Key Points
Question
What are the outcomes following aspirin vs clopidogrel for long-term maintenance in patients with diabetes who received percutaneous coronary intervention?
Findings
In this secondary analysis of the HOST-EXAM randomized clinical trial, clopidogrel was associated with lower rates of the 24-month composite end point of all-cause death, myocardial infarction, stroke, readmission due to acute coronary syndrome, and major bleeding in patients with and without diabetes.
Meaning
Clopidogrel could be considered over aspirin monotherapy regardless of the presence of diabetes in patients who have undergone coronary stenting and successfully completed dual antiplatelet therapy.
This secondary analysis of the HOST-EXAM randomized clinical trial evaluates clopidogrel vs aspirin for long-term maintenance after percutaneous coronary intervention in patients with diabetes.
Abstract
Importance
Selecting the optimal antiplatelet agent in patients who have received percutaneous coronary intervention is especially important in those with diabetes due to the heightened risk of ischemic events in this population. Studies on the efficacy and safety of clopidogrel vs aspirin for long-term maintenance after percutaneous coronary intervention in patients with diabetes are lacking.
Objective
To investigate cardiovascular outcomes with clopidogrel vs aspirin in patients with and without diabetes.
Design, Setting, and Participants
This was a post hoc analysis of the HOST-EXAM randomized clinical trial, an investigator-initiated, prospective, randomized, open-label, multicenter trial performed at 37 centers in Korea. Patients who received dual antiplatelet therapy without clinical events for 6 to 18 months after percutaneous coronary intervention with drug-eluting stents were enrolled from March 2014 to May 2018 with follow-up at 6, 12, 18, and 24 months. All 5438 patients in the original trial were included in this analysis, which was conducted from June to October 2021.
Interventions and Exposures
Enrolled patients were randomized 1:1 to clopidogrel or aspirin monotherapy. Subgroup analyses were performed by the presence of diabetes.
Main Outcomes and Measures
The main outcome was primary composite end point of all-cause death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and major bleeding (Bleeding Academic Research Consortium type 3 or 5) at 24-month follow-up.
Results
Of 5438 patients (mean [SD] age, 63.5 [10.7] years; 1384 [25.5%] female), 1860 (34.2%) had diabetes (925 in the clopidogrel arm and 935 in the aspirin arm), and 5338 (98.2%) completed follow-up. The rate of the primary composite end point was significantly lower in the clopidogrel group compared to the aspirin group in patients with diabetes (6.3% vs 9.2%; hazard ratio [HR], 0.69; 95% CI, 0.49-0.96; P = .03; absolute risk difference [ARD], 2.7%; number needed to treat [NNT], 37) and without diabetes (5.3% vs 7.0%; HR, 0.76; 95% CI, 0.58-1.00; P = .046; ARD, 1.6%, NNT, 63; P for interaction = .65). The presence of diabetes was not associated with a difference in benefit observed with clopidogrel monotherapy over aspirin for the thrombotic composite end point (HR, 0.68; 95% CI, 0.45-1.04 for patients with diabetes vs HR, 0.68; 95% CI, 0.49-0.93 for those without; P for interaction = .99) and any bleeding with Bleeding Academic Research Consortium 2, 3, or 5 (HR, 0.65; 95% CI, 0.39-1.09 for patients with diabetes vs HR, 0.74; 95% CI, 0.48-1.13 for those without; P for interaction = .71).
Conclusion and Relevance
In this study, clopidogrel monotherapy was associated with a lower rate of the primary composite end point compared to aspirin monotherapy as long-term maintenance therapy after dual antiplatelet therapy for coronary stenting in both patients with and without diabetes. Clopidogrel might thus be considered rather than aspirin in patients who have undergone coronary stenting and successfully completed dual antiplatelet therapy, regardless of diabetes status.
Trial Registration
ClinicalTrials.gov Identifier: NCT02044250
Introduction
Aspirin is the current standard for long-term maintenance antiplatelet monotherapy in patients who received coronary stenting and underwent an intended duration of dual antiplatelet therapy (DAPT) without clinical events.1 However, in the Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases-Extended Antiplatelet Monotherapy (HOST-EXAM) randomized clinical trial,2 clopidogrel was associated with better outcomes than aspirin in terms of the primary end point (a composite of all-cause death, nonfatal myocardial infarction [MI], stroke, readmission due to acute coronary syndrome [ACS], and major bleeding) 24 months after randomization in patients who had successfully completed due duration of DAPT after percutaneous coronary intervention (PCI) with drug-eluting stents.
Diabetes is a common comorbidity in patients with coronary artery disease. Patients with diabetes compared to those without diabetes who received PCI are at increased risk of ischemic events such as coronary or cerebrovascular events as well as mortality.3 Diabetes is regarded as a prothrombotic state, which is largely mediated by increased platelet turnover and P2Y12 expression.4 Thus, in this post hoc subgroup analysis of the HOST-EXAM trial, we sought to investigate the association of diabetes with the risk of clinical outcomes at 24 months and compare the outcomes between clopidogrel vs aspirin monotherapy.
Methods
Study Design and Participants
The trial protocol and statistical analysis plan are in Supplement 1. Detailed methods are described in the eMethods in Supplement 2. The HOST-EXAM trial2 was an investigator-initiated, prospective, randomized, open-labeled, multicenter trial performed at 37 centers in Korea. The detailed protocol and eligibility criteria of the HOST-EXAM trial have been reported previously.2,5 In brief, patients who maintained DAPT without clinical events for 6 to 18 months after PCI with drug-eluting stents were enrolled from March 2014 to May 2018. All participants were randomized in a 1:1 ratio to receive clopidogrel or aspirin monotherapy for 24 months after randomization and stratified by the enrollment center. The trial protocol was approved by the institutional review board at each participating center, and all participants provided written informed consent at the time of enrollment. The Seoul National University Hospital Clinical Trial Center and Medical Research Collaborating Center were responsible for the scientific conduct of the trial, data management, and independent analysis of the data. This study was conducted in accordance with the standards specified in the International Council for Harmonization Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. Data were analyzed from June to October 2021.
