Abstract
Context
MicroRNAs (miRNAs)—short, single-stranded, noncoding RNAs—regulate several biological processes, including bone metabolism.
Objective
We investigated circulating miRNAs as promising biomarkers for treatment monitoring in women with postmenopausal osteoporosis on denosumab (DMAB) therapy.
Methods
In this prospective, observational, single-center study, 21 postmenopausal women treated with DMAB were included for a longitudinal follow-up of 2 years. Next-generation sequencing (NGS) was performed to screen for serological miRNAs at baseline, month 6, and month 24. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was used to confirm NGS findings in the entire cohort. Bone turnover markers (BTM) P1NP and CTX, and bone mineral density (BMD) by dual x-ray absorptiometry were assessed and correlated to miRNAs.
Results
BMD at the hip (5.5%, P = 0.0006) and lumbar spine significantly increased (11.4%, P = 0.017), and CTX (64.1%, P < 0.0001) and P1NP (69.3%, P < 0.0001) significantly decreased during treatment. NGS analysis revealed significant changes in miRNAs after 2 years of DMAB treatment but not after 6 months. Seven miRNAs were confirmed by RT-qPCR to be significantly changed during a 2-year course of DMAB treatment compared to baseline. Four of these were mainly transcribed in blood cells, including monocytes. Correlation analysis identified significant correlation between change in miRNA and change in BTMs as well as BMD. Based on effect size and correlation strength, miR-454-3p, miR-26b-5p, and miR-584-5p were defined as top biomarker candidates, with the strongest association to the sustained effect of denosumab on bone in osteoporotic patients.
Conclusion
Two years of DMAB treatment resulted in upregulation of 7 miRNAs, 4 of which are mainly transcribed in monocytes, indicating a potential impact of DMAB on circulating osteoclast precursor cells. These changes were associated to BMD gain and BTM suppression and could therefore be useful for monitoring DMAB treatment response.
Keywords: miRNAs, denosumab, osteoporosis, postmenopausal osteoporosis, anti-osteoporotic therapy
Osteoporosis is a systemic bone disease associated with bone loss and structural changes in bone microarchitecture, resulting in an increased risk for fragility fractures (1). More than half of all fractures at age 50+ years are related to osteoporosis, with increasing risk in the elderly and a higher number of fragility fractures in females (2). Currently available pharmacological agents reduce fragility fracture risk (3). Denosumab (DMAB) is a human monoclonal antibody that binds Receptor Activator of Nuclear Factor Kappa B Ligand (RANKL) thereby blocking the interaction between RANKL and RANK, resulting in the inhibition of osteoclast activation and reduced survival (4). In postmenopausal women with osteoporosis, DMAB treatment resulted in a significant reduction of vertebral, nonvertebral, and hip fracture risk (5).
Bone turnover markers (BTMs) such as serum procollagen type I N propeptide (PINP) and serum C-terminal cross-linking telopeptide of type I collagen (CTX) are established tools to evaluate fracture risk on population level and can be helpful for monitoring the effectiveness and adherence to treatment (6). However, BTMs have failed to provide information on individual fracture risk and are not reliable for diagnosis of osteoporosis, which renders the search for novel diagnostic biomarkers necessary. Moreover, bone mineral density (BMD) measurements by dual energy x-ray absorptiometry (DXA) are used for follow-up examinations. However, it has to be considered, that changes in BMD by DXA are small under antiresorptive treatment, and that changes in bone metabolism are only partially reflected by DXA (7).
MicroRNAs (miRNAs) are short, noncoding RNAs, which act as posttranscriptional regulators of gene expression by manipulating protein translation and consequently protein synthesis. Since their discovery in 1993, the number of identified bona fide genome-encoded human miRNAs has steadily increased and is now estimated at 2500 mature miRNAs (8-10). Several of these have been shown to play a pivotal role in regulating developmental processes, including cell proliferation, apoptosis, and stem cell division (11). miRNA transcription is highly cell-type specific (12); several miRNAs have been identified to be high abundant in bone, and the relevance of miRNAs in the context of bone diseases has been extensively investigated (13, 14). A passive (upon cell death) or active release of miRNAs within protein complexes or extracellular vesicles results in stable extracellular presence of miRNAs in various biofluids, including cell-free blood (15). Importantly, miRNA quantification in biofluids is highly sensitive to pre-analytical and analytical biases and therefore requires careful standardization of sample collection as well as analytical methods (16-18). The assumption that variability in miRNA blood levels could inform about physiological and pathological processes in bone is the basis for the evaluation as bone biomarker candidates for diagnosis and prognosis of bone diseases (14, 19, 20). To date, only a few studies have investigated changes of miRNAs during anti-osteoporotic therapy (21-25). Using a representative animal model for postmenopausal osteoporosis, miRNA levels in bone tissue and serum under antiresorptive or osteo-anabolic therapy were investigated recently (21, 22). These findings indicate that miRNAs are changing during the course of anti-osteoporotic treatment. However, these published studies substantially differ in design, methods, and outcomes, and have only covered a follow-up period of 12 months. Moreover, only preselected miRNAs were analyzed rather than performing unbiased genome-wide screens of miRNA changes. Thus, the course and role of miRNAs during anti-osteoporotic therapy still remains unclear.
We hypothesize that circulating miRNAs could show a significant change and pattern in the course of denosumab therapy and therefore provide deeper insights into epigenetic adaptation to this type of osteoporosis therapy. Therefore, the aim of the MiRNA-Denosumab-Therapy Study (MiDeTe study) was to determine and quantify bone-specific miRNAs in the serum of postmenopausal women undergoing treatment with denosumab for an observational period of 24 months.
Materials and Methods
Study Design
The MiDeTe study is a prospective, observational, single-center study in women with postmenopausal osteoporosis. Recruitment was performed at the outpatient clinic of a specialized referral center for osteoporosis and bone diseases (St. Vincent Hospital Vienna, 2nd Department of Internal Medicine). The study was approved by the local Ethics Committee of the St. Vincent Hospital Vienna (201612_EK22). Oral and written consent was obtained from all study participants prior to any study-related procedure. No financial compensation was provided to the study participants.
Subjects
All patients included in the MiDeTe study were diagnosed and treated according to the current guideline of the DVO (Dachverband für Osteologie—Umbrella Organisation for Osteology) (26) Study involvement did not affect the choice of therapy. Participants received denosumab 60 mg subcutaneously every 6 months through their primary care physician for the observation period of 24 months. A maximum interval of 6 months (±2 weeks) between the injections was defined, as well as a defined timepoint with a tolerance of 2 weeks for every visit. Inclusion criteria combined postmenopausal osteoporosis, age from 60 to 80 years and (i) T-Score less than −2.5 in DXA or (ii) clinical indication for antiresorptive treatment. Exclusion criteria covered a former or ongoing diagnosis of secondary osteoporosis (eg, primary hyperparathyroidism, glucocorticoid induced osteoporosis), diabetes mellitus type 1, renal insufficiency III-V°, advanced liver dysfunction (defined as liver cirrhosis with Child Pugh Score B or C), alcohol abuse, rheumatological disease (rheumatoid arthritis, spondylarthritis, systemic lupus erythematosus), malignancy (over the past 5 years), and eating disorder (anorexia nervosa, bulimia nervosa). Prior therapy with bisphosphonates was tolerated, while any other bone-specific therapy with denosumab, teriparatide, strontium ranelate, or selective estrogen receptor modulators led to discharge.
Data on demographics, family history, comorbidities, lifestyle, history of fractures, and ongoing as well as former medication were gathered at baseline. Participants were rechecked at months 3, 6, 12, 18, and 24 to complete a self-developed questionnaire to request information about adverse events, hospitalization, new fractures, and changes in medication. Laboratory serum markers, including bone turnover markers (BTMs), and quantification of miRNAs were performed at defined time points (Fig. 1). DXA and radiographs of the thoracic and lumbar spine were collected at baseline and at the end of the study.
