Abstract
A state of the art lecture titled “Megakaryocytes in the Lung” was presented at the London International Society on Thrombosis and Haemostasis congress in 2022. This lecture highlighted that although most medical teaching presents platelets as bone marrow megakaryocyte-derived cellular mediators of thrombosis, platelets are also a critical part of the immune system with direct roles in responses to sterile tissue injury and pathogens. Bone marrow megakaryocytes differentiate from hematopoietic stem cells and package platelets with immune molecules. Activated platelets, therefore, initiate or accelerate the progression of vascular inflammatory pathologies, as well as being regulators of immune responses to infectious agents. Platelets are now known to have mechanistic roles in immune responses to disease processes, such as heart transplant rejection, myocardial infarction, aortic aneurysm, peripheral vascular disease, and infections. From these studies comes the concept that megakaryocytes are immune cell progenitors and recent emerging information highlights that megakaryocytes may themselves be immune cells. Despite megakaryocytes being described in the lung for >100 years, lung megakaryocytes have only recently been shown to be platelet producing and lung megakaryocytes are immune-differentiated in both phenotype and function. What is still not known is the origin of lung megakaryocytes and roles of lung megakaryocytes in health and disease. This review will discuss the long history of lung megakaryocytes in the literature and potential models for megakaryocyte origins and immune functions. Finally, we summarize relevant new data related to this topic that was presented during the 2022 International Society on Thrombosis and Haemostasis Congress.
Keywords: megakaryocyte, inflammation, platelet, lung, bone marrow
1. Lung Megakaryocytes—a History
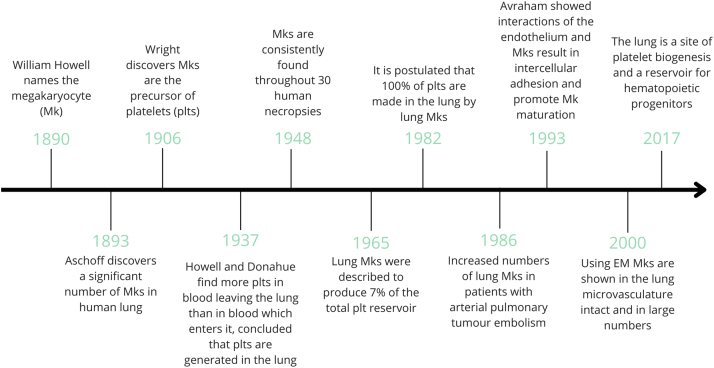
Megakaryocytes are platelet progenitors with central roles in hemostasis and thrombosis. In the last 20 years, a growing body of literature has shown that platelets and megakaryocytes also have critical roles in the immune system. Megakaryocytes are classically thought of as the largest hematopoietic cell in the bone marrow (BM). Although this is true, there are extensive publications documenting megakaryocytes also residing outside the BM with variable ploidy (Figure 1). In 1890, Howell [1] first named the megakaryocyte. Shortly thereafter in 1906, Wright [2] observed megakaryocytes producing platelets by forming long, arm-like, protrusions from the BM into the vasculature, with platelets budding off into the bloodstream. Over the last century, megakaryocytes have been found in human lungs, liver, spleen, peripheral blood, umbilical cord blood, yolk sac, and fetal liver [[3], [4], [5]]. After his initial characterization of megakaryocytes, Howell and Donahue [6] also began studying megakaryocytes outside of the BM, including the lung, where they described: “histological examination of the lungs with a technique adequate to give a differential staining of platelet material demonstrates the presence of giant cells in the lungs and supports the view that they are active in the production of platelets.” In 1911, lung megakaryocytes were described in Hodgkin’s disease, and in 1931, Medlar and Sasano [7] noted increased megakaryocytes in the lung during active tuberculosis infections. Aschoff [3] originally posited that megakaryocytes migrate to different tissues under chemotactic stimuli and proposed that lung megakaryocytes originate from the BM via migration through the blood. These findings began a century-long debate about megakaryocytes in the lung (summarized in Figure 1), including whether they produce circulating platelets and whether they are tissue resident or migrate from the BM.
Figure 1.
Megakaryocyte historical timeline. A timeline of significant contributions to understanding lung Megakaryocyte biology since the first description of Megakaryocytes in1890 through the Lefrancais et al. [16] study of Megakaryocytes in 2017.
Several studies have described compelling evidence indicating that megakaryocytes are present in multiple tissues, including the lung, and that lung megakaryocyte numbers increase during inflammation and injury [[7], [8], [9], [10]]. Kaufman et al. [11] performed studies on canines that had undergone thoracotomies in which one group had the right lung receive venous blood and the left lung received arterial blood. The other group of dogs received a mixture of arterial and venous blood in their left lung, whereas their right lung was perfused with venous blood only [11]. These intricate studies found that megakaryocytes were present in the lung without venous blood flow and found an increase in megakaryocyte number when venous blood flowed through the lung [11].
Although lung megakaryocytes were described more than a century ago, their study has been limited and often contentious. Soon after the discovery of lung megakaryocytes, Howell and Donahue [6] described that megakaryocytes in the lung had a different appearance than megakaryocytes found in the BM or the spleen, which might factor into the debate about lung megakaryocytes’ source and function. They observed that lung megakaryocytes’ nuclei did not have a “crown or basket” appearance and that lung megakaryocytes rarely were giant cells with a centrally located nucleus. Instead, Howell and Donahue [6] described the nucleus to be in an elongated “Y” shape. They noted that there were megakaryocytes with significantly smaller nuclei, but argued that the lung megakaryocytes had a variety of shapes and sizes and many of the megakaryocytes had abundant cytoplasm [7]. Another influential component of this study by Howell and Donahue [6] discovered the production of platelets in the lung by showing that arterial blood contained more platelets than venous blood, concluding that platelets must be generated in the lung. Their findings showed that in relation to erythrocytes, the platelet count was higher in the arteries than in the veins, suggesting platelet production in the lung [6]. Their histologic examination of the lungs demonstrated the presence of megakaryocytes and found platelets within the lung both alone and in groups so numerous that they were difficult to miss or mistake for another cell [6].