Study End Points and Definitions
The primary composite end point was the same as that of the original HOST-EXAM trial,2 which was defined as a composite of all-cause death, nonfatal MI, stroke, readmission due to ACS, and major bleeding (Bleeding Academic Research Consortium [BARC] type 3 or 5) at 24 months. Major secondary end points included a thrombotic composite end point (defined as cardiac death, nonfatal MI, ischemic stroke, readmission due to ACS, and definite or probable stent thrombosis), major bleeding, and any bleeding (defined as BARC type 2, 3, or 5). As an additional composite end point of this post hoc analysis, major adverse cardiovascular event was the composite of all-cause death, MI, and stroke at 24 months. Individual components of composite end points, target-vessel MI, hemorrhagic stroke, any repeat revascularization, target-vessel revascularization, target-lesion revascularization, and any minor gastrointestinal complications at 24 months were also analyzed.
The presence of diabetes was identified by each investigator at the time of enrollment. Participants were classified as having diabetes if they already had been taking nonpharmacologic or pharmacologic treatment for previously diagnosed diabetes or if they met any of the following diagnostic criteria using the American Diabetes Association definition6 at enrollment: hemoglobin A1c (HbA1c) 6.5% or greater (to convert to proportion of total hemoglobin, multiply by 0.01), fasting plasma glucose 126 mg/dL or greater (to convert to mmol/L, multiply by 0.0555), 2-hour plasma glucose 200 mg/dL or greater during an oral glucose tolerance test, or symptoms of hyperglycemia or hyperglycemic crisis with a random plasma glucose 200 mg/dL or greater.
All events were verified by the independent clinical event adjudication committee, whose members were blinded for the trial group assignments. Clinical follow-up was performed at 6, 12, 18, and 24 months (each with a window of ±3 months). The vital status of all patients was cross-checked using the National Health Insurance Service system of Korea and the Korea National Statistics System.
Statistical Analysis
Data were presented as numbers and frequencies for categorical variables, and as means and standard deviations for continuous variables. To compare between groups, the χ2 test was used for categorical variables, and an unpaired t test was used for continuous variables. Analyses were performed based on the intention-to-treat population. Cumulative incidences of the primary and secondary end points were compared between clopidogrel and aspirin monotherapy using Kaplan-Meier censoring estimates and the log-rank test. Hazard ratios (HRs) with 95% CIs of clopidogrel vs aspirin monotherapy were calculated using Cox proportional hazard models with interaction testing according to the presence of diabetes. Assuming a noninformative prior, a bayesian analysis for the thrombotic composite end point, any bleeding, primary composite end point, and major adverse cardiovascular event at 24 months according to the presence of diabetes was performed. Multivariable Cox proportional hazard models with stepwise selection method with a significance level of <.15 for entry and exit were used to identify independent predictors of 24-month primary composite end point for the diabetes and no diabetes groups. Subgroup analysis for primary composite end point was performed in the diabetes and no diabetes groups, respectively. In the diabetes group, subgroup analyses according to diabetes treatment and baseline HbA1c level were performed. P values were 2-sided, and a value <.05 was considered statistically significant. All analyses were conducted using Stata version 17.0 (StataCorp).
Results
Baseline Clinical and Angiographic Characteristics
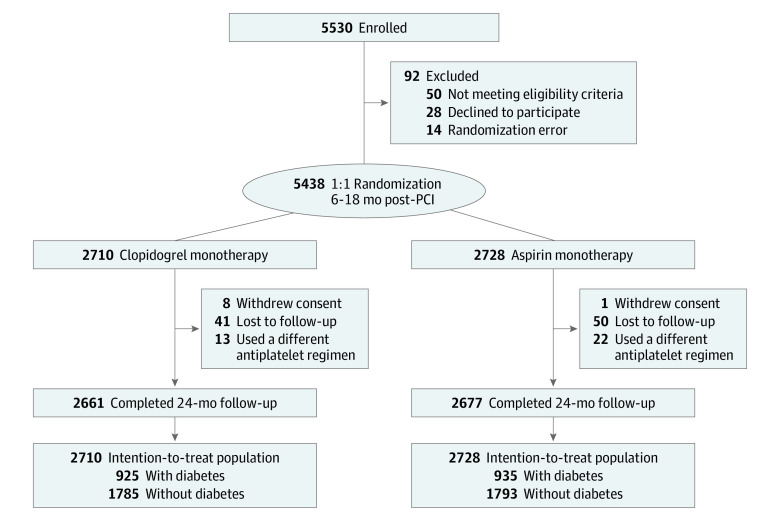
Of 5438 patients randomized (mean [SD] age, 63.5 [10.7] years; 1384 [25.5%] female), 1860 (34.2%) had diabetes at baseline (925 in the clopidogrel group and 935 in the aspirin group), and 5338 (98.2%) completed 24-month follow-up (Figure 1). The 2 groups were well balanced for overall baseline characteristics in both patients with and without diabetes (Table 1). Patients with hypertension, dyslipidemia, or chronic kidney disease and those presenting with silent ischemia were more prevalent in the diabetes group compared with the no diabetes group. The proportion of previous stroke was numerically higher in the aspirin arm compared with the clopidogrel arm of the diabetes subgroup. For the angiographic characteristics at index PCI, the diabetes group had higher proportion of 3-vessel disease and higher number of treated lesions and implanted stents with longer total length of implanted stents than the no diabetes group. Most patients with diabetes (1431 [76.9%]) were taking oral hypoglycemic agents, and 140 [7.5%] required insulin treatment. The diabetes subset with high baseline HbA1c level (≥9.0%) was 5.6% (n = 104), while 746 (40.1%) had an HbA1c level less than 7.0%. The proportions of baseline medications were evenly distributed in both the diabetes and no diabetes groups except for β-blockers in the diabetes group (eTable 1 in Supplement 2). For drug adherence, the proportion of patients who maintained the allocated medication per protocol was similar among all groups (eTable 2 in Supplement 2).
Figure 1. Study Flow.
Patients who underwent the intended duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents without any clinical events within 6 to 18 months were enrolled and underwent randomization. A primary composite end point of all-cause death, nonfatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and major bleeding at 24-month follow-up was analyzed in the intention-to-treat population stratified by the presence of diabetes at baseline. PCI indicates percutaneous coronary intervention.
