Abstract
Background and Objective
Traumatic injuries are amongst the leading causes of death and disability in the world across all age groups. This systematic review aimed to (1) describe the role of post‐traumatic stress symptoms (PTSS) on the development of chronic pain and/or pain‐related disability following musculoskeletal trauma and (2) report pain and or pain‐related disability by injury severity/type.
Database and Data Treatment
Electronic databases were searched, from inception to 31 November 2021 and updated on 10 May 2022, to identify studies in which: participants were adults aged ≥16 years sustaining any traumatic event that resulted in one or more musculoskeletal injuries; an outcome measure of PTSS was used within 3 months of a traumatic event; the presence of pain and/or pain‐related disability was recorded at a follow‐up of 3 months or more. Two reviewers independently screened papers and assessed the quality of included studies.
Results
Eight studies were included. Owing to between‐study heterogeneity, the results were synthesized using a narrative approach. Five studies investigated the relationship between PTSS and pain. Participants with PTSS were more likely to develop persistent pain for at least 12 months post‐injury. Six studies assessed the relationship between PTSS and pain‐related disability. The results suggest that patients with PTSS had significantly higher disability levels for at least 12 months post‐injury.
Conclusion
Findings from this comprehensive systematic review support a clear relationship between PTSS post‐injury and future pain/disability, with the potential importance of certain PTSS clusters (hyper‐arousal and numbing).
Significance
The findings of this systematic review indicate an association between PTSS reported within 3 months of a traumatic musculoskeletal injury and the development of longer‐term pain and disability. The PTSS clusters of ‘hyper‐arousal’ and ‘numbing’ appear to be of particular importance in this relationship.
Prospero Registration Number
CRD42021285243.
1. INTRODUCTION
Traumatic injuries are amongst the leading causes of death and disability across all age groups, accounting for 1 in 10 deaths worldwide (Krug et al., 2000; Norton & Kobusingye, 2013). In 2013, an estimated 973 million individuals sustained injuries that required some type of healthcare, of which 4.8 million died from their injuries (Haagsma et al., 2016). Approximately 1 in 10 of these deaths are caused by road trauma (Rosenbloom et al., 2013). Each year between 40,000 and 90,000 people in the UK sustain a traumatic injury; of these, 50% will have sustained a musculoskeletal injury (Herron et al., 2017; National Audit Office, 2010). These include injuries to bones, joints, ligaments, tendons and muscles that surround these structures (Clay et al., 2010). Some of the most common causes of traumatic musculoskeletal injuries include falls, road trauma, assaults and machinery‐related accidents (Moran et al., 2020). In the United States (US) approximately 2.8 million people were injured and 42,000 fatalities were recorded in 2004 (National Highway Traffic Safety Administration, 2005). Globally, traumatic injuries accounted for over 1.3 million fatalities and were the tenth leading cause of death in 2016 (World Health Organization, 2018).
Exposure to physical traumatic events can lead to the development of post‐traumatic stress symptoms (PTSS), such as intrusion, hyper‐arousal and avoidance (Regier et al., 2013). If these symptoms are significant within 1 month of a traumatic event, acute stress disorder (ASD) can be diagnosed (Bryant et al., 2011). If these symptoms are significant 1 month after the traumatic event, post‐traumatic stress disorder (PTSD) can be diagnosed (American Psychiatric Association, 2013). The two main diagnostic systems for PTSD are the 4th edition originating from the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) and the 10th edition of the World Health Organization (WHO) International Classification of Diseases (ICD‐10). Both define PTSD using three key symptom clusters: ‘re‐experiencing’, ‘avoidance’ and ‘hyper‐arousal’ (American Psychiatric Association, 1994; World Health Organization, 1992). However, these diagnostic systems have been controversial (Brewin et al., 2009). Several studies criticize the overlap of psychiatric disorders, such as anxiety and depression, with PTSD symptoms (Brewin et al., 2009; Maercker et al., 2013). As a result, the latest DSM edition (DSM‐V, American Psychiatric Association, 2013) contains an additional symptom cluster, ‘negative alterations in cognitions and mood’ (NACM), including, for example, guilt. In contrast, the revised ICD‐11 proposes two ‘sibling’ disorders: PTSD and ‘complex PTSD’ (Maercker et al., 2013). Symptoms are clustered into three groups namely: re‐experiencing’, ‘avoidance’ and ‘sense of current threat’. Furthermore, O'Donnell and colleagues (2014) used the modified version of the clinician‐administered structured interview for PTSD, which incorporated the new symptom cluster to estimate the prevalence of PTSD in a sample of hospital patients 72 h post‐discharge. They found that fewer patients met the ICD‐11 criteria (3.3%) compared with DSM‐V criteria (6.7%). Studies included in this review used current and previous PTSS diagnostic criteria developed to correspond to both the DSM‐IV/V or ICD‐10/11 symptom clusters.
PTSS often co‐occurs with pain and/or pain‐related disability (Bryant & Harvey, 1995; Dai et al., 2018; Guimmarra et al., 2017; Heron‐Delaney et al., 2013; Jenewein et al., 2009; Liedl et al., 2010). Several conceptual frameworks have been developed to explain the relationship between pain and/or disability and PTSS, and their influence on one other. A proposed mutual maintenance model suggests that attentional bias may be present in patients with both chronic pain and PTSS, as they both attend to threatening internal or external stimuli, anxiety and catastrophising when reminded of the traumatic event (Sharp & Harvey, 2001). The shared vulnerability model proposed by Asmundson et al. (2002) postulates that individuals with tendencies toward high anxiety sensitivity (tendency to respond with fear to anxiety or somatization) are at greater risk of both pain and PTSS. Furthermore, the diathesis‐stress model of disability addresses complex interactions between pre‐ and post‐traumatic cognitive risk factors and exposure to a traumatic event (Turk, 2002). Factors such as anxiety sensitivity, catastrophising, the anticipation of pain, self‐efficacy and fear avoidance beliefs are amongst the factors included in this model (Turk, 2002). The model was further expanded to include PTSS as a predictor of pain/disability and found that pain intensity accounted for 15% of the variance in PTSS. Furthermore, PTSS had an even greater association with pain‐related disability, accounting for 45% of the variance (Martin et al., 2010). These models validate the hypothesis that PTSS can have a negative effect on pain and disability, and vice versa, following a traumatic event. Several studies also suggest that PTSS plays a role in the development of chronic pain and disability following traumatic injuries (Pedler & Sterling, 2013; Ravn, Sterling, et al., 2018; Ravn, Vaegter, et al., 2018; Ruiz‐Párraga & López‐Martínez, 2014). Guimmarra et al. (2017) investigated the relationship between PTSS and pain in participants admitted to the hospital with orthopaedic and/or major trauma injuries. They found that hypersensitivity, fear of movement, catastrophising, low pain self‐efficacy and depression mediated the relationship between PTSS and pain 12 months following the traumatic event. By contrast, a recent reviewexamining the relationship between PTSS and changes in pain severity over time (Ravn, Hartvigsen, et al., 2018) found inconsistent evidence in support of a unidirectional or bidirectional association between pain and PTSS post‐trauma, which did not support the mutual maintenance model. More information is, therefore, needed regarding the influence of PTSS on the development of chronic post‐trauma pain and disability.
Two previous Scandinavian studies (Akerblom et al., 2017; Andersen et al., 2012) examined the prevalence of PTSS amongst patients with chronic pain consecutively referred for rehabilitation. In the first study, 23% of patients assessed for pain reported PTSS fulfilling the DSM‐IV criteria, whereas 29% of patients admitted for pain assessment reported PTSS at a level qualifying for a PTSD diagnosis in the second study. Furthermore, two systematic reviews reported mean PTSD prevalence rates between 11.7% and 19.1% amongst patients with chronic pain (Fishbain et al., 2016; Siqveland et al., 2017). Similarly, a Canadian study of people with chronic pain found PTSD rates of 7.7% and 46% amongst individuals with fibromyalgia and back pain respectively (Sareen et al., 2007). By contrast, variations in chronic pain prevalence rates (range 30% to 66%) have been reported in individuals with PTSD but research has mainly been carried out with war veterans (Beckham et al., 1997; Shipherd et al., 2007).
