Abstract
Rosuvastatin (RST) is a poorly water-soluble drug responsible for limited in vivo dissolution and subsequently low oral systemic absorption (poor bioavailability). The mole fraction solubility values of RST in various ratios of binary mixtures “{PEG400 (1) + water (2)}” at 298.15 K were employed to investigate the preferential solvation (PS) of RST (3) by the binary components. Moreover, the GastroPlus program predicted the drug dissolution/absorption rates, plasma drug concentration, and compartmental regional drug absorbed from a conventional tablet as compared to the RST-loaded (PEG400 + water) mixture (at x1 = 0.5) in healthy subjects (considering the fast condition). Fedors’ method was adopted to estimate the values of molar volume (314.8 cm3·mol–1) and Hildebrand solubility parameter (28.08 MPa1/2) of RST. The results of inverse Kirkwood–Buff integrals showed the PS of RST by PEG400 as observed in all studied ratios of the binary mixture. The highest PS value (δx1,3 = 1.65 × 10–2) for RST by PEG400 was attained at x1 = 0.5. Finally, the GastroPlus program predicted the maximum dissolution rate [20 mg within 15 min as compared to pure RST (1.5 mg within 15 min)]. Moreover, the program predicted increased in vivo oral absorption (1.2 μg/mL) and enhanced regional absorption (95.3%) of RST from upper segments of the gastrointestinal tract for the RST-loaded PEG400 + water mixture in humans as compared to conventional tablets (87.5% as total regional absorption and 0.88 μg/mL as in vivo absorption). Hence, the present binary system ferrying RST can be a promising strategy to control systemic dyslipidemia after oral or subcutaneous administration.
1. Introduction
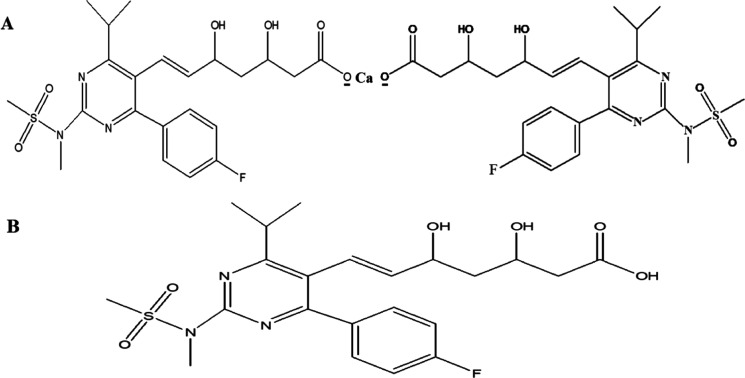
Rosuvastatin (RST) is a potential hypolipidemic agent that acts by selective and competitive inhibition of the HMG-CoA (hydroxymethyl-glutaryl co-enzyme A) reductase enzyme for conversion to mevalonate (a cholesterol precursor). In addition, the drug has also been reported for the treatment of Alzheimer’s, benign prostate hyperplasia, and osteoporosis.1 Chemically, the drug is named (6E)-7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl} hept-6-enoic acid. It has the molecular formula of C22H28FN3O6S, with a molar mass of 481.5 g·mol–1 and a pKa value of 4.0 (derived from hept-6-enoic acid) (Figure 1).1,2 RST is a lipophilic (log P = 2.4) candidate with low water solubility (0.33 mg/mL), poor in vivo dissolution in physiological fluids, and limited oral absorption resulting in least oral bioavailability (∼20%).3
Figure 1.
(A) Chemical structure of hemi calcium salt of RST used for solubility study and (B) structure of RST{(a synthetic statin and a conjugate acid of RST (1−)} used as the input parameter of GastroPlus simulation and prediction software.
In the published literature, several authors have reported the RST solubility in various neat solvents (water, ethanol, 2-butanol, 1-butanol, propylene glycol, isopropyl alcohol, and ethylene glycol) over a wide range of temperature (298.15–318.15 K), surfactants, co-surfactants, and lipids. Maximum solubility values of RST in propylene glycol (PG) and (polyethylene glycol 400) PEG400 were reported as 1.89 × 10–2 (mole fraction solubility at 318.15 K) and 65.7 mg/g, respectively.3,4 Several authors used ethanol, PEG200, and PEG400 as cosolvents with water to form new mixture ratios to improve lipophilic drug solubility in the explored temperature range.5−7 The concept of cosolvency technique has widely been used for formulation development, water treatment from wastewater treatment plant, and purification studies.
PEG400 is capable of solubilizing various lipophilic drugs with high safety (oral and parenteral). The reported oral dose of RST is approximately in the range of 5–40 mg daily for adults.3 The dose can also be delivered using the biocompatible {PEG400 (1) + water (2)} cosolvent mixture for the alternative route (such as subcutaneous) of drug administration to the oral dosage form. Hence, the present study examined the solubilization of RST in the aqueous solution of PEG400 at 25.0 °C and varied ratios of PEG400 in water. Thus, the PS study of RST would help investigate the dissolution-associated mechanisms in the binary system (PEG400 + water). This may establish a conclusive understanding of RST dissolution processes of the solute (RST) in the binary system and PS of RST by PEG400 in the same binary system using various solubility models.8−10 The PS and equilibrium solubility of RST are important parameters for standardization, formulation design, method development for quantification, and a thorough understanding of the molecular mechanisms [hydrogen bonding, free energy, and preferential solvation (PS)] involved in the physical stability during the dissolution process. In general, the solubility (equilibrium solubility) of an active ingredient and its physicochemical properties crucially dictate pharmacokinetic profiles of the dosage form intended for parenteral, oral, transdermal, and subcutaneous delivery.11 The data obtained from the equilibrium solubility study allow us to determine PS parameters of RST by each component of the binary system. The estimated parameters are powerful tools to investigate the molecular understanding of interaction for in vitro or in vivo dissolution mechanisms/processes using the targeted binary system.9,10
The drug is associated with limited aqueous solubility and solubility in buffer. We aimed to understand the PS behavior of polyethylene glycol 400 (as a cosolvent) in the binary mixture of different ratios. Moreover, the program was used to predict the optimized ratio of the binary mixture for in vivo behavior (increased in vivo dissolution and subsequent in vivo absorption for maximum oral bioavailability) in humans based on the solubility data. It was imperative to recognize the PS behavior of PEG400 in the binary system so that it can be administered in aqueous parenteral, subcutaneous, and oral dosage forms to control hyperlipidemia. Moreover, we attempted to identify a biocompatible and recommended water–soluble cosolvent for maximum drug solubility, stability, and within safe concentration. Therefore, an attempt has been made to investigate PS parameters of RST in the binary system {(PEG400 + water)} employing the equilibrium solubility data. This would be informative to understand the molecular interactions (hydrogen bonding, free energy, and PS) involved in the drug dissolution process at room temperature (25.0 °C) in the (PEG400 + water) mixtures. The equilibrium study of RST was carried out in pure solvent(s) and in various mass ratios (i.e., 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.5, 0.7, 0.8, 0.9, and 1.0) of PEG400 and water at 298.15 K. The PS parameter(s) of RST by the individual constituent of “PEG400 + water” system have not been studied so far. For this, the present study addressed the effect of the individual constituent in the targeted binary system on RST dissolution and subsequent determination of the PS parameters of RST in the binary system conformed by each solvent by applying several models. Furthermore, the GastroPlus program offered to simulate and predict in vivo performance of the binary system in humans and comparison against the conventional dosage form. The program is one of the best tools to predict bio-pharmacokinetics utilizing experimental data, by-default values, and literature-based findings.
2. Materials and Methods
2.1. Materials
Rosuvastatin calcium (RST, >98.0% pure) was obtained as a gift sample from Aurobindo Pharma, Hyderabad, India (Table 1) (provided by Dr Mohd Neyaz Ahsan, BIT, Mesra, Ranchi, India). RST (rosuvastatin hemicalcium) was chemically (E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl]-(3R,5S)-3,5-dihydroxyhept-6-enoic acid, calcium salt (2:1). Acetonitrile, methanol, PEG400, and phosphoric acid were used for the formulation studies, and these were purchased from Sigma-Aldrich, India. Phosphoric acid was used to set the final pH of the mobile phase. PEG400 and distilled water served as the binary mixture system in varied ratios. All reagents were of AR (analytical reagent) grade (Table 1).
