Abstract
Background
Data on respiratory syncytial virus (RSV) disease burden in adults remain scarce. We assessed the burden of confirmed RSV-acute respiratory infections (cRSV-ARIs) in community-dwelling (CD) adults and those in long-term care facilities (LTCFs).
Methods
In this prospective cohort study covering 2 RSV seasons (October 2019–March 2020 and October 2020–June 2021), RSV-ARIs were identified through active surveillance, in medically stable CD-adults ≥50 years (Europe) or adults ≥65 years in LTCFs (Europe and the United States). RSV infection was confirmed by polymerase chain reaction from combined nasal and throat swabs.
Results
Of 1981 adults enrolled, 1251 adults in CD and 664 LTCFs (season 1) and 1223 adults in CD and 494 LTCFs (season 2) were included in the analyses. During season 1, overall incidence rates ([IRs] cases/1000 person-years) and attack rates (ARs) for cRSV-ARIs were 37.25 (95% confidence interval [CI], 22.62–61.35) and 1.84% in adults in CD and 47.85 (CI, 22.58–101.4) and 2.26% in adults in LTCFs. Complications occurred for 17.4% (CD) and 13.3% (LTCFs) of cRSV-ARIs. One cRSV-ARI occurred in season 2 (IR = 2.91 [CI, 0.40–20.97]; AR = 0.20%), without complications. No cRSV-ARIs led to hospitalization or death. Viral pathogens were codetected in ≤17.4% of cRSV-ARIs.
Conclusions
RSV is an important cause of disease burden in adults in CD and LTCFs. Despite the observed low severity of cRSV-ARI, our results support the need for RSV prevention strategies among adults ≥50 years old.
Keywords: care facilities, community-dwelling, incidence/burden of disease, older adults, respiratory syncytial virus infection
In a prospective, multi-country, cohort study covering 2 consecutive RSV seasons (Oct2019/Mar2020, Oct2020/Jun2021), the incidence rate of confirmed RSV acute respiratory infections was 37.25 and 47.85 cases/1000 person-years in older adults in community-dwelling and long-term care facilities, respectively.
BACKGROUND
Human RSV is a ribonucleic acid virus of the family Pneumoviridae. Two antigenically distinct subtypes (RSV-A and RSV-B) exist [1] and cocirculate with alternating dominance, but without any clear pattern [2].
Because the clinical symptoms of RSV infection are not specific in adults, differentiation from other respiratory pathogens is challenging without laboratory confirmation. Moreover, virus titers in respiratory secretions are usually lower in adults compared with children and shedding is of shorter duration [3]. In addition, infections start with mild symptoms leading to delays in seeking medical advice and hence to lower likelihood to test positive for RSV [3]. Therefore, the burden of RSV infection continues to be underestimated in the older adult population.
Still, RSV is increasingly recognized as an important pathogen in adults because it can lead to severe lower respiratory tract disease in patients with comorbidities (in particular, immunocompromised adults and those with cardiopulmonary conditions) and in older adults, due to immunosenescence [4–8].
Globally, more than 1.5 million episodes of laboratory-confirmed acute respiratory infection due to RSV (cRSV-ARI), 336 000 hospitalizations, and 14 000 in-hospital deaths related to RSV-ARI were estimated in adults ≥65 years of age in 2015 [9]. However, more recently, approximately 5.2 million cRSV-ARI episodes, 470 000 related hospitalizations, and 33 000 in-hospital deaths were estimated to occur in 2019, in a meta-analysis assessing the burden of RSV-ARI in adults ≥60 years of age from high-income countries [10]. Other recent studies indicate that the burden of RSV disease may be even greater than that of influenza in hospitalized older adults [11–13]. Older adults living in long-term care facilities (LTCFs) are known to experience a high burden of respiratory diseases [14], and several studies have shown that the risk of severe RSV infection is higher among older adults living in LTCF settings than in adults in community-dwelling (CD) [15, 16].
Nevertheless, there are still few prospective surveillance studies performed in either LTCF or CD settings that provide incidence rate (IR) estimates for laboratory-cRSV-ARI. In addition, most studies report data for medically attended cases and therefore the incidence of RSV is most likely underestimated. Knowing the true burden of RSV disease in the general adult population and in older adults is crucial in the evaluation and future implementation of novel antiviral agents for treatment and vaccines for prevention of RSV, which are currently under development. We conducted a prospective study to provide robust estimates of the burden of RSV-ARI in adults ≥50 years of age in different settings, using the most sensitive and specific test, reverse-transcriptase polymerase chain reaction (RT-PCR), for confirmation of RSV cases.
METHODS
Study Design and Participants
We conducted a prospective, multicountry, multicenter cohort study between May 8, 2019 and July 29, 2021 in 5 European countries (Belgium, Estonia, Germany, Spain, United Kingdom) and in the United States (US).
We enrolled individuals aged ≥50 years living in the community (from European countries only) and ≥65 years of age living in LTCFs who were able to understand and comply with the study requirements, were medically stable in the investigators’ opinion, and had plans to remain in the same community or LTCF during 2 years from study start. Individuals with history of vaccination with an investigational RSV vaccine, or those with administration of an RSV-targeting drug or planned administration during the study, were not eligible for enrollment.
We observed all participants for approximately 2 years through active surveillance for potential ARI cases (see Case Definitions). A physical examination and physical frailty assessments were performed each year before the start of the RSV season, and influenza patient-reported outcome instrument (Flu-PRO) and health-related quality of life (HRQoL) questionnaires were completed by each participant. During the RSV seasons (October 2019 to March 2020 for season 1 and October 2020 to June 2021 for season 2), the investigator or study staff contacted the participants every 2 weeks to detect the occurrence of respiratory symptoms; participants were also instructed to contact the site spontaneously in case of ≥2 ARI symptoms (see Case Definitions) occurring between 2 planned surveillance contacts. ARI onset visits were scheduled preferably within 48–72 hours from detection of ARI symptoms and within 7 days of onset of the first symptom. Monthly surveillance contacts were planned between seasons.
