Abstract
Objectives:
In an evolving era of immunotherapeutic options for persistent or recurrent laryngeal squamous cell carcinoma (LSCC), there is a need for improved biomarkers of treatment response and survival to inform optimal treatment selection and prognostication. Herein, our primary objective was to explore correlations between tumor infiltrating lymphocytes (TILs) and PD-L1 Combined Positive Score (CPS). Secondarily, we sought to explore their combined association with survival outcomes in patients with persistent or recurrent LSCC treated with salvage surgery.
Materials and Methods:
This was a retrospective cohort study at a single academic medical center. Immunohistochemistry staining for TILs and PD-L1 was performed on a tissue microarray of persistent or recurrent LSCC pathologic specimens. Correlations between TIL subsets and PD-L1 CPS were examined using Pearson’s correlation coefficient and survival outcomes were analyzed with the Kaplan-Meier method and log-rank tests.
Results:
Only CD103+ TILs showed a statistically significant, weakly-positive correlation with PD-L1 CPS (r2 = 0.264, p < 0.015). No other TIL subsets correlated with PD-L1 CPS in our cohort. The most favorable survival outcomes were seen in patients with pathologic N0 tumors showing high CD103+ TILs and/or high PD-L1 CPS staining.
Conclusion:
Among patients with persistent or recurrent LSCC, CD103+ TILs only modestly correlated with PD-L1 CPS. A combined biomarker score incorporating CD103+ TILs and PD-L1 CPS greatly enhanced survival discrimination. This model may have additional utility in predicting the clinical benefit of immunotherapies in persistent or recurrent LSCC in the future.
Keywords: head and neck, squamous cell carcinoma, tumor-infiltrating lymphocytes, PD-L1, combined positive score, CD103, survival, larynx
Introduction
Recent clinical trials have examined the efficacy of immune checkpoint blockade with anti-programmed death protein-1/ligand-1 (PD-1/PD-L1) inhibitors in recurrent and metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) [1,2]. Particularly in patients with higher Combined Positive Score (CPS), a measure of tumor PD-L1 staining, such inhibitors are associated with improved overall survival compared to conventional cytotoxic chemotherapeutic regimens [3,4]. However, PD-L1 CPS poorly predicts response to immune checkpoint blockade overall. Thus, there is a need for improved biomarkers of treatment response and survival in patients with R/M HNSCC to improve patient selection for immune checkpoint blockade versus alternative therapies (e.g., salvage surgery, cytotoxic chemotherapy) [5].
PD-L1 expression on tumor cells is known to be stimulated by interferon gamma (IFN-ℽ) secreted by tumor-infiltrating helper and cytotoxic T-lymphocytes (TILs) [6]. TIL density, particularly the “resident memory” cytotoxic TIL subsets expressing CD103 (integrin alpha E), are prognostic of improved survival outcomes in both primary and recurrent HNSCC [7–9]. CD103 TILs are a subset of immune cells that reside in tissues without recirculating, serving critical roles in tumor immune surveillance and immunity to infection [10]. Examining correlations between PD-L1 CPS and CD103+ TIL density in R/M HNSCC would be valuable for two principal reasons. First, it may clarify whether IFN-ℽ secretion is the predominant mechanism driving PD-L1 expression and function in the HNSCC tumor microenvironment, and whether other signaling pathways contribute. Second, a combined biomarker incorporating both CD103+ TIL density and PD-L1 CPS may improve response prediction and treatment selection in R/M HNSCC, particularly for patients being considered for immunotherapy.
We have previously published on the prognostic relationship of CD103+ TILs in a large cohort of patients with locoregionally recurrent squamous cell carcinoma of the larynx [7–9]. Herein, in the same patient cohort, our primary objective was to examine how CD4+, CD8+, and CD103+ TIL subsets correlate with PD-L1 CPS. Our secondary objective was to examine how CD103+ TILs and PD-L1 CPS may predict survival outcomes in persistent or recurrent laryngeal squamous cell carcinoma. Uniquely, we used an FDA-approved, companion diagnostic PD-L1 CPS assay (22C3 clone, Agilent pharmDx kit) widely employed in pivotal clinical trials of immune checkpoint blockade in R/M HNSCC. In a population of patients with recurrent laryngeal squamous cell carcinoma specifically, we hypothesized that CD103+ TILs and PD-L1 CPS would positively correlate and together, would more robustly predict survival.