Despite this evidence, few studies have prospectively examined the role of PTSS in the development of chronic pain and pain‐related disability. Because of differences in sample size/study population, methods and widely differing PTSS criteria used to ascertain a diagnosis, estimates of pain and disability in individuals with PTSS vary considerably between studies. This warrants a thorough empirical examination of the role of PTSS and its impact on the development of pain and disability. A better understanding of this relationship should ultimately enable more targeted treatment.
1.1. Aims
This systematic review aims to:
Examine the longitudinal relationship between PTSS, measured within 3 months of a traumatic musculoskeletal injury, and persistent pain and/or pain‐related disability in adults aged ≥16 years.
Describe the variations in reported pain and/or pain‐related disability, following PTSS, characterized by severity or type of musculoskeletal trauma.
2. METHODS
This systematic review is reported in compliance with the Preferred Reporting Items for Systematic Review and Meta‐analysis (PRISMA) statement guidelines (Page et al., 2021; Methods S1). The review protocol is registered in PROSPERO (International Prospective Register of Systematic Reviews; registration number: CRD42021285243) and published (Jadhakhan et al., 2021). Assuming homogeneity between studies, we planned to conduct a random effect meta‐analysis with and without low‐quality studies.
2.1. Inclusion and exclusion criteria
The Population Intervention Comparator Outcome and Study type (PICOS) framework was utilized to define the inclusion and exclusion criteria of this review, as recommended by the Cochrane Handbook for systematic reviews (Higgins & Green, 2011). Specific interventions or comparators were not explored: only the population and outcome were identified in the inclusion and exclusion criteria, along with the type of study design.
2.1.1. Population
This review included studies where cases were adults (aged 16 or over) who sustained a traumatic event that was reported to result in at least one musculoskeletal injury. Musculoskeletal injuries were defined as damage to any bones, joints, ligaments (including intervertebral discs), tendons, muscles and the skin that surrounds these structures (Clay et al., 2010); a widely used definition in previous studies and reviews (Clay et al., 2010; Middlebrook et al., 2019). Common traumatic events included, but were not limited to, road trauma (including whiplash injuries), blunt‐force trauma, falls, sports injuries, occupational or work‐related injuries, stab wounds, gunshot wounds and violence. A broad range of musculoskeletal injuries was therefore included in this review. Studies with a heterogeneous population, where 90% of the sample (aged ≥ 16 years) sustained a musculoskeletal injury (Clay et al., 2010) were also included. Studies focusing solely on patients with traumatic brain injury (TBI) or studies that included burns or neurological injuries such as spinal cord injuries, and deliberate self‐injurious patient populations were excluded. In studies where a proportion of the population was less than 16 years old, the reported mean or median age (in years) of the sample population aged ≥16 years and a description of the distribution (SD or IQR range) were provided. In cases of insufficient data, authors were contacted by email to request this.
2.1.2. Patient‐reported outcome measures
This review included longitudinal studies in which a reported measure of PTSS was recorded at baseline (within 3 months of a traumatic event resulting in musculoskeletal injuries) and a recognized measure of the presence of pain and/or pain‐related disability was recorded at least 3 months following the same musculoskeletal traumatic event (Krismer & Van Tulder, 2007). We did not attempt to limit study eligibility to those attributing pain to the same event as PTSS. The presence of PTSS, pain and/or pain‐related disability was reported by either a diagnostic instrument or a validated questionnaire (Methods S2). Extracted outcome data were categorized by the time since injury.
2.1.3. Study design
Only prospective observational studies, secondary analysis of longitudinal data and record linkage studies (data linkage from longitudinal surveys) were eligible for inclusion. Included studies were published either in peer‐reviewed scientific journals, the Cochrane library or the grey literature. Only articles published in English were considered eligible.
2.2. Exclusion criteria
Studies investigating populations aged less than 16 years, single case studies, retrospective observational studies and randomized controlled trials were excluded. Review articles, letters, editorials, conference proceedings and studies with only abstracts (i.e. no available full text) were also excluded.
2.3. Search strategy
A comprehensive search strategy was developed to retrieve articles relevant to the aims of this review. The following citation databases were systematically searched from inception to 31 November 2021 and updated on 10 May 2022: MEDLINE (OVID), PsycINFO (OVID), EMBASE (OVID), CINAHL, Web of Science and PubMed. Ongoing studies, scientific literature and abstract proceedings were identified by searching the Cochrane Database of Systematic Reviews, PROSPERO, Google Scholar and ZETOC. Grey literature databases such as Grey Literature Report, OpenGrey, PubliCat and ScienceDaily.com were also examined. A search strategy was developed to retrieve relevant articles, using the following key terms and Medical Subject Heading (MeSH): post‐traumatic stress symptoms* (and all associated diagnoses and research terms), acute stress disorder* (ASD), pain* and disability*. The reference lists of any recent review articles and from any other eligible manuscript identified by the above search were hand‐searched. The Science Citation Index (SCI) was used to scan and track study titles. Search strategies for each electronic database are shown in Methods S3. In cases of insufficient data, we attempted to contact the corresponding authors of eligible studies up to three times by email. When authors did not respond to our emails, we tried to contact co‐authors by email at least twice. After 2 weeks if authors did not respond to our emails, we sent a final reminder email to the corresponding authors and co‐authors.
2.4. Preparing for eligibility screening
All records retrieved in the database search were imported into the literature management software EndNote V.X9 (Clarivate Analytics) to facilitate the management of references. Any duplicate articles were identified and removed at this stage.
2.5. Study selection
Two reviewers (F.J. and D.E.) independently reviewed the studies within the digital library identified by the search strategy in two phases. Retrieved titles and abstracts were initially reviewed to identify eligibility for full‐text screening. The full texts were then read to determine suitability for inclusion in the review. This was achieved by referring to an inclusion criteria checklist, based on study eligibility criteria and designed a priori (Table 1), to ensure studies were classified and interpreted appropriately. Any discrepancies or differences in opinion were resolved by consensus and by involving a third reviewer (D.F.) to arbitrate any disagreement. A PRISMA flow diagram (Figure 1) presents the included and excluded studies along with reasons for exclusions.
TABLE 1.
Review eligibility criteria checklist
| Study design | Prospective observational cohort study |
|---|---|
| Study characteristics |
Study identified via electronic database search, grey literature, research archive or reference lists of eligible studies Full text of available articles |
| Participants |
Experienced musculoskeletal trauma within 3 months of the baseline assessment >90% of participants are adults (aged ≥ 16 years) |
| Measures |
Post‐traumatic stress symptoms measured at baseline (no more than 3 months post‐trauma) Self‐reported pain and/or pain‐related disability measured at 3 months and or/longer following baseline |
FIGURE 1.
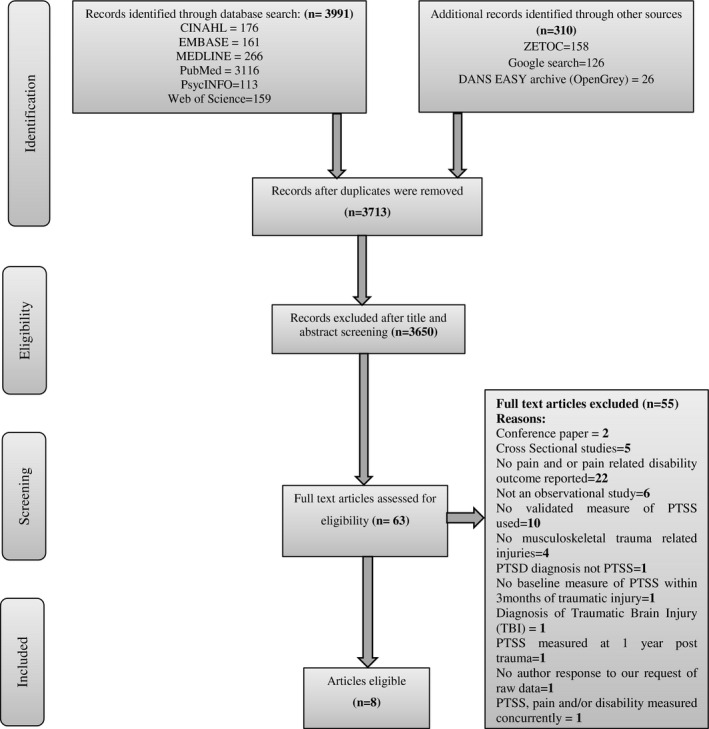
Study selection and reasons for exclusion.
