Key Points
Question
Is there an association between obesity after weight loss and breast cancer risk?
Findings
In this population-based, matched multiple cohort study including 69 260 women with obesity, there was no residual risk of breast cancer associated with weight loss after bariatric surgery, and surgical weight loss was associated with reduced risk of breast cancer development compared with maintaining body mass index greater than 25.
Meaning
Findings suggest that the association between substantial weight loss and breast cancer risk is sustained and appears to be associated with removal of the residual risk of obesity for women.
Abstract
Importance
Excess adiposity confers higher risk of breast cancer for women. For women who have lost substantial weight, it is unclear whether previous obesity confers residual increased baseline risk of breast cancer compared with peers without obesity.
Objectives
To determine whether there is a residual risk of breast cancer due to prior obesity among patients who undergo bariatric surgery.
Design, Setting, and Participants
Retrospective matched cohort study of 69 260 women with index date between January 1, 2010, and December 31, 2016. Patients were followed up for 5 years after bariatric surgery or index date. Population-based clinical and administrative data from multiple databases in Ontario, Canada, were used to match a cohort of women who underwent bariatric surgery for obesity (baseline body mass index [BMI] ≥35 with comorbid conditions or BMI ≥40) to women without a history of bariatric surgery according to age and breast cancer screening history. Nonsurgical controls were divided into 4 BMI categories (<25, 25-29, 30-34, and ≥35). Data were analyzed on October 21, 2021.
Exposures
Weight loss via bariatric surgery.
Main Outcomes and Measures
Residual hazard of breast cancer after washout periods of 1, 2, and 5 years. Comparisons were made between the surgical and nonsurgical cohorts overall and within each of the BMI subgroups.
Results
In total, 69 260 women were included in the analysis, with 13 852 women in each of the 5 study cohorts. The mean (SD) age was 45.1 (10.9) years. In the postsurgical cohort vs the overall nonsurgical cohort (n = 55 408), there was an increased hazard for incident breast cancer in the nonsurgical group after washout periods of 1 year (hazard ratio [HR], 1.40 [95% CI, 1.18-1.67]), 2 years (HR, 1.31 [95% CI, 1.12-1.53]), and 5 years (HR, 1.38 [95% CI, 1.21-1.58]). When the postsurgical cohort was compared with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different regardless of the washout period, whereas there was a reduced hazard for incident breast cancer among postsurgical patients compared with nonsurgical patients in all high BMI categories (BMI ≥25).
Conclusions and Relevance
Findings suggest that bariatric surgery was associated with a reduced risk of developing breast cancer for women with prior obesity equivalent to that of a woman with a BMI less than 25 and a lower risk when compared with all groups with BMI greater than or equal to 25.
This cohort study investigates whether surgical weight loss for women with obesity is associated with an elevated risk of breast cancer compared with that of women without obesity.
Introduction
Obesity rates continue to increase internationally. Risks associated with increased adiposity have been well documented, including increased risk of 13 types of cancer.1,2,3,4 Specifically, breast cancer is linearly associated with increased adiposity.5,6,7 Hypotheses for this interaction include obesity-related changes in both the insulin pathways and circulating sex hormone levels.8,9 Because breast cancer is the most common cancer among women, interventions to reduce the risk of breast cancer could yield significant public health benefits.9
Although weight gain and obesity are associated with increased risk of breast cancer, the associations of weight loss with risk of breast cancer are poorly defined. Recent studies have shown that weight loss via methods such as increased physical activity and dietary changes may have no association with breast cancer risk.8,10 However, studies including bariatric surgery as the means to weight loss have shown a protective association between weight loss and breast cancer risk,11,12,13 which may be due to the complex interplay of weight loss, weight regain, hormone levels before and after menopause, and the association with other risk factors, such as diabetes.8,11,14 Additionally, data from large studies adjusting for confounders such as breast cancer screening history are lacking.10 Although there is evidence of a beneficial association between surgical weight loss and breast cancer incidence, it is not known whether prior obesity confers a residual risk compared with risk for otherwise similar individuals without obesity.
Accordingly, the objective of this study was to examine whether surgical weight loss was associated with an elevated risk of breast cancer for women with obesity compared with otherwise similar patients without obesity, as well as to determine whether the strength of the association varied with body mass index (BMI; calculated as weight in kilograms divided by height in meters squared).
