. Journal of Molecular Biology, 394, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard, M. , Borch, J. , Jorgensen, M.G. & Gerdes, K. (2008) Messenger RNA interferase RelE controls relBE transcription by conditional cooperativity. Molecular Microbiology, 69, 841–857. [DOI] [PubMed] [Google Scholar]
- Pandey, D.P. & Gerdes, K. (2005) Toxin‐antitoxin loci are highly abundant in free‐living but lost from host‐associated prokaryotes. Nucleic Acids Research, 33, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.Y. , Kim, H.J. , Pathak, C. , Yoon, H.J. , Kim, D.H. , Park, S.J. et al. (2020) Induced DNA bending by unique dimerization of HigA antitoxin. IUCrJ, 7, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechstein, J. , Schulze‐Luehrmann, J. , Bisle, S. , Cantet, F. , Beare, P.A. , Olke, M. et al. (2020) The Coxiella burnetii T4SS effector AnkF is important for intracellular replication. Frontiers in Cellular and Infection Microbiology, 10, 559915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, K. , Zavialov, A.V. , Pavlov, M.Y. , Elf, J. , Gerdes, K. & Ehrenberg, M. (2003) The bacterial toxin RelE displays codon‐specific cleavage of mRNAs in the ribosomal A site. Cell, 112, 131–140. [DOI] [PubMed] [Google Scholar]
- Peters, J.M. , Koo, B.M. , Patino, R. , Heussler, G.E. , Hearne, C.C. , Qu, J. et al. (2019) Enabling genetic analysis of diverse bacteria with Mobile‐CRISPRi. Nature Microbiology, 4, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, S.E. , Goodman, A.G. & Omsland, A. (2021) Metabolic plasticity aids amphotropism of Coxiella burnetii . Infection and Immunity, e00135‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz, K.M. , Beare, P.A. , Cockrell, D.C. & Heinzen, R.A. (2016) Complementation of arginine auxotrophy for genetic transformation of Coxiella burnetii by use of a defined axenic medium. Applied and Environmental Microbiology, 82, 3042–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz, K.M. , Moore, R.A. , Beare, P.A. , Patel, A.V. , Smith, R.E. , Bern, M. et al. (2021) Beta‐barrel proteins tether the outer membrane in many gram‐negative bacteria. Nature Microbiology, 6, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz, K.M. , Sturdevant, D.E. , Hansen, B. & Heinzen, R.A. (2014) Developmental transitions of Coxiella burnetii grown in axenic media. Journal of Microbiological Methods, 96, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, W. , Schmidt, T. , Cordsmeier, A. , Borges, V. , Beare, P.A. , Pechstein, J. et al. (2020) The anti‐apoptotic Coxiella burnetii effector protein AnkG is a strain specific virulence factor. Scientific Reports, 10, 15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schureck, M.A. , Repack, A. , Miles, S.J. , Marquez, J. & Dunham, C.M. (2016) Mechanism of endonuclease cleavage by the HigB toxin. Nucleic Acids Research, 44, 7944–7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, C.F. & Bertram, R. (2013) Toxin‐antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiology Letters, 340, 73–85. [DOI] [PubMed] [Google Scholar]
- Socolovschi, C. , Audoly, G. & Raoult, D. (2013) Connection of toxin‐antitoxin modules to inoculation eschar and arthropod vertical transmission in Rickettsiales. Comparative Immunology, Microbiology and Infectious Diseases, 36, 199–209. [DOI] [PubMed] [Google Scholar]
- Song, S. & Wood, T.K. (2020) A primary physiological role of toxin/antitoxin systems is phage inhibition. Frontiers in Microbiology, 11, 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead, C.M. , Cockrell, D.C. , Beare, P.A. , Miller, H.E. & Heinzen, R.A. (2018) A Coxiella burnetii phospholipase A homolog pldA is required for optimal growth in macrophages and developmental form lipid remodeling. BMC Microbiology, 18, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, A. , Louveau, C. , Lepidi, H. , Ricci, F. , Baylac, P. , Davoust, B. et al. (2005) Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infection and Immunity, 73, 2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo Esquerra, E. , Yang, H. , Sanchez, S.E. & Omsland, A. (2017) Physicochemical and nutritional requirements for axenic replication suggest physiological basis for Coxiella burnetii niche restriction. Frontiers in Cellular and Infection Microbiology, 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth, D.E. , Beare, P.A. , Howe, D. , Sharma, U.M. , Samoilis, G. , Cockrell, D.C. et al. (2011) The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. Journal of Bacteriology, 193, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. & Wood, T.K. (2011) Toxin‐antitoxin systems influence biofilm and persister cell formation and the general stress response. Applied and Environmental Microbiology, 77, 5577–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, M.M. , Chen, C. , Rowin, K. , Mertens, K. , Galvan, G. , Zhi, H. et al. (2013) Identification of Coxiella burnetii type IV secretion substrates required for intracellular replication and Coxiella‐containing vacuole formation. Journal of Bacteriology, 195, 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Xu, W. , Shao, S. & Wang, Q. (2021) Gene silencing through CRISPR interference in bacteria: current advances and future prospects. Frontiers in Microbiology, 12, 635227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