2.6. Data extraction
Prior to data extraction, a standardized data extraction form was developed (Methods S4) based on the modified Checklist for critical Appraisal and data extraction for systematic Reviews of Prediction Modelling Studies (CHARMS‐PF checklist; Riley et al., 2019). This was developed iteratively with a focus on study location, study design, participant's characteristics, outcomes of interest (self‐reported pain and/or disability), predictor variables or symptom measurements, sample size, length of follow‐up, items associated with risk of bias, summary statistics and methods for statistical analysis, then pilot‐tested on known papers independently by two reviewers (F.J. and D.E.). Data from each study were extracted independently by two reviewers (F.J. and D.E.), and any differences were resolved by discussion. A third reviewer (D.F.) checked a random subset of the data extracted from the included studies to ensure that data had been extracted consistently without any deviation. Any discrepancies were resolved by discussion and re‐visiting the relevant study by the two reviewers (F.J. and D.E.). If any information was missing or incomplete, an initial attempt was made to contact the study authors and a follow‐up email was sent 2 weeks after to retrieve the missing data. Descriptive data extracted from included papers were summarized in a Microsoft Excel spreadsheet (Methods S5).
2.7. Quality assessment
Two reviewers (F.J. and D.E.) assessed the methodological quality of each included article independently to reduce bias. The quality assessment focussed on study participation, study attrition, outcome measurement and statistical analysis and reporting. All selected articles were assessed using the refined QUality In Prognosis Studies (QUIPS) appraisal tool (Hayden et al., 2013). Although the focus of this systematic review was not prognosis, we used the QUIPS appraisal tool because it covers the general quality criteria to assess the risk of bias adequately. We considered these general criteria as appropriate because of the wide variety of study designs included in this systematic review. QUIPS consists of six domains assessing potential biases in prognostic studies: study participation, study attrition, prognostic factor measurement, confounding factors, outcome measurement and statistical analysis and reporting (Hayden et al., 2013). Grading of each domain consisted of four options: high, moderate, low risk or unknown risk of bias. The stages and domains of this modified tool are shown in Methods S6. A third reviewer (D.F.) was available if needed. Any difference in opinion was resolved by further discussion and/or by involving the third reviewer. A narrative summary of the quality of each study is provided in Table 4.
TABLE 4.
Risk of bias assessment of the eight included studies according to the QUIPS tool
| Source | Study participation | Study attrition | Exposure measurement | Outcome measurement | Confounding | Statistical analyses and presentation | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Kongsted et al. (2008) | |||||||
| Maujean et al. (2017) | |||||||
| Pedler et al. (2016) | |||||||
| Ravn et al. (2019) | |||||||
| Ravn, Sterling, et al. (2018) | |||||||
| Ramchand et al. (2008) | |||||||
| Andersen et al. (2016) | |||||||
| Åsenlöf et al. (2013) |
Note: Green, yellow and red indicate low, moderate and high risk of bias respectively.
2.8. Synthesis of results
Estimates of pain and disability in relation to PTSS were considered separately by severity and or type of musculoskeletal trauma. Pain and disability were either reported as mean difference/standardized mean difference (SMD) with accompanying 95% CI and Odds Ratio (OR) or Risk Ratio (RR) with 95% CI between groups and subsequently extracted. The level of heterogeneity between study data was explored to determine whether the extracted data were sufficiently homogenous for a meta‐analysis. Two reviewers (F.J. and D.E.) independently assessed key items of the extracted data. A consensus was reached by discussion between the pair of reviewers. However, the studies differed considerably in terms of PTSS diagnostic measures, outcome measures (pain and or pain‐related disability measures), sample size, population, study setting and effect measures. The advice was sought from a senior member of the research team (D.F.) who supported the decision not to combine these studies in a meta‐analysis due to substantial heterogeneity between the studies. Pain and/or disability following musculoskeletal trauma in individuals (aged ≥ 16 years) with a recorded measure of PTSS were extracted for each study and a narrative summary of the outcome of the included studies was presented.
3. RESULTS
3.1. Identification of studies
The search was updated (10th May 2022) at the time of manuscript preparation to ensure the most recent and relevant studies were captured and recorded. In total, 3650 articles were screened by title and abstract. Title and abstract screening were performed independently by two reviewers (F.J. and D.E.) and resulted in the exclusion of 3587 articles. Of the 63 full‐text articles assessed, 55 were excluded at full‐text review, primarily because these articles did not report pain and/or pain‐related disability or were not primarily focussed on PTSS. Eight articles (Andersen et al., 2016; Åsenlöf et al., 2013; Kongsted et al., 2008; Maujean et al., 2017; Pedler et al., 2016; Ramchand et al., 2008; Ravn et al., 2019; Ravn, Sterling, et al., 2018) were included in the final analysis. A flow diagram of the study identification process is presented in Figure 1.
3.2. Study characteristics
The eight included studies involved 2108 individuals (aged ≥ 18 years sustaining any physically traumatic event that was reported to result in at least one musculoskeletal injury) from four countries were included in the final analysis (Figure 1). Considerable variation was found between studies regarding study design, including study setting, data source, measures used to define PTSS, pain and/or disability and summary effect measures employed to report the development of pain and/or pain‐related disability and injury type. Most eligible studies were conducted in Australia (n = 3; Maujean et al., 2017; Pedler et al., 2016; Ravn, Sterling, et al., 2018) and Denmark (n = 3; Kongsted et al., 2008; Ravn et al., 2019; Andersen et al., 2016), followed by Sweden (n = 1; Åsenlöf et al., 2013) and the United States (n = 1; Ramchand et al., 2008). Most studies recruited from emergency departments (n = 4; Maujean et al., 2017; Pedler et al., 2016; Ravn et al., 2019; Åsenlöf et al., 2013), followed by a combination of emergency department and general practice (n = 1; Kongsted et al., 2008), emergency department and primary care advertisement (n = 1; Ravn, Sterling, et al., 2018), trauma facility (n = 1; Ramchand et al., 2008) and using emergency department register data (n = 1) to define WAD grades and self‐reported questionnaires to measure PTSS and pain and pain‐related outcomes (Andersen et al., 2016). Participants were predominantly female (56.2%), with the ages of participants ranging from 18 to 70 years. Only one study reported ethnicity data (Ramchand et al., 2008). Five studies (Andersen et al., 2016; Åsenlöf et al., 2013; Maujean et al., 2017; Pedler et al., 2016; Ramchand et al., 2008) employed a purposive sampling strategy to recruit patients and three studies (Kongsted et al., 2008; Ravn et al., 2019; Ravn, Sterling, et al., 2018) recruited consecutive patients. The length of follow‐up ranged from 3 to 12 months. All studies had been published in English. An overview of study characteristics can be found in Table 2.
TABLE 2.