Methods
Overview of Study Design
Using a combination of clinical and administrative databases in Ontario, Canada, we conducted a population-based matched cohort study of weight loss and breast cancer risk. Study cohorts included women with obesity who underwent bariatric surgery compared with women without a history of bariatric surgery, matched for age and breast cancer screening status. The control cohort was subdivided into 4 BMI categories. The primary outcome was incidence of breast cancer after a 1-year washout period after the index date.
Setting
Created in 2009 by the Ontario Ministry of Health and Long-Term Care, the Ontario Bariatric Network is the centralized deliverer of bariatric care in Ontario.15 Bariatric surgery in Ontario is government funded, and access for eligibility is based on referral from a primary care physician.16,17 To be eligible, patients must meet the National Institute of Health criteria, including a BMI greater than 40 or greater than 35 and obesity-related comorbid conditions.18 In Ontario, Roux-en-Y gastric bypass accounts for nearly 80% of the procedures performed, whereas sleeve gastrectomy accounts for most of the remaining procedures.
Study Cohort
The 2 main study cohorts were women who underwent bariatric surgery from January 1, 2010, to December 31, 2016, and women with no history of bariatric surgery, matched for age and breast cancer screening status. Nonsurgical controls were identified from the linked Electronic Medical Record Primary Care database, which is composed of electronic medical records from 43 community-based family practice clinics across Ontario that have physician and patient characteristics that are a purposeful sample representative of the Ontario population. Nonsurgical controls were further subdivided into 4 categories based on BMI (<25, 25-29, 30-34, and ≥35). Women with a history of breast cancer were excluded from both cohorts. Linked databases were then used to obtain variables at an index date relevant to the study, including socioeconomic, clinical, and health care use rates, which were obtained via an independent, nonprofit research institute, ICES. Under Ontario’s health information privacy law, ICES’ legal status allows it to collect and analyze health care and demographic data, without informed consent, for health system evaluation and improvement. As such, this study did not require institutional review board or ethics committee approval, nor did it require the informed consent of study participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Matching Process and Covariates
Patients across the 5 cohorts (surgical and 4 BMI cohorts) were matched in a 1:1 ratio for age (±3 years) and breast cancer screening status within 2 years of the index date, using greedy nearest neighbor matching.19 The index date for the surgical cohort was the date of surgery, and the index date for the nonsurgical cohort was the BMI measurement date. Covariates included diabetes status, census-based neighborhood income quintile, and measures of health care use. Screening status was accounted for in a time-varying fashion in which patients were considered screened if their prior screening was within 2 years of their screening date. The lookback window for matching variables was 20 years; for clinical confounders, 5 years. Health care use had a lookback window of 1 year.
Outcomes
The primary outcome was the incidence of breast cancer after a 1-year washout period, with sensitivity analyses performed with washout periods of 2 and 5 years. Secondary outcomes were tumor characteristics and treatments undertaken by women who developed breast cancer during study follow-up. Cancer outcomes were determined from the Ontario Cancer Registry.20
Statistical Analysis
Statistical analysis was performed with SAS, version 9.4 (SAS Institute Inc). Analysis of variance was used for continuous variables and χ2 was used for categorical variables. Standardized differences of a set threshold of 0.1 were used to assess balance in the distribution of covariates and were used at this time in the analysis because they are insensitive to sample size.19 The primary outcome was assessed with extended cause-specific Cox regression models, with death as a competing event. Time-based interactions were used with the main exposure at 1, 2, and 5 years into the regression model because we expected the association between surgery and cancer incidence to vary over time. Adjustments were made for all covariates in Table 1 and each patient’s time-varying breast cancer screening status during follow-up. Model assumptions, such as proportionality of hazards, were tested by modeling residuals and by graphic observation of the dependence coefficients on time. The statistical significance was set a priori at α = .05, with 2-tailed P values. Data were analyzed on October 21, 2021.