Materials and Methods
Patient cohort
This was a retrospective analysis of patients with persistent or recurrent squamous cell carcinoma of the larynx (LSCC) presenting to our institution from 1997 to 2014. Included patients had 1) biopsy proven LSCC; 2) persistent or recurrent disease at the primary site after radiation or chemoradiation for early-stage versus late-stage tumors, respectively; 3) surgical salvage with total laryngectomy and neck dissection(s); and 4)sufficient tumor in our tissue microarray for TIL and PD-L1 analysis. Characteristics of included patients are shown in Table 1 [7,8]. Tumors were staged according to the American Joint Committee (AJCC) Staging System, 7th edition [11]. This study was approved by the University of Michigan Institutional Review Board (HUM00080561).
TABLE 1.
Characteristics of patient cohort. Data presented as n (%) or median (range).
| Cohort (n = 86) | |
|---|---|
|
| |
| Sex | |
| Male | 74 (86.0) |
| Female | 12 (14.0) |
|
| |
| Ethnicity | |
| Caucasian | 79 (91.9) |
| Black | 4 (4.7) |
| Other/Unk | 3 (3.4) |
|
| |
| Age at Initial Diagnosis, y | 56.5 (40.0 – 82.0) |
|
| |
| Primary Site | |
| Supraglottis | 41 (47.7) |
| Glottis | 45 (52.3) |
|
| |
| Initial Treatment | |
| Radiation | 46 (53.4) |
| Chemoradiation | 40 (46.6) |
|
| |
| Age at Recurrence, y | 58.5 (42.0 – 84.0) |
|
| |
| Time to Recurrence, mo | 12 (2.0 – 217.0) |
|
| |
| Recurrent Pathologic Stage | |
| I | 1 (1.2) |
| II | 18 (20.9) |
| III | 24 (27.9) |
| IV | 43 (50.0) |
Immunohistochemistry
A tissue microarray (TMA) of salvage laryngectomy tumor specimens was constructed, as previously described [7,8]. We previously performed and reported immunohistochemistry (IHC) staining for specific TIL subsets (i.e., CD4+, CD8+, and CD103+) using our lab’s established heat-induced epitope retrieval protocol and the following monoclonal antibodies: CD103–1:500 (Abcam Ab129202); CD4–1:250 (Abcam Ab486); CD8–1:40 (Novocastra VP-C320) [7,8]. Here, we performed IHC staining on independent TMA slides for PD-L1 content using the automated PD-L1 IHC 22C3 pharmDx kit (Agilent), according to manufacturer’s instructions [12]. This clinical PD-L1 assay is an FDA-approved companion diagnostic for Keytruda® (Merck & Co., Inc.) and has been employed in several pivotal trials of pembrolizumab for treatment of head and neck squamous cell carcinoma [3,13].
TIL and PD-L1 scoring
TMA cores consisting of < 50 % parenchymal tumor, those with extensive tumor necrosis, and partial cores were excluded from TIL and PD-L1 quantification [7,8]. Intratumoral CD4+, CD8+, and CD103+ TIL subsets were manually counted by two independent blinded reviewers (JDS, JEM) at 200x magnification (20x objective lens). Mean TIL counts per triplicate tumor cores were calculated and averaged and previously reported [7,8]. An expert head and neck pathologist (JBM) quantified PD-L1 expression using the validated Combined Positive Score (CPS), defined as: [number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages)/total number of viable tumor cells] x 100 %.3,4 CPS was averaged across tumor cores per individual patient for input into statistical analyses.
Statistical analysis
Independent correlations between specific TIL subsets (as continuous variable) and PD-L1 CPS were examined using Pearson’s correlation coefficient (negligible correlation: 0 < r2 ≤ 0.19; low correlation: 0.20 ≤ r2 ≤ 0.39; moderate correlation: 0.40 ≤ r2 ≤ 0.59; high correlation: 0.60 ≤ r2 ≤ 0.79; very high correlation: 0.80 ≤ r2 ≤ 1.0.) Wilcoxon Rank-Sum test was additionally used to assess correlations between TIL subsets and PD-L1 CPS with the latter dichotomized as PD-L1 CPS = 0 or PD-L1 CPS > 0. The Kaplan-Meier method and log-rank tests were employed to assess combinations of CD103+ TILs and PD-L1 CPS for prediction of overall survival (OS; time from salvage laryngectomy to death from any cause), disease-specific survival (DSS; time from salvage laryngectomy to death from any disease recurrence/persistence), and disease-free survival (DFS; time from salvage laryngectomy to any disease recurrence/persistence). These biomarkers were split into binary categories for statistical analyses: PD-L1 CPS > 0 was considered “high” and CD103+ TIL level > 11 was considered “high” based on previously published receiver operating characteristic (ROC) curve analysis on our cohort (Figure 1) [8]. Cox proportional hazards models were employed to explore associations with survival outcomes for CD103+ TILs and PD-L1 CPS individually, in combination with one another, and in multivariable models controlling for pathological nodal stage, (the strongest predictor of recurrence in prior studies) of the recurrent tumor [14].
