Abstract
Background
Rising incidence of hepatitis C virus (HCV) among people with HIV (PWH) in San Diego County (SDC) was reported. In 2018, the University of California San Diego (UCSD) launched a micro-elimination initiative among PWH, and in 2020 SDC launched an initiative to reduce HCV incidence by 80% across 2015–2030. We model the impact of observed treatment scale-up on HCV micro-elimination among PWH in SDC.
Methods
A model of HCV transmission among people who inject drugs (PWID) and men who have sex with men (MSM) was calibrated to SDC. The model was additionally stratified by age, gender, and HIV status. The model was calibrated to HCV viremia prevalence among PWH in 2010, 2018, and 2021 (42.1%, 18.5%, and 8.5%, respectively), and HCV seroprevalence among PWID aged 18–39 years, MSM, and MSM with HIV in 2015. We simulate treatment among PWH, weighted by UCSD Owen Clinic (reaching 26% of HCV-infected PWH) and non-UCSD treatment, calibrated to achieve the observed HCV viremia prevalence. We simulated HCV incidence with observed and further treatment scale-up (+/− risk reductions) among PWH.
Results
Observed treatment scale-up from 2018 to 2021 will reduce HCV incidence among PWH in SDC from a mean of 429 infections/year in 2015 to 159 infections/year in 2030. County-wide scale-up to the maximum treatment rate achieved at UCSD Owen Clinic (in 2021) will reduce incidence by 69%, missing the 80% incidence reduction target by 2030 unless accompanied by behavioral risk reductions.
Conclusions
As SDC progresses toward HCV micro-elimination among PWH, a comprehensive treatment and risk reduction approach is necessary to reach 2030 targets.
Keywords: HCV, HIV, PWH, modeling
Hepatitis C virus (HCV) and HIV share common transmission routes [1]. Although the most common HCV transmission form in the United States occurs due to sharing injection equipment among people who inject drugs (PWID) [2], sexual transmission of HCV is significant among people with HIV (PWH), especially among men who have sex with men (MSM) [3]. Hence, the coinfection of HCV among PWH is a major public health concern. According to 2009 data from the Centers for Disease Control and Prevention (CDC), 21% of all PWH were HCV seropositive [1]. As individuals coinfected with HIV and HCV experience accelerated liver disease progression [1], complications due to untreated HCV are a significant cause of morbidity and mortality among PWH who are otherwise well controlled on antiretroviral therapy [1].
In 2016, the World Health Organization (WHO) adopted its Global Health Sector Strategy (GHSS) and set the strategic goal to eliminate HCV as a public health threat [4]. The WHO targets include a 90% reduction in HCV and HBV incidence (with specific targets of 80% reduction for HCV and 95% reduction for HBV) and a 65% reduction in HCV mortality by 2030 compared with a 2015 baseline [4]. Because screening for HCV is part of the HIV standard of care and many PWH already have access to health services through integrated models of care [5], many consider that PWH comprise a population where HCV elimination (micro-elimination) is feasible [5]. Indeed, some countries with universal health care systems have reported significant progress toward HCV micro-elimination among PWH [6, 7]. Similar programs exist nationally, statewide, and county-wide in the United States. Our 2018 study on HCV burden in San Diego County estimated that 55 354 individuals are HCV seropositive in San Diego County [8]. The Eliminate Hepatitis C San Diego County Initiative is a public–private joint endeavor between the San Diego County Health Department and the American Liver Foundation to support the achievement of the WHO HCV elimination goal [9].
We recently reported that the rising HCV incidence among MSM with HIV indicates an urgent need for interventions to control the expanding HCV epidemic [10], with a projected number of 3500 PWH coinfected with HCV in San Diego in 2017 [11]. In 2018, the UCSD Owen Clinic launched a micro-elimination initiative to scale-up HCV treatment among PWH. While this program has achieved significant progress in HCV treatment uptake among PWH, the potential impact of the scale-up, particularly on meeting the 2030 elimination targets, is uncertain. Further, it is unclear whether San Diego is on track to achieve the HCV elimination goals among PWH. We aim to address that knowledge gap by using dynamic epidemic modeling of HCV transmission to assess the potential impact of observed treatment scale-up on HCV micro-elimination among PWH in San Diego County by 2030.
METHODS
Model Description
We developed a dynamic, deterministic compartmental model of HCV transmission and progression, stratified by HIV infection, which was calibrated to San Diego County. The model was stratified by hepatitis C infection and disease progression status (Figure 1A), age, gender, HIV status, and transmission risk, which included PWID or MSM (Figure 1B). The HCV infection and disease stages, represented by n, are (i) Susceptible, (ii) Spontaneous Clearance from no/mild liver disease, (iii) SVR from no/mild liver disease, (iv) SVR Moderate Liver Disease, (v) SVR Compensated Cirrhosis, (vi) SVR Decompensated Cirrhosis, (vii) SVR Hepatocellular Carcinoma, (viii) No/Mild Liver Disease, (ix) Moderate Liver Disease, (x) Compensated Cirrhosis, (xi) Decompensated Cirrhosis, and (xii) Hepatocellular Carcinoma. The age stages are 18–39, 40–54, 55–74, and 75+ years. The 6 population subtypes are MSM HIV-, MSM HIV+, PWID HIV- Male, PWID HIV+ Male, PWID HIV- Female, and PWID HIV+ Female. The model assumes that individuals enter the adult population at 18 years old, susceptible to primary HCV infection, and in one of the population subtype compartments stratified by gender, HIV status, PWID status, and MSM (Figure 1B). PWID enter the model in the first age stage (18–39 years) and progress through the age stages or discontinue injection drug use. The rate of permanent cessation of injecting was sampled widely with uncertainty using data on duration of injection among PWID from STAHR II [12]. We assume that HIV status is a fixed characteristic; the model does not simulate HIV transmission dynamically due to the stable HIV prevalence among PWID and MSM in San Diego County [13, 14].