Howell and Donahue [6] postulated that megakaryocytes originated in the lung because they found numerous megakaryocytes present in all normal lung tissue sections, not just in capillaries, suggesting that megakaryocytes do not solely migrate from the BM and become trapped in the lung vasculature. In their arguments, they pointed to a lack of data to support a BM migration theory and added that the lung capillaries are unlikely to allow “giant cells” to become “trapped” for extended periods of time. Beyond these seminal studies, others noted intrapulmonary megakaryocytes consistently across 30 human necropsies, with a density of 14 to 65 megakaryocytes/cm2 in lung [12], with the highest concentration found in the central zone of the right upper lung lobe [8,11]. Several studies observed an increase in megakaryocyte density (37 megakaryocytes/cm2) and significantly higher numbers of megakaryocytes, in the lungs from disease necropsies as compared with forensic necropsies (typically traumatic, 4 megakaryocytes/cm2) [8]. In those with cardiovascular or respiratory disease, the megakaryocyte count was also significantly increased [8,11]. It was hypothesized that the reason for the fluctuation in megakaryocyte numbers during hospital stays might be because of infection [9,10], leukocytosis [10], hemorrhage [10], postoperative states [13,14], shock [10], or acute respiratory distress syndrome [15], which stimulated the production of lung megakaryocytes. A study by Brill and Halpern [12] investigated the frequency of megakaryocytes in autopsies and found that lung megakaryocytes were present throughout all their human samples; however, they could not replicate this finding for any other organ they dissected, including spleen, liver, kidney, and heart. These data strongly indicated that megakaryocytes exist in the lung constitutively and that their numbers increase during disease or illness.
Other investigators have sought further information about platelet production in the lung and its relative contribution to circulating platelet numbers. As of today, there is no consensus on the percentage of circulating platelets that come from the lung, with calculations ranging between 7% and 50% of circulating platelets being lung-derived [11,16]. Howell and Donahue [6] argued that the lung was the “main source” of platelet production. Kaufman et al. [11] estimated a range of 20% to 50% of the platelet pool deriving from the lung. Studies in the early 1980s even postulated that all platelet production came from the lung, where investigators applied the mathematical description of fragmentation mechanism that leads to a log-normal volume distribution of platelet production from megakaryocyte cytoplasm [17,18]. In 2000, megakaryocytes were shown by electron microscopy (EM) in the lung microvasculature and were present in large numbers of intact, fragmented, and denuded megakaryocytes [19]. Lung megakaryocytes were found to have numerous demarcated platelet membranes and when mice were treated with thrombopoietin (TPO), a significant increase in platelets was found in the peripheral blood [19]. EM images from this study showed significant platelet release from megakaryocytes in the lung and the platelets released did not show signs of activation because they maintained a uniform shape and an even distribution of their granules [19]. Studies in 20 humans undergoing routine cardiac catheterizations showed that megakaryocytes in the lung had a full cytoplasm with demarcation membranes, granules, and mature nuclei [20], leading investigators to conclude that lung megakaryocytes were mature with the capacity to produce platelets.
In 2017, a pivotal article reinvigorated the century-old debate about platelet production in the lungs. The article by Lefrançais et al. [16] used several state of the art techniques to demonstrate that the lung is a significant site of platelet biogenesis and that lung progenitor cells can reconstitute platelet counts in thrombocytopenic mice. Lefrançais et al. [16] used intravital imaging to directly show that lung megakaryocytes contribute to platelet biogenesis, while also showing that the lung extravascular space is a reservoir for hematopoietic progenitors. Based on imaging, they estimated that lung megakaryocytes produced approximately 50% of platelets in the circulation [16]. The authors showed that with the addition of TPO, there was an increase in peripheral platelet counts, and an increase in lung megakaryocytes and proplatelet complexes, seen by intravital imaging [16] that was corroborated with EM [19]. In addition, this article characterized 2 populations of megakaryocytes: intravascular and extravascular. They provided evidence that intravascular megakaryocytes are responsible for the proplatelet release within the lung, whereas the extravascular population was termed sessile and accounted for up to 80% of the lung megakaryocyte population [16]. Importantly, the function of the extravascular megakaryocyte population was not characterized. Using RNA-sequencing of isolated BM and lung megakaryocytes, the authors found that megakaryocytes in the lungs express higher levels of cytokines, growth factors, and other host defense genes, which could contribute to inflammation during lung disease [16].
2. Current State of Lung Megakaryocyte Research and Future Directions
Despite a long history of studies describing megakaryocytes in the lung, tissue-specific megakaryocyte biology remains relatively unstudied. We now summarize critical concepts that are unknown or controversial in the lung megakaryocyte literature, including a focus on their platelet-producing capacity, their developmental origin, and immune-modulatory functions in health and disease.
2.1. Platelet production
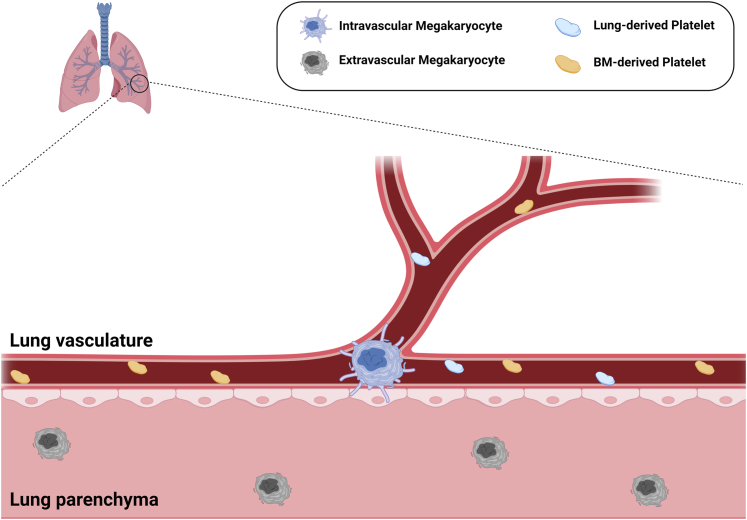
As described above, the study of lung-derived platelet production dates back almost a century. The literature describes a wide range of estimates for the lung-derived proportion of the platelet pool (7%-50%), making the overall contribution of lung megakaryocytes to the circulating platelet pool somewhat controversial. At present, there are no reliable biomarkers for lung-derived platelets, so any estimation of lung platelet biogenesis in physiologic conditions must rely on indirect evidence. Early evidence supporting lung-derived platelet production was based on higher arterial (postpulmonary) than venous (prepulmonary) platelet counts [6]. These studies were limited by the need for surgical intervention and an indirect inference in an anesthetized animal. More recently, intravital imaging was used to estimate the lung megakaryocyte contribution to the platelet pool [16]. This estimation was based on the imaging of lung megakaryocyte fragmentation under ventilation and quantifying platelets produced over a 2-hour period. These data were then extrapolated from the lung volume observed during the imaging, to the entire lung volume. There are limitations to this method of estimation. Lung megakaryocytes tend to localize to the distal lung alveolar interstitium [21], meaning that scaling up from a small imaging window to the entire lung and assuming equal megakaryocyte distribution over the entire lung, may overestimate the lung megakaryocyte contribution. In addition, it has been suggested that intravascular megakaryocytes produce platelets, so any imaging and extrapolation must account for a calculation of only the number of intravascular megakaryocytes because they make up only approximately 20% to 30% of the lung megakaryocyte pool [16,22] and the distribution of extravascular vs intravascular megakaryocytes may not be evenly distributed across the entire lung. In Figure 2, we summarize the current model that intravascular megakaryocytes produce platelets and that the extravascular megakaryocyte contribution to the platelet pool is unknown. An important question remaining is whether extravascular megakaryocytes migrate to become intravascular platelet-producing megakaryocytes. Further study using techniques that permit more physiologic conditions are required to fully understand the platelet biogenesis landscape in the lung to account for the unequal distribution and potential phenotypic heterogeneity of megakaryocytes throughout the lung.