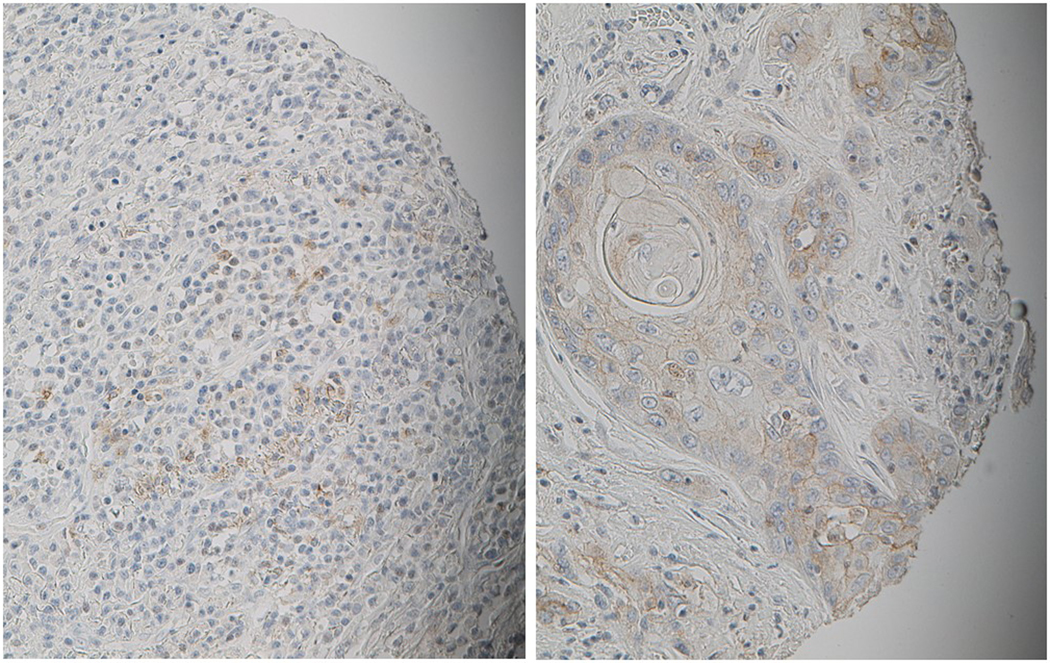
FIGURE 1.
Representative IHC images showing tumor cores staining PD-L1 CPS “high” (20x magnification).
Results
There was a total of 86 patients with persistent or recurrent LSCC and available tumor for PD-L1 CPS quantification (Table 1). Tumoral TIL counts (number of tumors, median [range]) were as follows: CD4+ TILs (n = 55, 0 [0–50]); CD8+ TILs (n = 76, 25 [0–150]); CD103+ TILs (n = 85, 13 [0 – 225]). Note that the n for TIL subsets was less than the total population (n = 86) due to depletion of available cores for TIL staining and quantification. Of 86 tumors, the median (range) PD-L1 CPS was 0 (0 – 100). Most tumors (n = 62, 72.1 %) had a CPS of 0. The remaining had CPS ≥ 1 (n = 24, 27.9 %) or CPS ≥ 20 (n = 17, 19.8 %).