Study characteristics
| Study details | Demographics | Participants | Patient‐reported outcome | Pain | Disability | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Setting (e.g. outpatient, inpatient, emergency department) | Age | Gender | Ethnicity | Sample size | Length of follow‐up | Individuals aged ≥16 years sustaining any physically traumatic event that was reported to result in at least one musculoskeletal injury within 3 months of the baseline assessment | Recorded measure of PTSS at baseline (within 3 months of the traumatic event in which injuries were sustained) definition of PTSS | Outcome measure | Effect measure | Outcome measure | Effect measure |
| Kongsted et al. | 2007 | Denmark | Emergency unit and GP | 18–70 years | M = 35.9%; F = 64.1% | Not reported | 668 | 3, 6 and 12 months | Whiplash injury grade (I–III): Within 3 days after the accident or a maximum of 10 days after the accident | Baseline PTSS collected using the Impact of Event Scale (IES) | SF‐36 | Unadjusted OR = 3.3 [1.8, 5.9, p < 0.001]; adjusted OR for age = 3.0 [1.6, 5.5, p < 0.001], adjusted OR for age and baseline pain intensity = 2.1 [1.1, 4.1, p < 0.05] | 15‐item Copenhagen Neck Functional Disability Scale | Unadjusted OR = 3.2 [1.7, 6.0, p < 0.001]; adjusted OR for age = 3.1 [1.6, 5.8, p < 0.01], adjusted OR for age and baseline pain intensity = 2.1 [1.1, 4.2, p < 0.05] |
| Maujean et al. | 2017 | Australia | Emergency department | 18–65 years | F = 64.4% | Not reported | 146 | 6 months | Acute Whiplash: (grade I–III) Within 1 month of injury | Post‐traumatic stress scale (PDS) within 1 month of injury | Not reported | Not reported | Neck Disability Index (NDI) | PTSS cluster (hyperarousal/numbing) significantly predicted future neck pain–related disability (unstandardized coefficient = 1.15; SE = 0.57, p = 0.043) |
| Pedler et al. | 2016 | Australia | Emergency department | 18–65 years | F = 71 | Not reported | 103 | 3 months | Whiplash‐associated disorder grade (I–III); symptoms less than 6 weeks following injury | The Posttraumatic Stress Diagnostic Scale (PDS) measured 30 days following a motor vehicle accident | (10‐cm visual analogue scale [VAS]) | Pearson correlation r = 0.349; p < 0.01 between PTSS and pain | Neck Disability Index (NDI) | Pearson correlation r = 0.624; p < 0.01 between PTSS and disability |
| Ravn et al. | 2019 | Denmark | Emergency department | ≥18 years | F = 62.9% | Not reported | 229 | 3 and 6 months | Whiplash grade (I–III): within 4 weeks to 6 months post‐injury | Harvard Trauma Questionnaire part IV | Numeric Rating Scale (NRS) | Recovering PTSS (high initial PTSS, then gradually recover): PAIN OR: 1.86 (1.26, 2.76); p = 0.002; Chronic PTSS (persist over 6 months): PAIN OR: 1.86 (1.27, 2.73); p = 0.001 | Pain Disability Questionnaire (PDQ) | MEAN (SD) Chronic PTSS V/S recovering PTSS: CHRONIC: Mean 34.8 (SD:28.7); RECOVERING: Mean: 16.5 (SD:20.4); p = 0.0009; Chronic PTSS V/S no PTSS: Chronic: 34.8 (28.7); no PTSS: 7.1 (13.1), p = 0.003 |
| Ravn et al. | 2018 | Australia | Emergency department; primary care; advertisement in newspaper | 18–65 years | F = 66.4% | Not reported | 253 | 3, 6 and 12 months | Acute Whiplash injury grade (I–III) within 4 weeks of injury | Post‐traumatic stress scale (PDS) | Visual Analogue Scale (VAS) | PTSS predicted an increase in pain from baseline to 3 months; standardized coefficient = (β = 0.24, p < 0.001); 6 months to 12 months = (β = 0.21, p < 0.001) | Not reported | Not reported |
| Ramchand et al. | 2008 | USA | hospitalized for injuries resulting from community violence | Mean = 25 (±5.8) | M = 94% | Seventy‐eight per cent self‐identified as Hispanic, 13% self‐identified as Black; 3% identified as non‐Hispanic Caucasian; 3% identified as Asian; and 4% identified as Native American, multiracial, or other | 413 | 3 and 12 months | Fifty‐nine per cent had sustained gunshot injuries and the remainder was injured from other penetrating or blunt objects. Between October 1998 and June 2000, all consecutively hospitalized young adults who were admitted to a large Level I trauma facility in Los Angeles for treatment of wounds from community violence were screened for eligibility. To be eligible for the study, participants had to (a) have sustained an injury inflicted by a person other than a family member or a former sexual partner, (b) be between 18 and 40 years of age and (c) be able to communicate fluently in English or Spanish. Individuals were screened for eligibility by trained lay interviewers only at such time as potential participants were capable of giving informed consent | Civilian Version of the Posttraumatic Stress Disorder (PTSD) Checklist (PCL) | Not reported | Not reported | Physical health and functioning were derived from 15 items drawn from the physical domain of the RAND‐36 Health Status Inventory. Questions from the physical domain cover four areas: general health perception, physical functioning, physical pain and physical health‐related role limitations | Our longitudinal measurement model including only PTSS and physical functioning fit the data well (χ 2 (174) = 286.7, CFI = 0.98, SRMR = 0.04) |
| Andersen et al. | 2016 | Denmark |
ED register data to define WAD grades. Participants were invited by post and Self‐reported questionnaires were used to measure PTSS and pain and pain‐related outcomes |
mean age for the patients was (M = 36.79, SD = 12.61) | 61.6% were women | Not reported | 198 | 3 months, 6 months | Consecutive patients from a large Danish emergency ward were all contacted by post within 3 weeks after their whiplash injury | To measure the severity of PTSS, we used the Harvard Trauma Questionnaire part IV (HTQ‐IV) The HTQ‐IV consists of 17 items on a 4‐point Likert scale (1 = not at all, 4 = very often) | Pain intensity was measured as the average score of four 11‐point Likert scales (NRS) Each scale measured pain intensity on a numerical rating scale ranging from 0 (no pain) to 10 (the worst possible pain). | The total effect (c) of PTSS symptoms at baseline on 6‐month pain intensity was positive and statistically significant of moderate size (R 2 = 29%), with no mediators in the model | Not reported | Not reported |
| Asenlof et al. | 2013 | Sweden | Ninety‐eight participants were recruited from the emergency wards at two hospitals in Uppsala (University hospital) and Västerås (Regional county hospital) in Sweden between January 2007 and December 2009 | 18 to 65 years | F = 52 (53.1%), M = 46 (46.9%) | Not reported | 98 | 3, 6 and 12 months | Eligibility criteria were; age 18 to 65 years, fulfilled criteria for the diagnosis of WAD grade I and II established by a physician on the emergency ward within 72 h from the accident, satisfactory Swedish language skills and subjective report of not being in need of further treatment due to mild pain and disability 2–4 weeks after the accident | Post‐traumatic stress symptoms were measured with the Impact of Event Scale (IES) | Pain intensity was operationalized as the average pain intensity experienced over the past two weeks, which was scored on a numerical rating scale (NRS) with a score of 0 (no pain) and 10 (worst pain imaginable/unbearable pain) | Not reported | Pain‐related disability was measured with the Swedish version of The Pain Disability Index (PDI) | IES did not predict disability at 6 months in the multivariate linear regression model: B = −0.12 (−0.28, 0.04), p = 0.15 |
Abbreviations: CFI, comparative fit index; HTQ, Harvard Trauma Questionnaire; IES, impact of events scale; NDI, neck disability index; NRS, numeric rating scale; OR, odds ratio; PCL, the posttraumatic stress disorder checklist; PDI, peri‐traumatic distress inventory; PSD, post traumatic diagnostic scale; PTSD, post traumatic stress disorder; PTSS, post traumatic stress symptoms; RMDQ, Roland Morris Disability Questionnaire; SEM, standard error of the mean; SRMR, standardized root mean squared; VAS: visual analogue scale; WAD, whiplash‐associated disorders.
3.3. Description of musculoskeletal trauma
The most common musculoskeletal injuries were whiplash injuries (grades I–III) established by a physician between 72 h from the accident and within 6 months following the injury (Andersen et al., 2016; Åsenlöf et al., 2013; Kongsted et al., 2008; Maujean et al., 2017; Pedler et al., 2016; Ravn et al., 2019; Ravn, Sterling, et al., 2018). Ramchand et al. (2008) included patients who had sustained gunshot injuries or were injured from other penetrating or blunt objects between October 1998 and June 2000. Most studies (Andersen et al., 2016; Åsenlöf et al., 2013; Kongsted et al., 2008; Maujean et al., 2017; Pedler et al., 2016; Ramchand et al., 2008; Ravn, Sterling, et al., 2018) reported a traumatic event that resulted in at least one musculoskeletal injury between 72 h and 3 weeks. In one study (Ravn et al., 2019), the injury was reported between 4 weeks and 6 months following a traumatic event.
