Abstract
Background
Due to the substantial increase in the use of disinfectants containing quaternary ammonion compounds (QACs) in healthcare and community settings during the COVID-19 pandemic, there is increased concern that heavy use might cause bacteria to develop resistance to QACs or contribute to antibiotic resistance. The purpose of this review is to briefly discuss the mechanisms of QAC tolerance and resistance, laboratory-based evidence of tolerance and resistance, their occurrence in healthcare and other real-world settings, and the possible impact of QAC use on antibiotic resistance.
Methods
A literature search was conducted using the PubMed database. The search was limited to English language articles dealing with tolerance or resistance to QACs present in disinfectants or antiseptics, and potential impact on antibiotic resistance. The review covered the period from 2000 to mid-Jan 2023.
Results
Mechanisms of QAC tolerance or resistance include innate bacterial cell wall structure, changes in cell membrane structure and function, efflux pumps, biofilm formation, and QAC degradation. In vitro studies have helped elucidate how bacteria can develop tolerance or resistance to QACs and antibiotics. While relatively uncommon, multiple episodes of contaminated in-use disinfectants and antiseptics, which are often due to inappropriate use of products, have caused outbreaks of healthcare-associated infections. Several studies have identified a correlation between benzalkonium chloride (BAC) tolerance and clinically-defined antibiotic resistance. The occurrence of mobile genetic determinants carrying multiple genes that encode for QAC or antibiotic tolerance raises the concern that widespread QAC use might facilitate the emergence of antibiotic resistance. Despite some evidence from laboratory-based studies, there is insufficient evidence in real-world settings to conclude that frequent use of QAC disinfectants and antiseptics has promoted widespread emergence of antibiotic resistance.
Conclusions
Laboratory studies have identified multiple mechanisms by which bacteria can develop tolerance or resistance to QACs and antibiotics. De novo development of tolerance or resistance in real-world settings is uncommon. Increased attention to proper use of disinfectants is needed to prevent contamination of QAC disinfectants. Additional research is needed to answer many questions and concerns related to use of QAC disinfectants and their potential impact on antibiotic resistance.
Keywords: Quaternary ammonium compounds, Disinfectants, Antiseptics, Side effects, Tolerance, Resistance, Antibiotic resistance
Introduction
Quaternary ammonium compounds (QACs) intended for preservative and antimicrobial applications are present in a wide variety of products, resulting in potential exposures in many settings [1, 2]. For example, QACs can be found in personal care products, ophthalmic medications, skin antiseptics, and disinfectants used in homes and healthcare facilities [1, 2]. QACs comprise a large number of chemical structures, with many variations on the basic structure, which includes a nitrogen (head) attached with four bonds to alkyl or aryl chains (tails) with varying number of carbon atoms. Examples of several types of commonly used QACs are listed in Table 1.
Table 1.
Examples of quaternary ammonium compounds commonly used in disinfectants
| Compound | Abbreviations | CAS Number |
|---|---|---|
| Akyl dimethyl benzyl ammonium chloride frequently called benzalkonium chloride (BAC) |
ADBAC C10-C18, or BAC C10-C18 |
8001-54-5 |
|
Didecyl dimethyl ammonium chloride Dioctyl dimethyl ammonium chloride |
DDAC C10 DDAC C8 |
7173-51-5 3026-69-5 |
| • Quaternary ammonium compounds, C12 − 18-alkyl[(ethylphenyl)methyl]dimethyl, chlorides | ADEBAC C10-C18 | 68956-79-6 |
| Alkyl trimethyl ammonium chloride | ATMAC C12-C18 | 68391-03-7 |
| Cetyl trimethyl ammonium chloride | CTAC | 112-02-7 |
| Cetylpyridinium chloride | CPC | 123-03-5 |
| Benzethonium chloride | BZT | 121-54-0 |
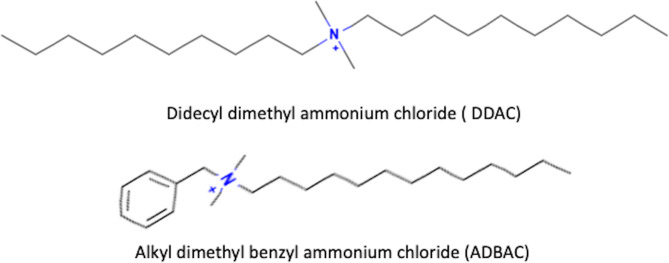
The chemical structures of two QACs commonly used in disinfectants (didecyl dimethyl ammonium chloride (DDAC) and alkyl dimethyl benzyl ammonium chloride (ADBAC or BAC) are shown in the Fig. 1.
Fig. 1.
Structure of (A) didecyl dimethyl ammonium chloride (DDAC) and (B) alkyl dimethyl benzyl ammonium chloride (ADBAC).
More complicated structures such as bis- and tris-QACs with two or three nitrogen atoms or QACs attached to polymers (polymeric QACs) are beyond the scope of this review.
Recently, interest in these compounds has intensified due to the large number of QAC disinfectants recommended for use against SARS-CoV-2 and their widespread use during the COVID-19 pandemic [2–4]. For example, QAC-containing disinfectants comprised 277/597 (46.3%) of the United States Environmental Protection Agency (EPA) List N products listed as effective against SARS-CoV-2 [5]. Concerns include misuse of disinfectants, a 331% increase in QAC levels of untreated wastewater in one area, QAC levels in household dust that were significantly higher than levels found before the pandemic, and the finding of QACs in blood and breast milk samples collected from individuals during the pandemic [3, 5–9]. Furthermore, marketing and ubiquitous use in community settings (e.g., households, offices) and healthcare facilities of QACs, including products with persistent activity due to silane moieties, during the pandemic might result in even higher or more prolonged sub-inhibitory concentrations on surfaces and in the environment, which may be a risk factor for emergence of QAC tolerance or resistance, and perhaps to an increase in antibiotic tolerance or resistance among pathogenic bacteria [1, 4, 10, 11]. One QAC-silane disinfectant was taken off the market because the manufacturer was selling and distributing the product in ways that were inconsistent with federal regulations [12]. This review focuses on mechanisms of tolerance and resistance of bacteria to QACs, and addresses concerns about the possible influence of QAC use on antibiotic resistance. Emphasis is placed on QAC compounds that are commonly used in disinfectants and antiseptics used in healthcare and community settings.
Methods
A literature search of the PubMed database was performed using the following search terms: quaternary ammonium compounds + disinfectants + tolerance; quaternary ammonium compounds + disinfectants + resistance; quaternary ammonium compounds + antiseptics + tolerance; quaternary ammonium compounds + antiseptic + resistance; and quaternary ammonium compounds + contaminated disinfectants. The search was limited to English language citations, and the search was confined to the period 2000 to mid-January 2023. A total of 1,161 unique citations were retreived from PubMed. Following a review of titles, 386 abstracts were reviewed, and 328 full-text articles were downloaded and reviewed. Additional citations were identified in bibliographies of reviewed articles. Citations dealing with issues related to the potential impact on the environmental pollution were not included. Data from 138 articles are included in the present review.
Results
Unfortunately, the terms such as QAC tolerance, reduced susceptibility, and resistance are not always used consistently by the scientific community, and can sometimes be misleading. As pointed out by Maillard, [13] the term “resistance” has often been defined as an increase in the in vitro minimum inhibitory concentration (MIC) or minimum bactericidal concentrations (MBC) of a disinfectant or an antiseptic. However, despite relatively small increases in the MIC (or MBC) of the QAC ingredient, products may still be very effective because the in-use concentration is often significantly higher than the in vitro MIC or MBC. For example, some Gram-negative bacteria have MICs to BAC of 12 to 60 µg per mL, [14–16] while QAC-based disinfectants may have in-use concentrations ranging from 200 to 16,000 ug/ml) [3, 17]. Accordingly, bacteria manifesting several-fold increase in the MIC of a disinfectant are more appropriately referred to as being tolerant or having reduced susceptibility to the disinfectant. For the purposes of this review, the term tolerance will be used to denote reduced susceptibility, and a biocide-resistant microorganism is defined as one that is not inactivated by an in-use concentration of a biocide [13, 18]. For more in-depth discussions of QAC tolerance and resistance and possible impact on antibiotic resistance, several reviews are available [1, 13, 19, 20].
Methods for studying QAC tolerance and resistance
A variety of methods have been used to study tolerance and resistance to QAC disinfectants and antiseptics [13, 21]. A few studies have evaluated bacteria recovered from surfaces which have been disinfected with QAC-based disinfectants [22, 23]. Two studies have evaluated the mechanisms of resistance in QAC-resistant strains recovered from contaminated QAC disinfectants or antiseptics [24, 25]. A much more common method is to study the adaptive changes that occur when bacteria are exposed in vitro to increasing, sublethal concentrations of QACs [15, 16, 26–30]. Although the extent to which such in vitro experiments yield the same findings that occur in nature has been questioned, considerable insight into the mechanisms of biocide resistance has been gained by studying such adaptive changes [24, 31].
Mechanisms of QAC tolerance and resistance
Intrinsic tolerance and resistance
Spore-forming organisms and mycobacteria possess intrinsic resistance to disinfectants and antiseptics due to their complex cell wall structures [32]. Gram-negative bacteria are generally less susceptible than Gram-positive bacteria to QACs due to their outer membrane, which can make it somewhat more difficult for biocides to reach their target site (cytoplasmic membrane) [32, 33]. In general, QACs are classified as fungicidal and viricidal against enveloped viruses, but have poor activity against mycobacteria and non-enveloped viruses such as norovirus [34, 35]. However, some QAC-based products are less active than some other disinfectants against Candida species including C. auris. [36] Intrinsic biocide resistance can also be due to the presence of chromosomally-encoded efflux pumps, the ability of organisms to produce biofilm, or degradation of QAC compounds [37, 38]. Examples of bacteria that often have intrinsic reduced susceptibility to QACs include species of Pseudomonas and Burkholderia, [32, 38–40] which may explain in part the frequency with which they have been reported to cause contamination of QAC-based antiseptic and disinfectant solutions [41]. The frequency and mechanisms of decreased susceptibility or resistance to QACs among fungi and viruses are outside the scope of this review.
