Abstract
Background
ADAMTS13 activity is one of the key investigations needed to diagnose thrombotic thrombocytopenic purpura, and there are a number of different assays available to measure it. HemosIL AcuStar, a chemiluminescent immunoassay, was developed and used as a quicker, automated test. In clinical practice, discrepancies between AcuStar and the gold standard FRETS-VWF73 have been documented in a manner that would affect diagnosis and treatment.
Objectives
We aimed to identify and highlight clinical situations where this discrepancy occurs and to attempt to determine the cause.
Method
Therefore, we undertook a study to compare the FRETS-VWF73 assay with AcuStar, the Technozym Activity ELISA, and Ceveron FRET assays using a mixture of 94 retrospective and prospective patient samples.
Results
We found that although the concordance between FRETS-VWF73 and the other methods was generally very good, discrepancies were found in a small number tested on AcuStar affecting diagnosis (5 of 32) and follow-up (7 of 51). A Wilcoxon test comparing FRETS-VWF73 to the AcuStar results suggested that the AcuStar results were significantly lower in 42 samples tested on all 4 platforms. We investigated potential causes for this difference by testing the impact of high vWF levels and addition of a monoclonal ADAMTS13 autoantibody (3H9) to samples. We found no impact of high vWF levels on interassay variability but found that 3H9 reduced ADAMTS13 activity levels much more in AcuStar and ELISA assays than in FRETS assays.
Conclusion
Based on our findings, we would suggest that when AcuStar is used upfront to guide management, a second testing method should be used in patients with an atypical thrombotic thrombocytopenic purpura presentation or unexpectedly slow ADAMTS13 recovery.
Keywords: AcuStar, ADAMTS13, ADAMTS13 monitoring, chemiluminescent assay, FRETS assay, thrombotic thrombocytopenic purpura
Essentials
-
•
Accurate ADAMTS13 activity testing is essential to diagnosing and monitoring thrombotic thrombocytopenic purpura.
-
•
We saw good concordance of several methods.
-
•
However, the AcuStar assay can underestimate ADAMTS13 activity in a small number of cases.
-
•
Therefore, it is important to consider the clinical context when utilizing the AcuStar assay.
1. Introduction
Measurement of the activity of ADAMTS13 enzyme in the blood plays an important role in diagnosis of thrombotic thrombocytopenic purpura (TTP) and in monitoring patients with TTP to prevent relapse. Severely reduced ADAMTS13 activity results in the persistence of ultra-large VWF multimers in the circulation, which can cause the formation of occluding thrombi in the microvasculature, with life-threatening consequences.
The diagnosis of TTP is confirmed by an ADAMTS13 activity of <10 IU/dL with a result of >20 IU/dL suggestive of an alternative diagnosis [1]. Between 10 and 20 IU/dL, clinical judgment is needed to make decisions regarding diagnosis and treatment. The current guidelines by the International Society for Thrombosis and Haemostasis do not recommend a particular ADAMTS13 assay; the same cut-off values for diagnosis have been used across assays, highlighting the importance of consistent results across testing methods.
Another area where the guidance is unclear is the ADAMTS13 activity level at which to commence elective rituximab treatment to prevent clinical relapse. We use an ADAMTS13 activity of ≤15 IU/dL as a cut-off for initiating elective rituximab treatment (as measured with FRETS-VWF73 assay) [2]. The French Thrombotic Microangiopathies Reference Centre has used <10% activity [3].
There have been large strides made in diagnosis and management of TTP over the last 2 decades with an increased availability of more accessible methods of ADAMTS13 activity testing, leading to more widespread use by laboratories. However, it is important to recognize the benefits and limitations of each assay. Additionally, with the recognition of long-term follow-up for TTP, the functionality of assays in ADAMTS13 activity monitoring needs to be evaluated.
The use of automated, quicker assays provides an attractive platform to aid prompt diagnosis of TTP. However, there have been examples of the HemosIL AcuStar assay resulting severe ADAMTS 13 deficiency (<5 IU/dL) and suggesting a diagnosis of TTP, being questioned clinically and TTP being ruled out by FRETS-VWF73. Indeed, the manufacturers, Werfen, issued a notification earlier in the year, highlighting this as an issue [4]. Therefore, we carried out ADAMTS13 testing for a range of clinical scenarios using a selection of testing platforms in use to compare their diagnostic and monitoring utility. This included retrospective cases, but also acute real-time sampling. Furthermore, we determined any potential causes for discrepancies between AcuStar and FRETS-VWF73 assays.
2. Methods
Ninety-four patient samples and 18 donor samples were used in this evaluation. Thirty-five consecutive samples with query thrombotic microangiopathy (TMA) received between August 2021 and October 2021 were analyzed as part of the normal diagnostic pathway, and 55 samples were of patients with TTP undergoing monitoring during remission over a 12-month period. Four severely unwell patients with COVID on the intensive care unit were also tested. Residual samples left from the analysis were tested using alternative ADAMTS13 platforms as part of service evaluation. The majority of samples were analyzed prospectively (79%) and the retrospective samples (21%) had been frozen for less than a year at the time of testing. The breakdown of samples analyzed is summarized in Table 1.