Table 1. Demographic Characteristics of Patientsa.
| Characteristic | Patients, No. (%) | P value | |||||
|---|---|---|---|---|---|---|---|
| BMI <25 | BMI 25-29 | BMI 30-34 | BMI ≥35 | Surgery group | Total | ||
| Age at index, mean (SD), y | 45.0 (10.9) | 45.1 (10.9) | 45.2 (11.2) | 45.2 (11.0) | 45.1 (10.8) | 45.1 (10.9) | .63 |
| New breast cancer | 159 (1.1) | 177 (1.3) | 188 (1.4) | 179 (1.3) | 125 (0.9) | 828 (1.2) | .004 |
| Smoking history | 879 (6.3) | 1037 (7.5) | 1209 (8.7) | 1195 (8.6) | 1181 (8.5) | 5501 (7.9) | <.001 |
| Diabetes history | 309 (2.2) | 602 (4.3) | 1188 (8.6) | 2194 (15.8) | 4987 (36.0) | 9280 (13.4) | <.001 |
| Cancer history | 104 (0.8) | 84 (0.6) | 104 (0.8) | 97 (0.7) | 78 (0.6) | 467 (0.7) | .19 |
| Rural | 1538 (11.1) | 2049 (14.8) | 2618 (18.9) | 2962 (21.4) | 2141 (15.5) | 11 308 (16.3) | <.001 |
| Neighborhood income quintile | |||||||
| 1 | 1798 (13.0) | 2081 (15.0) | 2429 (17.5) | 2757 (19.9) | 3285 (23.7) | 12 350 (17.8) | <.001 |
| 2 | 2224 (16.1) | 2497 (18.0) | 2604 (18.8) | 2771 (20.0) | 3224 (23.3) | 13 320 (19.2) | |
| 3 | 2490 (18.0) | 2754 (19.9) | 2856 (20.6) | 2854 (20.6) | 2866 (20.7) | 13 820 (20.0) | |
| 4 | 2994 (21.6) | 3032 (21.9) | 3032 (21.9) | 2907 (21.0) | 2560 (18.5) | 14 525 (21.0) | |
| 5 | 4347 (31.4) | 3489 (25.2) | 2932 (21.2) | 2564 (18.5) | 1918 (13.8) | 15 250 (22.0) | |
| Health care use | |||||||
| Rate, mean (SD) | |||||||
| ED | 0.70 (1.56) | 0.88 (2.16) | 1.16 (3.30) | 1.45 (3.50) | 2.11 (3.15) | 1.26 (2.88) | <.001 |
| Hospitalization | 0.41 (0.84) | 0.46 (0.91) | 0.53 (0.90) | 0.60 (1.08) | 1.51 (1.15) | 0.70 (1.06) | <.001 |
| PCP | 9.47 (12.49) | 10.05 (9.66) | 10.97 (13.13) | 12.18 (11.82) | 16.84 (12.83) | 11.90 (12.33) | <.001 |
| Breast screening | |||||||
| 2 y | 3501 (25.3) | 3501 (25.3) | 3501 (25.3) | 3501 (25.3) | 3501 (25.3) | 17 505 (25.3) | >.99 |
| Any | 5380 (38.8) | 5437 (39.2) | 5281 (38.1) | 5239 (37.8) | 5327 (38.5) | 26 664 (38.5) | .11 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ED, emergency department; PCP, primary care physician.
A total of 69 260 women were included. The 4 BMI subgroups and the surgery group included 13 852 women each.
Results
The study cohort characteristics are shown in Table 1. Overall, 69 260 women were included in this analysis. There were 55 408 women in the nonsurgical cohort and 13 852 women in the surgical cohort. Data on race and ethnicity were not collected, as is typical in Canada. Within the nonsurgical cohort, each of the 4 BMI subgroups included 13 852 women. The mean (SD) age across the study population was 45.1 (10.9) years. Groups were balanced for age, cancer history, and breast cancer screening status. Differences in baseline demographic characteristics were present for the remaining characteristics, including smoking history, diabetes history, and health care use rates.