Figure 2.
Current model of Megakaryocyte and platelet subpopulations. Intravascular lung megakaryocytes with pro-platelet protrusions produce platelets that enter the bloodstream along with bone marrow-derived platelets. Extravascular megakaryocytes have a smaller, more sessile morphology and their function has not been characterized. No direct comparisons of BM and lung derived platelets have been performed and there are no available biomarkers specific for lung platelets.
A recent study identified a spleen megakaryocyte-derived CD40Lhi platelet “subpopulation” in a murine model of sepsis [23]. Valet et al. [23] found that the CD40Lhi platelets from the spleen were increased in sepsis and had a protective effect by improving sepsis immune responses. This work highlighted the potential of identifying candidate markers that specify platelet tissue origin, as well as demonstrated the significance of considering platelet tissue origin in models of infectious/immunologic, cardiovascular, and pulmonary diseases in future studies. A challenge, however, is the specificity of the marker and assuming it is uniform across diseases. CD40Lhi platelets may not be spleen specific in another disease context, adding complexity to assuming platelet tissue origin based on shared platelet marker expression.
The identification of biomarkers for platelet tissue source, including lung, BM, and spleen, may lead to important insights for understanding sites of platelet production and platelet heterogeneity across tissue origins. At present, there are no markers to distinguish lung platelets from other tissue sources (shown in Figure 2). A biomarker for lung-derived platelets may provide direct evidence of the contribution of lung megakaryocytes to the total platelet pool and clarify this long-standing debate. More importantly, a lung platelet biomarker may create a means to study platelet pool heterogeneity in health and disease. Ideally, any new biomarkers for platelet tissue source will be translatable to humans in both health and disease and may serve as tools to modify platelet function in thrombosis or coordinated immune responses.
2.2. Developmental origin
BM megakaryocytes arise from a defined hierarchy of cells originating from long-term hematopoietic stem cells (HSCs) [[24], [25], [26], [27]]. Interestingly, there is mounting evidence of a megakaryocyte-biased HSC pathway where a subset of HSCs can bypass progenitor cells and differentiate directly to megakaryocytes [[28], [29], [30]]. In contrast to BM megakaryocytes, the literature regarding the origin of lung megakaryocytes is sparse. It is generally assumed, without experimental evidence, that lung megakaryocytes are “seeded” from immature circulating BM megakaryocytes or megakaryocyte progenitors [3,31]. Lefrançais et al. [16] showed the presence of GFP+ megakaryocytes in the lung vasculature when an mTmG lung was transplanted to a platelet factor 4 (PF4)-Cre-mTmG recipient; however, GFP+ megakaryocytes in the lung parenchyma were not discussed. The authors argued that the presence of GFP+ intravascular lung megakaryocytes demonstrated an extrapulmonary source. However, intravascular and extravascular lung megakaryocytes have morphological and perhaps functional differences. Extravascular lung megakaryocytes make up approximately 60% to 80% of all lung megakaryocytes, meaning that the majority of circulating megakaryocytes would have to leave the blood and tissues migrate to become lung resident. A hematopoietic progenitor pool is also present in the lung and can reconstitute megakaryocytes in the BM after lung transplantation [16]. Extramedullary hematopoiesis is known to occur posttransplantation, which may have influenced results from transplant models of Lefrançais et al. [16] [32]. Thus, the definitive origin of extravascular lung megakaryocytes remains unknown.
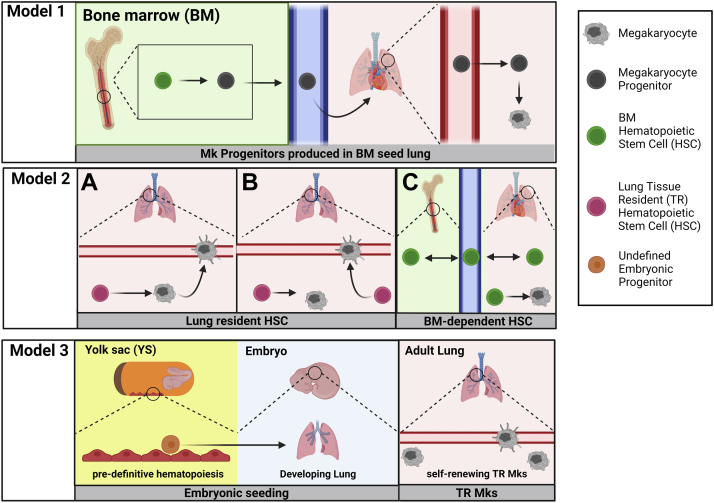
We propose 3 models to frame future studies of lung megakaryocyte developmental origin (Figure 3). The literature currently assumes model 1: BM megakaryopoiesis generates megakaryocytes or megakaryocyte progenitors that leave the BM and via the circulation seed the lung. As noted, there is no direct evidence for an extravascular lung megakaryocyte origin in the literature. There are reports of circulating megakaryocytes, particularly in disease states, such as COVID-19 infection [33,34]; however, there is no experimental evidence for their subsequent tissue migration and residence or platelet production in lung or other tissue. This leads us to propose 2 other alternative models for the origin of lung megakaryocytes.