Study procedures are summarized in Supplementary Figure 1. For each ARI episode detected during season, an onset visit, follow-up contacts (every 2 weeks until resolution), and a follow-up visit (at 28 days after the onset visit) were planned. Nasal and throat swab samples for the detection of RSV and other respiratory viruses were collected during the onset visit. All assays are described in Supplementary Table 1. The Flu-PRO [17] scores, HRQoL data (SF-12 [18] domain scores and EQ-5D [19] utility scores), healthcare resource utilization (HCRU), and workdays lost were also collected (Supplementary Figure 1).
Due to the COVID-19 pandemic, study procedures were amended for the second RSV season: RSV antibody detection was no longer performed; EQ-5D and SF-12 questionnaires were not used for LTCF participants (due to implementation challenges and increased workload during the pandemic); and RT-PCR testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was performed for all samples collected.
Patient Consent Statement
We conducted the study in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki, and all applicable regulatory requirements. Local or regional Independent Ethics Committees at each site (Supplementary Text 1) approved the study design. Informed consent was obtained from all participants.
Case Definitions
An ARI was defined as a respiratory infection when at least 2 of the following signs and/or symptoms occurred together: rhinorrhea/nasal congestion, sore throat, cough (new or increasing), sputum production (new or increasing), shortness of breath or dyspnea (new or increasing), wheezing (new or increasing), or feverishness or fever (temperature ≥37.5°C).
cRSV-ARI was defined as an ARI episode with detection of RSV by RT-PCR in a combined nasal and throat swab. Episodes with no swab collection or invalid RT-PCR results were classified as with missing RT-PCR results. An ARI episode for which a 4-fold increase in RSV antibody titer (as previously used for diagnosis of RSV infection in older adults [5]) from ARI onset visit to ARI follow-up visit was detected but with a combined nasal and throat swab with negative/invalid/missing RT-PCR result was considered a probable RSV-ARI (pRSV-ARI). Combined nasal and throat swabs were used to enhance the diagnostic yield of respiratory viruses [20, 21].
Study Objectives
Study objectives included determining the IRs and attack rates (ARs) of cRSV-ARI in older adults in CD (primary objective) and LTCF (secondary objective). Other secondary objectives were estimating the IR and AR of pRSV-ARI, the proportion of complications, hospitalizations, and case fatality among RSV-ARIs, the proportion of RSV codetection with other viral pathogens, physical frailty status at the start of each RSV season, as well as the impact of RSV-ARI on Flu-PRO scores, HRQoL, and HCRU, and workdays lost in participants with RSV-ARI and associated caregivers. All complications occurring during an ARI episode were recorded. Respiratory (e.g., leading to bronchitis, bronchopneumonia, pneumonia, worsening of chronic obstructive pulmonary disease, or asthma) and nonrespiratory complications were followed up to resolution.
Statistical Analyses
Sample size estimations were based on IRs of cRSV-ARI in adults aged ≥50 years ranging between 15 and 55 cases/1000 person-years during the RSV season depending on the age category [5, 22, 23]. With an overall sample size of 1000 evaluable adults in the CD cohort, exact Poisson confidence intervals (CIs) are 3.2–23.3 for an IR of 10 cases/1000 person-years and 40.5–85.7 for an IR of 60 cases/1000 person-years; corresponding CIs are 0.0–21.2 and 32.5–87.5 for the normal approximation with design effect assuming an intracluster correlation of 0.02 and 15 centers. With an overall sample size of 600 evaluable adults in the LTCF cohort, exact Poisson CIs are 2.1–29.2 for an IR of 10 cases/1000 person-years and 35.6–94.8 for an IR of 60 cases/1000 person-years; corresponding CIs are 0.0–23.3 and 27.4–92.6 for the normal approximation with design effect. The targets for enrollment were 1250 adults in the CD cohort and 667 adults in LTCF cohort, assuming a dropout rate of 20% among CD participants and 10% among LTCF participants over 2 RSV seasons.
IRs were calculated by dividing the number of older adults with first cRSV-ARI episodes over the sum of the follow-up period at risk for the participants and expressed as number of cases per 1000 person-years, with 95% CIs. The follow-up period at risk for a participant was defined as the duration from start of season until the first cRSV-ARI during the season (for participants with ≥1 cRSV-ARI episode) or end of season or the last follow up during the season, whichever comes first. ARs were calculated as the percentage of participants with ≥1 cRSV-ARI episode, with 95% CIs.
Proportions of complications, hospitalizations, case fatality among RSV-ARI cases, and of RSV codetection with other viral pathogens were estimated with 95% CIs. Flu-PRO and HRQoL data at each timepoint, as well as HCRU and workdays lost, were analyzed descriptively.
Univariate analysis of predictive and/or risk factors associated with development of RSV infection was performed based on participant characteristics, medical history, physical frailty status, and HRQoL at RSV preseason using Poisson regression model accounting for over dispersion. To identify predictive and/or risk factors for the development of RSV infection, a multivariable Poisson regression model accounting for overdispersion was fitted using backward elimination strategy with p-value ≤ 0.1 (level of significance). Analyses were performed on participants without any protocol deviations leading to exclusion (Figure 1). All statistical analyses were performed using SAS software, version 9.4.
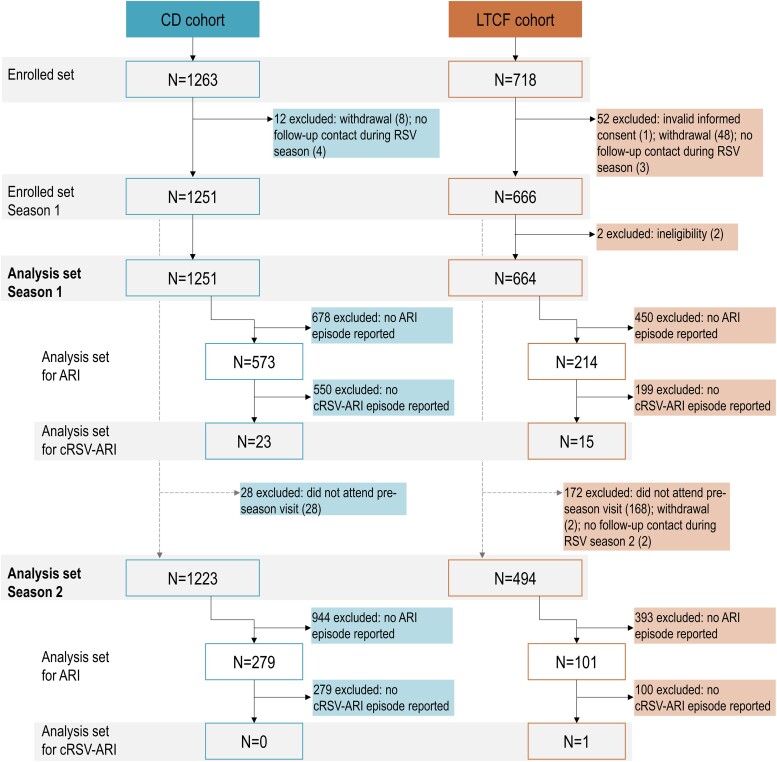
Figure 1.