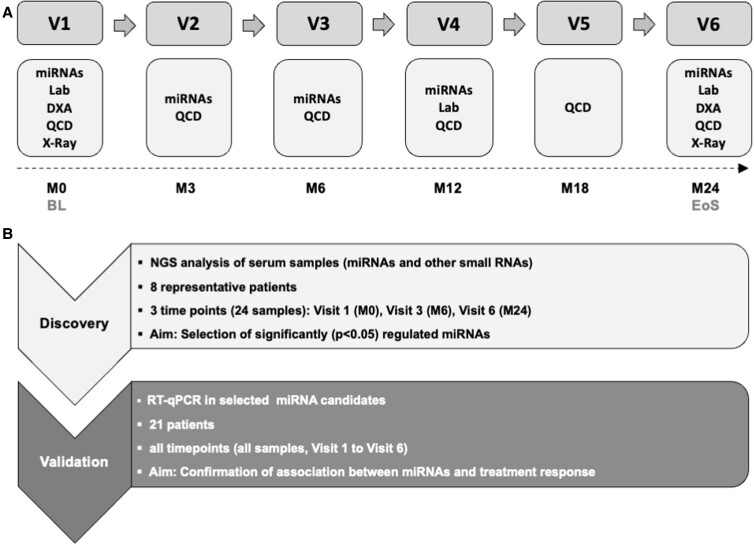
Figure 1.
Study design. Study Flow Chart. A, Postmenopausal women with osteoporosis were treated with denosumab for 2 years (n = 21). Abbreviations: BL, baseline; DXA, dual x-ray absorptiometry; EoS: end of study; Lab, serum markers of bone metabolism; M, month; miRNA: microRNA analysis; QCD: Questionnaire on demographic and clinical data; X-Ray: x-ray of thoracic and lumbar spine. Experimental Design B, MiRNA analysis was divided into 2 phases: a discovery phase and subsequent validation phase. In the discovery phase, next-generation sequencing (NGS) was performed to screen and select significantly regulated miRNAs at 3 time points in a subset of 8 (selected from 21) subjects. The validation phase utilized reverse transcription quantitative polymerase chain reaction (RT-qPCR) to quantify the selected miRNAs in all subjects and time points (except Visit 5), and perform correlation analysis to confirm the association between miRNAs and treatment response. In the validation phase, 8 patients from the discovery phase plus 13 additional patients were included.
Laboratory Analysis
Blood serum samples were drawn between 8 Am and 10 Am after an overnight fast and processed at room temperature. Clotting time was between 30 and 60 minutes, followed by centrifugation at 2500 × g for 10 minutes and storage at −70 °C for later analysis. Cross-linked C-telopeptide (CTX), procollagen type 1 N-terminal propeptide (P1NP), intact parathyroid hormone, and 25-hydroxyvitamin D (25-OH vitamin D) were measured via chemiluminescence on the IDS-iSYS microparticle immunoassay system (Immunodiagnostics Systems Ltd., Boldon, UK).
Bone Mineral Density
DXA measurements were performed at the hip and lumbar spine at baseline and after 24 months. DXA measurements were either performed at our study site or in an external radiological institute. Relative changes (in %) of BMD between baseline and 24 months were calculated and used for correlation analysis. In line with current International Society for Clinical Densitometry (ISCD) guidelines all levels (L1 to L4) at the lumbar spine were included, except for values above the standard variation. DXA measurements were only included in the analysis if at least 2 vertebrae were usable. Vertebral fractures were excluded from analysis. Radiographs of the spine were collected at the beginning and end of the study to rule out silent fractures during treatment.
miRNA Analysis
RNA extraction from serum
Total RNA was extracted from 200 μL serum using the miRNeasy Mini Kit (Qiagen, Germany) as described by Kocijan et al (27). Briefly, precisely 200 μL of each serum sample were mixed with 1000 μL Qiazol and 1 μL of a mix of 3 synthetic spike-in controls (miRCURY spike-in kit, Qiagen, Cat No. 339390). Following a 10-minute incubation at room temperature, 200 μL chloroform were added to the lysates followed by centrifugation at 12 000g for 15 minutes at 4 °C. Exactly 650 μL of the upper aqueous phase were transferred to a miRNeasy mini column where RNA was precipitated with 750 μL ethanol followed by automated washing with RPE and RWT buffer in a QiaCube liquid handling robot. Finally, total RNA was eluted in 30 μL nuclease-free water and stored at −80 °C.
Small RNA-Seq analysis
Small RNA-sequencing was performed using baseline, 6 months (visit 3 [V3]), and 24 months (visit 6 [V6]) serum samples from a subset of 8 patients (approximately one-third of the total cohort). The 8 patients were selected based on following exclusion criteria: diabetes mellitus type 2, parathyroid hormone treatment, incident fracture, body mass index (BMI) <20, duration of menopause >40 years, vitamin D <20 ng/mL, glycated hemoglobin (HbA1c) >6%.
Library preparation was performed as described by Khamina et al (28) using RealSeq-Biofluids Plasma/Serum miRNA Library kit for Illumina sequencing (RealSeq Biosciences, Santa Cruz, US, Cat No. 600-00048-SOM) and miND spike-in controls (TAmiRNA, Austria, Cat No. KT-041-MIND). Due to low RNA concentrations that render RNA quantifications inaccurate, RNA extraction efficiency was confirmed by reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis of spike-ins, and constant volumes of exactly 8.5 μL of extracted total RNA were used as input for library preparation. Adapter-ligated libraries were circularized, reverse transcribed, and amplified. Library PCR was performed using 18 cycles with Illumina primers included in the kit. In total, 24 miRNA libraries were prepared from serum samples as described in Fig. 1B, and the libraries were further analyzed for library fragment distribution using the Agilent DNA 1000 kit (Agilent Technologies, Cat No. 5067-1504) with Agilent DNA1000 reagents (Agilent Technologies, Cat No. 5067-1505). The generated libraries were pooled in an equimolar proportion and the obtained pool was size-selected with the BluePippin system using a 3% agarose cassette with a target range of 100 to 250 kb (Sage Science, Cat No. BDQ3010) to remove DNA fragments outside of the target range. The pooled and purified libraries were analyzed for fragment distribution on an Agilent High Sensitivity DNA kit (Agilent Technologies, Cat No. 5067-4626) with Agilent High Sensitivity DNA reagents (Agilent Technologies, Cat No. 5067-4627). The library pool was then sequenced on an Illumina NextSeq550 (single-read, 75bp) according to the manufacturer's protocol at the Vienna BioCenter Core Facilities (VBCF), Vienna, Austria.
Reverse transcription quantitative polymerase chain reaction analysis
Starting from total RNA samples, including the ones used for the discovery phase, cDNA was synthesized using the miRCURY LNA RT kit (Qiagen, Cat No. 339340). In total, 4 μL of total RNA were used per 10 μL reverse transcription (RT) reaction. To monitor RT efficiency and presence of impurities with inhibitory activity, a synthetic RNA spike-in (cel-miR-39-3p) was added to the RT reaction.
PCR amplification was performed in a customized 384-well plate format (Qiagen, Cat No. 339330) using miRCURY SYBR Green qPCR (Qiagen, Cat No. 339347) and miRCURY LNA miRNA PCR Assay products (Qiagen Cat No. 339306). qPCR was performed in a Roche LC480 II instrument (Roche, Germany). All steps were performed according to the manufacturer’s instructions.
To calculate the cycle of quantification values (Cq-values), the second derivative method was used. Spike-in control values were used for monitoring data quality. Cq-values of 2 endogenous miRNAs, miR-23a and miR-451a, were measured for monitoring hemolysis of the used serum samples. Spike-in controls showed acceptable variation and only 1 sample showed signs of inhibition and was therefore removed from the analysis. Cq-values of endogenous miRNAs were normalized to the RNA spike-in controls by subtracting the individual miRNA Cq-value from the RNA Spike-in Cq, thus obtaining delta-Cq (dCq) values that were used for the statistical analysis.
Statistical Analysis
RT-qPCR data analysis
GraphPad Prism v5.03 was used for analysis of changes in bone and metabolic data using a parametric paired t test.
GraphPad Prism v9.2.0 was used for the analysis of miRNA dCq-value changes over time. Statistical significance was assessed using 1-way analysis of variance (ANOVA) with repeated measures for comparisons between matched groups. All graphs were presented as the median percentage of change of all patients for each time point with the respective interquartile range.
Statistical differences in the percentage of change of miRNA levels at V4 and V6 between treatment-naïve patients and patients pretreated with bisphosphonate (BP) was assessed using the nonparametric Mann-Whitney test.
Spearman correlation analysis between the percentage of change from baseline to visit 6 of the different metabolic and bone parameters and the percentage of change of miRNA levels was performed using GraphPad Prism v9.2.0. Changes in miRNA levels were calculated as . P values were adjusted for multiple testing using the 2-stage set-up method of Benjamini, Krieger. and Yekutieli.
