Abstract
Background
Continuous-flow left ventricular assist devices (CF-LVADs) are an established therapy for advanced heart failure. Thrombosis and hemorrhage are common complications after CF-LVAD implantation, which may be explained by device-induced platelet activation. Few data on the effect of CF-LVAD implantation on platelets are available to date.
Objectives
The aim of this study was to characterize the change in the platelet activation status after CF-LVAD.
Methods
Platelet phenotype and reactivity were determined with flow cytometry in 32 adults with end-stage heart failure before and 4 to 6 weeks after CF-LVAD implantation. Sixteen adults with a biological aortic valve prosthesis (AVP) using the same antiplatelet regimen were included to discriminate between the effects of CF-LVAD and the antiplatelet regimen. Plasma markers for platelet activation were determined with enzyme-linked immunosorbent assay.
Results
Median (IQR) plasma levels of soluble P-selectin increased from 115.6 (79.1-142.7) ng/mL to 144.5 (100.4-197.5) ng/mL after CF-LVAD implantation (P < .001). Median (IQR) β-thromboglobulin levels were 60.5 (37.8-81.5) ng/mL before implantation and remained high after LVAD implantation [60.0 (42.0-69.5) ng/mL]. The platelet P-selectin expression after stimulation with ADP (30 and 60 μM) or PAR1-activating peptide (12.5 and 25 μM) was reduced by 17% to 21%, and fibrinogen binding was reduced by 37% to 86%. Platelet responses to agonists were similar in patients with a CF-LVAD and patients with an AVP, except for fibrinogen binding in response to 12.5 μM PAR1-AP, which was lower in patients with a CF-LVAD (P < .001).
Conclusions
Combined, these data provide evidence for systemic platelet activation and an acquired platelet disorder after CF-LVAD implantation. This might contribute to the risk of both hemorrhage and thrombosis associated with CF-LVADs.
Keywords: blood platelet disorder, hemorrhage, platelet activation, thrombosis, ventricular assist device
Essentials
-
•
Limited data on platelet activation after left ventricular assist device implantation are available.
-
•
We analyzed changes in platelet function after implantation of a left ventricular assist device.
-
•
Implantation was associated with systemic platelet activation and reduced platelet reactivity.
-
•
This might contribute to the increased risk of both bleeding and thrombosis after implantation.
1. Introduction
Implantation of a continuous-flow left ventricular assist device (CF-LVAD) is an established therapy in patients with end-stage heart failure. With advances in device technology and improvements in patient care, the average survival time for patients under permanent CF-LVAD therapy approaches that of patients who receive a heart transplantation. [1,2] However, thrombosis and hemorrhage frequently occur after CF-LVAD implantation and contribute significantly to morbidity and mortality. [[3], [4], [5], [6], [7]]
The CF-LVAD provides a prothrombotic environment that explains the observed thrombotic risk. Protein adsorption on the artificial surface of the CF-LVAD induces platelet adhesion, activation, and aggregation. Simultaneously, contact activation on the artificial surface initiates coagulation. Thrombin, the final enzyme of the coagulation cascade, is also a potent platelet agonist. [8] Furthermore, the CF-LVAD circulatory physiology results in increased shear stress, which promotes platelet activation. [9,10]
The 2019 Expert consensus of the European Association for Cardio-Thoracic Surgery recommends that all patients with a CF-LVAD should be treated with both a vitamin K antagonist and a platelet inhibitor, preferably aspirin, to mitigate the thrombotic risk associated with the CF-LVAD. [11] Although the combination of advances in device technology and antithrombotic management has decreased the incidence of pump thrombosis, the risk of thromboembolism and clinically relevant bleeds remains high. [12]
Device-induced platelet activation despite antiplatelet therapy may explain the increase in thrombotic risk associated with CF-LVAD implantation. Conversely, low-grade platelet activation could result in acquired platelet dysfunction and might explain the bleeding tendency. Until date, limited data on the effect of CF-LVAD implantation on platelet activation status are available. Some studies report a loss of platelet receptors (GPIbα, GPVI, and αIIbβ3) or reduced platelet reactivity toward agonists after implantation of a CF-LVAD in patients who bleed. [[13], [14], [15]] However, the interpretation of data is hampered by the use of antiplatelet drugs after CF-LVAD implantation, which makes it difficult to discriminate between the effects of CF-LVAD and the effects of the antiplatelet regimen.
Here, we performed a systematic analysis of the changes in platelet activation status after implantation of a CF-LVAD in patients with end-stage heart failure. Hereto, we analyzed plasma markers of platelet activation and platelet phenotype and platelet reactivity toward agonists before implantation and 4 to 6 weeks after implantation. To allow the differentiation between the effects of aspirin and the CF-LVAD on platelet phenotype and reactivity, we performed similar measurements in patients receiving a biological aortic valve prosthesis (AVP), who also received lifelong antiplatelet treatment after valve implantation.