Table 1. Summary of the Solvents for Purity and Source.
| name | source | purity (mass fraction) | analytical grade |
|---|---|---|---|
| PEG400a | Sigma-Aldrich | >0.99 | HPLCb |
| ACNc | Sigma-Aldrich | 0.989 | HPLCb |
| methanol | Sigma-Aldrich | 0.988 | HPLCb |
| RST | Aurobindo Pharma, India | >0.98 | HPLCb |
| orthophosphoric acid | Sigma-Aldrich | 0.95 | HPLCb |
PEG400 = polyethylene glycol 400.
HPLC = high-performance liquid chromatography.
ACN = acetonitrile. RST = rosuvastatin calcium.
2.2. Methods
2.2.1. Chemical Analysis Methodology
The procedure and method, for RST estimation, were adopted from the previous published report.12,13 In brief, analysis was conducted using the mobile phase composed of acetonitrile, methanol, and water in 40:40:20 ratios, respectively. The drug was quantified running isocratic high-performance liquid chromatography (HPLC) at 245 nm and a column temperature of 25.0 °C.12 Finally, the pH of the mobile phase was set at 4.0 using phosphoric acid. The freshly prepared mobile phase was filtered using a membrane filter, sonicated using a bath sonicator to remove gas bubbles, and then stored at room temperature for further use. The analysis process was performed at a constant flow rate (1.0 mL min–1) by injecting a low sample volume (10 μL) over a running time of 10 min. A calibration concentration range (5–50 μg/mL) was prepared for establishing the standard calibration curve with a high correlation coefficient for linearity (regression coefficient of 0.999 as r2). The lower limit of detection and limit of quantification were found to be 1.2 and 4.5 μg/mL, respectively. A summary (purity, source, and grade) of solvents has been presented in Table 1.
2.2.2. Solubility Assessment
For this, RST mole fraction solubility values were determined in varied mass fractions (m) (m = 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0) of PEG400 to water at T = 298.15 K and a fixed pressure of 0.1 MPa with slight modification in the reported method.4 Briefly, a weighed amount of the drug was constantly added to the (PEG400 + water) cosolvent binary system. The glass vials were placed (in platform) in a water-bath shaker maintained at 25.0 ± 1.0 °C. These glass vials were firmly closed to avoid loss of the cosolvent–water mixture during the experiment. The addition of RST was continued until the saturation/equilibrium was achieved. After establishment of equilibrium, the content was centrifuged at 2795×g for 10.0 min to separate out the precipitated RST.13 The supernatant was used to determine the drug content at saturation using the validated HPLC method at a λmax of 245 nm. A saturation time of three days was determined by measuring RST concentration (mole fraction) in neat water until a constant solubility value was achieved. The experiments were replicated for mean value ± SD (standard deviation) values (n = 3). Experimental solubility (xe) was calculated as (eq 1)
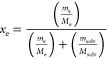 |
1 |
where me and Me are the mass and the molar mass of RST, respectively. Similarly, msolv and Msolv represent the mass and the pondered molar mass of the solvent, respectively.
2.2.3. Mathematical Models to Study PS
Theoretically, solubility of RST in a given solvent or multiple solvent combination is the result of various mechanistic processes involved at particular temperature. Hansen solubility, Hildebrand solubility parameters, and thermodynamics solubility parameter(s) are reported to investigate the mechanism involved in the drug dissolution process/mixing/miscibility. Interestingly, PS is considered as a phenomenon where solvent proportion of the mixed solution (a binary system) around a solute molecule differentiates from the “static proportion” when it is present in the bulk. Theoretically, it is important to disrupt cohesive forces existing (cohesive solvent–solvent interaction) among the solvent molecules surrounding (vicinity) the solute molecule rather than the interaction present between polymers and solvents.14,15 The behavior of the solute (diffusion, chemical shift, reactivity, and so on) depends upon the composition of the solvent in mixed solvents.16 In the present study, the PS parameter of RST by PEG400 (1) in the binary {PEG400 (1) + water (2)} mixtures can be defined as (eq 2)
| 2 |
where x1,3L and x1 are the local mole fractions of the prime component {PEG400 (1)} surrounding the solute {RST (3)} and the bulk mole fraction of the same (polyethylene glycol 400 designated as 1) in the initial binary system {(PEG400 + water)} free from RST, respectively. The calculated values of δx1,3 indicate the plausible chance of PS of RST through PEG400 (1) or water (2). The negative value of “δx1,3” (δx1,3 < 0) indicates PS of RST by water molecules, whereas the positive value of “δx1,3” (δx1,3 > 0) directs PS by the PEG400 (eq 3). Finally, the local (x1,3) and bulk (x1) compositions of PEG400 in the studied binary system need to be integrated with the standard thermodynamic functional parameter(s) (Gibbs free energy, G) using the inverse Kirkwood–Buff integrals model (eqs 4 and 5).
| 3 |
where
| 4 |
| 5 |
With the correlation volume (Vcor) as shown in eq 6
| 6 |
In eqs 4 and 5, κT represents
the isothermal compression
of the binary system calculated as an additive property employing
the explored compositions and κ value indicated for the pure
component. The partial molar volumes of each component of the binary
system were estimated, and these are 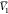 ,
, 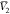 , and
, and 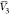 for PEG400 (1), water (2), and RST (3),
respectively. The estimated derivative function values of the standard
Gibbs free energy (G) of transfer from water (2)
to the binary system (PEG400 + water) with respective to the same
binary system without the solute (RST as 3) are represented as “D”, as shown in eq 7. Likewise, the second derivative function is represented
as “Q”, as shown in eq 8.
for PEG400 (1), water (2), and RST (3),
respectively. The estimated derivative function values of the standard
Gibbs free energy (G) of transfer from water (2)
to the binary system (PEG400 + water) with respective to the same
binary system without the solute (RST as 3) are represented as “D”, as shown in eq 7. Likewise, the second derivative function is represented
as “Q”, as shown in eq 8.
| 7 |
| 8 |
Eq 9 was used to estimate the molecular radius (r3) of the solute (RST), and NAv represents the Avogadro number.
| 9 |
Notably, the Vcor represents the definitive correlation volume, which depends upon the local mole fraction of each individual component of the binary system (PEG400 and water) in the vicinity of the solute. This needs iteration and is estimated by replacing δx1,3 and Vcor in eqs 2, 3, and 6 to re-estimate the value of x1,3L until Vcor becomes constant.
2.2.4. Predictive Study Using GastroPlus (Prediction and Simulation)
This is a program-based in silico simulation and prediction software used for pharmaceutical products (dosage form) in various animal models (rodents, primates, and animals). The software uses various models based on drug characteristics, formulation properties, and biopharmaceutics of the model drug.17−20 There are several data sets in the input tab as default values. The program offered three different tabs such as the compound tab for physicochemical properties of the model compound, the physiological tab to set the physiological condition for the outcome, and the pharmacokinetics tab for the pharmacokinetic profile of the drug. These tabs may need experimental, literature-based, and default suggested values for the running and optimization process.21 Notably, the program is associated with simulation and subsequent prediction in the target animal model using the ADMET (absorption, distribution, metabolism, and excretion) predictor model. Modeling and simulation studies required physicochemical and biopharmaceutical properties of RST. Initially, the physiological-based pharmacokinetic (PBPK) model has been advanced by incorporating the biopharmaceutics-related informative tab for oral products at various stages of product development in academic and industrial laboratories, which was later termed as physiological-based biopharmaceutics modeling (PBBM).22 GastroPlus version 9.6 was employed while developing the PBBM absorption model.23 GastroPlus uses the advanced compartmental absorption and transit (ACAT) mechanistic model to predict intestinal absorption in humans for oral products.24 For this, in vitro dissolution profile data and in vivo data are required for predicting in vivo dissolution and in vivo absorption in the investigated animal or human. Therefore, sufficient pharmacokinetic data are required for better simulation and prediction with a minimum fold error. To predict PK parameters, the compartmental PK model was used using a single oral dose (20 mg) of RST in the oral IR plasma concentration time profile of PKPlus for 12 h of simulation and prediction. The program was run to predict the plasma drug concentration time profile for the conventional tablet (RST). The prediction was repeated for the optimized binary mixture (x1 = 0.5) at the similar experimental condition. Notably, one compartmental model was the best-fit model based on the Akaike information criterion (AIC). Moreover, the regional compartmental absorption model includes nine compartments for oral absorption.13 These nine compartments are different from each other in terms of pH, radius, and lumen content (volume). The software predicts the overall total absorption as “AmtAbs”. IVIVR stands for in vitro and in vivo relationship in this model. The regional absorption study was studied to predict for both of them under similar experimental conditions (fast human subject of 70 kg).