Table 1.
Details of the 94 samples tested.
| Number of samples | |
|---|---|
| Total number of samples | 94 |
| Breakdown by method: | |
| • FRETS-VWF73 vs. Acustar | 88 |
| • FRETS-VWF73 vs. Technozym | 68 |
| • FRETS-VWF73 vs. Ceveron | 48 |
| • All 4 platforms | 42 |
| Breakdown by diagnosis: | |
| Acute immune TTP | 15 |
| Acute congenital TTP | 1 |
| TMAs and other diagnoses (HELLP syndrome, HUS, metastatic breast cancer, sepsis, PPH, unwell neonate) | 19 |
| TTP follow-up (this includes patients 2-4 days after acute TTP, those in remission and those with falling ADAMTS13 during monitoring i.e. planned for elective rituximab) | 55 |
| Severe COVID | 4 |
All the samples were assessed by FRETS-VWF73. The “Breakdown by method” shows the number of samples tested on other platforms. The second half of the table shows the breakdown of 94 samples by diagnosis.
TMA, thrombotic microangiopathy; HELLP, hemolysis, elevated liver enzymes and low platelets; HUS, hemolytic uremic syndrome; PPH, postpartum hemorrhage.
The retrospective cases and normal controls included in the study were consented to the UK TTP registry.
2.1. Blood collection
Citrated whole blood samples (3.2% sodium citrate; 1:9 with whole blood) were collected, centrifuged to obtain platelet-poor plasma, and tested immediately or frozen at −80°C and tested retrospectively by thawing 5 to 10 minutes at 37°C before testing.
All samples from TTP presentation cases were from the initial presentation and taken before the first plasma exchange. The remaining cases were run on the platforms as requested prospectively to confirm or exclude TTP. Platelet counts were established using an automated hematology analyser: Sysmex XN-2000 (Sysmex).
2.2. ADAMTS13 assays
Our in-house FRETS-VWF73 assay [5]. was used as part of the evaluation where ADAMTS13 activity assays were requested for confirmatory diagnosis of TTP, follow-up monitoring, but also in TMA scenarios to exclude TTP. This assay was adapted from Kokame et al. [6] using commercial recombinant FRETS-VWF73 peptide (Peptide Institute) and standardized against the World Health Organization International Standard. Our established normal range was 60 to 146 IU/dL, and linearity down to 2% was previously established.
Residual samples were run on 3 other commercial platforms, HemosIL AcuStar, (Instrumentation Laboratory) Technozyme ELISA (Technozym, Technoclone), and the TECHNOFLUOR ADAMTS13 kit run on Ceveron s100. These were performed according to the manufacturers’ package insert protocols.
To verify the performance of the HemosIL AcuStar ADAMTS13 activity assay under normal conditions, 10 normal donor samples were run, and linearity was tested using the International Standard ADAMTS13 plasma (12/252) at concentrations of 92%, 46%, 23%, 11.5%, 5.75%, and 2.88% and compared with the FRETS-VWF73 method.
In terms of assay turnaround times, the ELISA takes approximately 6 hours, FRETS-VWF73 takes 1.5 hours, and both the Ceveron and AcuStar take 1 hour.
2.3. ADAMST13 IgG and antigen
ADAMTS13 IgG antibody levels were measured by an in-house ELISA method as previously described [7] in which microtitre plates were precoated with purified full-length recombinant ADAMTS13 (donation from Takeda) for capture and Rabbit antibody against human IgG conjugated with HRP to detect bound patient IgG. Results were expressed as a percentage relative to an index plasma from a known TTP with established high titer ADAMTS13 IgG levels (normal range of <6% established locally).
ADAMTS13 antigen levels were quantified using an in-house developed enzyme-linked immunosorbent assay as described previously [8,9].
2.4. Interfering substances
High levels of endogenous VWF antigen in patient plasma could potentially be a limiting factor in the HemosIL AcuStar method. The manufacturer states that the results are not affected up to 200 IU/dL or 200%. To assess the impact of higher VWF antigen levels on ADAMTS13 activity analysis, anonymized samples from cases with a confirmed inflammatory response (i.e., raised CRP levels) were used to measure VWF antigen (Sysmex CS2500 analyzer/Siemens vWF Ag kit; part number OPAB03) as part of service evaluation. In addition, normal plasma was spiked with VWF (Voncento, 1200 IU, CSL Behring UK Limited) at various concentrations to yield final concentrations up to 1000% of normal and were run to determine ADAMTS13 activity on the FRETS-VWF73 method and compared with the HemosIL AcuStar method.