Table 1. Baseline Characteristics of Study Population.
| Diabetes group, No. (%) | P value | No diabetes group, No. (%) | P value | |||
|---|---|---|---|---|---|---|
| Clopidogrel (n = 925) | Aspirin (n = 935) | Clopidogrel (n = 1785) | Aspirin (n = 1793) | |||
| Demographic characteristics and comorbidities | ||||||
| Age, mean (SD), y | 64.8 (10.2) | 64.7 (10.0) | .85 | 62.8 (10.9) | 62.7 (11.0) | .81 |
| Female | 266 (28.8) | 255 (27.3) | .48 | 429 (24.0) | 434 (24.2) | .90 |
| Male | 659 (71.2) | 680 (72.7) | 1356 (76.0) | 1359 (75.8) | ||
| Hypertension | 630 (68.1) | 659 (70.5) | .27 | 1034 (57.9) | 1015 (56.6) | .43 |
| Dyslipidemia | 675 (73.0) | 663 (70.9) | .32 | 1209 (67.7) | 1220 (68.0) | .84 |
| Current smoking | 182 (19.7) | 185 (19.8) | .95 | 363 (20.3) | 396 (22.1) | .20 |
| Chronic kidney disease | 181 (19.6) | 178 (19.0) | .77 | 175 (9.8) | 159 (8.9) | .34 |
| Previous MI | 142 (15.4) | 150 (16.0) | .68 | 295 (16.5) | 285 (15.9) | .61 |
| Previous HF | 44 (4.8) | 56 (6.0) | .24 | 64 (3.6) | 60 (3.4) | .70 |
| Previous CVI | 46 (5.0) | 66 (7.1) | .059 | 74 (4.1) | 67 (3.7) | .53 |
| Clinical indication of PCI | ||||||
| Silent ischemia | 26 (2.8) | 29 (3.1) | .65 | 32 (1.8) | 41 (2.3) | .54 |
| Stable angina | 221 (23.9) | 238 (25.5) | 467 (26.2) | 463 (25.8) | ||
| Unstable angina | 339 (36.6) | 357 (38.2) | 636 (35.6) | 602 (33.6) | ||
| NSTEMI | 193 (20.9) | 174 (18.6) | 333 (18.7) | 354 (19.7) | ||
| STEMI | 146 (15.8) | 137 (14.7) | 317 (17.8) | 333 (18.6) | ||
| Laboratory results | ||||||
| White blood cells/μL, mean (SD) | 7.1 (2.1) | 7.1 (2.0) | .87 | 6.6 (1.8) | 6.7 (1.8) | .13 |
| Hemoglobin, mean (SD), g/dLa | 13.4 (1.9) | 13.5 (1.8) | .40 | 13.9 (1.5) | 13.9 (1.5) | .92 |
| Creatinine, mean (SD), mg/dLb | 1.1 (1.1) | 1.1 (0.8) | .28 | 0.9 (0.4) | 1.0 (0.6) | .47 |
| Total cholesterol, mean (SD), mg/dLc | 132.2 (31.4) | 134.4 (31.5) | .14 | 139.3 (28.6) | 140.2 (29.7) | .40 |
| Triglyceride, mean (SD), mg/dLd | 137.4 (101.0) | 134.7 (74.0) | .55 | 121.2 (77.3) | 120.1 (68.5) | .70 |
| HDL cholesterol, mean (SD), mg/dLc | 43.9 (11.5) | 44.6 (11.6) | .27 | 47.5 (12.1) | 47.5 (12.5) | .94 |
| LDL cholesterol, mean (SD), mg/dLc | 67.4 (22.9) | 69.1 (22.9) | .14 | 72.5 (23.9) | 73.6 (23.3) | .20 |
| Time from PCI to randomization, median (range), d | 386.0 (358.0-425.5) | 381.0 (360.0-422.0) | .34 | 381.0 (355.0-423.0) | 380.0 (357.0-420.0) | .91 |
| Angiographic data per patient | ||||||
| Extent of CAD | ||||||
| 1-Vessel disease | 389 (42.1) | 404 (43.2) | .54 | 978 (54.8) | 972 (54.2) | .95 |
| 2-Vessel disease | 313 (33.8) | 294 (31.4) | 542 (30.4) | 550 (30.7) | ||
| 3-Vessel disease | 223 (24.1) | 237 (25.3) | 265 (14.8) | 270 (15.1) | ||
| Left main disease | 55 (5.9) | 40 (4.3) | .10 | 87 (4.9) | 90 (5.0) | .84 |
| PCI for bifurcation lesion | 106 (11.5) | 97 (10.4) | .45 | 179 (10.0) | 198 (11.0) | .32 |
| PCI for CTO lesion | 97 (10.5) | 74 (7.9) | .055 | 160 (9.0) | 180 (10.0) | .27 |
| No. of treated lesions | 1.4 (0.6) | 1.4 (0.6) | .70 | 1.3 (0.6) | 1.3 (0.5) | .36 |
| Diameter of implanted stents, mean (SD), mm | 3.0 (0.4) | 3.0 (0.4) | .85 | 3.1 (0.4) | 3.1 (0.4) | .74 |
| Minimum diameter of implanted stents, mean (SD) mm | 2.9 (0.4) | 3.0 (0.4) | .78 | 3.0 (0.5) | 3.0 (0.5) | .86 |
| Total length of implanted stents, mean (SD) mm | 38.8 (26.1) | 38.2 (24.5) | .63 | 34.7 (23.0) | 34.4 (23.0) | .74 |
| Total No. of implanted stents, mean (SD) | 1.6 (0.9) | 1.6 (0.8) | .71 | 1.5 (0.8) | 1.4 (0.8) | .47 |
| Diabetes | 925 (100.0) | 935 (100.0) | NA | 0 | 0 | NA |
| HbA1c at baseline, mean (SD), %e | 7.1 (1.1) | 7.2 (1.2) | .53 | 5.7 (0.3) | 5.7 (0.3) | .67 |
| Treatment for diabetes | ||||||
| Newly diagnosed at baseline | 89 (9.6) | 95 (10.2) | .72 | NA | NA | NA |
| Lifestyle modification | 51 (5.5) | 54 (5.8) | NA | NA | ||
| Oral hypoglycemic agent | 721 (77.9) | 710 (75.9) | NA | NA | ||
| Insulin | 64 (6.9) | 76 (8.1) | NA | NA | ||
| Groups by baseline HbA1c levels | ||||||
| <7.0%e | 370 (40.0) | 376 (40.2) | .90 | NA | NA | NA |
| 7.0%-8.9%e | 269 (29.1) | 265 (28.3) | NA | NA | ||
| ≥9.0%e | 50 (5.4) | 54 (5.8) | NA | NA | ||
Abbreviations: CAD, coronary artery disease; CTO, chronic total occlusion; CVI, cerebrovascular incident; HDL, high-density lipoprotein; HbA1C, hemoglobin A1c; HF, heart failure; LDL, low-density lipoprotein; MI, myocardial infarction; NA, not applicable; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
To convert to g/L, multiply by 10.