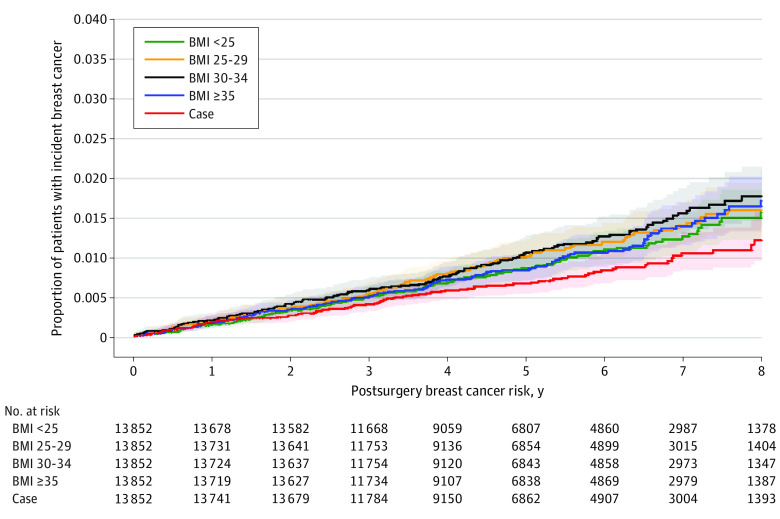
In total, 659 breast cancers were diagnosed in the study population of 69 260 women (0.95%) during the study period. In the BMI and surgical cohorts of 13 852 women each, there were 103 breast cancers in the surgical cohort (0.74%), 128 in the group with BMI less than 25 (0.92%), 143 in the group with BMI 25 to 29 (1.03%), 150 in the group with BMI 30 to 34 (1.08%), and 135 in the group with BMI greater than or equal to 35 (0.97%). The regression model results are presented in Table 2. When the adjusted hazard of developing breast cancer in the postsurgical group was compared with that of the entire nonsurgical cohort (n = 55 408), the postsurgical group had a lower risk of breast cancer development after a 1-year washout period (hazard ratio [HR], 1.40 [95% CI, 1.18-1.67]; P < .001), a 2-year washout period (HR, 1.31 [95% CI, 1.12-1.53]; P < .001), and a 5-year washout period (HR, 1.38 [95% CI, 1.21-1.58]; P < .001). The unadjusted cumulative incidence curve of incident breast cancer risk after a 1-year washout period is presented in the Figure. When the postsurgical groups with different BMI categories after a 1-year washout were compared, there was no difference in risk between the surgical group and the group with BMI less than 25 (HR, 1.07 [95% CI, 0.89-1.28]; P = .10). However, for the BMI categories greater than or equal to 25, the risk of breast cancer was increased compared with the risk in the postsurgical group. Specifically, compared with the surgical group, the group with BMI 25 to 29 had a 25% greater risk of breast cancer, the group with BMI 30 to 34 had a 42% greater risk, and the group with BMI less than 35 had a 35% greater risk. Similar results were observed after sensitivity analyses for 2-year and 5-year washouts.
Table 2. Point Estimates for Incident Breast Cancer.
| Point estimate | HR (95% Wald confidence limit) | P value |
|---|---|---|
| HR for surgery vs nonsurgery matcheda | ||
| At 1 y | 1.40 (1.18-1.67) | <.001 |
| At 2 y | 1.31 (1.12-1.53) | <.001 |
| At 5 y | 1.38 (1.21-1.58) | <.001 |
| HR for nonsurgery per BMI category vs surgery at 1 y | ||
| BMI | ||
| <25 | 1.07 (0.89-1.28) | .10 |
| 25-29 | 1.25 (1.06-1.49) | <.001 |
| 30-34 | 1.42 (1.21-1.67) | <.001 |
| ≥35 | 1.35 (1.15-1.59) | .005 |
| HR for nonsurgery per BMI category vs surgery at 2 y | ||
| BMI | ||
| <25 | 1.20 (0.99-1.47) | .48 |
| 25-29 | 1.32 (1.09-1.60) | <.001 |
| 30-34 | 1.54 (1.28-1.85) | <.001 |
| ≥35 | 1.47 (1.23-1.77) | .008 |
| Adjusted HR for nonsurgery per BMI category vs surgery at 5 y | ||
| BMI | ||
| <25 | 1.31 (0.94-1.82) | .19 |
| 25-29 | 1.42 (1.03-1.96) | <.001 |
| 30-34 | 1.63 (1.20-2.23) | <.001 |
| ≥35 | 1.55 (1.13-2.12) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio.
Adjusted.
Figure. Unadjusted Cumulative Incidence Curve of Incident Breast Cancer Risk After a 1-Year Washout Period.