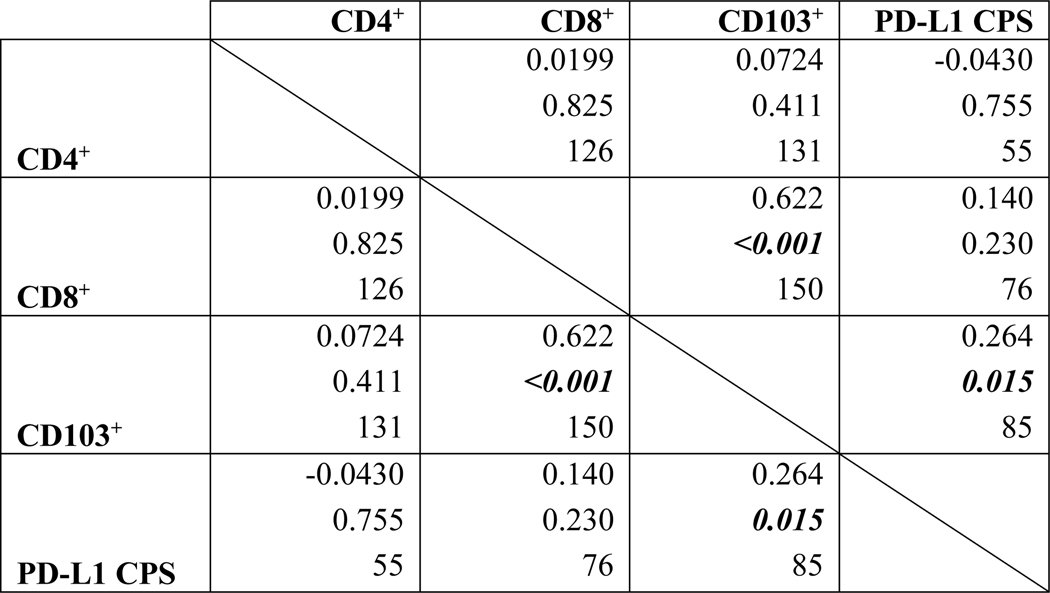
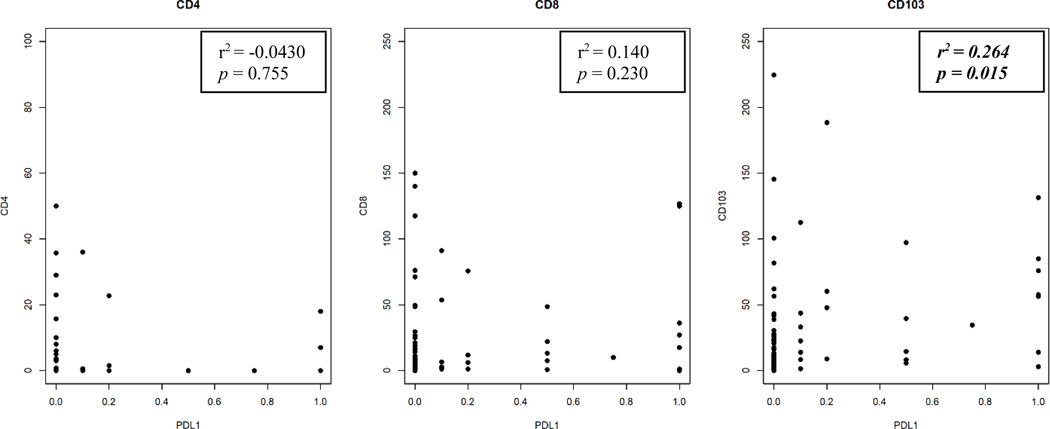
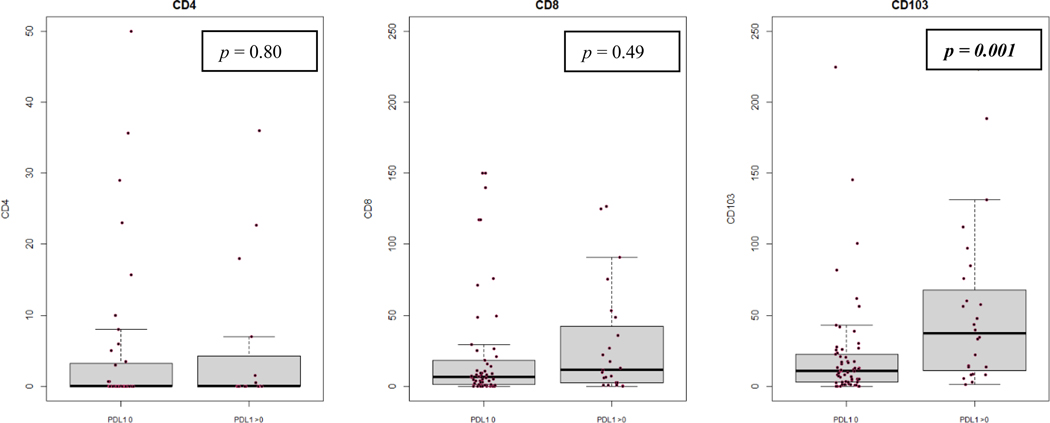
There is a statistically significant, positive but low (i.e., 0.20 ≤ r2 ≤ 0.39) correlation between CD103+ TILs and PD-L1 CPS in our cohort when both variables were analyzed as continuous variables (Pearson’s r2 = 0.264, p = 0.015) (Figures 2, 3). There remained a statistically significant difference in CD103+ TIL content when tumors were dichotomized into groups with PD-L1 CPS 0 or > 0 (p = 0.001) (Figure 4). PD-L1 CPS showed negligible correlation with either CD4+ or CD8+ TIL subsets in our cohort when analyzed as either a continuous variable (CD4+ TILs with PD-L1 Pearson’s r2 = − 0.0430, p = 0.755; CD8+ TILs with PD-L1 Pearson’s r2 = 0.140, p = 0.230) or categorical variable dichotomized at the median (CD4+ TILs p = 0.230, CD8+ TILs p = 0.490).