To convert to µmol/L, multiply by 88.4.
To convert to mmol/L, multiply by 0.0259.
To convert to mmol/L, multiply by 0.0113.
To convert to proportion of total hemoglobin, multiply by 0.01.
Primary and Secondary End Points at 24 Months According to the Presence of Diabetes
Comparisons of the risk of the primary and secondary end points between the clopidogrel and aspirin arms in the diabetes and no diabetes groups are presented in Table 2. The rate of the 24-month primary composite end point was significantly lower in the clopidogrel arm compared to the aspirin arm in both the diabetes group (6.3% vs 9.2%; HR, 0.69; 95% CI, 0.49-0.96; P = .03) (Figure 2A) and the no diabetes group (5.3% vs 7.0%; HR, 0.76; 95% CI, 0.58-1.00; P = .046) (Figure 2B), without significant interaction (P for interaction = .65). The absolute risk difference (ARD) for the primary composite end point with clopidogrel vs aspirin monotherapy was 2.7% in the diabetes group (number needed to treat [NNT] = 37) and 1.6% in the no diabetes group (NNT = 63).
Table 2. Clinical Outcomes According to Presence of Comorbid Diabetes.
| Diabetes group | No diabetes group | P value for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | HR (95% CI)a | P value | No. (%) | HR (95% CI)a | P value | ||||
| Clopidogrel (n = 925) | Aspirin (n = 935) | Clopidogrel (n = 1785) | Aspirin (n = 1793) | ||||||
| Composite end point | 58 (6.3) | 84 (9.2) | 0.69 (0.49-0.96) | .03 | 94 (5.3) | 123 (7.0) | 0.76 (0.58-1.00) | .046 | .65 |
| Primaryb | |||||||||
| Thromboticc | 36 (4.0) | 53 (5.8) | 0.68 (0.45-1.04) | .07 | 63 (3.6) | 93 (5.3) | 0.68 (0.49-0.93) | .02 | .99 |
| Bleeding | |||||||||
| Any (BARC type 2, 3, or 5) | 24 (2.7) | 37 (4.1) | 0.65 (0.39-1.09) | .11 | 37 (2.1) | 50 (2.8) | 0.74 (0.48-1.13) | .17 | .71 |
| Major (BARC type 3 or 5) | 14 (1.6) | 25 (2.7) | 0.57 (0.29-1.09) | .09 | 19 (1.1) | 28 (1.6) | 0.68 (0.38-1.22) | .20 | .68 |
| MACEd | 28 (3.1) | 50 (5.4) | 0.56 (0.35-0.89) | .01 | 55 (3.1) | 53 (3.0) | 1.04 (0.72-1.52) | .83 | .04 |
| All-cause death | 22 (2.4) | 18 (2.0) | 1.24 (0.66-2.30) | .51 | 29 (1.7) | 18 (1.0) | 1.62 (0.90-2.92) | .11 | .53 |
| Cardiac death | 11 (1.2) | 6 (0.7) | 1.85 (0.68-5.00) | .23 | 8 (0.5) | 8 (0.5) | 1.01 (0.38-2.68) | .99 | .39 |
| MI | 3 (0.3) | 12 (1.3) | 0.25 (0.07-0.89) | .03 | 15 (0.9) | 17 (1.0) | 0.89 (0.44-1.78) | .74 | .09 |
| Target-vessel MI | 0 | 8 (0.9) | 0.06 (0.00-0.47) | .003 | 9 (0.5) | 9 (0.5) | 1.01 (0.40-2.53) | .99 | .01 |
| Stroke | 3 (0.3) | 23 (2.5) | 0.13 (0.04-0.44) | .001 | 15 (0.9) | 20 (1.1) | 0.75 (0.39-1.47) | .41 | .01 |
| Ischemic | 3 (0.3) | 13 (1.4) | 0.23 (0.07-0.81) | .02 | 11 (0.6) | 13 (0.7) | 0.85 (0.38-1.90) | .69 | .09 |
| Hemorrhagic | 0 | 10 (1.1) | 0.05 (0.00-0.37) | <.001 | 4 (0.2) | 7 (0.4) | 0.58 (0.17-1.97) | .38 | .04 |
| Readmission due to ACS | 22 (2.4) | 35 (3.9) | 0.63 (0.37-1.08) | .09 | 44 (2.5) | 74 (4.2) | 0.59 (0.41-0.86) | .06 | .85 |
| Unstable angina | 18 (2.0) | 23 (2.5) | 0.79 (0.43-1.46) | .46 | 29 (1.7) | 56 (3.2) | 0.52 (0.33-0.81) | .004 | .28 |
| Urgent revascularization | 13 (1.4) | 19 (2.1) | 0.69 (0.34-1.40) | .30 | 31 (1.8) | 39 (2.2) | 0.80 (0.50-1.28) | .35 | .74 |
| Any revascularization | 15 (1.7) | 23 (2.5) | 0.66 (0.34-1.26) | .21 | 41 (2.3) | 46 (2.6) | 0.90 (0.59-1.37) | .61 | .43 |
| Target vessel revascularization | 7 (0.8) | 17 (1.9) | 0.41 (0.17-1.00) | .049 | 30 (1.7) | 31 (1.8) | 0.97 (0.59-1.61) | .92 | .10 |
| Target lesion revascularization | 4 (0.4) | 13 (1.4) | 0.31 (0.10-0.95) | .04 | 20 (1.2) | 23 (1.3) | 0.87 (0.48-1.59) | .66 | .11 |
| Definite or probable stent thrombosis | 1 (0.1) | 8 (0.9) | 0.13 (0.02-1.01) | .05 | 9 (0.5) | 8 (0.5) | 1.13 (0.44-2.94) | .80 | .06 |
| Any minor gastrointestinal complications | 95 (10.5) | 107 (11.7) | 0.89 (0.68-1.17) | .41 | 177 (10.1) | 213 (12.1) | 0.83 (0.68-1.01) | .07 | .68 |
Abbreviations: ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; HR, hazard ratio; MACE, major adverse cardiovascular event; MI, myocardial infarction.
