Abstract
Food allergy (FA) is increasingly reported in Europe, however, the latest prevalence estimates were based on studies published a decade ago. The present work provides the most updated estimates of the prevalence and trends of FA in Europe. Databases were searched for studies published between 2012 and 2021, added to studies published up to 2012. In total, 110 studies were included in this update. Most studies were graded as moderate risk of bias. Pooled lifetime and point prevalence of self‐reported FA were 19.9% (95% CI 16.6–23.3) and 13.1% (95% CI 11.3–14.8), respectively. The point prevalence of sensitization based on specific IgE (slgE) was 16.6% (95% CI 12.3–20.8), skin prick test (SPT) 5.7% (95% CI 3.9–7.4), and positive food challenge 0.8% (95% CI 0.5–0.9). While lifetime prevalence of self‐reported FA and food challenge positivity only slightly changed, the point prevalence of self‐reported FA, sIgE and SPT positivity increased from previous estimates. This may reflect a real increase, increased awareness, increased number of foods assessed, or increased number of studies from countries with less data in the first review. Future studies require rigorous designs and implementation of standardized methodology in diagnosing FA, including use of double‐blinded placebo‐controlled food challenge to minimize potential biases.
Keywords: epidemiology, Europe, food allergy, sensitization, systematic review
1. INTRODUCTION
The frequency of food allergy (FA) in Europe has been increasingly reported over the past decades. However, the data supporting an increase are mainly anecdotal, considering that the latest systematic report on FA epidemiology was published by the European Academy of Allergy and Clinical Immunology (EAACI) in 2014 based on the articles published between 2000 and 2012. 1 , 2 That report provided a detailed overview of the epidemiology of FA, including estimates of the incidence, prevalence, and time trends of any FA, as well as the so‐called eight big foods, i.e., cow's milk, egg, wheat, soy, peanut, tree nuts, fish, and shellfish.
It is now 10 years since the EAACI‐commissioned systematic review was completed. Several studies have been published since then, indicating that an update of the previous review is now warranted. By bringing together the evidence generated from the previous systematic review together with more recent studies, we have the opportunity to make clearer estimates of the incidence, prevalence, and time trends of FA in Europe. The update will also give greater opportunity to estimate the epidemiological burden of FA across various population subgroups (e.g., age and regions). Furthermore, this update is an excellent opportunity to identify and estimate the epidemiological burden of potentially “new” and “emerging” food allergy in Europe, beyond the so‐called eight big foods. The aim of the current work was to update the previously EAACI‐commissioned systematic review on the incidence, prevalence, and time trends of FA in Europe by identifying, critically appraising, and synthesizing evidence from studies now published since the previous systematic review was completed (2012). The current article reports on the estimates of the frequency of any FA.
2. METHODS
2.1. Protocol registration
The protocol for this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; reference CRD42021266657) prior to undertaking this review.
2.2. Search strategy
The search strategy was adapted from the previously published EAACI review. The two concepts of FA and epidemiology were combined to identify all relevant literature (including both articles, conference abstracts or posters, and theses) from the electronic databases. Six databases were searched: MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane Library, and Scopus. Compared with the EAACI review from 2014, Cochrane Library and Scopus were added among the databases in acknowledgement of the advancements that have occurred in the indexing of studies on the topic since the first review was done. A few more keywords were also included in the current review to ensure that it was updated with all the new and emerging keywords on FA. Experts on the topic of FA were consulted to ensure that the study identification procedure did not miss any relevant work. No language restrictions were applied in the database searches. When possible, studies published in languages different from English were translated by researchers fluent in the language to permit data extraction. When it was not possible to translate the article, but an English abstract was available, data extraction from the abstract was performed. The few cases for which neither abstract nor full article data extraction was possible have been reported. Detailed description of the search strategies employed are available in Box S1 of the Supporting Information section of the online version of this article.
2.3. Inclusion and exclusion criteria
The studies included in the current review comprised studies published from January 1, 2000 to June 30, 2021 (i.e., studies published in the previous systematic review, and studies identified in the current update). All studies that examined subjects with suspected FA, of any age and gender, and of any European country as defined by the United Nations (see Appendix 1) were considered eligible. Studies from Greenland and Turkey were also included, similarly to what was done in the previous EAACI review. The following types of studies were considered for inclusion: systematic reviews and meta‐analyses, prospective and retrospective cohort studies, cross‐sectional studies, case–control studies, clinical trials, and routine healthcare studies. Expert reviews or other reviews that are not systematic reviews, discussion papers, non‐research letters and editorials, qualitative studies, case studies, case series, and animal studies were excluded from the present work.
2.4. Study selection
All records obtained from the databases searches were exported to EndNote 20 (Clarivate Analytics, 2020) for de‐duplication. Following this, all relevant articles were exported to Rayyan (https://rayyan.ai) for titles and abstracts screening, and to manage all the retrieved records. Titles and abstracts screening was performed by four independent reviewers (SN/GS and YA/MA), working in pairs. Disagreements between reviewers were resolved with consensus when possible or by consultation with the project PI (BN). The full texts of the potentially eligible studies were then assessed by the same four independent reviewers, similarly to what was done for the titles and abstracts selection. The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram was used to document the screening process.
2.5. Risk of bias assessment
Risk of bias in individual studies was independently assessed for each study by the four reviewers (SN/GS and YA/MA), working in pairs, by employing the Critical Appraisal Skills Programme (CASP; http://www.casp‐uk.net) quality assessment tool. The same tool was also used in the previous EAACI systematic review. Accordingly, all studies were assigned an overall rating, along with a separate rating for each different components of the individual studies (i.e., appropriateness of the study design for the research question, risk of selection bias, exposure measurement, and outcome assessment). Any discrepancy was resolved by consensus or arbitrated by the project PI (BN).
2.6. Data extraction
Data were collected from included studies using a customized data extraction form. All data extracted were reported in a standardized and reproducible fashion. The developed form was first piloted with a small number of included studies and approved by all reviewers before it was employed to extract data from all studies. The form was stored on a Google Drive (Alphabet Inc., Mountain View, CA, USA) repository online to make all the information promptly available to all reviewers. Similar to the selection process, the extraction of all data was performed by four reviewers, working in pairs (SN/GS and YA/MA). Each pair conducted an independent extraction of the assigned records. After cross‐checking, all disagreement were addressed and further arbitrated by the project PI (BN).
2.7. Data analysis, synthesis, and reporting
We recalculated all the frequency estimates of FA occurrence if adequate data were provided by authors. If any discrepancies were observed between our recalculated estimates and those of the authors, we reported our recalculated estimates. Our recalculated estimates were based on minimal measured events rather than the extrapolated ones. The 95% confidence intervals (95% CI) were obtained by employing the Wilson score method without continuity correction. 3 Heterogeneity was assessed using I 2 statistics. For studies that presented missing data, thus not allowing estimates recalculation, we reported the estimates provided by the authors. Where needed and possible, we contacted authors of primary studies for clarifications. Countries outside the Organization for Economic Co‐operation and Development (OECD) definition of Europe were included in the systematic review but were not included in meta‐analysis, similarly to what was done in the previous version of the systematic review and meta‐analysis. An exception was made for Lithuania and Russia, which had recorded FA data for meta‐analysis also in the previous study. According to the criteria above, Albania, Bulgaria, Croatia, and Ukraine were included in the systematic review, but not in the current meta‐analysis.
Random‐effects meta‐analysis was performed for all studies that provided numerical data in order to derive pooled estimates across studies. The meta‐analysis was conducted using the software Stata (StataCorp. 2019. Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX, USA). The following outcomes were defined: 1. lifetime and point prevalence of self‐reported FA; 2. lifetime and point prevalence of self‐reported physician diagnosed FA (i.e., doctor‐diagnosed FA reported by a subject in a questionnaire); 3. point prevalence of sIgE positivity; 4. point prevalence of SPT positivity; 5. point prevalence of symptoms plus sIgE positivity; 6. point prevalence of symptoms plus SPT positivity; 7. point prevalence of clinical history or food challenge (OFC or DBPCFC)‐positivity; and 8. point prevalence of positive food challenge (OFC or DBPCFC). Meta‐analysis included the studies reporting on any FA published in the previous EAACI review and those obtained from the current updated searches. As was done in the previous EAACI review, data were also stratified by age category, in children (0–17 years) and adults (18 years and over), and by European region (Northern‐Eastern‐Southern‐Western Europe) following the classification by the United Nations (see Appendix 1). In case of overlap between the two age categories, or between groups, the estimate was included in either age group if the age distribution was skewed to that age group, following the approach used in the 2014 EAACI review. An exception was made for the United Kingdom, which was assigned to Western Europe instead of Northern Europe, as was done in the previous EAACI review. In the meta‐analysis, we estimated the updated prevalence of FA for the period 2000–2021. In addition, we also performed and reported meta‐analysis separately for the studies published during 2012–2021, which were compared with the estimate obtained in the previous review for the period 2000–2012.
3. RESULTS
3.1. Study selection and characteristics
The study selection and screening process of the current update are illustrated in the PRISMA flow chart presented in Figure 1. A total of 38,903 records were retrieved from the databases searched. After de‐duplication, 33,875 records were selected for screening. Based on titles and abstracts, 33,625 records were excluded due to being clearly ineligible or not fulfilling the inclusion criteria. Out of the remaining 250 records, two full‐text articles could not be retrieved, and the abstracts did not include any relevant information. They were therefore excluded. Of the remaining 248 records, 72 reports were included in this review. The new reports included were based on 54 newly identified studies, and on one study already included in the previous review but presenting updated data for the cohort enrolled in the study. Putting together the number of reports (and studies) included in the first systematic review from EAACI, with the records screened and included for the current review (September 2012 to June 2021), the total number of reports included was 147, which were based on 110 studies. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150
FIGURE 1.
