This cohort study investigates the clinical usefulness of cardiovascular magnetic resonance–based tissue characterization for cardiotoxicity surveillance in women with ERBB2 (formerly HER2)–positive breast cancer receiving anthracycline and trastuzumab therapy.
Key Points
Question
Is cardiovascular magnetic resonance (CMR)–based tissue characterization clinically useful for cardiotoxicity surveillance in women with ERBB2 (formerly HER2)–positive breast cancer receiving anthracycline and trastuzumab therapy?
Findings
In this cohort study including 136 women, serial CMR tissue characterization demonstrated peak myocardial inflammation and edema 3 months after trastuzumab initiation, and these changes were associated with left ventricular dilation and higher B-type natriuretic peptide. However, the observed values were mostly within the normal range, and the changes were small and not consistently associated with risk of cancer therapy–related cardiac dysfunction (CTRCD).
Meaning
Although changes in CMR tissue biomarkers occurred early during breast cancer therapy, these changes were not associated with CTRCD and are unlikely to be helpful in routine cardiotoxicity surveillance.
Abstract
Importance
There is a growing interest in understanding whether cardiovascular magnetic resonance (CMR) myocardial tissue characterization helps identify risk of cancer therapy–related cardiac dysfunction (CTRCD).
Objective
To describe changes in CMR tissue biomarkers during breast cancer therapy and their association with CTRCD.
Design, Setting, and Participants
This was a prospective, multicenter, cohort study of women with ERBB2 (formerly HER2)–positive breast cancer (stages I-III) who were scheduled to receive anthracycline and trastuzumab therapy with/without adjuvant radiotherapy and surgery. From November 7, 2013, to January 16, 2019, participants were recruited from 3 University of Toronto–affiliated hospitals. Data were analyzed from July 2021 to June 2022.
Exposures
Sequential therapy with anthracyclines, trastuzumab, and radiation.
Main Outcomes and Measures
CMR, high-sensitivity cardiac troponin I (hs-cTnI), and B-type natriuretic peptide (BNP) measurements were performed before anthracycline treatment, after anthracycline and before trastuzumab treatment, and at 3-month intervals during trastuzumab therapy. CMR included left ventricular (LV) volumes, LV ejection fraction (EF), myocardial strain, early gadolinium enhancement imaging to assess hyperemia (inflammation marker), native/postcontrast T1 mapping (with extracellular volume fraction [ECV]) to assess edema and/or fibrosis, T2 mapping to assess edema, and late gadolinium enhancement (LGE) to assess replacement fibrosis. CTRCD was defined using the Cardiac Review and Evaluation Committee criteria. Fixed-effects models or generalized estimating equations were used in analyses.
Results
Of 136 women (mean [SD] age, 51.1 [9.2] years) recruited from 2013 to 2019, 37 (27%) developed CTRCD. Compared with baseline, tissue biomarkers of myocardial hyperemia and edema peaked after anthracycline therapy or 3 months after trastuzumab initiation as demonstrated by an increase in mean (SD) relative myocardial enhancement (baseline, 46.3% [16.8%] to peak, 56.2% [18.6%]), native T1 (1012 [26] milliseconds to 1035 [28] milliseconds), T2 (51.4 [2.2] milliseconds to 52.6 [2.2] milliseconds), and ECV (25.2% [2.4%] to 26.8% [2.7%]), with P <.001 for the entire follow-up. The observed values were mostly within the normal range, and the changes were small and recovered during follow-up. No new replacement fibrosis developed. Increase in T1, T2, and/or ECV was associated with increased ventricular volumes and BNP but not hs-cTnI level. None of the CMR tissue biomarkers were associated with changes in LVEF or myocardial strain. Change in ECV was associated with concurrent and subsequent CTRCD, but there was significant overlap between patients with and without CTRCD.
Conclusions and Relevance
In women with ERBB2-positive breast cancer receiving sequential anthracycline and trastuzumab therapy, CMR tissue biomarkers suggest inflammation and edema peaking early during therapy and were associated with ventricular remodeling and BNP elevation. However, the increases in CMR biomarkers were transient, were not associated with LVEF or myocardial strain, and were not useful in identifying traditional CTRCD risk.
Introduction
Identifying early myocardial injury from cancer therapy remains a challenge. Current approaches, including measurements of left ventricular (LV) volumes, myocardial strain, and serum biomarkers, may detect later stages of injury.1,2,3 It is hypothesized that myocardial tissue changes (eg, inflammation and edema) noninvasively identified with serial cardiovascular magnetic resonance (CMR) scans may detect early myocardial injury and risk for cancer therapy–related cardiac dysfunction (CTRCD). Supporting this hypothesis, recent CMR animal studies suggested that edema may reflect the earliest anthracycline-related myocardial change preceding the decrease in LV ejection fraction (LVEF).4,5 However, there is limited human data on the longitudinal changes in CMR myocardial tissue biomarkers, their association with serum biomarkers (eg, high-sensitivity cardiac troponin I [hs-cTnI] and B-type natriuretic peptide [BNP]) and cardiac function, and their implication for the risk of CTRCD.6,7,8 Prior CMR studies have focused on patients treated with anthracyclines and have described small changes in myocardial tissue biomarkers during or after therapy with unclear clinical relevance.9,10,11
We conducted a prospective cohort study with detailed CMR characterization of women with ERBB2 (formerly HER2)–positive breast cancer receiving sequential anthracycline and trastuzumab therapy. Our objectives were to (1) use serial CMR scans to describe longitudinal changes in myocardial tissue biomarkers that reflect inflammation, edema, and/or fibrosis; (2) study the associations between these changes and LV remodeling, function, serum biomarkers, and CTRCD; and (3) assess the prognostic value of CMR tissue biomarker measures for future CTRCD.