The 95% CIs for secondary end points have not been adjusted for multiple testing and therefore no clinical inferences can be made from these analyses.
Primary composite end point was defined as a composite of all-cause death, nonfatal MI, stroke, readmission due to ACS, and major bleeding events (BARC type 3 or 5).
Thrombotic composite end point was defined as a composite of cardiac death, nonfatal MI, ischemic stroke, readmission due to ACS, and definite or probable stent thrombosis.
Major adverse cardiovascular event was defined as a composite of all-cause death, myocardial infarction, or stroke.
Figure 2. Cumulative Incidence of Primary Composite End Point in Diabetic and Nondiabetic Subgroups.
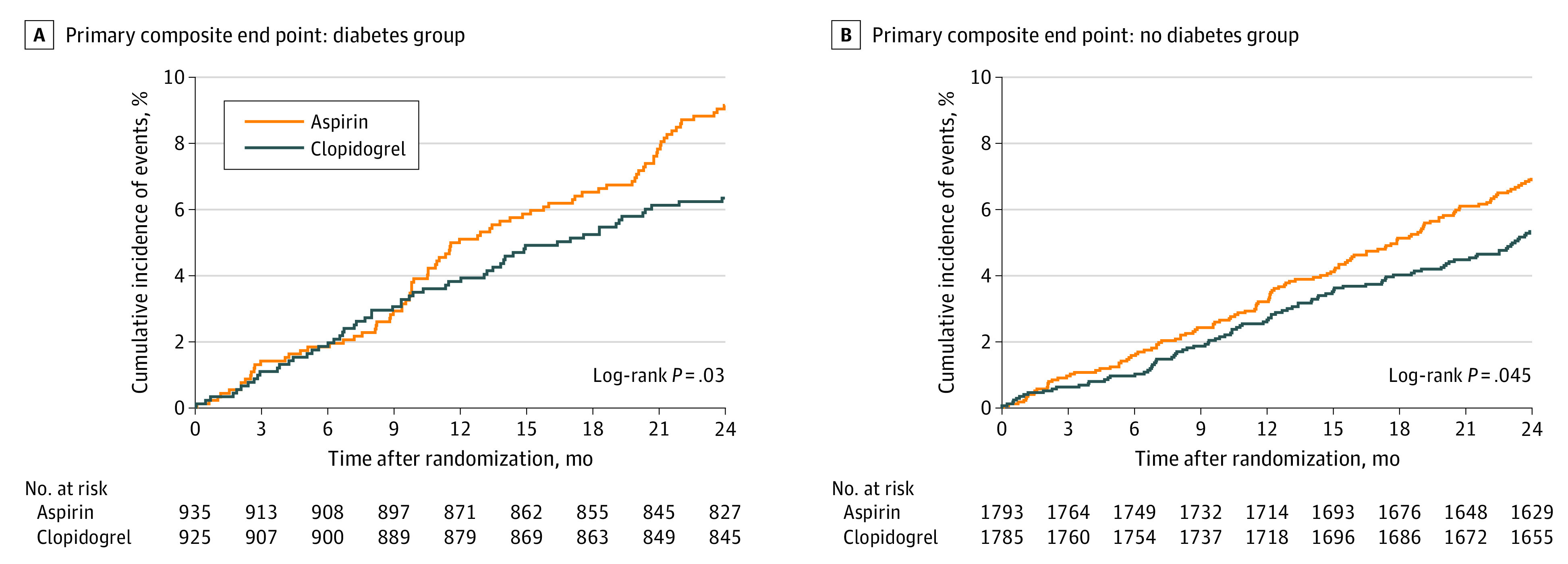
The primary composite end point consisted of all-cause death, myocardial infarction, stroke, readmission due to acute coronary syndrome, and major bleeding.
For the major secondary end points, the rates of the thrombotic composite end point (eFigure 1 in Supplement 2), any bleeding with BARC type 2, 3, or 5 (eFigure 2 in Supplement 2), and major bleeding with BARC type 3 or 5 at 24 months were all numerically lower in the clopidogrel arm with only the thrombotic composite end point showing statistical significance in the no diabetes group. All other comparisons were statistically marginal. The results did not differ by presence of diabetes for thrombotic composite end point (HR, 0.68; 95% CI, 0.45-1.04 for patients with diabetes vs HR, 0.68; 95% CI, 0.49-0.93 for those without; P for interaction = .99) or any bleeding with BARC type 2, 3, or 5 (HR, 0.65; 95% CI, 0.39-1.09 for patients with diabetes vs HR, 0.74; 95% CI, 0.48-1.13 for those without; P for interaction = .71). The rate of 24-month major adverse cardiovascular event was significantly lower in the clopidogrel arm compared to the aspirin arm (3.1% vs 5.4%; HR, 0.56; 95% CI, 0.35-0.89; P = .01) in the diabetes group, while the rates were not significantly different (3.1% vs 3.0%; HR, 1.04; 95% CI, 0.72-1.52; P = .83) in the no diabetes group (eFigure 3 in Supplement 2) with significant interaction (P for interaction = .04).
For individual end points, the rates of MI, target-vessel MI, stroke (both ischemic and hemorrhagic), target-vessel revascularization, and target-lesion revascularization were significantly lower in the clopidogrel arm than in the aspirin arm in the diabetes group but not in the no diabetes group. The risk of definite or probable stent thrombosis was numerically lower but not statistically significant in the clopidogrel arm of the diabetes group. In the no diabetes group, readmission due to ACS, including unstable angina, and any minor gastrointestinal complications were lower in the clopidogrel arm compared to the aspirin arm.