Figure 3.
Proposed models of lung megakaryopoiesis. In model 1, megakaryocyte progenitors (shown in figure) or mature megakaryocytes (as proposed in literature) are produced in the bone marrow (BM) and migrate through the circulation to the lung and must extravasate and tissue migrate to seed extravascular megakaryocytes. Model 2 includes various alternative models based on a lung hematopoietic stem cell (HSC) that produces lung megakaryocytes. A, lung HSCs produce extravascular megakaryocytes that then migrate to become intravascular megakaryocytes. B, lung HSCs produce extravascular megakaryocytes and intravascular megakaryocytes independently. Based on the known literature of BM HSCs, C, shows BM HSCs migrating bidirectionally between BM and blood. The 2C model includes bi-directional flow of an HSC pool between the circulation and lung, where the HSCs perform megakaryopoeisis locally. In model 3, an undefined pre-definitive hematopoiesis progenitor from the yolk sac produces megakaryocytes that then colonize the developing lung. During adulthood in model 3, these embryonically derived megakaryocytes are a self-renewing tissue resident population.
It was shown by Yeung et al. [21] that megakaryocytes are present in the lung on embryonic day 13 (E13) in mice, a developmental stage before the onset of BM hematopoiesis [35]. The work by the Palis laboratory has shown that platelet-producing megakaryocytes are present in mice by E8.5 [36]. This suggests the potential for a BM-independent origin of lung megakaryocytes, which combined with the presence of hematopoietic stem and progenitor cells (HSPCs) in the lung [16] leads us to propose 2 alternative models of lung megakaryocyte origins. Model 2: because a lung HSPC pool can reconstitute lung and BM megakaryocytes in genetically thrombocytopenic mice [16], model 2 hypothesizes that lung megakaryocytes derive from a local lung HSPC pool. In this second model, lung HSPCs are either tissue resident or alternatively derived from BM HSPC. To account for potential alternative sources of the lung HSPC pool, we include different versions of model 2 to either reflect a tissue-resident lung HSPC pool (shown in Figure 3A, B, model 2) or a lung HSPC pool seeded by BM HSPCs across the lifespan (Figure 3C, model 2). In model 2A and 2B, the local HSPC pool is tissue resident and established independent of BM HSPC. In model 2A, we show that intravascular megakaryocytes arise from extravascular megakaryocytes, similar to BM megakaryocyte maturation, in which more mature megakaryocytes reside in close proximity to BM sinusoids [37]. In model 2B, we propose an alternative model in which intravascular megakaryocytes arise from lung HSPC independent of extravascular megakaryocytes. However, there is significant experimental evidence that circulating HSPCs maintain an equilibrium across BM compartments by migrating out of tissues, into the circulation, and subsequently into new tissues [[38], [39], [40]]. Migrating BM HSPC may populate lung tissue across the lifespan in a similar manner to how disparate BM compartments are maintained. Therefore, we include model 2C in which lung HSPCs arise from migrating BM HSPC and subsequently produce megakaryocytes locally in the lung.
As of yet, there have not been detailed studies to characterize lung HSPCs, although the large body of research defining BM hematopoietic progenitors by their self-renewal potential, lineage bias, and immunophenotype may prove useful for the study of lung HSPC [27]. Lefrançais et al. [16] used conventional phenotypic markers for HSPC and showed that lung HSPCs were predominantly short-term HSCs and multipotent progenitor 2 subtypes [27,41]. The translatability of BM HSPC immunophenotypes and their corresponding function when applied to lung HSPC needs validation. Future studies to characterize lung HSPC may yield important insights into their contribution to lung megakaryocytes as well as other tissue sources of megakaryocytes. The BM HSPC niche is also highly regulated by megakaryocytes, including megakaryocytes supporting the proliferation and differentiation of HSCs via PF4 and transforming growth factor β [42]. The interactions between lung megakaryocytes and lung HSPCs, or other hematopoiesis-supporting cells [43], have not been investigated and may underlie key interactions in model 2.
Model 3: as a third model, lung megakaryocytes may be derived from the yolk sac during early embryonic development and self-renew in the lung. This model relies on substantial evidence describing HSC-independent hematopoiesis within the embryo [[44], [45], [46], [47], [48]] and the concept of tissue-resident hematopoietic cells [[48], [49], [50], [51], [52]]. Importantly, megakaryopoiesis in the embryo begins at E7.25 in the yolk sac during primitive HSC-independent hematopoiesis [53,54]. Progenitors with bipotential for megakaryocyte and erythroid lineages are present in the yolk sac [53]. In addition to the early presence of HSC-independent megakaryocyte progenitors in the developing embryo, the study of tissue-resident hematopoietic cells, particularly macrophages, may serve as a useful analog for the study of a tissue-resident megakaryocyte population as proposed in model 3. Lineage tracing experiments identified an HSC-independent origin of microglia [49] and self-renewing tissue-resident macrophages (Kupffer cells, alveolar macrophages, and Langerhans cells) that largely derive from fetal erythromyeloid progenitors [35,55]. These studies implicate that a local tissue-resident megakaryocyte population that is HSC independent may be worthy of future study.
Because of the lack of direct evidence for lung megakaryocyte origin, our future studies will focus on whether there is BM-independent megakaryopoiesis in the lung, either from a local HSPC pool or from a self-renewal mechanism, analogous to tissue-resident hematopoietic cells. Defining megakaryocyte origin is not just an academic endeavor; rather, megakaryocyte origin may prove important in determining how lung megakaryocytes acquire an immune-modulatory phenotype and how this may impact health and disease. By transferring BM megakaryocytes to the lung and showing an upregulation of immune surface markers, we showed that the immune-modulatory phenotype of lung megakaryocytes is at least in part regulated by the tissue environment [22]. However, the extent to which the developmental origin contributes to lung megakaryocytes’ immune-modulatory phenotype cannot be studied without more definitive description of lung megakaryocyte origin. Recent studies have also identified a BM megakaryocyte subset that has an immune-modulatory phenotype [34,56,57]. The process by which BM megakaryocytes immune differentiate and whether this is a static or transient megakaryocyte population also remains unknown. Therefore, studies of lung megakaryocyte origin and the potential developmental encoding of their immune differentiation may reveal mechanisms by which BM megakaryocyte subsets also develop an immune-modulatory phenotype.