Methods
Patients
Adult women with stage I to III ERBB2-positive breast cancer scheduled to receive anthracyclines and trastuzumab (eTable 1 in Supplement 1) with/without adjuvant radiotherapy and surgery, were recruited prospectively from November 7, 2013, to January 16, 2019, from 3 University of Toronto–affiliated hospitals as part of the Evaluation of Myocardial Changes During Breast Adenocarcinoma Therapy to Detect Cardiotoxicity Earlier With Magnetic Resonance Imaging (EMBRACE-MRI) study.12 Participants from the following self-identified race and ethnicity groups were included: Asian, Black, non-Black Hispanic, and White. Exclusion criteria were as follows: (1) general contraindications to CMR or to administration of gadolinium-based contrast agents; (2) life expectancy less than 12 months; (3) participation in another oncology clinical trial; (4) prior exposure to anthracyclines; and (5) history of current or prior cardiac disease (eg, myocardial infarction, heart failure). At every follow-up, symptom determination, physical examination, serum biomarker measurements, and CMR were performed. The institutional research ethics board of University Health Network approved the study, and all patients signed informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
CMR Acquisition and Analysis
Using a 1.5-T scanner (Magnetom Avanto Fit [Siemens Healthineers]), all patients had 5 CMR studies performed: before anthracycline therapy, after anthracycline but before trastuzumab therapy (hereon referred to as after anthracycline therapy), 3 and 6 months after trastuzumab initiation, and after trastuzumab completion (eFigure 1 in Supplement 1). CMR data acquisition included cine imaging for LV volumes and LVEF, T2 maps (3 slices) for assessing edema, T1 maps before and 15 minutes after contrast (3 slices) for assessing interstitial fibrosis and edema, mid–short axis T1-weighted turbo spin echo (TSE) image before and early after contrast for assessing hyperemia (a marker of inflammation), and late gadolinium enhancement (LGE) imaging to assess replacement fibrosis. Fellowship-trained radiologists blinded to all clinical data performed CMR analysis using cmr42 software, version 5.9.4 (Circle Cardiovascular Imaging). Peak systolic global longitudinal (GLS) and circumferential strain (GCS) were measured using cine images and reported as the percentage of shortening (positive values). Extracellular volume fraction (ECV) was calculated using T1 maps13 before and after contrast while early gadolinium enhancement ratio was calculated using T1-weighted TSE images before and after contrast.14 To better understand the potential driver for change in ECV, we calculated indexed ECV (iECV) and indexed intracellular volume (iICV) representing absolute values of total myocardial interstitial space and ICV, respectively. Details of CMR acquisition and analyses are summarized in eMethods and eTable 2 in Supplement 1. Normal values for our scanner and reproducibility of CMR tissue biomarkers in our laboratory have been previously reported.15
Serum Biomarkers and CTRCD Definitions
Both hs-cTnI and BNP were measured on blood samples collected on the day of imaging (eMethods in Supplement 1). CTRCD was defined using the Cardiac Review and Evaluation Committee criteria as either a reduction in CMR measured LVEF of 10% or greater (absolute) without heart failure symptoms or a reduction of 5% or greater (absolute) with heart failure symptoms from baseline to an LVEF of less than 55%.16 CTRCD was assessed at each time point during follow-up. Post hoc sensitivity analysis was performed using the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI; ≥10% reduction in LVEF to <53%) and the ESC (≥10% reduction in LVEF to <50%) definitions of CTRCD.17,18 When baseline LVEF was below the lower LVEF threshold for a given definition, a 10% or greater further reduction from baseline constituted CTRCD.
Statistical Analysis
For descriptive analysis, continuous variables were presented as mean and SD or median and IQR as appropriate. Categorical variables were summarized as frequencies and proportions. Differences in clinical variables between patients who did and did not develop CTRCD were compared using 2-sample t tests or Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables. Patient-specific profiles of clinical parameters, serum biomarkers, and CMR variables over follow-up were displayed using spaghetti plots. Given the serial nature of the measures, overall time trends were explored using natural cubic splines with 4 degrees of freedom in generalized estimating equation (GEE) with identity link functions. The 95% CIs were calculated based on robust sandwich estimators.
Concurrent Association With Continuous Outcomes
We applied fixed-effect models to quantify the association between CMR tissue biomarkers with ventricular volumes, mass, GLS, GCS, LVEF, and serum biomarkers. The models included time, modeled using natural cubic splines to allow for potentially nonlinear temporal variations, and other time-dependent covariates, including heart rate, cumulative doxorubicin-equivalent dose, and cardiac medication uses for CTRCD and for other reasons (eg, hypertension). The 95% CIs and the corresponding P values were calculated with t statistics. We used fixed-effect models because we were interested in how a patient’s variation in CMR tissue biomarkers affected other variables within the same patient during follow-up, and time-independent covariates (eg, age at baseline) were not included in the fixed-effect models because these models implicitly accounted for them.19
Association With Concurrent and Subsequent CTRCD
We performed 2 separate analyses using GEE to assess and quantify the association between CMR tissue biomarkers and CTRCD. Specifically, we quantified the associations of tissue biomarkers at time t with concurrent CTRCD (ie, at the same time as the tissue biomarkers) and separately with subsequent CTRCD (ie, at the next time point). In both analyses, we considered age at baseline, baseline cardiovascular risk factors (eg, diabetes, hypertension, dyslipidemia, and smoking histories), heart rate, cumulative doxorubicin-equivalent dose, and uses of cardiac medications for CTRCD and other reasons (eg, hypertension) as covariates. Independent working correlation matrices were specified in all aforementioned GEE analyses to avoid biased estimation due to the endogeneity of the CMR tissue biomarkers.20 The 95% CIs and P values were calculated using robust sandwich estimators. For the subsequent CTRCD analysis, serial observations were censored at the first occurrence of CTRCD or the end of follow-up, whichever was earlier.
Missing observations were imputed (eTable 3 in Supplement 1) using multivariate imputation by chained equations,21 and the regression results from the imputed data were combined using the Rubin rule.22 Statistical analyses were implemented using R software, version 4.0.323 (R Project for Statistical Computing) and assumed a statistical significance of <.05. Data were analyzed from July 2021 to June 2022.
Results
Patients
Among 160 enrolled patients, 24 withdrew consent or were withdrawn after baseline CMR due to claustrophobia (n = 3), visits being too lengthy (n = 8), a change to nonanthracycline cancer therapy (n = 10), or other (n = 3). Baseline characteristics, cancer treatment details, and feasibility of CMR analysis of the 136 patients (mean [SD] age, 51.1 [9.2] years) who completed the study are summarized in the Table and eTables 3, 4, and 5 in Supplement 1. Participants self-identified with the following race and ethnicity groups: 50 Asian (37%), 4 Black (3%), 6 non-Black Hispanic (4%), and 76 White (56%).
Table. Baseline Clinical, Oncological, Laboratory, and Cardiovascular Magnetic Resonance (CMR) Characteristics of the Entire Study Cohort and Patients With and Without Cancer Therapy–Related Cardiac Dysfunction (CTRCD).