FIGURE 2.
Correlations between specific TIL subsets and PD-L1 CPS in persistent or recurrent LSCC. Within each cell, the top line denotes Pearson’s r followed by the p-value, then number of observations. Statistically significant correlations bolded and italicized.
FIGURE 3.
Scatterplots for correlations between TIL subsets and PD-L1 CPS in persistent or recurrent LSCC. Dots represent individual tumors.
FIGURE 4.
Box and whisker plots for correlations between TIL subsets and PD-L1 CPS in persistent or recurrent LSCC, with PD-L1 CPS dichotomized into cutoff of 0 or > 0. Dots represent individual tumors.
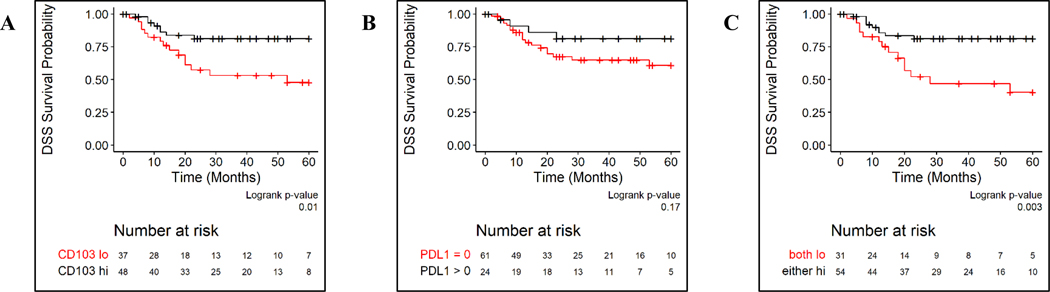
We then assessed the relationship between combinations of CD103+ TILs and PD-L1 CPS on survival outcomes in persistent or recurrent LSCC. Patients with PD-L1 CPS high tumors did not have statistically significant differences in OS (log rank p = 0.20), DSS (log rank p = 0.17) or DFS (p = 0.40) (Figure 5, Supplemental Figure). We split patients into two groups, those whose tumors were both CD103+ TILs and PD-L1 CPS low and those whose tumors were CD103+ TILs high and/or PD-L1 CPS high. This categorization significantly enhanced OS, DFS, and DSS discrimination in our cohort (Figure 5, Supplemental Figure). In Cox proportional hazards models, patients whose tumors were CD103+ TILs high and/or PD-L1 CPS high had the most favorable OS, DSS, and DFS (Table 2).
FIGURE 5.
Kaplan-Meier curves for disease-specific survival (DSS) based on CD103+ TILs (panel A), PD-L1 CPS (panel B), and combinations of CD103+ TILs and PD-L1 CPS (panel C).
TABLE 2.
Univariable and multivariable Cox proportional hazards models for associations between combinations of CD103+ TILs and PD-L1 CPS and survival outcomes. “Discordant” refers to CD103+ TILs high, PD-L1 CPS low and CD103+ TILs low, PD-L1 CPS high tumors. HR = hazard ratio.