2.2.5. Statistics Model and Software Tools
The experimentally obtained data were statistically tested employing “Dennʼs test” and “Kruskal–Wallis analysis”. The prediction and simulation were carried out using licensed version 9.7 of GastroPlus (Simulation Plus, Inc., Lancaster, United States of America) simulation and prediction software-predicted regional absorption of RST based on experimental, reported, and by default input data. A tested value was considered as significant when the value of p was <0.05. Expedients were repeated (triplicate) for the mean and standard deviation.
3.0. Results and Discussion
3.1. Solubility and Related Parameters
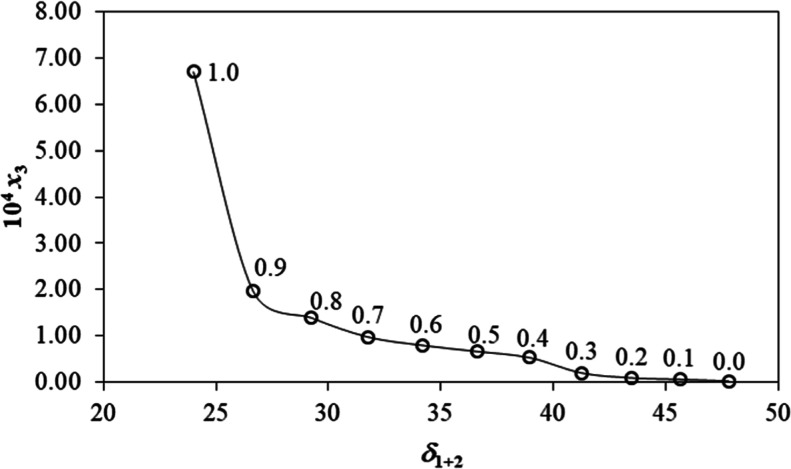
RST belongs to a statin family (super statin), and it contains two oxidrilic functional groups (linked to an asymmetric C-atom). The main problem of the compound is poor aqueous solubility (8.86 × 10–2 g/L as reported in NLM PubChem), which results in limited absorption from the gastrointestinal tract.2 RST is a poorly soluble drug in water and aqueous system due to high molecular weight and heterocyclic aromatic rings (Figure 1A,B). The result of RST solubility in different mass fractions of the (PEG400 + water) cosolvent system has been portrayed in Figure 2. The mole fraction solubility values of RST in pure water (m = 0.0) and neat PEG400 (neat m = 1.0) were observed as 2.15 × 10–6 and 6.7 × 10–4, respectively, at 298.15 K. Notably, our mole fraction solubility value in neat water is similar in magnitude to that reported before (as reported in NLM-PubChem), i.e., 3.32 × 10–6 (calculated from the value of 8.86 × 10–2 g/L).2 Furthermore, no solubility value of RST in PEG 400 or in its aqueous mixture has been reported so far, and therefore, no further comparison is possible. The mean relative apparent uncertainty in mole fraction solubility values was 0.02 (2.0%). Overall, mole fraction solubility of RST had been found to have linear dependence on PEG400 content in the studied ratios of the mixture (Figure 2). Water as a solvent has unique properties such as high dielectric point (80 at 20 °C), hydrogen bond forming ability, and high surface tension (71 dyn/cm), whereas PEG400 structurally possessed several hydroxyl functional groups responsible for forming hydrogen bonds. These interaction causes miscibility of PEG400 in water. Thus, the hydrophilic nature of PEG400 causes a linear increase in the solubility of RST in the binary system on the relative increase in the content of PEG400. PEG400 being hydrophilic and a good cosolvent has been well explored for various formulations.25 However, its salt (calcium salt of RST) in the neat solvent has been reported to execute non-linear dependency in solubility with the increase in temperature.4 Chemically, the drug is a member of pyrimidine, a sulfonamide, a dihydroxy monocarboxylic acid, and a member of monofluorobenzene.2
Figure 2.
Mole fraction solubility of RST (3) as a function of the Hildebrand solubility parameters of the {PEG400 (1) + water (2)} cosolvent mixture mass fractions at T = 298.15 K. Mass fractions of PEG400 are indicated near every point.
Figure 2 depicts the solubility (δ1+2 as the Hildebrand parameter) behavior of RST as a function of the polarity of the binary system “PEG400 + water”. The values of δ1+2 were individually estimated employing the Hildebrand parameters for pure PEG400 (δ1 = 24.0 MPa1/2) and pure water (δ2 = 47.8 MPa1/2) followed by estimating the volume fraction in the same binary system. Notably, the volume fraction was considered as the volume fraction additivity for mathematical calculation, as observed in eq 2.26−28 The density (eq 10) of the investigated binary system was used from the literature published before by us.29
| 10 |
Neat PEG400 exhibited maximum solubility of RST over an entire range of polarity (δ1 = 24.0 MPa1/2), as elicited Figure 2. It is noticeable that the difference of a solubility parameter (δd, δp, and δh) between a compound (like RST) and solvent (like PEG400 and water) (δd of solute – δd of solvent, or δp of solute – δp of solvent, or δh of solute – δh of solvent) in Hansen and Hildebrand models dictates miscibility or immiscibility depending upon the difference value. In general, a compound is considered to be soluble if the difference comes near zero or null (difference = 0).27,30 Thus, the δ3 value of RST in the studied binary system was predicted as 24.0 MPa1/2 for the highest solubility, which is close to the values estimated from Fedors’ method (Table 2, δ3 = 27.89 MPa1/2). Notably, the small discrepancy (3.89 MPa1/2) may be attributed to the specific solvation process not encountered in the Fedors’ method. The present findings may be correlated with the previous report with a similar pattern of discrepancy for the solubilization of a synthesized compound in the same system.11
Table 2. Hildebrand Solubility Parameters (δ3) of RST Using Fedors’ Method (Internal Energy as ΔU° and Molar Volume as V°).
| functional group | number | ΔU°/kJ·mol–1 | V°/cm3·mol–1 |
|---|---|---|---|
| –CH3 | 4.0 | 18.84 | 134 |
| –CH2– | 2.0 | 9.88 | 32.2 |
| >CH– | 3.0 | 10.29 | –3.0 |
| –CH= | 2.0 | 8.62 | 27.0 |
| >C= | 4.0 | 17.24 | –22.0 |
| phenylene | 1.0 | 31.90 | 52.4 |
| ring closure > 5 | 1.0 | 1.1 | 16.0 |
| conj. double bond | 4.0 | 6.68 | –8.8 |
| –F | 1.0 | 3.35 | 18.0 |
| –OH | 2.0 | 59.6 | 20.0 |
| –COOH | 1.0 | 27.6 | 28.5 |
| >N– | 1.0 | 4.2 | –9.0 |
| –N= | 2.0 | 23.4 | 10.0 |
| –SO2– | 1.0 | 25.55 | 19.5 |
| Σ | 246.53 | 317.0 | |
| δ3 = (246.53/317.0)1/2 = 27.89 MPa1/2 | |||
Furthermore, the thermodynamic functional parameters are important tools to know the mechanisms of drug dissolution processes. These are standard Gibbs free energy of dissolution (ΔsolnG°), standard enthalpy of dissolution (ΔsolnH°), and standard entropy of dissolution (ΔsolnS°). In general, the negative value of ΔsolnG° indicates the spontaneous dissolution/mixing process of the solute in the mixture. Similarly, negative and positive values of enthalpy indicate endothermic and exothermic solubilization/miscibility/mixing/dissolution processes, respectively.30 A summary of ΔsolnG° of RST in the binary system (at all mass fraction) is calculated using eq 11 (Table 3).
| 11 |
Table 3. Few Apparent Thermodynamic Properties of RST in the {PEG400 (1) + Water (2)} Cosolvent Mixture at 298.15 K and Pressure (p) = 0.1 MPad.