Membrane changes
In experiments involving exposure of a Pseudomonas aeruginosa strain to increasing concentrations of BAC-C14 or didecyldimethylammonium bromide (DDAB), changes in the cytoplasmic membrane fatty acid composition were associated with increases in the MIC and MBC to both compounds, which subsequently decreased when the organism was grown in QAC-free media [14, 42]. A Listeria monocytogenes strain exposed to BAC developed changes in cell surface fatty acid composition, which may have contributed to a two-fold increase in BAC MIC [43]. BAC can cause increases in membrane fluidity among some strains of bacteria recovered from food sources [44]. A study involving proteomic assays found that exposure to increasing concentrations of BAC resulted in changes in proteins that have been associated with acquired antimicrobial resistance, and decreased expression of porins and lipoproteins [45]. Strains of Pseudomonas species with reduced susceptibility to QACs have been reported to have changes in their outer membrane proteins and lipopolysaccharide [46, 47]. A 2018 study by Kim et al. [27] used genomic and transcriptomic methods to assess the impact of exposing Pseudomonas aeruginosa to increasing concentrations of BAC found that the strain was able to survive concentrations of BAC up to 1200 to 1600 ppm (ug/ml). Adaptation resulted in decreased expression of porins related to BAC transport, reduced growth rate, and reduction in the membrane negative charge. Similarly, analysis of a strain of Pseudomonas fluorescens recovered from a contaminated BAC solution revealed a reduction in adsorption of BAC to the cell surface due to a decrease negative cell surface charge [24]. Growth of Salmonella enterica serovar enteritidis in BAC resulted in decreased susceptibility to the agent, alterations in cell surface roughness and a shift in fatty acid composition [48]. Exposure of E. coli to increasing concentrations of BAC has been shown to produce a subpopulation of surviving cells with a decreased growth rate and lpxM gene mutation-related decrease in negative surface charge that likely decreased adsorption of BAC to its target site [31]. The authors concluded that their findings suggest that episodes of incomplete disinfection that result in a subpopulation of surviving cells could adversely affect the efficacy of disinfection practices.
Efflux pumps
Many bacteria contain efflux pumps that can remove substances such as QACs and various antibiotics from the cytoplasm and cytoplasmic membrane [37, 49]. There are several classes of efflux pumps, including resistance-nodulation-division (RND) superfamily, major facilitator superfamily (MFS), ATP-binding cassette (ABC) family, small multidrug resistance (SMR) family, multidrug and toxic compound extrusion (MATE) family, and proteobacterial antimicrobial compound efflux (PACE) family [50, 51]. RND efflux pumps, which are most commonly found in Gram-negative bacteria, are composed of a cytoplasmic membrane pump, a periplasmic protein, and an outer membrane protein channel [49]. For example, some clinical E. coli strains have been shown to contain a class 1 integron with cassettes containing genes encoding for trimethoprim-sulfamethoxazole resistance (drfA1/sul1) and the qacE∆1gene that encodes for QAC tolerance [52]. Reduced susceptibilities were due to overexpression of AcrAB-TolC efflux pump regulatory genes tolC and marOR, which was also associated with decreased susceptibility to ciprofloxacin. The MATE family pump (PmPM) can be found in P. aeruginosa, Acinetobacter baumannii, and Vibrio parahaemolyticus as well as in S. aureus. [50] MFS, ABC and SMR family efflux pumps are the most common in Gram-positive bacteria, although a MFS pump (SmfY) has also been found in Serratia marcescens [53]. In staphylococci, six different plasmid-mediated efflux pumps (QacA, QacB, QacC, QacG, QacH and QacJ) can reduce susceptibility to QACs, but do not result in QAC resistance [54]. The qacH gene has also been identified on a non-classic class 1 integron located on a conjugative plasmid in Proteus mirabilis [55]. The gene qacH and bcrABC cassette, which both encode for QAC efflux, have been found on transposable elements in different strains of Listeria monocytogenes. [56, 57] Some examples of the efflux pumps found in common pathogenic bacteria are listed in Table 2 [15, 49–51, 54, 58–65].
Table 2.
Examples of efflux pumps conferring increased tolerance to quaternary ammonium compounds. Cite at line 238
| Pathogen | RND | MFS | ABC | SMR | MATE | PACE |
|---|---|---|---|---|---|---|
| E. coli | AcrAB-TolC | MdfA(Cmr), EmrB, EmrD | EmrE, SugE | NorM, MdtK/YdhE | ||
| Pseudomonas | Mex-Opr | QacE△1 | PmPM | |||
| Serratia | SdeXY, SdeAB, SdeIJ, | SmfY | ||||
| Burkholderia | Mex-Opr-like | AceI | ||||
| Acinetobacter | QacA, QacB | QacE, QacE△1 | PmPM | AceI | ||
| Achromobacter | ||||||
| Proteus | PmPM | |||||
| Vibrio | PmPM | |||||
| Aeromonas | QacE2 | |||||
| Enterobacter | acrB | SugE | EmmdR/YeeO | |||
| Klebsiella | QacE | |||||
| Salmonella typhimurium | AcrAB-TolC | |||||
| MFS | ABC | SMR | ||||
| S. aureus |
NorA, QacA, Qac B; MepA MdeA |
EfrAB | QacC,QacG,QacH, QacJ, QacE△1 | |||
| L. monocytogenes | EmrELm | |||||
| Enterococcus | QacA/B | EfrAB | QacC, QacE△1 |
RND = resistance-nodulation-division; MFS = major facilitator superfamily; ABC = ATP-binding cassette; SMR = small multidrug resistance; PACE = proteobacterial antimicrobial compound efflux; MATE = multidrug and toxic compound extrusion
It is worth noting that decreased susceptibility, and occasionally resistance, to QACs is likely mediated by efflux pumps in addition to other mechanisms. For example, a number of studies suggest that efflux pumps may promote, directly or indirectly, biofilm production [66, 67].
Biofilm and reduced susceptibility to QACs
The presence of biofilm reduces the susceptibility to disinfectants in both Gram-positive and Gram-negative bacteria, with the greatest impact in Gram-negative bacteria [26, 68–71]. Reduced susceptibility to biocides in biofilms may be due to several factors [71]. Multiple layers of bacteria and the extracellular matrix may result in conditions in which effective biocide concentrations do not reach all internal layers of cells [72]. Diffusion of positively charged disinfectants within negatively charged biofilms could be hindered because of electrostatic interactions [73]. Biofilms may promote induction or upregulation of efflux pumps and increased mutations that may affect biocide susceptibility [66, 71, 74]. Decreased bacterial growth rates in biofilm may also result in reduced susceptibility to disinfectants [72]. As a result, bacteria present in biofilms may have minimum bactericidal concentrations 10-1000 times higher than planktonic organisms of the same strains, and in some instances may not be killed by biocides in concentrations recommended by the manufacturer [68]. Accordingly, it is important clean surfaces (i.e., remove organic and inorganic material) as part of the disinfection process [75].
Degradation of QACs
Studies of multiple strains of Burkholderia species have documented that these organisms can partially degrade BAC over a period of 7 days [38]. Multiple constitutively expressed enzymes were involved in degradation of BAC. In a few organisms such as Pseudomonas nitroreducens and Pseudomonas fluorescens, biocide resistance may also be due in part to enzymatic degradation of QACs [13, 38, 76, 77].
Adaptive studies
Multiple studies have demonstrated that exposure of bacteria to subinhibitory concentrations of QAC resulted in increased QAC MICs or MBCs, which has often been due to upregulation of QAC efflux pumps [15, 16, 26, 27, 29, 49, 78]. In a number of such studies, MICs or MBCs have increased to levels that are often far below in-use concentrations, with variable degrees of stability of the increased MICs when the organism is no longer exposed to the QAC [14, 15, 26, 29, 31, 43, 44, 69, 79]. However, some experts have argued that even temporary (a few days or weeks) increases in MICs to levels lower than in-use concentrations of disinfectants might affect the efficacy of standard surface disinfection protocols [29]. In contrast, a few adaptive studies found that some strains developed stable BAC MICs that are in the range of in-use concentrations [27, 30, 80]. For example, in one adaptive study in which Pseudomonas aeruginosa strains were exposed to increasing concentrations of BAC, several strains were able to survive concentrations from 1240 to 1640 µg/ml, which are concentrations higher than the recommended in-use concentrations of some QAC-based disinfectant products [3, 25, 27]. Although the MICs of a majority of strains reverted back to baseline levels within 150 generations or less when grown in the absence of BAC, several strains maintained high MICs. Additional studies, including transcriptome sequencing analysis, of a Pseudomonas aeruginosa strain exposed for 3 years to subinhibitory BAC concentrations and a similar unexposed strain revealed changes that included reduced growth rate, decreased expression of porins associated with BAC transport, and upregulation of efflux genes [81]. Mutations in the pmrB gene, which in other studies has been shown to result in reduction in the negative charge of the outer membrane, also occurred. Adaptive studies have also found that exposure of bacteria to QACs can promote biofilm formation [26].
A few adaptive studies have resulted in antibiotic tolerance or resistance [5, 78, 82–84].