Participant flowchart. ARI, acute respiratory infection; CD, community-dwelling; cRSV-ARI, confirmed RSV-ARI episode; LTCF, long-term care facility; N, number of participants; RSV, respiratory syncytial virus.
RESULTS
Demographics
The enrolled set comprised 1263 adults in the CD cohort and 718 in the LTCF cohort, 1251 and 664 of whom were included in the analyses at the start of season 1 and 1223 and 494 at the start of season 2 (Figure 1).
In the CD cohort, the mean age at enrollment was 65.1 years (standard deviation [SD] = 8.8), with most adults (869 [69.5%]) aged ≥60 years at enrollment. More than half of the participants (766 [61.2%]) were female (Table 1). Most participants (904 [72.3%]) were living at home without assistance, with only adults in the household.
Table 1.
Baseline Characteristics for Study Participants (Analysis Set)
| Characteristics | CD Cohort | LTCF Cohort |
|---|---|---|
| Season 1 | ||
| N | 1251 | 664 |
| Age at enrollment, mean ± SD (years) | 65.1 ± 8.8 | 82.7 ± 8.5 |
| Age Group at Enrollment (Years) | ||
| 50–59 | 382 (30.5%) | |
| 60–64 | 238 (19.0%) | |
| 65–69 | 242 (19.3%) | 59 (8.9%) |
| 70–79 | 315 (25.2%) | 172 (25.9%) |
| ≥ 80 | 74 (5.9%) | 433 (65.2%) |
| Female sex, n (%) | 766 (61.2%) | 430 (64.8%) |
| Race, n (%) | ||
| White-Caucasian/European heritage | 1245 (99.5%) | 629 (94.7%) |
| Other | 6 (0.5%) | 35 (5.3%) |
| Educational Status, n (%) | ||
| None | 2 (0.2%) | 14 (2.1%) |
| Elementary | 307 (24.6%) | 177 (26.7%) |
| High School | 340 (27.2%) | 228 (34.3%) |
| Vocational/Technical | 291 (23.3%) | 67 (10.1%) |
| College/University | 309 (24.7%) | 178 (26.8%) |
| Missing | 2 (0.1%) | 0 (0.0%) |
| Smoking Status, n (%) | ||
| Current smoker | 210 (16.8%) | 48 (7.2%) |
| Former smoker | 406 (32.5%) | 200 (30.1%) |
| Never smoked | 635 (50.8%) | 416 (62.7%) |
| Frailty Status* | ||
| Fit | 909 (72.7%) | 39 (5.9%) |
| Prefrail | 232 (18.5%) | 79 (11.9%) |
| Frail | 105 (8.4%) | 419 (63.1%) |
| Missing | 5 (0.4%) | 127 (19.1%) |
| Any past or current relevant medical conditions, n (%) | 1167 (93.3%) | 660 (99.4%) |
| Any Current Medical Conditions, n (%) | 1139 (91.0%) | 659 (99.2%) |
| Vascular disorders | 689 (55.1%) | 530 (79.8%) |
| Metabolism and nutrition disorders | 545 (43.6%) | 388 (58.4%) |
| Diabetes | 192 (15.3%) | 176 (26.5%) |
| Obesity | 71 (5.7%) | 20 (3.0%) |
| Respiratory, thoracic, and mediastinal disorders | 249 (19.9%) | 170 (25.6%) |
| Chronic obstructive pulmonary disease | 67 (5.4%) | 92 (13.9%) |
| Asthma | 83 (6.6%) | 35 (5.3%) |
| Cardiac disorders | 189 (15.1%) | 287 (43.2%) |
| Left ventricular failure | 4 (0.3%) | 1 (0.2%) |
| Coronary artery disease | 21 (1.7%) | 25 (3.8%) |
| Renal and urinary disorders | 112 (9.0%) | 181 (27.3%) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 68 (5.4%) | 45 (6.8%) |
| Hepatobiliary disorders | 47 (3.8%) | 15 (2.3%) |
| Received Any Influenza/Pneumococcal/Pertussis Vaccination, n (%) | 796 (63.6%) | 496 (74.7%) |
| Influenza vaccination | 711 (56.8%) | 480 (72.3%) |
| Pneumococcal vaccination | 247 (19.7%) | 208 (31.3%) |
| Pertussis vaccination | 177 (14.1%) | 21 (3.2%) |
| Season 2 | ||
| N | 1223 | 494 |
| Age at enrollment, mean ± SD (years) | 66.5 ± 8.8 | 83.4 ± 8.4 |
| Age Group at Enrollment (Years) | ||
| 50–59 | 294 (24%) | |
| 60–64 | 233 (19.1%) | |
| 65–69 | 255 (20.9%) | 34 (6.9%) |
| 70–79 | 345 (28.2%) | 126 (25.5%) |
| ≥80 | 96 (7.8%) | 334 (67.6%) |
| Female sex, n (%) | 749 (61.2%) | 318 (64.4%) |
| Race, n (%) | ||
| White-Caucasian/European heritage | 1218 (99.6%) | 464 (93.9%) |
| Other | 5 (0.4%) | 30 (6.1%) |
| Any past or current relevant medical conditions, n (%) | 1154 (94.4%) | 491 (99.4%) |
| Received Any Influenza/Pneumococcal/Pertussis Vaccination, n (%) | 838 (68.5%) | 410 (83.0%) |
| Influenza vaccination | 758 (62.0%) | 405 (82%) |
| Pneumococcal vaccination | 268 (21.9%) | 165 (33.4%) |
| Pertussis vaccination | 182 (14.9%) | 17 (3.4%) |
Abbreviations: CD, community-dwelling; LTCF, long-term care facility; N, number of participants in the analysis set; SD, standard deviation; n (%), number (percentage) of participants in a given category.