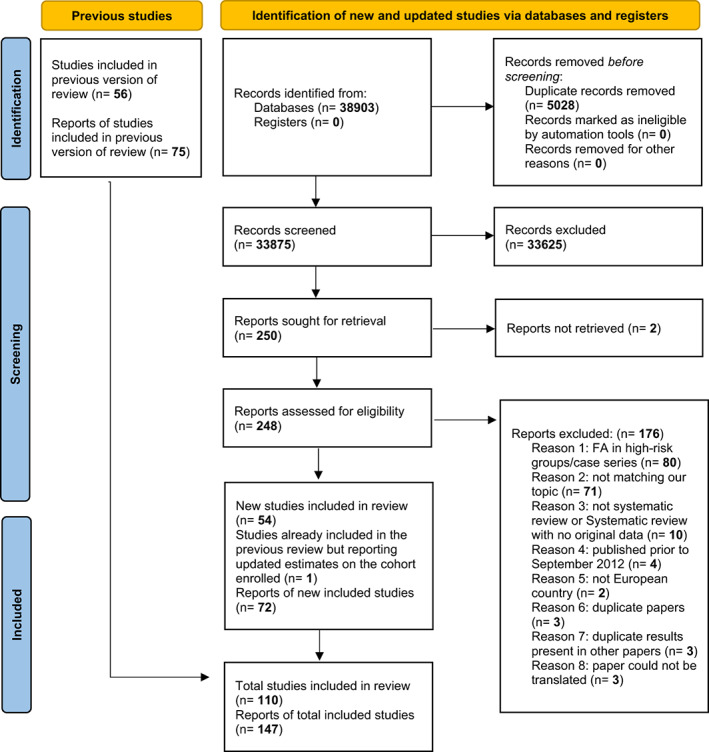
PRISMA flow diagram for updated systematic review on prevalence of food allergy in Europe, 2000–2021.
Of the 110 studies, 62 were cross‐sectional studies, 41 were cohort studies, three were case‐control studies (of which one nested‐control), three were systematic reviews, and one was a population‐based study. Most of the studies included only children (n = 76 studies). FA was investigated exclusively by self‐report in 26 studies, whereas in 17 studies FA was only investigated by sIgE and/or SPT positivity. Nine studies reported only on physician/clinician diagnosed FA (in three cases, self‐reported). The remaining 58 studies were a combination of self‐report, self‐report physician diagnosis, sIgE or SPT sensitization (with or without symptoms), clinical diagnosis/clinical history, and/or food challenge (OFC and/or DBPCFC). The majority of the studies (n = 94) reported point prevalence as the occurrence measure for assessing the frequency of FA, and in 69 of the studies point prevalence was the only occurrence measure provided. Lifetime prevalence was the second most reported type of estimate, while cumulative incidence was investigated in only 15 studies.
3.2. Risk of bias assessment
Overall, the risk of bias assessment for individual studies graded by the CASP quality assessment tool indicated that most of the studies had a moderate risk of bias (91 out of 110 studies). Table S2 summarizes the grading of the main CASP quality assessment features for all studies.
3.3. Frequency of any FA
The ranges of estimates for any FA categorized by age groups, and by different methods of assessment are presented in Table 1, based on the data extracted from the articles published between January 2000 and June 2021. Detailed results on the prevalence and incidence reported by each study are included in Tables S1–S6 of the Supporting Information section in the online version of this article. The pooled estimates for the prevalence of FA in Europe for the periods 2000–2021, 2000–2012, and 2012–2021 are presented in Figures 2, 3, 4, 5, 6 and further elaborated below. The forest plots for pooled data from studies published for the period 2000–2021, are included in the Supporting Information section of the online version of this article, along with the heterogeneity measurements for the studies included in the analysis (Figures S1–S28). As observed for the review published on data from 2000 to 2012, the heterogeneity between the studies was still significantly high (I 2 ≥ 80 in each case) in the updated pooled data for 2000–2021, regardless of age group and European region, which reflects the variations in estimate of prevalence of FA between studies and across places.
TABLE 1.
Summary of range of estimates of the frequency of FA in Europe by self‐report, self‐report physician diagnosis, skin prick test (SPT) positivity, sIgE positivity, symptoms plus sIgE positivity, symptoms plus SPT positivity, clinical history or food challenge, food challenges: estimates from all the studies published between 1 January 2000 and 30 June 2021
| Age bands (years) for each food allergy | Self‐report | Self‐report physician diagnosis | sIgE positivity | SPT positivity | Symptom plus positive sIgE | Symptom plus positive SPT | Clinical history or FC (OFC or DBPCFC) | Food challenge (OFC or DBPCFC) |
|---|---|---|---|---|---|---|---|---|
| Point prevalence, % | ||||||||
| ≤1 | 1.7–28.5 | 2.1–4.9 | 19.4–20.3 | 1.8–4.3 | 1.3–4.6 | 1.6–13.1 | 2.7–10.0 | 0.3–4.2 |
| 2–5 | 1.6–38.7 | 4.9–6.6 | 4.1–21.5 | 1.8–4.5 | 4.6 | 6.8–13.1 | 2.1–7.7 | 0.0–4.2 |
| 6–17 | 1.6‐47.5 | 2.3–7.6 | 0.1–52.0 | 0.1‐10.2 | 1.4–5.6 | 0.1–13.1 | 0.2–4.2 | 0.1–5.7 |
| ≥18 | 1.7–36.3 | 0.5–11.3 | 2.0–25.5 | 21.4 | 0.3–5.9 | — | — | 0.1–3.2 |
| Life–time prevalence, % | ||||||||
| ≤1 | 4.1–38.4 | 39.3 | — | — | — | — | 1.0 | — |
| 2–5 | 4.1–38.4 | — | — | — | — | — | 15.0 | — |
| 6–17 | 4.1–41.6 | 2.5–27.4 | — | 4.1–5.9 | — | — | — | — |
| ≥18 | 9.5–35.0 | 4.7 | — | — | — | — | — | — |
| Cumulative incidence, % | ||||||||
| ≤1 | 25.8 | 4.7 | — | — | — | — | 2.4–5.0 | 1.5 |
| 2–5 | 25.5‐28.1 | 4.7–9.8 | — | 5.3 | — | — | 5.0‐6.0 | 3.3 |
| 6–17 | 11.6‐21.4 | 4.7 | 47.3 | — | — | — | — | 3.6 |
| ≥18 | 11.7 | — | — | — | — | — | — | |
Abbreviations: DBPCFC, double‐bind placebo‐controlled food challenge; FA, food allergy; FC, food challenge; OFC, oral/open food challenge; sIgE, specific IgE; SPT, skin prick test for sensitization to specific food allergens.
FIGURE 2.
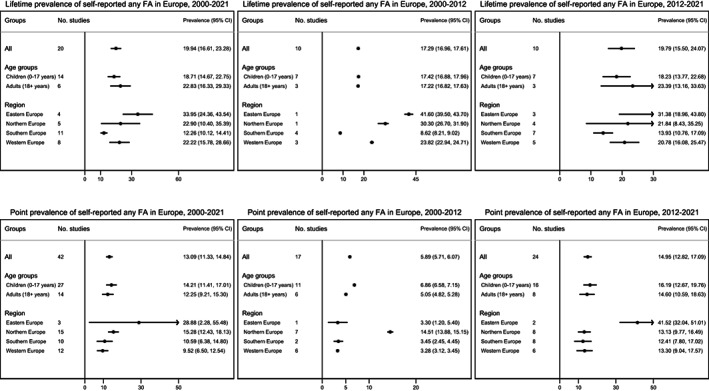
Pooled estimates for self‐reported any food allergy in Europe for lifetime (top) and point prevalence (bottom) between 2000 and 2021, 2000 and 2012, and 2012 and 2021.
FIGURE 3.
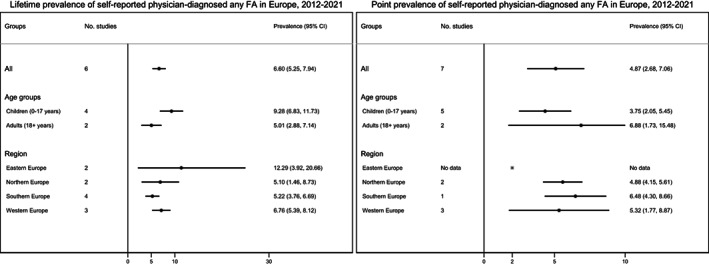
Pooled estimates for self‐reported physician diagnosed any food allergy (i.e., doctor‐diagnosed FA reported by a subject in a questionnaire) for lifetime (left) and point prevalence (right) between 2012 and 2021.
FIGURE 4.
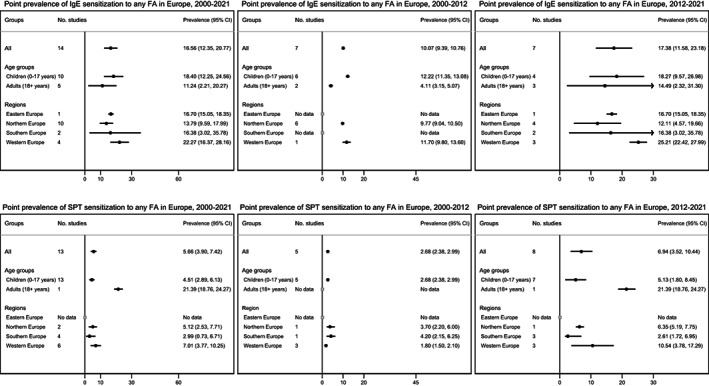
Pooled estimates for sIgE (top) or SPT (bottom) sensitization to any food allergy in Europe between 2000 and 2021, 2000 and 2012, and 2012 and 2021.
FIGURE 5.