Small RNA-Seq data analysis
Analysis of small RNA-Seq data was performed with the software package MiND, a data analysis pipeline that generates overall QC data, unsupervised clustering analysis, normalized miRNA count matrices, and differential expression analysis based on raw next-generation sequencing (NGS) data (29).
Overall quality of the NGS data was evaluated automatically and manually with fastQC v0.11.8 and multiQC v1.7. Reads from all passing samples were adapter trimmed and quality filtered using cutadapt v2.3 and filtered for a minimum length of 17nt. Mapping steps were performed with bowtie v1.2.2 and miRDeep2 v2.0.1.2, whereas reads were mapped first against the genomic reference GRCh38.p12 provided by Ensembl allowing for 2 mismatches and subsequently miRBase v22.1, filtered for miRNAs of hsa only, allowing for 1 mismatch. For a general RNA composition overview, non-miRNA mapped reads were mapped against RNAcentral and then assigned to various RNA species of interest. Statistical analysis of preprocessed NGS data was done with R v3.6 and the packages pheatmap v1.0.12, pcaMethods v1.78, and genefilter v1.68. Differential expression analysis with edgeR v3.28 used the quasi-likelihood negative binomial generalized log-linear model functions provided by the package. The independent filtering method of DESeq2 was adapted for use with edgeR to remove low abundant miRNAs and thus optimize the false discovery rate (FDR) correction.
Differential expression analysis from our NGS data uses statistical tests to find miRNAs that are over or underexpressed in a group. For this report, the well-established analysis toolkit edgeR was used.
miRNA target network construction using miRnet 2.0
Gene target networks were constructed using miRnet 2.0 (https://www.mirnet.ca/miRNet/upload/MirUploadView.xhtml) (30). Genes listed in miRTarBase v8.0 and TarBase v8.0 were selected for network construction and the degree filter was set to 3, meaning that target nodes with at least 3 connections remained in the network. The KEGG database (31) was used for pathway enrichment, using all genes identified in the network and hypergeometrical testing. Pathways of interest (with an FDR <20%) were selected based on their known role on bone biology or relation with bone quality and osteoporosis.
Cell-type enrichment analysis
FANTOM (Functional Annotation of the Mouse/Mammalian Genome) is an integrated expression atlas of miRNAs in various cell types. It is based on sRNA sequencing data that was generated using a wide variety of human and mouse cell types (32). Using the FANTOM5 database we analyzed the expression profile of the miRNAs selected in this study across a variety of cell types. For this, an average miRNA expression level was calculated for each cell type (regardless of the tissue of origin) that was available in the FANTOM5 database. Expression information from cells derived from male or female reproductive organs or cells obtained under a certain treatment were excluded from this analysis. The expression levels across cell types were visualized using a heatmap generated in ClustVis (33).
Results
Patient Characteristics
A total of 26 women (mean age 70.15 ± 6.41 years) were included in the MiDeTe study, of whom 21 completed the study. Four individuals could not be followed until the end of the study and 1 patient was excluded due to genetic disease. Therefore, the final analysis was performed in 21 patients.
BTMs were measured in all patients at baseline, after 12 months, and 24 months. miRNA analysis was missing in 1 patient at visit 6 (24 months). At baseline, all patients had at least one DXA measurement at 1 site; measurements were incomplete in 3 patients. At the end of the study, DXA was incomplete in 4 patients, and 2 women had no documented DXA measurement after 24 months of treatment. X-rays of the spine were collected at baseline in 20 patients; in 1 patient, magnetic resonance imaging of the spine was performed. At the end of the study, imaging of the spine was missing in 10 patients. Timing between DMAB injections and visits was differing in our cohort, leading to a variation regarding the blood sampling and the days since the last injection. In few patients, the exact information on the injection dates was missing (Supplementary Fig. S1) (34).
All patients were homogenous regarding age, height, weight, and BMI. Demographic and clinical data of the study group are summarized in Table 1. Fourteen patients had a history of fracture (66.1%: vertebral fractures 28.6%; peripheral fractures 66.1%). All patients received vitamin D and calcium supplementation throughout the study, while only 66.1% and 19% of patients had a documented preceding vitamin D and calcium intake, respectively. Twelve women (57.1%) had a history of bisphosphonate treatment (Supplementary Table S1) (34). BTMs at baseline showed values in line with postmenopausal state and revealed a significant reduction (CTX levels −64.1%, P1NP −69.3%, P < 0.01) after 24 months of treatment (Supplementary Fig. S2A and S2B) (34). BMD showed significant improvement after 2 years (Table 2, Supplementary Fig. S2C and S2D) (34). One patient suffered a fracture of the radius and acetabulum shortly after therapy initiation and underwent revision after 3 months. The patient remained in the study, because fractures occurred right after therapy initiation and there was no indication for treatment switch. Time between the fracture and visit 2 (3 months after DMAB) amounted to 90 days, and there were 60 days between surgical revision and visit 3 (6 months after DMAB). Bone turnover markers (BTMs) in this patient showed an ongoing suppression during the whole study period. Furthermore, 1 metatarsal fracture was reported after 18 months of treatment. The follow-up examinations of the spine by x-ray revealed no new vertebral fractures during treatment. Four patients showed divergent treatment response as measured by BMD and CTX levels at the end of the study: patient 105 showed a decrease in spine BMD of −7%, while combined T-Score at the spine and hip BMD improved (+10%) and CTX levels were suppressed (−70%). Patient 119 showed a decrease in spine BMD (−4%) and an increase in CTX levels (+3.9%), while combined T-Score at the spine was stable. Patient 302, who was diagnosed with diabetes mellitus type 2, showed a decrease in spine and hip BMD (−5%) while T-Score at the spine improved and BTMs were suppressed (−38%). Patient 101 showed a decrease in hip BMD (−2.5%) together with an increase in BMD at the radius (+3%) and suppressed CTX levels (−85%) (spine BMD was not available in this patient).
Table 1.
Demographic and clinical data of the study group (n = 21)
| Demographic and clinical data | Mean | ± SD |
|---|---|---|
| Age, years | 70.15 | 6.41 |
| Height, cm | 163.8 | 7.07 |
| Weight, kg | 67.69 | 9.71 |
| BMI, kg/cm2 | 25.39 | 4.18 |
| Menopause duration (years) | 21.82 | 8.00 |
| n | % of s.g. | |
| Diabetes mellitus type 2 | 2 | 9.52% |
| Current smoking | 1 | 4.76% |
| Previous smoking | 3 | 14.3% |
| Occasional alcohol intake | 8 | 38.1% |
| Fracture in general | 14 | 66.7% |
| Vertebral fracture | 6 | 28.6% |
| Hip fracture | 1 | 4.76% |
| Humerus fracture | 6 | 28.6% |
| Radius fracture | 2 | 9.52% |
| Pelvis fracture | 2 | 9.52% |
| Other fracture | 3 | 14.3% |
| Medication | ||
| Bisphosphonates (in general) | 12 | 57.1% |
| Bisphosphonates (oral) | 7 | 33.3% |
| Bisphosphonates (intravenous) | 7 | 33.3% |
| Oral anticoagulation | 1 | 4.76% |
| Oral calcium intake | 4 | 19.0% |
| Vitamin D supplementation | 14 | 66.7% |
| Proton pump inhibitors | 14 | 66.7% |
| Hormone replacement therapy | 2 | 9.52% |
Abbreviations: BMI, body mass index; s.g., study group.
Table 2.