NOTE: Frailty status was determined based on the short physical performance battery total scores: fit 10–12 points; prefrail 8–9 points; frail ≤7 points.
In the LTCF cohort, the mean age was 82.7 years (SD = 8.5), and 433 (65.2%) of participants were ≥80 years old at enrollment; most (430 [64.8%]) were female (Table 1). Approximately half (305 [45.9%]) lived alone in the room, 191 (28.8%) had 1 roommate, 103 (15.5%) had 2 roommates, and 65 (9.8%) had ≥3 roommates.
Outcomes
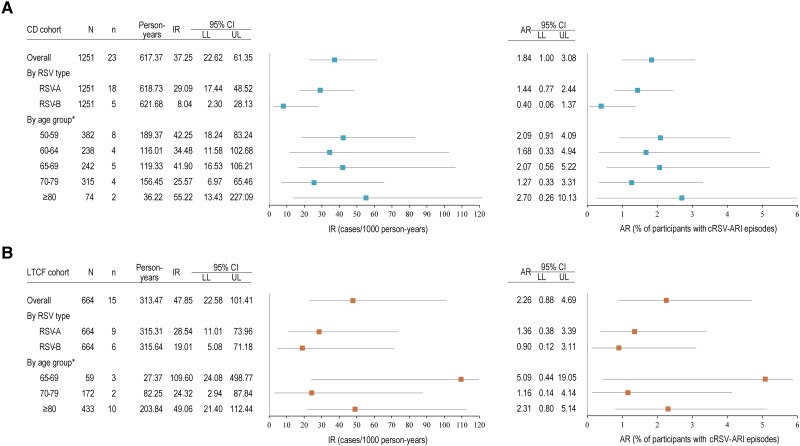
During season 1, we identified 38 cRSV-ARI episodes: 23 (4.0% of all ARIs) in the CD cohort and 15 (7.0% of all ARIs) in the LTCF cohort. IRs for cRSV-ARI were 37.25 cases/1000 person-years (95% CI, 22.62–61.35) in the CD cohort and 47.85 cases/1000 person-years (95% CI, 22.58–101.41) in the LTCF cohort, with corresponding ARs of 1.84% (CD cohort) and 2.26% (LTCF cohort). In both cohorts, higher IR and AR point estimates were observed for RSV-A than RSV-B ARI cases (Figure 2, Supplementary Table 2). In season 2, there was only 1 case of cRSV-ARI (RSV-A), an 87-year-old woman in the LTCF cohort, corresponding to an IR of 2.91 cases/1000 person-years (95% CI, .40–20.97) and an AR of 0.20%.
Figure 2.
Incidence rate (IR) and attack rate (AR) for confirmed respiratory syncytial virus-acute respiratory infection episode (cRSV-ARI) in the community-dwelling (CD) (A) and long-term care facility (LTCF) (B) cohorts during season 1, overall, by RSV type and by age group at enrollment (analysis set). Note: Error bars represent 95% confidence intervals (CIs). For IRs, Wald 95% CIs accounting for clustered data were calculated using Poisson regression with Rao-Scott transformation; when the design effect was either ≤1 or could not be estimated, the exact Poisson 95% CIs were calculated instead. For ARs, extended Clopper-Pearson exact 95% CIs accounting for clustered data were calculated; when the adjusted effective sample size was greater than the actual sample size or the design effect could not be estimated, Clopper-Pearson exact 95% CIs not extended for clustered data were calculated instead. LL, lower limit; N, number of participants in the analysis set; n, number of participants with cRSV-ARI; UL, upper limit.
When using a 4-fold increase threshold in anti-RSV antibody titers, during season 1, 3 pRSV-ARI cases were identified in each cohort (Supplementary Figure 2), corresponding to total ARs of 2.00% (CD cohort) and 2.71% (LTCF cohort) for cRSV-ARI + pRSV-ARI. No recurrent RSV-ARI episodes were reported during the same season. The participant with cRSV-ARI in season 2 did not have cRSV-ARI in season 1. Of participants with cRSV-ARI, ≥95.7% had various medical conditions (Table 1, Supplementary Table 3) at season start. The duration and outcomes of ARI episodes varied by season and cohort (Supplementary Table 4).
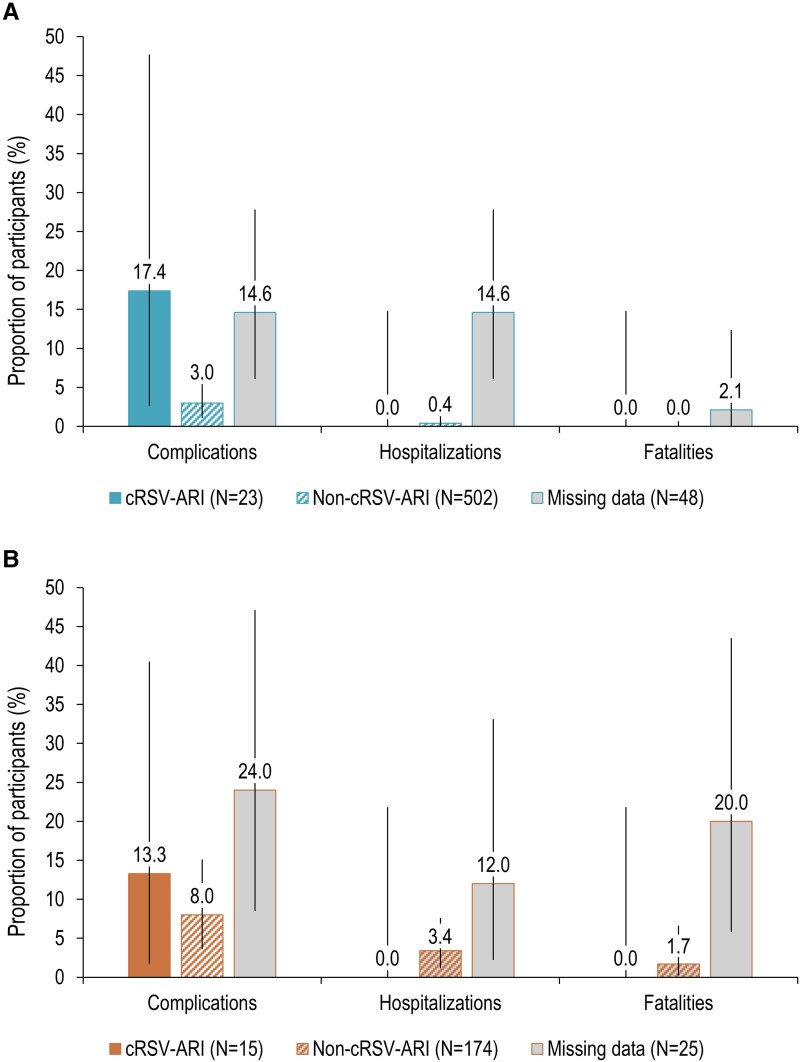
In season 1, complications tended to be more frequent in participants with cRSV-ARI than those without: 17.4% versus 3.0% in the CD cohort and 13.3% versus 8.0% in the LTCF cohort. Most complications were respiratory complications. None of the cRSV-ARI episodes led to hospitalization or death (Figure 3). No complications were reported for the single case of cRSV-ARI occurring during season 2. During RSV interseason, 56 (4.5%) participants in the CD cohort and 49 (8.5%) in the LTCF cohort reported presence of ≥2 ARI symptoms (not caused by RSV).