Continuous exposure of Pseudomonas aeruginosa to BAC resulted in an increase in BAC MIC to 350 ug/ml and of ciprofloxacin to 32 ug/ml [78]. The latter change was attributed to a mutation in the nfxB gene, which is an efflux pump regulator. In another study, step-wise exposure of E. coli to DDAC resulted in tolerance to DDAC and BAC, and development of clinically-defined resistance to chloramphenicol, ampicillin, ciprofloxacin, cefotaxime and ceftazidime [84]. In some strains, an increase expression of acrB gene likely contributed to emergence of antibiotic resistance. In studies by Jia et al., [5] exposure to DDAC was more likely than BAC to result in resistance to some antibiotics in a laboratory strain of E. coli, albeit some of the resistance was to antibiotics seldom used to treat E. coli infections. Whole genome sequencing revealed that mutations affecting RNA transcription, efflux pump regulators, and cell membrane synthesis had occurred [5]. Rakic-Martinez et al. [82] found that exposure of Listeria monocytogenes to BAC yielded strains that developed efflux pump-mediated resistance to gentamicin, which is used for treatment of invasive Listeria infections. Another study of L. monocytogenes found that exposure to BAC or DDAC caused increased tolerance to ciprofloxacin MICs, [85] which was later shown to be caused by mutations in the fepR repressor that affects the fluoroquinolone efflux pump FepA [86]. It should be noted that the stability of antibiotic resistance demonstrated in the above studies was tested over short time periods in some studies, but none evaluated the persistence of resistance over prolonged time periods.
A proposed protocol that exposes pathogens to in-use concentrations of products has been developed to better predict bacterial resistance to biocides [87]. The protocol recommends that if an organism develops a stable increase in MIC or MBC, several avenues of investigation should be performed since biocide resistance is usually due to several mechanisms. An alternative approach that warrants further attention is to study the impact of QAC exposure on the adaptive changes in biocide susceptibility by exposing multi-organism biofilms (such as those found in sink drains) to in-use concentrations of formulated disinfectant products, rather than exposure to pure QAC compounds [88].
QAC tolerance in real-world settings
Some authors have mentioned that it is arguable whether biocides can develop tolerance in non-laboratory settings [69]. However, there are a few examples of QAC tolerance in real-world settings. One study found that hospital isolates of Pseudomonas aeruginosa had increased BAC tolerance, and some of the isolates also had clinically-significant resistance to antibiotics [89]. He et al. [22] reported that 63 isolates of Staphylococcus spp. recovered from fitness centers and school dormitories had slightly increased tolerance to BAC, which was attributed to the presence of one or more qac genes. A study of 199 A. baumannii clinical isolates from four teaching hospitals found that some isolates had BAC MICs and MBCs as high as 640 to 1280 ug/ml, respectively [90].
QAC resistance in real-world settings: contaminated in-use disinfectants and antiseptics
QAC-resistant microorganisms have been defined as those which are capable of surviving in an in-use concentration of a product [13]. If one adopts this definition, then examples of QAC-resistant bacteria include those recovered from contaminated in-use disinfectants or antiseptic solutions in clinical settings [25, 41, 91–115]. Product contamination by QAC-resistant pathogens can also be affected by user errors, including use of outdated products, substantial over-dilution of concentrated solutions, or introduction of organic material or prolonged soaking of wipes with strong QAC-binding affinity in disinfectant solutions [13, 41, 116, 117]. Of 43 reported episodes of contaminated disinfectants or antiseptics, authors described outbreaks of infection (26), single cases of infection (3), pseudo-outbreaks (4) surveys of disinfection buckets (3), contamination without reported confirmed consequences (6), and an episode with unclear outcome (1) (see Table 3).
Table 3.
Reported episodes of contaminated quaternary ammonium disinfectant or antiseptic. Cite at line 341
| Year | Author | Pathogen | Product Type | Type of Report |
|---|---|---|---|---|
| 1951 | Lowbury | Pseudomonas pyocyanea | Antiseptic | Infection outbreak |
| 1957 | Keown | Pseudomonas aeruginosa | Disinfectant | Infection outbreak |
| 1958 | Plotkin | Pseudomonas | Antiseptic | Infection outbreak |
| 1959 | Shickman | Pseudomonas aeruginosa | Disinfectant | Single infection |
| 1960 | Malizia | Enterobacter aerogenes | Antiseptic | Infection outbreak |
| 1961 | Lee | Pseudomonas/ Achromobacter group | Antiseptic | Infection outbreak |
| 1967 | Burdon | Pseudomonas multivorans | Antiseptic | No confirmed infections |
| 1969 | CDC* | Pseudomonas kingii | Antiseptic | Infection outbreak |
| 1970 | Hardy | Pseudomonas EO-1 | Disinfectant | Infection outbreak |
| 1970 | Bassett | Burkholderia cepacia ** | Antiseptic | Infection outbreak |
| 1970 | Gilardi | Pseudomonas EO-1 | Antiseptic | Outcome unclear |
| 1976 | Dixon | Pseudomonas species | Disinfectant | Infection outbreak |
| 1976 | Kaslow | Burkholderia cepacia; Enterobacter | Antiseptic | Pseudo-outbreak |
| 1976 | Frank | Burkholderia cepacia | Antiseptic | Infection outbreak |
| 1976 | Morris | Burkholderia cepacia | Antiseptic | Single infection |
| 1976 | Guinness | Burkholderia cepacia | Antiseptic | Infection outbreak |
| 1976 | Wishart | Stenotrophomonas maltophilia | Antiseptic | Infection outbreak |
| 1980 | Ehrenkranz | Serratia marcescens | Disinfectant | Infection outbreak; contaminated surfaces |
| 1981 | Fox | Serratia marcescens | Antiseptic | Infection outbreak (dogs and cats) |
| 1982 | Van Damme | Serratia marcescens | Antiseptic | Outbreak of bovine mastitis infections |
| 1984 | Sautter | Serratia marcescens | Antiseptic | Single infection |
| 1987 | Nakashima | Serratia marcescens | Antiseptic | Infection outbreak |
| 1988 | Gahrn-Hansen | Achromobacter xylosoxidans | Disinfectant | Infection outbreak |
| 1990 | Georgia DPH | Mycobacterium chelonae | Antiseptic | Infection outbreak |
| 1996 | Nagai | Pseudomonas fluorescens | Disinfectant | No infections |
| 1996 | Oie | Burkholderia cepacia; Pseudomonas aeruginosa; Pseudomonas fluorescens |
Antiseptic and Disinfectant |
No Infections |
| 1999 | Olson | Pseudomonas aeruginosa | Disinfectant | Infection outbreak |
| 2000 | Kaitwatcharachai | Burkholderia cepacia | Disinfectant | Infection outbreak |
| 2002 | Lehours | Achromobacter xylosoxidans | Disinfectant | Infection outbreak |
| 2003 | Tiwari | Mycobacterium abscessus | Antiseptic | Infection outbreak |
| 2003 | Gajadhar | Pseudomonas | Antiseptic | Survey |
| 2005 | Ebner | Burkholderia cepacia | Disinfectant | Pseudo-outbreak |
| 2005 | Fisher | Pseudomonas aeruginosa | Antiseptic | Infection outbreak |
| 2006 | Lo Cascio | Burkholderia cenocepacia | Disinfectant | Infection outbreak |
| 2007 | Siebor | Pseudomonas fluorescens; Achromobacter xylosoxidans | Disinfectant | Pseudo-outbreak |
| 2008 | Lee CS | Burkholderia cepacia | Antiseptic | Infection outbreak |
| 2010 | Hakuno | Pseudomonas fluorescens;Burkholderia cepacia; Aeromonas species | Antiseptic | No infections |
| 2014 | Kampf | Achromobacter species; Serratia marcescens | Disinfectant | Survey |
| 2015 | Kupfahl | Achromobacter species | Disinfectant | Survey |
| 2015 | Hugon | Achromobacter denitificans | Disinfectant | Infection outbreak; contaminated surfaces |
| 2016 | Tandel | Burkholderia cepacia | Antiseptic | Pseudo-outbreak |
| 2021 | FDA |
Burkholderia cepacia complex Ralstonia pickettii |
Hand sanitizer | No reported infections |
| 2022 | Boyce | Serratia marcescens; Achromobacter xylosoxidans | Disinfectant | No infections; contaminated high-touch surfaces |
* Centers for Disease Control and Prevention
** Originally classified as Pseudomonas cepacia (mulivorans)
Episodes of contamination involved skin antiseptics (24), surface disinfectants (17), a product used as both antiseptic and disinfectant (1), and a hand sanitizer (1). The most common QAC-resistant microorganisms responsible for contaminated disinfectants and antiseptics were Pseudomonas species (17), Burkholderia species (13), Achromobacter species (8), and Serratia marcescens (7) (Table 3). The types of infections reported in 29 episodes of contamination included bloodstream infections (15 episodes), wound infections (5), skin abscesses (2), septic arthritis (2), meningitis (2), urinary tract (3), ear cartilage infections (1), respiratory tract (1), and IV catheters (1), with more than one type of infection reported in several episodes [91, 92]. Contamination of surfaces in patient rooms occurred in three episodes [25, 113]. In one of the three episodes, bloodstream infections may have resulted from contamination of intravascular catheters by aerosols created by spraying of the implicated disinfectant, or by contamination of catheter materials from contaminated surfaces [113]. Contamination of surfaces in healthcare environments has also led to healthcare personnel hand contamination [96].
The frequency of reported episodes of contaminated disinfectants is probably an underestimate of the actual frequency. Kampf et al. [111] surveyed disinfectant buckets in 15 German hospitals and found that 42% of disinfectant buckets (often containing QAC-based products) from 11 hospitals were contaminated with Achromobacter spp. or Serratia. Kupfahl et al. [112] reported that 47% of 30 buckets containing QAC disinfectants in four medical centers were contaminated with up to 1 × 104 CFU of Achromobacter spp. During the time period when buckets were sampled, an Achromobacter strain with the same pulsed-field gel electrophoresis (PFGE) pattern as that recovered from several contaminated disinfectant buckets was responsible for a nosocomial infection. The direction of spread (buckets to patient vs. patient to buckets) was not established.