| w1a | f1a | x1a | x3c | δ1+2b | ΔsolnG°/kJ·mol–1c |
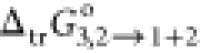 /kJ·mol–1c /kJ·mol–1c
|
|---|---|---|---|---|---|---|
| 0.00 | 0.0 | 0.0 | 2.15 × 10–6 | 47.8 | 32.36 | 0.00 |
| 0.10 | 0.0898 | 0.0050 | 5.63 × 10–6 | 45.66 | 29.97 | –2.39 |
| 0.20 | 0.1817 | 0.0111 | 8.92 × 10–6 | 43.48 | 28.83 | –3.53 |
| 0.30 | 0.2757 | 0.0189 | 1.97 × 10–5 | 41.24 | 26.86 | –5.49 |
| 0.40 | 0.3719 | 0.0292 | 5.27 × 10–5 | 38.95 | 24.42 | –7.93 |
| 0.50 | 0.4704 | 0.0431 | 6.59 × 10–5 | 36.60 | 23.87 | –8.49 |
| 0.60 | 0.5713 | 0.0633 | 7.91 × 10–5 | 34.20 | 23.42 | –8.94 |
| 0.70 | 0.6745 | 0.0951 | 9.75 × 10–5 | 31.75 | 22.90 | –9.46 |
| 0.80 | 0.7804 | 0.1527 | 1.39 × 10–4 | 29.23 | 22.02 | –10.34 |
| 0.90 | 0.8888 | 0.2885 | 1.97 × 10–4 | 26.65 | 21.15 | –11.20 |
| 1.00 | 1.0 | 1.0 | 6.70 × 10–4 | 24.0 | 18.12 | –14.24 |
w1, f1, and x1 are the mass, volume, and mole fractions of PEG 400 (1) in the {PEG 400 (1) + water (2)} mixtures free of RST (3). Volume fractions of PEG400 were calculated assuming additive behavior from density values reported by Rodriguez et al.29
δ1+2 is the Hildebrand solubility parameter of {PEG 400 (1) + water (2)} mixtures free of RST at 298.15 K.
x3 is the mole fraction solubility of RST.
Standard uncertainties u are u (T) = 0.02 K for temperature and u (p) = 0.001 MPa for pressure with 0.95% level of confidence. x3 = mole fraction solubility at standard uncertainties u of u (T) = 0.02 K for temperature and u (p) = 0.001 MPa for pressure with 0.95% level of confidence.
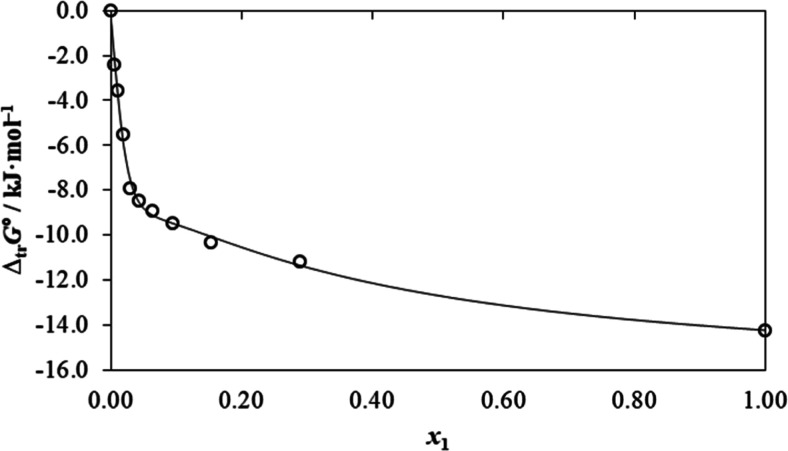
It is clear from Table 3 that the negative values of ΔsolnG° (all mixture ratio) and free energy transfer
(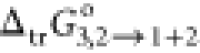 ) from water to PEG-containing mixtures
suggested the spontaneous dissolution process of RST at the explored
temperature. Furthermore, the value of Gibbs free energy is encouraging
{low +ve value (18.12 kJ·mol–1) in pure PEG400}
for assisted dissolution/solubilization of RST owing to the increased
amount of PEG400 in the progressive proportion.
) from water to PEG-containing mixtures
suggested the spontaneous dissolution process of RST at the explored
temperature. Furthermore, the value of Gibbs free energy is encouraging
{low +ve value (18.12 kJ·mol–1) in pure PEG400}
for assisted dissolution/solubilization of RST owing to the increased
amount of PEG400 in the progressive proportion.
3.2. Preferential Solvation
The result
of PS parameters has been illustrated in Tables 3 and 4. The values
of 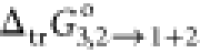 (the Gibbs energy transfer) of RST from
neat water (2) to the {PEG400 (1) + water (2)} mixtures are summarized
in Table 3, and
(the Gibbs energy transfer) of RST from
neat water (2) to the {PEG400 (1) + water (2)} mixtures are summarized
in Table 3, and 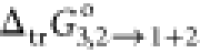 behavior has been exhibited in Figure 3 at 298.15 K. Table 3 shows the values
of
behavior has been exhibited in Figure 3 at 298.15 K. Table 3 shows the values
of 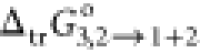 , and these were found to be in the range
from −2.39 to −14.24 kJ·mol–1, estimated from Table 3 (mole fraction solubility data of RST). It is quite clear that the
negative values of
, and these were found to be in the range
from −2.39 to −14.24 kJ·mol–1, estimated from Table 3 (mole fraction solubility data of RST). It is quite clear that the
negative values of 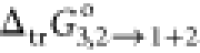 were regularly increasing with an augmented
proportional increment of PEG400 in the (PEG400 + water) binary system.
So, the highest −ve (negative) value of
were regularly increasing with an augmented
proportional increment of PEG400 in the (PEG400 + water) binary system.
So, the highest −ve (negative) value of 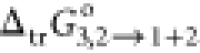 of RST from water (2) to the (PEG400 +
water) binary system was found as −11.2 kJ·mol–1 (Table 3). The value
of
of RST from water (2) to the (PEG400 +
water) binary system was found as −11.2 kJ·mol–1 (Table 3). The value
of 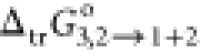 in pure PEG400 was achieved to be about
1.27 times greater than the same parameter calculated at w1 = 0.9 (x1 = 0.2885). The
values of
in pure PEG400 was achieved to be about
1.27 times greater than the same parameter calculated at w1 = 0.9 (x1 = 0.2885). The
values of 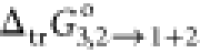 were calculated by eq 12.
were calculated by eq 12.
| 12 |
Table 4. Various Calculated Parameters of RST-Related PS in the Explored Binary system{PEG400 (1) + Water (2)} (298.15 K and p = 0.1 MPa)a.
| x1b | D/kJ·mol–1 | G1,3/cm3·mol–1 | G2,3/cm3·mol–1 | Vcor/cm3·mol–1 | 100 δx1,3 |
|---|---|---|---|---|---|
| 0.00 | –328.34 | –2707.6 | –313.7 | 1163 | 0.00 |
| 0.05 | –31.52 | –488.9 | –491.2 | 1589 | 0.01 |
| 0.10 | –9.90 | –358.4 | –412.8 | 1980 | 0.31 |
| 0.15 | –10.32 | –353.1 | –458.2 | 2343 | 0.71 |
| 0.20 | –9.89 | –346.4 | –492.0 | 2680 | 1.05 |
| 0.25 | –8.96 | –339.9 | –513.6 | 2997 | 1.29 |
| 0.30 | –7.95 | –334.6 | –529.0 | 3297 | 1.45 |
| 0.35 | –7.01 | –330.5 | –542.0 | 3585 | 1.54 |
| 0.40 | –6.19 | –327.6 | –554.7 | 3863 | 1.60 |
| 0.45 | –5.48 | –325.4 | –568.4 | 4133 | 1.64 |
| 0.50 | –4.88 | –323.8 | –584.0 | 4396 | 1.65 |
| 0.55 | –4.36 | –322.8 | –601.8 | 4652 | 1.64 |
| 0.60 | –3.92 | –322.0 | –621.8 | 4901 | 1.61 |
| 0.65 | –3.54 | –321.5 | –643.4 | 5144 | 1.55 |
| 0.70 | –3.21 | –321.0 | –664.5 | 5379 | 1.46 |
| 0.75 | –2.92 | –320.4 | –682.1 | 5607 | 1.31 |
| 0.80 | –2.67 | –319.6 | –691.4 | 5827 | 1.09 |
| 0.85 | –2.45 | –318.5 | –687.6 | 6039 | 0.83 |
| 0.90 | –2.26 | –317.0 | –668.0 | 6246 | 0.54 |
| 0.95 | –2.08 | –315.3 | –633.9 | 6447 | 0.25 |
| 1.00 | –1.93 | –313.7 | –590.9 | 6645 | 0.00 |
Standard uncertainties u are u (T) = 0.02 K for temperature u (p) = 0.001 MPa for pressure with 0.95% level of confidence.
x1 is the mole fraction of PEG 400 (1) in the {PEG 400 (1) + water (2)} mixtures free of RST (3).