Figure 3.
Proportions of complications, hospitalizations, and fatalities among adults with acute respiratory infection episode (ARI) in the community-dwelling (CD) (A) and long-term care facility (LTCF) (B) cohorts during season 1, by respiratory syncytial virus (RSV) status (analysis set). Notes: Error bars represent 95% confidence intervals (CIs). Extended Clopper-Pearson exact 95% CIs accounting for clustered data were calculated; when the adjusted effective sample size was greater than the actual sample size or the design effect could not be estimated, Clopper-Pearson exact 95% CIs not extended for clustered data were calculated instead. cRSV-ARI, participants with confirmed RSV-ARI episode (by reverse-transcriptase polymerase chain reaction [RT-PCR]); missing data, participants with missing and/or invalid RT-PCR RSV results for at least 1 ARI episode and without any RT-PCR RSV-positive ARI episode; N, number of participants in the analysis set; non-cRSV, participants with ARI episodes with either no respiratory viral pathogen or a non-RSV viral pathogen identified by RT-PCR.
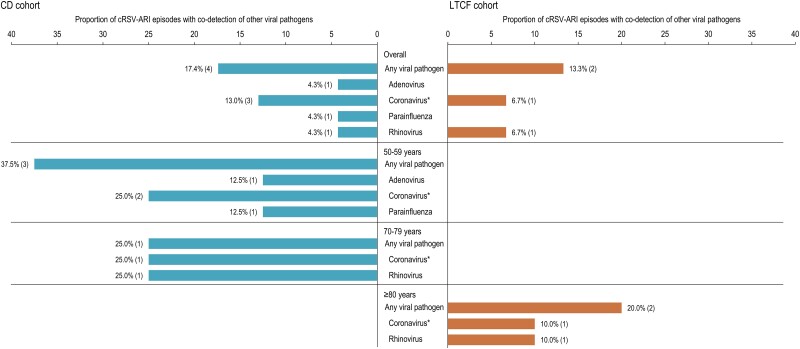
Viral pathogens were codetected for 17.4% of cRSV-ARI episodes in the CD cohort and 13.3% of episodes in the LTCF cohort. Codetected viruses were seasonal coronavirus, adenovirus, parainfluenza, and rhinovirus among CD participants and seasonal coronavirus and rhinovirus among LTCF participants (Figure 4). No other viral pathogens were codetected in the case of cRSV-ARI occurring during season 2. Any viral pathogens were detected in 46.8% and 48.1% of the non-RSV-ARI episodes in the CD and LTCF cohorts during season 1; the most detected virus was rhinovirus. A lower proportion (12.3% for CD and 10.2% for LTCF adults) was observed during season 2, when rhinovirus and SARS-CoV-2 were the most frequently detected pathogens.
Figure 4.
Proportion of confirmed respiratory syncytial virus-acute respiratory infection episode (cRSV-ARI) episodes with codetection of other viral pathogens during season 1, overall and by age group at enrollment (analysis set for cRSV-ARI). Notes: The age groups for which no other viral pathogens were codetected are not shown. The numbers in the brackets are cRSV-ARI episodes with codetection of other viral pathogens. *Seasonal coronaviruses: 229E, OC43, NL63, and HKU1. CD, community-dwelling; LTCF, long-term care facility.
Overall, 99.6% and 98.8% of participants in the CD cohort and 80.9% and 67.1% of those in the LTCF cohort completed the short physical performance battery test pre-RSV season 1 and season 2. Among participants with cRSV-ARI episodes, most older adults (69.6%) in CD were classified as fit, whereas most older adults (69.2%) in LTCFs were classified as frail pre-RSV season 1 (Supplementary Table 5). In the first season, 1.9% (CD cohort) and 2.1% (LTCF cohort) of participants with frail status had cRSV-ARI (Supplementary Table 6). The LTCF participant with the only cRSV-ARI episode during season 2 was classified as frail.
Overall, ≥65.0% of participants completed all surveillance contacts during each RSV season; compliance decreased over time (Supplementary Figure 3). In the combined CD + LTCF cohort, adherence to completion of daily questionnaires during the cRSV-ARI episode was >90% for the Flu-PRO between days 3 and 9; all participants completed ≥1 questionnaire. Completion rates were 100% at day 7 and 80% at day 14 for the weekly SF-12 and EQ-5D questionnaires (Supplementary Table 7, Supplementary Figure 4). The Flu-PRO domains most impacted as demonstrated by the mean maximum (worst) score during cRSV-ARI were nose (mean, 1.97; interquartile range [IQR], 1.25–2.75), chest/respiratory (mean, 1.76; IQR, 1.00–2.57), and throat (mean, 1.51; IQR, 0.67–2.33). Mean worst scores appeared higher for participants with cRSV-ARI versus with non-RSV-ARI (Supplementary Table 8). Supplementary Table 9 presents the SF-12 and EQ-5D domain scores at days 7 and 14 of the cRSV-ARI episode and the corresponding mean change from preseason values. At day 7, the domains with the largest change from preseason were physical functioning (mean = −11.1; SD = 28.3), role emotional (mean = −8.4; SD = 21.1), and vitality (mean = −6.6; SD = 20.7). At day 14, the values were closer to the preseason values except for vitality (mean = −12.1; SD = 23.5). The mean change in EQ5D utility at day 7 was −0.05 (SD = 0.26), whereas the observed mean change in the visual analog scale score was −16.2 (SD = 22.2).