Bone mineral density (BMD) and laboratory markers of the study group (n = 21)
| Bone parameters | Baseline data | End of study | Absolute change V1-V6 | P value | ||
|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | |||
| DXA-Hip | ||||||
| Total hip BMD, g/cm² | 0.76 | 0.09 | 0.80 | 0.08 | 0.04 | 0.0006 |
| Total hip T-Score | −1.79 | 0.78 | −1.53 | 0.76 | 0.26 | 0.0032 |
| Femoral neck BMD, g/cm² | 0.72 | 0.09 | 0.74 | 0.07 | 0.02 | 0.15 |
| Femoral neck T-Score | −1.81 | 0.72 | −1.68 | 0.70 | 0.13 | 0.021 |
| DXA-Lumbar spine | ||||||
| Lumbar spine BMD, g/cm² | 0.81 | 0.11 | 0.89 | 0.12 | 0.08 | 0.0017 |
| Lumbar spine (L1-L4) T-score | −2.80 | 0.67 | −1.83 | 1.36 | 0.97 | 0.011 |
| Laboratory serum markers | Relative change V1-V6% | |||||
| Calcium (mmol/L) | 2.35 | 0.10 | 2.30 | 0.11 | 2.15 | 0.028 |
| Phosphate (mmol/L) | 1.07 | 0.11 | 1.02 | 0.16 | 4.43 | 0.18 |
| Parathyroid hormone (mg/mL) | 67.1 | 22.8 | 90.2 | 30.6 | 34.4 | 0.075 |
| CTX (ng/mL) | 0.42 | 0.24 | 0.15 | 0.15 | 64.1 | <0.0001 |
| Vitamin D (ng/mL) | 37.0 | 12.9 | 41.5 | 11.8 | 12.2 | 0.11 |
| P1NP (µg/L) | 57.8 | 31.6 | 17.7 | 8.49 | 69.3 | <0.0001 |
miRNA Analysis
Discovery phase
Small RNA-Seq analysis was performed using serum samples of 8 patients at baseline, 6 months, and 24 months. Small RNA-Seq data quality was checked on the basis of total reads obtained per sample and relative reads mapping against miRNA reads (Supplementary Fig. S3A and S3B) (34). Total reads >17nt (after quality filtering and adapter trimming) ranged between 6 and 10 million reads, with on average 20% reads mapping against miRNAs. Overall, we were able to detect more than 600 miRNAs per sample, of which >200 showed a read count of >10 reads (Supplementary Fig. S3C) (34).
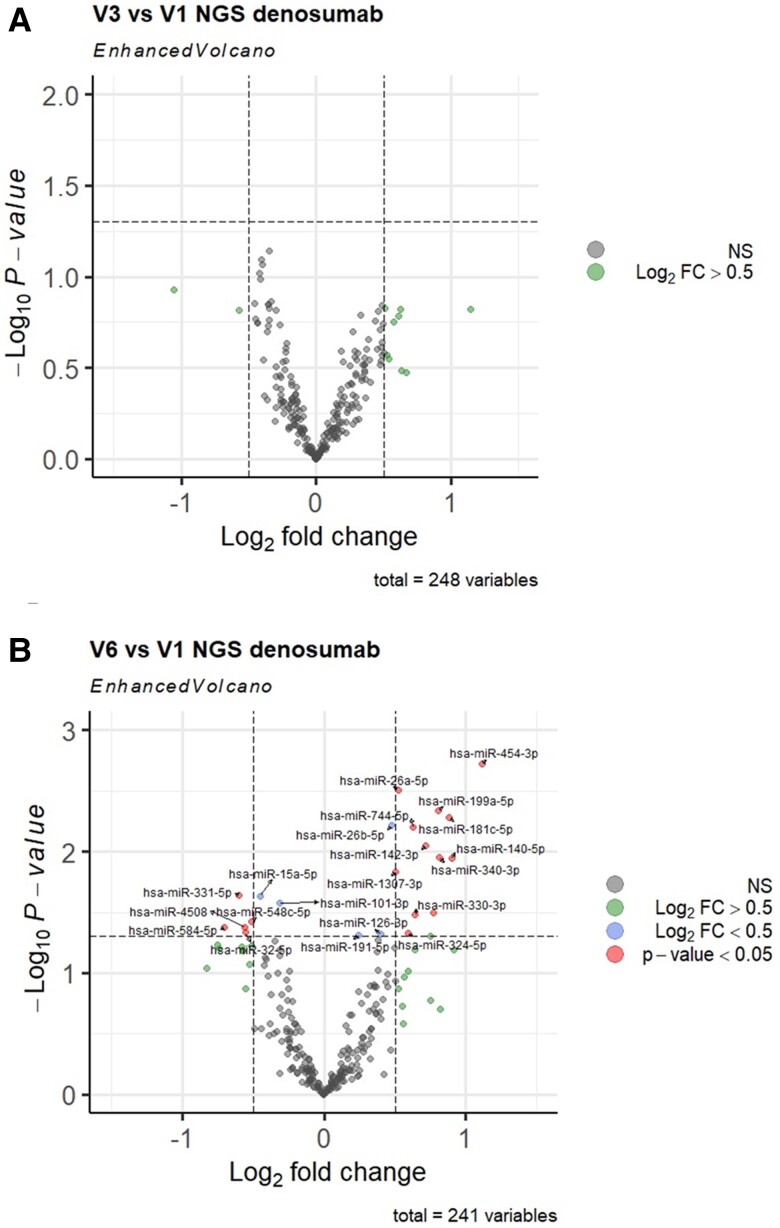
Differential expression analysis identified no significant changes at V3 (month 6) (Fig. 2A). At V6 (month 24) 7 downregulated and 15 upregulated miRNAs were identified (P < 0.05, Fig. 2B). Based on these findings, 22 significantly dysregulated miRNAs were selected for RT-qPCR validation in all subjects and all available time points.
Figure 2.
Next-generation sequencing discovery of circulating miRNA changes compared with baseline (V1) after 6 months (V3) and 24 months (V6) of denosumab therapy in postmenopausal women. Volcano plots depict the log2 transformed fold-change and log10 transformed P values. A, Visit 3 vs Visit 1; B, Visit 6 vs Visit 1. The total number of miRNAs included in the statistical analysis after filtering is indicated below each plot.
Validation phase
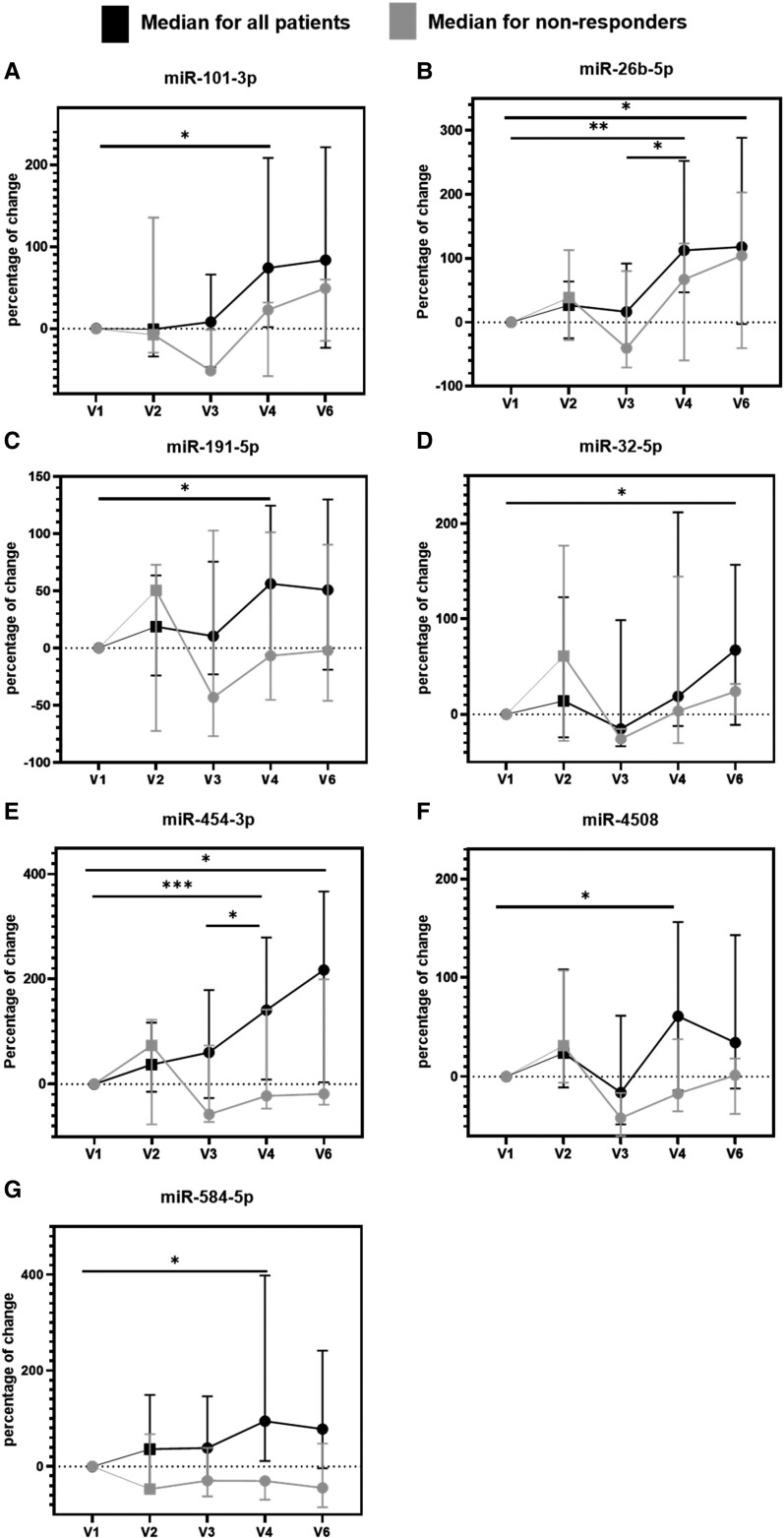
Twenty-two miRNA candidates and 4 quality controls were quantified in 105 serum samples (N = 21 patients, 5 time points). One sample showed RT-qPCR inhibition, and 4 samples were found to be hemolytic (Supplementary Fig. S4A and S4B) (34). Of the 22 selected miRNAs, 16 were detected in 100% of samples and 4 miRNAs were detected in >93% of samples. miR-7704 and miR-331-5p showed low abundance (detected in < 40% of samples) and were excluded from further analysis. While 13 miRNAs did not show significant changes, we were able to confirm a time-dependent regulation of 7 miRNAs (miR-101-3p, miR-191-5p, miR-26b-5p, miR-32-5p, miR-4508, miR-454-3p, and miR-584-5p) (Fig. 3), which were all upregulated.