3.4. Diagnostic tool/measures used
The majority of studies (n = 3) used the Post Traumatic Diagnostic Scale (PDS) to assess PTSS, followed by the Impact of Event Scale (IES; n = 2) and Harvard Trauma Questionnaire (HTQ‐part IV; n = 2) and one study used the Posttraumatic Stress Disorder Checklist (PCL). A diversity of methods was used to ascertain the presence of pain and/or disability. Two studies used a visual analogue scale (VAS) to determine pain intensity experienced by individuals following musculoskeletal trauma whereas three studies used a numeric rating scale (NRS). Two studies used the Neck Disability Index (NDI) to assess the self‐rated disability of patients with neck pain. The Swedish version of the Pain Disability Index (PDI) was used in one study to investigate the magnitude of self‐reported pain‐related disability. In one study, the Short Form‐36 Health Survey (SF‐36) standardized quality‐of‐life assessment tool was used and in another study, the Copenhagen Neck Functional Disability Scale (CNFDS) was used to measure the level of functional disabilities in patients with neck pain following a whiplash injury. The Pain Disability Questionnaire (PDQ) was used in one study to assess the perception of disability in relation to pain following whiplash injury and another, the RAND‐36 item health status inventory questionnaire was used to assess the physical functioning of patients following multiple traumatic injuries. Table 3 provides an overview of the diagnostic tools used.
TABLE 3.
Diagnostic/measures used to ascertain PTSS, pain and/or disability
| Study/year | Diagnostic tool/measures | ||
|---|---|---|---|
| PTSS | Pain | Disability | |
| Maujean et al. (2017) | PDS | NDI | |
| Pedler et al. (2016) | PDS | 10 cm VAS | NDI |
| Ravn, Sterling, et al. (2018) | PDS | 10 cm VAS | |
| Kongsted et al. (2008) | IES | SF‐36 | CNFDS |
| Åsenlöf et al. (2013) | IES | 0–10 NRS | PDI |
| Ramchand et al. (2008) | PCL | RAND‐36 | |
| Ravn et al. (2019) | HTQ (part IV) | 0–10 NRS | PDQ |
| Andersen et al. (2016) | HTQ (part IV) | 0–10 NRS | |
3.5. Follow‐up assessment points
Most studies included in this review had follow‐up outcome assessments between 3, 6 and 12 months intervals. The remaining studies measured outcomes at 3 and 6 months post‐injury and another assessed outcomes at 3‐ and 12‐month intervals. The follow‐up assessment point for each study is provided in Table 2.
3.6. Risk of bias
None of the included studies had an overall low risk of bias in every domain covered by the refined QUIPS appraisal tool (Hayden et al., 2013). Six of the eight (75%) studies adequately described the study population (low risk of bias), and clearly defined the selection of participants, with an adequate description of the target population and inclusion/exclusion criteria. Population and outcome were universally well described; all of the eight studies included in this review presented valid measures for ascertaining the presence of PTSS and the development of pain and/or disability. In seven studies (88%), the analytical approach utilized was considered appropriate. Three (38%) of the studies reported adequate information about attrition. However, five (62%) studies did not report attrition clearly enough or no reasons for participants withdrawing were given (moderate/high risk of bias). Seven studies (88%) described adjustment of confounders in the analysis. Table 4 displays the review quality scores per item based on the refined QUIPS appraisal tool (Hayden et al., 2013). Across all eight studies, four (50%) had a moderate risk of bias and four (50%) had a low risk of bias (Figure 2).
FIGURE 2.
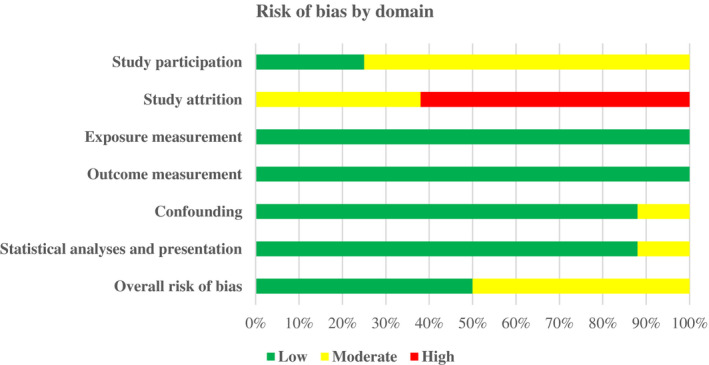
Overall risk of bias.
3.7. Relationship between PTSS and pain post‐trauma
Five of the eight (63%) studies (Andersen et al., 2016; Kongsted et al., 2008; Pedler et al., 2016; Ravn et al., 2019; Ravn, Sterling, et al., 2018) investigated the relationship between pain and PTSS (Table 2). Overall, the results were highly heterogeneous. All studies reported follow‐ups and the development of persistent pain but the follow‐up times varied considerably from 3, 6 and 12 months after injury. Furthermore, the definition of pain and the measure of association between PTSS and pain varied significantly between studies, rendering direct comparability between studies difficult.
3.8. Pain by injury type
3.8.1. Whiplash injury
One study (Kongsted et al., 2008) reported odds ratios to summarize the difference between those with PTSS compared to those without PTSS and the development of pain in people with a whiplash injury (grades I–III). Another study (Ravn et al., 2019) examined predictors and functional outcomes associated with PTSS trajectories following a whiplash injury. People with PTSS were approximately twice as likely (OR: 1.86, 95% CI [1.27–2.73], p < 0.001) to develop pain after a whiplash injury (Ravn et al., 2019). Furthermore, people classed as having ‘chronic severe’ PTSS had a significantly higher level of pain‐related disability at 6 months compared to those classed as having ‘resilient’ PTSS (p = 0.003) and ‘recovering’ (p = 0.009).
Kongsted et al. (2008) found that individuals with post‐traumatic stress reactions were more than three times (unadjusted OR: 3.3, 95% CI [1.8–5.9], p < 0.001) likely to develop long‐term pain after a whiplash injury. After adjusting for age and baseline pain intensity, this association was slightly attenuated (adjusted OR: 2.1: 95% CI [1.1–4.1], p < 0.05). Another study (Ravn, Sterling, et al., 2018), examined the longitudinal association between PTSS and pain amongst a cohort of people who sustained whiplash injury (grades I–III) and found that PTSS at baseline predicted an increase in pain at 3 months (standardized coefficient [β] = 0.24, p < 0.001) and that PTSS at 6 months predicted an increase in pain at 12 months (standardized coefficient [β] = 0.21, p < 0.001). Pedler et al. (2016) explored the relationship between measures of PTSS and pain/disability outcomes in people with a whiplash injury (grades I–III) and found that PTSS was significantly correlated with pain (Pearson's correlation coefficient r = 0.349, p < 0.01) at their 3‐month follow‐up. Andersen et al. (2016) investigated the longitudinal effect of PTSS on pain in a whiplash injury cohort (grades I–III) and found that PTSS was associated with pain at 6 months with moderate size (r 2 = 29%, p < 0.001).
3.9. Relationship between PTSS and disability post‐trauma
Six of the eight (75%) studies (Åsenlöf et al., 2013; Kongsted et al., 2008; Maujean et al., 2017; Pedler et al., 2016; Ramchand et al., 2008; Ravn et al., 2019) assessed the relationship between PTSS and disability (Table 2). The assessment methods and follow‐up period of PTSS and the development of disability varied considerably across studies. Furthermore, the definition of injury type varied with some studies defining specific injuries (e.g., whiplash injury grades I–III) and those including multiple injury types (e.g., gunshot injuries, penetrating/blunt objects).
3.10. Disability by injury type
3.10.1. Whiplash injury
One study (Kongsted et al., 2008) reported (OR) to describe the likely association of PTSS and disability in people with a whiplash injury (grades I–III). People with PTSS were more than three times (OR: 3.2, 95% CI [1.70–6.0], p < 0.001) likely to develop disability 3 months following a whiplash injury (Kongsted et al., 2008). After controlling for age and pain intensity, PTSS was still significantly associated with disability (OR: 2.1, 95% CI [1.1, 4.2], p < 0.05). Another study (Maujean et al., 2017) assessed which PTSS symptomology best predicted long‐term neck pain‐related disability in a whiplash‐injured population (grade I–III); the finding showed that hyper‐arousal and numbing significantly predicted neck pain‐related disability (un‐standardized coefficient = 1.15, SE = 0.57; p = 0.043). In another study, Pedler et al. (2016) found that PTSS influenced neck pain‐related disability (Pearson's correlation coefficient r = 0.624, p < 0.01) 12 weeks post whiplash injury (grades I–III). Another study (Ravn et al., 2019) evaluating pain‐related disability in patients with PTSS following whiplash injury (grades I–III), found three different trajectories of recovery: chronic (PTSS), resilient (no PTSS) and recovered (PTSS at baseline but recovered during follow‐up). Patients with chronic PTSS had significantly higher disability levels [mean [SD] = 34.8 (±28.8)] compared to patients who were resilient [mean [SD] = 7.1 (±13.1), p = 0.003]. On the other hand, a study by Åsenlöf et al. (2013), examining the relationship between PTSS and pain‐related disability in a cohort of patients with whiplash injury (grade II–III), reported a small and non‐significant regression coefficient (β = −0.12, 95%CI [−0.34 to 0.147], p = 0.15).