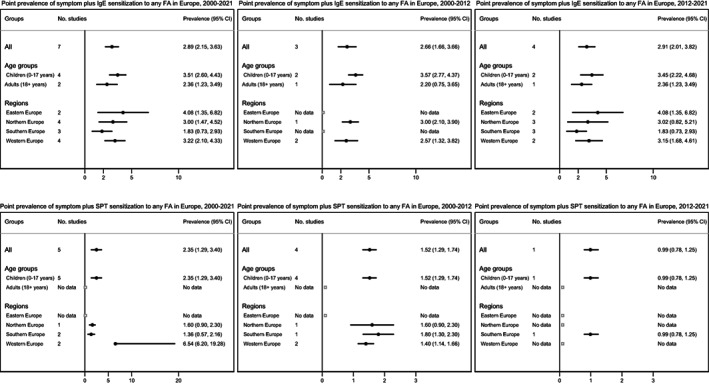
Pooled estimates for symptoms plus sIgE (top) or SPT (bottom) sensitization to any food allergy in Europe between 2000 and 2021, 2000 and 2012, and 2012 and 2021.
FIGURE 6.
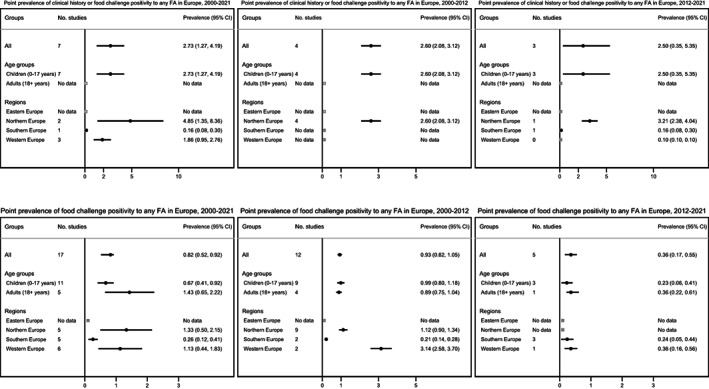
Pooled estimates for clinical history or food challenge positive any food allergy (top) and for food‐challenged verified any food allergy (bottom) in Europe between 2000 and 2021, 2000 and 2012, and 2012 and 2021.
3.3.1. Self‐reported FA
The overall pooled estimate of self‐reported lifetime prevalence of any FA was 19.9% (95% CI 16.6–23.3%); 18.7% vs. 22.8% for children and adults, respectively. Self‐reported point prevalence of any FA was 13.1% (95% CI 11.3–14.8%); 14.2% vs. 12.3% for children and adults, respectively. The lifetime prevalence of any FA was lowest in Southern Europe and highest in Eastern Europe. The point prevalence was lowest in Western Europe and highest in Eastern Europe. However, for both lifetime and point prevalence, Eastern Europe was also the region with the lowest number of studies reporting on FA prevalence. Overall, the lifetime prevalence did not substantially differ between the estimates in 2000–2012 (17.3%) and 2012–2021 (19.8%). However, there was almost three times increase in point prevalence between 2000 and 2012 (5.9%) and 2012 and 2021 (14.9%) (Figure 2).
3.3.2. Self‐reported physician diagnosed FA
The overall pooled estimate for self‐reported physician‐diagnosed lifetime prevalence of any FA was 6.6% (95% CI 5.2–7.9%); 9.3% vs. 5.0% for children and adults, respectively. Self‐reported physician‐diagnosed point prevalence of any FA was 4.9% (95% CI 2.7–7.1%); 3.8% vs. 6.9% for children and adults, respectively. The lifetime prevalence of any FA was lowest in Southern Europe and highest in Eastern Europe, while the point prevalence was lowest in Western Europe and highest in Southern Europe (although based on only one study). For point prevalence, no data were available on Eastern Europe. No estimates for self‐reported physician‐diagnosed FA were available for the previous systematic review for the period 2000–2012, thus the calculated estimates were based only on the studies obtained for the period 2012–2021 (Figure 3).
3.3.3. Food sensitization (FS) by positive sIgE and SPT
The overall pooled estimate for point prevalence of sIgE positivity to any FA was 16.6% (95% CI 12.3–20.8%); 18.4% vs. 11.2% for children and adults, respectively, lowest in Northern Europe and highest in Western Europe. Specific IgE positivity to any FA was 10.1% during 2000–2012 and 17.4% during 2012–2021 (Figure 4).
The overall pooled estimate for point prevalence of SPT positivity to any FA was 5.7% (95% CI 3.9–7.4%); 4.5% vs. 21.4% for children and adults, respectively, lowest in Southern Europe and highest in Western Europe. No data were available for the Eastern European region. SPT positivity to any FA was 2.7% during 2000–2012 and 6.9% during 2012–2021 (Figure 4).
3.3.4. Symptom plus sIgE or SPT positivity FA
The overall pooled estimate for prevalence of symptom plus sIgE positivity to any FA was 2.9% (95% CI 2.1–3.6%); 3.5% and 2.4% for children and adults, respectively. The prevalence was lowest in Southern Europe and highest in Eastern Europe. Estimates for symptom plus sIgE positivity to any FA was similar during 2000–2012 (2.7%) and 2012–2021 (2.9%).
The overall pooled estimate for prevalence of symptom plus SPT positivity to any FA was 2.4% (95% CI 1.3–3.4%), all studies being only available for children and none for adults. The prevalence was lowest in Southern Europe and highest in Western Europe, but data were unavailable for Eastern Europe. Estimates for symptom plus specific SPT positivity to any FA was 1.5% during 2000–2012 and 1% during 2012–2021 (Figure 5).
3.3.5. FA defined by clinical history or food challenge
The overall pooled estimate for prevalence of clinical history or food challenge (OFC or DBPCFC) positivity to any FA was 2.7% (95% CI 1.3–4.2%), all studies being only available for children and none for adults. The prevalence was lowest in Southern Europe and highest in Northern Europe. No data were available for Eastern Europe region. Between 2000 and 2012 and 2012 and 2021, the prevalence of clinical history or food challenge FA was 2.6% and 2.5%, respectively (Figure 6).
3.3.6. Food challenge‐verified FA
The overall pooled estimate for prevalence of food challenge (OFC or DBPCFC) positivity to any FA was 0.8% (95% CI 0.5–0.9%); 0.7% vs. 1.4% for children and adults, respectively. The prevalence was lowest in Southern Europe and highest in Northern Europe. There were no estimates from Eastern Europe. The prevalence was 0.9% during 2000–2012 and 0.4% during 2012–2021 (Figure 6).
3.4. Time trends of frequency of FA
Data on time trends of FA in Europe are reported in Table 2. In addition to the three studies originally reported in the previous systematic review, two more studies were identified and included in this update, giving a total of five studies. 29 , 55 , 56 , 57 , 65 , 76 , 136 , 137 , 138 , 141 Four out of the five studies were undertaken in the United Kingdom, 29 , 65 , 76 , 136 , 137 , 138 , 141 while the remaining one took place in Finland. 65 We also added updated trends on the frequency of FA for one of the studies already reported in the review from 2014. 136 , 137 , 138 , 141 Three of the studies reported trends for both any FA and specific FA. One study reported the trends of hospital admission rate for FA, 55 , 56 , 57 while one study reported trends for doctor‐diagnosed peanut allergy. 76 While the available data remained limited to allow clear conclusion on the current time trends in the incidence or prevalence of FA, the additional data from this updated review may suggest a slight but progressive increase of clinician‐diagnosed FA in the United Kingdom, with reported prevalence going from 0.6% in 2000 to 1.3% in 2015, 29 but a slight decrease in point prevalence self‐reported FA, with estimates going from 8.5% to 7.2% at 1 year, from 9.2% to 8.4% at 2 years, and from 9.1% to 8.3% at 3–4 years in two birth cohort born in 1989–1990 and in 2001–2002, respectively. 136 , 137 , 138 , 141 In Finland, the prevalence of self‐reported FA was not relevantly changed from 2009 to 2013 (2.7% vs. 2.5%). 65
TABLE 2.