To investigate if antibodies that inhibit ADAMTS13 enzymatic activity could cause discrepant results across the platforms, spiking experiments were performed where the monoclonal inhibitory anti-ADAMTS13 antibody 3H9 [10] was added at various concentrations to normal control plasma and incubated for 1 hour at room temperature.
2.5. Statistical analysis
The correlation between the different ADAMTS13 activity testing platforms was calculated using the Pearson’s method. Wilcoxon matched-pairs signed rank test was carried out to assess if there was a statistically significant difference between the FRETS-VWF73 and the other assays’ results. Statistical analysis was performed using Prism version 9.4.1 (GraphPad Software). The effect size for the Wilcoxon matched-pairs signed rank test was measured using the formula r = z/√N. For this study, ADAMTS13 activity results from all platforms were extrapolated to zero to allow statistical evaluation between methods.
3. Results
3.1. Overall comparison of ADAMTS13 activity assays
To evaluate the real-life utility of ADAMTS13 activity testing platforms in clinical practice, we analyzed 94 samples for which ADAMTS13 activity had been requested by the treating physician (see Table 1 for details).
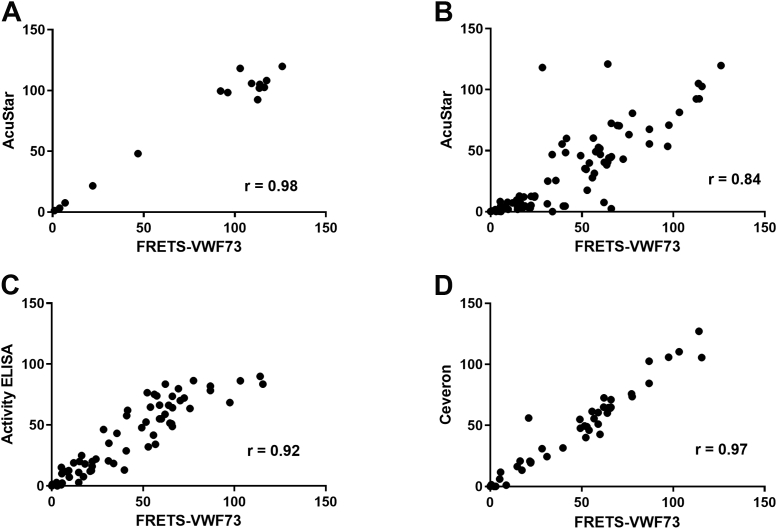
We compared how the results of different platforms correlated overall with the gold standard FRETS-VWF73. The Pearson correlation coefficient between AcuStar and FRETS-VWF73 using normal control samples, pooled normal plasma, and a range of dilutions was r = 0.98 (Figure 1A). However, the inclusion of clinical samples led to a fall in Pearson’s r to 0.84 (Figure 1B). The Technozym Activity ELISA correlated better with FRETS-VWF73 (r = 0.92; Figure 1C) and as expected, the Ceveron FRETS assay was the most comparable (r = 0.97; Figure 1D). The Technozym Activity ELISA and AcuStar had a Pearson’s r = 0.86.
Figure 1.
Pearson correlation between ADAMTS13 activity as measured by FRETS-VWF73 and commercial assays. (A) Eight healthy control samples, 1 pooled normal plasma sample and a range of dilutions of this on the AcuStar (Hemosil) platform. (B) Patient samples on the AcuStar. (C) Patient samples on Technozym activity ELISA. (D) Patient samples on the Ceveron Technofluor (FRETS) platform.