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All patients (N = 136) | No CTRCD (n = 99) | CTRCD (n = 37) | ||
| Age, mean (SD), y | 51.1 (9.2) | 50.5 (9.0) | 52.6 (9.8) | .25 |
| Race | ||||
| Asian | 50 (37) | 37 (37) | 13 (35) | .34 |
| Black | 4 (3) | 2 (2) | 2 (5) | |
| Non-Black Hispanic | 6 (4) | 3 (3) | 3 (8) | |
| White | 76 (56) | 57 (58) | 19 (51) | |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 118 (16) | 117 (16) | 119 (16) | .41 |
| Diastolic | 75 (10) | 74 (9) | 78 (11) | .08 |
| Heart rate, mean (SD), bpm | 66 (9) | 65 (8) | 69 (11) | .09 |
| Diabetes | 5 (3.7) | 2 (2.0) | 3 (8.1) | .12 |
| Hypertension | 21 (15.4) | 16 (16.2) | 5 (13.5) | .80 |
| Dyslipidemia | 14 (10.3) | 6 (6.1) | 8 (21.6) | .02 |
| Smoking | 33 (24.3) | 21 (21.2) | 12 (32.4) | .18 |
| Baseline medications | ||||
| ACE inhibitor | 6 (4.4) | 3 (3.0) | 3 (8.1) | .34 |
| Angiotensin receptor blocker | 7 (5.1) | 7 (7.1) | 0 (0.0) | .19 |
| β-Blocker | 7 (5.1) | 5 (5.1) | 2 (5.4) | <.99 |
| Statin | 9 (6.6) | 4 (4.0) | 5 (13.5) | .06 |
| Any cardiac medication | 22 (16.2) | 14 (14.1) | 8 (21.6) | .30 |
| Breast cancer stage | .53 | |||
| 1 | 16 (11.8) | 12 (12.1) | 4 (10.8) | |
| 2 | 81 (59.6) | 60 (60.6) | 21 (56.8) | |
| 3 | 38 (27.9) | 27 (27.3) | 11 (29.7) | |
| 4a | 1 (0.7) | 0 (0.0) | 1(2.7) | |
| Breast cancer side | .84 | |||
| Left | 79 (58.1) | 56 (56.6) | 23 (23.2) | |
| Right | 55 (40.4) | 41 (41.4) | 14 (37.8) | |
| Bilateral | 2 (1.5) | 2 (2.0) | 0 (0) | |
| CMR measures, mean (SD) | ||||
| LVEDV, mL | 128.1 (22.4) | 127.1 (20.3) | 130.8 (27.4) | .45 |
| LVEDV indexed, mL/m2 | 74.4 (11.6) | 74.5 (11.0) | 74.2 (13.3) | .88 |
| LVESV, mL | 47.5 (10.7) | 46.7 (9.9) | 49.5 (12.4) | .21 |
| LVESV indexed, mL/m2 | 27.6 (5.8) | 27.4 (5.5) | 28.1 (6.4) | .54 |
| LVEF, % | 63.2 (4.0) | 63.4 (4.0) | 62.4 (4.0) | .17 |
| LV mass index, g/m2 | 35.9 (6.0) | 36.2 (6.2) | 35.2 (5.4) | .37 |
| GLS, % | 18.5 (1.9) | 18.8 (1.9) | 17.8 (1.8) | .01 |
| GCS, % | 20.7 (2.1) | 20.9 (2.0) | 20.0 (2.1) | .03 |
| EGEr, median (IQR) | 1.95 (1.55-2.69) | 1.89 (1.55-2.60) | 2.06 (1.72-2.85) | .33 |
| T1, milliseconds | 1012.0 (26.4) | 1013.1 (25.7) | 1009.0 (28.5) | .45 |
| T2, milliseconds | 51.4 (2.2) | 51.5 (2.1) | 51.0 (2.3) | .24 |
| ECV, % | 25.3 (2.4) | 25.4 (2.4) | 24.9 (2.6) | .30 |
| Indexed ECV, mL/m2 | 8.7 (1.7) | 8.8 (1.7) | 8.4 (1.7) | .25 |
| Indexed ICV, mL/m2 | 25.6 (4.5) | 25.8 (4.7) | 25.2 (3.8) | .46 |
| Serum biomarkers, mean (SD) | ||||
| BNP level, pg/mL | 21.0 (13.8) | 21.1 (12.8) | 20.9 (16.4) | .96 |
| hs-cTnI Level, ng/L | 3.3 (3.8) | 3.7 (4.4) | 2.0 (1.0) | .001 |
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; BNP, B-type natriuretic peptide; bpm, beats per minute; ECV, extracellular volume fraction; EGEr, early gadolinium enhancement ratio; GCS, global circumferential strain; GLS, global longitudinal strain; hs-cTnI, high-sensitivity cardiac troponin I; ICV, intracellular volume; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume.
One patient had stage 3 disease at study enrollment, but early during treatment was found to have solitary metastasis to the liver. She continued the same cancer regimen and therefore was kept in the study.
During the study, 37 (27%) patients (Table) developed CTRCD (2 with heart failure symptoms): 1 after anthracycline therapy, 14 at 3 months after trastuzumab initiation, 14 at 6 months after trastuzumab initiation, and 8 after trastuzumab completion. LVEF at baseline, nadir, and after trastuzumab completion for these patients is illustrated in eFigure 2 in Supplement 1. Temporal changes in heart rate and blood pressure are illustrated in eFigure 3 in Supplement 1. At the time of diagnosis of CTRCD, trastuzumab was interrupted (1-2 cycles) in 10 patients and β-blockers and/or angiotensin-converting enzyme inhibitor or angiotensin receptor blocker were started or titrated up in 19 patients. Reasons for failure to receive cardiac medications or to have trastuzumab interrupted included the following: LVEF between 50% and 55% at the time of CTRCD, refusal of cardiac medications, treating oncologist decided to continue trastuzumab, or CTRCD not identified during routine screening.
Temporal Changes in CMR Tissue Biomarkers
Early after contrast myocardial relative enhancement peaked after anthracycline therapy (mean [SD] relative myocardial enhancement: baseline, 46.3% [16.8%] to peak 56.2% [18.6%]) and remained elevated 3 months into trastuzumab therapy, whereas skeletal muscle relative enhancement peaked 3 months after trastuzumab initiation with both changes recovering during follow-up (Figure 1; eTable 6 in Supplement 1). There was no significant change in early gadolinium enhancement ratio over follow-up. There was an increase in native global T1, T2, and ECV compared with before anthracycline therapy with values peaking at 3 months after trastuzumab initiation (mean [SD] native T1: baseline, 1012 [26] milliseconds to peak, 1035 [28] milliseconds; T2: 51.4 [2.2] milliseconds to 52.6 [2.2] milliseconds; ECV: 25.2% [2.4%] to 26.8% [2.7%]) and recovering subsequently (Figure 1; eTable 6 in Supplement 1). Normal values for T1, T2, and ECV for the CMR sequences used on our scanner are shown in Figure 1, and the changes were small (mean peak change in T1, T2, and ECV were 23 milliseconds, 1.2 milliseconds, and 1.5%, respectively). Eleven patients (8%) had a midwall linear pattern of LGE before anthracycline treatment (basal slice: 8 of 11 [73%]) that was minimal and remained unchanged after trastuzumab completion, constituting 1.8% (IQR, 0.7%-3.0%) and 2.0% (IQR, 1.3%-5.0%) of LV myocardial mass, respectively. None of the patients developed new LGE.
Figure 1. Cardiovascular Magnetic Resonance (CMR) Tissue Biomarkers at Each Study Visit.
