Abstract
Background
Avatrombopag is an orally administered second-generation thrombopoietin receptor agonist (TPO-RA) approved for the treatment of chronic immune thrombocytopenia (ITP). However, increased thrombogenicity in patients with ITP after initiation of TPO-RA treatment has been reported.
Key Clinical Question
We report a case of a patient with ITP who developed a catastrophic antiphospholipid antibody syndrome (CAPS), following treatment with avatrombopag.
Clinical Approach
A20-year-old known chronic patient with ITP presented at the emergency department with a 2-week history of headache, nausea, and abdominal pain, 3 weeks after initiating avatrombopag. In-hospital diagnostic work-up revealed multiple microvascular thrombotic events, including myocardial, cerebrovascular, and pulmonary infarctions. Laboratory test results showed a triple-positive antiphospholipid antibodies serology.
Conclusion
The diagnosis of probable avatrombopag-associated CAPS was made.
Essentials
-
•
Antiphospholipid antibodies (APLAs) are prevalent in immune thrombocytopenia (ITP).
-
•
Thromboembolism has been reported after initiation of thrombopoietin receptor agonists (TPO-RA).
-
•
APLA testing in a patient with ITP is of potential utility before start of TPO-RA.
-
•
Patients with ITP and (triple) positive APLAs should be monitored closely for thromboembolic events.
1. Case Presenttaion
A 20-year-old man presented at the emergency department with a 2-week history of fatigue, headache, nausea, vomiting, and abdominal pain. At the age of 9, he had been diagnosed with primary immune thrombocytopenia (ITP), in a setting of purpura and low platelet count. Treatment with high-dose corticosteroids and intravenous immunoglobins (IVIG) did not result in long-term responses and led to the development of chronic ITP, not requiring therapy for the last 10 years. Three weeks before admission, treatment with the thrombopoietin receptor agonist (TPO-RA) avatrombopag, 20 mg daily was initiated at a platelet count of 29.000/μL in the presence of subtle bruising. The patient was a nonsmoker and did not use drugs. He had no arterial hypertension. His family history was negative for arterial or venous thromboembolic events.
Upon admission, the patient was in respiratory distress, receiving 10 L of oxygen per minute via a face mask. Lung auscultation revealed bilateral crackles. The abdomen was soft but tender on palpation. Heart auscultation was normal without signs of congestion or bleeding tendency. Neurologic examination remained normal. Blood analyses showed normalization of the platelet count (213.000/μL) with normal hemoglobin and white blood cell count. Prothrombin time and activated partial thromboplastin time (APTT) were markedly prolonged at 27.3 seconds (reference range, 9.4-12.5 seconds) and 88.1 seconds (reference range, 25.1-36.6seconds), respectively. There was no diffuse intravascular coagulopathy (high fibrinogen levels 9.05 g/L and no schistocytes on peripheral blood smear). Troponin T levels (2137 ng/L) and C-reactive protein (276 mg/L) were elevated.
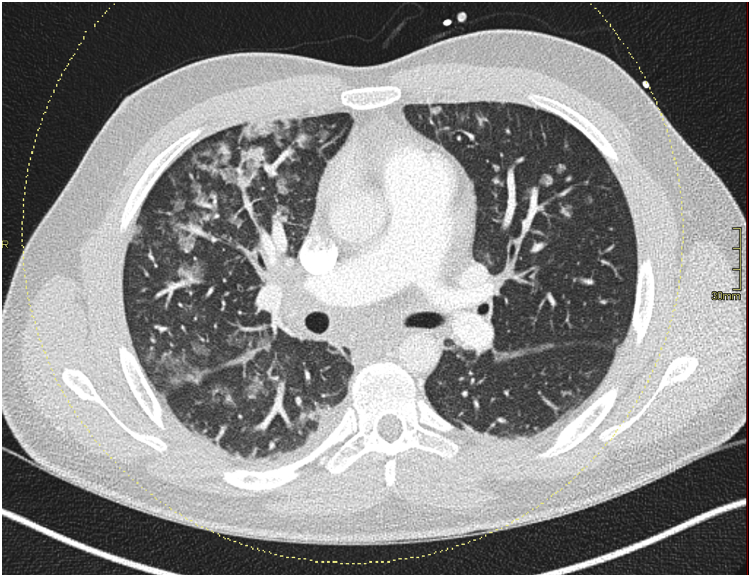
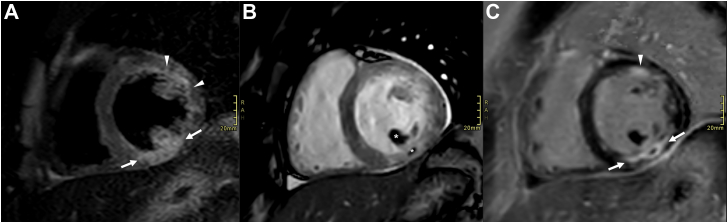
Thoracic computed tomography showed diffuse ground-glass opacities (Figure 1). Lateral ST depressions were noted on electrocardiogram, in the absence of any cardiovascular risk factor. Transthoracic echocardiography confirmed hypokinesia in the inferolateral segments with a mildly reduced left ventricular ejection fraction (47%). Coronary angiography showed no signs of coronary artery disease. Endomyocardial biopsy could not find any signs of myocarditis. Cardiac magnetic resonance imaging showed transmural late gadolinium enhancement in the inferior and anterolateral segments with areas of no-reflow, compatible with acute transmural myocardial infarction (Figure 2).