Figure 3.
Gibbs energy of transfer of RST (3) from neat water (2) to {PEG 400 (1) + water (2)} mixtures at 298.15 K.
Furthermore, the values of 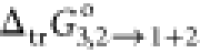 were correlated to the coefficient of the
model, as illustrated in eq 13. These calculated coefficients have been calculated as a = −0.21, b = 4.77, c = −329.3, d = 863.9, e =
−4043.0, f = 31.62, and g = −53,024, with a regression coefficient (r2) value of 0.995, typical error = 0.46,
and F = 138.4.
were correlated to the coefficient of the
model, as illustrated in eq 13. These calculated coefficients have been calculated as a = −0.21, b = 4.77, c = −329.3, d = 863.9, e =
−4043.0, f = 31.62, and g = −53,024, with a regression coefficient (r2) value of 0.995, typical error = 0.46,
and F = 138.4.
| 13 |
Thus, the values of D are presented in Table 4. Additionally, other
input parameters (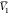 ,
, 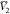 , RT·κT, and Q) have been employed from our reported values
for the same binary system {PEG400 (1) + water (2)} studied before.31 The calculated molar volume of RST was 314.8
cm3·mol–1 (Table 2), using Fedors’ method.32 It is noteworthy that the values of G1,3 and G2,3 were
negative in all compositions and suggested a good affinity of RST
with both solvents (PEG400 and water). This might be due to hydrogen
boding of the drug with water and facilitated interaction by PEG400
working as the cosolvent with water. This may be correlated with a
high number of hydrogen bond acceptor counts (total 10) in RST responsible
for hydrogen bond formation for improved RST solubility in the mixture.
This may be rationalized based on the increasing content of PEG400
(relative content of PEG400 + water mixture) in the mixture and subsequent
progressive increment in the
, RT·κT, and Q) have been employed from our reported values
for the same binary system {PEG400 (1) + water (2)} studied before.31 The calculated molar volume of RST was 314.8
cm3·mol–1 (Table 2), using Fedors’ method.32 It is noteworthy that the values of G1,3 and G2,3 were
negative in all compositions and suggested a good affinity of RST
with both solvents (PEG400 and water). This might be due to hydrogen
boding of the drug with water and facilitated interaction by PEG400
working as the cosolvent with water. This may be correlated with a
high number of hydrogen bond acceptor counts (total 10) in RST responsible
for hydrogen bond formation for improved RST solubility in the mixture.
This may be rationalized based on the increasing content of PEG400
(relative content of PEG400 + water mixture) in the mixture and subsequent
progressive increment in the 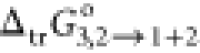 value of RST from neat water to the binary
system (Table 3). The
value of
value of RST from neat water to the binary
system (Table 3). The
value of 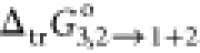 of RST in neat PEG400 was comparatively
maximum than any composition of the system.
of RST in neat PEG400 was comparatively
maximum than any composition of the system.
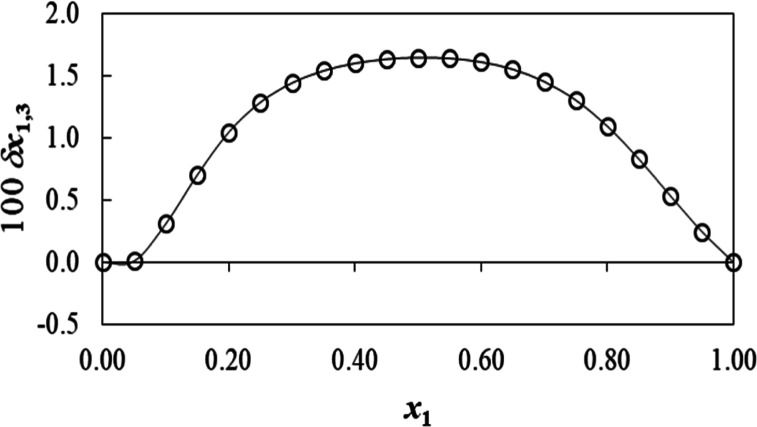
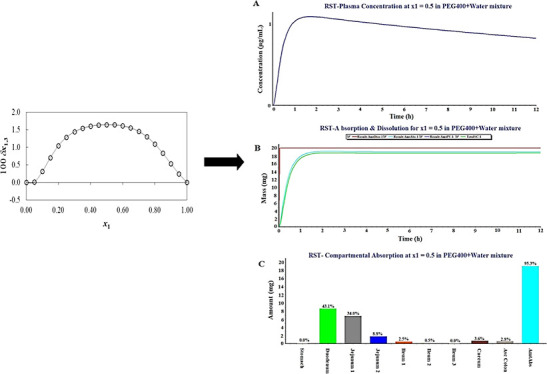
The value of RST radius (r3) was calculated from eq 10, and it was found as 0.5 nm. The value of Vcor was iterated (n = 3) in eqs 2, 3, and 6, and values are shown in Table 4. In Table 4, the PS parameter(s) of RST (3) by PEG400 are presented and represented as δx1,3.
Figure 4 and Table 4 show the values of δx1,3 of RST (3) changing nonlinear as the proportion of PEG400 increases in the binary system. Moreover, adding PEG400 (1) into water (2) causes positivity of δx1,3 values of RST (3) from x1 = 0.0 (pure water) to 1.0 (pure PEG400) in all explored composition ratios. Considering the above parameters, the local mole fraction of PEG400 (1) in the vicinity of RST (3) is substantially higher than the value in the bulk mixture free from RST. It is obvious that the highest positive value of δx1,3 was attained at x1 = 0.5 (δx1,3 = 1.65 × 10–2) which corroborated the tangible PS effects of PEG400 molecules on RST.33,34 The drug is a conjugate Lewis acid of RST (1−), and it is derived from hept-6-enoic acid. The increased solubility of RST by PEG400 might be attributed to hydrogen-bond formation preferentially over solute–solvent polar interactions, decreased surface tension, improved wettability by PEG400, and other factors. Recently, Vemuri and Lankalapalli improved RST solubility using amino acids through hydrogen-bonding interaction for co-crystal formation.35 Additionally, RST possessed the carboxylic functional group which may impart surface propensity (surface behavior) of the aqueous solution and binary system for the air/water interface by reducing surface tension to some extend at explored temperature.36 There are several factors responsible to influence surface (surface propensities) behavior of molecules bearing carboxylic groups such as (a) molecular size, (b) saturation and unsaturation in the compound, (c) hydrophobicity/hydrophilicity, (d) molecular density, (e) number of carboxylic groups in the molecules, (f) strength of interaction (weak surface propensity due to strong interaction via three hydrogen bonds in citric acid compared to oxalic acid), and (g) orientation of molecules at the surface/interface.36 Mahiuddin et al. investigated that stronger hydrophobic characteristics of a molecule and its orientation at the surface/interface due to unsaturation (−C=C– in maleic acid compared to succinic acid) led to weak capability to form firm H-bonds with water.36 There may be a relative competition between hydrophilic and hydrophobic interactions of the charged functional group (−COO–) and flexible aliphatic part of the molecule, resulting in conformation changes in the large solvated cluster. Furthermore, the molecular size difference also results in varying degrees of solvation behavior (smaller molecule preferably solvated in the bulk as compared to the larger one).37,38 Thus, RST may have surface propensity due to the hydrophobic moiety and polar side chain for reduced surface tension in water as an additive effect which can be explored in detail at varied molar concentration.
Figure 4.
Values of δx1,3 of RST (3) in {PEG 400 (1) + water (2)} mixtures at 298.15 K.