In the combined CD + LTCF cohort, cRSV-ARI episodes required 14 general practitioner and 1 emergency room visits (Table 2, Supplementary Table 10) and 63.2% of episodes required medication. Lost workdays for cRSV-ARI episodes were reported by 2 of 13 (15.4%) participants in active employment and no caregiver support was required (Table 2). The cRSV-ARI episode in season 2 did not require medical visits or caregiver support.
Table 2.
Summary of Healthcare Resource Utilization for ARI Episodes During RSV Season 1, by RSV Status (Analysis Set for ARI, Combined CD + LTCF Cohort)
| HCRU/Workdays Lost | cRSV-ARI (N = 38) | Non-cRSV-ARI (N = 900) | Missing cRSV-ARI (N = 75) | Overall (N = 1013) |
|---|---|---|---|---|
| General practitioner visits, n (%) | 14 (36.8%) | 462 (51.3%) | 52 (69.3%) | 528 (52.1%) |
| Emergency room visits, n (%) | 1 (2.6%) | 26 (2.9%) | 12 (16.0%) | 39 (3.8%) |
| Specialist visits, n (%) | 1 (2.6%) | 14 (1.6%) | 4 (5.3%) | 19 (1.9%) |
| Medication Taken to Treat ARI, n (%) | ||||
| Yes | 24 (63.2%) | 584 (64.9%) | 48 (64.0%) | 656 (64.8%) |
| No | 14 (36.8%) | 316 (35.1%) | 23 (30.7%) | 353 (34.8%) |
| Missinga | 0 (0.0%) | 0 (0.0%) | 4 (5.3%) | 4 (0.4%) |
| Intensive care unit admissions number | 0 | 0 | 1 | 1 |
| Days spent in intensive care unit | 0 | 2 | 2 | |
| Participants with lost days, n (%) | 2 (5.3%) | 79 (8.8%) | 8 (11.0%) | 89 (8.8%) |
| Days lost, mean ± SD | 0.75 ± 0.35 | 6.98 ± 9.41 | 6.00 ± 4.04 | 6.75 ± 8.99 |
| Participants with no lost days, n (%) | 11 (28.9%) | 266 (29.6%) | 10 (13.7%) | 287 (28.4%) |
| Not applicable, n (%) | 25 (65.8%) | 555 (61.7%) | 55 (75.3%) | 635 (62.8%) |
| Missing, n (%) | 0 (0.0%) | 0 (0.0%) | 2 (2.7%) | 2 (0.2%) |
| Caregivers with lost days, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Caregivers with no lost days, n (%) | 5 (13.2%) | 129 (14.3%) | 4 (5.5%) | 138 (13.6%) |
| Not applicable, n (%) | 33 (86.8%) | 771 (85.7%) | 69 (94.5%) | 873 (86.4%) |
| Missing,a n (%) | 0 (0.0%) | 0 (0.0%) | 2 (2.7%) | 2 (0.2%) |
Abbreviations: ARI, acute respiratory infection; CD, community-dwelling; cRSV-ARI, participants with confirmed RSV-ARI episode (by reverse-transcriptase polymerase chain reaction [RT-PCR]); HCRU, healthcare resource utilization; LTCF, long-term care facility; N, number of ARI episodes/participants in the analysis set; n (%), number (percentage) of episodes/participants in a given category; non-cRSV, participants with ARI episodes with either no respiratory viral pathogen or a non-RSV viral pathogen identified by RT-PCR; RSV, respiratory syncytial virus; SD, standard deviation.
Missing data: participants with missing and/or invalid RT-PCR RSV results for at least 1 ARI episode and without any RT-PCR RSV-positive ARI episode.
No risk factors for developing RSV infections were identified through univariate analysis (Supplementary Table 11), using the data during season 1. No independent variable was selected in the final multivariable Poisson regression model in either cohort. However, these results should be interpreted with caution, given the relatively small sample size and low ARs observed for cRSV-ARI, which might have hindered the identification of risk factors.
DISCUSSION
Our results from this observational multicountry study confirm that RSV disease is prevalent among the older adult population, in both community dwellers and those in LTCF settings.
We observed a lower incidence and prevalence of RSV disease in CD settings than that reported in other observational studies conducted in Europe. The proportion of participants with cRSV-ARI among all CD participants was 1.84% in the current study during 2019–2020, whereas in the REspiratory Syncytial virus Consortium in EUrope (RESCEU) study, 2.1% and 4.9% of ≥60-year-old CD adults had confirmed PCR RSV disease in 2 consecutive seasons during 2017–2019 [24]. The proportion of participants with ARI episodes was also lower than that observed in the RESCEU study (45.8% versus 59.2%) [24]. However, our study included ≥50-year-old adults and partially overlapped with the COVID-19 pandemic, when restrictions were in place. In another study conducted in the United States between 2013 and 2015, 3.8% of CD adults ≥65 years of age tested positive for RSV [25]. Lower RSV-ARI rates (2.4%), similar to those in our study, were observed in a study in Japanese adults aged ≥65 years living mostly in CD settings during the 2019–2020 season [26]. Higher IRs and ARs were observed in our study in LTCF participants ≥65 years of age than in the ≥50-year-old CD adults. Other studies in older adults living in LTCF settings reported incidence proportions of 1.1%–10.8% (compared to 2.25% in the current study) for RSV infections [14]. In our study, serology increased the diagnostic yield for RSV by 13% in the CD and 20% in the LTCF ARs. This is lower than the 34%–64% increase reported in a recent systematic review, but which only included studies conducted in medically attended RSV infections among US adults [27]. Of note, any comparisons between studies are hindered by varying case definitions, methodology, difference in the age group of study populations, RSV testing method, and timing and other factors (eg, geography, duration of RSV season). Only 1 case of cRSV-ARI was detected in our study during season 2, when RSV virtually disappeared. This disappearance was due to nonpharmaceutical interventions implemented during the COVID-19 pandemic, which led to disruptions in the transmission and seasonality of respiratory viruses, including RSV [28–30]. These disruptions ultimately led to off-season RSV epidemics after lifting of nonpharmaceutical interventions, as documented in several countries in 2021–2022 mostly in children [31–33] but also in adults [34]. The COVID-19 pandemic started in March 2020; therefore, we expect some underestimation of RSV IR/ARs in season 1 in our study because RSV surveillance was scheduled to finish at the end of March 2020.