| OS | DSS | DFS | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Univariable Models | ||||||
| CD103+ TILs high | 0.54 (0.28, 1.02) | 0.06 | 0.35 (0.15, 0.82) | 0.03 | 0.44 (0.20, 0.94) | 0.03 |
| PD-L1 CPS high | 0.61 (0.28, 1.34) | 0.22 | 0.48 (0.16, 1.41) | 0.18 | 0.70 (0.28, 1.74) | 0.44 |
| Multivariable Models | ||||||
| Discordant vs. Both Low | 0.49 (0.24, 0.99) | 0.05 | 0.31 (0.12, 0.80) | 0.02 | 0.52 (0.23, 1.20) | 0.52 |
| Both High vs. Both Low | 0.45 (0.18, 1.12) | 0.09 | 0.29 (0.08, 1.03) | 0.05 | 0.40 (0.13, 1.23) | 0.11 |
| Discordant and Both High vs. Both Low | 0.48 (0.25, 0.90) | 0.02 | 0.30 (0.13, 0.70) | 0.005 | 0.48 (0.22, 1.02) | 0.06 |
The clinical variable most strongly associated with survival outcomes in our previous studies was pathological nodal stage of the recurrent tumor (pN0 vs. pN+) [7,8]. We performed a multivariable Cox proportional hazards model for associations between combinations of CD103+ TILs and PD-L1 CPS and survival outcomes adjusted for recurrent pathologic nodal stage. In this model, patients whose tumors were CD103+ TILs high and/or PD-L1 CPS high continued to show better OS (HR: 0.45 [95 % CI: 0.24 – 0.86] p = 0.02, DSS (HR: 0.28 [95 % CI: 0.12 – 0.64] p = 0.003, DFS (HR: 0.40 [95 % CI: 0.19 – 0.86] p = 0.02.
Discussion
In a rapidly evolving era of immunotherapy for persistent or recurrent LSCC, predicting which patients will benefit from anti-PD-L1/PD-1 therapy remains challenging [15,16]. PD-L1 CPS is predictive of a survival benefit to pembrolizumab in first-line treatment of metastatic HNSCC, including laryngeal subsites [1,3,4,13]. However, it is not predictive of response to immunotherapy in a clinically meaningful way. The cellular mechanisms influencing intratumoral heterogeneity in PD-L1 expression and function in persistent or recurrent LSCC and R/M HNSCC more broadly remain enigmatic. A better understanding of these mechanisms likely holds the key to improving the rate and durability of immunotherapy response in these patients. This study adds to a body of literature supporting the utility of anti-PD-1 therapy in recurrent HNSCC populations. Combining CD103+ TILs with PD-L1 CPS may identify a more specific cohort in which anti-PD-1 therapy may be associated with better responses and outcomes.
In the tumor microenvironment of HNSCC, a subset of cytotoxic CD8+ TILs co-express CD103 (integrin αE), a protein that localizes CD8+ TILs to the epithelium and confers potent recognition of and reactivity against tumor cells [17–19]. Traditionally, CD8+ TILs have been considered to be the main secretors of IFNℽ that increases antigen expression and pro-inflammatory cytokine production in the tumor microenvironment to promote tumor cell kill [18]. In this model, IFNℽ effects are countered by a pro-carcinogenic, concomitant increase in PD-L1 expression on tumor cells promoting T-cell exhaustion and tumor immune evasion [20].
This relationship suggests a strong positive correlation between CD8+ TILs and PD-L1 expression in individual tumors. In two large retrospective studies of human papillomavirus-negative HNSCC, CD8+ TIL density strongly correlated with increased PD-L1 expression, and higher numbers of both portended improved overall- and disease-specific survival [21,22]. However, other authors have found no relationship between these biomarkers in similarly designed studies [23,24]. The true relationship may require a more nuanced understanding of the tumor-immune microenvironment. For instance, how co-expression of CD103 on CD8+ TILs may impact tumoral PD-L1 expression and function in HNSCC remain unexplored. Our results suggest that CD8+ TILs co-expressing CD103 may have a central role in IFNℽ-mediated upregulation of PD-L1 in the tumor microenvironment.
Alternatively, the low positive correlation between CD103+ TILs and PD-L1 CPS seen in our study may support the existence of alternative cellular mechanisms of PD-L1 regulation in the LSCC tumor microenvironment, independent of TIL-mediated IFNℽ signaling. Concha-Benavente et al showed that epidermal growth factor receptor (EGFR) activation is a potent inducer of PD-L1 expression in HNSCC cells in a Janus kinase (JAK2)-dependent manner [25]. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) correlates with PD-L1 overexpression and an immunosuppressive tumor phenotype [26]. Further, we have recently shown that tumor infiltrating microbes have the potential to modify tumoral PD-L1 expression in a TLR2-dependent manner in vitro, suggesting a more complex and dynamic landscape for PD-L1 regulation and immune evasion in HNSCC [27].