2. Methods
2.1. Healthy controls
Blood from healthy participants was obtained through a blood donation facility of University Medical Center Utrecht (UMCU). Healthy controls provided written informed consent in accordance with the declaration of Helsinki.
2.2. Patients
We performed a single-center, prospective observational study among patients with end-stage heart failure who received a CF-LVAD or a biological AVP at the UMCU. Permission from the local medical ethics review board was obtained, and all participants provided written informed consent in accordance with the declaration of Helsinki.
2.2.1. Patients with end-stage heart failure who received a CF-LVAD
Patients (aged > 18 years) were eligible for inclusion if they were willing and able to provide informed consent and were scheduled for an elective or urgent CF-LVAD implantation. Patients were excluded from this study if they had a known coagulation disorder or if they had received extracorporeal mechanical circulatory support before CF-LVAD implantation to avoid confounding effects on coagulation markers. All patients were routinely followed up in our clinic. During the study period, our institutional anticoagulation protocol for CF-LVAD patients included the administration of the platelet inhibitor aspirin (80-100 mg) or clopidogrel (75 mg) on indication and a vitamin K antagonist, acenocoumarol, or phenprocoumon, with a target INR of 2.0 to 3.0 after implant for HM3 patients and 2.5 to 3.5 for patients with HVAD.
2.3. Platelet activation, platelet phenotype, platelet responsiveness, and platelet reactivity
Platelet activation is associated with secretion of compounds from storage organelles in the platelet and surface expression of P-selectin, which can subsequently be cleaved from the platelet surface. Plasma levels of these markers therefore reflect in vivo platelet activation. Healthy controls were used to measure platelet activation status as a control for patients with end-stage heart failure receiving a CF-LVAD.
To determine whether CF-LVAD implantation altered the phenotype of circulating platelets, the surface expressions of the collagen receptors α2β1 and GPVI, the fibrinogen receptor αIIbβ3, and the VWF receptor GPIbα were analyzed in the absence of agonists. Platelet responsiveness toward stimulation with an intermediate and high concentration of either ADP (30 μM and 60 μM) or PAR1-AP (12.5 μM and 25 μM) was assessed with flow cytometry before and 4 to 6 weeks after implantation of a CF-LVAD.
2.3.1. Patients with aortic valve stenosis receiving a biological AVP
To be able to discriminate between the effects of antiplatelet therapy and CF-LVAD implantation in platelet reactivity, platelet responsiveness toward agonists was also investigated in patients with aortic stenosis at 4 to 6 weeks after implantation of a biological AVP.
Patients (aged > 18 years) were eligible for inclusion if they were willing and able to provide informed consent and were scheduled for elective aortic valve replacement surgery. They were excluded from this study if they had a known coagulation disorder. During the study period, patients with a biological AVP received lifelong treatment with the platelet inhibitor aspirin (80-100 mg).
2.3.2. Study follow-up
Study follow-up consisted of 2 venipunctures, one between 0 and 8 days before implantation (t = 0) and one at 4 to 6 weeks after implantation (t = 1). Blood was collected in trisodium citrate tubes and used for flow cytometry within 1 to 6 hours after collection. The remainder was centrifuged at 2000 g for 15 minutes to obtain plasma, which was aliquoted and stored at −80 °C until use.
Baseline demographic data were collected from electronic patient files. Baseline hemodynamic parameters in patients with a CF-LVAD were obtained with echocardiography.
2.4. Assays
2.4.1. Flow cytometry
Whole blood was diluted at a ratio of 1:10 (v:v) in 10 mM HEPES, 150 mM NaCl, 1 mM MgSO4, and 5 mM KCl pH 7.4 containing fluorescent antibodies, with or without platelet agonists. Platelet expressions of the fibrinogen receptor αIIbβ3 (HIP8-PE; BD Biosciences), the von Willebrand factor receptor GPIbα (VhH clone 17-PE), and the collagen receptors α2β1 (AK7-FITC; BD Biosciences) and GPVI (HY101-eFluor660; Thermo Fisher Scientific) were measured in the absence of agonists. Platelet reactivity toward adenosine diphosphate (ADP; 60 and 30 μM) and protease-activated receptor (PAR)-1 agonist peptide SFLLRN (PAR1-AP; 25 and 12.5 μM) was assessed by measuring the platelet P-selectin expression (VhH clone B10.6-alexa647) as a proxy for α-granule secretion and fibrinogen binding (VhH clone C3-alexa488) as a proxy for αIIbβ3 activation. The background signal was assessed with alexa647- and alexa488-conjugated isotype control VhH (clone R2). Samples were incubated for 10 minutes at 37°C, fixated with 1.11% formaldehyde, 137 mM NaCl, 2.7 mM KCl, 1.12 mM NaH2PO4, 10.2 mM Na2HPO4, 1.15 mM KH2PO4, and 4 mM EDTA, pH 6.8 for 20 minutes and stored in the dark at 4°C until analysis on a BD FACSCantoII. Platelets were gated based on the forward and side scatters, as well as the expression of GPIbα (VhH Clone 17-RPE) or αIIbβ3. Fluorescent signals were expressed as median fluorescent intensity after subtraction of the background signal.