Figure 1.
Model schematic of (A) HCV disease progression and (B) stratification by age, HIV status, and risk. Abbreviations: HCV, hepatitis C virus; MSM, men who have sex with men; PWID, people who inject drugs.
Individuals enter the model susceptible to primary HCV infection. HCV transmission is simulated among PWID and MSM groups separately (without transmission between these groups) because very few MSM report injecting drugs in San Diego (1% of HIV-negative and 3% of MSM diagnosed with acute HIV) [15, 16], and based on phylogenetic analyses in other settings indicating that the MSM-IDU and PWID epidemics are distinct [17]. Therefore, although a small fraction of MSM may inject drugs, we classify these individuals as MSM and assume they inject with other MSM. The model also assumes assortative mixing among MSM by HIV status.
Once acutely infected, individuals can either transition to the no/mild liver disease compartment or they may spontaneously clear HCV infection and move to the spontaneous clearance from the no/mild liver disease compartment. The fraction of individuals who spontaneously clear infection, which is reduced for those with HIV. From the no/mild liver disease compartment, individuals continue to progress through the disease stages as if they continue to have chronic persistent infection unless successfully treated. Individuals can be treated from all stages. Those who have been successfully treated move into the equivalent SVR stage and are susceptible to HCV reinfection. Successful treatment stops any HCV-related disease progression unless an individual has already reached the compensated cirrhosis stage or beyond, where disease progression occurs at a slower rate compared with those without SVR. HCV-related mortality occurs from the decompensated cirrhosis compartments and hepatocellular carcinoma compartments. All baseline model parameters, sampling distributions, and sources are listed in Supplementary Table 1.
Model Parameterization and Calibration
The model was parameterized mainly from the published literature (Supplementary Table 1). For model calibration and parameterization, we used data from our 2018 HCV burden estimation (population sizes and HCV seroprevalence) among adults over age 18 in San Diego County [8] and 3 time points of HCV viremia prevalence among PWH from UCSD Owen Clinic [18]. By varying 7 predetermined parameters (transmission rate among MSM, transmission rate among PWID, degree of assortative mixing among MSM by HIV status, county-wide annual treatment rates among HIV/HCV-coinfected individuals treated from 1996 to 2010 and 2011 to 2017, non-UCSD treatment rates among HIV/HCV-coinfected individuals from 2018 to 2021, and number of PWID at model initialization in 1955), the model was calibrated to HCV seroprevalence estimates in 2015 among MSM (4.6% among all MSM, 16.5% among MSM with HIV) and PWID aged 18–39 years (49.5%) [8, 12], HCV viremia prevalence among HCV-seropositive PWH of 42.1% (2010), 18.5% (2018), and 8.5% (2021) [18], and number of PWID in 2007 [19]. Calibrated parameters are listed in Supplementary Table 2. The calibrated model was then validated against HCV seroprevalence among PWID aged 40–54 (68%) and 55–74 (88%) in 2015 (age-stratified data unpublished, but overall prevalence published in Horyniak et al. [12]).
Historic (pre-2017) HCV treatment rates among PWH were unknown in San Diego County and calibrated to observed HCV viremia prevalence trends among PWH. To obtain the observed declines in viremic prevalence among PWH, we simulated a piece-wise treatment function from 1996 to 2010, 2011 to 2017, and 2018 to 2021. The time period of 1996–2010 represents the pegylated interferon (IFN) plus ribavirin era; 2011–2017 represents the era of the first generation of direct-acting antiviral (DAA) therapy, such as telaprevir (which still required IFN and was available in February 2011 at UCSD through compassionate access programs), along with the DAA era. The UCSD Owen Clinic cares for ∼3200 PWH every year. As we had data from the UCSD Owen Clinic on treatment rates from 2018 onwards, we simulated a weighted treatment rate during this period between UCSD and other (non-UCSD) clinics, with the UCSD Owen Clinic providing care for an estimated 26% of PWH in San Diego County in the last 5 years [11, 20], and our model assumed a conservative estimate of a similar proportion of PWH coinfected with HCV. At the Owen Clinic, a rapid scale-up of HCV treatment among PWH occurred from 2018 onwards, achieving an average rate of 54% HCV-infected PWH treated per year between 2018 and 2021, reaching a maximum treatment rate of 71% in 2021. HCV treatment rates among patients without HIV were obtained from studies in San Francisco and nationally [21, 22]. As the model does not explicitly model diagnosis, the data informing our treatment rates are based on treatment among the entire population, and calibrated treatment rates among PWH outside the Owen Clinic implicitly incorporate undiagnosed individuals.