Time trends in the frequency of FA in Europe: estimates from studies published between 1 January 2000 and 30 June 2021
| Reference, country | Age(s) of subjects | Frequency of FA | Comments | ||||
|---|---|---|---|---|---|---|---|
| Diwaker et al. 2017, United Kingdom 29 | Children 0–17 years old |
Year 2000 Point prevalence of physician diagnosed FA: ‐ any FA: 0.6% ‐ eggs: 0.2% ‐ nuts: 0.1% |
Year 2015 Point prevalence of physician diagnosed FA: ‐ any FA: 1.3% ‐ eggs: 0.3% ‐ nuts: 0.5% |
Data were extracted from a conference abstract. The abstract reports on a population study based on routine primary care data. The objective was to "estimate the trends in prevalence of General Practitioner (GP) diagnosed allergies between 2000 and 2015 among United Kingdom (UK) children (0–17 years)”. To the scope, a primary care database representing 6% of the entire UK population was screened. Point prevalence of physician‐diagnosed FA was also measured for the following specific foods: nuts and eggs. |
|||
| Gupta et al. 2004‐a, 2004‐b, and 2007, United Kingdom 55 , 56 , 57 | All ages |
Years 1991/92 Admissions rate for FA: All ages: 0.5% 0–14 age group: 1.6% 15–44 age group: 0.5% 45+ age group: 0.0% |
Years 2000/01 Admissions rate for FA: All ages: 2.9% 0–14 age group: 11.8 15–44 age group: 1.1% 45+ age group 0.5% |
Years 2003/04 Admissions rate for FA: All ages: 2.6% 0–14 age group: 10.7% 15–44 age group: 9.0% 45+ age group: 0.6% |
The increasing trends of hospital admissions for FA between the study years were statistically significant. These admission data do not include period accident and emergency departments for observation and are therefore likely to underestimate the actual incidence or prevalence. | ||
| Järvenpää et al. 2014, Finland 65 | Children 6–7 years old |
Year 2009 Point prevalence self‐reported FA to: ‐ basic foods: 2.7% (1.9–3.5) ‐ cow's milk allergy: 1.5% (0.9–2.1) ‐ eggs: 1.1% (0.6–1.6) ‐ grains: 1% (0.5–1.5) ‐ fruit and vegetables: 5.8% (4.7–7.0) ‐ nuts: 3.1% (2.2–4.0) ‐ legumes: 0.7% ‐ spices: 0.6% ‐ fish: 0.8% (0.4–1.3) |
Year 2013 Point prevalence self‐reported FA to: ‐ basic foods: 2.5% (1.9–3.4) ‐ cow's milk: 1.3% (0.9–2.0) ‐ eggs: 1.5% (1.0–2.2) ‐ grains: 1% (0.6–1.6) ‐ fruit and vegetables: 3.2% (2.5–4.2) ‐ nuts: 1.8% (1.3–2.6) ‐ legumes: 0.9% (0.5–1.4) ‐ spices: 0.5% (0.3–1.0) ‐ fish: 0.7% (0.4–1.3) |
Children attending the first year of elementary school at 29 different schools in the Tampere (Finland) district were screened for the study. The objective was to assess the prevalence of self‐reported FA in the Tampere district. Basic foods according to the authors’ definition include milk, eggs, and grains. Point prevalence for the following specific foods was also measured: cow milk, eggs, grain, nuts, fruits and vegetables, and fish. |
|||
|
Kotz et al. 2011, United Kingdom 76 |
All ages |
Lifetime prevalence physician diagnosed peanut allergy per 1000 patients: |
All estimates were age‐ and sex‐standardized. During the study period, while the lifetime prevalence of peanut allergy doubled, the incidence rate of peanut allergy remained fairly stable. Sex‐specific, age‐specific, and SES‐specific estimates are also reported in the table. Only data regarding the prevalence trends of peanut allergy were reported by the authors. |
||||
|
Year 2001: 0.24% (0.22–0.26) |
Year 2002: 0.32% (0.30–0.34) |
Year 2003: 0.39% (0.37–0.42) |
Year 2004: 0.45% (0.43–0.48) |
Year 2005: 0.51% (0.49–0.54) |
|||
| Incidence rate of physician diagnosed peanut allergy per 1000 person‐years: | |||||||
|
Year 2001: 0.06% (0.05–0.07) |
Year 2002: 0.08% (0.07–0.09) |
Year 2003: 0.08% (0.07–0.09) |
Year 2004: 0.08% (0.07–0.09) |
Year 2005: 0.08% (0.07–0.09) |
|||
| Venkataraman et al. 2017, 136 Venter et al. 2010, 137 Venter et al. 2008, 138 Venter et al. 2016, 141 United Kingdom | Children 0–18 years old | Point prevalence of self‐reported FA, SPT positive FA, and clinical history or OFC positive FA at 1 year |
The data presented come from three different birth cohorts of children, which were born in the Isle of Wight 1989–1990, 1994–1996, and 2001–2002. All three cohorts have been reviewed at 3–4 years after birth in 1993, 1998–2000, and 2004–2005, respectively. Two of the cohorts have been followed up for more years: the Isle of Wight‐IOW birth cohort for subjects born in 1989–1990, and The Food Allergy and Intolerance Research‐FAIR birth cohort for subjects born in 2001–2002, respectively. Overall, the prevalence of self‐reported FA has slightly decreased, while the prevalence of peanut allergy sensitization seems increased in children living in the Isle of Wight. |
||||
|
Year 1990–1991 Self‐reported FA: ‐ any FA: 8.5% (7.1–10.2) SPT positive FA ‐ peanut allergy: 0.3% |
Year 1995–1997 Self‐reported FA: ‐ any FA: N/A SPT positive FA: ‐ peanut allergy: N/A |
Year 2002–2003 Self‐reported FA: ‐ any FA: 7.2% (5.7–9.1) SPT positive FA: ‐ peanut allergy: 0.4% (0.1–1.2) |
|||||
| Point prevalence of self‐reported FA and of SPT positive FA at 2 years | |||||||
|
Year 1991–1992 Self‐reported FA: ‐ any FA: 9.2% (7.6–10.9) SPT positive FA: ‐ peanut allergy: 1.0% |
Year 1996–1998 Self‐reported FA: ‐ any FA: N/A SPT positive FA: ‐ peanut allergy: N/A |
Year 2003–2004 Self‐reported FA: ‐ any FA: 8.4% (6.7–10.4) SPT positive FA: ‐ peanut allergy: 2.0% (1.2–3.4) |
|||||
| Point prevalence of self‐reported FA, SPT positive FA, and of clinical history or FC positive FA at 3–4 years | |||||||
|
Year 1993–1994 Self‐reported FA: ‐ any FA: 9.1% (7.6–10.9) SPT positive FA: ‐ any FA 3.2% (2.2–4.5) ‐ peanut allergy: 1.3% (0.6–1.8) Clinical history or OFC positive FA: ‐ peanut allergy: 0.5% (0.2–1.1) |
Year 1998–2000 Self‐reported FA: ‐ any FA: N/A SPT positive FA: ‐ any FA: N/A ‐ peanut allergy: 3.3% (2.4–4.4) Clinical history or OFC positive FA: ‐ peanut allergy: 1.4% (0.9–2.2) |
Year 2004–2005 Self‐reported FA: ‐ any FA: 8.3% (6.7–10.3) SPT positive FA: ‐ any FA: 4.5% (3.2–6.4) ‐ peanut allergy: 2.0% (1.2–3.4) Clinical history or OFC positive FA: ‐ peanut allergy: 1.2% (0.7–2.2) |
|||||
| Point prevalence of SPT positive FA at 10 years | |||||||
|
Year 1999–2000 ‐ any FA: 4.4% (3.4–5.9) ‐ peanut allergy: 1.84% |
Year 2004–2006 N/A |
Year 2011–2012 ‐ any FA: 2.7% (1.7–4.4) ‐ peanut allergy: 2.4% (1.4–4.0) |
|||||
Note: Diwakar et al. was extracted from a conference abstract.
Abbreviations: FA, food allergy; OFC, oral/open food challenge; SPT, skin prick test for sensitization to specific food allergens.
4. DISCUSSION
4.1. Statement of principal findings
The current systematic review and meta‐analysis provides the most updated estimates of the frequency of FA in Europe for the period 2000–2021. We estimate that the lifetime and point prevalence of self‐reported any FA currently stand at 20% and 13%, respectively. The point prevalence of sensitization as assessed by sIgE stands at 17%, skin prick test 6%, and food challenge positivity 1%. While the lifetime prevalence of self‐reported FA and point prevalence of food challenge positivity only slightly changed during the period 2000–2012 and 2012–2021, the point prevalence of self‐reported FA, sIgE and SPT positivity increased between the same periods. However, based on clinical history or positive food challenge (OFC or DBPCFC), FA increased from 2.6% in 2000–2012 to 3.5% in 2012–2021. Overall, there was no apparent pattern in the frequency of FA in children and adults across the different measures of assessment, nor a consistent pattern by European regions.
4.2. Strengths, limitations, and implications of the current review update
As with the previous EAACI‐led systematic review 1 , 2 we followed recommended rigor in undertaking this updated review, which included a comprehensive search of the extant literature and a systematic approach at every stage of the review process. Additional databases were included in the current update, more than were included in the previous review to ensure that we did not miss any relevant study given the advancement made in the literature collection over the last decade. In keeping with the previous review that included studies investigating frequency of FA using all possible methods to measure FA (e.g., self‐report, specific sensitization to foods, food challenge, and their various combinations), as well as including different measures of occurrence of FA (e.g., point prevalence, lifetime prevalence, and incidence), the current review remains so far the most comprehensive in providing a clear picture of the burden of FA in Europe.
As observed in the previous review, 1 most studies in this update also failed to make any distinction between IgE or non‐IgE FA phenotypes, thus it was impossible to present estimates of prevalence of FA by its IgE‐mediated and non‐IgE‐mediated phenotypes. Overall, the quality of studies included in the review remained moderate as it was in the previous review, indicating that in going forward, the quality of the underlying evidence needs to be improved. Although the previous review highlighted the need for improved assessment of FA through increased use of the gold standard DBPCFC measurement, we did not observe such improvement in this update, regardless of the European region. This is an aspect that still requires further attention. However, OFC is traditionally more commonly used than DBPCFC, since DBPCFC can be challenging in its application. Many symptomatic individuals may be excluded from DBPCFC due to co‐existing disease, labor intensity, lack of validated and blinded challenge materials, or refusal of the individuals, which usually leads to an underestimation of actual frequency of FA. In this case, we highlighted in the previous review that using estimates based on convincing clinical history or positive food challenge may represent the best objective estimates. 1 Based on these estimates, FA remained stable between 2000 and 2012 (2.6%) and 2012 and 2021 (2.5%). Still, there was no improvement over the past decade in the use and definition of FA using convincing clinical history or positive food challenge as only a negligible number of studies used it to define FA. There was also no improvement in time trends of FA as only two additional studies 29 , 65 were found in this update, adding to the three studies 55 , 56 , 57 , 76 , 137 , 138 , 141 found in the previous review. More time trend studies are required to provide a clearer picture of time trends of FA overtime.