2.3. Lung megakaryocyte immune-modulatory roles
Our understanding of immune roles for both BM and lung megakaryocytes remains limited to largely descriptive analysis. Lung megakaryocytes have an immune-modulatory phenotype that includes higher surface expression of immune regulatory molecules for antigen uptake, processing, and presentation to CD4+ T cells and subsequent CD4+ T-cell activation in a lung bacterial infection mouse model [22]. Multiple studies have demonstrated an immune-differentiated phenotype of lung megakaryocytes based on RNAseq and single cell RNA sequencing (scRNA)-seq data [16,21,22]. However, many questions remain regarding the extent to which lung megakaryocytes may contribute to immune responses in specific disease processes. Lung megakaryocytes are at an interface with the external environment and therefore may have additional roles in maintaining immune tolerance in healthy conditions, but these potential functions are unexplored.
The role of lung megakaryocytes in disease presents an important area of study. Our laboratory and others have shown that megakaryocytes express many immunomodulatory molecules at higher levels compared with BM megakaryocytes [16,21,22]. Megakaryocytes contain numerous cytokines and chemokines that are important for the innate and adaptive immune system, as well as in hematopoietic development [16,21,22,[58], [59], [60], [61], [62], [63], [64], [65]]. Notably, PF4 and transforming growth factor-β are stored in megakaryocyte alpha granule, and PF4−/ mice demonstrate skewed neutrophil [66,67], monocyte [66,67] and T-cell responses [68,69]. In addition, granulocyte-colony stimulating factor elicits the production of TPO that stimulates megakaryocytes to produce CXCL1 and CXCL2 that can facilitate neutrophil BM egress [70]. BM megakaryocytes, defined as CD41+ CD151+ cells, were found to induce Th17 responses in lupus-prone mouse strains through the production of cytokines needed for Th17 differentiation [59]. Zufferey et al. [71] showed that BM megakaryocytes were not only capable of processing full-length ovalbumin (OVA), but were also able to cross-present OVA-peptide via major histocompatibility complex (MHC)-I to CD8+ T cells, ultimately triggering a CD8 T-cell response [60]. In addition, they showed that proplatelets from megakaryocytes that processed OVA contained OVA-MHC-I complexes [60]. Whether lung megakaryocytes are a source to disseminate antigen, both for immune tolerance and immune responses, is not known, but may provide functional logic for their lung residence. Our laboratory has provided evidence that lung megakaryocytes also process and present antigen. We found that the majority of the megakaryocytes in the lung reside extravascular, and using in vitro techniques showed that the lung megakaryocytes were capable of antigen processing and bacteria phagocytosis [22]. Using scRNA-seq of the BM and lung megakaryocytes, we found that lung megakaryocytes had phenotypically distinct gene expression that was similar to dendritic cells, the classic antigen-presenting cells. Lung megakaryocytes were capable of presenting the OVA-peptide via MHC-II to CD4+ T cells both in vitro and in vivo [22]. Two recent scRNA-seq datasets described immune-differentiated BM megakaryocytes and showed that this subpopulation also expressed MHC-II [56,34]. Sun et al. [56] showed that this immune-differentiated subset of BM megakaryocytes was also low ploidy, raising the question of whether low ploidy itself is associated with immune differentiation because lung megakaryocytes are also predominantly low ploidy.
Our data, along with a growing field, show that lung and BM megakaryocytes share many similar characteristics, including similar cell markers and platelet production capacity, but lung megakaryocytes have immune cell characteristics and capabilities that distinguish them from their BM counterpart. Further investigation of lung megakaryocytes as immune cells, as well as how lung megakaryocytes may shape platelet immune functions, will be a fertile area of research in understanding a variety of diseases.
3. Highlights From International Society on Thrombosis and Haemostasis 2022
A growing interest in understanding megakaryocyte responses to infection and immune disease was evident at the 2022 International Society on Thrombosis and Haemostasis Congress. A number of abstracts clearly presented these emerging concepts. A few are now highlighted.
The Nagy laboratory presented an abstract demonstrating a role for RhoB in megakaryocyte transendothelial migration. This was presented in the context of megakaryocyte membrane protrusions into the blood flow that drives platelet production; however, it may also have mechanistic significance if circulating megakaryocytes can exit the circulation. Using cell-specific mice lacking megakaryocyte RhoA, the investigators found a compensatory increase in RhoB expression, whereas RhoB-mutant mice had microthrombocytopenia and no evidence of transendothelial transmigration, indicating a potential role for RhoB in these important end-stage steps of platelet production [72].
As noted in this review, there is increasing evidence that megakaryocytes are functionally impacted by immune stimuli, including infection. Megakaryocytes and HSPCs express toll-like receptors (TLRs), such as TLR7 and 8, that can recognize single-stranded RNA viruses such as coxsackievirus B3 (CVB3). Therefore, a team of investigators sought to determine whether CVB3 infection of human CD34+ cells affected their ability to differentiate into megakaryocytes and produce platelets [73]. Their data suggested a role for TLR7/8 in limiting megakaryopoiesis during viral infections that might contribute to a better understanding of the molecular basis underlying thrombocytopenia during CVB3 infection as well as many other viral infections.
Lipids are essential cell components and are stored as energy sources. Lipids can also have cell-signaling roles and contribute to cell fate decisions during hematopoiesis. However, the contribution of lipids to megakaryopoiesis is less explored, so investigators sought to determine the role of key lipids in megakaryocyte differentiation and platelet production [74]. The analysis of HSPC lipidomes revealed that cell populations clustered into distinct populations with HSPC, with enrichment in polyunsaturated fatty acids distinguishing mature from immature megakaryocytes. Inhibitors of fatty acid metabolism and lipogenesis decreased megakaryocyte differentiation and mice fed a high-fat diet with increased palmitic acid and reduced polyunsaturated fatty acids had larger megakaryocytes, but reduced platelet counts compared with mice fed a standard chow diet, revealing that fatty acid metabolism and synthesis are critical for megakaryocyte differentiation. It is intriguing to consider how hyperlipidemia and oxidized lipids that drive inflammation and atherosclerosis may also affect megakaryocyte differentiation.