Bayesian Analysis for the ARD in Thrombotic Composite End Point and Any Bleeding in Patients With Diabetes
In bayesian analysis, the mean and 95% credible interval (CrI) for the ARD in the thrombotic composite end point at 24 months between clopidogrel and aspirin monotherapy was 1.78% (−0.17 to 3.74) in the diabetes group (Figure 3A), and 1.66% (0.31 to 3.02) in the no diabetes group (Figure 3B). The probability that the risk of the thrombotic composite end point would be lower in the clopidogrel arm was 96.3% for the diabetes group and 99.2% for the no diabetes group. For any bleeding in the diabetes group (ARD, 1.36%; 95% CrI, −0.27 to 3.00) (Figure 3C) and no diabetes group (ARD, 0.72%; 95% CrI, −0.30 to 1.74) (Figure 3D), the probability that the risk would be lower in the clopidogrel arm was 94.9% for those with diabetes and 91.7% for those without. Bayesian analysis for the primary composite end point and major adverse cardiovascular event is shown in eFigures 4 and 5 in Supplement 2.
Figure 3. Bayesian Approach for Absolute Risk Difference of 24-Month Thrombotic and Bleeding Events in Patients With Diabetes.
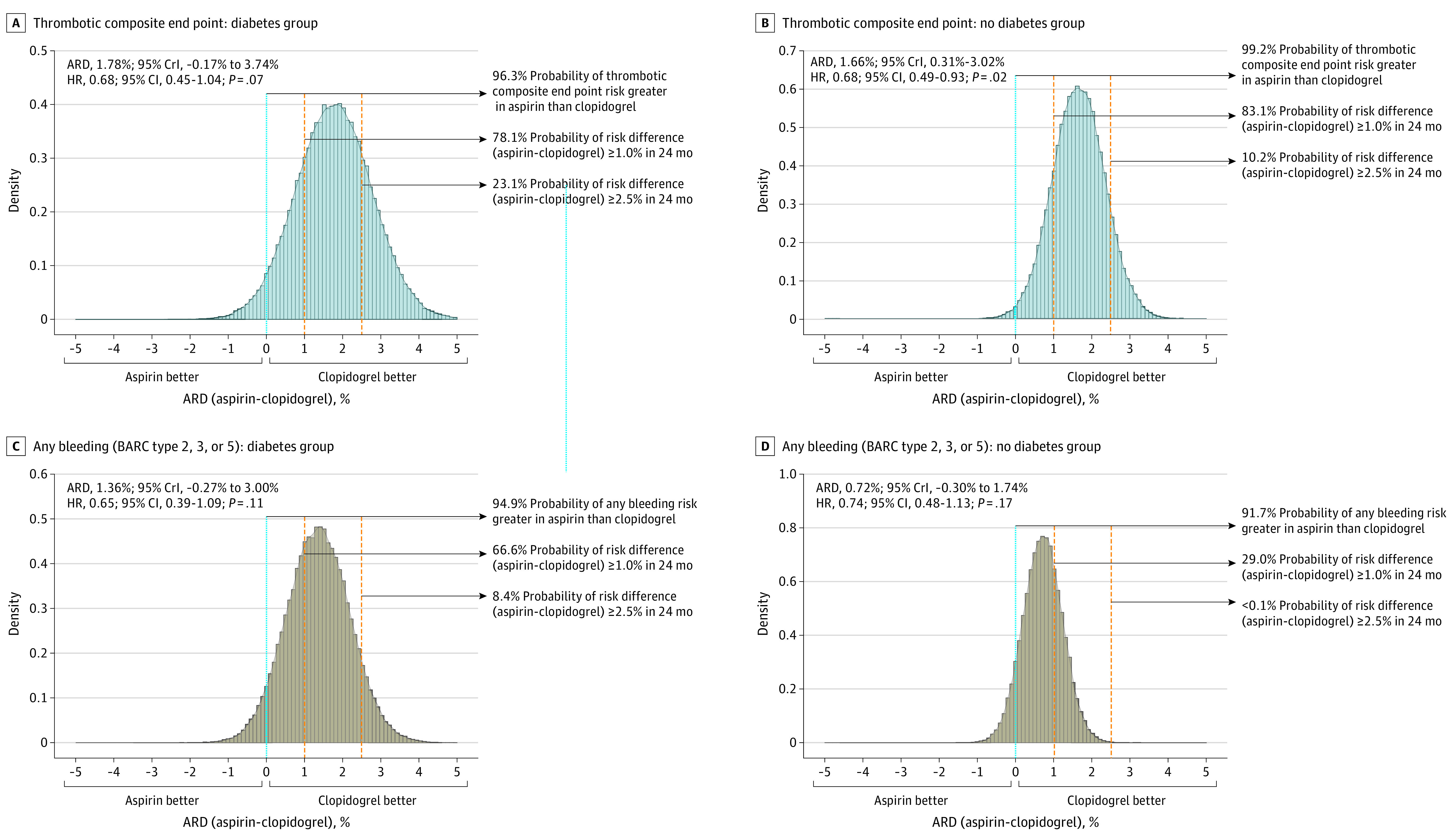
ARD indicates absolute risk difference; BARC, Bleeding Academic Research Consortium; CrI, credible interval; HR, hazard ratio.
Independent Predictors of Primary Composite End Point According to the Presence of Diabetes
Predictors of the primary composite end point were compared between the diabetes and no diabetes groups (eTable 3 in Supplement 2). Increasing age and aspirin monotherapy (compared with clopidogrel monotherapy) were independently associated with risk of the primary composite end point in both the diabetes and no diabetes groups. In the diabetes group, high-density lipoprotein (HDL) cholesterol level less than 40 mg/dL (to convert to mmol/L, multiply by 0.0259) was significantly associated with the primary composite end point (vs HDL cholesterol level ≥40 mg/dL; HR, 1.60; 95% CI, 1.08-2.36; P = .02), while male sex was significantly associated with outcomes in the no diabetes group.