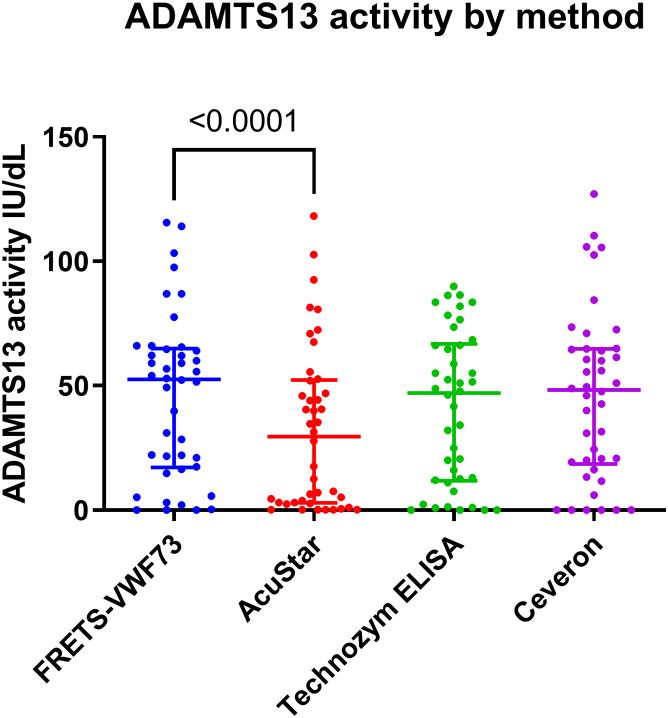
Next, a comparison of 42 samples tested on all 4 platforms was undertaken. This consisted of 10 samples referred from other hospitals and 32 samples from our center: 28 were from patients with TTP at various timepoints in their disease course, including presentation and other TMAs, and 4 were from severely ill patients with COVID on the intensive care unit (Figure 2). We found that the median results on the AcuStar were lower than that for the other methods, which had comparable levels. A Wilcoxon test comparing FRETS-VWF73 to the AcuStar suggested that the AcuStar results were significantly lower in this sample cohort (P < .0001) with an effect size of 0.7. This statistically significant difference persisted while analyzing all 88 samples tested on these 2 platforms. In contrast, although FRETS-VWF73 vs Technozym initially suggested a significantly lower ADAMTS13 activity (P = .0084) in 42 samples, this significance was lost when analysis was performed on all 68 samples for which Technozym testing was undertaken (data not shown). As expected, there was no significant difference between FRETS-VWF73 and the Ceveron assay. A comparison between Technozym and AcuStar also showed that AcuStar had significantly lower results (P < .0001) similar to the comparison between FRETS-VWF73 and AcuStar.
Figure 2.
ADAMTS13 activity results tested on all four platforms. For all 42 samples that were tested across the four different platforms, the individual results are plotted with the median and interquartile range shown as long and short lines respectively.
3.2. Discrepant results
In total, 32 TMA presentation samples were tested, of which TTP was confirmed in 13 cases. All 4 platforms showed results <10 IU/dL for the TTP samples tested, suggesting comparable sensitivity (Table 2).
Table 2.
Acute TTP presentation samples.
| TTP presentation sample | FRETS-VWF73 (IU/dL) | AcuStar (IU/dL) | Technozym ELISA (IU/dL) | Ceveron (IU/dL) | Anti-ADAMTS13 IgG (RR<6.1%) | ADAMTS13 antigen (RR 74-134%) |
|---|---|---|---|---|---|---|
| 1 | <2 | <0.2 | <0.7 | <0.4 | 11 | 4 |
| 2 | <2 | <0.2 | <0.7 | <0.4 | 100 | 6 |
| 3 | <2 | <0.2 | 1 | <0.4 | 16 | 1 |
| 4 | <2 | <0.2 | <0.7 | <0.4 | 64 | 2 |
| 5 | <2 | <0.2 | <0.7 | <0.4 | 19 | 3 |
| 6 | <2 | <0.2 | N.A | N.A | 28 | 14 |
| 7 | <2 | <0.2 | <0.7 | <0.4 | 137 | 2 |
| 8 | <2 | <0.2 | <0.7 | <0.4 | 71 | 10 |
| 9 | 5 | 0.3 | N.A | N.A | 42 | 12 |
| 10 | 9.6 | 2 | 7 | N.A | 114 | 5 |
| 11 | 2.1 | 1 | 1 | <0.4 | 13 | N.A. |
| 12 | <2 | 0.2 | <0.7 | <0.4 | 73 | 2 |
| 13 | 8 | 4 | 12 | N.A. | 5 | 15.5 |
ADAMTS13 activity results from the 4 ADAMTS13 activity assays with corresponding ADAMTS13 autoantibody (IgG) and ADAMTS13 antigen measurements [with reference range (RR) given].
N.A., sufficient residual sample not available.
However, 5 of the 19 (26%) remaining TMA samples had discrepant results between AcuStar and FRETS-VWF73, suggesting TTP in the AcuStar assay (Table 3). As these cases were not confirmed as TTP, it suggests a somewhat lower specificity of the AcuStar assay. Three of these samples were from patients that were diagnosed with sepsis, who had thrombocytopenia and red cell fragmentation but no disseminated intravascular coagulation at presentation. As VWF levels can be very high in patients with sepsis, we tested the hypothesis that VWF can interfere with the assays experimentally by spiking VWF into normal control samples at high levels. However, this showed no impact on the results (Supplementary Table S1A), suggesting that high VWF levels do not explain the discrepancies found between the assays. Similarly, we found that raised VWF antigen levels in pathological samples could not explain the identified discrepancies (Supplementary Table S1B).
Table 3.
