Abstract
OBJECTIVE--To investigate the production of the matrix metalloproteinase (MMP), collagenase (MMP-1), and its natural inhibitor, the tissue inhibitor of metalloproteinases (TIMP) by diseased human tendon samples in organ culture. METHODS--Portions of tendons were excised from the shoulders of patients undergoing shoulder surgery, classified as either proximal to the lesion (abnormal) or distal to the lesion (normal) according to their macroscopic appearance at surgery, and placed in organ culture for periods of up to 28 days. The release of collagenase and TIMP activity in the conditioned culture medium was measured. RESULTS--Procollagenase and TIMP were both produced by all the tendon samples for an extended period of time. The levels of enzyme and inhibitor varied between patients, but in most of them TIMP levels were greater than collagenase levels. In one sample of calcified tendon, procollagenase levels were greater than those of TIMP. The mean level of collagenase produced by tendon proximal to the lesion and tendon distal to the lesion were not significantly different (95.2 (SD 106.8) U/g and 34.0 (45.3) U/g, respectively), while the corresponding figures for TIMP were 109.7 (62.3) U/g and 53.0 (27.9) U/g (p = < 0.05), although there was considerable variation in some samples. Western blotting and collagen fragment analysis confirmed that the collagenolytic activity detected was attributable to the metalloproteinase fibroblast collagenase (MMP-1). CONCLUSIONS--Tendon tissue can actively secrete procollagenase, an enzyme that, once activated, is capable of remodelling collagen, the major connective tissue component of tendon. Collagenase is produced even in unstimulated cultures, although the concentrations