The mechanisms responsible for the QAC resistance of the contaminating pathogens were not determined in most episodes, due to the lack of appropriate molecular methods or laboratory resources when contamination was discovered. In one study, resistance was attributed to decreased adsorption of BAC to the cell surface and changes consistent with an efflux pump [24]. S. marcescens isolates recovered from contaminated footbaths in several dairy farms were not killed when inoculated into a fresh in-use concentration of BAC, which reduced S. marcescens ATCC 13,880 by > 5 log10 [118]. The study suggested that reduced susceptibility was not due to decreased membrane permeability, but may have been due in part to the ability to form biofilm. In a recent study, whole genome sequencing revealed that S. marcescens recovered from an in-use hospital disinfectant contained sdeXY, sdeAB, smfY, and a sugE-like gene, which have been shown in earlier studies to encode for efflux pumps that confer reduced susceptibility to QAC compounds [25, 118]. The potential role of other mechanisms of resistance including biofilm formation was not assessed.
Combined tolerance or resistance to QACs and antibiotics
Publications dealing with combined resistance or tolerance to both QACs and antibiotics have often used the terms cross-resistance or co-resistance. Cross-resistance is due to one mechanism that results in reduced susceptibility to two or more dissimilar agents. Examples include a single multidrug efflux pump that exports both QACs and antibiotics, or changes in cell membrane structure that affect both types of compounds [119, 120]. Co-resistance occurs when dissimilar mechanisms of reduced susceptibility are linked by two or more resistance genes that encode for unrelated resistance mechanisms. Examples would be linkage of genes encoding for QAC efflux pumps and genes encoding antibiotic resistance by production of an inactivating enzyme or by an altered intracellular target [27, 52, 121]. However, it is important to note that articles referring to cross-resistance and co-resistance often describe pathogens with QAC tolerance combined with antibiotic resistance.
Combined QAC and antibiotic resistance in nonlaboratory settings
Several investigators who obtained pathogens from community settings or clinical isolates from hospitals have described isolates with tolerance (reduced susceptibility) to QACs plus clinically-defined antibiotic resistance [22, 40, 52, 89, 122, 123]. A 2008 study found a significant correlation between BAC tolerance and clinically-defined antibiotic resistance among Gram-negative bacteria found in home settings [124]. A number of these studies did not establish if the combined QAC tolerance and antibiotic resistance was due to cross-resistance or co-resistance. Instances of combined resistance to QACs and antibiotics have occasionally resulted in clinically significant consequences. For example, QAC resistance combined with antibiotic resistance (based on currently existing Clinical Laboratory Standards Institute breakpoints) has occurred in bacteria recovered in several episodes of contamination of in-use antiseptics or disinfectants [25, 103, 104, 113, 125–127]. In one episode caused by S. marcescens, co-resistance was due at least in part to the presence of multiple QAC efflux pumps and antibiotic resistance that was most likely due to an inducible chromosomally-mediated AmpC beta-lactamase, which is common in S. marcescens [25, 128]. In several of the above-mentioned episodes, contaminating pathogens with combined resistance to QACs and antibiotics caused healthcare-associated infections [103, 113, 125, 126].
Hospital sink drains contaminated with pathogens with high BAC or DDAC MICs and clinically-defined antibiotic resistance is another example of how combined resistance to QACs and antibiotics may be of clinical significance. For example, in one hospital, multidrug resistant extended-spectrum beta-lactamase producing Enterobacter cloacae strains with elevated BAC and DDAC MICs (64–512 ug/ml) recovered from hospital sink drain biofilms had the same PFGE type as strains responsible for healthcare-associated infections among patients cared for on the ward with contaminated sinks [129]. When the practice of pouring a DDAC-based disinfectant down sink drains was replaced by using a bleach-based solution and biofilms were removed from sinks, the incidence of infections decreased shortly thereafter, suggesting that the sinks may have been the source of healthcare-associated infections [129]. Sink-related outbreaks of infection are likely due at least in part to droplet transmission of pathogens from sinks to the hands of healthcare personnel, with subsequent spread to patients [130].
Potential role of QAC use on antibiotic resistance
It has been stated that there is no evidence that use of antiseptics or disinfectants selects for antibiotic-resistant microorganisms in nature, or that such mutants survive in nature [34, 131]. Nonetheless, the widespread use of QAC disinfectants and growing frequency of multidrug-resistant healthcare-associated pathogens has increased concerns about whether or not use of QAC-based disinfectants promotes the emergence of tolerance or resistance to antibiotics, or to combined resistance to both antibiotics and QACs. Evidence cited in support of this concern includes the in vitro adaptive studies mentioned above wherein repeated or prolonged exposure to QACs resulted in increases in QAC and antibiotic MICs, sometimes to levels equal to in-use disinfectant concentrations and clinically relevant increases in antibiotic MICs [5, 78, 81–84].
For example, an adaptive study found that exposing Ps. aeruginosa to BAC resulted in both increased BAC MICs and mutations in the polymixin resistance gene pmrB and increased tolerance to polymixin [27]. A recent adaptive study repeatedly exposed E. coli to BAC in liquid media in a manner that was designed to mimic repeated application of disinfectant on surfaces yielded BAC-tolerant presistent mutants that had no change in BAC MIC, but had 2-fold increases in MICs to ampicillin or ciprofloxacin, or both [31]. Although the persistent cells had a slower growth rate, they had improved ability to survive sub-inhibitory concentrations of antibiotics, suggesting that the BAC-tolerant persistent mutants may have a selective advantage in environments where antibiotics are present [31]. It may be useful to conduct similar experiments on hard surfaces using commerical disinfectants to see if similar mutant persisters also occur in real-world settings.
The occurrence of mobile genetic determinants (e.g. plasmids, transposons, integrons) that carry genes that encode for tolerance to QACs and others that encode for tolerance to antibiotics also raises the concern that QAC use might lead to decreased susceptibility to antibiotics [52, 55, 132]. A study that utilized an E. coli donor strain carrying an RP4 plasmid carrying resistance to ampicillin, kanamycin and tetracycline evaluated the effect of exposure to dodecyl dimethyl benzyl ammonium chloride (DDBAC) on plasmid conjugation [132]. Exposure to DDBAC promoted conjugative transfer of the antibiotic-resistance plasmid to a recipient E. coli strain.
Buffet-Bataillon et al. [52] identified clinical strains of E. coli that contained a class 1 integron containing genes encoding for reduced susceptibility to trimethoprim-sulfamethoxazole and QACs (dfrA/Sul1 and qacE∆1, respectively). The same strains also showed overexpression of the efflux pump element tolC and its regulators, which was associated with reduced susceptibility to QACs and ciprofloxacin [52]. The presence of both qacH and a gene (sul3) encoding increased tolerance to sulfomamides on a conjugative plasmid in Proteus mirabilis raises the possibility of co-transfer of QAC and antibiotic tolerance [55]. E. coli strains recovered from retail meats in China have been shown to contain plasmids with integrons that carry genes encoding for tolerance to both QACs and antibiotics [133]. The authors of the latter study demonstrated that the plasmids could be horizontally transferred to an E. coli recipient strain. While the occurrence of genes that confer tolerance or resistance to both QACs and antibiotics (co-resistance) raises the possibility that widespread use of QACs may promote antibiotic resistance, dissemination of clinically-significant antibiotic resistance by this mechanism has not been established in non-laboratory settings.
It seems likely that emergence of QAC-resistant pathogens in contaminated in-use disinfectants occurs when bacteria with intrinsic mechanisms of QAC resistance and ability to form biofilms (e.g., Pseudomonas spp. and Burkholderia cepacia) are repeatedly exposed to sublethal QAC concentrations due to suboptimal disinfection practices. It is not clear if exposure to the QAC selects for clinically-relevant levels of antibiotic resistance similar to what has been shown in in vitro studies, or if the pathogens were already antibiotic-resistant when inadvertently introduced into disinfectant containers.
In vitro studies suggest that sink drain biofilms may provide a milieu in which repeated or prolonged exposure of mixed communities of bacteria to QACs may result in enrichment of Gram-negative organisms (e.g., Pseudomonas spp.) with intrinsic tolerance to QACs, the ability to encode for efflux pumps and form biofilm, and perhaps to degrade QACs, resulting in sub-inhibitory concentrations of QACs that allow the bacteria to survive and develop resistance to both QACs and antibiotics [21, 27, 37, 39, 77, 83]. The same may be true for Burkholderia spp., which also possess many of the same microbial properties [38, 70, 134]. Alternatively, exposure of pathogens to QACs in this setting may result solely in emergence of QAC tolerance or resistance in pathogens which were already antibiotic-resistant when they became trapped in sink drain biofilm.
Currently, there is no evidence that accumulation on hard surfaces of sub-inhibitory concentrations of QACs has resulted in selection of antibiotic-resistant pathogens. In fact, there are very little published data regarding QAC levels on surfaces routinely disinfected with QAC-based products. A recent study found that a single application to surfaces previously exposed to a QAC disinfectant resulted in only minor increases in the QAC concentrations on school desks [135]. Repeated application of BAC to surfaces in buses led to increasing concentrations that peaked after one to two weeks [136]. A one-time sampling of surfaces in a hospital nursing station revealed BAC levels ranging from 6.9 to 76.6 ug/100 cm [2, 10].
Research gaps
Although QAC-based disinfectants and antiseptics have been widely used for decades, there are issues related to their use that are poorly understood and require additional research. A brief list of issues that would benefit from additional research is noted below:
Our understanding of QAC tolerance and resistance would benefit from greater utilization of standardized definitions of these terms by investigators.
Development and adoption of standard protocols for exposing pathogens to in-use concentrations of products may yield better estimates of bacterial resistance to biocides [87].
Longitudinal studies of the level of QAC accumulation on surfaces repeatedly disinfected with QAC-containing disinfectants and the pathogens recovered from such surfaces may provide data regarding whether or not sub-inhibitory QAC concentrations on surfaces may promote QAC resistance or antibiotic resistance, as noted in some laboratory-based adaptive experiments.
Data are needed regarding the tolerance and resistance of newer QAC compounds, such as bis- and tris-QACs and polymeric QACs, where other moieties such as organosilanes, an oxazoline homopolymer, or silver are attached to mono- or bis-QACs [137–139].
Additional field studies are needed to establish the frequency with which in-use solutions of dilutable QAC-based disinfectants are contaminated.