Figure 3.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) confirmation of miRNA serum levels in 21 subjects and 5 time points. Line plots for the 7 miRs that were confirmed to change over time are shown as the median percentage of change in miRNA levels together with the interquartile range (n = 18). Data for patients with a decrease in spine BMD after 2 years (n = 3) is highlighted in gray. One-way ANOVA with RM using the normalized Cq-values: *adjusted P values between 0.01 and 0.05, **adjusted P values between 0.01 and 0.001, ***adjusted P values between 0.001 and 0.0001.
Two miRNAs, miR-26b-5p and miR-454-3p, showed a significant upregulation after 12 and 24 months (miR-26b-5p: V4 +167% with P value of 0.0013, V6 +182% with P value of 0.045; miR-454-3p: V4 +196% with P value of 0.0009, V6 +213% with P value of 0.018).
miR-101-3p, miR-584-5p, miR-191-5p, and miR-4508 were significantly upregulated after 12 months with increases of 90% (P value of 0.048), 125% (P value of 0.020), 65% (P value of 0.022), and 87% (P value of 0.028), respectively, while miR-32-5p was significantly upregulated after 24 months with an increase of 86% (P value of 0.049).
Of note, the 3 patients 105, 119, and 302 with a decrease in the spine BMD after 2 years of denosumab treatment had a lower increase or even a decrease in the levels of these miRNAs after 12 and 24 months of denosumab treatment compared with the median miRNA level increase (Fig. 3). Patient 119, who also showed an increase in CTX, had a decrease after 12 and 24 months of treatment in 6 of the 7 confirmed miRNAs. The diabetic patient 302, who also showed a decrease in both spine and hip BMD after 2 years, showed a decrease after 12 and 24 months of treatment in 3 of the 7 confirmed miRNAs while the other 4 miRNAs showed a lower increase compared with the average. Last, patient 105 had a lower increase after 24 months of treatment in 4 of the 7 confirmed miRNAs.
Correlation analysis
The 20 miRNAs showing good measurability as outlined above were included in a correlation analysis where the percentage of change in CTX, P1NP, total hip BMD, and spine BMD between V1 and V6 was compared to the percentage of change in miRNA levels (Table 3). Interestingly, all miRNAs showed a significant (FDR < 0.05) negative correlation with CTX levels, while correlation with P1NP was observed for 16/20 miRNAs. BMD changes measured at the total hip were significantly correlated with 11 miRNAs, whereas BMD at the lumbar spine was significantly correlated with 7 miRNAs. MiR-454-3p and miR-584-5p revealed significant correlation with BMD changes at the hip and spine, as well as with CTX- and P1NP levels. The 5 remaining significantly upregulated miRNAs showed correlation only with BTMs.
Table 3.
Spearman correlation assessing the association between the percentage of change in BMD and BTMs and the percentage of change in miRNA levels (V1 vs V6, N = 20)
| miRNAs | Total hip BMD | Mean spine BMD | CTX | PINP | ||||
|---|---|---|---|---|---|---|---|---|
| Correlation value | FDR | Correlation value | FDR | Correlation value | FDR | Correlation value | FDR | |
| miR-101-3p | 0.107 | 0.295 | 0.025 | 0.865 | −0 .446 | <0 .0001 | −0 .360 | 0 .005 |
| miR-191-5p | 0.266 | 0.088 | 0.134 | 0.596 | −0 .399 | <0 .0001 | −0 .316 | 0 .013 |
| miR-26b-5p | 0.311 | 0.057 | 0.181 | 0.514 | −0 .615 | <0 .0001 | −0 .465 | 0 .002 |
| miR-32-5p | 0.202 | 0.156 | 0.159 | 0.533 | −0 .239 | 0 .003 | −0.045 | 0.307 |
| miR-4508 | 0.132 | 0.259 | 0.074 | 0.747 | −0 .349 | <0 .0001 | −0.177 | 0.087 |
| miR-454-3p | 0 .536 | 0 .001 | 0 .402 | 0 .051 | −0 .665 | <0 .0001 | −0 .434 | 0 .002 |
| miR-584-5p | 0 .490 | 0 .003 | 0 .499 | 0 .022 | −0 .477 | <0 .0001 | −0 .362 | 0 .005 |
| miR-126-3p | 0 .639 | <0 .0001 | 0 .423 | 0 .051 | −0 .456 | <0 .0001 | −0 .257 | 0 .029 |
| miR-140-5p | 0 .338 | 0 .042 | 0.124 | 0.604 | −0 .351 | <0 .0001 | −0 .407 | 0 .003 |
| miR-142-3p | 0 .584 | <0 .0001 | 0 .395 | 0 .051 | −0 .495 | <0 .0001 | −0 .288 | 0 .019 |
| miR-181c-5p | −0.158 | 0.221 | −0.041 | 0.854 | −0 .295 | 0 .001 | −0 .366 | 0 .005 |
| miR-199a-5p | 0 .610 | <0 .0001 | 0 .401 | 0 .051 | −0 .296 | 0 .001 | −0 .286 | 0 .019 |
| miR-26a-5p | 0 .644 | <0 .0001 | 0 .401 | 0 .051 | −0 .582 | <0 .0001 | −0 .413 | 0 .003 |
| miR-324-5p | 0 .495 | 0 .003 | 0.369 | 0.069 | −0 .443 | <0 .0001 | −0 .245 | 0 .033 |
| miR-330-3p | 0.293 | 0.067 | 0.096 | 0.689 | −0 .323 | 0 .001 | −0.138 | 0.137 |
| miR-340-3p | 0 .502 | 0 .002 | 0.288 | 0.177 | −0 .269 | 0 .002 | −0.127 | 0.148 |
| miR-548c-5p | 0.258 | 0.091 | 0.157 | 0.533 | −0 .356 | <0 .0001 | −0 .276 | 0 .021 |
| miR-744-5p | 0 .539 | 0 .001 | 0.292 | 0.177 | −0 .476 | <0 .0001 | −0 .288 | 0 .019 |
| miR-1307-3p | 0 .728 | <0 .0001 | 0 .556 | 0 .010 | −0 .561 | <0 .0001 | −0 .365 | 0 .005 |
| miR-15a-5p | 0.075 | 0.349 | 0.019 | 0.865 | −0 .289 | 0 .001 | −0 .223 | 0 .046 |
Bold data indicates that the correlations are significant (FDR < 0.05).
Five miRNAs (miR-126-3p, miR-142-3p, miR-199a-5p, miR-26a-5p, and miR-1307-3p) showed a significant correlation with both BMD parameters and BTMs, although they were not significantly upregulated at 12 or 24 months. No correlation with vitamin D levels was observed. One miRNA correlated with serum calcium levels and 6 miRNAs with parathyroid hormone (Supplementary Table S2) (34).
Subanalysis: pretreated vs treatment-naïve patients
Twelve women (57.1%) had a history of bisphosphonate treatment, 5 women received their treatment less than 1 year ago, 1 patient had a wash out period of more than 18 months, and in 4 women the wash out period was more than 48 months. In 2 patients, detailed data on bisphosphonate treatment was missing. Accordingly, a subgroup analysis regarding the effect of bisphosphonate pretreatment was performed. Comparison of treatment-naïve patients and pretreated patients revealed no difference in the change of miRNAs during DMAB treatment (Supplementary Table S3) (34).