3.10.2. Multiple injuries
Only one (Ramchand et al., 2008) of the eight studies examined the relationship between PTSS and disability in individuals who sustained multiple injuries. Ramchand et al. (2008) examined the relationship between PTSS and physical functioning in people who sustained gunshot injuries and/or were injured from other penetrating or blunt objects in a longitudinal measurement model and found that PTSS and physical functioning fitted the data well Chi‐Square (χ 2) (174) = 286.7, Comparative Fit Index [CFI] = 0.98 (threshold for acceptable model fit = ≥90) and Square Root Mean Residual [SRMR] = 0.04 (threshold for acceptable model fit = ≤0.08). Results indicate that individuals with PTSS at 1‐week post‐trauma have worse physical functioning at 3 months follow‐up.
4. DISCUSSION
The purpose of this review was to systematically investigate the existing literature to determine the role of PTSS on the development of chronic pain and/or pain‐related disability following musculoskeletal trauma. Few studies to date have reported on the relationship between PTSS and the development of persistent pain and pain‐related disability. Estimates of pain and disability vary considerably in such studies and may be related to methods and the PTSS criteria used for diagnosis, sample size, length of follow‐up and effect measures to report outcome (pain and disability). Our findings demonstrate wide variation amongst studies on several different parameters, including definitions and methods of identifying pain and pain‐related disability, definitions and methods used to identify the population with PTSS, length of follow‐up, injury type, country and type of health service where the study was undertaken and clinical setting (primary, secondary or emergency department).
Most studies adequately described the study population and inclusion/exclusion criteria. Exposure and outcome were almost universally well described and most studies used valid measures for ascertaining the presence of PTSS and outcome measures (pain and pain‐related disability). Musculoskeletal trauma was well described across all studies. However, there was a ‘moderate/high’ risk of bias in most studies due to the attrition rate and a lack of adequate consideration of power analysis. Another frequently observed limitation was the lack of consecutive sampling reported in two of the eight studies, which could be explained by practical difficulties in reaching the target population. The extent of variation across the studies, combined with our quality findings that most studies have varying degrees of risk of bias, meant that the results could not be pooled for meta‐analysis. Furthermore, the link between pain and/or disability and PTSS may be confounded by the occurrence of specific types of traumatic events affecting pain thresholds and disability differently indicating the presence of different sub‐groups (Tesarz et al., 2020).
5. SUMMARY OF EVIDENCE
5.1. Pain estimates
The longitudinal nature of the studies included in this review allowed us to investigate the natural interactions of PTSS and pain over time, and the results yielded important insights into the relationship between pain and PTSS in the context of musculoskeletal injuries. The analyses revealed that those with PTSS were more likely to develop persistent pain compared to those without PTSS. However, these estimates varied considerably between studies. This is unsurprising given the variability in diagnostic measures to ascertain PTSS/pain and assessment time points across studies. Notably, the development of pain was relatively consistent across time (at 3, 6 and 12 months intervals), suggesting that pain continues to be a significant problem 12 months following injury in people with PTSS. One study (Ravn, Sterling, et al., 2018) provided pain estimates at two time points and showed that pain occurs consistently from baseline to 3 months and then from 6 months post‐injury (as baseline) to 12 months. A study by (Liedl et al., 2010) found that the PTSS cluster ‘arousal’ measured at 3 months following injury contributed to the severity of pain at 12 months post‐injury. Only one study (Kongsted et al., 2008), investigated the influence of selected covariates on pain development in people with PTSS. Sex (female) and pain intensity (high) at baseline were approximately twice more likely to be associated with pain development in people with PTSS compared to those without.
5.2. Disability estimates
Six studies (Åsenlöf et al., 2013; Kongsted et al., 2008; Maujean et al., 2017; Pedler et al., 2016; Ramchand et al., 2008; Ravn et al., 2019) examined the relationship between pain‐related disability and the presence of PTSS in people with musculoskeletal injuries. Although they provided estimates of the effect of PTSS on disability following a traumatic event, the comparability of these studies was limited by heterogeneity in terms of the follow‐up period, outcome measures and definitions, PTSS measurement and type of injury. Four of these studies (Åsenlöf et al., 2013; Kongsted et al., 2008; Pedler et al., 2016; Ravn et al., 2019) examined the effect of PTSS on disability after a whiplash injury which was classified as grade I–III or grade I–II, and unsurprisingly all of these studies reported increased levels of pain‐related disability mediated by PTSS following injury. The other study (Ramchand et al., 2008) examined the link between PTSS and disability in cases of multiple injuries and reported significant associations at 12‐month follow‐up. In a sample of patients who had sustained a whiplash injury, Maujean et al. (2017) found significant associations between PTSS clusters (hyper‐arousal and numbing) and disability at 6 months. Our findings provide preliminary evidence to suggest that PTSS is associated with greater long‐term disability and suggest that interventions aimed at identifying and treating PTSS early could help to reduce the development of these chronic conditions and improve patient outcomes. Our results concur with those of previous studies showing that PTSS is significantly associated with disability, pain intensity, absenteeism/presenteeism and low self‐esteem in whiplash patients' one‐year post‐accident (Åhman & Stålnacke, 2008; Buitenhuis et al., 2006; Ehlers et al., 1998; Stålnacke, 2009).
5.3. Strengths and limitations
Several recent studies have described the relationship of PTSS with increased levels of pain and pain‐related disability across populations with traumatic injuries (Buitenhuis et al., 2006; Geisser et al., 1996; Jenewein et al., 2009; Katz et al., 2009). It is important, however, to begin to understand the link between PTSS and pain/disability following a traumatic injury to plan effective interventions. This is the first systematic review to identify and present an in‐depth synthesis of all available evidence describing the role of PTSS and its impact on the development of persistent pain and pain‐related disability. The strengths of this systematic review include the rigorous methodological approach employed using an established methodological framework (Hayden et al., 2013; Page et al., 2021; Riley et al., 2019). Two independent reviewers were involved in study selection, data extraction and quality assessment and a third reviewer was available to ensure overall methodological consistency and to resolve any disagreements. To ensure an exhaustive review of the available literature, a comprehensive search strategy was implemented with broad inclusion criteria. The search was repeated at the time of manuscript preparation to capture recent and relevant studies. We also looked for evidence in the grey literature. There are however some limitations to the present study. First, the quality of these studies was variable because of the large methodological differences, statistical power, reliability of measures and information about effect measures making interpretations of the findings difficult. Second, given the limitations of the reported data, the high risk of bias amongst the included studies and the wide heterogeneity between them, we were unable to combine data in a meta‐analysis, which would have added significantly to a narrative synthesis (Fagard et al., 1996). Whilst this is a stronger methodology, we do not think it would have impacted our conclusions. Thirdly, due to limited data on ethnicity and sex, we were unable to adequately measure the effect of these variables, although this represents an important area for future research. We also intended to assess publication bias but were unable to do so owing to the wide heterogeneity between the included studies. The generalisability and applicability of these findings may be reduced, although each setting is inevitably unique and researchers may use different assessment criteria to ascertain PTSS, traumatic event and definitions to identify pain and disability.