In total, 659 breast cancers were diagnosed in the study population of 69 260 women (0.95%) during the study period: 103 in the surgical cohort (0.74%), 128 in the group with body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) less than 25 (0.92%; hazard ratio [HR], 1.25; 95% CI, 0.97-1.62), 143 in the group with BMI 25 to 29 (1.03%; HR, 1.39; 95% CI, 1.08-1.80), 150 in the group with BMI 30 to 34 (1.08%; HR, 1.46; 95% CI, 1.14-1.88), and 135 in the group with BMI greater than or equal to 35 (0.97%; HR, 1.31; 95% CI, 1 [Reference]). Gray K-sample test P = .04. Median (95% CI) values were not estimable.
Tumor characteristics are presented in Table 3 for women who developed breast cancer during study follow-up. Across all cohorts, stage I cancers were most commonly diagnosed (65 for BMI <25, 76 for BMI 25-29, 65 for BMI 30-34, 67 for BMI ≥35, and 60 for the surgery group). Most tumors were medium grade, estrogen receptor positive, progesterone receptor positive, and ERBB2 (formerly HER2) negative. There were no significant differences across the groups for the distribution of stage, grade, or hormone status data.
Table 3. Characteristics of Breast Cancersa.
| Characteristic | No. (%) of patients | P value | |||||
|---|---|---|---|---|---|---|---|
| BMI <25 | BMI 25-29 | BMI 30-34 | BMI ≥35 | Surgery | Total | ||
| Stage | |||||||
| I | 65 (56.0) | 76 (57.1) | 65 (50.4) | 67 (58.3) | 60 (67.4) | 333 | .29 |
| II | 41 (35.3) | 40 (30.1) | 42 (32.6) | 33 (28.7) | 24 (27.0) | 180 | |
| III | <10 | 14 (10.5) | 14 (10.9) | <10 | <10 | 48 | |
| IV | <5 | <5 | <10 | <10 | <5 | 21 | |
| Grade | |||||||
| Low | 12 (17.6) | 9 (11.8) | 7 (9.2) | 9 (13.2) | 17 (30.9) | 54 | .07 |
| Medium | 24 (35.3) | 36 (47.4) | 32 (42.1) | 30 (44.1) | 23 (41.8) | 145 | |
| High | 17 (25.0) | 21 (27.6) | 23 (30.3) | 21 (30.9) | 10 (18.2) | 92 | |
| Unknown | 15 (22.1) | 10 (13.2) | 14 (18.4) | <10 | <10 | 52 | |
| ER status | |||||||
| Positive | 55 (56.7) | 61 (56.0) | 60 (58.3) | 56 (57.8) | 48 (63.2) | 280 | .94 |
| Negative | 13 (13.4) | 12 (11.0) | 13 (12.6) | <10 | <10 | 53 | |
| Unknown | 29 (29.9) | 36 (33.0) | 30 (29.1) | 31 (32.0) | 23 (30.3) | 149 | |
| PR status | |||||||
| Positive | 49 (50.5) | 55 (50.5) | 51 (49.5) | 52 (53.6) | 43 (56.6) | 250 | .90 |
| Negative | 19 (19.6) | 18 (16.5) | 22 (21.4) | 14 (14.4) | 10 (13.2) | 83 | |
| Unknown | 29 (28.9) | 36 (33.0) | 30 (29.1) | 31 (32.0) | 23 (30.3) | 149 | |
| ERBB2 (formerly HER2) status | |||||||
| Positive | 15 (14.0) | 10 (9.2) | 17 (16.5) | 10 (10.3) | 6 (7.9) | 58 | .65 |
| Negative | 53 (49.5) | 60 (55.0) | 54 (52.4) | 54 (55.7) | 46 (60.5) | 267 | |
| Unknown | 29 (27.1) | 39 (35.8) | 32 (31.1) | 33 (34.0) | 24 (31.6) | 157 | |
Abbreviations: BMI (calculated as weight in kilograms divided by height in meters squared); ER, estrogen receptor; PR, progesterone receptor.
The privacy policy for the data use agency prohibits the publication of cells with fewer than 5 patients.
Table 4 presents the breast cancer treatments undertaken according to BMI category. More than 93% of patients underwent breast cancer surgery, including 98.1% in the bariatric surgery group and 118 of 135 patients (87.4%) in the group with the highest BMI. Distributions of radiation therapy, chemotherapy, and endocrine therapy were not assessed for statistical significance between the groups.