As discussed above, scRNA-seq from many groups has now indicated that megakaryocyte functions go beyond platelet production, with evidence for immunoregulatory and stem cell niche supporting subpopulations of megakaryocytes. However, the relationship between the spatial organization of megakaryocytes and the megakaryocyte “subpopulations” in the marrow environment is unclear. Therefore, Barrachina et al. sought to map the molecular, cellular, and spatial composition of megakaryocytes in the murine femur using spatial transcriptomics [74]. These authors found a clustering of megakaryocytes based on the proximal versus distal axes of the bone, with megakaryocytes in the proximal side enriched for proplatelet basic protein and PF4. How function tracks with location is not known, but this abstract provides technical insight valuable to the field.
MicroRNAs (miRNA) expression is evident in platelets and megakaryocytes, but miRNA activities in megakaryocyte differentiation and function have received little investigation. Chen et al. [75] developed an miRNA switch technology that reflects the endogenous activity of miRNA and can be used to identify specific cell populations. They sought to use this technology to determine whether induced pluriopotent stem cell (iPSC)-derived immortalized megakaryocyte progenitor cell lines contain immune-biased megakaryocytes and identified 2 subpopulations with differential activity of let-7a-5p and let-7g-5p in the proliferation phase of iPSC-derived megakaryocytes. Let-7 low megakaryocytes had an immune-skewed transcriptional signature and as the cells matured, interferon-responsive genes were enriched in let-7 low megakaryocytes, whereas platelet activation signaling was enriched in let-7 high cells. Their data indicated an immune-skewed subpopulation of megakaryocytes in iPSC/embryonic stem cell-derived megakaryocytes that may be very significant as teams seek to make platelets in vitro from iPSCs and provide insights into the potential to limit their immune differentiation.
Tumor necrosis factor α (TNFα) is an inflammatory cytokine common to many immune pathologies; however, the effects of TNFα on platelet production are not known. Chu et al., [76] therefore, sought to determine the effect of TNFα on megakaryocyte and platelet production in a dose-dependent manner. They reported a significantly higher TNFα level in patients with normal platelet reconstitution after a stem cell transplant compared with those with prolonged thrombocytopenia. Studies in tissue culture showed that a low concentration of TNFα-promoted megakaryocyte maturation, and platelet production, but concentrations of >10 ng/mL inhibited platelet production. The authors investigated the transcriptome of cultured megakaryocytes treated with high- and low-dose TNFα and found differentially expressed cytoskeletal molecules as well as MAPK-ERK1/2 and PI3K-Akt signaling pathways. In vivo, megakaryocyte number and location in TNFα-treated groups were also altered. This work provided insight into the differences between mild vs severe inflammation and how it may affect platelet production in systemic vascular inflammation.
New ex vivo systems need to be developed to advance our understanding of platelet production from both lung and BM megakaryocytes under physiologic conditions. The research team of Zhao et al. [77] presented an abstract in which they established an ex vivo mouse heart–lung model for perfusion of murine megakaryocytes, while performing confocal microscopy to image platelet production in real time. They found that megakaryocytes can pass multiple times through the lung vasculature, leading to the generation of physiological levels of functional platelets through a process involving nuclear marginalization and enucleation. This ex vivo mouse heart–lung model may allow for the study of platelet generation, as well as a possibly establishing a role for TPM4-dependent fragmentation in platelet generation.
A deeper understanding of megakaryocyte and platelet disorders also demands the development of more advanced comparative model systems, including models for diseases, such as gray platelet syndrome. In addition to platelet defects, gray platelet syndrome presents with dysfunctional neutrophils, a proinflammatory plasma proteome, autoantibodies, and autoimmune disorders. However, the pathogenesis of many of the clinical manifestations such as splenomegaly, emperipolesis, and bone marrow fibrosis (BMF) are not understood. Using the Nbeal2−/− mouse model, Collin et al. found that the percentage of megakaryocytes containing neutrophils increased in Nbeal2−/− mice, with 40% of Nbeal2−/− mice older than 10 months also having BMF [78]. The authors concluded that although splenomegaly and emperipolesis developed in Nbeal2−/− mice at an early age, BMF is a later-life onset disease complication. The authors also found using in vitro models that the absence of Nbeal2 had a smaller effect on the loss of megakaryocyte α-granules and their content compared with platelets, suggesting that α-granule loss might occur after proplatelet formation or platelet release. This may provide deeper insights into the disease as well as insights into the mechanisms of platelet granule formation and cargo loading.
Despite its clinical importance and extensive research focus, a mechanistic understanding of immune-mediate thrombocytopenia purpura (ITP) pathogenesis is still lacking. Franzoso et al. [79] examined megakaryocytes and platelets from the peripheral blood of healthy controls as well as from the patients with acute and chronic ITP and found increased expression of apoptotic genes such as CLU, cytochrome-c, Bax, and Bak in ITP megakaryocytes and platelets. This may indicate that a defect in apoptosis regulation at the megakaryocyte level may lead to increased platelet destruction in some patients with ITP.
Acknowledgments
Funding
This study was funded by NIH/NHLBI - R01 HL160610.
Author contributions
A.C.L., D.N.P., and C.N.M. all wrote and revised this manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Prof Yotis Senis
Alison C. Livada and Daphne N. Pariser contributed equally to this study.
References
- 1.Howell WH. Observations upon the occurrence, structure, and function of the giant cells of the marrow. J Morphol. 180; 4:117–130.