Figure 1.
Dual-energy contrast-enhanced thoracic computed tomography shows multifocal peribronchovascular ground-glass opacities (right>left) without interlobular septal thickening, possibly corresponding to pulmonary microthrombi with alveolar hemorrhage.
Figure 2.
Cardiac magnetic resonance imaging. Short-axis plane of the basal left ventricular segments. A = short tau inversion recovery (STIR) sequence before intravenous gadolinium administration. B = cine sequence and C = inversion recovery sequence after intravenous gadolinium administration. Images show a multifocal acute myocardial infarction, transmural in the basal inferior wall (arrows in A and C) and near transmural in the basal anterolateral wall (arrowheads in A and C). Subendocardial microvascular obstruction in the basal inferior wall and inferior papillary muscle (asterisks in B).
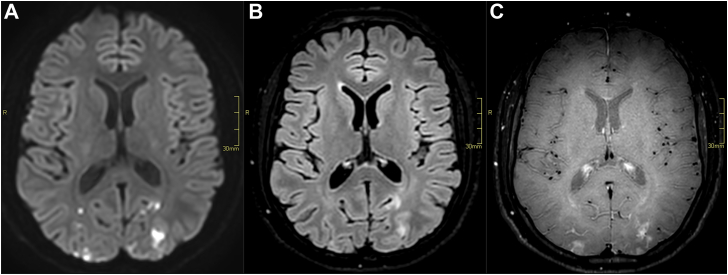
During hospitalization, the patient experienced binocular blurred vision. Subsequent brain magnetic resonance imaging showed multiple acute subcortical infarctions in the parietal, occipital, and frontal lobes, indicated by diffusion-weighted images (Figure 3).
Figure 3.
Brain magnetic resonance imaging. A = diffusion-weighted sequence (b-value = 1000 s/mm2). B = fluid-attenuated inversion recovery (FLAIR) sequence. C = black blood T1 weighted sequence after intravenous gadolinium administration. Images show multiple cortical and subcortical, FLAIR hyperintense, enhancing lesions with diffusion restriction in the occipital lobes, in keeping with multifocal acute infarctions.
Further work-up for multiple organ microvascular thrombosis revealed a strongly positive lupus anticoagulant (LA) and very high titers of anticardiolipin immunoglobulin G (IgG) 895 U/mL (reference range: <20 U/mL) and β2-glycoprotein I IgG >6100 U/mL (reference range: <20 U/mL). The diagnosis of probable catastrophic antiphospholipid antibody syndrome (CAPS) was made on the basis of these laboratory results and the thrombotic involvement of at least 3 end organs. Retrospectively, the patient had high-risk antiphospholipid antibody (APLA) positive serology at the age of 9 (positive LA and anticardiolipin IgG 135 U/mL). Nevertheless, no thromboembolic events had occurred so far.
Treatment with heparin, overlapped and followed by a vitamin K antagonist, was immediately started with a target international normalized ratio of 3-4, while the therapy with avatrombopag was interrupted. IVIGs and high-dose IV corticosteroids were added and administered over 3 days. The patient promptly improved and fully recovered within days. His platelet count remained within the reference levels of 200.000/μL to 400.00/μL. In the past, our patient had rapid responses to IVIGs and corticosteroids, only no durable response.
Two months later, the platelet count dropped to 13.000/μL with limited bruising after trauma, while fully anticoagulated. His APLA serology remained positive (LA strongly positive, anticardiolipin IgG 2536 U/mL and β2-glycoprotein I IgG >6100 U/mL). Avatrombopag was successfully reinitiated, maintaining platelets between 30.000/μL and 50.000/μL without venous or arterial thrombosis recurrence.