Table 4. Breast Cancer Treatments Undertakena.
| Treatment | No. (%) of patients | P value | ||
|---|---|---|---|---|
| Did not receive | Received | Total | ||
| Radiation therapy | ||||
| BMI | ||||
| <25 | 36 (28.1) | 92 (71.9) | 128 | .23 |
| 25-29 | 29 (20.3) | 114 (79.7) | 143 | |
| 30-34 | 33 (22.0) | 117 (88.0) | 150 | |
| ≥35 | 40 (29.6) | 95 (70.4) | 135 | |
| Surgery | 21 (20.6) | 82 (79.4) | 103 | |
| Cancer surgery | ||||
| BMI | ||||
| <25 | 6 (4.7) | 122 (95.3) | 128 | .009 |
| 25-29 | 7 (4.9) | 136 (95.1) | 143 | |
| 30-34 | 13 (8.7) | 137 (91.3) | 150 | |
| ≥35 | 17 (12.6) | 118 (87.4) | 135 | |
| Surgery | <5 | >95 | 103 | |
| Chemotherapy | ||||
| BMI | ||||
| <25 | 96 (75.0) | 32 (25.0) | 128 | .21 |
| 25-29 | 123 (86.0) | 20 (14.0) | 143 | |
| 30-34 | 117 (78.0) | 33 (22.0) | 150 | |
| ≥35 | 107 (79.3) | 28 (20.7) | 135 | |
| Surgery | 84 (81.6) | 19 (18.4) | 103 | |
| Endocrine therapy | ||||
| BMI | ||||
| <25 | 112 (87.5) | 16 (12.5) | 128 | .48 |
| 25-29 | 124 (86.7) | 19 (13.3) | 143 | |
| 30-34 | 122 (81.3) | 28 (18.7) | 150 | |
| ≥35 | 111 (82.2) | 24 (17.8) | 135 | |
| Surgery | 84 (79.8) | 19 (20.2) | 103 | |
Abbreviation: BMI (calculated as weight in kilograms divided by height in meters squared).
The privacy policy for the data use agency prohibits the publication of cells with fewer than 5 patients.
Discussion
This population-based study including 69 260 women demonstrated that at 1-, 2-, and 5-year washout periods, weight loss after bariatric surgery was associated with an observed breast cancer risk reduction to a level equivalent to that of a woman with a BMI less than 25 and a lower risk when compared with all groups with BMI greater than or equal to 25. This finding suggests that there is no major residual breast cancer risk associated with obesity for these patients. When compared with all nonsurgical patients, the postbariatric surgery group also had a lower hazard of developing breast cancer after a 1-year washout and at each subsequent point compared with all nonsurgical controls. Last, the distribution of cancer characteristics was similar across the groups except for rate of cancer surgery, which was highest in the bariatric surgery groups and lowest in the group with BMI greater than or equal to 35. This study was able to account for breast cancer screening in a time-varying fashion, in addition to adjustments for other major risk factors such as diabetes. Accordingly, this study addresses limitations often encountered in weight loss and breast cancer risk research. Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25.
Unfortunately, we did not have access to reliable follow-up data on weight over time and therefore were unable to determine whether a dose-response association existed. Data elsewhere in the literature suggest expected weight loss of 20% to 30% of total body weight after bariatric surgery in similar cohorts, whereas nonsurgical groups tend to have stable weight over time.20 However, evidence suggests that reductions in incident breast cancer have been observed with sustained weight loss of as little as 2 kg, with greater risk reductions with losses greater than 5% of body weight.4,8,21 The findings in the literature are typically suggestive of a positive correlation between weight loss and reduced risk of breast cancer, with greater weight loss conferring more protection.6,21 Weight loss at any time in premenopausal adulthood has been shown to provide a reduced risk of breast cancer, which is a time typically of weight gain instead of weight loss.22 Taken together with the results of this study, efforts for early interventions to reduce weight, especially when weight gain is expected (such as in our population with a mean [SD] age of 45.1 [10.9] years), may provide the most effective method of reducing breast cancer risk.6
Mechanistic studies investigating the reduced risk of breast cancer among women with sustained weight loss have demonstrated associations with the reduction in circulating sex hormones after weight loss.1,21,23,24 Studies have shown that not only are circulating levels of endogenous estrogens positively associated with BMI and breast cancer risk but also these levels and the associated breast cancer risk respond to a decrease in weight.1,21,23,24 This finding is further reinforced by a 2020 study demonstrating a reduced incidence of breast cancer after weight loss that found a stronger association among women not receiving hormone therapy in the postmenopausal period compared with those who did,21 which further strengthens the association between increased breast cancer risk and higher circulating estrogen levels in women with obesity and those receiving hormone therapy.21,24 Additional mechanisms of reduced risk include changes in inflammatory markers, such as C-reactive protein, interleukin 6, and tumor necrosis factor, as well as changes in the insulin pathway, including insulinlike growth factor 1 and insulinlike growth factor binding protein.21,24 For example, using the homeostatic model assessment of insulin resistance, a 2020 study demonstrated a positive association between insulin resistance and breast cancer incidence.25 Control of diabetes and glucose metabolism likely also play roles in the pathogenesis of breast cancer across all of these groups.