of TIMP are usually greater than that of collagenase in most samples. Some activation of collagenase appeared to have occurred. These results indicate that tendon tissue cells are capable of producing a remodelling response, even in end stage tendon disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews H. J., Plumpton T. A., Harper G. P., Cawston T. E. A synthetic peptide metalloproteinase inhibitor, but not TIMP, prevents the breakdown of proteoglycan within articular cartilage in vitro. Agents Actions. 1992 Sep;37(1-2):147–154. doi: 10.1007/BF01987904. [DOI] [PubMed] [Google Scholar]
- Becker H., Graham M. F., Cohen I. K., Diegelmann R. F. Intrinsic tendon cell proliferation in tissue culture. J Hand Surg Am. 1981 Nov;6(6):616–619. doi: 10.1016/s0363-5023(81)80146-3. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Murphy G., Mercer E., Galloway W. A., Hazleman B. L., Reynolds J. J. The interaction of purified rabbit bone collagenase with purified rabbit bone metalloproteinase inhibitor. Biochem J. 1983 May 1;211(2):313–318. doi: 10.1042/bj2110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard M. D., Cawston T. E., Riley G. P., Gresham G. A., Hazleman B. L. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994 Jan;53(1):30–34. doi: 10.1136/ard.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard M. D., Wright J. K., Hazleman B. L. Isolation and growth characteristics of adult human tendon fibroblasts. Ann Rheum Dis. 1987 May;46(5):385–390. doi: 10.1136/ard.46.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. S., Halverson P. B., McCarty D. J. Release of collagenase, neutral protease, and prostaglandins from cultured mammalian synovial cells by hydroxyapatite and calcium pyrophosphate dihydrate crystals. Arthritis Rheum. 