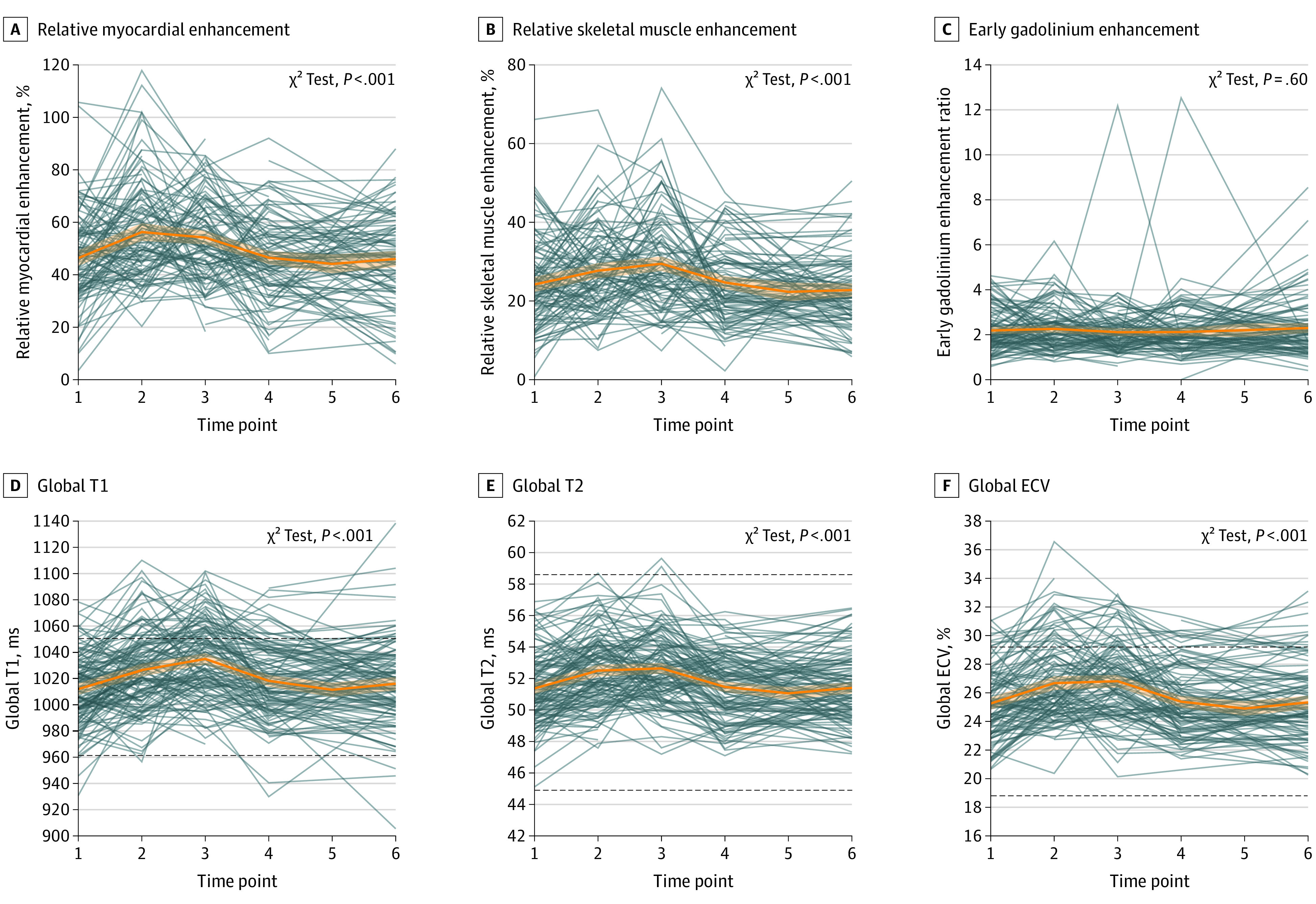
Individual patient trajectories and overall trajectories (orange line) with corresponding 95% CI (orange shading) are shown for relative myocardial enhancement (A), relative skeletal enhancement (B), early gadolinium enhancement (C), global T1 (D), global T2 (E), and global extracellular volume fraction (ECV) (F). The P values assess if the estimated trajectory is different from a horizontal line (ie, no changes from baseline). Relative myocardial and skeletal muscle enhancement, T1, T2, and ECV all peaked after anthracycline (before trastuzumab) therapy or 3 months into trastuzumab therapy and returned to baseline by end of trastuzumab therapy (eFigures 10 and 11 and eTable 6 in Supplement 1 for grouping based on cancer therapy–related cardiac dysfunction status). Time points on the x-axis are as follows: 1, before anthracycline therapy; 2, after anthracycline but before trastuzumab therapy; 3, three months after trastuzumab initiation; 4, six months after trastuzumab initiation; and 6, after trastuzumab completion. No CMR data was collected at time point 5. Normal ranges for same CMR sequences and scanner from our prior publication15 is provided as dashed lines for T1, T2, and ECV.
Concurrent Association Between CMR Tissue Biomarkers and Ventricular Remodeling, Function, and Serum Biomarkers
Nadir LVEF, GLS, and GCS levels were seen between 3 and 6 months after trastuzumab initiation coinciding with the largest LV end-systolic and end-diastolic volume index and LV mass index (Figure 2). Changes in ventricular volumes were associated with change in T1, T2, and/or ECV (Figure 3). No significant associations were seen between any of the CMR tissue biomarkers and LVEF (Figure 2), GLS/GCS (eFigure 4 in Supplement 1), or LV mass index (eFigure 5 in Supplement 1). hs-cTnI and BNP levels peaked after anthracycline treatment and then recovered (eFigure 6 in Supplement 1). Patients who had a larger increase in T1, T2, and ECV had a larger increase in BNP, but no association was seen with hs-cTnI (eFigure 7 in Supplement 1).
Figure 2. Left Ventricular (LV) Volumes, Mass, and Function at Each Visit.
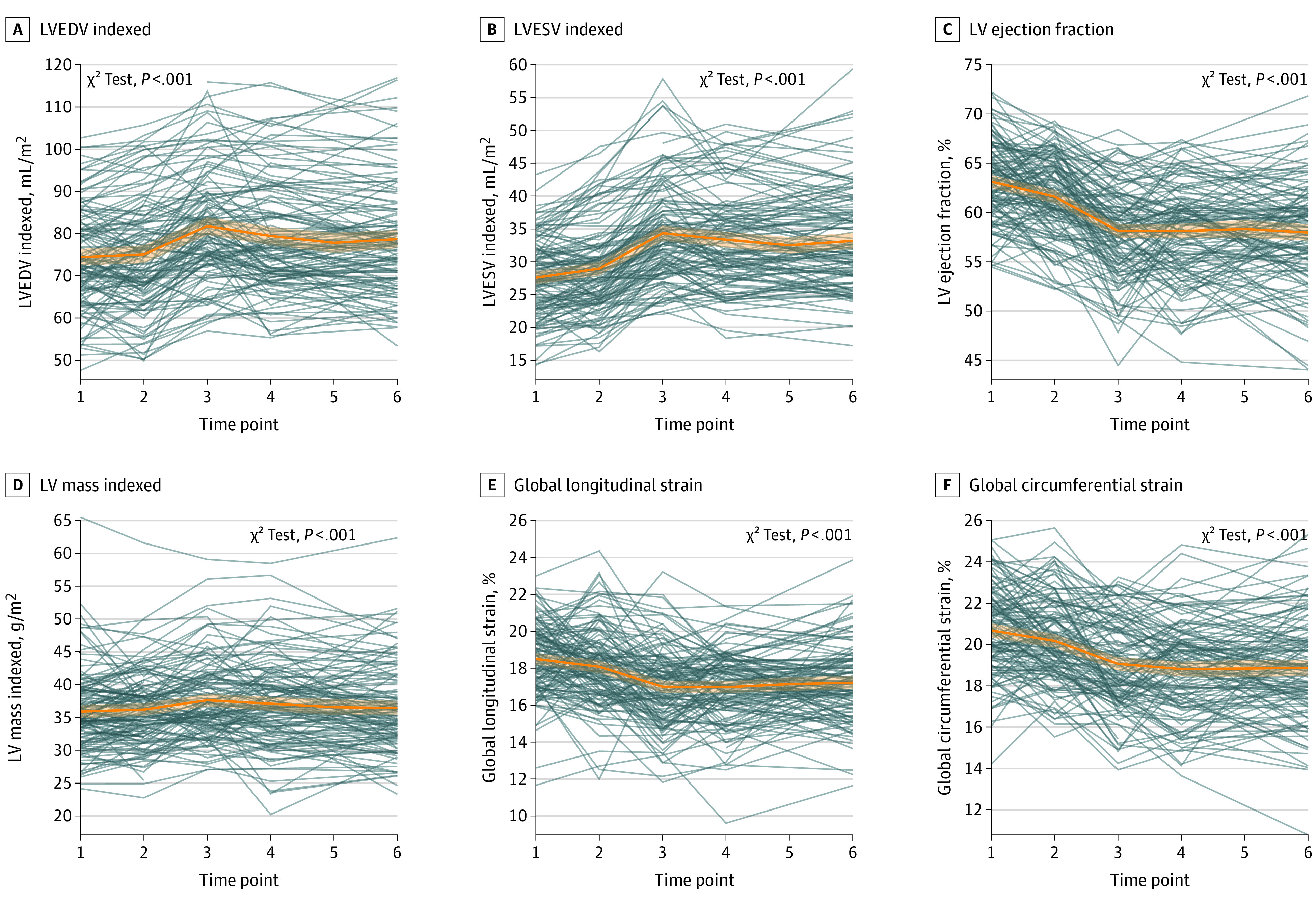
Individual patient trajectories and overall trajectories (orange line) with corresponding 95% CI (orange shading) are shown for LV end-diastolic volume (EDV) (A), LV end-systolic volume (ESV) (B), LV ejection fraction (C), LV mass indexed (D), global longitudinal strain (E), and global circumferential strain (F). On average, ventricular volumes and mass reached their largest, while LVEF and both strain measures reached their minimum first at 3 months into trastuzumab therapy with incomplete or no recovery by end of follow-up. The P values assess if the estimated trajectory is different from a horizontal line (ie, no changes from baseline). Figure 1 contains the definition of the time points. eFigures 8 and 9 in Supplement 1 display the same measures grouped based on cancer therapy–related cardiac dysfunction status.