Pathogens with combined tolerance/resistance to QACs plus antibiotic resistance that have been recovered from contaminated in-use disinfectants should be examined using methods that can establish the molecular mechanisms for combined resistance to determine the likelihood that exposure to QACs co-selected for or induced antibiotic resistance, or if antibiotic resistance was due solely to the presence of preexisting mechanisms of resistance resulting from widespread antibiotic use.
The potential role that sink drain biofilms may contribute to the spread of antibiotic-resistant pathogens requires further studies [129]. Longitudinal studies of sink drains utilizing whole genome sequencing and metagenomic methods similar to those conducted by Johnson RC et al. [140] and Constantinides B et al. [141] might yield information regarding possible co-selection or induction of antibiotic resistance by QACs if the studies include identification of resistance mechanisms of both QACs and antibiotics. Ideally, such studies should include measurement of QAC levels in biofilm and QAC MICs/MBCs of pathogens imbedded in biofilm, given the fact that one in vitro study found that exposure of a sink biofilm microcosm to very low levels of BAC or disinfectant solution did not result in detection of significantly increased QAC or antibiotic resistance levels [88].
Discussion
QAC-containing disinfectants are widely used due to a number of factors, including their activity against many bacteria, fungi and enveloped viruses, low cost (especially in concentrated forms), relatively good materials compatibility, and much less offensive odor when compared to sodium hypochorite (bleach) or peracetic acid-containing products. However, spore-forming organisms and mycobacteria are intrinsically resistant to QACs, and Gram-negative bacteria are generally less susceptible to QACs than Gram-positive bacteria. In vitro studies involving exposure of pathogens to increasing concentrations of QACs have shown that bacteria can utilize a variety of mechanisms to develop tolerance, or less commonly, resistance to QACs and/or antibiotics. To date, there are relatively limited examples of de novo development of QAC tolerance or resistance in real-world settings. Compared to other disinfectants and antiseptics, those containing QACs as the sole active agent are more likely to become contaminated with Gram-negative bacteria. Multiple episodes of contamination of in-use antiseptic and disinfectant solutions by pathogens with resistance to QACs (sometimes combined with antibiotic resistance) have resulted in clusters or outbreaks of healthcare-associated infections. Combined resistance to both QACs and antibiotics has also been reported in pathogens imbedded in sink biofilms exposed to a QAC disinfectant, with possible transmission of pathogens to patients.
To avoid contamination of in-use disinfectants, re-usable buckets in which dilutable QAC-based disinfectants are used should be thoroughly cleaned and dried before new disinfectant is added [25, 41]. It is important to test the in-use concentrations of dilutable QAC disinfectants following manual dilution, and after new containers of concentrated product are attached to automated dilution systems. Daily testing the concentration of dilutable QAC solutions in buckets should be considered if cotton or microfiber cloths are used, especially if multiple wipes are allowed to soak in buckets [117]. Exposure of pathogens to low and ineffective concentrations may also occur by failing to remove organic material (including biofilms), adsorption of QAC to the wipe material, or application of products at a suboptimal pH [29, 69, 117, 142]. QAC-based disinfectants should not be used to wipe the tops of blood culture bottles prior to injecting blood into culture bottles [105]. Because multiple outbreaks related to contaminated BAC-based antiseptics have occurred, the Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare no longer lists QAC-based antiseptics as one of the recommended uses of QACs in healthcare settings [34]. Of note, contaminated disinfectant/antiseptic products have been based on either BAC, DDAC, BAC + DDAC, or cetrimide-chorhexidine gluconate [25, 101, 104, 108, 113, 114, 125, 126, 143]. Disinfectants containing one or more QACs plus additional active agents (e.g., ≥ 15% alcohol) have not been reported to be contaminated.
Conclusions
The increased use of QAC-containing disinfectants during the COVID-19 pandemic has heightened concerns regarding the possible impact that widespread QAC use and environmental build-up may have on promoting antimicrobial resistance among pathogens in healthcare and other settings. However, despite some evidence from laboratory-based studies, currently there is a lack of conclusive evidence that QAC use has contributed to widespread emergence of antimicrobial resistance in real-world settings. Additional studies of the evolution of pathogens with combined QAC tolerance/resistance and antibiotic resistance in real-world settings and of the mechanisms that may be responsible for combined resistance are needed to better understand the possible role that QAC use may play in promoting clinically-significant antibiotic resistance.
Acknowledgements
The author thanks GOJO Scientists James W. Arbogast, PhD, Charles Crawford, PhD, Michael J. Dolan, David R. Macinga, PhD, Clyde S. Manuel, PhD, and Antonio Quinones-Rivera, PhD for providing a critical review of the manuscript.
Author Contribution
JMB performed the literature searches, distilled and interpreted the information presented in downloaded articles, and was the sole author who wrote the manuscript.
Funding
This literature review was funded by GOJO Industries. GOJO employees played no role in the design of the study, or data collection, or analysis. GOJO scientists made a limited number of suggestions regarding wording of the penultimate manuscript. JMB had complete control and approval of the final content.
Data Availability
Data used to form tables and figures are available in articles available in the PubMed database.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JMB is a consultant to, has received travel support from, and has spoken at conferences supported by GOJO Industries and by Diversey, and has been a consultant to Sodexo Healthcare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Merchel Piovesan Pereira BT. Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl Environ Microbiol. 2019;85:e00377–00319. doi: 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewey HM. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem Health Saf. 2022;29:27–38. doi: 10.1021/acs.chas.1c00026. [DOI] [Google Scholar]
- 3.Zheng G. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ Sci Technol Lett. 2020;7:760–5. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hora PI. Increased use of quaternary ammonium compounds during SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7:622–31. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 5.Jia Y, Lu H, Zhu L. Molecular mechanism of antibiotic resistance induced by mono- and twin-chained quaternary ammonium compounds. Sci Total Environ. 2022;832:155090. doi: 10.1016/j.scitotenv.2022.155090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharpure R, Hunter CM, Schnall AH, et al. Knowledge and practices regarding safe household cleaning and disinfection for COVID-19 prevention - United States, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:705–9. doi: 10.15585/mmwr.mm6923e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng G, Webster TF, Salamova A. Quaternary ammonium compounds: Bioaccumulation potentials in humans and levels in blood before and during the Covid-19 pandemic. Environ Sci Technol. 2021;55:14689–98. doi: 10.1021/acs.est.1c01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hrubec TC, Seguin RP, Xu L, et al. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol Rep. 2021;8:646–56. doi: 10.1016/j.toxrep.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng G, Schreder E, Sathyanarayana S, Salamova A. The first detection of quaternary ammonium compounds in breast milk: implications for early-life exposure. J Expo Sci Environ Epidemiol. 2022;32:682–8. doi: 10.1038/s41370-022-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBouf RF, Virji MA, Ranpara A, Stefaniak AB. Air and surface sampling method for assessing exposures to quaternary ammonium compounds using liquid chromatography tandem mass spectrometry. Ann Work Expo Health. 2017;61:724–36. doi: 10.1093/annweh/wxx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren BG, Barrett A, Graves A, King C, Turner NA, Anderson DJ. An enhanced strategy for daily disinfection in acute care hospital rooms: a randomized clinical trial. JAMA Netw Open. 2022;5:e2242131. doi: 10.1001/jamanetworkopen.2022.42131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Environmental Protection Agency. EPA orders Allied BioScience to stop selling and distributing SurfaceWise2. https://www.epa.gov/newsreleases/epa-orders-allied-bioscience-stop-selling-and-distributing-surfacewise2. Accessed March 23, 2023.
- 13.Maillard JY. Impact of benzalkonium chloride, benzethonium chloride and chloroxylenol on bacterial antimicrobial resistance. J Appl Microbiol. 2022;133:3322–46. doi: 10.1111/jam.15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerin-Mechin L, Dubois-Brissonnet F, Heyd B, Leveau JY. Specific variations of fatty acid composition of Pseudomonas aeruginosa ATCC 15442 induced by quaternary ammonium compounds and relation with resistance to bactericidal activity. J Appl Microbiol. 1999;87:735–42. doi: 10.1046/j.1365-2672.1999.00919.x. [DOI] [PubMed] [Google Scholar]
- 15.Maseda H, Hashida Y, Konaka R, Shirai A, Kourai H. Mutational upregulation of a resistance-nodulation-cell division-type multidrug efflux pump, SdeAB, upon exposure to a biocide, cetylpyridinium chloride, and antibiotic resistance in Serratia marcescens. Antimicrob Agents Chemother. 2009;53:5230–5. doi: 10.1128/AAC.00631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moen B, Rudi K, Bore E, Langsrud S. Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of Escherichia coli with inheritable characteristics. Int J Mol Sci. 2012;13:4101–23. doi: 10.3390/ijms13044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Environmental Protection Agency. Alkyl dimethyl benzyl ammonium chloride (ADBAC) final work plan. 2017:1–81. https://www.turi.org/content/download/13430/205412/file/ADBAC_EPA_final_workplan_2017_EPA-HQ-OPP-2015-0737-0004.pdf. Accessed 3.10.22
- 18.Russell AD. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect Dis. 2003;3:794–803. doi: 10.1016/S1473-3099(03)00833-8. [DOI] [PubMed] [Google Scholar]
- 19.Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:e00088–17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mc Carlie S, Boucher CE, Bragg RR. Molecular basis of bacterial disinfectant resistance. Drug Resist Updat. 2020;48:100672. doi: 10.1016/j.drup.2019.100672. [DOI] [PubMed] [Google Scholar]
- 21.Tezel U, Pavlostathis SG. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol. 2015;33:296–304. doi: 10.1016/j.copbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 22.He GX, Landry M, Chen H, et al. Detection of benzalkonium chloride resistance in community environmental isolates of staphylococci. J Med Microbiol. 2014;63:735–41. doi: 10.1099/jmm.0.073072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz S, Lopez-Alonso V, Rodriguez P, Martinez-Suarez JV. The connection between persistent, disinfectant-resistant Listeria monocytogenes strains from two geographically separate Iberian pork processing plants: evidence from comparative genome analysis. Appl Environ Microbiol. 2016;82:308–17. doi: 10.1128/AEM.02824-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai K, Murata T, Ohta S, Zenda H, Ohnishi M, Hayashi T. Two different mechanisms are involved in the extremely high-level benzalkonium chloride resistance of a Pseudomonas fluorescens strain. Microbiol Immunol. 2003;47:709–15. doi: 10.1111/j.1348-0421.2003.tb03440.x. [DOI] [PubMed] [Google Scholar]
- 25.Boyce JM, Havill NL. In-use contamination of a hospital-grade disinfectant. Am J Infect Control. 2022;50:1296–301. doi: 10.1016/j.ajic.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Pagedar A, Singh J, Batish VK. Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J Dairy Res. 2012;79:383–9. doi: 10.1017/S0022029912000295. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Weigand MR, Oh S, et al. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl Environ Microbiol. 2018;84:e01201–18. doi: 10.1128/AEM.01201-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sousa-Silva M, Simoes M, Melo L, Machado I. Pseudomonas fluorescens tolerance to benzyldimethyldodecyl ammonium chloride: altered phenotype and cross-resistance. J Glob Antimicrob Resist. 2018;15:188–95. doi: 10.1016/j.jgar.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Kampf G. Adaptive microbial response to low-level benzalkonium chloride exposure. J Hosp Infect. 2018;100:e1–e22. doi: 10.1016/j.jhin.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Loughlin MF, Jones MV, Lambert PA. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J Antimicrob Chemother. 2002;49:631–9. doi: 10.1093/jac/49.4.631. [DOI] [PubMed] [Google Scholar]
- 31.Nordholt N, Kanaris O, Schmidt SBI, Schreiber F. Persistence against benzalkonium chloride promotes rapid evolution of tolerance during periodic disinfection. Nat Commun. 2021;12:6792. doi: 10.1038/s41467-021-27019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denyer SP, Maillard JY. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J Appl Microbiol. 2002;92(Suppl):35S–45S. doi: 10.1046/j.1365-2672.92.5s1.19.x. [DOI] [PubMed] [Google Scholar]
- 34.Rutala WA, Weber DJ, the Healthcare Infection Practices Advisory Committee (HICPAC). and. Guideline for disinfection and sterilization in healthcare facilities, 2008 - Update 2019:1-163. https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf Accessed March 10, 2022.