1981 Nov;24(11):1338–1344. doi: 10.1002/art.1780241102. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Wright J. K., Cawston T. E., Hazleman B. L. Monoclonal antibodies against human fibroblast collagenase and the design of an enzyme-linked immunosorbent assay to measure total collagenase. Matrix. 1992 Dec;12(6):475–480. doi: 10.1016/s0934-8832(11)80092-2. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Wright J. K., Cawston T. E. Polyclonal and monoclonal antibodies against human tissue inhibitor of metalloproteinases (TIMP) and the design of an enzyme-linked immunosorbent assay to measure TIMP. Matrix. 1991 Apr;11(2):76–85. doi: 10.1016/s0934-8832(11)80211-8. [DOI] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J., Amiel D., Harper E. Collagenase production by rabbit ligaments and tendon. Connect Tissue Res. 1988;17(4):253–259. doi: 10.3109/03008208809017476. [DOI] [PubMed] [Google Scholar]
- Harper J., Amiel D., Harper E. Collagenases from periarticular ligaments and tendon: enzyme levels during the development of joint contracture. Matrix. 1989 Jun;9(3):200–205. doi: 10.1016/s0934-8832(89)80051-4. [DOI] [PubMed] [Google Scholar]
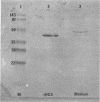
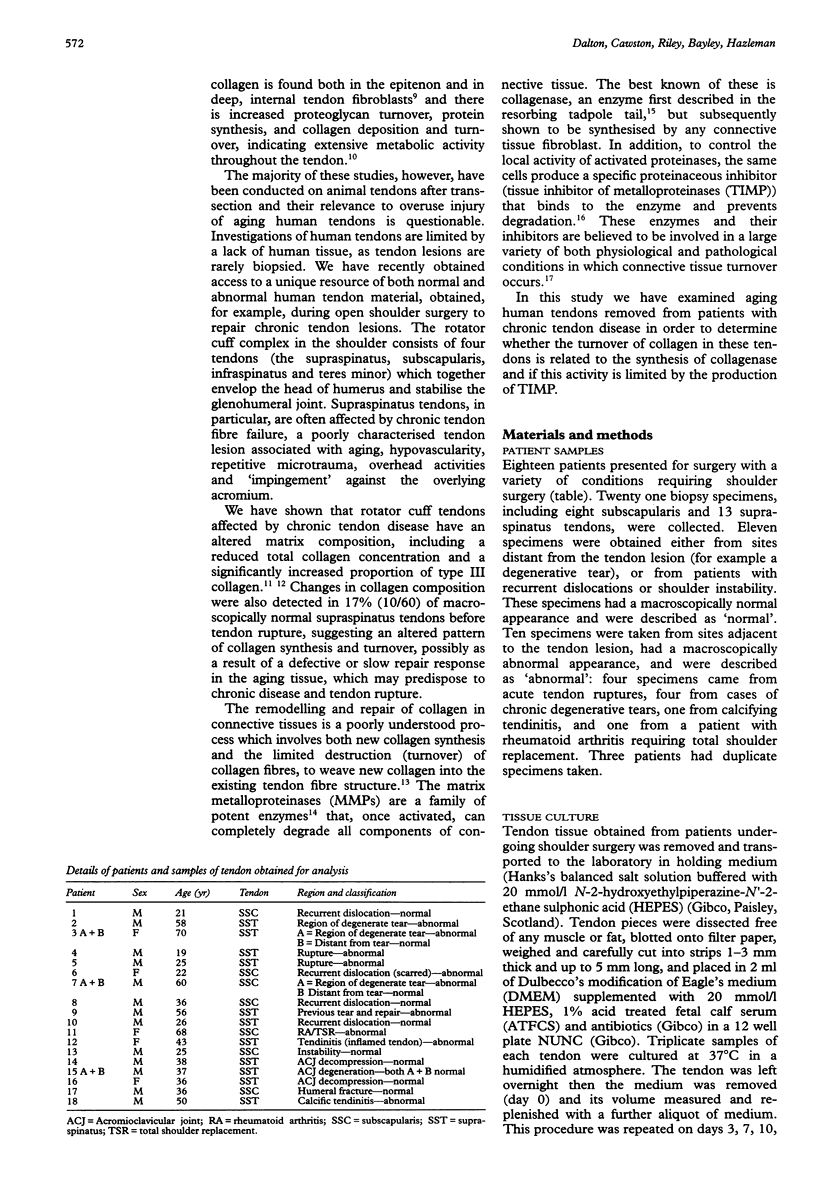
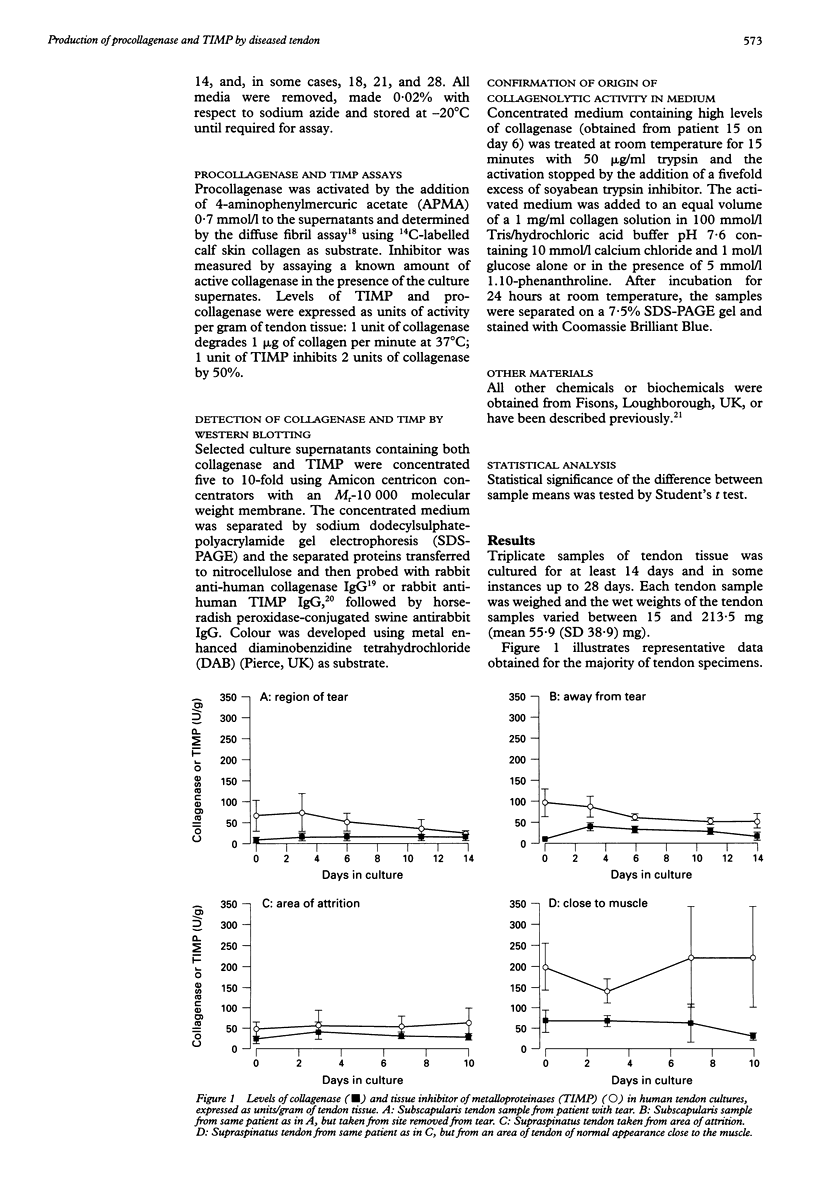
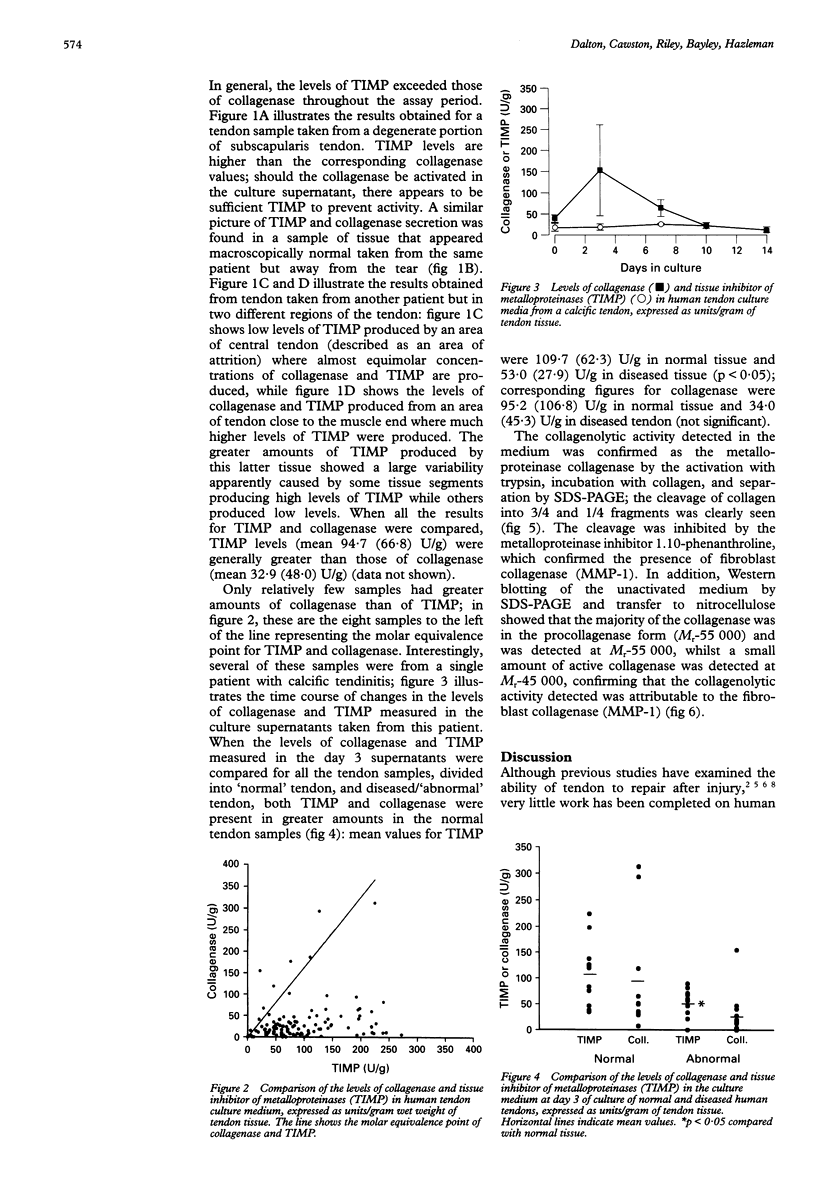
- Harper J., Amiel D., Harper E. Inhibitors of collagenase in ligaments and tendons of rabbits immobilized for 4 weeks. Connect Tissue Res. 1992;28(4):257–261. doi: 10.3109/03008209209016819. [DOI] [PubMed] [Google Scholar]
- Kannus P., Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991 Dec;73(10):1507–1525. [PubMed] [Google Scholar]