Discrepant results on the AcuStar platform.
| Sample | FRETS-VWF73 (IU/dL) | AcuStar (IU/dL) | Technozym ELISA (IU/dL) | Ceveron (IU/dL) | Anti-ADAMTS13 IgG RR (<6.1%) | ADAMTS13 antigen (RR 74%–134%) |
|---|---|---|---|---|---|---|
| Neonate with anemia & schistocytes on film | 66 | 2 | 49 | 65 | 2 | 94 |
| Sepsis | 40 | 5 | 13 | 32 | 1 | 51 |
| HUS | 41 | 4 | 29 | N.A. | 1 | 35 |
| Sepsis | 62 | 8 | 59 | 65 | 5 | 62 |
| Sepsis | 16 | 10 | N.A. | N.A. | 1 | N.A. |
| TTP 6 week follow-up | 17 | 3 | 8 | 13 | 107 | 47 |
| TTP 6 week follow-up | 53 | 18 | 32 | 49 | 10 | 54 |
| TTP 8 week follow-up | 34 | 0 | 18 | N.A. | N.A. | N.A. |
| TTP 4 week follow-up | 22 | 5 | 16 | 19 | 25 | 14 |
| TTP 1 week follow-up | 15 | 2 | 3 | N.A. | N.A. | 26 |
| TTP 2 week follow-up | 22 | 3 | 13 | 21 | 18 | 29 |
| TTP 3 week follow-up | 21 | 3 | 12 | 56 | 11 | 38 |
Examples of very low ADAMTS13 activity results as measured by AcuStar that could pose a challenge for the treating physician. All non-TTP cases had thrombocytopenia, and TTP follow-up samples were from patients in clinical remission.
N.A., sufficient residual sample not available.
We also tested 51 samples from patients with TTP undergoing monitoring during remission, and in 7 cases (14%), there was a discrepancy between AcuStar and FRET-VWF73 that would impact treatment decisions (e.g., further immunosuppression or continuation of caplacizumab) (Table 3).
3.3. Longitudinal analysis following plasma exchange
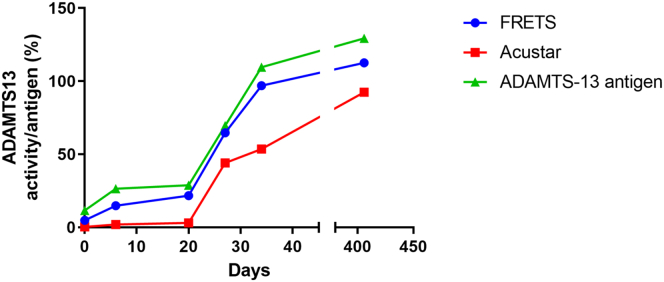
A longitudinal evaluation of 6 patients with iTTP post standard of care treatment further highlighted the apparent slow normalization of ADAMTS13 activity when measured by AcuStar, where on average 5 weeks after initial presentation of iTTP, the ADAMTS13 activity results remained very low at 5.3 IU/dL compared with 16.5 IU/dL with the ELISA method and 28.2 IU/dL with our FRETS-VWF73 method. All 6 of these patients were in clinical remission with a significant reduction in ADAMTS13 IgG antibodies and showed an ADAMTS13 antigen level (%) comparable to FRET-VWF73 activity levels throughout the recovery process (Table 3 and Supplementary Data Table S2).
Additionally, an illustration of ADAMTS13 activity results of AcuStar and FRETS over time to ADAMTS13 antigens levels is shown in Figure 3. ADAMTS13 antigen levels could prove useful in supporting ADAMTS13 activity results to illustrate full recovery in which activity levels were inconsistent with the clinical picture.
Figure 3.
Longitudinal ADAMTS13 activity as measured by FRETS-VWF73 (blue circles) or AcuStar (red squares) and ADAMTS13 antigen levels (green triangles) from initial presentation to follow up during remission in a patient.
3.4. Inhibitory antibodies
We hypothesized that following PEX and recovery of ADAMTS13 antigen levels, the enzymatic activity may be inhibited by persistent inhibitory autoantibodies, which could affect these assays differently. To test this experimentally, we spiked the monoclonal inhibitory antibody 3H9 [10] into normal control samples and tested the activity on 4 different platforms. On all 4 platforms, the activity for this sample in the absence of the inhibitory antibody was >100 IU/dL. However, spiking 5.2 μg/mL of the 3H9 antibody affected the results on AcuStar and the Technozym Activity ELISA (<5 IU/dL) to a much greater extent than the 2 FRETS-based assays (>39 IU/dL; Table 4). Increasing the concentration of 3H9 to 16.7 μg/mL further lowered the activity on all platforms, but a large discrepancy was maintained between the FRETS assays compared with AcuStar and Technozym Activity ELISA, showing that antibodies that inhibit ADAMTS13 activity affect the latter 2 assays more dramatically. However, a discrepancy caused by these inhibitory antibodies would be less likely in presentation samples, as ADAMTS13 antigen levels are typically very low and antibody levels very high (Table 2), leading to very low activity results in all assays.
Table 4.