Moreover, RST has 3 and 10 H-bond donor counts and H-bond acceptor counts, respectively, which suggested facilitated interaction with PEG400 for improved solubilization with the progressive increase of PEG400 content in each mixture ratio. Aryafard et al. reported that the solubility of compounds (probes) depends upon the functional groups and the number of benzene rings present in the compound. Moreover, authors exemplified that nitrobenzene (no hydrogen bond acceptor/donor counts) is neither soluble in water nor in ethanol (short-chain alkyl alcohol), whereas aniline (−NH2 as hydrogen bond acceptor/donor counts) is freely soluble in both solvents. This was rationalized based on the number and type of hydrogen donor/acceptor counts.39 Moreover, PEGs have both hydrophilic and hydrophobic character as either liquid or solid melt depending on the molecular weight and considered as a good cosolvent for the compound possessing hydrogen bond acceptor/donor groups (counts) as observed in RST.39,40 Thus, improved solvation behavior of PEG 400 may be prudent to correlate with the present functional groups in RST responsible to form hydrogen bonding in the binary mixture (PEG400 + water). Alshora et al. reported that the solubility of RST salt (calcium salt) exhibited a non-linear progressive increment with the increase in temperature in propylene glycol, ethylene glycol, ethanol, butanol, and isopropyl alcohol.4
3.3. GastroPlus-Based Simulation and Prediction
Considering experimental solubility data of the drug, PEG400 was found to have good solubility as compared to others. Therefore, PEG400 was selected as a good cosolvent for the binary mixture. PS study confirmed a convincing impact of PEG400 in the binary mixture for maximum RST solubility. For oral, parenteral, and subcutaneous drug delivery, it is mandatory to formulate an aqueous formulation. It must be soluble and stable in the aqueous system for successful in vivo performance after administration. The ratio “x = 0.5” was selected to predict in vivo performance using the GastroPlus program. The program assisted industries for reduced developmental cost, product development timeline, and clinical burden. Therefore, we used in vitro data, literature data, and by-default values for predicting in vivo performance (oral administration) of the binary construct in the human body at fast conditions.
From the reported literature, several physicochemical properties of RST, compound-related input parameters (solubility, log P, pKa, particle density, apparent permeability coefficient, and pH), dosage form (IR tablet for oral delivery, dose, and dosing frequency), and pharmacokinetics input parameters (area under the curve as AUC, maximum plasma concentration as Cmax, and the time required to attain Cmax as Tmax) for the RST-loaded IR (immediate release) tablet were fed in the respective tabs. Similarly, the studied data were used to compare between the conventional RST tablet and RST loaded in the (PEG400 + water) cosolvent mixture system at x1 = 0.5, keeping rest of the parameters unchanged. All of the required input parameters are summarized in Table 5.19
Table 5. Various Input Parameters Related to RST for GastroPlus-Based Predictive and Simulation Studies in Humans.
| s. no | required input parameters | values |
|---|---|---|
| 1 | chemical formula of RST | C22H28FN3O6S |
| 2 | hydrogen-bonding donor counts | 3 |
| 3 | hydrogen-bonding acceptor counts | 10 |
| 4 | molecular weight (g/mol) | 481.5 |
| 5 | log P (−COOH) | 0.13 |
| 6 | pKa | 4 |
| 7 | melting point (°C) | 173–184 |
| 8 | experimental solubility (mg/mL) in PEG400 at 25 (°C) | 6.5 |
| 9 | solubility (mg/mL) in water at 25 (°C) | 0.33 |
| 10 | dose (mg)d20 | 40.0 |
| 11 | dosing volume (mL) | 250 |
| 12 | apparent permeability (cm/s)a17 | 4.87 × 10–4 |
| 13 | total clearance (L/h)c19 | 0.455 |
| 14 | volume of distribution (L/kg)b18 | 2.23 |
| 15 | elimination half-life (h)d20 | 19 |
| 16 | protein binding capacity (%)b18 | 88 |
| 17 | body weight as input parameter (kg) | 60 |
| 18 | simulation run time (h) | 12 |
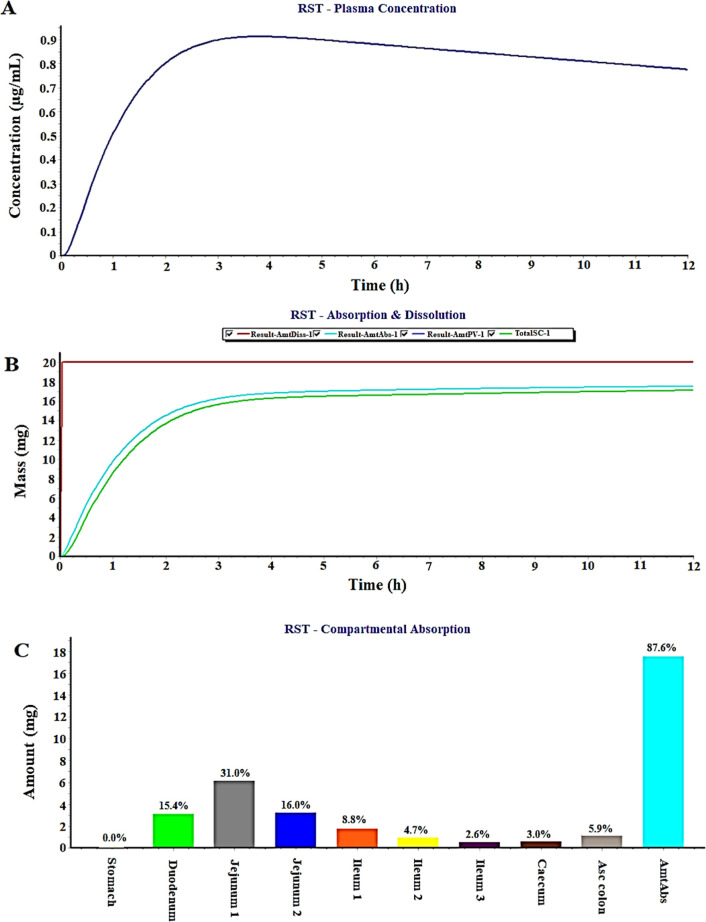
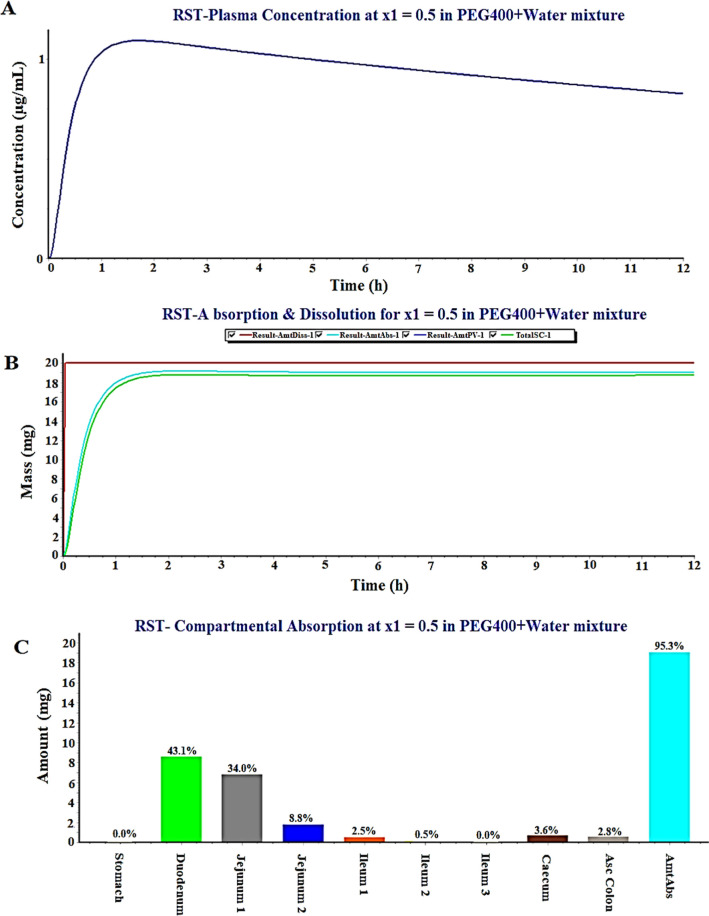
The GastroPlus program predicted plasma-drug concentration, in vitro dissolution in physiological medium, compartmental absorption of RST from nine different intestinal segments of humans for the oral conventional dosage form possessing poor aqueous solubility (0.33 mg/mL), and RST dissolved in (PEG400 + water) the cosolvent mixture system (4.14 mg/mL). Thus, the program permits us to investigate improved in vivo performance of RST containing the (PEG400 + water) mixture than the conventional dosage form in humans.41 In general, poor aqueous solubility of a drug results in a slow dissolution process and subsequently low oral bioavailability. The present study aimed to increase RST solubility using biocompatible PEG400 as a cosolvent which may augment oral absorption and bioavailability in a normal healthy human (70 kg). Figure 5A illustrates a predicted value (0.87 μg/mL) of RST-plasma concentration after oral delivery of 20 mg of the RST tablet (selecting immediate release in tab) to a human subject of 70 kg body weight, whereas 16.5 mg of RST was predicted to be absorbed within 4 h, as shown in Figure 5B. However, this value is significantly different from real absorption of the commercial RST tablet, as reported in the literature.19 This difference can be correlated with poor aqueous solubility and physicochemical properties of the drug responsible to be degraded in gastric lumen.41 The drug is acidic in nature due to bearing the carboxylic functional group, and the pKa value is 4.0 (Table 5). In vitro dissolution behavior of RST and predicted in vivo absorption established as good IVIVR, as illustrated in Figure 5C in terms of percent drug absorption from different regions.41 It is quite clear that the major site of the drug absorption is the upper portion of GIT such as duodenum, jejunum, and ileum. The total percent of the drug administered predicted drug absorption as 15.4, 47, and 16.1% from duodenum, jejunum, and ileum, respectively. This may be due to the unionized form of the drug in this pH range of human intestine. Moreover, total drug absorption of the drug from an IR tablet was predicted to be approximately 86%. However, this value was predicted as 26% (not shown here) when RST suspension is taken in the software tab, which is a quite approximate value of the reported oral bioavailability (∼20%) in the literature.3 For comparison with RST loaded in the PEG400 + water cosolvent mixture system, similar prediction study was performed, and the results are presented in Figure 6A–C. Figure 6A illustrates a predicted value (1.2 μg/mL) of RST-plasma concentration after oral delivery of the RST-loaded (PEG400 + water) cosolvent mixture (selecting IR solution in tabs) to a human subject of 70 kg body weight where 19.0 mg of RST was predicted to be absorbed within 1.5 h, as shown in Figure 6A,B. Thus, absorbed RST (19 mg) within the reduced time period (1.5 h) than tablet formulation suggested that increased solubility/miscibility/drug dissolution of RST by the PEG400-mediated PS phenomenon could be an encouraging strategy for increased drug absorption and subsequent improved oral or subcutaneous bioavailability.19 There is no observed lag time of the drug dissolution and absorption due to the IR product {(PEG400 + water) mixture as the solution form} in both cases. Figure 6C reveals relatively higher percent drug absorption (95.5%) as compared to the IR tablet (87%) and RST suspension (26%) (not given here).21 Conclusively, RST solubilization in the (PEG400 + water) binary system can be considered as a suitable carrier for safe delivery to control DM.