To capture uncertainty in input parameters, a sample of 500 parameter sets was drawn from uncertainty distributions for each parameter. These sampled parameter sets were then used to generate 500 model fits to the observed data. Calibration was achieved using a least-squares minimization solver, lsqnonlin, in MATLAB (Optimization Toolbox). The lsqnonlin function uses the Levenberg-Marquardt minimization algorithm, and we use the function MultiStart to start from multiple initial guess points within the parameter priors to ensure a global minimum is found. We assign wide prior bounds to the unknown parameters and assess our posterior estimates to ensure our priors were sufficiently wide (Supplementary Table 2 shows priors and posteriors).
Calibrated model runs were then excluded if the fits fell outside the 95% CI of the calibration data for HCV seroprevalence in 2015 among (i) all MSM, (ii) PWID aged 18–39 years, and (iii) MSM with HIV [8, 12], generating a total of 182 model fits to the data.
Simulation Scenarios
We simulated 3 scenarios of HCV treatment. For all of them, we assumed a treatment rate of 2%/year from 1996 to the end of 2012. It increased to 5%/year from 2013 onwards for all non-PWH populations [21, 22]. Among PWH, our calibrated model fits generated estimates of mean treatment rates of 25.4%/year from 1996–2010 and 28.8%/year from 2011 to 2017. From 2018 onwards, the following scale-up among PWH was simulated:
Scenario 1 (counterfactual, no scale-up from 2018 onwards): continuation of pre-2018 treatment rates [mean 28.8% of chronically infected PWH treated/year].
Scenario 2 (observed): UCSD [53.7%/year], other sites [calibrated 60.6%/year].
Scenario 3 (enhanced scale-up from 2021): as in scenario 2, then all scale-up to maximum UCSD treatment rate [in 2021, 71% of chronically infected PWH treated/year] starting in 2021.
Scenario 4 (enhanced scale-up plus halved transmission risk from 2021 onwards): as in scenario 3, with the addition of a halving of transmission risk starting in 2021.
We outputted the incidence of HCV infections among PWH, HCV viremia prevalence among PWH, and the percent reduction in HCV incidence among PWH from 2015 to 2030.
Sensitivity Analyses
We performed a partial rank coefficient correlation (PRCC) analysis to understand how sensitive the model prediction of HCV incidence reductions (2015–2030) was to uncertainty in the underlying parameters.
We perform several 1-way sensitivity analyses to test the importance of model assumptions. First, we examine variations in assumptions of treatment among people without HIV. For our baseline analysis, we assume historic treatment rates, but we perform 2 additional sensitivity analyses assuming (i) treatment scale-up at the same relative percent increase as HCV-infected PWH compartments and (ii) 2-fold relative percent increase as compared with HCV-infected PWH compartments.
Second, there is uncertainty regarding the effect of the COVID-19 pandemic on treatment rates in San Diego County, but national analyses showed a drop in treatment by 20% during 2020, which rebounded through the end of 2020 but did not achieve pre-COVID levels. We performed 2 sensitivity analyses where treatment rates were reduced by a relative 20% from (iii) 2020 to 2021 (then returning to pre-COVID levels in 2022) and (iv) 2020 to 2024 (returning to pre-COVID levels in 2025).
Lastly, our baseline analysis assumes similar SVR rates by risk, as a recent meta-analysis found that SVR rates among PWID or people on opiate substation therapy were high (90%) and were not significantly different from patients without a history of injecting drugs [23]. However, we additionally performed a sensitivity analysis, where SVR rates among PWID were reduced by a relative 10% (re-calibrated parameters listed in Supplementary Table 3).
Statistical Analysis
The model was created using MATLAB (version R2022b).
RESULTS
The model calibration resulted in 182 runs that fit the data. The calibrated model accurately matched 2015 HCV seroprevalence estimates for MSM and PWID aged 18–39 years and HCV viremia prevalence among PWH for 2010, 2018, and 2021 (Table 1). The model was validated against HCV seroprevalence estimates among PWID aged 40–54 (model estimate, 71.5%; 95% CI, 61.9%–77.9%; compared with 68% target) and PWID aged 55–74 (84.3%; 95% CI, 75.7%–89.4%; compared with 88% target) in 2015. In 2015, the model projects 429 (95% CI, 238–777) incident HCV infections among PWH (Figure 2), with an HCV incidence rate of 2.6 per 100 person-years (/100py; 95% CI, 1.4–4.6). HCV viremia prevalence among HCV-seropositive PWH was estimated at 27.2% (95% CI, 21.7%–32.3%) (Figure 3). Details on HCV incident infections (total) among PWH in San Diego County are provided in Supplementary Table 4.
Table 1.