As for the possible differences in FA between children (0–17 years) and adults (18 years and over), although in most of the cases frequency estimates for FA differed between children and adults, there was no clear pattern across the different measures of assessment by age category. For the estimates of FA across the European regions, estimates were mostly comparable for all the methods of assessment included in the analysis. However, for self‐reported FA, prevalence estimates were frequently higher in Northern and Eastern regions compared with Southern and Western regions. Nevertheless, it is impossible to infer if the reported data were due to an actual increase in FA in the specific European population examined or if the higher (or lower) estimates could result from the different approaches used, framing of the questionnaire or subjective interpretation of the studied populations. This is especially true when considering that the definition of self‐reported FA per se can be challenging to establish. Given that the differences in European regions were far smaller when FA outcome was defined by objective measures such as SPT or sIgE tests, the probable differences observed for self‐reported FA may depend more on over or under‐reporting of the phenomena across the different regions. Indeed, the observed rise in the point prevalence estimated for SPT and sIgE FA between 2000 and 2012 and 2012 and 2021 could be partially explained by the fact that more food allergens are now being investigated by sIgE testing, leading to a higher number of positive cases found.
A highly significant heterogeneity was observed in the updated review for the pooled prevalence estimates of studies published on FA in Europe in the past two decades, regardless of age and European region. While such heterogeneity may indicate inherent methodological differences across the studies regarding study implementation and definition of FA, it may also reflect the fact that indeed estimates of prevalence of FA varies across places in Europe. Consequently, harmonization of protocols, implementation, and definition of FA across studies in future studies may not resolve the heterogeneity between studies.
5. CONCLUSIONS
This updated systematic review shows that the lifetime prevalence of FA in Europe has slightly increased since the previous review was published in 2014. Similarly, the point prevalence, especially for self‐reported and sIgE positive FA increased during the same period, while clinical history and OFC or DBPCFC confirmed FA remained stable. However, the prevalence of food challenge positivity slightly decreased during this period. The observed increase may reflect a real increase, increased awareness, increased number of food allergens assessed or increased number of studies from countries that had less data in the first review. The frequency of FA differed in children and adults, but there were no consistent patterns by age category across the outcomes investigated. Likewise, no consistent pattern was observed by European regions, although prevalence of FA was frequently higher in Northern and Eastern regions compared with Southern and Western regions. As there seemed to be important methodological and diagnostic differences within and across the European regions, interpretation of the findings requires caution, especially considering the high heterogeneity among the studies still observed in this updated review.
Overall, there was no improvement in the design of studies and diagnostic approaches used between the current update and the previous review. There is still a need to improve this evidence base in order to better understand the frequency of FA across Europe, through which its healthcare and societal burden can be clearer explained. As indicated in the previous review, future studies still require rigorous designs and implementation of standardized methodology in diagnosing FA, including use of DBPCFC to minimize potential biases.
CONFLICT OF INTEREST
Carina Venter reports: grants (Reckitt Benckiser, Food Allergy Research and Education, and National Peanut Board) and personal fees (Reckitt Benckiser, Nestle Nutrition Institute, Danone, Abbott Nutrition, Else Nutrition, Sifter, and Before Brands). Ronald van Ree reports: consultancies (HAL Allergy BV, Citeq BV, Angany Inc., Reacta Healthcare Ltd., Mission MightyMe, and AB Enzymes), speaker's fees (HAL Allergy BV, ThermoFisher Scientific, and ALK), and stock options (Angany Inc.). Margitta Worm reports: grants and personal fees (Stallergens, HAL Allergie, Bencard Allergie, Allergopharma, ALK‐Abello, Mylan Germany, Actelion Pharmaceuticals Deutschland, Biotest, AbbVie Deutschland, Lilly Deutschland Aimmune, DBV Technologies SA, Regeneron Pharmaceuticals, Sanofi Aventis, Leo Pharma, Novartis, and Viatris) outside of the submitted work and being past WAO co‐chair of the anaphylaxis committee and past chair of the food allergy interest group of EAACI. Berber Vlieg‐Boerstra reports: personal fees (Marfo Food Group, Nestlé, and Nutricia) and grants (Nutricia). Antonella Muraro reports: grants and speaker's fees (Aimmune), speaker's fees (DVB Technologies SA, Viatris [Mylan], ALK, and Nestlé), and being member of the Executive Committee of GA2LEN and past president of EAACI. Graham Roberts reports grants (Asthma UK and National Institutes of Health Research). Bright I, Nwaru reports unrestricted grants and personal fees from DBV Technologies and AstraZeneca, respectively. Giulia C.I. Spolidoro, Yohannes Tesfaye Amera, Mohamed Mustafa Ali, Sungkutu Nyassi, Daniil Lisik, Athina Ioannidou report fee from ACT Institutet Sweden. The other authors report no conflicting interests related to this work. The funder played no role in the content and decision to submit this manuscript.
Supporting information
Data S1
Figures S1‐S28
ACKNOWLEDGEMENTS
The study was funded through an unrestricted grant from DBV Technologies SA. The funders had no influence on the design of the study, interpretation of findings, or the decision to publish. BN acknowledges the support of Knut and Alice Wallenberg Foundation, the Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Sweden, and the VBG Group Herman Krefting Foundation on Asthma and Allergy.
APPENDIX 1.
| Geoscheme of European countries by UN | |||
| Eastern Europe | Northern Europe | Southern Europe | Western Europe |
| Belarus | Åland* | Albania | Austria |
| Bulgaria | Channel Islands (Guernsey, Jersey, Sark) | Andorra | Belgium |
| Czech Republic | Denmark | Bosnia and Herzegovina | France |
| Hungary | Estonia | Croatia | Germany |
| Poland | Faroe Islands | Gibraltar | Liechtenstein |
| Moldova | Finland | Greece | Luxembourg |
| Romania | Iceland | Holy See (Vatican City) | Monaco |
| Russia | Ireland | Italy | Netherlands |
| Slovakia | Isle of Man | Kosovo* | Switzerland |
| Ukraine | Latvia | Malta | |
| Lithuania | Montenegro | ||
| Norway | (North) Macedonia | ||
| Svalbard and Jan Mayen Islands* | Portugal | ||
| Sweden | San Marino | ||
| UK (England, Scotland, Wales, and Northern Ireland) | Serbia | ||
| Slovenia | |||
| Spain | |||
| Turkey* | |||
| Yugoslavia (historical)* | |||
| Adapted version from https://cies2018.org/wp‐content/uploads/List‐of‐Countries‐by‐Region‐UN‐Annex‐II.pdf | |||
| * Appended | |||
Spolidoro GCI, Amera YT, Ali MM, et al. Frequency of food allergy in Europe: An updated systematic review and meta‐analysis. Allergy. 2023;78:351‐368. doi: 10.1111/all.15560
REFERENCES
- 1. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(1):62‐75. [DOI] [PubMed] [Google Scholar]
- 2. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(8):992‐1007. [DOI] [PubMed] [Google Scholar]
- 3. Newcombe R. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857‐872. [DOI] [PubMed] [Google Scholar]
- 4. Arshad SH, Patil V, Mitchell F, et al. Cohort profile update: the Isle of Wight whole population birth cohort (IOWBC). Int J Epidemiol. 2020;49(4):1083‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baccioglu A, Sogut A, Kilic O, Beyhun E. The prevalence of allergic diseases and associated risk factors in school‐age children and adults in Erzurum, Turkey. Turk Thorac J. 2015;16(2):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bant A, Kruszewski J. Increased sensitization prevalence to common inhalant and food allergens in young adult Polish males. Ann Agric Environ Med. 2008;15:21‐27. [PubMed] [Google Scholar]
- 7. Baricic TV, Catipovic M, Cetinic EL, Krmek V, Horvat I. Parental perception, prevalence and primary care physicians' knowledge on childhood food allergy in Croatia. Children (Basel). 2015;2(3):305‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barlik F, Guner SN, Barlik M, Sugut A, Sancak R. Prevalence of food allergy in nursery and kindergarten children in Samsun. Türk Pediatri Arşivi. 2013;48(4):288‐293. [Google Scholar]
- 9. Bobrowska‐Korzeniowska M, Kapszewicz K, Jerzynska J, et al. Early life environmental exposure in relation to new onset and remission of allergic diseases in school children: polish mother and child cohort study. Allergy Asthma Proc. 2019;40(5):329‐337. [DOI] [PubMed] [Google Scholar]