2.4.2. β-thromboglobulin (βTG; CXCL7) and soluble P-selectin (sP-selectin; CD62P)
βTG levels were determined with enzyme-linked immunosorbent assay as described. [16,17] Mouse anti-human-CXCL7 IgG1 (R&D systems; 1 μg/mL) or human P-selectin/CD62P coating antibody (R&D systems; 1 μg/mL) was coated on Nunc Maxisorp microtiter plates in 15 mM Na2CO3, 35 mM NaHCO3, and 3 mM NaN3, pH 9.6 o/n at 4°C. Plates were blocked with 150 μL per well of 137.0 mM NaCl, 2.7 mM KCl, 9.2 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4 (PBS) supplemented with 2% (w/v) bovine serum albumin (BSA). Plasma samples were diluted either 160-fold (CXCL7) or 10-fold (CD62P) in PBS with 0.1% (w/v) Tween-20 and 1% (w/v) BSA and incubated on the plate. Then, βTG was detected with biotinylated goat anti-human CXCL7 antibodies (R&D systems; 56 ng/mL) and streptavidin-HRP (Dako; 0.1 μg/mL), and sP-selectin was detected with human P-selectin/CD62P capturing antibody (R&D systems; 3.6 μg/mL) and streptavidin-HRP (Dako; 0.1 μg/mL). Plates were developed with TMB, after which colorimetric reactions were stopped with H2SO4 or Supersignal Westpico Chemoluminescent Substrate (Thermo Scientific). Absorbance was measured in a Versamax M2e microtiter plate reader (Molecular Devices) at 450 nm. Luminescence was measured in a Spectramax M2e microtiter plate reader. Plasma levels of βTG and sP-selectin were deduced from a calibration curve with known levels and are expressed in ng/ml. All samples were run in duplicates. The analytical coefficients of variation (CV) were 10% for βTG and 11.8% for sP-selectin. The interassay CVs were 6.1% for βTG and 6.3% for sP-selectin.
2.5. Statistical analysis
The data are presented as medians and interquartile ranges. Differences between pre- and post-implantation levels of plasma markers were assessed with Wilcoxon’s-signed rank test for non-normally distributed values with a Holm-Sidak correction for multiple comparisons. Differences between patients and healthy controls were analyzed with the Mann–Whitney U tests, as were the differences between patients who received a CF-LVAD and patients who received a biological aortic valve replacement. Differences were considered statistically notable at P < .05.
3. Results
The study population comprised 40 adult patients who received a CF-LVAD between September 2019 and July 2021. After exclusion of 8 patients (3 patients died before the second venipuncture could occur, one patient declined further participation within the study before the second venipuncture could occur, and 4 patients underwent a clinical follow-up on remote due to COVID-19 (Coronavirus Disease 2019) measure; therefore, the second venipuncture could not occur within the clinic), 32 patients were eligible for further analysis (Table). Thirty patients received a HeartMate 3 (HM3, Abbott laboratories) and 2 patients received a HeartWare (HVAD, HeartWare Inc). The median age at CF-LVAD implantation was 57.0 years, and 24/32 (75.0%) patients were men. Three patients received antiplatelet therapy before CF-LVAD implantation. In the postoperative course, transfusion requirements and postoperative blood loss were similar for all patients. Patients received mechanical ventilation for 2 days (median, IQR 1-5 days) and stayed in the intensive care unit for 3 days (median, IQR 2-5 days).
Table.