HCV Calibration Data for San Diego County and Model Outputs
| Year | Observed Calibration Data [95% CI] | Calibrated Model Output [95% CI] |
Reference | |
|---|---|---|---|---|
| HCV seroprevalence among MSM with HIV | 2015 | 0.165 [0.155–0.176] | 0.163 [0.156–0.172] | Wynn et al. [8] |
| HCV seroprevalence among all MSM | 2015 | 0.046 [0.030–0.061] | 0.054 [0.047–0.061] | Wynn et al. [8] |
| HCV seroprevalence among PWID aged 18–39 y | 2015 | 0.495 [0.428–0.563] | 0.494 [0.433–0.552] | Horyniak et al. [12] |
| HCV viremia prevalence among HCV-seropositive PWH | 2010 | 0.4211 [0.3791–0.4643] | 0.4212 [0.4128–0.4444] | UCSD Owen Clinic [18] |
| 2018 | 0.1849 [0.1534–0.2212] | 0.1844 [0.1321–0.2384] | UCSD Owen Clinic [18] | |
| 2021 | 0.0857 [0.0636–0.1146] | 0.0857 [0.0857–0.0857] | UCSD Owen Clinic [18] | |
| Number of PWID (total) | 2007 | 24 991 [3751–49 503] | 24 996 [24 991–25 031] | Tempalski et al. [19] |
Abbreviations: HCV, hepatitis C virus; MSM, men who have sex with men; PWID, people who inject drugs.
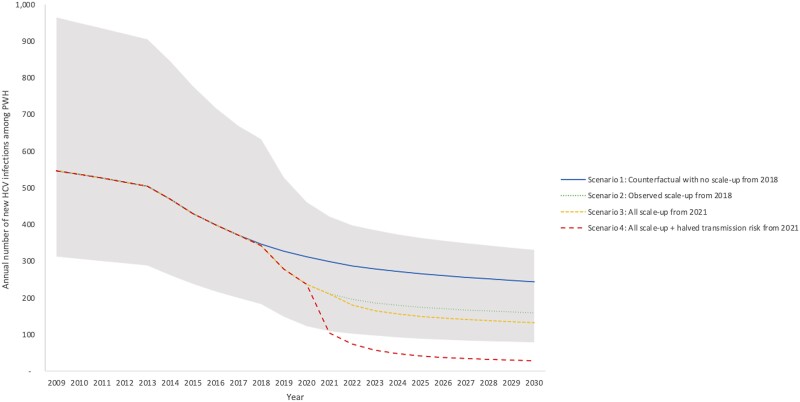
Figure 2.
Annual number of new HCV infections among PWH in San Diego County. Scenarios shown are (1) counterfactual continuation of pre-2018 treatment rates (29%/year, blue solid line); (2) observed scale-up from 2018 (to 54% at UCSD and 61%/year non-UCSD, green round dotted line); (3) county-wide scale-up to peak treatment achieved by UCSD (71%/year) from 2021 (71%/year, yellow square dotted line); (4) as in Scenario #3 plus halved transmission risk behavior from 2021 onwards (red dashed line). Mean model projections (lines), with shading denoting the 95% uncertainty interval around the observed scenario (Scenario 2). Abbreviations: HCV, hepatitis C virus; PWH, people with HIV; UCSD, University of California San Diego.
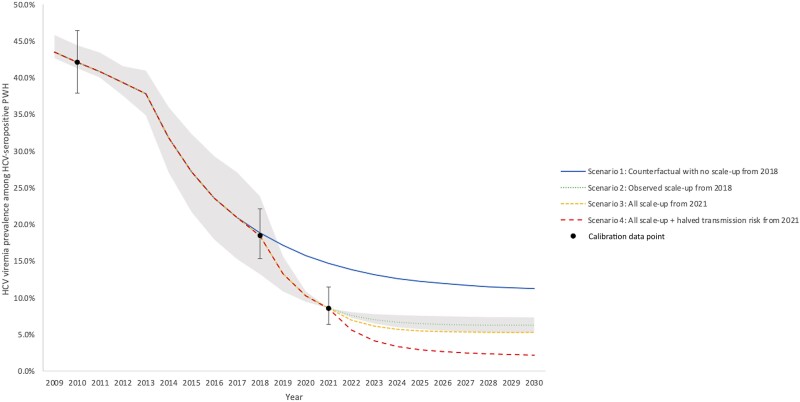
Figure 3.
HCV viremia prevalence among HCV-seropositive PWH in San Diego County. Scenarios shown are (1) counterfactual continuation of pre-2018 treatment rates (29%/year, blue solid line); (2) observed scale-up from 2018 (to 54% at UCSD and 61%/year non-UCSD, green round dotted line); (3) county-wide scale-up to peak treatment achieved by UCSD (71%/year) from 2021 (71%/year, yellow square dotted line); (4) as in Scenario 3 plus halved transmission risk behavior from 2021 onwards (red dashed line). Mean model projections (lines), with shading denoting the 95% uncertainty interval around the observed scenario (Scenario 2). Abbreviations: HCV, hepatitis C virus; PWH, people with HIV; UCSD, University of California San Diego.