The results of this study could have significant public health implications, given the increasing rates of both obesity and breast cancer. Given the increasingly strong evidence supporting a reduced risk of breast cancer after weight loss, even among women at high risk of breast cancer, breast cancer risk reduction should be discussed as a benefit of weight loss.5 Additionally, according to this study, the association with breast cancer risk should be seen as another major health benefit to bariatric surgery. Weight loss interventions, including a healthy diet, regular exercise, and bariatric surgery, should be presented to women at high risk of breast cancer as a potential means for reducing risk. Women with obesity who are at high risk of breast cancer because of family history or personal health factors have shown similar risk reductions with weight loss and should be counseled on ways to reduce weight.5 When weight loss programs are offered to women at high risk for breast cancer as a means to reduce cancer risk, there are higher rates of uptake, retention, and overall weight loss.10 When these methods are framed as preventive medicine, access to them empowers women and affords them the ability to control their own risk, a strong motivating factor in sustaining weight loss.9,10 Overall, promotion of a healthy lifestyle and achievement or maintenance of a healthy body weight via lifestyle modifications or bariatric surgery should be conducted in part as a public health intervention to reduce the risk of breast cancer among women.4
Limitations
The limitations to this study include the inability to adjust for healthy user bias because the bariatric surgery cohort may have been relatively healthier in unmeasured ways or made other changes to their lives that reduced their breast cancer risk.26 Secondary limitations include the method of assessment of BMI in the cohorts because weight data are not systemically entered for patients in family physician practices and may introduce some variability. Moreover, although attempts were made to adjust for major potential confounders, we were limited to data collected and were therefore unable to assess the additional variables shown to be associated with breast cancer risk, such as alcohol intake and ethnicity. Finally, because of few data for cancer characteristics and treatments undertaken, statistical analysis of these findings could not be completed.
Conclusions
Substantial and sustained weight loss, such as that observed after bariatric surgery, is associated with a reduction in the risk of breast cancer among women with obesity. The findings in this cohort study suggest that bariatric surgery was associated with a reduced risk of developing breast cancer for women with prior obesity equivalent to that of a woman with a BMI less than 25 and a lower risk when compared with all groups with BMI greater than or equal to 25. This finding suggests that the carcinogenic associations between obesity and cancer risk are reversible and warrant public health attention given the current obesity crisis.