Complications, mostly respiratory in nature, tended to be more frequent for cRSV-ARI than other ARI episodes. However, they were not severe, and no hospitalizations or deaths were observed among adults with cRSV-ARI in both CD and LTCF cohorts in this study. This was an expected finding, because the study included medically stable participants with access to high-quality healthcare services; similarly, in the RESCEU study, severe RSV disease was rare, and no hospitalizations or deaths were observed [24]. In a recent meta-analysis conducted in adults ≥60 years from United States, Canada, European countries, Japan, and South Korea between 2000 and 2021, pooled hospitalization AR of 0.15% and in-hospital case-fatality ratio of 7.13% were estimated for RSV-ARI [10].
Other pathogens were codetected with RSV in <18% of participants in both cohorts in our study, in line with recently reported low viral coinfection rates in adults (7.4%) [35]. RSV-influenza coinfections in adults [36] have been previously shown to be associated with poorer outcomes, including mortality, compared with monoinfections. Most participants in our study had influenza vaccination before RSV season, likely reducing the risk of coinfections. In addition, viral interference between RSV and influenza could also have led to lower codetection of influenza in our study [37].
Most participants with cRSV-ARI in the CD cohort in our study were classified as fit or prefrail. However, most of those in the LTCF cohort were classified as frail. The symptomatology of cRSV-ARI was demonstrated by the mean worst scores observed during the episode in the Flu-PRO nose, throat, chest/respiratory, and eyes domains and the consistently higher mean worst scores in participants with cRSV-ARI. The impact of cRSV-ARI on HRQoL was apparent during the first 7 days of the episode with notable worsening (from preseason) of the participants’ physical functioning and ability to carry out normal activities due to emotional impact. This emotional impact was also observed in a concept elicitation study of the effects of RSV on HRQoL [38]. The cRSV-ARI episode had a notable longer impact on the participants’ vitality with worsening fatigue evident during the first 14 days.
The HCRU attributable to cRSV-ARI episodes was similar to that for all ARI episodes. Although to date there are no studies comparing pathogen-specific HCRU, a recent systematic review and meta-analysis showed that ARI management costs are high in older adults [39].
The results of our study support the need for a vaccine and the adoption of prevention strategies to reduce the burden of RSV disease in older adults. Currently, no licensed vaccine is available, but several candidates have reached phase 3 trials [40, 41].
This study's prospective design allowed full description of RSV disease outcome and course. Other study strengths include the collection of paired nasopharyngeal swabs and blood samples to maximize RSV detection. However, the study has several limitations. The case definition (excluding infections with mild respiratory symptoms) may have led to an underestimation of RSV incidence. In addition, only the first season contributed to the estimation of RSV-ARI incidence. Generalization of study results to the population ≥50 years is not possible because study participants had to be medically stable. Moreover, it is not guaranteed that the sample population enrolled in the study are representative of the general population of the country or region from which older adults were enrolled. Some selection bias and health-seeking behavior bias cannot be excluded. Finally, the relatively small sample size and healthier condition of the study population might explain the fact that RSV complications leading to hospitalization were not reported.
CONCLUSIONS
In conclusion, despite the limited number of participants, this study showed that RSV causes disease burden in CD and LTCF older adults. Although hospitalizations and fatalities were not frequent in this population, the observed disease burden supports the need for prevention strategies against RSV among the older adults.
Supplementary Material
Acknowledgments
We thank all participants and their families and study staff. We also thank Akkodis Belgium c/o GSK for medical writing support (Petronela M. Petrar) and manuscript coordination.
Author contributions. CV, DL, J-YP, JSTB, RD, and SD were involved in the conception or the design of the study. ABM, AH, AA, AS, CV, DM, IL-R, J-YP, JSTB, JMRT, KS, LLH, LP-B, RP, RGF, SM, and SNP participated in the collection or generation of the study. AP, AH, AA, AS, CV, DM, DL, IL-R, J-YP, JSTB, JMRT, KS, LLH, LP-B, RP, RGF, SM, and SNP performed the study. DL and RD contributed materials/analysis/reagent tools. DL, IL-R, J-YP, JMRT, LP-B, RD, SD, and SM were involved in the analyses or interpretation of the data.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA . GlaxoSmithKline Biologicals SA covered all costs associated with the development and publishing of the present manuscript.
Contributor Information
Sílvia Narejos Pérez, CAP Centelles, Centelles, Barcelona, Spain.
Josep María Ramón Torrell, Hospital de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain.
Airi Põder, Clinical Research Center, Tartu, Estonia.
Isabel Leroux-Roels, Center for Vaccinology, Ghent University Hospital, Ghent, Belgium.
Lina Pérez-Breva, Fundación para el Fomento de la Investigación Sanitaria y Biomédica, Valencia, Spain.
Katie Steenackers, Centre for the Evaluation of Vaccination, University of Antwerp, Antwerp, Belgium.
Corinne Vandermeulen, KU Leuven, Leuven University Vaccinology Center, Department of Public Health and Primary Care, Leuven, Belgium.
Sandra Meisalu, Innomedica Medical Center, Tallinn, Estonia.
Damien McNally, Ormeau Health Centre, Belfast, UK.
Jordan S T Bowen, John Radcliffe Hospital, Oxford University Hospitals NHS Trust, Oxford, UK.
Amardeep Heer, Lakeside Surgery, Lakeside Healthcare, Corby, UK.
Adrian Beltran Martinez, Ashgate Medical Practice, Chesterfield, UK.
Laura L Helman, MOC Research, Mishawaka, Indiana, Indiana, USA.
Amit Arora, Haywood Community Hospital—Midlands Partnership NHS Foundation Trust, Stoke-on-Trent, UK.
Robert G Feldman, Senior Clinical Trials, Laguna Hills, California, California, USA.