Without treatment scale-up in 2018 (scenario 1), incident HCV infections among PWH were expected to decrease to 342 (95% CI, 183–633) incident infections by 2018, with an HCV incidence rate of 2.0/100py (95% CI, 1.1–3.7). By 2030, incident infections decline to 347 (95% CI, 185–636), but the HCV elimination target is not reached—with an estimated 43% (95% CI, 33%–52%) reduction in HCV incidence from 2015 to 2030 (Figure 2). By 2030, the HCV incidence rate declines to 1.4/100py (95% CI, 0.7–3.0). HCV viremia prevalence among PWH declines to 18.8% (95% CI, 13.2%–25.1%) in 2018 and 11.2% (95% CI, 8.1%–17.4%) in 2030.
With observed treatment scale-up from 2018 onwards (scenario 2), the model projects a sharp decline in incident HCV infections and HCV viremia prevalence among PWH that slowly tapers off as the model approaches 2030. By 2021, the number of incident infections drops to 211 (95% CI, 109–421), the HCV incidence rate drops to 1.2/100py (95% CI, 0.6–2.4), and viremia prevalence among HCV-seropositive PWH drops to 8.6% (95% CI, 8.6%–8.6%). By 2030, the number of incident infections drops to 159 (95% CI, 78–331), the HCV incidence rate drops to 0.9/100py (95% CI, 0.4–1.9), and viremia prevalence drops to 6.3% (95% CI, 5.2%–7.3%). Between 2015 and 2030, this would achieve a reduction in HCV incidence among PWH of 63% (95% CI, 57%–67%).
If non-UCSD sites scale-up to the maximum levels achieved at UCSD from 2021 onwards (scenario 3), the model predicts 132 (95% CI, 59–309) incident HCV infections among PWH in 2030, with an HCV incidence rate of 0.7/100py (95% CI, 0.3–1.8), just falling short of the HCV elimination goal (achieving a decrease in HCV incidence among PWH of 69%; 95% CI, 60%–75%). If in addition to treatment scale-up to the maximum level there is a reduction in the behavioral risk starting in 2021 (Scenario 4), the model predicts 28 (95% CI, 13–68) incident infections among PWH in 2030, with an HCV incidence rate of 0.002/100py (95% CI, 0–0.004), meeting the HCV elimination goal (achieving a decrease in HCV incidence among PWH of 94% (95% CI, 91%–95%).
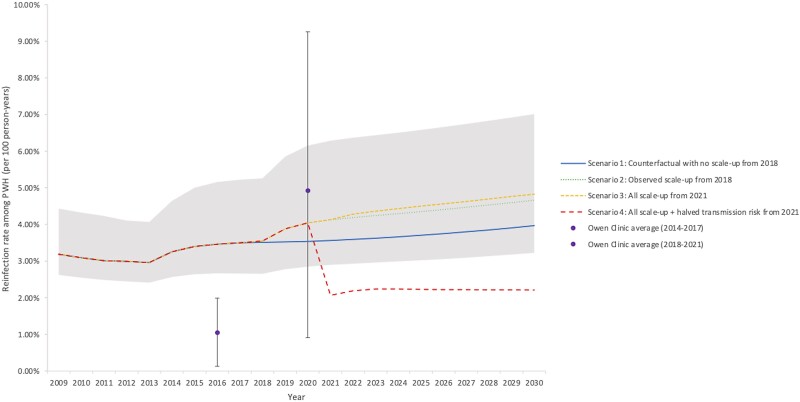
Reductions in incidence can be tracked through corresponding reductions in HCV viremia prevalence among HCV-seropositive PWH (Figure 3) to monitor elimination progress, with scenarios 2 and 3 projecting a 4% to 7% viremia prevalence among PWH by 2030. The HCV reinfection rate among previously treated PWH is projected to remain steady at around 4%, with treatment scale-up scenarios 2 and 3 projecting a slight increase from 3.4% in 2015 to above 4.5% by 2030. Only the scenario with behavioral risk reductions results in reduced reinfection incidence in 2030. Results on HCV reinfection rate among previously treated PWH and HCV-infected PWH under treatment in San Diego County are presented in Figure 4 and Supplementary Figure 1, respectively.
Figure 4.
HCV reinfection rate among previously treated PWH in San Diego County. Mean model projections (lines) and observed data from UCSD Owen Clinic (dots and whiskers), to which the model was not calibrated. Shading denotes the 95% uncertainty interval around the observed scenario (Scenario 2). Scenarios shown are (1) counterfactual continuation of pre-2018 treatment rates (29%/year, blue solid line); (2) observed scale-up from 2018 (to 54% at UCSD and 61%/year non-UCSD, green round dotted line); (3) county-wide scale-up to peak treatment achieved by UCSD (71%/year) from 2021 (71%/year, yellow square dotted line); (4) as in Scenario 3 plus halved transmission risk behavior from 2021 onwards (red dashed line). Abbreviations: PWH, people with HIV; UCSD, University of California San Diego.
Sensitivity and Uncertainty Analysis
There is uncertainty in treatment rates among HCV-infected persons without HIV, which we explored through sensitivity analysis (Supplementary Figures 2, 3, and 4). Assuming an increase in background treatment among those without HIV, the model projects greater reductions in HCV incidence among PWH (Supplementary Figure 5, which looks at sensitivity analyses on relative reduction in HCV incidence among PWH from 2015 to 2030 [%]). However, our models still predict that status quo treatment rates will not be able to achieve elimination with this level of scale-up, unless there was a 2-fold relative increase in treatment rates among non-PWH populations compared with PWH populations (mean 82% reduction). In contrast, if treatment rates for PWH were scaled up among non-UCSD providers and treatment rates for people infected with HCV mono-infection also increased (at the same relative rate or above), the HCV elimination targets could be met, and we may even be able to achieve a 90% reduction in incidence (Supplementary Figure 5).