Baseline characteristics of patients with end-stage heart failure undergoing LVAD implantation.
| Patients with end-stage heart failure (n = 32) | |
|---|---|
| Age, median years (IQR) | 57 (44-64) |
| Men, n (%) | 24 (75.0) |
| BMI, median kg/m2 (IQR) | 25.3 (22.7-29.4) |
| Race | |
| White | 32 (100.0) |
| Etiology | |
| Ischemic cardiomyopathy, n (%) | 8 (25.0) |
| Non-ischemic cardiomyopathy, n (%) | 24 (75.0) |
| INTERMACS, n (%) | |
| Level II: Progressive decline | 6 (18.7) |
| Level III: Stable but inotrope dependent | 10 (31.3) |
| Level IV: Recurrent advanced HF | 11 (34.4) |
| Level V: Exertion intolerant | 4 (12.5) |
| Level VI: Exertion limited | 1 (3.2) |
| Medical history, n (%) | |
| Hypertension | 8 (25.0) |
| Atrial fibrillation | 10 (31.3) |
| Peripheral arterial disease | 1 (3.2) |
| Cerebral vascular accident | 4 (12.3) |
| Pulmonary embolism | 3 (9.4) |
| Deep venous thrombosis | 3 (9.4) |
| ICD | 23 (71.9) |
| Implanted LVAD type, n (%) | |
| HeartMate 3 | 30 (93.7) |
| HeartWare | 2 (6.3) |
| Antithrombotic therapy prior to LVAD implantation, n (%) | |
| Aspirin | 2 (6.3) |
| Clopidogrel | 1 (3.1) |
| Vitamin K antagonist | 10 (31.3) |
| Factor X inhibitor | 10 (31.3) |
| Factor II inhibitor | 1 (3.2) |
| Antithrombotic therapy post LVAD implantation, n (%) | |
| Vitamin K antagonist + aspirin | 30 (92.7) |
| Vitamin K antagonist + clopidogrel | 2 (6.3) |
BMI, body mass index; CABG, coronary artery bypass grafting; ICD, implantable cardioverter defibrillator; HF, heart failure; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device.
During the 4- to 6-week follow-up after CF-LVAD implantation, one patient (3.1%) experienced recurrent episodes of epistaxis. Because of the severity of the bleeding events, aspirin therapy was discontinued, and treatment in the operation room performed by the otolaryngology specialist was necessitated. All patients had a normal platelet count before CF-LVAD implantation (median 203, IQR 169-253 × 109/L), which increased after implantation (median 351; IQR 273-412 × 109/L) (P = < .001), but remained within the normal range (150-450 × 109/L).
Sixteen patients who received a biological aortic valve prostheses between September 2019 and January 2021 were included. The median age at aortic valve implantation was 67.0 years, and 7/16 (43.8%) patients were men. Five patients received additional coronary bypass grafting during the same operation procedure. Twelve patients received aspirin treatment, 3 patients received aspirin in combination with a factor X inhibitor, and one patient received aspiring together with a factor II inhibitor.
3.1. Evidence for systemic platelet activation after LVAD implantation
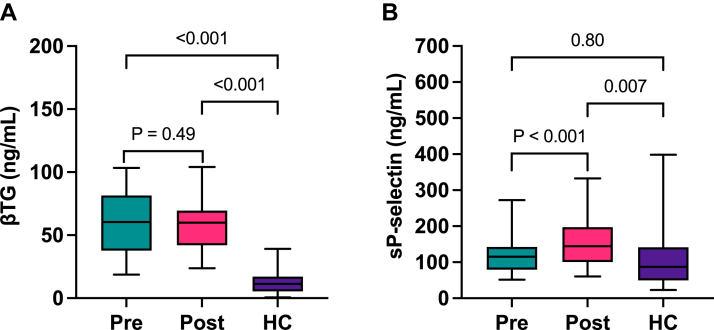
At baseline, patients with end-stage heart failure had elevated levels of alpha granule protein βTG (median 60.5, IQR 37.8-81.5 ng/mL) compared with healthy controls (median 11.4, IQR 5.5-17.2 ng/mL) (P < .001) (Figure 1A). Plasma levels of βTG did not change after CF-LVAD implantation and remained high after 4 to 6 weeks (median 60.0, IQR 42.0-69.5 ng/mL) (P = .49). Median baseline sP-selectin plasma levels in patients with end-stage heart failure (115.6, IQR 79.1-142.7 ng/mL) were similar to levels in healthy controls (median 87.1, IQR 49.9-141.5 ng/mL) (P = .80). After CF-LVAD implantation, sP-selectin plasma levels were increased compared with baseline (median 144.5, IQR 100.4-197.5 ng/mL) (P < .001) and higher than that in healthy controls (Figure 1B) (P = .007). These data are in line with continuous platelet activation after LVAD implantation.
Figure 1.
Plasma levels of platelet activation markers before and after implantation of a CF-LVAD Plasma levels of platelet activation marker β-thromboglobulin (A) and soluble P-selectin (B) were measured in the plasma samples from patients with end-stage heart failure before (pre) and after (post) LVAD implantation (n = 32) and in healthy controls (HC, n = 28) with enzyme-linked immunosorbent assay. Data are presented as median and interquartile ranges. βTG, β-thromboglobulin; sP-selectin, soluble P-selectin
3.2. Expressions of platelet GPVI, αIIbβ3, and GPIbα are reduced after CF-LVAD implantation
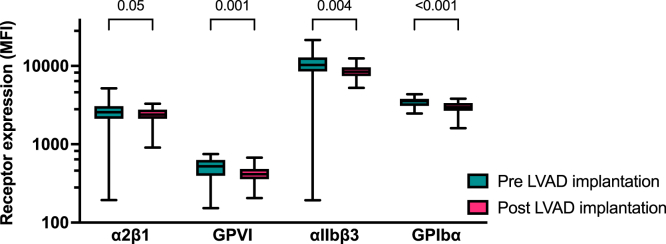
Expression of α2β1 slightly changed after CF-LVAD implantation (median decrease 10%; IQR 0-27, P = .05), whereas the decrease of GPVI expression was more pronounced (median decrease 15%; IQR 5-26, P = .001). A similar decrease was observed for the expression of αIIbβ3, which decreased by 24% (IQR -1-37%, P = .004) after implantation and GPIbα, which decreased by 17% (IQR 3-27, P < .001) after implantation (Figure 2).
Figure 2.
Surface expression of platelet receptors before and after implantation of a CF-LVAD Platelet surface expression of the collagen receptors α2β1and GPVI, the fibrinogen receptor αIIbβ3, and the Von Willebrand receptor GPIbα was measured in the absence of platelet agonists in whole blood before and 4 to 6 weeks after CF-LVAD implantation with flow cytometry (n = 32). Data are presented as median and interquartile ranges. MFI, median fluorescent intensity
3.3. Platelet reactivity toward PAR1-AP is reduced after CF-LVAD implantation
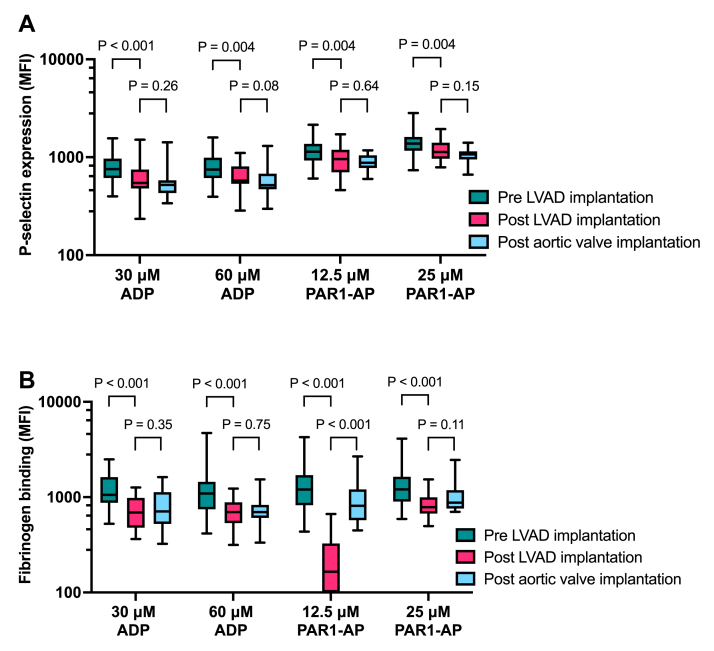
After LVAD implantation, P-selectin expression in response to platelet stimulation was reduced by 21% (IQR 2-37) for 30 μM ADP (P < .001), 16% (IQR 1-33) for 60 μM ADP (P = .004), 18% (IQR 3-35) for 12.5 μM PAR1-AP (P = .004), and 17% (IQR 4-21) for 25 μM PAR1-AP (P = .004) compared with responses before implantation (Figure 3A). P-selectin expression after biological aortic valve implantation was similar compared with responses after CF-LVAD implantation, suggesting that this observed change in P-selectin expression after CF-LVAD implantation reflects antiplatelet therapy. Fibrinogen binding after platelet stimulation was reduced by 40% (IQR 30-56) for 30 μM ADP (P < .001), 37% (IQR 9-55) for 60 μM ADP (P < .001), 86% (IQR 71-92) for 12.5 PAR1-AP (P < .001), and 37% |(IQR 15-54) for 25 μM PAR1-AP (P < .001) compared with responses before implantation (Figure 3B). Responses toward ADP after CF-LVAD implantation were similar to responses in patients who received a biological aortic valve. However, although fibrinogen binding after stimulation with an intermediate dose of PAR1-AP was almost absent after CF-LVAD implantation, fibrinogen binding was much higher after aortic valve implantation (P < .001). No differences in fibrinogen binding were observed after stimulation with a high dose of PAR1-AP.
Figure 3.