Normal control (NC) plasma was spiked with the inhibitory antibody 3H9.
| Sample | FRETS-VWF73 (IU/dL) | AcuStar (IU/dL) | Technozym ELISA (IU/dL) | Ceveron (IU/dL) |
|---|---|---|---|---|
| NC (no 3H9) | 106.3 | 150 | 104 | 141 |
| NC-spiked 3H9 (5.2 μg/mL) | 39.4 | 3.8 | 1.7 | 39.9 |
| NC-spiked 3H9 (16.7 μg/mL) | 23.7 | 1.6 | 1.3 | 27.3 |
The monoclonal mouse antibody 3H9, raised against human ADAMTS13 binds to the active site of ADAMTS13 and inhibits its activity [10]. Here it was spiked into NC plasma at the indicated concentrations to experimentally test the hypothesis that inhibitory antibodies cause a discrepancy in activity results between the AcuStar and Technozym ELISA compared with FRETS-VWF73-based assays.
3.5. Monitoring of patients with TTP
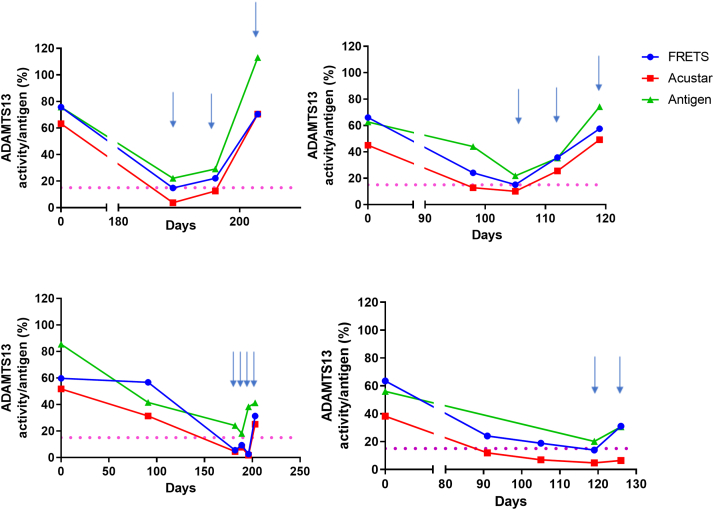
Another clinical scenario where ADAMTS13 activity levels are used to guide treatment is the prophylactic use of rituximab to prevent clinical relapse when ADAMTS13 activity has dropped to critically low levels. Figure 4 shows 4 patients treated with elective rituximab once their ADAMTS13 activity by FRETS-VWF73 was <15 IU/dL. It illustrates the differences between ADAMTS13 activity by FRETS-VWF73 and AcuStar and shows how they relate to ADAMTS13 antigen levels. All the elective treatment episodes show that AcuStar activity drops earlier than that of FRETS-VWF73, which could potentially alter the timing of prophylactic treatment with rituximab.
Figure 4.
Longitudinal ADAMTS13 activity measurements prior to elective Rituximab treatment as measured by FRETS-VWF73 (blue circles) or AcuStar (red squares). Light blue arrows indicate when a dose of Rituximab was given. Rituximab is usually offered when the ADAMTS13 activity as measured with FRETS-VWF73, is approaching or lower than 15IU/dL (horizontal pink dotted line). ADAMTS13 Antigen levels are also indicated (green triangles).
4. Discussion
Our study confirmed that AcuStar, Technozym Activity ELISA, FRETS-VWF73, and Ceveron all have comparable sensitivity when it comes to the diagnosis of TTP, with all samples of patients with acute TTP showing values <10 IU/dL in all 4 assays. Other studies comparing AcuStar and FRETS-VWF73 have all reported an acceptable level of correlation (Pearson’s r = 0.78-0.956) [11,12], not dissimilar to our result of 0.84, and the overall consensus is that AcuStar has excellent sensitivity in the diagnosis of acute TTP.
However, AcuStar showed values <10 IU/dL for 5 samples that were confirmed to have an alternative diagnosis based on analysis by FRETS and the clinical phenotype, accounting for 15% of the 32 presentation samples that were tested. The clinical implications of this are that a patient may be undergoing unnecessary and invasive treatments with plasma exchange until the confirmatory result is available.
Other scenarios in which ADAMTS13 activity is measured clinically include follow-up of patients with TTP after an acute TTP episode and longer-term follow-up. In 7 of 51 follow-up samples (14%), AcuStar showed much lower values to an extent that could impact on treatment. Other studies have also noted this discrepancy in some follow-up samples; however, details regarding the timing of the follow-up samples has not been available [[12], [13], [14]]. We found that this discrepancy was found in patients in clinical remission after an acute episode (defined as platelets >150 × 109/L, LDH <1.5 × ULN, no new/progressive ischemic injury [15]). During this time, key decisions are based on ADAMTS13 activity and the lower ADAMTS13 activity as measured by AcuStar may prompt clinicians to consider additional immunosuppression, which may not be needed and continuation of caplacizumab for longer, which has cost implications.