Figure 3. Regression-Adjusted Nonlinear Concurrent Associations Between Cardiovascular Magnetic Resonance (CMR) Tissue Biomarkers and Left Ventricular (LV) Volumes and Ejection Fraction.
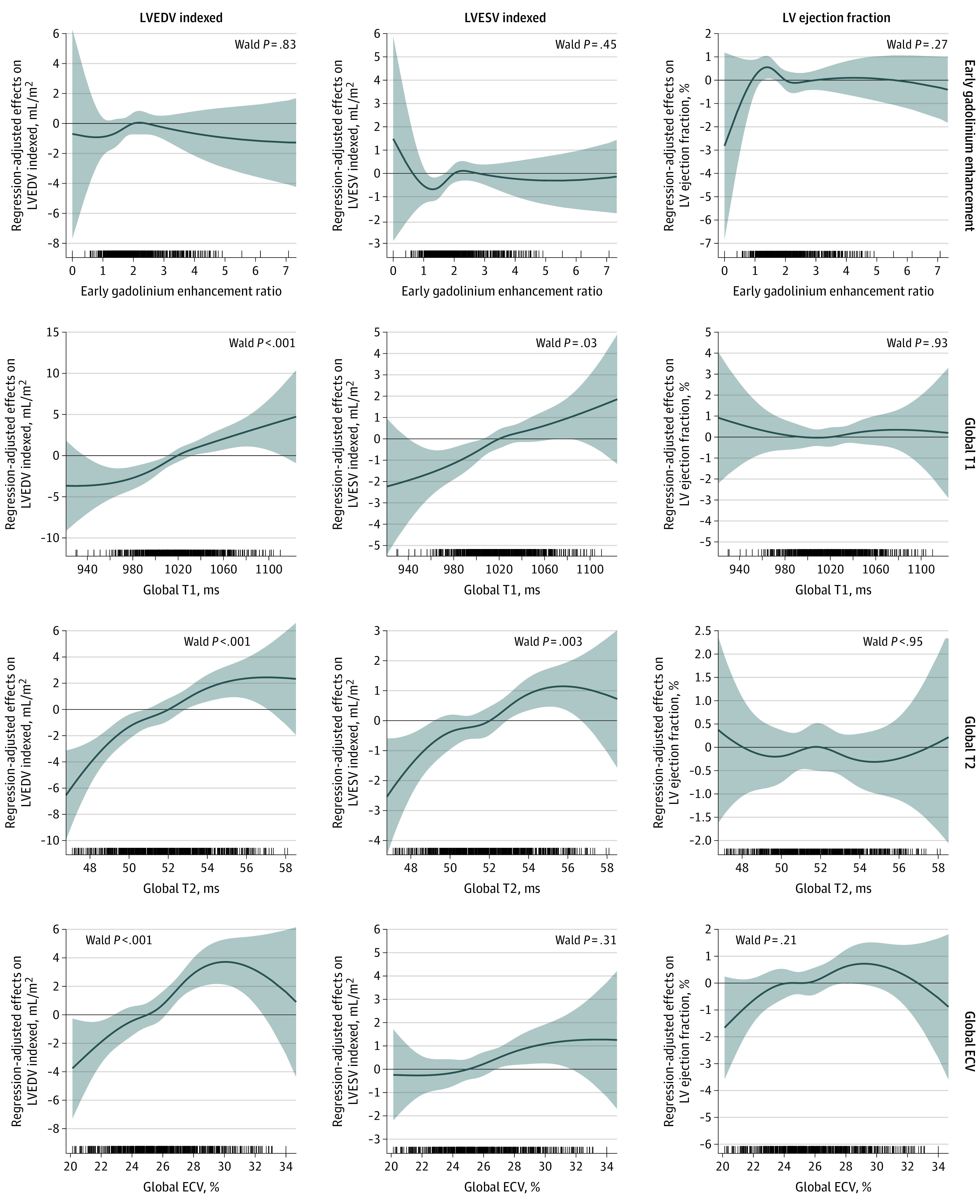
The graphs show the expected changes in outcome variables (y-axis) associated with changes in CMR tissue biomarkers (x-axis). For example, a change in T1 from 1000 to 1050 milliseconds was associated with an increase in LV end-diastolic volume (EDV) index of 3.12 (95% CI, 1.99-4.25) mL/m2 and LV end-systolic volume (ESV) index of 1.07 (95% CI, 0.47-1.67) mL/m2. The tick marks on the x-axis reflect individual observed measurements. The shaded region represents the 95% CI of the estimated association. P values <.05 suggest statistically significant associations. ECV indicates extracellular volume fraction.