- 10. Boehmer D, Schuster B, Krause J, Darsow U, Biedermann T, Zink A. Prevalence and treatment of allergies in rural areas of Bavaria, Germany: a cross‐sectional study. World Allergy Organ J. 2018;11(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bröms K, Norbäck D, Eriksson M, Sundelin C, Svärdsudd K. Prevalence and co‐occurrence of parentally reported possible asthma and allergic manifestations in pre‐school children. BMC Public Health. 2013;13:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burney P, Summers C, Chinn S, Hooper R, van Ree R, Lidholm J. Prevalence and distribution of sensitization to foods in the European Community Respiratory Health Survey: a EuroPrevall analysis. Allergy. 2010;65(9):1182‐1188. [DOI] [PubMed] [Google Scholar]
- 13. Woods RK, Abramson M, Fau‐Bailey M, Bailey M, Fau‐Walters EH, Walters EH. International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991‐1994. Eur J Clin Nutr. 2001;55:298‐304. [DOI] [PubMed] [Google Scholar]
- 14. Burney PG, Potts J, Kummeling I, et al. The prevalence and distribution of food sensitization in European adults. Allergy. 2014;69(3):365‐371. [DOI] [PubMed] [Google Scholar]
- 15. Lyons SA, Burney PGJ, Ballmer‐Weber BK, et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019;7(6):1920‐1928.e11. [DOI] [PubMed] [Google Scholar]
- 16. Butiene I, Dubakiene R, Rudzeviciene O. Prevalence of sensitization to cow‘s milk in EuroPrevall Lithuanian birth cohort. Clinical and Translational Allergy. 2013;3(S3):O21. [Google Scholar]
- 17. Caffarelli C, Coscia A, Fau‐Ridolo E, et al. Parents' estimate of food allergy prevalence and management in Italian school‐aged children. Pediatr Int. 2011;53:505‐510. [DOI] [PubMed] [Google Scholar]
- 18. Chafen JJ, Newberry SJ, Fau‐Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848‐1856. [DOI] [PubMed] [Google Scholar]
- 19. Chytiroglou E, Gkavogiannakis N, Potika M, et al. Prevalence of atopic diseases and sensitization profile in pediatric population: comparative data in Greek islands. Allergy. 2015;70:298. [Google Scholar]
- 20. Clausen I, Goksör E, Alm B, Wennergren G. Food Allergy at 12 Years of Age in Western Sweden – Risk Factors and Protective Factors. University of Iceland; 2017. [Google Scholar]
- 21. Goksor E, Loid P, Alm B, Aberg N, Wennergren G. The allergic march comprises the coexistence of related patterns of allergic disease not just the progressive development of one disease. Acta Paediatr. 2016;105(12):1472‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goksör E, Lougheide‐Camejo N, Göran Wennergren B. Cow's milk allergy from infancy to school age. Clinical and Translational Allergy. 2018;8(S2):O4. [Google Scholar]
- 23. Colver AF, Nevantaus H Fau‐Macdougall CF, Macdougall Cf Fau‐Cant AJ, Cant AJ. Severe food‐allergic reactions in children across the UK and Ireland, 1998‐2000. Acta Paediatr. 2005;94:689‐695. [DOI] [PubMed] [Google Scholar]
- 24. de Jong NW, Elbert NJ, Mensink‐Bout SM, et al. Parental and child factors associated with inhalant and food allergy in a population‐based prospective cohort study: the Generation R Study. Eur J Pediatr. 2019;178(10):1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Depner M, Ege MJ, Genuneit J, et al. Atopic sensitization in the first year of life. J Allergy Clin Immunol. 2013;131(3):781‐788. [DOI] [PubMed] [Google Scholar]
- 26. Dereci S, Orhan F, Koca T, Akcam M. Prevalence of blueberry allergy in a Turkish population. Ann Allergy Asthma Immunol. 2015;114:259‐260. [DOI] [PubMed] [Google Scholar]
- 27. Dereci S, Koca T, Akcam M. The incidence and clinical characteristics of IgE‐mediated hazelnut allergy in children living in the eastern Black Sea region of Turkey. Pediatr Allergy Immunol Pulmonol. 2016;29(1):24‐28. [Google Scholar]
- 28. Haktanir Abul M, Dereci S, Hacisalihoglu S, Orhan F. Is kiwifruit allergy a matter in kiwifruit‐cultivating regions? A population‐based study. Pediatr Allergy Immunol. 2017;28(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 29. Diwakar L, Ryan R, Cummins C, Roberts T. Increasing prevalence of GP diagnosed childhood allergies in the United Kingdom: an analysis of The Health Information Network (THIN) database. Clin Exp Allergy. 2017;47(12):1678‐1721. [Google Scholar]
- 30. Dogruel D, Bingol G, Altintas DU, Yilmaz M, Guneser Kendirli S. Clinical features of food allergy during the 1st year of life: the ADAPAR birth cohort study. Int Arch Allergy Immunol. 2016;169(3):171‐180. [DOI] [PubMed] [Google Scholar]
- 31. Karakoc G, Karagoz D, Altintas D, Yılmaz M, Duyuler G, Kont A. Natural course of food allergy at the end of the 4 years of age: results of birth cohort study from Turkey. Allergy. 2015;70(1477):527‐613. [Google Scholar]
- 32. Du Toit G, Katz Y, Fau‐Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984‐991. [DOI] [PubMed] [Google Scholar]
- 33. Dubakiene R, Rudzeviciene O, Fau‐Butiene I, et al. Studies on early allergic sensitization in the Lithuanian birth cohort. Sci World J. 2012;2012:909524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eckers N, Grabenhenrich L, McBride D, et al. Frequency and development of hen's egg allergy in early childhood in Germany: the EuroPrevall birth cohort. Allergologie. 2015;38(10):507‐515. [Google Scholar]
- 35. Eggesbø M, Botten G, Fau‐Stigum H, et al. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112:420‐426. [DOI] [PubMed] [Google Scholar]
- 36. Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of CMA/CMPI in young children: the validity of parentally perceived reactions in a population‐based study. Allergy. 2001;56(5):393‐402. [DOI] [PubMed] [Google Scholar]
- 37. Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population‐based study in young children. Allergy. 2001;56(5):403‐411. [DOI] [PubMed] [Google Scholar]
- 38. Eller E, Kjaer HF, Høst A, Andersen KE, Bindslev‐Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64(7):1023‐1029. [DOI] [PubMed] [Google Scholar]
- 39. Kjaer HF, Eller E, Høst A, Andersen KE, Bindslev‐Jensen C. The prevalence of allergic diseases in an unselected group of 6‐year‐old children. The DARC birth cohort study. Pediatr Allergy Immunol. 2008;19(8):737‐745. [DOI] [PubMed] [Google Scholar]
- 40. Jøhnke H, Norberg LA, Vach W, Høst A, Andersen KE. Patterns of sensitization in infants and its relation to atopic dermatitis. Pediatr Allergy Immunol. 2006;17(8):591‐600. [DOI] [PubMed] [Google Scholar]
- 41. Erhard SM, Bellach J, Yurek S, et al. Primary and pollen‐associated hazelnut allergy in school‐aged children in Germany: a birth cohort study. Allergol Int. 2021;70(4):463‐470. [DOI] [PubMed] [Google Scholar]
- 42. Falcão H, Lunet N, Lopes C, Barros H. Food hypersensitivity in Portuguese adults. Eur J Clin Nutr. 2004;58(12):1621‐1625. [DOI] [PubMed] [Google Scholar]
- 43. Fedorova O, Fedotova M, Ogorodova L, et al. Fish allergy prevalence in children of West Siberia, Russia. Allergy. 2014;69:269.24266692 [Google Scholar]
- 44. Fedorova O, Ogorodova L, Fedotova M, Evdokimova TA. The prevalence of food allergy to peanut and hazelnut in children in Tomsk Region. Voprosy Pitaniia. 2014;83(1):48‐54. [PubMed] [Google Scholar]
- 45. Fedorova O, Fedotova M, Yazdanbakhsh M, et al. The prevalence of food allergy to hen's egg in schoolchildren in Western Siberia (Russian Federation). Allergy. 2016;71:300‐389. [Google Scholar]
- 46. Flokstra‐de Blok BM, Doriene van Ginkel C, Roerdink EM, et al. Extremely low prevalence of epinephrine autoinjectors in high‐risk food‐allergic adolescents in Dutch high schools. Pediatr Allergy Immunol. 2011;22(4):374‐377. [DOI] [PubMed] [Google Scholar]
- 47. Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123(2):417‐423. [DOI] [PubMed] [Google Scholar]
- 48. Frongia O, Bellomo A, Di Giorgio G, Fiumalbi C, Frizza J, Maresca C. Food allergies and intolerance in infants and children. [Italian] Intolleranze e allergie alimentari nella prima infanzia. Medico e Bambino. 2005;24:533‐538. [Google Scholar]
- 49. Gaspar‐Marques J, Carreiro‐Martins P, Papoila AL, et al. Food allergy and anaphylaxis in infants and preschool‐age children. Clin Pediatr (Phila). 2014;53(7):652‐657. [DOI] [PubMed] [Google Scholar]
- 50. Gelincik A, Büyüköztürk S, Gül H, et al. Confirmed prevalence of food allergy and non‐allergic food hypersensitivity in a Mediterranean population. Clin Exp Allergy. 2008;38(8):1333‐1341. [DOI] [PubMed] [Google Scholar]
- 51. Gómez‐Galán C, Peña‐Peloche M, Ferré‐Ybarz L, et al. Incidence of Cow's milk allergy in the first year of life in Central Catalonia. Pediatria Catalana. 2017;77(1):15‐19. [Google Scholar]
- 52. Grabenhenrich L, Trendelenburg V, Bellach J, et al. Frequency of food allergy in school‐aged children in eight European countries‐the EuroPrevall‐iFAAM birth cohort. Allergy. 2020;75(9):2294‐2308. [DOI] [PubMed] [Google Scholar]
- 53. Grimshaw KE, Bryant T, Oliver EM, et al. Incidence and risk factors for food hypersensitivity in UK infants: results from a birth cohort study. Clin Transl Allergy. 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110(5):784‐789. [DOI] [PubMed] [Google Scholar]
- 55. Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62(1):91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clin Exp Allergy. 2004;34(4):520‐526. [DOI] [PubMed] [Google Scholar]
- 57. Gupta R, Sheikh A, Strachan D, Anderson HR. Increasing hospital admissions for systemic allergic disorders in England: analysis of national admissions data. BMJ. 2003;327(7424):1142‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haftenberger M, Laussmann D, Ellert U, et al. Prevalence of sensitization to aeraoallergens and food allergens: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5‐6):687‐697. [DOI] [PubMed] [Google Scholar]
- 59. Langen U, Schmitz R, Steppuhn H. Prevalence of allergic diseases in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5‐6):698‐706. [DOI] [PubMed] [Google Scholar]