We investigated the potential causes for the lower results in the AcuStar assay. As AcuStar manufacturer’s instructions suggested that interference by VWF was tested only up to 200 IU/dL, we spiked normal plasma with Voncento, up to 600 IU/dL, which showed no interference (Supplementary Table S1A). Secondly, we established whether inhibitory antibodies could account for the lower results by spiking normal control plasma with the mouse monoclonal inhibitory antibody 3H9, which showed that this had a much bigger impact on AcuStar and Technozym ELISA assays than FRETS-based assays. We tested whether this could be because of the lower pH in the FRETS assay (pH 6) potentially minimizing the inhibitory effect in FRETS-VWF73, but increasing the pH to 7.6 did not reduce the ADAMTS13 activity result (data not shown). Favaloro et al. [13] have previously shown that AcuStar is more sensitive to inhibitory antibodies compared with Technozym ELISA but did not compare to FRETS-VWF73. The concentration of VWF73 (which competes with inhibitory antibody) in the AcuStar assay is not disclosed and could explain this discrepancy in theory. Although these different effects of inhibitory antibodies on AcuStar vs FRETS-VWF73 could potentially explain the discrepancies in TTP follow-up samples, they do not explain the aberrantly low results in non-TTP samples with the AcuStar. For these, further investigation of possible causes is necessary to clarify the limitations of the assay. The Ceveron platform uses assay conditions identical to FRETS-VWF73, explaining the high correlation in results. In contrast, AcuStar uses magnetic particles coated with an anti-GST-tag antibody to capture VWF73 fused to an N-terminal GST tag. ADAMTS13 cleaves GST-VWF73 that is captured on these magnetic particles, not in the solution. As the cleavage site is 9 amino acids from the N-terminus of VWF73, the proximity of the VWF cleavage site to anti-GST IgG and the magnetic particles could potentially cause steric hindrance when ADAMTS13 needs to interact with VWF73. This could potentially be aggravated by large bulky autoantibodies bound to the N-terminal domains of ADAMTS13 or a larger dependency on N-terminal exosites if ADAMTS13 is cleaved by proteases present in sepsis samples.
In our cohort, at least 3 of 5 false-positive results in the presentation diagnostic samples were in patients who had severe sepsis. Pascual et al. [14] also found that at least of one of their false positives had sepsis. The cause for this is unclear.
In general, we have found that the AcuStar underestimated ADAMTS13 activity compared with FRETS-VWF73 (Figure 2), similar to Pascual et al. [14] In contrast, Valsecchi et al. [11] found that after correction for samples with ADAMTS13 activity levels below the limit of quantitation of the FRETS-VWF73 assay, AcuStar overestimated ADAMTS13 activity, and Beranger et al.’s findings also supported this [12]. The overestimations in AcuStar compared with the FRETS-VWF73 method in these cases may be because of variations in the specific protocols for the test and the analysis of FRETS-VWF73 in local laboratories.
Much of the initial data on the performance of AcuStar has been obtained through retrospective studies. However, real-time assessment of this platform is now highlighting evidence of some clinically discordant results. For example, Beranger et al. [12] found that the level of correlation dropped in prospective samples (0.78) compared with retrospective samples (0.90).
There are some limitations to our study. For example, the sample size is not as large as other studies. Secondly, as one of the aims of the study was to investigate the scenarios where ADAMTS13 activity discrepancies occur and the possible causes, a degree of bias was unavoidable as we performed more selected testing (e.g., longitudinal testing on patients with discrepant results) after prospective testing. We did not have data on the race and/or ethnicity of all study participants.
Based on our findings, we would propose that centers utilizing AcuStar to measure ADAMTS13 activity should test via an alternative method (e.g., FRETS-VWF73 or Technozym ELISA) in any presenting patients in whom the clinical picture is atypical for TTP (e.g., severe sepsis). They should also consider an alternative testing method in cases in which ADAMTS13 activity is not recovering as expected after clinical remission to guide further treatment decisions and potentially avoid unnecessary intervention.
Acknowledgments
Funding
No funding was provided for the preparation of this article or for any part thereof.
Author contributions
D.S. designed experiments, analyzed data, and wrote the manuscript. M.O.S. analyzed data and wrote the manuscript. R.d.G. designed the experiments, analyzed data, and wrote the manuscript. A.Z. and B.D. performed the experiments. K.V. provided 3H9 mAB, analyzed data, and wrote the manuscript. M.S. designed experiments, analyzed data, and wrote the manuscript.
Relationship Disclosure
M.S. has received research funding from Takeda, Alexion, and Novartis and is a member of the advisory board at Takeda, Sanofi, and Alexion. She has received consultancy fees and speaker honoraria from Takeda, Sanofi, Alexion, Novartis, and Octapharma. The other authors have no conflicts of interest to report.