Rajul Patel, Royal South Hants Hospital, Clinical Research Office, Solent NHS Trust, Southampton, UK.
Amit Shah, Piedmont Research Partners, LLC, Fort Mill, South Carolina, USA.
Raghavendra Devadiga, GSK, Bangalore, India.
Silvia Damaso, GSK, Wavre, Belgium.
Sean Matthews, Freelance c/o GSK, Wavre, Belgium.
Jean-Yves Pirçon, GSK, Wavre, Belgium.
Dominique Luyts, GSK, Wavre, Belgium.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 1966; 83:299–313. [DOI] [PubMed] [Google Scholar]
- 2. Staadegaard L, Caini S, Wangchuk S, et al. . Defining the seasonality of respiratory syncytial virus around the world: national and subnational surveillance data from 12 countries. Influenza Other Respir Viruses 2021; 15:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets 2012; 12:98–102. [DOI] [PubMed] [Google Scholar]
- 4. Belongia EA, King JP, Kieke BA, et al. . Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis 2018; 5:ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 6. Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol 2018; 71:1574–83. [DOI] [PubMed] [Google Scholar]
- 7. Kumar R, Dar L, Amarchand R, et al. . Incidence, risk factors, and viral etiology of community-acquired acute lower respiratory tract infection among older adults in rural north India. J Glob Health 2021; 11:04027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malosh RE, Martin ET, Callear AP, et al. . Respiratory syncytial virus hospitalization in middle-aged and older adults. J Clin Virol 2017; 96:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi T, Denouel A, Tietjen AK, et al. . Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–83. [DOI] [PubMed] [Google Scholar]
- 10. Savic M, Penders Y, Shi T, Branche A, Pirçon J-Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses 2022; 17:e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ackerson B, Tseng HF, Sy LS, et al. . Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis 2019; 69:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atamna A, Babich T, Froimovici D, et al. . Morbidity and mortality of respiratory syncytial virus infection in hospitalized adults: comparison with seasonal influenza. Int J Infect Dis 2021; 103:489–93. [DOI] [PubMed] [Google Scholar]
- 13. Sharp A, Minaji M, Panagiotopoulos N, Reeves R, Charlett A, Pebody R. Estimating the burden of adult hospital admissions due to RSV and other respiratory pathogens in England. Influenza Other Respir Viruses 2022; 16:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Childs A, Zullo AR, Joyce NR, et al. . The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr 2019; 19:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging 2015; 32:261–9. [DOI] [PubMed] [Google Scholar]
- 16. Goldman CR, Sieling WD, Alba LR, et al. . Severe clinical outcomes among adults hospitalized with respiratory syncytial virus infections, New York City, 2017–2019. Public Health Rep 2021; 137:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powers JH, Guerrero ML, Leidy NK, et al. . Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware JJ, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–33. [DOI] [PubMed] [Google Scholar]
- 19. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33:337–43. [DOI] [PubMed] [Google Scholar]
- 20. Ek P, Böttiger B, Dahlman D, Hansen KB, Nyman M, Nilsson AC. A combination of naso- and oropharyngeal swabs improves the diagnostic yield of respiratory viruses in adult emergency department patients. Infect Dis (Lond) 2019; 51:241–8. [DOI] [PubMed] [Google Scholar]
- 21. Kim C, Ahmed JA, Eidex RB, et al. . Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One 2011; 6:e21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falloon J, Yu J, Esser MT, et al. . An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McClure DL, Kieke BA, Sundaram ME, et al. . Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS One 2014; 9:e102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korsten K, Adriaenssens N, Coenen S, et al. . Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J 2021; 57:2002688. [DOI] [PubMed] [Google Scholar]
- 25. Smithgall M, Maykowski P, Zachariah P, et al. . Epidemiology, clinical features, and resource utilization associated with respiratory syncytial virus in the community and hospital. Influenza Other Respir Viruses 2020; 14:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurai D, Natori M, Yamada M, Zheng R, Saito Y, Takahashi H. Occurrence and disease burden of respiratory syncytial virus and other respiratory pathogens in adults aged ≥65 years in community: a prospective cohort study in Japan. Influenza Other Respir Viruses 2022; 16:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis 2022; 9: ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi HJ, Kim NY, Eom SA, et al. . Effects of non-pharmacological interventions on respiratory viruses other than SARS-CoV-2: analysis of laboratory surveillance and literature review from 2018 to 2021. J Korean Med Sci 2022; 37:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tempia S, Walaza S, Bhiman JN, et al. . Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill 2021; 26:2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Summeren J, Meijer A, Aspelund G, et al. . Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill 2021; 26:2001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li ZJ, Yu LJ, Zhang HY, et al. . Broad impacts of COVID-19 pandemic on acute respiratory infections in China: an observational study. Clin Infect Dis 2021; 75:e1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis 2021; 27:2969–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinberger Opek M, Yeshayahu Y, Glatman-Freedman A, Kaufman Z, Sorek N, Brosh-Nissimov T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Euro Surveill 2021; 26:2100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fourgeaud J, Toubiana J, Chappuy H, et al. . Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis 2021; 40:2389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mandelia Y, Procop GW, Richter SS, Worley S, Liu W, Esper F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin Microbiol Infect 2021; 27:e1–6. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Zhao J, Zou X, et al. . Severity of influenza virus and respiratory syncytial virus coinfections in hospitalized adult patients. J Clin Virol 2020; 133:104685. [DOI] [PubMed] [Google Scholar]
- 37. Piret J, Boivin G. Viral interference between respiratory viruses. Emerg Infect Dis 2022; 28:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Curran D, Cabrera ES, Bracke B, et al. . Impact of respiratory syncytial virus disease on quality of life in adults aged ≥50 years: a qualitative patient experience cross-sectional study. Influenza Other Respir Viruses 2022; 16:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang S, Wahi-Singh P, Wahi-Singh B, Chisholm A, Keeling P, Nair H. Costs of management of acute respiratory infections in older adults: a systematic review and meta-analysis. J Glob Health 2022; 12:04096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drysdale SB, Barr RS, Rollier CS, Green CA, Pollard AJ, Sande CJ. Priorities for developing respiratory syncytial virus vaccines in different target populations. Sci Transl Med 2020; 12:eaax2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. PATH . RSV vaccine and mAb snapshot. Available at: http://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Accessed 20 June 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.