Figure 5.
In silico prediction using GastroPlus: (a) plasma-drug concentration in humans, (b) dissolution-absorption of the RST-IR tablet, and (c) regional absorption after RST tablet delivery when dosed 20 mg (feeding solubility value as 0.33 mg/mL as the input parameter).
Figure 6.
GastroPlus-based prediction of (a) plasma-drug concentration in humans, (b) dissolution and absorption of the RST-IR tablet, and (c) regional absorption after RST tablet delivery when dosed 20 mg (feeding solubility value as 0.33 mg/mL as the input parameter).
4. Conclusions
RST is a poorly water-soluble
hypolipidemic agent with low oral
bioavailability. Several approaches have been implemented to improve
its solubility and dissolution for increased absorption. Most of them
are nanocarrier-based drug delivery systems employing large concentrations
of surfactant and co-surfactant. The present study addressed the mechanistic
perspective in terms of solubility parameters occurring in the drug
solubilization/dissolution processes using PEG400 in the (PEG400 +
water) cosolvent mixture. Chemically, RST is a conjugate acid of RST
(1−) and possessed a carboxylic acid moiety. RST contains 10
as the H-bond acceptor count (as described before) and is expected
to form hydrogen bonding with water and PEG400-mediated increased
PS. Therefore, it is quite simple to explain the PS phenomenon using
the Hildebrand model of RST by PEG400 in the binary system. The finding
suggested that the values of 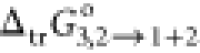 of RST by PEG400 from water (2) to the
binary system were observed to be decreased on increasing the content
of PEG400 in the same binary system. Furthermore, the +ve (positive)
values of δx1,3 corroborated the
PS of RST by PEG400 in the “PEG400 + water”. Additionally,
GastroPlus-based ACAT and PKPlus models were quite fit for simulation
and prediction for in vivo performance of the RST-loaded (PEG400 +
water) binary system as compared to RST conventional tablet. The program
is good for predicting in vivo performance of the developed product
using experimental and literature-based information to reduce the
burden of clinical studies (an alternative strategy for the oral product).
of RST by PEG400 from water (2) to the
binary system were observed to be decreased on increasing the content
of PEG400 in the same binary system. Furthermore, the +ve (positive)
values of δx1,3 corroborated the
PS of RST by PEG400 in the “PEG400 + water”. Additionally,
GastroPlus-based ACAT and PKPlus models were quite fit for simulation
and prediction for in vivo performance of the RST-loaded (PEG400 +
water) binary system as compared to RST conventional tablet. The program
is good for predicting in vivo performance of the developed product
using experimental and literature-based information to reduce the
burden of clinical studies (an alternative strategy for the oral product).
Acknowledgments
Authors would like to extend their appreciation to the Deanship of Scientific Research at King Khalid University supporting this research through a small group program under grant number RGP.1/58/43.
Author Contributions
Credit Authorship contribution statements: Afzal Hussain: writing: original draft, software, and conceptualization; Obaid Afzal: review and conceptualization; Sabina Yasmin: writing, funding acquisition, and methodology, Nazima Haider: visualization, data curation, and extensive review, Abdulmalik S.A. Altamimi: analysis; Fleming Martinez: analysis and software; William E. Acree, Jr.: data curation and review; and Mohhammad Ramzan: data curation and validation.
The authors declare no competing financial interest.
References
- Akbari B. V.; Valaki B. P.; Maradiya V. H.; Akbari A. K.; Vidyasagar G. Optimization of super disintegrants and subliming agent on dissolution rate of rosuvastatin orodispersible tablets by using a 32 factorial design. Int. J. Comp. Pharm. 2011, 1, 1–6. [Google Scholar]
- Pubchem search engine; National Library of Medicine, Rosuvastatin. https://pubchem.ncbi.nlm.nih.gov/compound/Rosuvastatin accessed 2 Aug, 2021.
- Ahsan M. N.; Verma P. R. P.; Singh S. K.; Verma S.; Yashpal M. Formulation of rosuvastatin-loaded self-nanoemulsifying drug delivery system using Box-Behnken Design. Part. Sci. Technol. 2013, 32, 46–60. 10.1080/02726351.2013.800929. [DOI] [Google Scholar]
- Alshora D. H.; Haq N.; Alanazi F. K.; Ibrahim M. A.; Shakeel F. Solubility of rosuvastatin calcium in different neat solvents at different temperatures. J. Chem. Thermodyn. 2016, 94, 230–233. 10.1016/j.jct.2015.11.019. [DOI] [Google Scholar]
- Soltanpour S.; Nazemi V. Solubility of ketoconazole in binary and ternary solvents of polyethylene glycols 200, 400 or 600 with ethanol and water at 298.2 K. Data report and analysis. J. Solution Chem. 2018, 47, 65–79. 10.1007/s10953-017-0708-6. [DOI] [Google Scholar]
- Zadaliasghar S.; Rahimpour E.; Ghafourian T.; Martinez F.; Barzegar-Jalali M.; Jouyban A. Measurement and mathematical modeling of ketoconazole solubility in propylene glycol + water mixtures at various temperatures. J. Mol. Liq. 2019, 291, 111246. 10.1016/j.molliq.2019.111246. [DOI] [Google Scholar]
- Jouyban-Gharamaleki V.; Jouyban A.; Martinez F.; Zhao H.; Rahimpour E. A laser monitoring technique for solubility study of ketoconazole in propylene glycol and 2-propanol mixtures at various temperatures. J. Mol. Liq. 2020, 320, 114444. 10.1016/j.molliq.2020.114444. [DOI] [Google Scholar]
- Yalkowsky S. H.Solubility and Solubilization in Aqueous Media; Oxford University Press: New York (NY), 1999. [Google Scholar]
- Marcus Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008, 140, 61–67. 10.1016/j.molliq.2008.01.005. [DOI] [Google Scholar]
- Marcus Y. Preferential solvation of drugs in binary solvent mixtures. Pharm. Anal. Acta 2017, 8, 1000537. 10.4172/2153-2435.1000537. [DOI] [Google Scholar]
- Jouyban A.; Shakeel F.; Bhat M. A.; Acree W. E. Jr.; Martínez F. Preferential solvation of 4-(4-ethoxyphenyl)-5-(3,4,5-trimethoxybenzoyl)-3,4-Dihydropyrimidin-2(1H)-one in {PEG 400 (1) + water (2)} mixtures. Phys. Chem. Liq. 2021, 59, 423–430. 10.1080/00319104.2020.1731811. [DOI] [Google Scholar]
- Sankar G. D.; Babu J. P.; Kumar A. B.; Krishna V. M. RP-HPLC method for the estimation of rosuvastatin calcium in bulk and pharmaceutical dosage form. Acta Cienc. Indica, Chem. 2007, 33, 1. [Google Scholar]
- Haq N.; Shakeel F.; Alanazi F.; Alshora D. H.; Ibrahim M. A. Development and validation of a green RP-HPLC method for the analysis of rosuvastatin: a step towards making liquid chromatography environmentally benign. Green Process. Synth. 2018, 7, 160–169. 10.1515/gps-2017-0023. [DOI] [Google Scholar]
- Özal T. A.; van der Vegt N. F. A. Confusing cause and effect: energy–entropy compensation in the preferential solvation of a nonpolar solute in dimethyl sulfoxide/water mixtures. J. Phys. Chem. B 2006, 110, 12104–12112. 10.1021/jp061608i. [DOI] [PubMed] [Google Scholar]
- Morisue M.; Ueno I. Preferential solvation unveiled by anomalous conformational equilibration of porphyrin dimers: Nucleation growth of solvent–solvent segregation. J. Phys. Chem. B 2018, 122, 5251–5259. 10.1021/acs.jpcb.8b02558. [DOI] [PubMed] [Google Scholar]
- Ben-Naim A. Theory of preferential solvation of nonelectrolytes. Cell Biophys. 1988, 12, 255–269. 10.1007/bf02918361. [DOI] [PubMed] [Google Scholar]
- Diril M.; Karasulu Y.; Toskas M.; Nikolakakis I. Development and permeability testing of self-emulsifying atorvastatin calcium pellets and tablets of compressed pellets. Processes 2019, 7, 365. 10.3390/pr7060365. [DOI] [Google Scholar]
- Luvai A.; Mbagaya W.; Hall A. S.; Barth J. H. Rosuvastatin: A review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin. Med. Insights: Cardiol. 2012, 6, CMC.S4324. 10.4137/cmc.s4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir S.; Iqbal Z.; Shah Y.; Ahmad L.; Khan A. Pharmacokinetic study of rosuvastatin in males and females. Eur. J. Drug Metab. Pharmacokinet. 2014, 40, 313–318. 10.1007/s13318-014-0211-z. [DOI] [PubMed] [Google Scholar]
- US-FDA . CRESTOR (rosuvastatin calcium) tablets, Full prescribing information: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021366s016lbl.pdf (accessed Oct 25, 2022).