In our previous studies examining the prognostic value of TILs in persistent or recurrent LSCC, high CD103+ TIL density was the strongest predictor of improved overall-, disease-free, and disease-specific survival [7,8]. Independently, PD-L1 CPS did not reach statistical significance for prognostication in our cohort. In two recent studies of primary LSCC, PD-L1 CPS > 1 was independently associated with improved recurrence rates and disease-free survival, but not overall survival [28, 29]. That PD-L1 CPS was not independently predictive or prognostic in our cohort suggests a more complex tumor immune microenvironment with dynamic mechanisms of immune-evasion in persistent or recurrent LSCC. In our multivariable models, combining CD103+ TILs and PD-L1 CPS greatly enhanced survival discrimination, suggesting a synergistic impact of these variables on immune-reactivity and tumor control that merits validation in a different cohort. This may provide an opportunity for more personalized, accurate prediction of survival outcomes in persistent or recurrent LSCC beyond our current paradigms. Future investigation into the utility of a combined biomarker incorporating both CD103+ TIL density and PD-L1 CPS for predicting immunotherapy response in persistent or recurrent LSCC is also promising. For instance, our biomarker could potentially serve as a companion diagnostic in persistent or recurrent LSCC to select patients for immunotherapy-based approaches in place of, or as an adjuvant to, salvage surgery.
Our study is strengthened by using a clinically validated PD-L1 IHC platform (Agilent 22C3 PD-L1 pharmDx kit) with scoring done by an expert head and neck pathologist [3,13]. Previous studies employing PD-L1 IHC in HNSCC are characterized by inconsistent PD-L1 staining patterns, methodology, and scoring with resultant limitations on reproducibility and generalizability of findings [30]. Our methodology for PD-L1 CPS quantification enhances the translational relevance of our findings. Our study is limited by lack of correlation of PD-L1 CPS with genomic and transcriptomic alterations that may impact the tumor-immune microenvironment and survival outcomes, as we have previously published these data in this cohort [7,8,31]. All patients in our cohort were treated with salvage laryngectomy and tumor tissue for this study was obtained at the time of surgery. None had been treated with immunotherapy prior to study enrollment and too few were later treated with immunotherapy to warrant valid statistical comparisons. Thus, our biomarker model is solely prognostic of survival outcomes, not predictive of immunotherapy response, and will require validation in this setting. Finally, it is important to note that our findings were demonstrated using tissue microarray staining ensuring greater technical consistency among patient samples. Such findings in whole tissue sections have not been reported previously and correlations of TMA TIL counts and whole section counts have not been reported.
Conclusions
In a large cohort of persistent or recurrent LSCC, CD103+ TILs correlated positively with PD-L1 CPS. Thus, CD103 may define a subset of cytotoxic TILs with enhanced capacity for IFNℽ-mediated PD-L1 upregulation in the tumor microenvironment. However, the overall weak correlations between TIL subsets and PD-L1 CPS seen in our study supports further exploration of alternative, potentially targetable cellular mechanisms of PD-L1 regulation in LSCC. A combined biomarker model incorporating CD103+ TILs and PD-L1 CPS enhanced prognostication with potential implications for personalized patient counseling and immunotherapy treatment selection. However, our findings require validation in larger prospective cohorts.
Supplementary Material
SUPPLMENTAL FIGURE. Kaplan-Meier curves for overall survival (OS) based on CD103+ TILs (panel A), PD-L1 CPS (panel B), and combinations of CD103+ TILs and PD-L1 CPS (panel C). Curves additionally shown for disease-free survival (DFS) based on CD103+ TILs (panel D), PD-L1 CPS (panel E), and combinations of CD103+ TILs and PD-L1 CPS (panel F).