Informed patient consent
Retrospective TTP cases and normal controls included in the study were consented to the UK TTP registry.
Footnotes
Funding information No funding was provided for the preparation of this article or for any part thereof.
Handling Editor: Johnny Mahlangu
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100108
Contributor Information
Deepak Singh, Email: deepak.singh@tdlpathology.com.
Maryam Owais Subhan, Email: maryam.subhan@nhs.net.
Supporting Information
References
- 1.Zheng X.L., Vesely S.K., Cataland S.R., Coppo P., Geldziler B., Iorio A., et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18:2486–2495. doi: 10.1111/jth.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westwood J.-P., Thomas M., Alwan F., McDonald V., Benjamin S., Lester W.A., et al. Rituximab prophylaxis to prevent thrombotic thrombocytopenic purpura relapse: outcome and evaluation of dosing regimens. Blood Adv. 2017;1:1159–1166. doi: 10.1182/bloodadvances.2017008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hie M., Gay J., Galicier L., Provôt F., Presne C., Poullin P., et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124:204–210. doi: 10.1182/blood-2014-01-550244. [DOI] [PubMed] [Google Scholar]
- 4.Urgent field safety notice for HemosIL AcuStar ADAMTS13-activity assay by Instrumentation Laboratory Co., Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) https://www.bfarm.de/SharedDocs/Kundeninfos/EN/08/2022/04426-22_kundeninfo_en.html?nn=986784
- 5.Mackie I., Langley K., Chitolie A., Liesner R., Scully M., Machin S., Peyvandi F. Discrepancies between ADAMTS13 activity assays in patients with thrombotic microangiopathies. Thromb Haemost. 2013;109:488–496. doi: 10.1160/TH12-08-0565. [DOI] [PubMed] [Google Scholar]
- 6.Kokame K., Nobe Y., Kokubo Y., Okayama A., Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 7.Starke R., Machin S., Scully M., Purdy G., Mackie I. The clinical utility of ADAMTS13 activity, antigen and autoantibody assays in thrombotic thrombocytopenic purpura. Br J Haematol. 2007;136:649–655. doi: 10.1111/j.1365-2141.2006.06471.x. [DOI] [PubMed] [Google Scholar]
- 8.Alwan F., Vendramin C., Vanhoorelbeke K., Langley K., McDonald V., Austin S., et al. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2017;130:466–471. doi: 10.1182/blood-2016-12-758656. [DOI] [PubMed] [Google Scholar]
- 9.Dekimpe C., Roose E., Kangro K., Bonnez Q., Vandenbulcke A., Tellier E., et al. Determination of anti-ADAMTS-13 autoantibody titers in ELISA: Influence of ADAMTS-13 presentation and autoantibody detection. J Thromb Haemost. 2021;19:2248–2255. doi: 10.1111/jth.15297. [DOI] [PubMed] [Google Scholar]
- 10.Feys H.B., Roodt J., Vandeputte N., Pareyn I., Lamprecht S., van Rensburg W.J., et al. Thrombotic thrombocytopenic purpura directly linked with ADAMTS13 inhibition in the baboon (Papio ursinus) Blood. 2010;116:2005–2010. doi: 10.1182/blood-2010-04-280479. [DOI] [PubMed] [Google Scholar]
- 11.Valsecchi C., Mirabet M., Mancini I., Biganzoli M., Schiavone L., Faraudo S., et al. Evaluation of a new, rapid, fully automated assay for the measurement of ADAMTS13 activity. Thromb Haemost. 2019;119:1767–1772. doi: 10.1055/s-0039-1696718. [DOI] [PubMed] [Google Scholar]
- 12.Beranger N., Benghezal S., Joly B.S., Capdenat S., Delton A., Stepanian A., et al. Diagnosis and follow-up of thrombotic thrombocytopenic purpura with an automated chemiluminescent ADAMTS13 activity immunoassay. Res Pract Thromb Haemost. 2021;5:81–93. doi: 10.1002/rth2.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favaloro E.J., Mohammed S., Chapman K., Swanepoel P., Zebeljan D., Sefhore O., et al. A multicenter laboratory assessment of a new automated chemiluminescent assay for ADAMTS13 activity. J Thromb Haemost. 2021;19:417–428. doi: 10.1111/jth.15157. [DOI] [PubMed] [Google Scholar]
- 14.Pascual C., Nieto J.M., Fidalgo T., Seguí I.G., Díaz-Ricart M., Docampo M.F., et al. Multicentric evaluation of the new HemosIL Acustar® chemiluminescence ADAMTS13 activity assay. Int J Lab Hematol. 2021;43:485–493. doi: 10.1111/ijlh.13414. [DOI] [PubMed] [Google Scholar]
- 15.Cuker A., Cataland S.R., Coppo P., de la Rubia J., Friedman K.D., George J.N., et al. Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021;137:1855–1861. doi: 10.1182/blood.2020009150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.