- 2.Wright J.H. Die Entstehung der Blutplättchen. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin. 1906;186:55–63. [Google Scholar]
- 3.Aschoff L. Uber capillare Embolie von riesenkernhaltigen Zellen. Virchows Arch Path Anat Phys. 1893;134:11–26. [Google Scholar]
- 4.Orbison J.C., Laipply T.C. Pneumococcal lobar pneumonia associated with megakaryocytic and leukoblastic hyperplasia. Ohio State MJ. 1945;41:148. [Google Scholar]
- 5.Downey H., Nordland M. Hematologic and histologic study of a case of myeloid megakaryocytic hepatosplenomegaly. Folia Heamat. 1939;62:1. [Google Scholar]
- 6.Howell W.H., Donahue D.D. The production of blood platelets in the lungs. J Exp Med. 1937;65:177–203. doi: 10.1084/jem.65.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medlar E.M., Sasano K.T. The significance of lesions resembling Hodgkin's disease in tuberculosis. Am J Pathol. 1931;7:491–498.7. [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma G.K., Talbot I.C. Pulmonary megakaryocytes: “missing link” between cardiovascular and respiratory disease? J Clin Pathol. 1986;39:969–976. doi: 10.1136/jcp.39.9.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith E.B., Butcher J. The incidence, distribution and significance of megakaryocytes in normal and diseased human tissues. Blood. 1952;7:214–224. [PubMed] [Google Scholar]
- 10.Aabo K., Hansen K.B. Megakaryocytes in pulmonary blood vessels. I. Indicence at autopsy, clinicopathological relations especially to disseminated intravascular coagulation. Acta Pathol Microbiol Scand A. 1978;86:285–291. [PubMed] [Google Scholar]
- 11.Kaufman R.M., Airo R., Pollack S., Crosby W.H. Circulating megakaryocytes and platelet release in the lung. Blood. 1965;26:720–731. [PubMed] [Google Scholar]
- 12.Brill R., Halpern M.M. The frequency of megakaryocytes in autopsy sections. Blood. 1948;3:286–291. [PubMed] [Google Scholar]
- 13.Sharnoff J.G., Kim E.S. Evaluation of pulmonary megakaryocytes. AMA Arch Pathol. 1958;66:176–182. [PubMed] [Google Scholar]
- 14.Sharnoff J.G. Increased pulmonary megakaryocytes; probable role in postoperative thromboembolism. J Am Med Assoc. 1959;169:688–691. doi: 10.1001/jama.1959.03000240026006. [DOI] [PubMed] [Google Scholar]
- 15.Hasleton P.S. Adult respiratory distress syndrome—a review. Histopathology. 1983;7:307–332. doi: 10.1111/j.1365-2559.1983.tb02247.x. [DOI] [PubMed] [Google Scholar]
- 16.Lefrancais E., Ortiz-Munoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M., et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater D.N., Trowbridge E.A., Martin J.F. The megakaryocyte in thrombocytopenia: a microscopic study which supports the theory that platelets are produced in the pulmonary circulation. Thromb Res. 1983;31:163–176. doi: 10.1016/0049-3848(83)90017-8. [DOI] [PubMed] [Google Scholar]
- 18.Trowbridge E.A., Martin J.F., Slater D.N. Evidence for a theory of physical fragmentation of megakaryocytes, implying that all platelets are produced in the pulmonary circulation. Thromb Res. 1982;28:461–475. doi: 10.1016/0049-3848(82)90163-3. [DOI] [PubMed] [Google Scholar]
- 19.Zucker-Franklin D., Philipp C.S. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157:69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine R.F., Eldor A., Shroff P.K., Kirwin S., Tenza D., Cramer E.M. Circulating megakaryocytes: delivery of large numbers of intact, mature megakaryocytes to the lungs. Eur J Haematol. 1993;51:233–246. doi: 10.1111/j.1600-0609.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 21.Yeung A.K., Villacorta-Martin C., Hon S., Rock J.R., Murphy G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020;4:6204–6217. doi: 10.1182/bloodadvances.2020002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariser D.N., Hilt Z.T., Ture S.K., Blick-Nitko S.K., Looney M.R., Cleary S.J., et al. Lung megakaryocytes are immune modulatory cells. J Clin Invest. 2021;131 doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valet C., Magnen M., Qiu L., Cleary S.J., Wang K.M., Ranucci S., et al. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J Clin Invest. 2022;132 doi: 10.1172/JCI153920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas S., Trumpp A., Milsom M.D. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22:627–638. doi: 10.1016/j.stem.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Cabezas-Wallscheid N., Klimmeck D., Hansson J., Lipka D.B., Reyes A., Wang Q., et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Pietras E.M., Reynaud D., Kang Y.A., Carlin D., Calero-Nieto F.J., Leavitt A.D., et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H., Zheng Z., Cheng T. New paradigms on hematopoietic stem cell differentiation. Protein Cell. 2020;11:34–44. doi: 10.1007/s13238-019-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noetzli L.J., French S.L., Machlus K.R. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler Thromb Vasc Biol. 2019;39:1288–1300. doi: 10.1161/ATVBAHA.119.312129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanjuan-Pla A., Macaulay I.C., Jensen C.T., Woll P.S., Luis T.C., Mead A., et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto R., Morita Y., Ooehra J., Hamanaka S., Onodera M., Rudolph K.L., et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Lefrançais E., Looney M.R. Platelet biogenesis in the lung circulation. Physiology (Bethesda) 2019;34:392–401. doi: 10.1152/physiol.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlitt H.J., Schafers S., Deiwick A., Eckardt K.U., Pietsch T., Ebell W., et al. Extramedullary erythropoiesis in human liver grafts. Hepatology. 1995;21:689–696. doi: 10.1002/hep.1840210314. [DOI] [PubMed] [Google Scholar]
- 33.Bernardes J.P., Mishra N., Tran F., Bahmer T., Best L., Blasé J.I., et al. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells, and plasmablasts as hallmarks of severe COVID-19. Immunity. 2020;53:1296–1314.e9. doi: 10.1016/j.immuni.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Xie J., Wang D., Han X., Chen M., Shi G., et al. CXCR4(high) megakaryocytes regulate host-defense immunity against bacterial pathogens. Elife. 2022;11 doi: 10.7554/eLife.78662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGrath K.E., Frame J.M., Palis J. Early hematopoiesis and macrophage development. Semin Immunol. 2015;27:379–387. doi: 10.1016/j.smim.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potts K.S., Sargeant T.J., Markham J.F., Shi W., Biben C., Josefsson E.C., et al. A lineage of diploid platelet-forming cells precedes polyploid megakaryocyte formation in the mouse embryo. Blood. 2014;124:2725–2729. doi: 10.1182/blood-2014-02-559468. [DOI] [PubMed] [Google Scholar]