Data Sharing Statement
References
- 1.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378-397. doi: 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KR, Hwang IC, Han KD, Jung J, Seo MH. Waist circumference and risk of breast cancer in Korean women: a nationwide cohort study. Int J Cancer. 2018;142(8):1554-1559. doi: 10.1002/ijc.31180 [DOI] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Luo J, Anderson GL, et al. Weight loss and breast cancer incidence in postmenopausal women. Cancer. 2019;125(2):205-212. doi: 10.1002/cncr.31687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer. 1994;74(2):632-639. doi: [DOI] [PubMed] [Google Scholar]
- 6.Trentham-Dietz A, Newcomb PA, Egan KM, et al. Weight change and risk of postmenopausal breast cancer (United States). Cancer Causes Control. 2000;11(6):533-542. doi: 10.1023/A:1008961931534 [DOI] [PubMed] [Google Scholar]
- 7.Heshmati K, Harris DA, Rosner B, et al. Association of bariatric surgery status with reduced HER2+ breast cancers: a retrospective cohort study. Obes Surg. 2019;29(4):1092-1098. doi: 10.1007/s11695-018-03701-7 [DOI] [PubMed] [Google Scholar]
- 8.Hardefeldt PJ, Penninkilampi R, Edirimanne S, Eslick GD. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2018;18(4):e601-e612. doi: 10.1016/j.clbc.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Wright CE, Harvie M, Howell A, Evans DG, Hulbert-Williams N, Donnelly LS. Beliefs about weight and breast cancer: an interview study with high risk women following a 12 month weight loss intervention. Hered Cancer Clin Pract. 2015;13(1):1-9. doi: 10.1186/s13053-014-0023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvie M, Pegington M, French D, et al. Breast cancer risk status influences uptake, retention and efficacy of a weight loss programme amongst breast cancer screening attendees: two randomised controlled feasibility trials. BMC Cancer. 2019;19(1):1089. doi: 10.1186/s12885-019-6279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovrics O, Butt J, Lee Y, et al. The effect of bariatric surgery on breast cancer incidence and characteristics: a meta-analysis and systematic review. Am J Surg. 2021;222(4):715-722. doi: 10.1016/j.amjsurg.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? a systematic review. Obes Surg. 2017;27(11):3014-3020. doi: 10.1007/s11695-017-2901-5 [DOI] [PubMed] [Google Scholar]
- 13.Feigelson HS, Caan B, Weinmann S, et al. Response to comment on “Bariatric Surgery Is Associated With Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women”. Ann Surg. 2020;271(2):e19-e20. doi: 10.1097/SLA.0000000000003603 [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Hendryx M, Manson JE, et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectr. 2019;3(4):pkz054. 31737862 doi: 10.1093/jncics/pkz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doumouras AG, Saleh F, Gmora S, Anvari M, Hong D. Regional variations in the public delivery of bariatric surgery: an evaluation of the center of excellence model. Ann Surg. 2016;263(2):306-311. doi: 10.1097/SLA.0000000000001129 [DOI] [PubMed] [Google Scholar]
- 16.Doumouras AG, Anvari S, Breau R, Anvari M, Hong D, Gmora S. The effect of an online referral system on referrals to bariatric surgery. Surg Endosc. 2017;31(12):5127-5134. doi: 10.1007/s00464-017-5578-x [DOI] [PubMed] [Google Scholar]
- 17.Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. The effect of health system factors on outcomes and costs after bariatric surgery in a universal healthcare system: a national cohort study of bariatric surgery in Canada. Surg Endosc. 2017;31(11):4816-4823. doi: 10.1007/s00464-017-5559-0 [DOI] [PubMed] [Google Scholar]
- 18.Chair MG, Barondess JM, Bellegie NJ, et al. Gastrointestinal surgery for severe obesity: consensus statement. Nutr Today. 1991;26(5):32-35. doi: 10.1097/00017285-199109000-00007 [DOI] [Google Scholar]
- 19.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation. 2021;143(15):1468-1480. doi: 10.1161/CIRCULATIONAHA.120.052386 [DOI] [PubMed] [Google Scholar]
- 21.Teras LR, Patel AV, Wang M, et al. Sustained weight loss and risk of breast cancer in women 50 years and older: a pooled analysis of prospective data. J Natl Cancer Inst. 2020;112(9):929-937. doi: 10.1093/jnci/djz226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai M, Minami Y, Kuriyama S, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Br J Cancer. 2010;103(9):1443-1447. doi: 10.1038/sj.bjc.6605885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gemert WAM, Schuit AJ, van der Palen J, et al. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: the SHAPE-2 trial. Breast Cancer Res. 2015;17(1):120. doi: 10.1186/s13058-015-0633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dragano NRV, Fernø J, Diéguez C, López M, Milbank E. Reprint of: recent updates on obesity treatments: available drugs and future directions. Neuroscience. 2020;447:191-215. doi: 10.1016/j.neuroscience.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Pan K, Chlebowski RT, Mortimer JE, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126(16):3638-3647. doi: 10.1002/cncr.33002 [DOI] [PubMed] [Google Scholar]
- 26.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546-550. doi: 10.1007/s11606-010-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement