Abstract
OBJECTIVE--To determine the distribution, activation, and tryptase/chymase phenotype of mast cells (MCs) in the rheumatoid lesion. METHODS--MC tryptase and chymase were studied by immunohistochemistry using monoclonal antibodies and examination by brightfield, interference, and fluorescent microscopy. Thirty four specimens of cartilage-pannus junction and 26 specimens of rheumatoid synovium, all derived from knee surgery, were examined. RESULTS--MCs were identified in all specimens examined, but their distribution and local concentrations varied, both within and between specimens. As a proportion of total synovial cells, there were more MCs in fibrous synovial tissues than in those with active inflammatory cell infiltrations; MCs usually showed a peripheral distribution around lymphocytic/mononuclear cell infiltrations. Most cartilage-pannus specimens demonstrated local concentrations of MCs at, or close to, sites of cartilage erosion, a significant proportion of which showed extracellular tryptase indicative of MC degranulation. MC degranulation was often associated with localised oedema and disruption of the stromal matrix. Two MC phenotypes were identified: one population contained tryptase alone (MCT) whilst another contained both tryptase and chymase (MCTC). The ratio MCT:MCTC approximated 8:1. CONCLUSIONS--This histological study demonstrated that local concentrations of MCs and their activation/degranulation are commonly observed in the rheumatoid lesion, and especially at sites of cartilage erosion. Such observations add weight to the concept that MCs contribute to the processes of inflammation, matrix degradation and tissue remodelling.
Full text
PDF


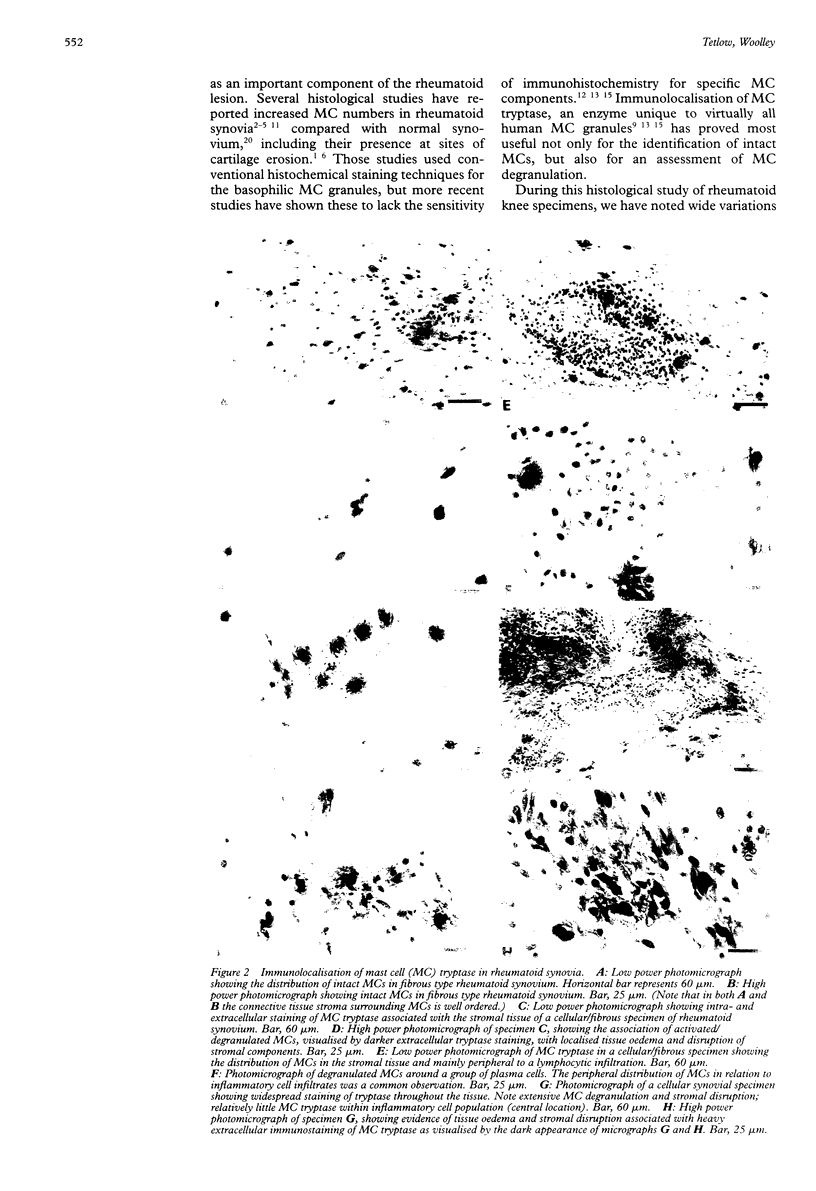
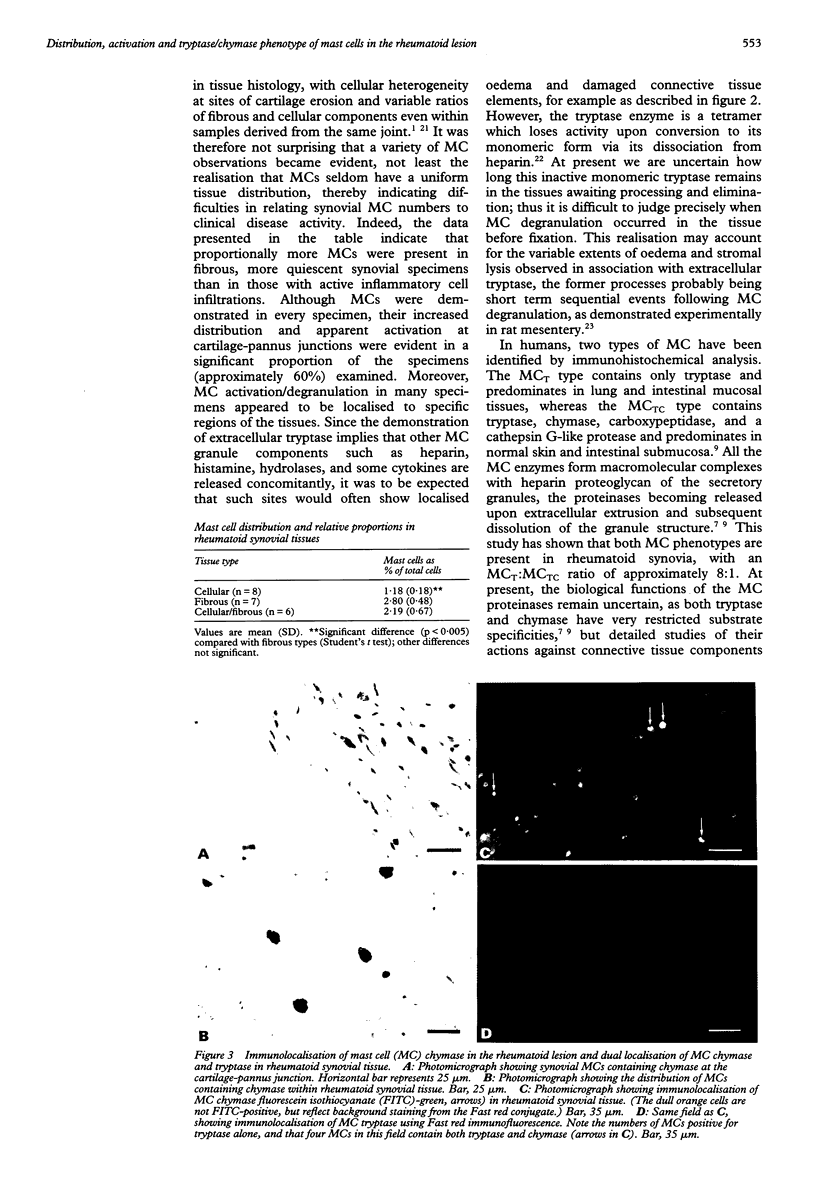


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Horisberger U., Martin U. Phagocytosis of mast cell granules by mononuclear phagocytes, neutrophils and eosinophils during anaphylaxis. Int Arch Allergy Appl Immunol. 1982;67(3):219–226. doi: 10.1159/000233022. [DOI] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Wilson S., Bardin P. G., Heusser C. H., Holgate S. T., Howarth P. H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993 Oct 1;151(7):3853–3865. [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. TNF alpha--a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992 May;31(5):293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- Bridges A. J., Malone D. G., Jicinsky J., Chen M., Ory P., Engber W., Graziano F. M. Human synovial mast cell involvement in rheumatoid arthritis and osteoarthritis. Relationship to disease type, clinical activity, and antirheumatic therapy. Arthritis Rheum. 1991 Sep;34(9):1116–1124. doi: 10.1002/art.1780340907. [DOI] [PubMed] [Google Scholar]
- CASTOR C. W. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960 Apr;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchat J. F., Henchoz S., Mazzei G., Aubry J. P., Brunner T., Blasey H., Life P., Talabot D., Flores-Romo L., Thompson J. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993 Sep 23;365(6444):340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Ilardi C., Engber W., Graziano F. M. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984 Aug;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Suzuki K., Schwartz L. B., Okada Y., Nagase H., Ramamurthy N. S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989 Nov;84(5):1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber B., Poznansky M., Boss E., Partin J., Gorevic P., Kaplan A. P. Characterization and functional studies of rheumatoid synovial mast cells. Activation by secretagogues, anti-IgE, and a histamine-releasing lymphokine. Arthritis Rheum. 1986 Aug;29(8):944–955. doi: 10.1002/art.1780290802. [DOI] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani A. M., Bradford T. R., Kepley C. L., Schechter N. M., Schwartz L. B. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989 Oct;37(10):1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- Johansson O., Virtanen M., Hilliges M., Yang Q. Histamine immunohistochemistry is superior to the conventional heparin-based routine staining methodology for investigations of human skin mast cells. Histochem J. 1994 May;26(5):424–430. doi: 10.1007/BF00160055. [DOI] [PubMed] [Google Scholar]
- Kopicky-Burd J. A., Kagey-Sobotka A., Peters S. P., Dvorak A. M., Lennox D. W., Lichtenstein L. M., Wigley F. M. Characterization of human synovial mast cells. J Rheumatol. 1988 Sep;15(9):1326–1333. [PubMed] [Google Scholar]
- Lees M., Taylor D. J., Woolley D. E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994 Jul 1;223(1):171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Vikingsson A., Seebruch J. S., Verbsky J. W., Dolan P. W. In vivo effects of nonsteroidal antiinflammatory drugs on rat skin and synovial mast cell-induced vasopermeability. Arthritis Rheum. 1991 Feb;34(2):164–170. doi: 10.1002/art.1780340206. [DOI] [PubMed] [Google Scholar]
- Norrby K., Eneström S. Cellular and extracellular changes following mast-cell secretion in avascular rat mesentery. An electron-microscopic study. Cell Tissue Res. 1984;235(2):339–345. doi: 10.1007/BF00217858. [DOI] [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen J., Kalkkinen N., Welgus H. G., Kovanen P. T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994 Jul 8;269(27):18134–18140. [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Taylor D. J., Yoffe J. R., Brown D. M., Woolley D. E. Histamine stimulates prostaglandin E production by rheumatoid synovial cells and human articular chondrocytes in culture. Arthritis Rheum. 1986 Feb;29(2):160–165. doi: 10.1002/art.1780290202. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Lees M., Ogata Y., Nagase H., Woolley D. E. Differential expression of gelatinase B (MMP-9) and stromelysin-1 (MMP-3) by rheumatoid synovial cells in vitro and in vivo. Rheumatol Int. 1993;13(2):53–59. doi: 10.1007/BF00307734. [DOI] [PubMed] [Google Scholar]
- Walsh L. J., Trinchieri G., Waldorf H. A., Whitaker D., Murphy G. F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Austen K. F. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. 1993 Mar;189(2):156–162. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Wooley D. E. Mast cell products stimulate collagenase and prostaglandin E production by cultures of adherent rheumatoid synovial cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):270–276. doi: 10.1016/0006-291x(84)90470-4. [DOI] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Woolley D. E. Mast-cell products and heparin stimulate the production of mononuclear-cell factor by cultured human monocyte/macrophages. Biochem J. 1985 Aug 15;230(1):83–88. doi: 10.1042/bj2300083. [DOI] [PMC free article] [PubMed] [Google Scholar]