In additional scenario analyses assuming a 20% reduction in HCV treatment rates during the COVID-19 pandemic (from 2020 to 2021 or 2020 to 2025), the model projects more HCV incident cases during the reduced treatment rate period (Supplementary Figures 2, 3, and 4). However, the relative reduction in HCV incidence among PWH from 2015 to 2030 is similar to the baseline analysis (Supplementary Figure 5).
The model findings were additionally insensitive to analyses assuming that PWID have reduced SVR rates of a relative 10% lower than non-PWID (Supplementary Figures 2, 3, 4, and 5).
The outcomes from the partial rank correlation coefficient uncertainty analysis found that the predicted relative reductions in HCV incidence among PWH from 2015 to 2030 are most sensitive to the relative HCV susceptibility for PWH compared with individuals without HIV, with greater susceptibility among PWH resulting in lower incidence reductions (Supplementary Figure 6).
DISCUSSION
Our analyses used epidemic modeling with available surveillance data from an HCV micro-elimination initiative among PWH in San Diego County to determine whether we are on track to achieve 2030 HCV elimination targets. Our analysis indicates that despite a substantial increase in HCV treatment provided to PWH at the UCSD Owen Clinic, current levels of treatment are unlikely to achieve the HCV incidence elimination target of 80% reduction between 2015 and 2030 among PWH in San Diego County. We found that achieving HCV micro-elimination among PWH will likely require both a scale-up of HCV treatment among PWH and reductions in transmission risk. In response to this observation, the Owen Clinic expanded services in 2022 to treat HCV in people who use drugs without HIV in conjunction with integrated substance use treatment and HIV prevention services. The expansion of evidence-based harm reduction interventions such as medications for opioid use disorder and syringe service programs (which together have been shown to reduce risk by 75% among PWID) [24] and the development of interventions to reduce HCV risk among MSM are both needed. Yet, there is insufficient evidence on effective interventions to mitigate the risk of sexual HCV transmission among MSM. Our clinic protocol includes periodic STI testing during and after DAA treatment as a surrogate marker of ongoing condomless behaviors. This strategy provides us with opportunities for reinforcement counseling on sexual behaviors associated with HCV infection (such as condomless anal sex, fisting, and multiple partners) [25–27] to prevent HCV reacquisition and forward transmission [28]. Additionally, our findings highlight that reinfection may be an important consideration among PWH, with both modeling and observational studies showing a potential rise in reinfection rates with expanded treatment [28].
Strengths and Limitations
The Lancet Gastroenterology & Hepatology Commission on Accelerating the Elimination of Viral Hepatitis point out that HCV elimination data are limited [29]. Traditional surveillance data on new diagnoses are hampered by underascertainment and late diagnoses, so longitudinal data from at-risk populations may provide better insights regarding changes in reinfection incidence and chronic prevalence and treatment scale-up impacts [29]. This is one of the strengths of our analysis because it uses recent data on HCV treatment and chronic prevalence from a large cohort of PWH at the UCSD Owen Clinic in San Diego.
However, the model does have several limitations. First, we lacked recent and robust data on HCV treatment rates among the population without HIV in San Diego. We explored the implications through sensitivity analyses, but this warrants further exploration. The COVID-19 pandemic led to reduced HCV testing and treatment nationally [30], and it is possible that HCV treatment rates declined both among PWH and overall in San Diego County, even though at the UCSD Owen Clinic treatment rates increased in 2021. Nevertheless, even in our simulations with more pronounced disruptions until 2025, our key results were unchanged, that scaled-up treatment is required to achieve HCV elimination. Although our modeling focuses on a single county, to our knowledge, there are few funded projects explicitly aimed to facilitate larger-scale data accrual tracking HCV elimination among PWH with a similar level of detail in the United States. Investing in better surveillance systems for PWH that track and routinely report data such as HCV diagnoses, treatment numbers, outcomes, and reinfections would facilitate more robust modeling, validation, and analytics to determine whether communities are on track for HCV elimination.
Second, our model only implicitly included the impact of harm reduction impact in our calibrated transmission rates. We believe this is justifiable because there has been no evidence of a recent scale-up of harm reduction efforts in San Diego County. However, lifting the ban on syringe service programs in 2021 provided an important opportunity for the scale-up of these important prevention measures. If scaled up, harm reduction could support HCV elimination efforts as explored in our behavior change scenario, prevent infection/reinfection among PWID, and have other benefits related to preventing HIV and other health harms among PWID [31–33].
Third, our model neglects immigration and emigration as we lack data on migration into and out of San Diego County by HIV and HCV status. However, we note that the San Diego–Tijuana border is one of the busiest land border crossings in the world. While import of HCV infections could hamper elimination efforts, HCV treatment efforts in San Diego could aid in prevention of transmission from individuals who emigrate to other areas.