Subanalysis: radius and acetabulum fracture
One patient reported a fracture of the radius and acetabulum a few days after the first DMAB injection. MiRNA regulation in this patient differed during the course of the study when compared to the median of the nonfractured patients. At visit 2 (month 3) and visit 3 (month 6), a pronounced upregulation was detected, while at visit 4 and visit 6 (after 12 months and 24 months), the miRNA levels remained constant (Supplementary Fig. S5) (34).
Subanalysis: diabetes mellitus type 2
We conducted a subgroup analysis after excluding 2 patients with type 2 diabetes. Three miRNAs (miR-101-3p, miR-32-5p, and miR-4508) lost significance in the time-course analysis, while 3 other miRNAs (miR-324-5p, miR-26a-5p, and miR-1307-3p) showed a significant upregulation in this subgroup. The top candidates miR-26b-5p, miR-584-5p, and miR-454-3p remained significantly upregulated after 12 months and/or 24 months in this subgroup (Supplementary Fig. S6) (34).
Subanalysis nonresponders
Three patients showed a decrease in BMD after 24 months compared to baseline. Of these, 1 patient revealed an increase in CTX levels, indicating a poor response to therapy. To address this issue, we performed a subgroup analysis after exclusion of these patients. Six out of 7 miRNAs remained significantly upregulated in this subgroup, including the top candidates. Six additional miRNAs showed a significant upregulation after 12 months and/or 24 months (Supplementary Fig. S7) (34).
Gene target and network analysis
The 7 significantly upregulated miRNAs (miR-101-3p, miR-191-5p, miR-26b-5p, miR-32-5p, miR-4508, miR-454-3p, miR-584-5p) were mapped to their experimentally verified target genes. Thirteen genes with at least 3 miRNA interactions were identified (Supplementary Fig. S8) (34). Gene set enrichment analysis identified several significant KEGG pathways (P < 0.05, Supplementary Table S4) (34) relevant to bone biology and disease, including WNT, TGF-beta signaling, and osteoclast differentiation pathways via the targets CCND2, GSK3B, JUN, VANGL1, FZD6, TBL1Xr1, SMAD7, TGFBr2, SMAD5, SP1, FYN, and FOSL2. All of the identified target genes are at least targeted by 4 of the 7 miRNAs, which were significantly upregulated under DMAB treatment, and 3 genes (IGF1r, TBL1Xr1, and VANGL1) are targeted by 5 of the significantly dysregulated miRNAs (Supplementary Table S4) (34). Of note, all the miRNAs were found to target at least 9 of the 13 genes revealed by miRNet, with the exception of miR-584-5p and miR-4508, which exclusively target the genes FYN and IGF1r, respectively (Supplementary Table S4) (34).
Cell-type enrichment analysis
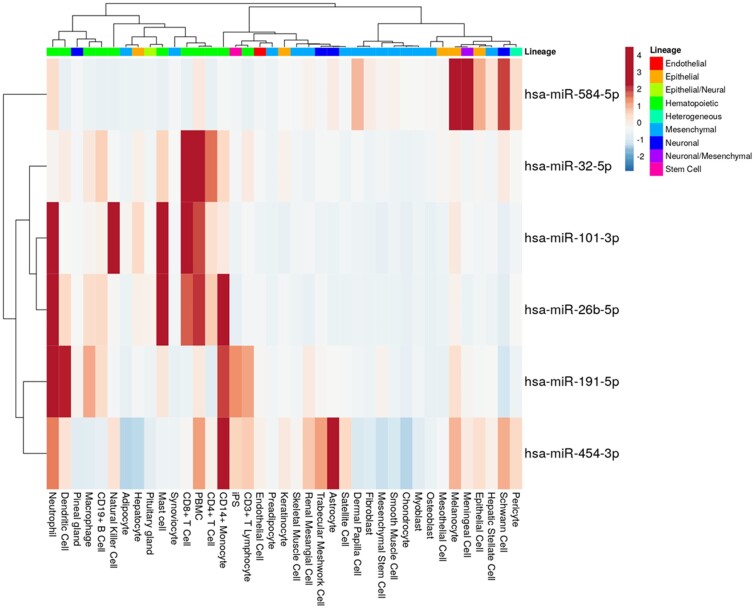
In order to explore the potential cellular origin of the 7 circulating miRNAs regulated under DMAB therapy, we obtained miRNA expression profiles across a large variety of cell types using the FANTOM5 public data resource and visualized the data in the form of a heatmap (Fig. 4). miR-4508 was excluded from the analysis because no data were available. For each of the 6 upregulated miRNAs, all cell types with their respective miRNA average expression value (CPM, count per million) were selected. miR-584-5p expression was highly enriched in epithelial and meningeal cells. The other 5 upregulated miRNAs (miR-101-3p, miR-191-5p, miR-26b-5p, miR-32-5p, miR-454-3p) were all found to be enriched in hematopoietic cells like lymphocytes and/or myeloid leukocytes. For instance, miR-101-3p appeared to be enriched in T cells and natural killer (NK) cells, miR-191-5p in neutrophils and dendritic cells (a known osteoclast precursor), miR-26b-5p in mast cells and neutrophils, and miR-32-5p in T cells and PBMCs. All of these miRNAs were also highly expressed in CD14+ monocytes, the main precursor of osteoclasts (Fig. 4).
Figure 4.
Heatmap illustration the cellular expression profiles of top microRNA candidates. The average miRNA expression levels of each cell type that appears in the FANTOM5 database were obtained and used to draw this heatmap. No data were available for miR-4508 in FANTOM5.
Discussion
The investigation of miRNAs as biomarkers in osteoporosis has moved into the focus of translational research in recent years. However, only a few studies have addressed miRNA regulation under osteoporosis therapy. This study performed an untargeted, NGS-based analysis of circulating miRNAs in the serum of postmenopausal women undergoing treatment with denosumab over a period of 2 years.
In the NGS discovery study, serum samples collected at 3 time points from 8 patients were analyzed and searched for suitable miRNA candidates that could be validated in the entire cohort. We selected 22 miRNA candidates and confirmed a significant time-dependent upregulation for 7 miRNAs in all patients (miR-101-3p, miR-191-5p, miR-26b-5p, miR-32-5p, miR-4508, miR-454-3p, miR-584-5p). Correlation analysis revealed significant associations between percentage of change in miRNA serum levels and treatment defined as the percentage of change in BMD (gain) and BTM (suppression). Among all significantly upregulated miRNAs, miR-454-3p, miR-26b-5p, and miR-584-5p were defined as top candidates with respect to significant correlation with BMD, BTMs, and the highest degree of upregulation during treatment. MiR-454-3p and miR-584-5p were the only miRNAs that showed a significant correlation with hip BMD, spine BMD, and both BTMs. Furthermore, they showed the highest and second highest upregulation after 12 months, respectively, and the highest and third highest upregulation after 24 months of therapy. miR-26b-5p was significantly upregulated after 12 and 24 months, was correlated with both BTMs, and showed the second highest upregulation after 12 and 24 months of therapy. In addition, these miRNAs also showed a significant upregulation in our subgroup analysis after exclusion of 2 patients with diabetes mellitus type 2 and exclusion of nonresponders.
Three patients showed signs of reduced treatment response based on a decrease in spine BMD alone, or in spine and hip BMD, or in spine BMD together with an increase in CTX (after 24 months). We observed that these patients showed a generally lower increase or a decrease in the 7 confirmed miRNAs after the treatment. We did not observe any specific parameters in these 3 subjects that could be related to DMAB resistance. This result indicates that miRNAs could contribute to, or block the effect of, denosumab treatment through their epigenetic function.
Prior anti-osteoporotic treatment could have an influence on the serological miRNA profile during DMAB treatment. A subanalysis of bisphosphonate-pretreated vs treatment-naïve patients was performed, which revealed that bisphosphonate pretreatment did not affect serological miRNA changes during DMAB therapy. In line with this result, CTX baseline levels in these patients gave no evidence for an ongoing suppression caused by bisphosphonate pretreatment.
One patient had sustained a radius and acetabulum fracture shortly after DMAB initiation. In this patient, the serological miRNA levels showed a different pattern until approximately 6 months, when compared with nonfractured patients on DMAB. It can be postulated that fractures exert a short-term impact on miRNA expression, yet the sustained effect of DMAB treatment remains.