Association Between CMR Tissue Biomarkers and CTRCD
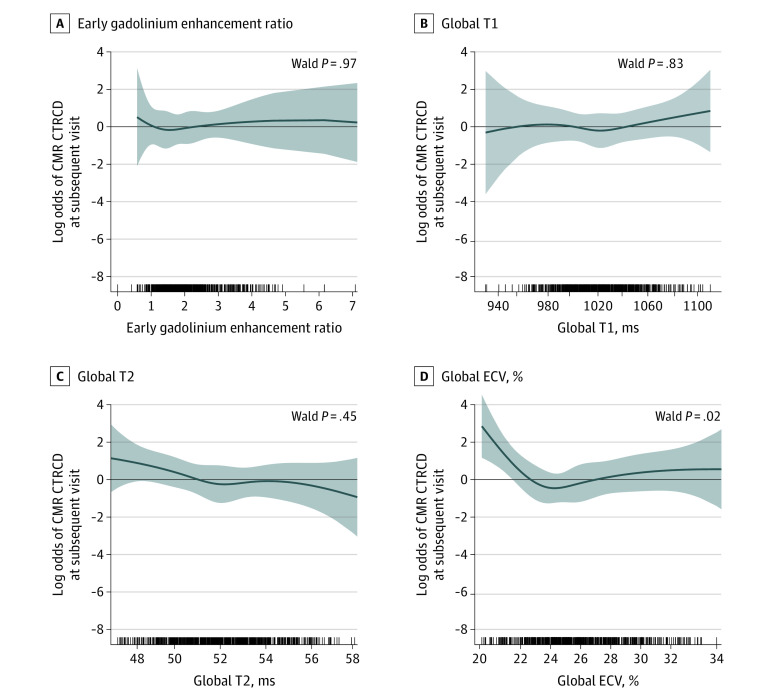
Trajectories of the absolute values and changes in LV volumes, LVEF, LV mass, GLS, and GCS over the treatment period in patients with and without CTRCD are illustrated in eFigures 8 and 9 in Supplement 1. There was significant overlap in the absolute values and changes in all of the CMR tissue biomarkers between patients with and without CTRCD over the treatment period (eFigures 10 and 11 in Supplement 1). On regression analysis, only ECV was significantly associated with CTRCD at the same visit (P = .009) (eFigure 12 in Supplement 1) and subsequent visit (P = .02) (Figure 4). A total of 36% of patients (4 of 11) with LGE had CTRCD compared with 27% of patients (33 of 122) without LGE (P = .50). Baseline LGE could not be determined in 3 patients. On sensitivity analysis using ASE or ESC CTRCD definition, all estimated associations remained similar (eFigures 13 and 14 in Supplement 1).
Figure 4. Regression-Adjusted Nonlinear Associations Between Cancer Therapy–Related Cardiac Dysfunction (CTRCD) at the Next Visit and Cardiovascular Magnetic Resonance (CMR) Tissue Biomarkers in Terms of Log Odds.
The graphs show the log-odds of CTRCD (y-axis) associated with changes in the x-axis CMR tissue biomarkers: early gadolinium enhancement ratio (A), global T1 (B), global T2 (C), and global extracellular volume fraction (ECV) (D). For example, a change in ECV from 24% to 22% increased the log odds of CTRCD at the next visit by 1.232 (95% CI, 0.381-2.082), which translates to an odds ratio of 3.427 ( = 101.232). In other words, an absolute decrease from 24% to 22% in ECV was associated with a 242.7% increase in the likelihood of CTRCD at the next visit. The tick marks on the x-axis reflect individual observed measurements. The shaded region represents the 95% CI of the estimated association. P values <.05 suggest statistically significant associations.
Post hoc Analysis
Total myocardial interstitial space (iECV) first increased after anthracycline therapy and peaked 3 months after trastuzumab initiation whereas iICV increased 3 months after trastuzumab initiation and remained higher than baseline; however, these changes did not differ between patients with and without CTRCD (eFigure 15, eTable 6 in Supplement 1). LVEF trended lower with higher intracellular volumes (P = .08) (eFigure 16 in Supplement 1), but no associations were seen with interstitial space.
Discussion
Using serial CMR scans, we assessed myocardial tissue changes in women with ERBB2–positive breast cancer during sequential therapy with anthracyclines and trastuzumab. Our study results suggest that an increase in CMR tissue biomarkers occurred early during treatment, was transient, and was associated with changes in LV volumes and BNP. Only ECV was associated with the traditional LVEF definition of CTRCD.
CMR Tissue Biomarkers to Monitor Patients Receiving Cancer Therapy
There has been a growing interest in using CMR tissue biomarkers to monitor patients receiving potentially cardiotoxic cancer therapy, but prospective multicenter data to assess its clinical usefulness are limited. Although CMR tissue biomarker changes are not equivalent to pathology data, previous work in inflammatory disease and cardiomyopathy have suggested that hyperemia identified by early gadolinium enhancement imaging is a marker of inflammation24; increase in T1 values with simultaneous increase in T2 likely reflects (intracellular/extracellular) edema, and ECV in the setting of elevated T2 highlights interstitial edema.25 In women with ERBB2–positive breast cancer receiving sequential anthracyclines and trastuzumab, our study suggests diffuse muscle inflammation as indicated by increased early myocardial enhancement after anthracycline therapy and early skeletal muscle enhancement peaking 3 months later coinciding with taxane therapy. Myocardial T1, T2, and ECV values peaked at 3 months after trastuzumab initiation supporting the hypothesis of injury-related myocardial edema with anthracyclines followed by delayed repair or progression with trastuzumab.26 However, the observed CMR tissue biomarker values were mostly within the normal range (Figure 1), the changes were small (mean peak change in T1, T2, and ECV were 23 milliseconds, 1.2 milliseconds, and 1.5%, respectively) and on average within previously reported expected temporal variability using the same scanner and sequences (29 milliseconds for T1, 3.0 milliseconds for T2, and 2.2% for ECV) (eFigure 11 in Supplement 1),15 and biomarker values returned to baseline by the end of treatment. Our findings were similar to prior small studies of patients treated with anthracyclines that have also described only a small increase in combination of T1, T2, and/or ECV early after treatment compared with baseline27,28or healthy controls.29 Overall, these data suggest that there is likely limited value of these CMR tissue biomarkers for routine surveillance of myocardial injury from the studied cancer therapies in similar patient cohorts.
Drivers of CMR Tissue Biomarker Changes
Without histopathology, the mechanisms driving the CMR tissue biomarker changes detected in patients are difficult to determine; however, recent anthracycline animal model studies provide some insights. In a primate model with imaging prior to and 15 weeks after the human equivalent of doxorubicin, 240 mg/m2, significant increases in ECV and T2 were seen.30 These changes correlated with histopathological findings of interstitial edema and collagen deposition and increased cardiomyocyte cross-sectional area but a decrease in cardiomyocyte numbers. Similarly, in a rabbit model of doxorubicin CTRCD, ECV increase was the earliest finding and was associated with interstitial edema.5 In a pig model of doxorubicin CTRCD with intracoronary injection, an increase in T2 was the earliest myocardial change and correlated with edema.4 Based on these studies, the increase in early myocardial enhancement, T1, T2, and ECV after anthracycline therapy and/or early during trastuzumab therapy in our study, along with increase in LV mass, suggests a combination of inflammation and both intracellular and extracellular edema. This is further supported by increase in both iECV and iICV in the patients in our study. It is also important to note that none of the patients in our study developed new LGE or had an increase in the burden of preexisting LGE, despite a 27% incidence of CTRCD, suggesting that the treatments studied at their respective doses do not contribute to replacement fibrosis. Other concomitant processes, such as interstitial fibrosis or myocyte loss with compensatory hypertrophy of residual myocytes,30 may have occurred but are difficult to determine without histopathology.
Associations Between CMR Tissue Biomarkers and Ventricular Remodeling, Function, and Serum Biomarkers
In the patients in our study, the timing of peak measures of T1, T2, and ECV and ventricular volumes coincided, and the tissue biomarkers and ventricular volumes were associated, which suggests that myocardial inflammation and edema were contributors to ventricular remodeling. The lack of association with hs-cTnI implies that this remodeling was not associated with myocyte necrosis. The association with BNP suggests that this remodeling may be contributing to higher LV filling pressures. Despite these findings, we did not identify a statistically significant association between CMR tissue biomarkers and change in LVEF or strain. Furthermore, among the CMR tissue biomarkers, only ECV had an association with CTRCD. However, the between-group difference in ECV was small, the observed values were mostly within the normal range, and the changes overlapped between patients with and without CTRCD. The reason for the U-shape association seen with ECV (Figure 4) alone is difficult to explain biologically. However, given that the significance was likely driven by decreased ECV values, a possible explanation is the need for a certain threshold increase in intracellular edema or compensatory myocyte hypertrophy resulting in a reduction in interstitial space (ie, reduced ECV) before a significant decrease in LVEF (ie, >10% to define CTRCD). In support of this hypothesis, the total iICV peaked and remained higher than before anthracycline treatment, whereas the interstitial volume peaked and subsequently started to decrease at the time when most CTRCD events began occurring.