Change in platelet reactivity after implantation of a CF-LVAD or a biological aortic valve prosthesis Platelet reactivity toward the agonists ADP (30 and 60 μM) and PAR1-AP (12.5 and 25 μM) was analyzed in whole blood before implantation of a CF-LVAD and 4 to 6 weeks thereafter (n = 32) with flow cytometry measuring P-selectin expression (A) or fibrinogen binding (B). Platelet reactivity at 4 to 6 weeks after implantation of a biological aortic valve prosthesis (n = 16) was used as a control for antiplatelet therapy. Data are presented as median and interquartile ranges. MFI, median fluorescent intensity
4. Discussion
This study provides evidence of systemic platelet activation after CF-LVAD implantation despite antiplatelet therapy. Our data indicate the elevated plasma levels of βTG and sP-selectin after LVAD implantation, which coincided with a reduced expression of platelet surface markers and a more severe reduction in platelet reactivity toward PAR1-AP than can be explained by antiplatelet therapy. Combined, our data suggest that the implantation of a CF-LVAD causes low-grade platelet activation, which leads to acquired platelet dysfunction.
Our data indicate that βTG levels were already elevated in patients with end-stage heart failure before CF-LVAD implantation. Dilated cardiomyopathy is associated with abnormal rheology in the heart due to the dilated left ventricle. This causes stasis and the formation of intracardiac thrombi [18,19], which might explain this observation. Although stasis is resolved after CF-LVAD implantation, platelets are subsequently exposed to device-induced shear stress, causing shear-induced platelet activation. The observed sustained elevation of βTG levels and the increase in sP-selectin levels after implantation fit with the notion of shear-induced platelet degranulation and receptor shedding. [20] Our data fit well with other reports of platelet activation after CF-LVAD implantation. Several studies have reported elevated βTG levels [21,22] or sP-selectin levels after LVAD implantation. [21,23,24]
The analysis of changes in platelet reactivity in patients who receive intracardiac implants is hampered by the antiplatelet therapy necessary to prevent thromboembolism after implantation of such devices. Our data indicate that both P-selectin expression and fibrinogen binding on platelets in response to agonists decreased after CF-LVAD implantation, which might reflect both antiplatelet therapy and device-associated effects. To be able to differentiate between these 2 causes, we analyzed platelet reactivity in a cohort of patients who received a biological aortic valve and a similar antiplatelet regimen. Our data indicate that most of the observed changes in platelet reactivity are similar after implantation of either a CF-LVAD or a biological heart valve, suggesting that these changes are associated with antiplatelet therapy. Nevertheless, fibrinogen binding in response to stimulation of the thrombin receptor PAR-1 was 4-fold lower in patients with a CF-LVAD than in patients with a biological aortic valve, providing evidence for platelet dysfunction that cannot be explained by antiplatelet therapy alone.
Our data are in line with the reported changes in platelet reactivity toward PAR1-AP after exposure of platelets to high shear conditions. [20] We hypothesize that shear-induced platelet activation “exhausts” the platelet storage pool, leading to the reduced granule content. As the activation of the fibrinogen receptor αIIbβ3 after stimulation of PAR-1 requires co-stimulation by ADP [25] secreted from these granules, this might explain the observed decrease in reactivity toward PAR1-AP. Further studies are required to determine the mechanism behind the reduced platelet reactivity after CF-LVAD implantation.
Loss of platelet surface receptors has been linked to non-surgical bleeding in patients with a CF-LVAD. [[13], [14], [15]] In addition to the evidence of shedding of GPIbα, αIIbβ3, and GPVI, our data show reduced platelet reactivity toward agonists, offering an additional explanation for hemorrhage risk in patients with a CF-LVAD.
The strength of this study is the inclusion of patients in a stable condition, thus not in the midst of acute thrombosis or a bleeding complication that could influence platelet function. The monocentric design and the limited size of our patient cohort with a relatively short follow-up of 4 to 6 weeks are the limitations of our study. We cannot fully exclude type 1 errors because of the relatively small sample size. Sample collection was not performed at the same time of the day for all participants. Although we are not aware of diurnal variation in any of the laboratory markers, we cannot exclude this contributing to the variability between subjects. Moreover, we cannot exclude shear-dependent effects of the prosthetic valve on platelet reactivity, potentially leading to reduced platelet reactivity in the control group. This might have caused an underestimation of the effects of LVAD implantation on platelet reactivity. In addition, all but 2 patients with heart failure who were included in our study received an HM3. Although platelet reactivity was similar in patients with an HVAD and an HM3, it remains to be determined whether our data also applied to patients with a HVAD. Another potential limitation is the lack of racial heterogeneity, which might affect the generalizability of our results to a wider population. We cannot exclude that responses to intravascular medical devices differ between the White European population of our study and other racial and ethnic groups.
Despite these limitations, our data clearly provide evidence of chronic platelet activation in response to the LVAD and altered blood hemodynamics, which might result in an elevated risk of thrombosis and bleeding complications.