- Lundborg G., Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg Am. 1978 Jan;3(1):21–31. doi: 10.1016/s0363-5023(78)80114-2. [DOI] [PubMed] [Google Scholar]
- Manske P. R., Gelberman R. H., Vande Berg J. S., Lesker P. A. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984 Mar;66(3):385–396. [PubMed] [Google Scholar]
- POTENZA A. D. CRITICAL EVALUATION OF FLEXOR-TENDON HEALING AND ADHESION FORMATION WITHIN ARTIFICIAL DIGITAL SHEATHS. J Bone Joint Surg Am. 1963 Sep;45:1217–1233. [PubMed] [Google Scholar]
- Piening C., Riederer-Henderson M. A. Neutral metalloprotease from tendons. J Orthop Res. 1989;7(2):228–234. doi: 10.1002/jor.1100070210. [DOI] [PubMed] [Google Scholar]
- Riley G. P., Harrall R. L., Constant C. R., Chard M. D., Cawston T. E., Hazleman B. L. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994 Jun;53(6):367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G. P., Harrall R. L., Constant C. R., Chard M. D., Cawston T. E., Hazleman B. L. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994 Jun;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. E., Manske P. R. Collagen synthesis during primate flexor tendon repair in vitro. J Orthop Res. 1990 Jan;8(1):13–20. doi: 10.1002/jor.1100080103. [DOI] [PubMed] [Google Scholar]
- Squier C. A., Magnes C. Spatial relationships between fibroblasts during the growth of rat-tail tendon. Cell Tissue Res. 1983;234(1):17–29. doi: 10.1007/BF00217399. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Inhibitor of human collagenase from cultures of human tendon. J Biol Chem. 1979 Apr 25;254(8):3045–3053. [PubMed] [Google Scholar]
- Williams I. F., McCullagh K. G., Silver I. A. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12(3-4):211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]