Our findings are comparable with those of prior small studies of patients treated with anthracycline that reported lack of association between T1, T2, and/or ECV early after initiation of anthracyclines (approximately 3-6 months) and LVEF,27 circumferential strain,27 or poor association with LVEF or GLS-defined CTRCD.8,10 However, our findings also differ from those of other studies. In a high-risk cohort of older adult patients receiving anthracycline therapy, T2 values after cycle 2 were associated with CTRCD (mostly concurrent) in unadjusted analyses.6 Another study of patients with sarcoma imaged 48 hours after the first anthracycline dose identified an association between a larger reduction in T1 values and the risk of CTRCD.7 No association was seen with T2 values or ECV. More recently, in a small group of patients with breast cancer who were treated with epirubicin and/or radiation, in unadjusted analysis, an increase in T1 but not T2 values at approximately 3 months after anthracycline therapy discriminated (area under the curve, 0.71) patients (n = 9) who developed either a significant LVEF or GLS change at 13-month follow-up.28 Although heterogenous in their findings, the differences between the 3 studies6,7,28 and ours may be associated with (1) lack of sequential anthracycline and trastuzumab use; (2) inclusion of patients at higher CTRCD risk6,7,28; (3) earlier imaging during anthracycline therapy6,7; (4) variable CTRCD definitions28; (5) no adjustment for potential confounders6,7,28; and (6) assumption that the associations would have a linear pattern in the prior studies.
Clinical Implications
Our study suggests that in patients receiving sequential anthracycline and trastuzumab therapy, myocardial inflammation and edema were ubiquitous during early therapy reflecting a potentially vulnerable period for the myocardium. These findings may help future studies that are focused on defining the optimal timing for cardiac surveillance (eg, echocardiography) during cancer therapy. However, CMR tissue biomarkers are unlikely to be useful at the individual patient level for routine CTRCD risk surveillance given that the values were mostly in the normal range, and there was significant overlap in the observed values and their changes between patients with and without CTRCD. Our findings may relate to the population studied as the patients in our study would be considered to be at low to medium risk of CTRCD as per recently published guidelines.3 Therefore, whether CMR tissue biomarkers may have a diagnostic and prognostic value in patients at higher risk for cardiotoxicity remains to be studied.
Limitations
This study has some limitations. It is possible that alternative timing of CMR imaging (eg, after first or second dose of anthracycline) may identify different associations with ventricular function and CTRCD. However, we took a pragmatic approach by performing imaging at commonly used time points. Although our study included a large sample size with over 650 CMR studies, the number of patients who developed CTRCD was only 37. Therefore, larger studies such as the ongoing Understanding and Predicting Fatigue, Cardiovascular Decline, and Events After Breast Cancer (UPBEAT) study31 or those that include patients at higher risk of developing cardiotoxicity may help further define associations between CMR tissue biomarkers and the risk of CTRCD. We do not have cardiac biopsy data to correlate with CMR tissue biomarkers. However, we have used findings from animal-model studies to hypothesize on the potential pathophysiological mechanisms driving our findings.
Conclusions
Results of this cohort study suggest that in women receiving anthracyclines and trastuzumab therapy for ERBB2-positive breast cancer, the observed CMR tissue biomarker measurements were predominantly within the normal range. The changes were mostly small, on average within previously reported temporal variability, and overlapped between patients with and without CTRCD. Although CMR tissue biomarkers were associated with ventricular remodeling and an increase in BNP, these changes were transient, were not associated with LVEF or myocardial strain, and were not useful in identifying traditional CTRCD risk. These data suggest that CMR tissue characterization may be of limited value in routine CTRCD surveillance.
eMethods.
eTable 1. Cancer Treatment Regimens and Doses Used in the Included Patients
eTable 2. Typical CMR Imaging Parameters
eTable 3. Frequency of Measurements That Were Not Feasible or Available and Hence Imputed at the Various Time Points
eTable 4. Cancer Treatment Summary
eTable 5. Percentage of Segments Among Evaluable Segments That Were Excluded for the Various CMR Tissue Biomarker Measurements Due to Artifacts
eTable 6. CMR Tissue and Serum Biomarkers Over Follow-up Period for the Whole Cohort and Dichotomized by CTRCD Status
eFigure 1. CMR Imaging Time Points and Relationship to Cancer Treatment
eFigure 2. Absolute Left Ventricular Ejection Fraction and Its Change From Baseline at Each Study Visit in the 37 Patients Who Developed CTRCD
eFigure 3. Blood Pressure and Heart Rate at Each Study Visit
eFigure 4. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and Myocardial Strain
eFigure 5. Regression-Adjusted Nonlinear Concurrent Association Between CMR Tissue Biomarkers and LV Mass
eFigure 6. Absolute BNP and Troponin at Each Study Visit and Changes Compared to Baseline
eFigure 7. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and B-type Natriuretic Peptide (BNP) and High Sensitivity Troponin I
eFigure 8. CMR Left Ventricular Volumes, Mass, and Function at Each Study Visit by the Development of CTRCD During Follow-up
eFigure 9. Changes Compared to Baseline in Indexed LVEDV, LVESV, and LV Mass, LVEF, Global Longitudinal Strain and Global Circumferential Strain at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 10. CMR Tissue Biomarkers at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 11. Changes Compared to Baseline in EGE Measures, T1, T2, and ECV at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 12. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and CTRCD in Terms of Log-Odds
eFigure 13. Sensitivity Analysis Examining the Concurrent Association, in Terms of Log-Odds, Between Changes in CMR Tissue Biomarkers and CTRCD Using the ASE and ESC CTRCD Definitions
eFigure 14. Sensitivity Analysis Examining the Association, in Terms of Log-Odds, Between CMR Tissue Biomarkers and CTRCD at the Next Visit Using the ASE and ESC CTRCD Definitions
eFigure 15. Indexed Extracellular (iECV) and Intracellular (iECV) Volumes at Each Visit
eFigure 16. Regression-Adjusted Nonlinear Concurrent Associations Between Indexed Extracellular and Intracellular Volume and Left Ventricular Ejection Fraction (LVEF)
eReferences
Data Sharing Statement
References
- 1.Houbois CP, Nolan M, Somerset E, et al. Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. JACC Cardiovasc Imaging. 2021;14(5):962-974. doi: 10.1016/j.jcmg.2020.09.039 [DOI] [PubMed] [Google Scholar]
- 2.Pudil R, Mueller C, Čelutkienė J, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966-1983. doi: 10.1002/ejhf.2017 [DOI] [PubMed] [Google Scholar]
- 3.Lyon AR, López-Fernández T, Couch LS, et al. ; ESC Scientific Document Group . 2022 ESC Guidelines on cardiooncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 4.Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73(7):779-791. doi: 10.1016/j.jacc.2018.11.046 [DOI] [PubMed] [Google Scholar]