- Martin P. D.; Warwick M. J.; Dane A. L.; Brindley C.; Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin. Therapeut. 2003, 25, 2553–2563. 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- The use of Physiologically Based Pharmacokinetic Analyses—Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls. Guidance for Industry. Draft Guidance; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2020.
- Guideline on Reporting of Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation; Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency (EMA), December, 2018. accessed on 28th February 2023.
- Sjögren E.; Thörn H.; Tannergren C. In Silico Modeling of Gastrointestinal Drug Absorption: Predictive Performance of Three Physiologically Based Absorption Models. Mol. Pharmaceutics 2016, 13, 1763–1778. 10.1021/acs.molpharmaceut.5b00861. [DOI] [PubMed] [Google Scholar]
- Handbook of Pharmaceutical Excipients, 5th ed.; Rowe R. C., Sheskey P. J., Owen S. C., Eds.; Pharmaceutical Press and American Pharmacists Association: Grayslake (IL), 2006. [Google Scholar]
- Barton A. F. M.Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: New York (NY), 1991. [Google Scholar]
- Martin A.; Bustamante P.; Chun A. H. C.. Physical Chemical Principles in the Pharmaceutical Sciences, 4th ed.; Lea & Febiger: Philadelphia (PA), 1993. [Google Scholar]
- Connors K. A.Thermodynamics of Pharmaceutical Systems: an Introduction for Students of Pharmacy; Wiley-Interscience: Hoboken (NJ), 2002. [Google Scholar]
- Rodríguez G. A.; Holguín A. R.; Martínez F.; Khoubnasabjafari M.; Jouyban A. Volumetric properties of (PEG 400 + water) and (PEG 400 +ethanol) mixtures at several temperatures and correlation with the Jouyban-Acree model. Rev. Colomb. Cienc. Quim.-Farm. 2012, 41, 187–202. [Google Scholar]
- Mahdi W. A.; Hussain A.; Altamimi M. A.; Alshehri S.; Bukhari S. I.; Ahsan M. N. Experimental solubility, thermodynamic/computational validations, and GastroPlus-based In Silico prediction for subcutaneous delivery of rifampicin. AAPS PharmSciTech 2021, 22, 116. 10.1208/s12249-021-01987-y. [DOI] [PubMed] [Google Scholar]
- Mohammadian E.; Rahimpour E.; Martinez F.; Jouyban A. Budesonide solubility in polyethylene glycol 400 + water at different temperatures: experimental measurement and mathematical modelling. J. Mol. Liq. 2019, 274, 418–425. 10.1016/j.molliq.2018.10.088. [DOI] [Google Scholar]
- Fedors R. F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. 10.1002/pen.760140211. [DOI] [Google Scholar]
- Marcus Y. Solubility and solvation in mixed solvent systems. Pure Appl. Chem. 1990, 62, 2069–2076. 10.1351/pac199062112069. [DOI] [Google Scholar]
- Kamlet M. J.; Taft R. W. The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. 10.1021/ja00418a009. [DOI] [Google Scholar]
- Vemuri V. D.; Lankalapalli S. Rosuvastatin cocrystals: an attempt to modulate physicochemical parameters. Future J. Pharm. Sci. 2021, 7, 64. 10.1186/s43094-021-00213-7. [DOI] [Google Scholar]
- Mahiuddin S.; Minofar B.; Borah J. M.; Das M. R.; Jungwirth P. Propensities of oxalic, citric, succinic, and maleic acids for the aqueous solution/vapour interface: Surface tension measurements and molecular dynamics simulations. Chem. Phys. Lett. 2008, 462, 217–221. 10.1016/j.cplett.2008.07.085. [DOI] [Google Scholar]
- Minofar B.; Mucha M.; Jungwirth P.; Yang X.; Fu Y.-J.; Wang X.-B.; Wang L.-S. Bulk versus Interfacial Aqueous Solvation of Dicarboxylate Dianions. J. Am. Chem. Soc. 2004, 126, 11691–11698. 10.1021/ja047493i. [DOI] [PubMed] [Google Scholar]
- Minofar B.; Vrbka L.; Mucha M.; Jungwirth P.; Yang X.; Wang X.-B.; Fu Y.-J.; Wang L.-S. Interior and Interfacial Aqueous Solvation of Benzene Dicarboxylate Dianions and Their Methylated Analogues: A Combined Molecular Dynamics and Photoelectron Spectroscopy Study. J. Phys. Chem. A 2005, 109, 5042–5049. 10.1021/jp050836u. [DOI] [PubMed] [Google Scholar]
- Aryafard M.; Abbasi M.; Řeha D.; Harifi-Mood A. R.; Minofar B. Experimental and theoretical investigation of solvatochromic properties and ion solvation structure in DESs of reline, glyceline, ethaline and their mixtures with PEG 400. J. Mol. Liq. 2019, 284, 59–67. 10.1016/j.molliq.2019.03.149. [DOI] [Google Scholar]
- Hussain A.; Afzal O.; Altamimi A. S. A.; Ali A.; Ali A.; Martinez F.; Usman Mohd Siddique M.; Acree W. E.; Ali N. Preferential solvation study of (Z)-N-benzyl-2-{5-(4-hydroxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetamide (3) in {NMP (1) + Water (2)} co-solvent mixture and GastroPlus software based in vitro simulation. J. Mol. Liq. 2022, 349, 118491. 10.1016/j.molliq.2022.118491. [DOI] [Google Scholar]
- Hussain A.; Altamimi M. A.; Afzal O.; Altamimi A. S. A.; Ali A.; Ali A.; Martinez F.; Mohd Siddique M. U.; Acree W. E. Jr.; Jouyban A. Preferential Solvation Study of the Synthesized Aldose Reductase Inhibitor (SE415) in the {PEG 400 (1) + Water (2)} Cosolvent Mixture and GastroPlus-Based Prediction. ACS Omega 2022, 7, 1197–1210. 10.1021/acsomega.1c05788. [DOI] [PMC free article] [PubMed] [Google Scholar]