The current ARIES HM3 trial [26], an international, randomized, controlled trial, investigates whether aspirin may be removed safely from the antithrombotic regimen with the HM3 in an effort to reduce bleeding complications. Considering the evidence of shear-induced blood platelet disorder provided by our study, it remains to be determined whether the omission of aspirin from the antithrombotic regimen in patients with a CF-LVAD will mitigate the hemorrhage risk. The reduced platelet reactivity we observed in the peripheral blood likely reflects increased platelet activation within the CF-LVAD device, which could also contribute to the increased risk of thrombosis associated with CF-LVAD-implantation. One might argue that increasing the dose of antiplatelet therapy rather than reducing antiplatelet therapy might be required to reduce the bleeding risk owing to shear-induced acquired platelet dysfunction and thrombotic risk due to platelet activation within the CF-LVAD.
In conclusion, these data provide evidence for shear-induced platelet activation during CF-LVAD therapy and reduced platelet reactivity toward PAR1-AP after CF-LVAD implantation, which cannot be explained by antiplatelet therapy alone. We hypothesize that shear-induced platelet activation “exhausts” the platelet storage pool, leading to reduced granule content. The resulting blood platelet disorder may contribute to both the increased hemorrhage risk and the risk of thrombosis associated with CF-LVAD implantation.
Acknowledgments
We would like to thank C. Maas for his constructive feedback during writing of this manuscript.
Funding
This work was not supported by any funding agency.
Author contributions
O.C.D.L., R.T.U., S.A.E.S, J.J.R., and R.E.G.S. performed the research and analyzed data. O.C.D.L., R.T.U., L.M. de H., W.J.L.S., and R.E.G.S. designed the study and interpreted the data. O.C.D.L., R.T.U., L.M. de H., F.Z.R., W.J.L.S., and R.E.G.S. wrote the manuscript. All authors read and approved the final version of the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: M. Cushman
References
- 1.Molina E.J., Shah P., Kiernan M.S., Cornwell W.K., 3rd, Copeland H., Takeda K., et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021;111:778–792. doi: 10.1016/j.athoracsur.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 2.Fukunaga N., Rao V. Left ventricular assist device as destination therapy for end stage heart failure: the right time for the right patients. Curr Opin Cardiol. 2018;33:196–201. doi: 10.1097/HCO.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 3.Colombo P.C., Mehra M.R., Goldstein D.J., Estep J.D., Salerno C., Jorde U.P., et al. Comprehensive analysis of stroke in the long-term cohort of the MOMENTUM 3 study. Circulation. 2019;139:155–168. doi: 10.1161/CIRCULATIONAHA.118.037231. [DOI] [PubMed] [Google Scholar]
- 4.Zimpfer D., Gustafsson F., Potapov E., Pya Y., Schmitto J., Berchtold-Herz M., et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE Registry. Eur Heart J. 2020;41:3801–3809. doi: 10.1093/eurheartj/ehaa639. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin J.K., Naftel D.C., Pagani F.D., Kormos R.L., Myers S., Acker M.A., et al. Pump thrombosis in the Thoratec HeartMate II device: an update analysis of the INTERMACS Registry. J Heart Lung Transplant. 2015;34:1515–1526. doi: 10.1016/j.healun.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Mehra M.R., Goldstein D.J., Uriel N., Cleveland J.C., Jr., Yuzefpolskaya M., Salerno C., et al. MOMENTUM 3 Investigators. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. doi: 10.1056/NEJMoa1800866. [DOI] [PubMed] [Google Scholar]
- 7.Rogers J.G., Pagani F.D., Tatooles A.J., Bhat G., Slaughter M.S., Birks E.J., et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954. [DOI] [PubMed] [Google Scholar]
- 8.Jaffer I.H., Fredenburgh J.C., Hirsh J., Weitz J.I. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015;13(Suppl. 1):S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 9.Apostoli A., Bianchi V., Bono N., Dimasi A., Ammann K.R., Moiia Y.R., et al. Prothrombotic activity of cytokine-activated endothelial cells and shear-activated platelets in the setting of ventricular assist device support. J Heart Lung Transplant. 2019;38:658–667. doi: 10.1016/j.healun.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlöglhofer T., Aigner P., Migas M., Beitzke D., Dimitrov K., Wittmann F., et al. Inflow cannula position as risk factor for stroke in patients with HeartMate 3 left ventricular assist devices. Artif Organs. 2022;46:1149–1157. doi: 10.1111/aor.