- 35.Tung G, Macinga D, Arbogast J, Jaykus LA. Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. J Food Prot. 2013;76:1210–7. doi: 10.4315/0362-028X.JFP-12-532. [DOI] [PubMed] [Google Scholar]
- 36.Cadnum JL, Shaikh AA, Piedrahita CT, et al. Relative resistance of the emerging fungal pathogen Candida auris and other Candida species to killing by ultraviolet light. Infect Control Hosp Epidemiol. 2018;39:94–6. doi: 10.1017/ice.2017.239. [DOI] [PubMed] [Google Scholar]
- 37.Poole K. Efflux pumps as antimicrobial resistance mechanisms. Ann Med. 2007;39:162–76. doi: 10.1080/07853890701195262. [DOI] [PubMed] [Google Scholar]
- 38.Ahn Y, Kim JM, Kweon O, et al. Intrinsic resistance of Burkholderia cepacia complex to benzalkonium chloride. mBio. 2016;7:e01716–16. doi: 10.1128/mBio.01716-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tattawasart U, Maillard JY, Furr JR, Russell AD. Comparative responses of Pseudomonas stutzeri and Pseudomonas aeruginosa to antibacterial agents. J Appl Microbiol. 1999;87:323–31. doi: 10.1046/j.1365-2672.1999.00811.x. [DOI] [PubMed] [Google Scholar]
- 40.Rose H, Baldwin A, Dowson CG, Mahenthiralingam E. Biocide susceptibility of the Burkholderia cepacia complex. J Antimicrob Chemother. 2009;63:502–10. doi: 10.1093/jac/dkn540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber DJ, Rutala WA, Sickbert-Bennett E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51:4217–24. doi: 10.1128/AAC.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechin L, Dubois-Brissonnet F, Heyd B, Leveau JY. Adaptation of Pseudomonas aeruginosa ATCC 15442 to didecyldimethylammonium bromide induces changes in membrane fatty acid composition and in resistance of cells. J Appl Microbiol. 1999;86:859–66. doi: 10.1046/j.1365-2672.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 43.To MS, Favrin S, Romanova N, Griffiths MW. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl Environ Microbiol. 2002;68:5258–64. doi: 10.1128/AEM.68.11.5258-5264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadea R, Fernandez Fuentes MA, Perez Pulido R, Galvez A, Ortega E. Effects of exposure to quaternary-ammonium-based biocides on antimicrobial susceptibility and tolerance to physical stresses in bacteria from organic foods. Food Microbiol. 2017;63:58–71. doi: 10.1016/j.fm.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 45.Machado I, Coquet L, Jouenne T, Pereira MO. Proteomic approach to Pseudomonas aeruginosa adaptive resistance to benzalkonium chloride. J Proteom. 2013;89:273–9. doi: 10.1016/j.jprot.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Tattawasart U, Maillard JY, Furr JR, Russell AD. Outer membrane changes in Pseudomonas stutzeri resistant to chlorhexidine diacetate and cetylpyridinium chloride. Int J Antimicrob Agents. 2000;16:233–8. doi: 10.1016/S0924-8579(00)00206-5. [DOI] [PubMed] [Google Scholar]
- 47.Tabata A, Nagamune H, Maeda T, Murakami K, Miyake Y, Kourai H. Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR. Antimicrob Agents Chemother. 2003;47:2093–9. doi: 10.1128/AAC.47.7.2093-2099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangalappalli-Illathu AK, Vidovic S, Korber DR. Differential adaptive response and survival of Salmonella enterica serovar enteritidis planktonic and biofilm cells exposed to benzalkonium chloride. Antimicrob Agents Chemother. 2008;52:3669–80. doi: 10.1128/AAC.00073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buffet-Bataillon S, Tattevin P, Maillard JY, Bonnaure-Mallet M, Jolivet-Gougeon A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016;11:81–92. doi: 10.2217/fmb.15.131. [DOI] [PubMed] [Google Scholar]
- 50.Meade E, Slattery MA, Garvey M. Biocidal resistance in clinically relevant microbial species: a major public health risk. Pathogens. 2021;10:598. doi: 10.3390/pathogens10050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slipski CJ, Zhanel GG, Bay DC. Biocide selective TolC-independent efflux pumps in Enterobacteriaceae. J Membr Biol. 2018;251:15–33. doi: 10.1007/s00232-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buffet-Bataillon S, Le Jeune A, Le Gall-David S, Bonnaure-Mallet M, Jolivet-Gougeon A. Molecular mechanisms of higher MICs of antibiotics and quaternary ammonium compounds for Escherichia coli isolated from bacteraemia. J Antimicrob Chemother. 2012;67:2837–42. doi: 10.1093/jac/dks321. [DOI] [PubMed] [Google Scholar]
- 53.Shahcheraghi F, Minato Y, Chen J, et al. Molecular cloning and characterization of a multidrug efflux pump, SmfY, from Serratia marcescens. Biol Pharm Bull. 2007;30:798–800. doi: 10.1248/bpb.30.798. [DOI] [PubMed] [Google Scholar]
- 54.Wassenaar TM, Ussery D, Nielsen LN, Ingmer H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol (Bp) 2015;5:44–61. doi: 10.1556/EuJMI-D-14-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang X, Yu T, Liu L, et al. Examination of quaternary ammonium compound resistance in Proteus mirabilis isolated from cooked meat products in China. Front Microbiol. 2017;8:2417. doi: 10.3389/fmicb.2017.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller A, Rychli K, Zaiser A, Wieser C, Wagner M, Schmitz-Esser S. The Listeria monocytogenes transposon Tn6188 provides increased tolerance to various quaternary ammonium compounds and ethidium bromide. FEMS Microbiol Lett. 2014;361:166–73. doi: 10.1111/1574-6968.12626. [DOI] [PubMed] [Google Scholar]
- 57.Dutta V, Elhanafi D, Kathariou S. Conservation and distribution of the benzalkonium chloride resistance cassette bcrABC in Listeria monocytogenes. Appl Environ Microbiol. 2013;79:6067–74. doi: 10.1128/AEM.01751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toba S, Minato Y, Kondo Y, et al. Comprehensive analysis of resistance-nodulation-cell division superfamily (RND) efflux pumps from Serratia marcescens, Db10. Sci Rep. 2019;9:4854. doi: 10.1038/s41598-019-41237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bay DC, Turner RJ. Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants. J Bacteriol. 2012;194:5941–8. doi: 10.1128/JB.00666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovacevic J, Ziegler J, Walecka-Zacharska E, Reimer A, Kitts DD, Gilmour MW. Tolerance of Listeria monocytogenes to quaternary ammonium sanitizers is mediated by a novel efflux pump encoded by emrE. Appl Environ Microbiol. 2016;82:939–53. doi: 10.1128/AEM.03741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Mukherjee MM, Varela MF. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int J Bacteriol 2013;2013. 10.1155/2013/204141. [DOI] [PMC free article] [PubMed]
- 62.Jennings MC, Minbiole KP, Wuest WM. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect Dis. 2015;1:288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 63.Deus D, Krischek C, Pfeifer Y, et al. Comparative analysis of the susceptibility to biocides and heavy metals of extended-spectrum beta-lactamase-producing Escherichia coli isolates of human and avian origin, Germany. Diagn Microbiol Infect Dis. 2017;88:88–92. doi: 10.1016/j.diagmicrobio.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Kazama H, Hamashima H, Sasatsu M, Arai T. Distribution of the antiseptic-resistance gene qacE delta 1 in Gram-positive bacteria. FEMS Microbiol Lett. 1998;165:295–9. doi: 10.1111/j.1574-6968.1998.tb13160.x. [DOI] [PubMed] [Google Scholar]