Fourth, our model assumes a stable PWID population size due to a lack of data to suggest otherwise, although we note that the PWID population size estimate is old and uncertain. National data indicate an increase in PWID population size, mainly driven by increases in rural areas [34]. If the PWID population size is increasing in San Diego, it will likely further hamper elimination efforts.
Fifth, our work focuses on PWH and therefore does not consider what is needed for HCV elimination more broadly, nor does it assess the cost implications of elimination. Future work should examine the most cost-effective HCV elimination strategies and the budgetary impact of achieving HCV elimination.
CONCLUSIONS
San Diego County is progressing toward HCV micro-elimination among PWH, but increased HCV screening, treatment scale-up, and monitoring of HCV viremia among PWH are required to reach the 2030 targets. To ensure treatment rates can be maintained, efforts to identify remaining treatment barriers among PWH are necessary. Scale-up of harm reduction services, including medications for opioid use disorder, syringe exchange, and supervised consumption sites among PWID both with and without HIV, are needed to reduce the risk of infection/reinfection.
Supplementary Material
Acknowledgments
Financial support . This study was funded by the NoCo Grant funded by Gilead Sciences (IN-US-334-4481). The funders played no role in the design or interpretation of this study. N.M., E.C., and S.J. were additionally funded by the San Diego Center for AIDS Research (SD CFAR), an NIH-funded program (P30 AI036214), which the following NIH Institutes and Centers support: NIAID, NCI, NHLBI, NIA, NICHD, NIDA, NIDCR, NIDDK, NIGMS, NIMH, NIMHD, FIC, and OAR. Data from the STAHR study were obtained with support from the National Institute of Drug Abuse (R01 DA031074).
Patient consent. Our study does not include factors necessitating patient consent.
Contributor Information
Jaskaran S Cheema, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
William C Mathews, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Adriane Wynn, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Laura B Bamford, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Francesca J Torriani, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Lucas A Hill, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Amutha V Rajagopal, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Jeffrey Yin, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Sonia Jain, Herbert Wertheim School of Public Health and Human Longevity Science, University of California San Diego, San Diego, California, USA.
Richard S Garfein, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Edward R Cachay, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA.
Natasha K Martin, Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, California, USA; Population Health Sciences, University of Bristol, Bristol, United Kingdom.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . People coinfected with HIV and viral hepatitis. Updated September 21, 2020. Available at: https://www.cdc.gov/hepatitis/populations/hiv.htm. Accessed May 22, 2021.
- 2. Centers for Disease Control and Prevention . Hepatitis C questions and answers for health professionals. Updated August 7, 2020. Available at: https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed May 22, 2021.
- 3. Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29:2335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Consolidated strategic information guidelines for viral hepatitis: planning and tracking progress towards elimination. 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/310912/9789241515191-eng.pdf. Accessed January 5, 2021.
- 5. Cachay ER, Hill L, Ballard C, et al. Increasing hepatitis C treatment uptake among HIV-infected patients using an HIV primary care model. AIDS Res Ther 2013; 10:9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kusejko K, Salazar-Vizcaya L, Shah C, et al. Sustained effect on hepatitis C elimination among men who have sex with men in the Swiss HIV Cohort Study: a systematic re-screening for hepatitis C RNA two years following a nation-wide elimination program. Clin Infect Dis 2022; 75:1723–31. [DOI] [PubMed] [Google Scholar]
- 7. Chen G-J, Ho S-Y, Su L-H, et al. Hepatitis C microelimination among people living with HIV in Taiwan. Emerg Microbes Infect 2022; 11:1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynn A, Tweeten S, McDonald E, et al. The estimated hepatitis C seroprevalence and key population sizes in San Diego in 2018. PLoS One 2021; 16:e0251635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suckow S, Ramers C. Eliminate Hepatitis C San Diego county initiative. Live Well San Diego. 2019. Available at: https://www.sandiegocounty.gov/content/dam/sdc/hhsa/programs/bhs/documents/NOC/bhab/Eliminate%20Hepatitis%2°C%20San%20Diego%20County%20Inititiative%20PPT_BHAB. Accessed May 22, 2021.
- 10. Chaillon A, Sun X, Cachay ER, et al. Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. San Diego Office of HIV and AIDS Statistics . Reports and statistics. 2021. Available at: https://www.sandiegocounty.gov/content/sdc/hhsa/programs/phs/hiv_aids_epidemiology_unit/reports_and_statistics.html. Accessed October 15, 2021.
- 12. Horyniak D, Wagner KD, Armenta RF, Cuevas-Mota J, Hendrickson E, Garfein RS. Cross-border injection drug use and HIV and hepatitis C virus seropositivity among people who inject drugs in San Diego, California. Int J Drug Policy 2017; 47:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. San Diego County . HIV/AIDS epidemiology report—2016. 2017. Available at: https://www.sandiegocounty.gov/content/dam/sdc/hhsa/programs/phs/documents/EpiReport2017final.pdf. Accessed October 15, 2021.
- 14. Centers for Disease Control and Prevention . NCHHSTP AtlasPlus. Updated October 1, 2021. Available at: https://gis.cdc.gov/grasp/nchhstpatlas/charts.html. Accessed January 5, 2021.