Recurrent larynx cancers vary in tumor-infiltrating lymphocytes and PD-L1 staining
CD103+ lymphocyte infiltration only modestly correlates with PD-L1 staining
CD4+ and CD8+ lymphocyte infiltration does not correlate with PD-L1 staining
CD103+ lymphocytes and PD-L1 staining predict survival in recurrent larynx cancer
References
- 1.Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomized, open-label, phase 3 study. Lancet. 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 2.Sacco AG, Chen R, Worden FP, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–92. [DOI] [PubMed] [Google Scholar]
- 3.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomized, open-label, phase 3 study. Lancet. 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 4.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol. 2019;99:104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoesli R, Birkeland AC, Rosko AJ, et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol. 2018;77:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JE, Smith JD, Birkeland AC, et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol Immunother. 2019;68(2):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spector ME, Bellile E, Amlani L, et al. Prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145(11):1012–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rischin D, Mehanna H, Young RJ, et al. Prognostic stratification of HPV-associated oropharyngeal cancer based on CD103+ immune cell abundance in patients treated on TROG 12.01 and De-ESCALaTE randomized trials. Ann Oncol. 2022;33(8):804–13. [DOI] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer (2010) AJCC Cancer Staging Manual. 7th. Springer Press, Chicago. [Google Scholar]
- 12.PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2020. [Google Scholar]
- 13.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicenter, phase 1b Trial. Lancet Oncol. 2016;17(7):956–65. [DOI] [PubMed] [Google Scholar]
- 14.Birkeland AC, Beesley L, Bellile E, et al. Predictors of survival after total laryngectomy for recurrent/persistent laryngeal squamous cell carcinoma. Head Neck. 2017;39(12):2512–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dy Evrard, Hourseau M, Couvelard A, et al. PD-L1 expression in the microenvironment and the response to checkpoint inhibitors in head and neck squamous cell carcinoma. Oncoimmunology. 2020;9(1):1844403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wondergem NE, Nauta IH, Muijlwijk T, Leemans CR, van de Ven R. The immune microenvironment in head and neck squamous cell carcinoma: on subsets and subsites. Curr Oncol Rep. 2020;22(8):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesan A, Clarke J, Wood O, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nature Immunol. 2017;18:940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duhen T, Duhen R, Montler R, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9(1):2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younghoon K, Shin Y, Kang GH. Prognostic significance of CD103+ immune cells in solid tumor: a systematic review and meta-analysis. Sci Rep. 2019;9:3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Canteli M, Granda-Diaz R, del Rio-Ibisate N, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2020;69:2089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, Tian S, Lin L, Zhang J, Ding H. Prognostic and clinicopathological significance of PD-L1 and tumor infiltrating lymphocytes in hypopharyngeal squamous cell carcinoma. Oral Oncol. 2020;102:104560. [DOI] [PubMed] [Google Scholar]
- 23.Lechner A, Schlober H, Rothschild SI, et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8:44418–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngamphaiboon N, Chureemas T, Siripoon T, et al. Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and p16 status in head and neck squamous cell carcinoma. Med Oncol. 2019;36(2):21. [DOI] [PubMed] [Google Scholar]
- 25.Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNℽ that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bu LL, Yu GT, Wu L, et al. STAT3 induces immunosuppression by upregulating PD-1/PD-L1 in HNSCC. J Dent Res. 2017;96(9):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann JE, Ludwig ML, Kulkarni A, et al. Microbe-mediated activation of toll-like receptor 2 drives PDL1 expression in HNSCC. Cancers (Basel); 2021;13(19):4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franz L, Alessandrini L, Calvanese L, Crosetta G, Chiara Frigo A, Marioni G. Angiogenesis, programmed death ligand 1 (PD-L1) and immune microenvironment association in laryngeal carcinoma. Pathology. 2021;53(7):844–51. [DOI] [PubMed] [Google Scholar]
- 29.Alessandrini L, Franz L, Ottaviano G, et al. Prognostic role of programmed death ligand 1 (PD-L1) and the immune microenvironment in laryngeal carcinoma. Oral Oncol. 2020;108:104836. [DOI] [PubMed] [Google Scholar]
- 30.De Keukeleire SJ, Vermassen T, Hilgert E, Creytens D, Ferdinande L, Rottey S. Immuno-oncological biomarkers for squamous cell cancer of the head and neck: Current state of the art and future perspectives. Cancers (Basel). 2021;13:1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heft Neal ME, Bhangale AD, Birkeland AC, et al. Prognostic significance of oxidation pathway mutations in recurrent laryngeal squamous cell carcinoma. Cancers (Basel). 2020;12(11):3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLMENTAL FIGURE. Kaplan-Meier curves for overall survival (OS) based on CD103+ TILs (panel A), PD-L1 CPS (panel B), and combinations of CD103+ TILs and PD-L1 CPS (panel C). Curves additionally shown for disease-free survival (DFS) based on CD103+ TILs (panel D), PD-L1 CPS (panel E), and combinations of CD103+ TILs and PD-L1 CPS (panel F).