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potapov E.V., Antonides C., Crespo-Leiro M.G., Combes A., Färber G., Hannan M.M., et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg. 2019;56:230–270. doi: 10.1093/ejcts/ezz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah P., Yuzefpolskaya M., Hickey G.W., Breathett K., Wever-Pinzon O., Ton V.K., et al. Twelfth Interagency Registry for Mechanically Assisted Circulatory Support Report: Readmissions After Left Ventricular Assist Device. Ann Thorac Surg. 2022;113:722–737. doi: 10.1016/j.athoracsur.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arias K., Sun W., Wang S., Sorensen E.N., Feller E., Kaczorowski D., et al. Acquired platelet defects are responsible for nonsurgical bleeding in left ventricular assist device recipients. Artif Organs. 2022;46:2244–2256. doi: 10.1111/aor.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaeske K., Dieterlen M.T., Eifert S., Scholz U., Garbade J., Jawad K., et al. Device-induced platelet dysfunction in patients after left ventricular assist device implantation. J Thromb Haemost. 2021;19:1331–1341. doi: 10.1111/jth.15279. [DOI] [PubMed] [Google Scholar]
- 15.Mondal N.K., Chen Z., Trivedi J.R., Sorensen E.N., Pham S.M., Slaughter M.S., et al. Association of oxidative stress and platelet receptor glycoprotein gpibα and gpvi shedding during nonsurgical bleeding in heart failure patients with continuous-flow left ventricular assist device support. ASAIO J. 2018;64:462–471. doi: 10.1097/MAT.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Bladel E.R., Roest M., de Groot P.G., Schutgens R.E. Up-regulation of platelet activation in hemophilia A. Haematologica. 2011;96:888–895. doi: 10.3324/haematol.2011.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snoep J.D., Roest M., Barendrecht A.D., De Groot P.G., Rosendaal F.R., Van Der Bom J.G. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost. 2010;8:906–913. doi: 10.1111/j.1538-7836.2010.03786.x. [DOI] [PubMed] [Google Scholar]
- 18.Rossini L., Braun O.Ö., Brambatti M., Benito Y., Mizeracki A., Miramontes M., et al. Intraventricular flow patterns in patients treated with left ventricular assist devices. ASAIO J. 2021;67:74–83. doi: 10.1097/MAT.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 19.Craft M., Jani V., Bliamptis J., Barnes B.T., Erickson C.C., Schuster A., et al. Characterization of left ventricular cavity flow, wall stress and energy loss by color doppler vector flow mapping in children and adolescents with cardiomyopathy. Int J Cardiol Heart Vasc. 2020;32 doi: 10.1016/j.ijcha.2020.100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roka-Moiia Y., Miller-Gutierrez S., Palomares D.E., Italiano J.E., Sheriff J., Bluestein D., et al. Platelet dysfunction during mechanical circulatory support: elevated shear stress promotes downregulation of αIIbβ3 and GPIb via microparticle shedding decreasing platelet aggregability. Arterioscler Thromb Vasc Biol. 2021;41:1319–1336. doi: 10.1161/ATVBAHA.120.315583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granja T., Magunia H., Schüssel P., Fischer C., Prüfer T., Schibilsky D., et al. Left ventricular assist device implantation causes platelet dysfunction and proinflammatory platelet-neutrophil interaction. Platelets. 2022;33:132–140. doi: 10.1080/09537104.2020.1859101. [DOI] [PubMed] [Google Scholar]
- 22.Koster A., Loebe M., Hansen R., Potapov E.V., Noon G.P., Kuppe H., et al. Alterations in coagulation after implantation of a pulsatile Novacor LVAD and the axial flow MicroMed DeBakey LVAD. Ann Thorac Surg. 2000;70:533–537. doi: 10.1016/s0003-4975(00)01404-1. [DOI] [PubMed] [Google Scholar]
- 23.Tscharre M., Wittmann F., Kitzmantl D., Lee S., Eichelberger B., Wadowski P.P., et al. Platelet activation and aggregation in different centrifugal-flow left ventricular assist devices. Platelets. 2022;33:249–256. doi: 10.1080/09537104.2021.1881950. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z., Zhang J., Kareem K., Tran D., Conway R.G., Arias K., et al. Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif Organs. 2019;43:745–755. doi: 10.1111/aor.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leunissen T.C., Wisman P.P., van Holten T.C., de Groot P.G., Korporaal S.J., Koekman A.C., et al. The effect of P2Y12 inhibition on platelet activation assessed with aggregation- and flow cytometry-based assays. Platelets. 2017;28:567–575. doi: 10.1080/09537104.2016.1246713. [DOI] [PubMed] [Google Scholar]
- 26.Mehra M.R., Crandall D.L., Gustafsson F., Jorde U.P., Katz J.N., Netuka I., et al. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo-controlled, non-inferiority ARIES HM3 trial. Eur J Heart Fail. 2021;23:1226–1237. doi: 10.1002/ejhf.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]