5.4. Implications of results
The findings suggest a clear link between PTSS and pain and pain‐related disability following traumatic injuries. Given the likely development of pain and disability following a traumatic injury in people with PTSS and the likely impact on function and quality of life, it is important to identify/prioritize individuals with early‐onset PTSS following injury and initiate early preventative efforts. Phifer et al. (2011) argue that PTSS, pain and pain‐related functional impairment co‐occur and impact enormously on quality of life. In terms of future research, our findings demonstrate a clear need for a standardized approach to manage PTSS at an early stage following injury and subsequent management of both pain and pain‐related disability at an early stage. There was a great deal of variety of methods used to ascertain the presence of PTSS and to identify those with pain and pain‐related disability. An agreed core set of diagnostic definitions of PTSS is needed to allow better comparison and synthesis of findings derived from academic literature (Bryant, 2019). Similarly, a core set of standardized definitions of pain and pain‐related disability would be helpful to have a clear idea of the longitudinal association of these comorbidities. The Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT) recommends a core set of outcome domains when conducting RCT's concerning interventions for chronic pain when pain assessment tools and methods to ascertain pain vary considerably. These outcome domains include: pain dimension, physical functioning, emotional functioning, participant's ratings of global improvement, symptoms and adverse events and participant's disposition (Dworkin et al., 2005; Turk et al., 2003).
The term PTSS is often used interchangeably with PTSD symptoms. For example, Guimmarra et al. (2017) used the Posttraumatic Stress Disorder Checklist (PCL‐C) to ascertain PTSD symptoms (PTSS) from a trauma cohort derived from the registered Victorian Orthopaedic Trauma Outcomes Registry (VOTOR).
Recent changes in DSM‐V and ICD‐11 have marked discordance in how these two classification systems diagnose PTSD. Because of these differences, prevalence rates of PTSD can differ considerably when the two systems are used in the same sample. In general, lower prevalence rates of PTSD are found using the ICD‐11 compared to the DSM‐V (Brewin et al., 2017; Schellong et al., 2019; Shevlin et al., 2018). Due to its more restrictive definition, ICD‐11 could be failing to detect cases with PTSD symptoms; on the other hand, the DSM‐V may be inflating the number of cases. This difference could therefore have implications for patient management. Future research should explore the relative sensitivity of these two diagnostic tools in identifying PTSD and assess the longitudinal association with pain and/or pain‐related disability following a traumatic injury.
6. CONCLUSION
The findings of this review suggest that there is a clear relationship between PTSS post‐injury and future pain/disability, with the potential importance of certain PTSS clusters (hyperarousal and numbing). Significant heterogeneity was found between the included studies, particularly in relation to the PTSS measurement, description of the traumatic event and measures of pain and/or disability, in addition to the high risk of bias amongst the studies. These factors precluded meta‐analysis. Nonetheless, this review indicates that people with PTSS tend to develop pain and disability at an early stage following injury and these symptoms persist for at least 12 to 24 months post‐injury. This should be considered when developing appropriate treatment and planning long‐term care. Variations across studies and lack of separate analysis regarding age, ethnicity and sex meant that we were unable to conduct sub‐group analyses. Hence, our findings should be considered with a degree of caution. A standardized methodology and a core set of validated measures may also explore the association of PTSS and pain/or pain‐related disability after minor injuries or surgical procedures to further validate the relationship between PTSS and pain/disability following a traumatic event.
AUTHOR CONTRIBUTIONS
Ferozkhan Jadhakhan, David W. Evans and Deborah Falla contributed equally to the conception and design of this review. Ferozkhan Jadhakhan and David W. Evans drafted the first version of this review and this was reviewed/revised by Deborah Falla. The final version was drafted by Ferozkhan Jadhakhan. The search strategy was developed by Ferozkhan Jadhakhan and iteration was discussed with David W. Evans and Deborah Falla. The final version was approved by Deborah Falla and David W. Evans. The search was performed by Ferozkhan Jadhakhan. Ferozkhan Jadhakhan and David W. Evans performed screening for study selection. Ferozkhan Jadhakhan and David W. Evans collected data from the included studies and conducted the quality assessment. Ferozkhan Jadhakhan and David W. Evans performed data analysis/synthesis. Deborah Falla ensured data extraction consistency. Ferozkhan Jadhakhan, David W. Evans and Deborah Falla critically reviewed the manuscript and approved the final version. Deborah Falla is the guarantor.
FUNDING INFORMATION
This project is funded by the National Institute for Health Research (NIHR) Surgical Reconstruction and Microbiology Research Centre (SRMRC). SRMRC is a research infrastructure for trauma research jointly funded by the NIHR, and the ministry of defence. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Jadhakhan, F. , Evans, D. W. , & Falla, D. (2023). The role of post‐trauma stress symptoms in the development of chronic musculoskeletal pain and disability: A systematic review. European Journal of Pain, 27, 183–200. 10.1002/ejp.2048
REFERENCES
- Åhman, S. , & Stålnacke, B. (2008). Post‐traumatic stress, depression, and anxiety in patients with injury‐related chronic pain: A pilot study. Neuropsychiatric Disease and Treatment, 4, 1245–1249. [PMC free article] [PubMed] [Google Scholar]
- Akerblom, S. , Perrin, S. , Fischer, R. M. , & McCracken, L. M. (2017). The impact of PTSD on functioning in patients seeking treatment for chronic pain and validation of the posttraumatic diagnostic scale. International Journal of Behavioural Medicine, 24, 249–259. 10.1007/s12529-017-9641-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (1994). Diagnostic and statistical manual of mental disorders (4th ed.). (DSM‐IV). American Psychiatric Association. https://psychiatry.org/psychiatrists/practice/dsm [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. https://psychiatry.org/psychiatrists/practice/dsm [Google Scholar]
- Andersen, E. T. , Andersen, P. G. , Vakkala, M. A. , & Elklit, A. (2012). The traumatised chronic pain patient‐prevalence of posttraumatic stress disorder‐ PTSD and pain sensitization in two Scandinavian samples referred for pain rehabilitation. Scandinavian Journal of Pain, 3, 39–43. 10.1016/j.sjpain.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Andersen, T. E. , Karstoft, K. I. , Brink, O. , & Elklit, A. (2016). Pain‐catastrophizing and fear‐avoidance beliefs as mediators between post‐traumatic stress symptoms and pain following whiplash injury ‐ A prospective cohort study. European Journal of Pain, 20, 1241–1252. 10.1002/ejp.848 [DOI] [PubMed] [Google Scholar]
- Åsenlöf, P. , Bring, A. , & Söderlund, A. (2013). The clinical course over the first year of whiplash associated disorders (WAD): Pain‐related disability predicts outcome in a mildly affected sample. BMC Musculoskeletal Disorder, 21, 361. 10.1186/1471-2474-14-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson, G. J. , Coons, M. J. , Taylor, S. , & Katz, J. (2002). PTSD and the experience of pain: Research and clinical implications of shared vulnerability and mutual maintenance models. Canadian Journal of Psychiatry, 47, 930–937. 10.1177/070674370204701004 [DOI] [PubMed] [Google Scholar]
- Beckham, J. C. , Crawford, A. L. , Feldman, M. E. , Kirby, A. C. , Hertzberg, M. A. , Davidson, J. R. , & Moore, S. D. (1997). Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. Journal of Psychosomatic Research, 43, 379–389. 10.1016/S0022-3999(97)00129-3 [DOI] [PubMed] [Google Scholar]