- 15. Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med 2015; 13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoenigl M, Green N, Mehta SR, Little SJ. Risk factors for acute and early HIV infection among men who have sex with men (MSM) in San Diego, 2008 to 2014: a cohort study. Medicine (Baltimore) 2015; 94:e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vanhommerig JW, Bezemer D, Molenkamp R, et al. Limited overlap between phylogenetic HIV and hepatitis C virus clusters illustrates the dynamic sexual network structure of Dutch HIV-infected MSM. Aids 2017; 31:2147–58. [DOI] [PubMed] [Google Scholar]
- 18. Cachay E, Torriani FJ, Hill L, et al. Progress and real-life challenges for HCV elimination in people living with HIV. Paper presented at: Conferences on Retrovirus and Opportunistic Infections; March 8–11, 2020; Boston, USA. [Google Scholar]
- 19. Tempalski B, Pouget ER, Cleland CM, et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS One 2013; 8:e64789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cachay ER, Hill L, Torriani F, et al. Predictors of missed hepatitis C intake appointments and failure to establish hepatitis C care among patients living with HIV. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas DL. State of the hepatitis C virus care cascade. Clin Liver Dis (Hoboken) 2020; 16:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Facente SN, Grebe E, Burk K, et al. Estimated hepatitis C prevalence and key population sizes in San Francisco: a foundation for elimination. PLoS One 2018; 13:e0195575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graf C, Mücke MM, Dultz G, et al. Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis 2020; 70:2355–65. [DOI] [PubMed] [Google Scholar]
- 24. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newsum AM, Matser A, Schinkel J, et al. Incidence of HCV reinfection among HIV-positive MSM and its association with sexual risk behavior: a longitudinal analysis. Clin Infect Dis 2021; 73:460–7. [DOI] [PubMed] [Google Scholar]
- 26. Vanhommerig JW, Lambers FA, Schinkel J, et al. Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-infected men who have sex with men: a case-control study. Open Forum Infect Dis 2015; 2:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volk JE, Marcus JL, Phengrasamy T, Hare CB. Incident hepatitis C virus infections among users of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 2015; 60:1728–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill LA, Martin NK, Torriani FJ, et al. Screening for sexually transmitted infections during hepatitis C treatment to predict reinfection among people with HIV. Open Forum Infect Dis 2020; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology commission. Lancet Gastroenterol Hepatol 2019; 4:135–84. [DOI] [PubMed] [Google Scholar]
- 30. Hoenigl M, Abramovitz D, Flores Ortega RE, Martin NK, Reau N. Sustained impact of the COVID-2019 pandemic on HCV treatment initiations in the United States. Clin Infect Dis 2022;75:e955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol 2014; 43:235–48. [DOI] [PubMed] [Google Scholar]
- 33. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradley H, Hall EW, Asher A, et al. Estimated number of people who inject drugs in the United States. Clin Infect Dis 2023; 76:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borroni G, Andreoletti M, Casiraghi MA, et al. Effectiveness of pegylated interferon/ribavirin combination in ‘real world’ patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther 2008; 27:790–7. [DOI] [PubMed] [Google Scholar]
- 36. Scotto R, Buonomo AR, Moriello NS, et al. Real-world efficacy and safety of pangenotypic direct-acting antivirals against hepatitis C virus infection. Rev Recent Clin Trials 2019; 14:173–82. [DOI] [PubMed] [Google Scholar]
- 37. Hézode C. Treatment of hepatitis C: results in real life. Liver Int 2018; 38(Suppl 1):21–7. [DOI] [PubMed] [Google Scholar]
- 38. Davies A, Singh KP, Shubber Z, et al. Treatment outcomes of treatment-naïve hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PLoS One 2013; 8:e55373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006; 13:34–41. [DOI] [PubMed] [Google Scholar]
- 40. Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014; 59:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women's Interagency HIV Study (WIHS). AIDS Patient Care STDS 2009; 23:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin NK, Vickerman P, Miners A, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 2012; 55:49–57. [DOI] [PubMed] [Google Scholar]
- 43. Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007; 11:1–205, iii. [DOI] [PubMed] [Google Scholar]
- 44. Walker JG, Kuchuloria T, Sergeenko D, et al. Interim effect evaluation of the hepatitis C elimination programme in Georgia: a modelling study. Lancet Glob Health 2020; 8:e244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 46. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158(5 Pt 1):329–37. [DOI] [PubMed] [Google Scholar]
- 47. Arias E, Xu J. United States Life Tables, 2018. Vol. 69. National Vital Statistics Reports;2020. [PubMed]
- 48. San Diego County . HIV disease among adult/adolescent males, 2017. 2017. Available at: https://www.sandiegocounty.gov/content/dam/sdc/hhsa/programs/phs/documents/Adult_Adol_Male_Through_12312017_final.pdf. Accessed October 15, 2021.
- 49. San Diego County . HIV disease among females in San Diego County, 2017. 2017. Available at: https://www.sandiegocounty.gov/content/dam/sdc/hhsa/programs/phs/documents/Females_Through_12312017v04-11-19.pdf. Accessed October 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.