- 37.Machlus K.R., Italiano J.E., Jr. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Méndez-Ferrer S., Lucas D., Battista M., Frenette P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 40.Wright D.E., Wagers A.J., Gulati A.P., Johnson F.L., Weissman I.L. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 41.Ng A.P., Alexander W.S. Haematopoietic stem cells: past, present and future. Cell Death Discov. 2017;3 doi: 10.1038/cddiscovery.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller A.M., Medvinsky A., Strouboulis J., Grosveld F., Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 45.Palis P., Robertson S., Kennedy M., Wall C., Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 46.McGrath K.E., Frame J.M., Fegan K.H., Bowen J.R., Conway S.J., Catherman S.C., et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumaravelu P., Hook L., Morrison A.M., Ure J., Zhao S., Zuyev S., et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimoto M., Porayetter P., Glosson N.L., Conway S.J., Carlesso N., Cardoso A.A., et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz C., Perdiguero E.G., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 51.Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C.A., Azzoni E., et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi M., Shelley W.C., Seo W., Vemula S., Lin Y., Liu Y., et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc Natl Acad Sci U S A. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tober J., Koniski A., McGrath K.E., Vemishetti R., Emerson R., de Mesy-Bentley K.K., et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M.J.X., Matsuoka S., Yang F.C., Ebihara Y., Manabe A., Tanaka R., et al. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97:2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 55.Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Sun S., Jin C., Si J., Lei Y., Chen K., Cui Y., et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood. 2021;138:1211–1224. doi: 10.1182/blood.2021010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas S., Hansson J., Klimmeck D., Loeffler D., Velten L., Uckelmann H., et al. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17:422–434. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Finkielsztein A., Schlinker A.C., Zhang L., Miller W.M., Datta S.K. Human megakaryocyte progenitors derived from hematopoietic stem cells of normal individuals are MHC class II-expressing professional APC that enhance Th17 and Th1/Th17 responses. Immunol Lett. 2015;163:84–95. doi: 10.1016/j.imlet.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang H.K., Chiang M.Y., Ecklund D., Zhang L., Ramsey-Goldman R., Datta S.K. Megakaryocyte progenitors are the main APCs inducing Th17 response to lupus autoantigens and foreign antigens. J Immunol. 2012;188:5970–5980. doi: 10.4049/jimmunol.1200452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zufferey A., Speck E.R., Machlus K.R., Aslam R., Gui L., McVey M.J., et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunin P., Penke L.R., Thon J.N., Monach P.A., Jones T., Chang M.H., et al. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J Clin Invest. 2017;127:1714–1724. doi: 10.1172/JCI84598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beaulieu L.M., Lin E., Morin K.M., Tanriverdi K., Freedman J.E. Regulatory effects of TLR2 on megakaryocytic cell function. Blood. 2011;117:5963–5974. doi: 10.1182/blood-2010-09-304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D'Atri L.P., Etulain J., Rivadeneyra L., Lapponi M.J., Centurion M., Cheng K., et al. Expression and functionality of toll-like receptor 3 in the megakaryocytic lineage. J Thromb Haemost. 2015;13:839–850. doi: 10.1111/jth.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crist S.A., Elzey B.D., Ahmann M.T., Ratliff T.L. Early growth response-1 (EGR-1) and nuclear factor of activated T cells (NFAT) cooperate to mediate CD40L expression in megakaryocytes and platelets. J Biol Chem. 2013;288:33985–33996. doi: 10.1074/jbc.M113.511881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunin P., Nigrovic P.A. Megakaryocytes as immune cells. J Leukoc Biol. 2019;105:1111–1121. doi: 10.1002/JLB.MR0718-261RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapchak P.H., Loannou A., Rani P., Lieberman L.A., Yoshiya K., Kannan L., et al. The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava K., Field D.J., Aggrey A., Yamakuchi M., Morrell C.N. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava K., Cockburn I.A., Swaim A., Thompson L.E., Tripathi A., Fletcher C.A., et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi G., Field D.J., Ko K.A., Ture S., Srivastava K., Levy S., et al. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124:543–552. doi: 10.1172/JCI71858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohler A., Filippo K.D., Hasenberg M., van den Brandt C., Nye E., Hosking M.P., et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117:4349–4357. doi: 10.1182/blood-2010-09-308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zufferey A., Speck E.R., Guo L., Aslam R., Kapur R., Semple J.W. Murine bone marrow-derived megakaryocytes are capable of antigen cross-presentation on major histocompatibility class (MHC) I molecules. Blood. 2015;126:3465. [Google Scholar]
- 72.Englert M., Aurbach K., Becker I.C., Gerber A., Heib T., Wackerbarth L.M., et al. Impaired microtubule dynamics contribute to microthrombocytopenia in RhoB-deficient mice. Blood Adv. 2022;6:5184–5197. doi: 10.1182/bloodadvances.2021006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schattner M., Rodriguez C., Charo N., Tatti S., Gomez R., D’Atri L. Coxsackievirus B3 infection of CD34+ cells impairs megakaryocyte and platelet production through activation of Toll-like receptors 7 and 8 [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]
- 74.Barrachina M., Pernes G., Becker I., Freire D., Groeneveld D., Luyendyk J., et al. Thrombopoiesis has a unique lipidomic profile enriched in polyunsaturated fatty acids that facilitates megakaryocyte maturation and platelet production [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]
- 75.Chen S., Yuzuriha A., Fujio K., Hashimoto K., Fujita Y., Paul S., et al. Synthetic microRNA switch technology enables to detect the immune-biased megakaryocytes from heterogenous iPSC-derived megakaryocyte progenitor cell lines [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]
- 76.Chu T., Hu S., Qi J., Han Y., Wu D. Bifunctional effect of inflammatory cytokine TNFα on human megakaryopoiesis and platelet production [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) doi: 10.1111/jth.15891. [DOI] [PubMed] [Google Scholar]
- 77.Zhao X., Alibhai D., Walsh T., Tarassova N., Birol S., Williams C., et al. Platelet generation from circulating megakaryocytes is triggered in the lung vasculature [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]
- 78.Collin L., Mayer L., Kollipara L., Sickmann A., Zhou H., Hoffman G., et al. Modeling gray platelet syndrome: longitudinal mouse studies and in vitro human iPSC-derived megakaryocytes to investigate clinical and cellular features [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]
- 79.Franzoso F., Schmuge M., Gowin C., Stein T. Molecular mechanisms of apoptosis in platelets and megakaryocytes in pediatric immune thrombocytopenia [abstract] Res Pract Thromb Haemost. 2022;6(Suppl 1) [Google Scholar]