- 65.Hassan KA, Liu Q, Henderson PJ, Paulsen IT. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio. 2015;6:e01982–14. doi: 10.1128/mBio.01982-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol. 2008;74:7376–82. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alav I, Sutton JM, Rahman KM. Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother. 2018;73:2003–20. doi: 10.1093/jac/dky042. [DOI] [PubMed] [Google Scholar]
- 68.Smith K, Hunter IS. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966–73. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- 69.Condell O, Iversen C, Cooney S, et al. Efficacy of biocides used in the modern food industry to control salmonella enterica, and links between biocide tolerance and resistance to clinically relevant antimicrobial compounds. Appl Environ Microbiol. 2012;78:3087–97. doi: 10.1128/AEM.07534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rushton L, Sass A, Baldwin A, Dowson CG, Donoghue D, Mahenthiralingam E. Key role for efflux in the preservative susceptibility and adaptive resistance of Burkholderia cepacia complex bacteria. Antimicrob Agents Chemother. 2013;57:2972–80. doi: 10.1128/AAC.00140-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27:1017–32. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 72.Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol. 2002;92(Suppl):98S–110S. doi: 10.1046/j.1365-2672.92.5s1.5.x. [DOI] [PubMed] [Google Scholar]
- 73.Ganeshnarayan K, Shah SM, Libera MR, Santostefano A, Kaplan JB. Poly-N-acetylglucosamine matrix polysaccharide impedes fluid convection and transport of the cationic surfactant cetylpyridinium chloride through bacterial biofilms. Appl Environ Microbiol. 2009;75:1308–14. doi: 10.1128/AEM.01900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol. 2008;190:4447–52. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rutala WA, Weber DJ. Best practices for disinfection of noncritical environmental surfaces and equipment in health care facilities: a bundle approach. Am J Infect Control. 2019;47S:A96–A105. doi: 10.1016/j.ajic.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Nishihara T, Okamoto T, Nishiyama N. Biodegradation of didecyldimethylammonium chloride by Pseudomonas fluorescens TN4 isolated from activated sludge. J Appl Microbiol. 2000;88:641–7. doi: 10.1046/j.1365-2672.2000.01007.x. [DOI] [PubMed] [Google Scholar]
- 77.Oh S, Kurt Z, Tsementzi D, et al. Microbial community degradation of widely used quaternary ammonium disinfectants. Appl Environ Microbiol. 2014;80:5892–900. doi: 10.1128/AEM.01255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mc Cay PH, Ocampo-Sosa AA, Fleming GTA. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiol (Reading) 2010;156:30–8. doi: 10.1099/mic.0.029751-0. [DOI] [PubMed] [Google Scholar]
- 79.Langsrud S, Sundheim G, Holck AL. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J Appl Microbiol. 2004;96:201–8. doi: 10.1046/j.1365-2672.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- 80.Braoudaki M, Hilton AC. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J Clin Microbiol. 2004;42:73–8. doi: 10.1128/JCM.42.1.73-78.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim M, Hatt JK, Weigand MR, Krishnan R, Pavlostathis SG, Konstantinidis KT. Genomic and transcriptomic insights into how bacteria withstand high concentrations of benzalkonium chloride biocides. Appl Environ Microbiol. 2018;84:e00197–18. doi: 10.1128/AEM.00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rakic-Martinez M, Drevets DA, Dutta V, Katic V, Kathariou S. Listeria monocytogenes strains selected on ciprofloxacin or the disinfectant benzalkonium chloride exhibit reduced susceptibility to ciprofloxacin, gentamicin, benzalkonium chloride, and other toxic compounds. Appl Environ Microbiol. 2011;77:8714–21. doi: 10.1128/AEM.05941-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tandukar M, Oh S, Tezel U, Konstantinidis KT, Pavlostathis SG. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ Sci Technol. 2013;47:9730–8. doi: 10.1021/es401507k. [DOI] [PubMed] [Google Scholar]
- 84.Soumet C, Meheust D, Pissavin C, et al. Reduced susceptibilities to biocides and resistance to antibiotics in food-associated bacteria following exposure to quaternary ammonium compounds. J Appl Microbiol. 2016;121:1275–81. doi: 10.1111/jam.13247. [DOI] [PubMed] [Google Scholar]
- 85.Guerin A, Bridier A, Le Grandois P, et al. Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in Listeria monocytogenes. Pathogens. 2021;10:220. doi: 10.3390/pathogens10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Douarre PE, Sevellec Y, Le Grandois P, Soumet C, Bridier A, Roussel S. FepR as a central genetic target in the adaptation to quaternary ammonium compounds and cross-resistance to ciprofloxacin in Listeria monocytogenes. Front Microbiol. 2022;13:864576. doi: 10.3389/fmicb.2022.864576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knapp L, Amezquita A, McClure P, Stewart S, Maillard JY. Development of a protocol for predicting bacterial resistance to microbicides. Appl Environ Microbiol. 2015;81:2652–9. doi: 10.1128/AEM.03843-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forbes S, Cowley N, Humphreys G, Mistry H, Amezquita A, McBain AJ. Formulation of Biocides increases Antimicrobial Potency and mitigates the Enrichment of nonsusceptible Bacteria in Multispecies Biofilms. Appl Environ Microbiol. 2017;83:303054–16. doi: 10.1128/AEM.03054-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romao CM, Faria YN, Pereira LR, Asensi MD. Susceptibility of clinical isolates of multiresistant Pseudomonas aeruginosa to a hospital disinfectant and molecular typing. Mem Inst Oswaldo Cruz. 2005;100:541–8. doi: 10.1590/S0074-02762005000500015. [DOI] [PubMed] [Google Scholar]
- 90.Khosravi AD, Montazeri EA, Maki SR. Antibacterial effects of Octenicept, and benzalkonium chloride on Acinetobacter baumannii strains isolated from clinical samples and determination of genetic diversity of isolates by RAPD-PCR method. Mol Biol Rep. 2021;48:7423–31. doi: 10.1007/s11033-021-06758-3. [DOI] [PubMed] [Google Scholar]
- 91.Lowbury EJ. Contamination of cetrimide and other fluids with Pseudomonas pyocyanea. Br J Ind Med. 1951;8:22–5. doi: 10.1136/oem.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keown KK, Gilman RA, Bailey CP. Open heart surgery; anesthesia and surgical experiences. JAMA. 1957;165:781–7. doi: 10.1001/jama.1957.02980250015004. [DOI] [PubMed] [Google Scholar]
- 93.Shickman MD, Guze LB, Pearce ML. Bacteremia following cardiac catheterization; report of a case and studies on the source. N Engl J Med. 1959;260:1164–6. doi: 10.1056/NEJM195906042602304. [DOI] [PubMed] [Google Scholar]
- 94.Burdon DW, Whitby JL. Contamination of hospital disinfectants with Pseudomonas species. Brit Med J. 1967;2:153–5. doi: 10.1136/bmj.2.5545.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gilardi GL. Characterization of EO-1 strains (Pseudomonas kingii) isolated from clinical specimens and the hospital environment. Appl Microbiol. 1970;20:521–2. doi: 10.1128/am.20.3.521-522.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ehrenkranz NJ, Bolyard EA, Wiener M, Cleary TJ. Antibiotic-sensitive Serratia marcescens infections complicating cardiopulmonary operations: contaminated disinfectant as a reservoir. Lancet. 1980;2:1289–92. doi: 10.1016/S0140-6736(80)92349-1. [DOI] [PubMed] [Google Scholar]
- 97.Van Damme DM. Mastitis caused by contaminated teat dip and dipping cup.VM/SAC Vet Med/Small Animal Clinician1982:541–544.