2. Discussion
Antiphospholipid syndrome (APS) is an acquired autoimmune disorder characterized by vascular thromboses and/or pregnancy loss associated with persistently positive APLA [1]. APLA is a heterogeneous group of antibodies against phospholipid-binding proteins, with anticardiolipin antibodies, β2-glycoprotein I antibodies, and LA being the diagnostic markers [2,3]. APLA activation leads to upregulation of endothelial cell adhesion molecules, activation of complement and platelets, stimulation of monocytes and neutrophils to secrete extracellular traps, and release of interleukin-8 and tissue factor, all resulting in vasculopathy of both arterial and venous thrombosis [3]. Determining the APLA profile has diagnostic and therapeutic implications and helps in risk stratification. A high-risk profile is defined as a positive LA with or without a moderate to high titer of anticardiolipin antibodies or β2-glycoprotein I antibodies [3].
According to the revised Sapporo criteria, definite APS is present if at least one of the clinical criteria and one of the laboratory criteria are met [4]. These criteria do not incorporate the full spectrum of non-thrombotic clinical findings. APS is often associated with mild thrombocytopenia with an incidence ranging from 20% to 40% [2,5]. The pathophysiology of thrombocytopenia can be explained by the binding of antiphospholipid antibodies to circulating platelets, initiating a consumption coagulopathy as well as immune-mediated destruction of platelets [6]. Normally, thrombocytopenia causes an increased bleeding risk. However, in APLA-positive patients, thrombocytopenia is a potential risk factor for thrombosis, due to continuous stimulation, activation, and aggregation of platelets [7].
ITP is an acquired autoimmune disorder characterized by a low platelet count resulting from impaired megakaryocyte production and platelet destruction. Autoantibodies in ITP generally recognize platelet membrane glycoproteins [2]. ITP is classified as primary when idiopathic and secondary when occurring with an associated underlying disorder. Although mucocutaneous bleeding is the main symptom of ITP, there is increasing epidemiologic evidence suggesting a higher risk of venous thromboembolism [8]. The mechanism of thrombosis is multifactorial. Furthermore, the disease-related factors include increased levels of circulating immature hyperactive platelets and prothrombotic microparticles as well as the activation of endothelial surfaces by autoantibodies, leading to greater von Willebrand factor exposure [8].
Our patient had severe thrombocytopenia since the age of 9, diagnosed as primary ITP. There were no clinical signs of an underlying rheumatological autoimmune disorder and antinuclear antibodies were negative. APLA was documented at diagnosis, but at that time he did not fulfill the revised Sapporo criteria for APS. Hence, according to the guidelines, this patient was diagnosed with primary ITP [9]. However, his high-risk APLA profile might suggest the diagnosis of a secondary ITP caused by APLA, although no clinically overt thromboembolic events had occurred in his past medical history. At the time of his thrombotic event, the diagnosis of primary ITP was subsequently reconsidered as secondary ITP.
APLAs are prevalent in ITP, but only very few data are available on their clinical relevance. In the absence of population-based studies, little is known about APLA prevalence and its transient expression in healthy individuals [2]. Given the broad heterogeneity of APLA, not all APLA are pathogenic. APLA in ITP seems to have qualitative differences from APLA in APS. In APS, antibodies against β2-glycoprotein I predominate, in combination with a positive LA. In ITP, antibodies mainly target phospholipids and are less associated with LA [10]. This different APLA profile might explain the opposite clinical manifestation of APS and ITP. The presence of APLA does not seem to influence the treatment outcome in ITP. In addition, APLA levels do not change significantly with immunosuppressive therapy, suggesting that these antibodies do not play a major role in the pathophysiology of thrombocytopenia [11].
Furthermore, its thromboembolic risk remains controversial. There is increasing data suggesting a higher risk of venous thromboembolism and developing APS, especially in patients with persistent LA positivit [5]. However, there is insufficient data to establish these results for other APLAs. The thromboembolic risk in patients with ITP still remains multifactorial and the correction of cardiovascular risk factors is primordial. Further prospective studies are required.
CAPS is the most severe and rare (<1%) form of APS requiring prompt recognition and management due to its life-threatening nature. Definite CAPS is defined as arterial and/or venous thromboembolism in at least 3 organs, developing in less than a week, histopathological confirmation of microthrombosis in at least 1 organ, and persistent APLA positivity [12]. As our patient had no histopathologic proof of microthrombosis (although we should keep sampling error of the non-affected right ventricle into account), he was diagnosed with probable CAPS.