Subgroup Analysis Within Diabetes Group
When patients with diabetes were subdivided according to the type of diabetes treatment and baseline HbA1c levels, clopidogrel monotherapy was associated with lower rates of the primary composite end point in those taking an oral hypoglycemic agent without insulin and in those with a baseline HbA1c level less than 7.0% (eTable 4 in Supplement 2). The benefit with clopidogrel vs aspirin was not observed in patients treated with insulin or in those with baseline HbA1c 9.0% or greater. However, the interaction was not statistically significant.
Discussion
In this post hoc analysis of the HOST-EXAM randomized clinical trial,2 the results of the head-to-head comparison between aspirin and clopidogrel monotherapy according to the presence of diabetes can be summarized as follows. Clopidogrel showed lower rates of the primary composite end point as well as the thrombotic composite end point and any bleeding at 24 months regardless of the presence of diabetes. Clopidogrel was especially beneficial in patients with diabetes in terms of 24-month major adverse cardiovascular event, a composite of all-cause death, MI, and stroke. Bayesian analysis showed 96.3% and 99.2% probability that thrombotic risk would be lower in the clopidogrel arm vs the aspirin arm, respectively, and 94.9% and 91.7% probability that any bleeding risk would be lower in the clopidogrel arm vs the aspirin arm, respectively, in both patients with and without diabetes. In multivariable analysis, clopidogrel monotherapy (compared with aspirin monotherapy) was independently associated with lower risk of primary composite end point irrespective of the presence of diabetes.
Diabetes is regarded as a prothrombotic state, which is largely mediated by increased platelet turnover and P2Y12 expression.4 Patients with diabetes who have received PCI have a high risk of long-term cardiovascular events.3 Thus, choosing the optimal antiplatelet therapy is important for secondary prevention in these patients. However, limited randomized data are available regarding the optimal antiplatelet regimen beyond the acute phase after coronary revascularization with focus on patients with diabetes.7
The results of our study suggest that clopidogrel could be considered over aspirin for long-term maintenance antiplatelet therapy after PCI in both patients with and without diabetes. Although not significant due to insufficient statistical power in each group, the association of clopidogrel with reduced risk compared with aspirin was consistent for both thrombotic and bleeding outcomes in both the diabetes and no diabetes groups. The anticipated tradeoff between ischemic and bleeding risks was not observed in this study even in patients with diabetes. In line with our results, the risk of any bleeding event as well as the ischemic end points was significantly lower in the clopidogrel group (relative risk reduction of 37.0%; P = .03) in the diabetes subgroup analysis of the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.8 A recent observational study9 also reported a reduced risk of cardiovascular adverse events without increase in bleeding complications with clopidogrel maintenance monotherapy compared to aspirin. Such counterintuitive result may be partly explained by medication adherence. Since both ischemic and bleeding outcomes interact with adherence to antiplatelet medication, frequent interruption due to complications or drug-related adverse effects may be closely related to the increased overall risk of adverse events.10,11 Notably, a numerically higher rate of gastrointestinal bleeding in the aspirin arm may have contributed to the worse outcomes compared to the clopidogrel arm in the current study. The comparison of adherence between aspirin and clopidogrel according to the presence of diabetes will be informative in longer-term follow-up studies.
Another finding that we observed was a stronger benefit with clopidogrel in patients with diabetes vs those without for end points such as MI, stent thrombosis, and ischemic and hemorrhagic stroke. Of note, although interaction was nonexistent, the absolute risk decrease in the primary composite end point with clopidogrel was also slightly greater in the diabetes group than in the no diabetes group, showing a smaller NNT. Such results are similar to what was observed in the CAPRIE trial,8 which was conducted decades ago in a non-PCI population and without widespread use of high-dose, high-intensity statins. The potential relative benefit with clopidogrel over aspirin in patients with diabetes has been reported in a variety of research. Diabetes may be associated with decreased efficacy in the antiplatelet effects of aspirin.12 Platelet reactivity is increased in patients with diabetes due to reduced endothelial nitric oxide generation, increased platelet turnover, and a disproportionate increase of calcium concentration inside the platelet.13 Increased platelet-erythrocyte interactions and platelet exposure or responsiveness to adenosine diphosphate may also contribute to the poor response to aspirin in patients with diabetes.14 Therefore, clopidogrel, an adenosine diphosphate receptor antagonist, may be more effective than aspirin in reducing ischemic risk in patients with diabetes.
A multivariable regression analysis of the present study showed that HDL cholesterol level less than 40 mg/dL was significantly associated with increased risk of the primary composite end point, especially in patients with diabetes. Lower HDL cholesterol, 1 of the key components in diagnosing metabolic syndrome, is a risk factor for adverse cardiovascular outcomes even in individuals receiving optimal medical therapy with well-controlled low-density lipoprotein cholesterol level.15 Although there is lack of evidence that supports a benefit with increased HDL cholesterol levels,16 HDL cholesterol level can be at least a surrogate marker for secondary prevention in patients with diabetes, emphasizing the need for more aggressive lipid-lowering therapy as well as intensive lifestyle modification (eg, healthy diet, regular physical activity, smoking cessation, and maintaining optimal body weight) in those with HDL cholesterol less than 40 mg/dL.17
In subgroup analysis, the association of clopidogrel with reduced risk of the primary composite end point was not prominent in patients with insulin-treated diabetes or high baseline HbA1c in the present study. Although the study was not powered to analyze subgroups of subgroups and thus should be taken as hypothesis generating at best, reduced clopidogrel effects with adenosine diphosphate–induced platelet aggregation have been reported in patients with insulin-treated type 2 diabetes receiving DAPT compared to patients with non–insulin-treated diabetes.18 Therefore, the efficacy of clopidogrel monotherapy may be relatively limited in patients with insulin-treated diabetes. The possible benefit with more potent P2Y12 inhibitors in insulin-treated diabetes should be further investigated.