Several nonclinical studies were performed to investigate bone and/or serum microRNA levels during anti-osteoporotic therapy: a study with ovariectomized rats reported a significant regulation of miRNAs in the femoral head after administration of teriparatide compared with sham surgery resulting in partial reestablishment of the miRNA pattern of sham-operated animals. Effects of antiresorptive treatment with zoledronic acid had a weaker effect on bone miRNA expression. By comparing microRNA expression in femoral head with serum microRNA levels, Weigl et al observed for some but not all miRNAs a significant correlation, indicating the possibility of analyzing circulating miRNAs as a surrogate for bone tissue expression (21). Another study in an ovariectomized rat model of postmenopausal osteoporosis investigated the effect of estrogen administration on miRNA levels. Plasma levels of miR-29a-3p, miR-93-5p, and miR-486 were significantly decreased in comparison with the control group, and estrogen administration significantly reversed these effects after 1 month of treatment (23). Finally, treatment of diabetic rats with anti-Sclerostin as well as insulin had a significant impact on serum microRNA levels, resulting in a partial rescue of microRNA changes observed in untreated diabetic animals (35). Overall, these data suggest that anti-osteoporotic treatments, specifically osteoanabolic treatments, induce changes in bone miRNA expression as well as circulating miRNA levels. However, none of these studies investigated the effect of anti-RANKL treatment using denosumab, being the probable reason why they did not show any miRNA in common with the miRNAs upregulated by denosumab in this study.
Clinical studies investigating the effects of denosumab on miRNA expression levels were focused on short term (<12 months) effects. Anastasilakis et al did not observe any significant changes in miRNA levels after 3 and 12 months of denosumab treatment (25). This is in line with the present study, since no significant changes within the first 6 months of DMAB treatment have been observed. However, between 12 and 24 months of treatment, we observed a continuous increase in the levels of selected circulating miRNAs, reaching significance at 12 and/or 24 months after DMAB therapy, respectively. In a prospective study on postmenopausal women, the relative expression of miRNAs was significantly lower after 6 months of DMAB initiation in patients previously treated with teriparatide. In contrast, no significant changes were found in zoledronate-pretreated or treatment-naïve patients (24). Anastasilakis et al further observed an upregulation of markers of osteoclast formation in patients with vertebral fractures after DMAB-discontinuation compared with treatment-naïve fracture patients alongside downregulation of 2 miRNAs (miR-503 and miR-222-2) in serum (36). These 2 miRNAs are known to regulate the activity of osteoclasts via RANK, but were not detected in our study cohort. However, in postmenopausal women, to our knowledge, this is the first time that a significant change in miRNA expression was observed under DMAB therapy and highlights that changes of miRNA levels do not occur in the short run, but in the long term. This could mean that the immediate change in bone turnover observed in DMAB treated patients is not measured on circulating miRNA level, but rather changes in cell populations or adaptations in coding and noncoding gene expression that occur over longer periods of DMAB treatment.
miRnet target network analysis revealed several verified gene targets for the identified miRNA candidates that are involved in pathways relevant to bone physiology, such as Wnt and TGF-beta signaling pathways as well as osteoclast differentiation, in accordance with the molecular mechanism by which DMAB improves bone mass. As previously mentioned, miRNAs inhibit the translation of specific target mRNAs. In this way, our upregulated miRNA candidates could affect the posttranslational gene expression of the gene targets detected by miRnet, altering the pathways of bone development in which those genes are involved. Fitting with these data and also with the RANKL inhibition by DMAB, the associated cell ontology terms in our cell type enrichment analysis indicated that miRNAs upregulated during DMAB treatment are enriched in CD8+ T cells, neutrophils and osteoclast precursors such as dendritic cells and CD14+ monocytes. We hypothesize that the miRNAs upregulated in serum during DMAB could be released from osteoclast precursors such as monocytes and dendritic cells, as well as other immune cells that interact with them like T cells. This means that “long-term” inhibition of RANKL could either result in an adaptation of coding and noncoding gene expression in osteoclast hematopoietic precursor cells or the accumulation of a certain hematopoietic cell population. Given the fact that discontinuation of DMAB treatment was shown to result in a rebound effect in some patients, putative adaptations in gene expression in blood cells requires further investigation. Furthermore, due to the lack of data on the biological function of the identified miRNAs in monocytes and osteoclasts further mechanistic studies are required to unravel a potential role of miRNAs in the response to denosumab treatment.
Our study has several strengths and limitations. Firstly, exact data on dates of the injections were lacking and timing of injections and the timing of the visits varied. Variable time differences between injections and blood collection could influence the variability in miRNA levels. However, our cohort showed a significant response in terms of suppressed BTMs and/or improvement of BMD through the whole observational period, therefore a sustained effect of DMAB treatment still could be assumed in our patients. No rebound effect occurred. Moreover, previous studies have demonstrated that miRNAs do not change significantly after 3 and/or 6 months of DMAB treatment (24, 25). This is in line with our findings and indicates that miRNAs reflect a persistent and long-lasting effect of DMAB after 1 and/or 2 years of treatment.
Secondly, 57% of our patients were not treatment-naïve to bisphosphonates. However, subanalysis did not show a significant effect of prior bisphosphonate treatment in the present study. Similar miRNA regulations in bisphosphonate-pretreated and treatment-naïve postmenopausal women under DMAB therapy were found by others (24).
Thirdly, radiographs of the vertebral spine were missing in 11 patients, thus exclusion of silent fractures was incomplete. Nevertheless, there were no indirect signs of vertebral fractures. All patients reported a good quality of life and were free of back pain or changes in mobility.
Fourthly, our cohort included 2 patients with type 2 diabetes as well as patients with questionable or poor response to DMAB therapy. Anyhow, subgroup analysis, after exclusion of these patients, revealed a significant upregulation of our top candidates, miR-26b-5p, miR-584-5p, and miR-454-3p, after 12 and/or 24 months.
Conclusion
We report changes in circulating miRNAs under DMAB treatment over a period of 24 months in postmenopausal women. We have used an untargeted analytical approach (NGS) for genome-wide discovery of circulating microRNA changes in a subset of patients and applied RT-qPCR in the validation phase to the entire cohort. We were able to identify several differentially regulated miRNAs after 24 months of treatment and report their association to BMD and established BTMs, their cell-type enrichment, and potential biological function in the context of bone metabolism. Our data suggest that DMAB treatment may result in long-term changes in microRNA expression in hematopoietic cells.
Acknowledgments
The authors acknowledge funding support by the MSCA Innovative Training Network FIDELIO under Grant Agreement no. 860898. Furthermore, this project was funded and awarded with the project award by the Austrian Society for Bone and Mineral Research.
Abbreviations
- BMD
bone mineral density
- BTM
bone turnover marker
- Cq
cycle of quantification
- CTX
C-terminal cross-linking telopeptide of type I collagen
- DMAB
denosumab
- DXA
dual-energy x-ray absorptiometry
- FDR
false discovery rate
- MiDeTe (study)
MiRNA-Denosumab-Therapy Study
- miRNA
micro RNA
- NGS
next-generation sequencing
- PINP
procollagen type I N propeptide
- RANKL
Receptor Activator of Nuclear Factor Kappa B Ligand
- RT
reverse transcription
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
Contributor Information
Zora Messner, 2nd Department of Internal Medicine—VINFORCE, St. Vincent Hospital, 1060 Vienna, Austria.
David Carro Vázquez, Department of Biotechnology , University of Natural Resources and Life Sciences Vienna, 1180 Vienna, Austria; TAmiRNA GmbH, 1110 Vienna, Austria.
Judith Haschka, 1st Med. Dept. Hanusch Hospital, Ludwig Boltzmann Institute of Osteology at Hanusch Hospital of OEGK and AUVA, Trauma Centre Meidling, 1140 Vienna, Austria.
Johannes Grillari, Ludwig Boltzmann Institute for Traumatology, The Research Centre in Cooperation with AUVA, 1200 Vienna, Austria; Austrian Cluster for Tissue Regeneration, 1200 Vienna, Austria; University of Natural Resources and Life Science [BOKU], Institute of Molecular Biotechnology, 1180 Vienna, Austria.
Heinrich Resch, 2nd Department of Internal Medicine—VINFORCE, St. Vincent Hospital, 1060 Vienna, Austria; Metabolic Bone Diseases Unit, Sigmund Freud University Vienna, School of Medicine, 1020 Vienna, Austria.