Avatrombopag is a TPO-RA used as second-line therapy in patients with ITP who are unresponsive or had a relapse after initial corticosteroid treatment. TPO-RA activates the TPO-R c-MPL, a glycoprotein that induces megakaryocytic maturation and platelet production leading to an increased platelet count [13]. Patients treated with TPO-RA have response rates of 59%-88% [14]. Despite its pathophysiological effects, several studies, including the pivotal phase 2 and 3 clinical trials of TPO-RA, did not find a statistically significant relation between thrombosis and TPO-RA use [8,13]. Thrombogenicity and even the triggering of CAPS in APLA-positive patients by TPO-RA treatment with romiplostim and eltrombopag have been reported [11,15]. Our case suggests that this can also occur with avatrombopag, as no other risk factors for CAPS were identified.
Our patient was initiated on avatrombopag 20mg 3 weeks before admission at a platelet count of 29.000/μL without bleeding signs or other symptoms. Subsequently, his platelet count significantly increased to 213.000/μL. This TPO-RA-induced raise in platelet count might promote cross-reaction between APLA and platelet-specific antigens, resulting in platelet activation and aggregation [10]. APLA binds platelet membrane anionic phospholipids, usually hidden in the inner membrane but exposed after platelet activation [16]. This can explain why the patient only developed thrombosis after stimulation and activation of platelets by TPO-RA. In our patient, avatrombopag was successfully reinitiated at a lower dose 2 months after hospitalization, in combination with full anticoagulation therapy. Maintaining his platelet count between 30.000/μL and 50.000/μL, causing less antibody cross-reaction and subsequently less platelet activation.
According to the results of the CAPS registry, clinical recovery was associated with anticoagulation, forming the cornerstone of CAPS treatment. Although immunomodulatory agents can help control the APS non-criteria manifestations, the currently available literature is limited to case reports and small series [17].
3. Conclusion
Here, we want to illustrate the potential risk of developing life-threatening thrombosis in patients with APLA.
Primary ITP remains a diagnosis of exclusion. The management of thrombocytopenia depends on the underlying disease, and no treatment is needed for patients with platelets >30.000/μL in the absence of bleeding symptoms.
Current ITP guidelines suggest that the presence of APLA has no impact on clinical management and do not routinely recommend APLA testing in the absence of APS symptoms [9]. Even when APLA is detected in a patient with ITP without a history of thrombosis or obstetric complications, it will not change the diagnosis of primary ITP or the recommended treatment [9]. However, APLA testing is of potential utility, when there is a concern for thrombosis or an underlying autoimmune disorder. In the absence of these risk factors, the initiation of TPO-RA in our patient was a justifiable option. However, taking the high-risk triple APLA profile into account, the patient should have been monitored closely for persistent positive LA and thromboembolic events. In retrospect, the prolonged APTT at diagnosis of primary ITP and before initiation of TPO-RA was a warning for the presence of LA, a major risk factor for thrombosis in APS. For this reason, we advise adding standard screening coagulation tests before starting treatment with TPO-RA and consider testing for APLA when the APTT is prolonged or when APS is suspected. The persistent presence of APLA may identify a subgroup of patients with ITP at risk of thromboembolism. Close monitoring of thrombotic events in these patients after TPO-RA initiation should be recommended.
The causality between avatrombopag and the occurrence of CAPS in the absence of any other risk factor is unknown, but physicians should be aware of this possibility. Using TPO-RA in APLA-positive patients with ITP is not contraindicated, especially with a low or moderate thrombotic risk APLA profile. However, in high-risk patients, we advise close monitoring. The goal of therapy in this specific setting is even more to maintain hemostatic platelet levels and to prevent bleeding with minimal toxicity rather than platelet normalization. Our patient was successfully retreated with avatrombopag, without recurrence of venous or arterial thrombosis so far.
Acknowledgments
Funding
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
S.V.de.-V. drafted the manuscript and conducted a review the literature. C.V., P.V., and A.J. performed a critical revision of the manuscript. G.G. contributed to figures and figure legends. All authors read and approved the final paper.