- 60. Hicke‐Roberts A, Wennergren G, Hesselmar B. Late introduction of solids into infants' diets may increase the risk of food allergy development. BMC Pediatr. 2020;20(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(s15):23‐28. [DOI] [PubMed] [Google Scholar]
- 62. Hourihane JO, Aiken R, Briggs R, et al. The impact of government advice to pregnant mothers regarding peanut avoidance on the prevalence of peanut allergy in United Kingdom children at school entry. J Allergy Clin Immunol. 2007;119(5):1197‐1202. [DOI] [PubMed] [Google Scholar]
- 63. Isolauri E, Huurre A, Salminen S, Impivaara O. The allergy epidemic extends beyond the past few decades. Clin Exp Allergy. 2004;34(7):1007‐1010. [DOI] [PubMed] [Google Scholar]
- 64. Ivakhnenko O, Nyankovskyy S. Nutritional status of babies and influence of unmodified cow's milk on allergic reactions according to the epidemiological study from Ukraine. Pediatria Polska. 2013;88(2):138‐143. [Google Scholar]
- 65. Jarvenpaa J, Paassilta M, Salmivesi S, Sannisto T, Niitty S, Korppi M. Stability of parent‐reported food allergy in six and 7‐year‐old children: the first 5 years of the Finnish allergy programme. Acta Paediatr. 2014;103(12):1297‐1300. [DOI] [PubMed] [Google Scholar]
- 66. Johansson SG, Nopp A, Florvaag E, et al. High prevalence of IgE antibodies among blood donors in Sweden and Norway. Allergy. 2005;60(10):1312‐1315. [DOI] [PubMed] [Google Scholar]
- 67. Jorge A, Soares E, Sarinho E, Lorente F, Gama J, Taborda‐Barata L. Prevalence and clinical features of adverse food reactions in Portuguese children. Allergy Asthma Clin Immunol. 2017;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Julge K, Vasar M, Björkstén B. Development of allergy and IgE antibodies during the first five years of life in Estonian children. Clin Exp Allergy. 2001;31(12):1854‐1861. [DOI] [PubMed] [Google Scholar]
- 69. Vasar M, Julge K, Bjökstö B. Development of atopic sensitization and allergic diseases in early childhood. Acta Paediatr. 2000;89(5):523‐527. [DOI] [PubMed] [Google Scholar]
- 70. Jurado‐Escobar R, P Erez‐Sanchez N, Victorio L et al. Sensitization and allergy patterns to inhalant and food allergens in a population from the Mediterranean area. Allergy 2017; 72: 383‐757. [Google Scholar]
- 71. Jurisson M, Julge K, Voor T, Vasar M, Rebane T, Vorobjov S. Trends of prevalence of atopic dermatitis and sensitization during the first twoyears of life in Estonian children. Allergy. 2015;70:507‐526. [Google Scholar]
- 72. Kanny G, Moneret‐Vautrin DA, Flabbee J, Beaudouin E, Morisset M, Thevenin F. Population study of food allergy in France. J Allergy Clin Immunol. 2001;108(1):133‐140. [DOI] [PubMed] [Google Scholar]
- 73. Kaya A, Erkocoglu M, Civelek E, Cakir B, Kocabas CN. Prevalence of confirmed IgE‐mediated food allergy among adolescents in Turkey. Pediatr Allergy Immunol. 2013;24(5):456‐462. [DOI] [PubMed] [Google Scholar]
- 74. Kelleher M, Cullinane C, Dunn Galvin A, Murray D, Hourihane J. Prevalence of food allergy in Irish children during their first 2 years: results from the Cork BASELINE birth cohort study. Allergy. 2014;69:269‐270.24266692 [Google Scholar]
- 75. Kose S, Mandiracioglu A, Cavdar G, Ulu Y, Senger SS. Prevalence of allergic diseases in Izmir Province, Turkey. Int Forum Allergy Rhinol. 2014;4(3):232‐238. [DOI] [PubMed] [Google Scholar]
- 76. Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner‐recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011;127(3):623‐630.e1. [DOI] [PubMed] [Google Scholar]
- 77. Krause TG, Koch A, Poulsen LK, Kristensen B, Olsen OR, Melbye M. Atopic sensitization among children in an arctic environment. Clin Exp Allergy. 2002;32(3):367‐372. [DOI] [PubMed] [Google Scholar]
- 78. Kristinsdóttir H, Clausen M, Ragnarsdóttir HS, et al. Prevalence of food allergy in Icelandic infants during first year of life. Laeknabladid. 2011;97(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 79. Kucukosmanoglu E, Yazi D, Yesil O, et al. Prevalence of egg sensitization in Turkish infants based on skin prick test. Allergol Immunopathol (Madr). 2008;36(3):141‐144. [PubMed] [Google Scholar]
- 80. Kurukulaaratchy RJ, Matthews S, Arshad SH. Defining childhood atopic phenotypes to investigate the association of atopic sensitization with allergic disease. Allergy. 2005;60(10):1280‐1286. [DOI] [PubMed] [Google Scholar]
- 81. Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108(2):E33. [DOI] [PubMed] [Google Scholar]
- 82. Tariq SM, Matthews SM, Hakim EA, Arshad SH. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol. 2000;11(3):162‐167. [DOI] [PubMed] [Google Scholar]
- 83. Kvedariene V, Sitkauskiene B, Tamasauskiene L, et al. Prevalence of self‐reported drug hypersensitivity reactions among Lithuanian children and adults. Allergol Immunopathol (Madr). 2019;47(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 84. Kvenshagen B, Halvorsen R, Jacobsen M. Is there an increased frequency of food allergy in children delivered by caesarean section compared to those delivered vaginally? Acta Paediatr. 2009;98(2):324‐327. [DOI] [PubMed] [Google Scholar]
- 85. Le TM, van Hoffen E, Kummeling I, et al. Food allergy in the Netherlands: differences in clinical severity, causative foods, sensitization and DBPCFC between community and outpatients. Clin Transl Allergy. 2015;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lozoya‐Ibanez C, Morgado‐Nunes S, Rodrigues A, et al. Prevalence and clinical features of adverse food reactions in Portuguese adolescents. World Allergy Organ J. 2020;13(8):100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lyons SA, Knulst AC, Le T‐M, et al. Prevalence of food sensitization and food allergy in children across Europe. J Allergy Clin Immunol Pract. 2020;8(8):2736. [DOI] [PubMed] [Google Scholar]
- 88. Majkowska‐Wojciechowska B, Wardzynska A, Luczynska M, Kowalski M, Makowska J, Kowalski M. Food hypersensitivity in the population of school children in Lodz ‐ results of the “EuroPrevall” surveys. Alergia Astma Immunologia. 2009;14:35‐44. [Google Scholar]
- 89. Marklund B, Ahlstedt S, Nordström G. Health‐related quality of life among adolescents with allergy‐like conditions ‐ with emphasis on food hypersensitivity. Health Qual Life Outcomes. 2004;2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Matricardi PM, Bockelbrink A, Beyer K, et al. Primary versus secondary immunoglobulin E sensitization to soy and wheat in the Multi‐Centre Allergy Study cohort. Clin Exp Allergy. 2008;38(3):493‐500. [DOI] [PubMed] [Google Scholar]
- 91. Matsyura O, Lesya B. Food intolerance and food allergy in children in Lviv region (Ukraine). Allergy: Eur J Allergy Clin Immunol. 2017;72:770. [Google Scholar]
- 92. Mortz CG, Andersen KE, Bindslev‐Jensen C. Allergy to sesame‐prevalence in an unselected population and relation to pollen sensitization. Allergy: Eur J Allergy Clin Immunol. 2013;68:146. [Google Scholar]
- 93. Mossakowska M, Pawlinska‐Chmara R, Broczek KM. Asthma, allergy, and respiratory symptoms in centenarians living in Poland. J Physiol Pharmacol. 2008;59(Suppl 6):483‐489. [PubMed] [Google Scholar]
- 94. Mustafayev R, Civelek E, Orhan F, Yuksel H, Boz AB, Sekerel BE. Similar prevalence, different spectrum: IgE‐mediated food allergy among Turkish adolescents. Allergol Immunopathol (Madr). 2013;41(6):387‐396. [DOI] [PubMed] [Google Scholar]
- 95. Nicolaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component‐resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191‐197.e1‐13. [DOI] [PubMed] [Google Scholar]
- 96. Niggemann B, Schmitz R, Schlaud M. The high prevalence of peanut sensitization in childhood is due to cross‐reactivity to pollen. Allergy. 2011;66(7):980‐981. [DOI] [PubMed] [Google Scholar]
- 97. Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E‐mediated food allergy in 6‐9‐year‐old urban schoolchildren in the eastern Black Sea region of Turkey. Clin Exp Allergy. 2009;39(7):1027‐1035. [DOI] [PubMed] [Google Scholar]
- 98. Ostblom E, Lilja G, Ahlstedt S, van Hage M, Wickman M. Patterns of quantitative food‐specific IgE‐antibodies and reported food hypersensitivity in 4‐year‐old children. Allergy. 2008;63(4):418‐424. [DOI] [PubMed] [Google Scholar]
- 99. Ostblom E, Lilja G, Pershagen G, van Hage M, Wickman M. Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin Exp Allergy. 2008;38(8):1325‐1332. [DOI] [PubMed] [Google Scholar]
- 100. Ostblom E, Wickman M, van Hage M, Lilja G. Reported symptoms of food hypersensitivity and sensitization to common foods in 4‐year‐old children. Acta Paediatr. 2008;97(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 101. Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005;35(5):612‐618. [DOI] [PubMed] [Google Scholar]
- 102. Osterballe M, Hansen TK, Mortz CG, Høst A, Bindslev‐Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16(7):567‐573. [DOI] [PubMed] [Google Scholar]
- 103. Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev‐Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20(7):686‐692. [DOI] [PubMed] [Google Scholar]
- 104. Patelis A, Gunnbjornsdottir M, Borres MP, et al. Natural history of perceived food hypersensitivity and IgE sensitization to food allergens in a cohort of adults. PLoS ONE. 2014;9(1):e85333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pawlińska‐Chmara R, Teul I. Food hypersensitivity in the population of school children in Opole. Pom J Life Sci. 2015;61(1):120‐123. [PubMed] [Google Scholar]