Limitations
Several limitations to this study should be discussed. First, the open-labeled design of the trial may be a source of bias in outcome reporting. However, all study end points had standardized definitions and were verified by an independent event adjudication committee that was blinded to the randomization results. Second, since the randomization was not stratified according to diabetes status, the study was not powered to compare outcomes between the 2 monotherapies in patients with or without diabetes. The lower-than-expected event rates may also have contributed to the relatively limited statistical power of this analysis.2,5 Furthermore, correction for multiple testing was not performed. Notably, the bayesian analysis conducted in this study was not prespecified, and the sample size for the diabetes group was particularly insufficient to detect differences in thrombotic events. Therefore, the results from this analysis should be interpreted as hypothesis generating at best, especially for the association of clopidogrel with a reduction in risk of MI or stroke. Further follow-up with the patients (HOST-EXAM-EX, ClinicalTrials.gov identifier: NCT05567536) may shed light on the validity of such observations. Third, this trial was performed solely in a Korean population, which limits the ethnic generalizability of the study results. Fourth, since the randomized study population included only patients who were event free during DAPT 6 to 18 months post-PCI, extrapolation of the results to those receiving a shorter duration of DAPT cannot be supported by the current study. Fourth, there was lack of information on the detailed types of oral antidiabetic medications.
Conclusions
In this post hoc analysis of the HOST-EXAM randomized clinical trial, clopidogrel monotherapy was associated with a lower risk of the primary composite end point in both patients with and without diabetes. These results may inform decision making for long-term therapy following PCI.
Trial protocol and statistical analysis plan
eMethods
eTable 1. Baseline medications of study population
eTable 2. Information on drug adherence and anticoagulation during follow-up
eTable 3. Predictors of primary composite endpoint according to presence of diabetes mellitus
eTable 4. Risk of primary composite endpoint according to subsets related with diabetes mellitus
eFigure 1. Cumulative Incidence of Thrombotic Composite Endpoint in Diabetic and Non-diabetic Subgroups
eFigure 2. Cumulative Incidence of Any Bleeding in Diabetic and Non-diabetic Subgroups
eFigure 3. Cumulative Incidence of MACE in Diabetic and Non-diabetic Subgroups
eFigure 4. Bayesian Approach for Absolute Risk Difference of 24-Month Primary Composite Endpoint in Patients with Diabetes Mellitus
eFigure 5. Bayesian Approach for Absolute Risk Difference of 24-Month MACE in Patients with Diabetes Mellitus
eReferences
The HOST-EXAM trial members
Data sharing statement
References
- 1.Neumann F-J, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 2.Koo BK, Kang J, Park KW, et al. ; HOST-EXAM investigators . Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021;397(10293):2487-2496. doi: 10.1016/S0140-6736(21)01063-1 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Eagle KA, Ohman EM, et al. ; REACH Registry Investigators . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350-1357. doi: 10.1001/jama.2010.1322 [DOI] [PubMed] [Google Scholar]
- 4.Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res. 2015;116(9):1579-1598. doi: 10.1161/CIRCRESAHA.114.303505 [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Koo BK, Park KW, et al. ; HOST-EXAM investigators . A randomized clinical trial comparing long-term clopidogrel vs aspirin monotherapy beyond dual antiplatelet therapy after drug-eluting coronary stent implantation: design and rationale of the Harmonizing Optimal Strategy for Treatment of coronary artery stenosis-Extended Antiplatelet Monotherapy (HOST-EXAM) trial. Am Heart J. 2017;185:17-25. doi: 10.1016/j.ahj.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Association AD; American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1)(suppl 1):S67-S74. doi: 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiarito M, Sanz-Sánchez J, Cannata F, et al. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet. 2020;395(10235):1487-1495. doi: 10.1016/S0140-6736(20)30315-9 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90(6):625-628. doi: 10.1016/S0002-9149(02)02567-5 [DOI] [PubMed] [Google Scholar]
- 9.Park TK, Song YB, Ahn J, et al. Clopidogrel Versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(1):e002816. doi: 10.1161/CIRCINTERVENTIONS.115.002816 [DOI] [PubMed] [Google Scholar]
- 10.Kang J, Park KW, Lee H, et al. Aspirin vs. clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention: the HOST-EXAM extended study. Circulation. 2023;147(2):108-117. doi: 10.1161/CIRCULATIONAHA.122.062770 [DOI] [PubMed] [Google Scholar]
- 11.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. [DOI] [PubMed] [Google Scholar]
- 12.Ajjan R, Storey RF, Grant PJ. Aspirin resistance and diabetes mellitus. Diabetologia. 2008;51(3):385-390. doi: 10.1007/s00125-007-0898-3 [DOI] [PubMed] [Google Scholar]
- 13.Ajjan R, Grant PJ. Coagulation and atherothrombotic disease. Atherosclerosis. 2006;186(2):240-259. doi: 10.1016/j.atherosclerosis.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54(8):2430-2435. doi: 10.2337/diabetes.54.8.2430 [DOI] [PubMed] [Google Scholar]
- 15.Acharjee S, Boden WE, Hartigan PM, et al. Low levels of high-density lipoprotein cholesterol and increased risk of cardiovascular events in stable ischemic heart disease patients: a post-hoc analysis from the COURAGE trial (clinical outcomes utilizing revascularization and aggressive drug evaluation). J Am Coll Cardiol. 2013;62(20):1826-1833. doi: 10.1016/j.jacc.2013.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a Report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong KN, Fuster V, Rosenson RS, Rosendorff C, Bhatt DL. How low to go with glucose, cholesterol, and blood pressure in primary prevention of CVD. J Am Coll Cardiol. 2017;70(17):2171-2185. doi: 10.1016/j.jacc.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo DJ, Bernardo E, Ramírez C, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48(2):298-304. doi: 10.1016/j.jacc.2006.03.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods
eTable 1. Baseline medications of study population
eTable 2. Information on drug adherence and anticoagulation during follow-up
eTable 3. Predictors of primary composite endpoint according to presence of diabetes mellitus
eTable 4. Risk of primary composite endpoint according to subsets related with diabetes mellitus
eFigure 1. Cumulative Incidence of Thrombotic Composite Endpoint in Diabetic and Non-diabetic Subgroups
eFigure 2. Cumulative Incidence of Any Bleeding in Diabetic and Non-diabetic Subgroups
eFigure 3. Cumulative Incidence of MACE in Diabetic and Non-diabetic Subgroups
eFigure 4. Bayesian Approach for Absolute Risk Difference of 24-Month Primary Composite Endpoint in Patients with Diabetes Mellitus
eFigure 5. Bayesian Approach for Absolute Risk Difference of 24-Month MACE in Patients with Diabetes Mellitus
eReferences
The HOST-EXAM trial members
Data sharing statement