Christian Muschitz, 2nd Department of Internal Medicine—VINFORCE, St. Vincent Hospital, 1060 Vienna, Austria.
Peter Pietschmann, Center for Pathophysiology, Infectiology and Immunology, Institute of Pathophysiology and Allergy Research, Medical University of Vienna, 1090 Vienna, Austria.
Jochen Zwerina, 1st Med. Dept. Hanusch Hospital, Ludwig Boltzmann Institute of Osteology at Hanusch Hospital of OEGK and AUVA, Trauma Centre Meidling, 1140 Vienna, Austria.
Matthias Hackl, TAmiRNA GmbH, 1110 Vienna, Austria.
Roland Kocijan, 1st Med. Dept. Hanusch Hospital, Ludwig Boltzmann Institute of Osteology at Hanusch Hospital of OEGK and AUVA, Trauma Centre Meidling, 1140 Vienna, Austria; Metabolic Bone Diseases Unit, Sigmund Freud University Vienna, School of Medicine, 1020 Vienna, Austria.
Funding
This project was funded and awarded by the Austrian Society for Bone and Mineral Research and by the MSCA Innovative Training Network FIDELIO.
Disclosure Summary
Peter Pietschmann has received research support and/or honoraria from: Amgen GmbH, Biomedica GmbH, Fresenius Kabi Austria, Takeda Pharma GesmbH, TAmiRNA GmbH, and UCB Biopharma Srl/UCB Pharma; Roland Kocijan has received support and/or honoraria from Amgen GmbH.
Data Availability
The sequencing data are available in the Gene Expression Omnibus repository (GSE206540).
All supplementary materials and figures are located in a digital repository https://doi.org/10.6084/m9.figshare.21347745.v1.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention D and Therapy . Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285(6):785‐795. [DOI] [PubMed] [Google Scholar]
- 2. Muschitz C, Hummer M, Grillari J, et al. . Epidemiology and economic burden of fragility fractures in Austria. Osteoporos Int. 2022; 33(3):637‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanis JA, Cooper C, Rizzoli R, Reginster JY, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) . European Guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyle W, Simonet W, Lacey D. Osteoclast differentiation and activation. Nature. 2003;423(6937):337‐342. [DOI] [PubMed] [Google Scholar]
- 5. Cummings SR, Martin JS, McClung MR, et al. . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756‐765. [DOI] [PubMed] [Google Scholar]
- 6. Vasikaran S, Eastell R, Bruyère O, et al. . Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011; 22(2):391‐420. [DOI] [PubMed] [Google Scholar]
- 7. Paschalis EP, Krege JH, Gamsjaeger S, et al. . Teriparatide treatment increases mineral content and volume in cortical and trabecular bone of iliac crest: a comparison of infrared imaging with X-ray-based bone assessment techniques. J Bone Miner Res. 2018;33(12):2230‐2235. [DOI] [PubMed] [Google Scholar]
- 8. Bhaskaran M, Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol. 2014;51(4):759‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843‐854. [DOI] [PubMed] [Google Scholar]
- 10. Alles J, Fehlmann T, Fischer U, et al. . An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019;47(7):3353‐3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(suppl_1):R17‐R29. [DOI] [PubMed] [Google Scholar]
- 12. Keller A, Gröger L, Tschernig T, et al. . miRNATissueAtlas2: an update to the human miRNA tissue atlas. Nucleic Acids Res. 2022;50(D1):D211‐D221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases—complex signatures for multifactorial diseases? Mol Cell Endocrinol. 2016;432:83‐95. [DOI] [PubMed] [Google Scholar]
- 14. Ciuffi S, Donati S, Marini F, Palmini G, Luzi E, Brandi ML. Circulating MicroRNAs as novel biomarkers for osteoporosis and fragility fracture risk: is there a use in assessment risk? IJMS. 2020;21(18):6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37(11):460‐465. [DOI] [PubMed] [Google Scholar]
- 16. Blondal T, Jensby Nielsen S, Baker A, et al. . Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1‐S6. [DOI] [PubMed] [Google Scholar]
- 17. Sourvinou IS, Markou A, Lianidou ES. Quantification of circulating miRNAs in plasma. J Mol Diagn. 2013;15(6):827‐834. [DOI] [PubMed] [Google Scholar]
- 18. Mussbacher M, Krammer TL, Heber S, et al. . Impact of anticoagulation and sample processing on the quantification of human blood-derived microRNA signatures. Cells. 2020;9(8):1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weber JA, Baxter DH, Zhang S, et al. . The MicroRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733‐1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grillari J, Mäkitie RE, Kocijan R, et al. . Circulating miRNAs in bone health and disease. Bone. 2021;145:115787. [DOI] [PubMed] [Google Scholar]
- 21. Kocijan R, Weigl M, Skalicky S, et al. . MicroRNA levels in bone and blood change during bisphosphonate and teriparatide therapy in an animal model of postmenopausal osteoporosis. Bone. 2020;131:115104. [DOI] [PubMed] [Google Scholar]
- 22. Weigl M, Kocijan R, Ferguson J, et al. . Longitudinal changes of circulating miRNAs during bisphosphonate and teriparatide treatment in an animal model of postmenopausal osteoporosis. J Bone Miner Res. 2021;36(6):1131‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Zhang P, Li X, et al. . MicroRNA expression profiling in an ovariectomized rat model of postmenopausal osteoporosis before and after estrogen treatment. Am J Transl Res. 2020;12(8):4251‐4263. [PMC free article] [PubMed] [Google Scholar]
- 24. Yavropoulou MP, Anastasilakis AD, Makras P, et al. . Serum profile of microRNAs linked to bone metabolism during sequential treatment for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2020;105(8):e2885‐e2894. [DOI] [PubMed] [Google Scholar]
- 25. Anastasilakis AD, Makras P, Pikilidou M, et al. . Changes of circulating MicroRNAs in response to treatment with teriparatide or denosumab in postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(3):1206‐1213. [DOI] [PubMed] [Google Scholar]
- 26. Dachverband Osteologie e.V . Prophylaxe, Diagnostik Und Therapie Der Osteoporose Bei Postmenopausalen Frauen Und Bei Männern—Leitlinie Des Dachverbands Der Deutschsprachigen Wissenschaftlichen Osteologischen Gesellschaften e.V. 2017—Langfassung.; 2017. Accessed November 29, 2022.https://www.dv-osteologie.org/uploads/Leitlinie%202017/Finale%20Version%20Leitlinie%20Osteoporose%202017_end.pdf
- 27. Kocijan R, Muschitz C, Geiger E, et al. . Circulating microRNA signatures in patients with idiopathic and postmenopausal osteoporosis and fragility fractures. J Clin Endocrinol Metab. 2016;101(11):4125‐4134. [DOI] [PubMed] [Google Scholar]
- 28. Khamina K, Diendorfer AB, Skalicky S, et al. . A MicroRNA next-generation-sequencing discovery assay (miND) for genome-scale analysis and absolute quantitation of circulating MicroRNA biomarkers. Int J Mol Sci. 2022;23(3):1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diendorfer A, Khamina K, Pultar M, Hackl M. miND (miRNA NGS discovery pipeline): a small RNA-seq analysis pipeline and report generator for microRNA biomarker discovery studies [version 1; peer review: 1 approved with reservations]. F1000Res. 2022;11:233. [Google Scholar]
- 30. Chang L, Zhou G, Soufan O, Xia J. miRNet 2.0: network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48(W1):W244‐W251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Rie D, Abugessaisa I, Alam T, et al. . An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat Biotechnol. 2017;35(9):872‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Metsalu T, Vilo J. Clustvis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566‐W570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carro Vázquez D, Messner Z. Supplementary data for: circulating miRNAs respond to denosumab treatment after two years in postmenopausal women with osteoporosis—the MiDeTe-study, figshare, dataset. Published online October 17, 2022. https://figshare.com/articles/journal_contribution/MiDeTe_Supplementary_Material_for_Peer_Review_docx/21347745/1 [DOI] [PMC free article] [PubMed]
- 35. Vázquez D C, Emini L, Rauner M, et al. . Effect of anti-osteoporotic treatments on circulating and bone MicroRNA patterns in osteopenic ZDF rats. Int J Mol Sci. 2022;23(12):6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anastasilakis AD, Yavropoulou MP, Makras P, et al. . Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol. 2017;176(6):677‐683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data are available in the Gene Expression Omnibus repository (GSE206540).
All supplementary materials and figures are located in a digital repository https://doi.org/10.6084/m9.figshare.21347745.v1.