- 5.Hong YJ, Park HS, Park JK, et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using T1-mapping cardiac magnetic resonance imaging: an animal study. Sci Rep. 2017;7(1):2663. doi: 10.1038/s41598-017-02627-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Garcia A, Diaz-Pelaez E, Lopez-Corral L, et al. T2 mapping identifies early anthracycline-induced cardiotoxicity in elderly patients with cancer. JACC Cardiovasc Imaging. 2020;13(7):1630-1632. doi: 10.1016/j.jcmg.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Muehlberg F, Funk S, Zange L, et al. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail. 2018;5(4):620-629. doi: 10.1002/ehf2.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giusca S, Korosoglou G, Montenbruck M, et al. Multiparametric early detection and prediction of cardiotoxicity using myocardial strain, T1 and T2 mapping, and biochemical markers: a longitudinal cardiac resonance imaging study during 2 years of follow-up. Circ Cardiovasc Imaging. 2021;14(6):e012459. doi: 10.1161/CIRCIMAGING.121.012459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giusca S, Steen H, Montenbruck M, et al. Multiparametric assessment of left ventricular hypertrophy using late gadolinium enhancement, T1 mapping and strain-encoded cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23(1):92. doi: 10.1186/s12968-021-00775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustberg MB, Reinbolt R, Addison D, et al. Early detection of anthracycline-induced cardiotoxicity in breast cancer survivors with T2 cardiac magnetic resonance. Circ Cardiovasc Imaging. 2019;12(5):e008777. doi: 10.1161/CIRCIMAGING.118.008777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan JH, Vasu S, Morgan TM, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9(8):e004325. doi: 10.1161/CIRCIMAGING.115.004325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evaluation of Myocardial Changes During Breast Adenocarcinoma Therapy to Detect Cardiotoxicity Earlier With MRI (EMBRACE-MRI). ClinicalTrials.gov Identifier: NCT02306538. Updated February 14, 2023. Accessed Month Day, Year. https://clinicaltrials.gov/ct2/show/NCT02306538
- 13.Arheden H, Saeed M, Higgins CB, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211(3):698-708. doi: 10.1148/radiology.211.3.r99jn41698 [DOI] [PubMed] [Google Scholar]
- 14.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141(6):1007-1013. doi: 10.1067/mhj.2001.115436 [DOI] [PubMed] [Google Scholar]
- 15.Altaha MA, Nolan M, Marwick TH, et al. Can quantitative CMR tissue characterization adequately identify cardiotoxicity during chemotherapy—impact of temporal and observer variability. JACC Cardiovasc Imaging. 2020;13(4):951-962. doi: 10.1016/j.jcmg.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 16.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215-1221. doi: 10.1200/JCO.2002.20.5.1215 [DOI] [PubMed] [Google Scholar]
- 17.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911-939. doi: 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. ; ESC Scientific Document Group . 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. John Wiley & Sons; 2012. [Google Scholar]
- 20.Diggle P, Haegerty P, Liang K, Zeger S. The Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 21.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 23.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 24.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97(18):1802-1809. doi: 10.1161/01.CIR.97.18.1802 [DOI] [PubMed] [Google Scholar]
- 25.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Mohan N, Endo Y, Shen Y, Wu WJ. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget. 2017;9(5):6095-6108. doi: 10.18632/oncotarget.23543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meléndez GC, Jordan JH, D’Agostino RB Jr, Vasu S, Hamilton CA, Hundley WG. Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging. 2017;10(6):708-709. doi: 10.1016/j.jcmg.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahir E, Azar M, Shihada S, et al. Myocardial injury detected by T1 and T2 mapping on CMR predicts subsequent cancer therapy-related cardiac dysfunction in patients with breast cancer treated by epirubicin-based chemotherapy or left-sided RT. Eur Radiol. 2022;32(3):1853-1865. doi: 10.1007/s00330-021-08260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haslbauer JD, Lindner S, Valbuena-Lopez S, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. 2019;275:179-186. doi: 10.1016/j.ijcard.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 30.Meléndez GC, Vasu S, Lesnefsky EJ, et al. Myocardial extracellular and cardiomyocyte volume expand after doxorubicin treatment similar to adjuvant breast cancer therapy. JACC Cardiovasc Imaging. 2020;13(4):1084-1085. doi: 10.1016/j.jcmg.2019.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan JH, D’Agostino RB Jr, Avis NE, et al. Fatigue, cardiovascular decline, and events after breast cancer treatment: rationale and design of UPBEAT study. JACC CardioOncol. 2020;2(1):114-118. doi: 10.1016/j.jaccao.2020.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Cancer Treatment Regimens and Doses Used in the Included Patients
eTable 2. Typical CMR Imaging Parameters
eTable 3. Frequency of Measurements That Were Not Feasible or Available and Hence Imputed at the Various Time Points
eTable 4. Cancer Treatment Summary
eTable 5. Percentage of Segments Among Evaluable Segments That Were Excluded for the Various CMR Tissue Biomarker Measurements Due to Artifacts
eTable 6. CMR Tissue and Serum Biomarkers Over Follow-up Period for the Whole Cohort and Dichotomized by CTRCD Status
eFigure 1. CMR Imaging Time Points and Relationship to Cancer Treatment
eFigure 2. Absolute Left Ventricular Ejection Fraction and Its Change From Baseline at Each Study Visit in the 37 Patients Who Developed CTRCD
eFigure 3. Blood Pressure and Heart Rate at Each Study Visit
eFigure 4. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and Myocardial Strain
eFigure 5. Regression-Adjusted Nonlinear Concurrent Association Between CMR Tissue Biomarkers and LV Mass
eFigure 6. Absolute BNP and Troponin at Each Study Visit and Changes Compared to Baseline
eFigure 7. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and B-type Natriuretic Peptide (BNP) and High Sensitivity Troponin I
eFigure 8. CMR Left Ventricular Volumes, Mass, and Function at Each Study Visit by the Development of CTRCD During Follow-up
eFigure 9. Changes Compared to Baseline in Indexed LVEDV, LVESV, and LV Mass, LVEF, Global Longitudinal Strain and Global Circumferential Strain at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 10. CMR Tissue Biomarkers at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 11. Changes Compared to Baseline in EGE Measures, T1, T2, and ECV at Each Study Visit Grouped by the Development of CTRCD During Follow-up
eFigure 12. Regression-Adjusted Nonlinear Concurrent Associations Between CMR Tissue Biomarkers and CTRCD in Terms of Log-Odds
eFigure 13. Sensitivity Analysis Examining the Concurrent Association, in Terms of Log-Odds, Between Changes in CMR Tissue Biomarkers and CTRCD Using the ASE and ESC CTRCD Definitions
eFigure 14. Sensitivity Analysis Examining the Association, in Terms of Log-Odds, Between CMR Tissue Biomarkers and CTRCD at the Next Visit Using the ASE and ESC CTRCD Definitions
eFigure 15. Indexed Extracellular (iECV) and Intracellular (iECV) Volumes at Each Visit
eFigure 16. Regression-Adjusted Nonlinear Concurrent Associations Between Indexed Extracellular and Intracellular Volume and Left Ventricular Ejection Fraction (LVEF)
eReferences
Data Sharing Statement