- 98.Gahrn-Hansen B, Alstrup P, Dessau R, et al. Outbreak of infection with Achromobacter xylosoxidans from contaminated intravascular pressure transducers. J Hosp Infect. 1988;12:1–6. doi: 10.1016/0195-6701(88)90115-6. [DOI] [PubMed] [Google Scholar]
- 99.Nagai K, Ohta S, Zenda H, Matsumoto H, Makino M. Biochemical characterization of a Pseudomonas fluorescens strain isolated from a benzalkonium chloride solution. Biol Pharm Bull. 1996;19:873–5. doi: 10.1248/bpb.19.873. [DOI] [PubMed] [Google Scholar]
- 100.Oie S, Kamiya A. Microbial contamination of antiseptics and disinfectants. Am J Infect Control. 1996;24:389–95. doi: 10.1016/S0196-6553(96)90027-9. [DOI] [PubMed] [Google Scholar]
- 101.Olson RK, Voorhees RE, Eitzen HE, Rolka H, Sewell CM. Cluster of postinjection abscesses related to corticosteroid injections and use of benzalkonium chloride. West J Med. 1999;170:143–7. [PMC free article] [PubMed] [Google Scholar]
- 102.Kaitwatcharachai C, Silpapojakul K, Jitsurong S, Kalnauwakul S. An outbreak of Burkholderia cepacia bacteremia in hemodialysis patients: an epidemiologic and molecular study. Am J Kidney Dis. 2000;36:199–204. doi: 10.1053/ajkd.2000.8295. [DOI] [PubMed] [Google Scholar]
- 103.Lehours P, Rogues AM, Occhialini A, Boulestreau H, Gachie JP, Megraud F. Investigation of an outbreak due to Alcaligenes xylosoxydans subspecies xylosoxydans by random amplified polymorphic DNA analysis. Eur J Clin Microbiol Infect Dis. 2002;21:108–13. doi: 10.1007/s10096-001-0669-x. [DOI] [PubMed] [Google Scholar]
- 104.Gajadhar T, Lara A, Sealy P, Adesiyun AA. Microbial contamination of disinfectants and antiseptics in four major hospitals in Trinidad. Rev Panam Salud Publica. 2003;14:193–200. doi: 10.1590/S1020-49892003000800006. [DOI] [PubMed] [Google Scholar]
- 105.Ebner W, Meyer E, Schulz-Huotari C, Scholz R, Zilow G, Daschner FD. Pseudocontamination of blood components with Burkholderia cepacia during quality controls. Transfus Med. 2005;15:241–2. doi: 10.1111/j.1365-3148.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 106.Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204–9. doi: 10.1016/j.amepre.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Lo Cascio G, Bonora MG, Zorzi A, et al. A napkin-associated outbreak of Burkholderia cenocepacia bacteraemia in haemodialysis patients. J Hosp Infect. 2006;64:56–62. doi: 10.1016/j.jhin.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 108.Siebor E, Llanes C, Lafon I, et al. Presumed pseudobacteremia outbreak resulting from contamination of proportional disinfectant dispenser. Eur J Clin Microbiol Infect Dis. 2007;26:195–8. doi: 10.1007/s10096-007-0260-1. [DOI] [PubMed] [Google Scholar]
- 109.Lee CS, Lee HB, Cho YG, Park JH, Lee HS. Hospital-acquired Burkholderia cepacia infection related to contaminated benzalkonium chloride. J Hosp Infect. 2008;68:280–2. doi: 10.1016/j.jhin.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Hakuno H, Yamamoto M, Oie S, Kamiya A. Microbial contamination of disinfectants used for intermittent self-catheterization. Jpn J Infect Dis. 2010;63:277–9. doi: 10.7883/yoken.63.277. [DOI] [PubMed] [Google Scholar]
- 111.Kampf G, Degenhardt S, Lackner S, Jesse K, von Ostermeyer BH. Poorly processed reusable surface disinfection tissue dispensers may be a source of infection. BMC Infect Dis. 2014;14:37. doi: 10.1186/1471-2334-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kupfahl C, Walther M, von Wendt C. Identical Achromobacter strain in reusable surface disinfection tissue dispensers and a clinical isolate. Infect Control Hosp Epidemiol. 2015;36:1362–4. doi: 10.1017/ice.2015.176. [DOI] [PubMed] [Google Scholar]
- 113.Hugon E, Marchandin H, Poiree M, Fosse T, Sirvent N. Achromobacter bacteraemia outbreak in a paediatric onco-haematology department related to strain with high surviving ability in contaminated disinfectant atomizers. J Hosp Infect. 2015;89:116–22. doi: 10.1016/j.jhin.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Tandel KB, Sen P, Gupta S, Hazra RM, Praharaj N, Kumar AK, Sahni S. Burkholderia cepacia pseudobacteremia traced to contaminated antiseptic used for skin antisepsis prior to blood collection. Rev Med Microbiol. 2016;27:136–40. doi: 10.1097/MRM.0000000000000082. [DOI] [Google Scholar]
- 115.Food US, DrugAdministration. FDA updates on hand sanitizers consumers should not use. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use. Accessed March 10, 2023.
- 116.Bassett DC, Stokes KJ, Thomas WR. Wound infection with Pseudomonas multivorans. A water-borne contaminant of disinfectant solutions. Lancet. 1970;1:1188–91. doi: 10.1016/S0140-6736(70)91783-6. [DOI] [PubMed] [Google Scholar]
- 117.Boyce JM, Sullivan L, Booker A, Baker J. Quaternary ammonium disinfectant issues encountered in an environmental services department. Infect Control Hosp Epidemiol. 2016;37:340–2. doi: 10.1017/ice.2015.299. [DOI] [PubMed] [Google Scholar]
- 118.Langsrud S, Moretro T, Sundheim G. Characterization of Serratia marcescens surviving in disinfecting footbaths. J Appl Microbiol. 2003;95:186–95. doi: 10.1046/j.1365-2672.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 119.Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds–a critical review. Int J Antimicrob Agents. 2012;39:381–9. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 120.Wales AD, Davies RH. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiot (Basel) 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sidhu MS, Heir E, Leegaard T, Wiger K, Holck A. Frequency of disinfectant resistance genes and genetic linkage with beta-lactamase transposon Tn552 among clinical staphylococci. Antimicrob Agents Chemother. 2002;46:2797–803. doi: 10.1128/AAC.46.9.2797-2803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu WJ, Fu L, Huang M, et al. Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem-resistant Acinetobacter baumannii. J Med Microbiol. 2017;66:13–7. doi: 10.1099/jmm.0.000403. [DOI] [PubMed] [Google Scholar]
- 123.Boutarfi Z, Rebiahi SA, Morghad T, et al. Biocide tolerance and antibiotic resistance of Enterobacter spp. isolated from an algerian hospital environment. J Glob Antimicrob Resist. 2019;18:291–7. doi: 10.1016/j.jgar.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Carson RT, Larson E, Levy SB, Marshall BM, Aiello AE. Use of antibacterial consumer products containing quaternary ammonium compounds and drug resistance in the community. J Antimicrob Chemother. 2008;62:1160–2. doi: 10.1093/jac/dkn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fox JG, Beaucage CM, Folta CA, Thornton GW. Nosocomial transmission of Serratia marcescens in a veterinary hospital due to contamination by benzalkonium chloride. J Clin Microbiol. 1981;14:157–60. doi: 10.1128/jcm.14.2.157-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sautter RL, Mattman LH, Legaspi RC. Serratia marcescens meningitis associated with a contaminated benzalkonium chloride solution. Infect Control. 1984;5:223–5. doi: 10.1017/S019594170006015X. [DOI] [PubMed] [Google Scholar]
- 127.Clinical Laboratory Standards Institute. CLSI M100-ED:20220 Performance Standards for Antimicrobial Susceptibility Testing, 39th Edition. 2020. https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf
- 128.Tamma PD, Doi Y, Bonomo RA, Johnson JK, Simner PJ, Antibacterial Resistance Leadership Group A primer on AmpC beta-lactamases: necessary knowledge for an increasingly multidrug-resistant world. Clin Infect Dis. 2019;69:1446–55. doi: 10.1093/cid/ciz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapuis A, Amoureux L, Bador J, et al. Outbreak of extended-spectrum beta-lactamase producing Enterobacter cloacae with high MICs of quaternary ammonium compounds in a hematology ward associated with contaminated sinks. Front Microbiol. 2016;7:1070. doi: 10.3389/fmicb.2016.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kotay SM, Donlan RM, Ganim C, Barry K, Christensen BE, Mathers AJ. Droplet- rather than aerosol-mediated dispersion is the primary mechanism of bacterial transmission from contaminated hand-washing sink traps. Appl Environ Microbiol. 2019;85:e01997–18. doi: 10.1128/AEM.01997-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Russell AD. Biocides and pharmacologically active drugs as residues and in the environment: is there a correlation with antibiotic resistance? Am J Infect Control. 2002;30:495–8. doi: 10.1067/mic.2002.124676. [DOI] [PubMed] [Google Scholar]
- 132.Han Y, Zhou ZC, Zhu L, et al. The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes. Environ Sci Pollut Res Int. 2019;26:28352–60. doi: 10.1007/s11356-019-05673-2. [DOI] [PubMed] [Google Scholar]
- 133.Jiang X, Xu Y, Li Y, et al. Characterization and horizontal transfer of qach-associated class 1 integrons in Escherichia coli isolated from retail meats. Int J Food Microbiol. 2017;258:12–7. doi: 10.1016/j.ijfoodmicro.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 134.Lucero CA, Cohen AL, Trevino I, et al. Outbreak of Burkholderia cepacia complex among ventilated pediatric patients linked to hospital sinks. Am J Infect Control. 2011;39:775–8. doi: 10.1016/j.ajic.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 135.Boles C, Maier A, Vincent M, Stewart C, Attar S, Yeomans D. Multi-route exposure sampling of quaternary ammonium compounds and ethanol surface disinfectants in a K-8 school. Indoor Air. 2022;32:e13036. doi: 10.1111/ina.13036. [DOI] [PubMed] [Google Scholar]
- 136.Lazofsky A, Doherty C, Szary P, Buckley B. A surface sampling and liquid chromatography mass spectrometry method for the analysis of quaternary ammonium compounds collected from public transportation buses in New Jersey. Emerg Contam. 2022;8:318–28. doi: 10.1016/j.emcon.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oblak E, Piecuch A, Rewak-Soroczynska J, Paluch E. Activity of gemini quaternary ammonium salts against microorganisms. Appl Microbiol Biotechnol. 2019;103:625–32. doi: 10.1007/s00253-018-9523-2. [DOI] [PubMed] [Google Scholar]
- 138.Kim M, Linstadt RTH, Ahn Ando K, Ahn J. Gemini-mediated self-disinfecting surfaces to address the contact transmission of infectious diseases. Langmuir. 2022;38:2162–73. doi: 10.1021/acs.langmuir.1c03401. [DOI] [PubMed] [Google Scholar]
- 139.Saseendran Nair S, Anand V, De Silva K, Wiles S, Swift S. The antibacterial potency and antibacterial mechanism of a commercially available surface-anchoring quaternary ammonium salt (SAQAS)-based biocide in vitro. J Appl Microbiol. 2022;133:2583–98. doi: 10.1111/jam.15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johnson RC, Deming C, Conlan S, et al. Investigation of a cluster of Sphingomonas koreensis infections. N Engl J Med. 2018;379:2529–39. doi: 10.1056/NEJMoa1803238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Constantinides B, Chau KK, Quan TP, et al. Genomic surveillance of Escherichia coli and Klebsiella spp. in hospital sink drains and patients. Microb Genom. 2020;6:mgen000391. doi: 10.1099/mgen.0.000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pascoe MJ, Mandal S, Williams OA, Maillard JY. Impact of material properties in determining quaternary ammonium compound adsorption and wipe product efficacy against biofilms. J Hosp Infect. 2022;126:37–43. doi: 10.1016/j.jhin.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Tiwari TS, Ray B, Jost KC, Jr, et al. Forty years of disinfectant failure: outbreak of postinjection Mycobacterium abscessus infection caused by contamination of benzalkonium chloride. Clin Infect Dis. 2003;36:954–62. doi: 10.1086/368192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to form tables and figures are available in articles available in the PubMed database.