Relationship Disclosure
S.V.de.-V., C.V., and P.V. declare no conflicts of interest. A.J. receives and participates in Abbvie: speakers bureau, travel grants; Amgen: speakers bureau, travel grants; Astra Zeneca: consultancy, speakers bureau; Beigene: speakers bureau, travel grants; Janssen: consultancy, speakers bureau; Incyte: speaker board; Novartis: consultancy, speaker board; Roche: consultancy, travel grants; Sanofi Genzyme: consultancy, speaker board; Sobi: speaker board, and Takeda: consultancy.
References
- 1.Chaturvedi S., McCrae K.R. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017;31:406–417. doi: 10.1016/j.blre.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomasello R., Giordano G., Romano F., Vaccarino F., Siragusa S., Lucchesi A., et al. Immune thrombocytopenia in antiphospholipid syndrome: is it primary or secondary? Biomedicines. 2021;9:1–12. doi: 10.3390/biomedicines9091170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia D., Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378:2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 4.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 5.Diz-Kucukkaya R., Hacihanefioglu A., Yenerel M., Turgut M., Keskin H., Nalçaci M., et al. Antiphospholipid antibodies and antiphospholipid syndrome in patients presenting with immune thrombocytopenic purpura: a prospective cohort study. Blood. 2001;98:1760–1764. doi: 10.1182/blood.v98.6.1760. [DOI] [PubMed] [Google Scholar]
- 6.Uthman I., Godeau B., Taher A., Khamashta M. The hematologic manifestations of the antiphospholipid syndrome. Blood Rev. 2008;22:187–194. doi: 10.1016/j.blre.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Demetrio Pablo R., Munoz P., López-Hoyos M., Calvo V., Riancho L., Martínez-Taboada V.M. Thrombocytopenia as a thrombotic risk factor in patients with antiphospholipid antibodies without disease criteria. Med Clin (Barc) 2017;148:394–400. doi: 10.1016/j.medcli.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Swan D., Newland A., Rodeghiero F., et al. Thrombosis in immune thrombocytopenia - current status and future perspectives. Br J Haematol. 2021;194:822–834. doi: 10.1111/bjh.17390. [DOI] [PubMed] [Google Scholar]
- 9.Provan D., Arnold D.M., Bussel J.B., Chong B.H., Cooper N., Gernsheimer T., et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidot C., Jy W., Horstman L.L., Ahn E.R., Yaniz M., Ahn Y.S., et al. Antiphospholipid antibodies (APLA) in immune thrombocytopenic purpura (ITP) and antiphospholipid syndrome (APS) Am J Hematol. 2006;81:391–396. doi: 10.1002/ajh.20571. [DOI] [PubMed] [Google Scholar]
- 11.Dichtwald S., Meyer A., Ifrach N. Catastrophic anti-phopholipid syndrome with Libman-Sacks endocarditis following eltrombopag therapy for immune thrombocytopenic purpura: a case report. Lupus. 2021;30:2304–2309. doi: 10.1177/09612033211065140. [DOI] [PubMed] [Google Scholar]
- 12.Kalmanti L., Lindhoff-Last E. Treatment of vascular thrombosis in antiphospholipid syndrome: an update. Hamostaseologie. 2020;40:31–37. doi: 10.1055/s-0040-1701473. [DOI] [PubMed] [Google Scholar]
- 13.Tjepkema M., Amini S., Schipperus M. Risk of thrombosis with thrombopoietin receptor agonists for ITP patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022;171 doi: 10.1016/j.critrevonc.2022.103581. [DOI] [PubMed] [Google Scholar]
- 14.Ghanima W., Cooper N., Rodeghiero F., Godeau B., Bussel J.B., et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112–1123. doi: 10.3324/haematol.2018.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMoreaux B., Barbar-Smiley F., Ardoin S., Madhoun H. Two cases of thrombosis in patients with antiphospholipid antibodies during treatment of immune thrombocytopenia with romiplostim, a thrombopoietin receptor agonist. Semin Arthritis Rheum. 2016;45:e10–e12. doi: 10.1016/j.semarthrit.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Stasi R., Stipa E., Masi M., Oliva F., Sciarra A., Perrotti A., et al. Prevalence and clinical significance of elevated antiphospholipid antibodies in patients with idiopathic thrombocytopenic purpura. Blood. 1994;84:4203–4208. [PubMed] [Google Scholar]
- 17.Cervera R., Bucciarelli S., Espinosa G., Gomez-Puerta J.A., Font J., et al. Catastrophic antiphospholipid syndrome: lessons from the ‘CAPS Registry. Autoimmun Rev. 2006;6:81–84. doi: 10.1016/j.autrev.2006.06.009. [DOI] [PubMed] [Google Scholar]