- 106. Pénard‐Morand C, Raherison C, Kopferschmitt C, et al. Prevalence of food allergy and its relationship to asthma and allergic rhinitis in schoolchildren. Allergy. 2005;60(9):1165‐1171. [DOI] [PubMed] [Google Scholar]
- 107. Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol. 2005;116(4):884‐892. [DOI] [PubMed] [Google Scholar]
- 108. Protudjer JLP, Olen O, Vetander M, et al. Milk‐related symptoms and immunoglobulin E reactivity in Swedish children from early life to adolescence. Nutrients. 2018;10(5):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pyrhönen K, Hiltunen L, Kaila M, Näyhä S, Läärä E. Heredity of food allergies in an unselected child population: an epidemiological survey from Finland. Pediatr Allergy Immunol. 2011;22(1 Pt 2):e124‐e132. [DOI] [PubMed] [Google Scholar]
- 110. Pyrhönen K, Näyhä S, Kaila M, Hiltunen L, Läärä E. Occurrence of parent‐reported food hypersensitivities and food allergies among children aged 1‐4 yr. Pediatr Allergy Immunol. 2009;20(4):328‐338. [DOI] [PubMed] [Google Scholar]
- 111. Pyziak K, Kamer B. Natural history of IgE‐dependent food allergy diagnosed in children during the first three years of life. Adv Med Sci. 2011;56(1):48‐55. [DOI] [PubMed] [Google Scholar]
- 112. Raciborski F, Samel‐Kowalik P, Tomaszewska A, et al. Food allergy in children aged 6‐8 years in Poland. Allergy: Eur J Allergy Clin Immunol. 2012;67:610‐611. [Google Scholar]
- 113. Rancé F, Grandmottet X, Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clin Exp Allergy. 2005;35(2):167‐172. [DOI] [PubMed] [Google Scholar]
- 114. Rentzos G, Johanson L, Goksor E, Telemo E, Lundback B, Ekerljung L. Prevalence of food hypersensitivity in relation to IgE sensitization to common food allergens among the general adult population in West Sweden. Clin Transl Allergy. 2019;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Roberts G, Peckitt C, Northstone K, et al. Relationship between aeroallergen and food allergen sensitization in childhood. Clin Exp Allergy. 2005;35(7):933‐940. [DOI] [PubMed] [Google Scholar]
- 116. Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977‐985. [DOI] [PubMed] [Google Scholar]
- 117. Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: a meta‐analysis. J Allergy Clin Immunol. 2007;120(3):638‐646. [DOI] [PubMed] [Google Scholar]
- 118. Ronchetti R, Jesenak M, Trubacova D, Pohanka V, Villa MP. Epidemiology of atopy patch tests with food and inhalant allergens in an unselected population of children. Pediatr Allergy Immunol. 2008;19(7):599‐604. [DOI] [PubMed] [Google Scholar]
- 119. Sandin A, Annus T, Björkstén B, et al. Prevalence of self‐reported food allergy and IgE antibodies to food allergens in Swedish and Estonian schoolchildren. Eur J Clin Nutr. 2005;59(3):399‐403. [DOI] [PubMed] [Google Scholar]
- 120. Schäfer T, Böhler E, Ruhdorfer S, et al. Epidemiology of food allergy/food intolerance in adults: associations with other manifestations of atopy. Allergy. 2001;56(12):1172‐1179. [DOI] [PubMed] [Google Scholar]
- 121. Schnabel E, Sausenthaler S, Schaaf B, et al. Prospective association between food sensitization and food allergy: results of the LISA birth cohort study. Clin Exp Allergy. 2010;40(3):450‐457. [DOI] [PubMed] [Google Scholar]
- 122. Shytaj KM, Deda L, Hoxha S, et al. Prevalence of allergies among children in the district of Gjirokastra, Albania. Allergy: Eur J Allergy Clin Immunol. 2013;68:536‐537. [Google Scholar]
- 123. Skypala IJ, Bull S, Deegan K, et al. The prevalence of PFS and prevalence and characteristics of reported food allergy; a survey of UK adults aged 18‐75 incorporating a validated PFS diagnostic questionnaire. Clin Exp Allergy. 2013;43(8):928‐940. [DOI] [PubMed] [Google Scholar]
- 124. Soost S, Leynaert B, Almqvist C, Edenharter G, Zuberbier T, Worm M. Risk factors of adverse reactions to food in German adults. Clin Exp Allergy. 2009;39(7):1036‐1044. [DOI] [PubMed] [Google Scholar]
- 125. Zuberbier T, Edenharter G, Worm M, et al. Prevalence of adverse reactions to food in Germany ‐ a population study. Allergy. 2004;59(3):338‐345. [DOI] [PubMed] [Google Scholar]
- 126. Roehr CC, Edenharter G, Reimann S, et al. Food allergy and non‐allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004;34(10):1534‐1541. [DOI] [PubMed] [Google Scholar]
- 127. Stefanaki E, Margetaki A, Roumeliotaki T, Chatzi L. Incidence of parent reported food hypersensitivity in Greek children at 4 and 6 years of age: results from a birth cohort study in Crete. Clin Transl Allergy. 2018;8(S2):118. [Google Scholar]
- 128. Steinke M, Fiocchi A, Kirchlechner V, et al. Perceived food allergy in children in 10 European nations. A randomised telephone survey. Int Arch Allergy Immunol. 2007;143(4):290‐295. [DOI] [PubMed] [Google Scholar]
- 129. Sterner T, Uldahl A, Svensson A, et al. The Southern Sweden Adolescent Allergy‐Cohort: prevalence of allergic diseases and cross‐sectional associations with individual and social factors. J Asthma. 2019;56(3):227‐235. [DOI] [PubMed] [Google Scholar]
- 130. Sterner T, Uldahl A, Svensson A, et al. IgE sensitization in a cohort of adolescents in southern Sweden and its relation to allergic symptoms. Clin Mol Allergy. 2019;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Strinnholm A, Winberg A, West C, Hedman L, Ronmark E. Food hypersensitivity is common in Swedish schoolchildren, especially oral reactions to fruit and gastrointestinal reactions to milk. Acta Paediatr. 2014;103(12):1290‐1296. [DOI] [PubMed] [Google Scholar]
- 132. Winberg A, West CE, Strinnholm A, Nordstrom L, Hedman L, Ronmark E. Assessment of allergy to milk, egg, cod, and wheat in Swedish Schoolchildren: a population based cohort study. PLoS ONE. 2015;10(7):e0131804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Topcu ZIK, Kaklikkaya N, Baki A, Orhan F. Characteristics of beef allergy in schoolchildren in Turkey. Allergy Asthma Proc. 2018;39(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 134. Treudler R, Simon JC, Ahnert P, Walther F. High prevalence of self‐reported allergies in adults: second interim analysis on 4088 subjects of Leipzig Interdisciplinary Research Cluster (LIFE). Allergy: Eur J Allergy Clin Immunol. 2014;69:543. [Google Scholar]
- 135. Van Den Hoogen SCTA, Van De Pol AC, Meijer Y, Toet J, Van Klei C, De Wit NJ. Suspected cow's milk allergy in everyday general practice: a retrospective cohort study on health care burden and guideline adherence. BMC Res Notes. 2014;7(1):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Venkataraman D, Erlewyn‐Lajeunesse M, Kurukulaaratchy RJ, et al. Prevalence and longitudinal trends of food allergy during childhood and adolescence: results of the Isle of Wight Birth Cohort study. Clin Exp Allergy. 2018;48(4):394‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Venter C, Hasan Arshad S, Grundy J, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65(1):103‐108. [DOI] [PubMed] [Google Scholar]
- 138. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63(3):354‐359. [DOI] [PubMed] [Google Scholar]
- 139. Venter C, Pereira B, Grundy J, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117(5):1118‐1124. [DOI] [PubMed] [Google Scholar]
- 140. Dean T, Venter C, Pereira B, et al. Patterns of sensitization to food and aeroallergens in the first 3 years of life. J Allergy Clin Immunol. 2007;120(5):1166‐1171. [DOI] [PubMed] [Google Scholar]
- 141. Venter C, Maslin K, Patil V, et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol. 2016;27(8):804‐811. [DOI] [PubMed] [Google Scholar]
- 142. Venter C, Maslin K, Arshad SH, et al. Very low prevalence of IgE mediated wheat allergy and high levels of cross‐sensitization between grass and wheat in a UK birth cohort. Clin Transl Allergy. 2016;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six‐year‐old children: a population‐based study. Pediatr Allergy Immunol. 2006;17(5):356‐363. [DOI] [PubMed] [Google Scholar]
- 144. von Hertzen L, Mäkelä MJ, Petäys T, et al. Growing disparities in atopy between the Finns and the Russians: a comparison of 2 generations. J Allergy Clin Immunol. 2006;117(1):151‐157. [DOI] [PubMed] [Google Scholar]
- 145. Vrbova M, Dorociakova P, Vyskovsky R, et al. Dynamics of allergy development during the first 5 years of life. Eur J Pediatr. 2018;177(9):1317‐1325. [DOI] [PubMed] [Google Scholar]
- 146. Westerlaken‐Van Ginkel CD, Sprikkelman AB, Koppelman GH, Dubois AEJ, Vonk JM, Flokstra‐De Blok BMJ. Likely questionnaire‐diagnosed food allergy in 78, 890 adults from the northern Netherlands. PLoS ONE. 2020;15(5):e0231818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xepapadaki P, Fiocchi A, Grabenhenrich L, et al. Incidence and natural history of hen's egg allergy in the first 2 years of life‐the EuroPrevall birth cohort study. Allergy. 2016;71(3):350‐357. [DOI] [PubMed] [Google Scholar]
- 148. Zeyrek D, Cakmak A, Koruk I, Kara B, Demir C. Prevalence of IgE mediated cow's milk and egg allergy in children under 2 years of age in Sanliurfa, Turkey: the city that isn't almost allergic to cow's milk. Minerva Pediatrica. 2015;67(6):465‐472. [PubMed] [Google Scholar]
- 149. Zivic H, Strizic H. Prevalence of cow's‐Milk protein allergy in a primary paediatric practice in Croatia. J Pediatr Gastroenterol Nutr. 2018;66:403. [Google Scholar]
- 150. Zuidmeer L, Goldhahn K, Rona RJ, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121(5):1210‐1218.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1‐S28