- Brewin, C. R. , Cloitre, M. , Hyland, P. , Shevlin, M. , Maercker, A. , Bryant, R. A. , Humayun, A. , Jones, L. M. , Kagee, A. , Rousseau, C. , Somasundaran, D. , Suzuki, Y. , Wessely, S. , van Ommeren, M. , & Reed, G. M. (2017). A review of current evidence regarding the ICD‐11 proposals for diagnosing PTSD and complex PTSD. Clinical Psychology Review, 58, 1–15. 10.1016/j.cpr.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Brewin, C. R. , Lanius, R. A. , Novac, A. , Schnyder, U. , & Galea, S. (2009). Reformulating PTSD for DSM‐V: Life after criterion A. Journal of Traumatic Stress, 22, 366–373. 10.1002/jts.20443 [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. (2019). Post‐traumatic stress disorder: A state of the art review of evidence and challenges. World Psychiatry, 18, 259–269. 10.1002/wps.20656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, R. A. , Friedman, M. J. , Spiegel, D. , Ursano, R. , & Strain, J. (2011). A review of acute stress disorder in DSM‐5. Depression & Anxiety, 28, 802–817. 10.1002/da.20737 [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , & Harvey, A. G. (1995). Avoidant coping style and post‐traumatic stress following motor vehicle accidents. Behaviour Research and Therapy, 33, 631–635. 10.1016/0005-7967(94)00093-Y [DOI] [PubMed] [Google Scholar]
- Buitenhuis, J. , Jong, P. J. , Jaspers, J. P. C. , & Groothoff, J. W. (2006). Relationship between posttraumatic stress disorder symptoms and the course of whiplash complaints. Journal of Psychosomatic Research, 61, 681–689. 10.1016/j.jpsychores.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Clay, F. J. , Newstead, S. V. , & McClure, R. J. (2010). A systematic review of early prognostic factors for return to work following acute orthopaedic trauma. Injury, 41, 787–803. 10.1016/j.injury.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Dai, W. , Liu, A. , Kaminga, A. C. , Deng, J. , Lai, Z. , Yang, J. , & Wen, S. W. (2018). Prevalence of acute stress disorder among road traffic accident survivors: A meta‐analysis. BMC Psychiatry, 18, 188. 10.1186/s12888-018-1769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin, R. H. , Turk, D. C. , Farrar, J. T. , Haythornthwaite, J. A. , Jensen, M. P. , & Katz, N. P. (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113, 9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Ehlers, A. , Mayou, R. A. , & Bryant, B. (1998). Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. Journal of Abnormal Psychology, 107, 508–519. 10.1037/0021-843X.107.3.508 [DOI] [PubMed] [Google Scholar]
- Fagard, R. H. , Staessen, J. A. , & Thijs, L. (1996). Advantages and disadvantages of the meta‐analysis approach. Journal of Hypertension, 14, 9–12. 10.1097/00004872-199609002-00004 [DOI] [PubMed] [Google Scholar]
- Fishbain, D. A. , Pulikal, A. , Lewis, J. E. , & Gao, J. (2016). Chronic pain types differ in their reported prevalence of posttraumatic stress disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: An evidence‐based structured systematic review. Pain Medicine, 18, 711–735. 10.1093/pm/pnw065 [DOI] [PubMed] [Google Scholar]
- Geisser, M. E. , Roth, R. S. , Bachman, J. E. , & Eckert, T. A. (1996). The relationship between symptoms of post‐traumatic stress disorder and pain, effective disturbance and disability among patients with disability and non‐accident related pain. Pain, 66, 207–214. 10.1016/0304-3959(96)03038-2 [DOI] [PubMed] [Google Scholar]
- Guimmarra, M. J. , Casey, S. L. , Devlin, A. , Ioannou, L. J. , Gibson, S. J. , Goergiou‐Karistianis, N. , Jennings, P. A. , Cameron, P. A. , & Ponsford, J. (2017). Co‐occurrence of posttraumatic stress symptoms, pain, and disability 12 months after traumatic injury. Pain, 2, e622. 10.1097/PR9.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma, J. A. , Graet, Z. N. , Bollinger, I. , Naghavi, M. , Higashi, H. , Mullany, E. C. , Abera, S. F. , Abraham, J. P. , Adofo, K. , Alsharif, U. , Ameh, E. A. , Ammar, W. , Antonio, C. A. T. , Barrero, L. H. , Bekele, T. , Bose, D. , Brazinova, A. , Catalá‐López, F. , Dandona, L. , … Vos, T. (2016). The global burden of injury: Incidence, mortality, disability‐adjusted life years and time trends from the Global Burden of Disease study 2013. Injury Prevention, 22, 3–18. 10.1136/injuryprev-2015-041616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, J. A. , Van Der Windt, D. A. , Cartwright, J. L. , Côté, P. , & Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Annals of Internal Medicine, 158, 280–286. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- Heron‐Delaney, M. , Kenardy, J. , Charlton, E. , & Matsuoka, Y. (2013). A systematic review of predictors of posttraumatic stress disorder (PTSD) for adult road traffic crash survivors. Injury, 44, 1413–1422. 10.1016/j.injury.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Herron, J. , Hutchinson, R. , Lecky, F. , Bouamra, O. , Edwards, A. , Woodford, M. , & Eardley, W. G. P. (2017). The impact of age on major orthopaedic trauma: An analysis of the United Kingdom trauma audit research network database. The Bone & Joint Journal, 12, 1677–1680. 10.1302/0301-620X.99B12.BJJ-2016-1140.R2 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org [Google Scholar]
- Jadhakhan, F. , Evans, D. , & Falla, D. (2021). Role of post‐ trauma stress symptoms in the evelopment of chronic musculoskeletal pain and disability: A protocol for a systematic review. BMJ Open, 11, e058386. 10.1136/bmjopen-2021-058386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenewein, J. , Moergeli, H. , Wittmann, L. , Buchi, S. , Kraemer, B. , & Schnyder, U. (2009). Development of chronic pain following severe accidental injury. Results of a 3‐year follow‐up study. Journal of Psychosomatic Research, 66, 119–126. 10.1016/j.jpsychores.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Katz, J. , Asmundson, G. J. , McRae, K. , & Halket, E. (2009). Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoracotomy. European Journal of Pain, 13, 870–878. 10.1016/j.ejpain.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Kongsted, A. , Bendix, T. , Qerama, E. , Kasch, H. , Bach, F. W. , Korsholm, L. , & Jensen, T. S. (2008). Acute stress response and recovery after whiplash injuries. A one‐year prospective study. European Journal of Pain, 12, 455–463. 10.1016/j.ejpain.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Krismer, M. , & Van Tulder, M. (2007). Low back pain (non‐specific). Best Practice & Research Clinical Rheumatology, 21, 77–91. 10.1016/j.berh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Krug, E. G. , Sharma, G. K. , & Lozano, R. (2000). The global burden of injuries. American Journal of Public Health, 90, 523–526. 10.2105/ajph.90.4.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedl, A. , O'Donnell, M. , Creamer, M. , Silove, D. , McFarlane, A. , Knaevelsrud, C. , & Bryant, R. A. (2010). Support for the mutual maintenance of pain and post‐traumatic stress disorder symptoms. Psychological Medicine, 40, 1215–1223. 10.1017/S0033291709991310 [DOI] [PubMed] [Google Scholar]
- Maercker, A. , Brewin, C. R. , Bryant, R. A. , Cloitre, M. , Reed, G. M. , van Ommeren, M. , Humayun, A. , Jones, L. M. , Kagee, A. , Llosa, A. E. , Rousseau, G. , Somasundaram, D. J. , Souza, R. , Suzuki, Y. , Weissbecker, I. , Wessely, S. C. , First, M. B. , & Saxena, S. (2013). Proposals for mental disorders specifically associated with stress in the International Classification of Diseases‐11. Lancet, 11, 1683–1685. 10.1016/S0140-6736(12)62191-6 [DOI] [PubMed] [Google Scholar]
- Martin, A. L. , Halket, e. , Asmundson, G. J. G. , Flora, D. B. , & Katz, J. (2010). Posttraumatic stress symptoms and the diathesis‐stress model of chronic pain and disability in patients undergoing major surgery. The Clinical Journal of Pain, 26, 518–527. 10.1097/AJP.0b013e3181e15b98 [DOI] [PubMed] [Google Scholar]
- Maujean, A. , Gullo, M. J. , Andersen, T. E. , Ravn, S. L. , & Sterling, M. (2017). Post‐traumatic stress symptom clusters in acute whiplash associated disorder and their prediction of chronic pain‐related disability. Pain Reports, 2, e631. 10.1097/PR9.0000000000000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook, N. , Rushton, A. B. , Heneghan, N. R. , & Falla, D. (2019). Measures of central sensitisation and their measurement properties in the adult musculoskeletal trauma population: A protocol for a systematic review and data synthesis. BMJ Open, 9, e023204. 10.1136/bmjopen-2018-023204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, M. E. , Hodgson, J. L. , Jensen, J. F. , & Wood, T. L. (2020). Musculoskeletal injury survivors' resiliency: A systematic review. Disability & Health Journal, 14, 100987. 10.1016/j.dhjo.2020.100987 [DOI] [PubMed] [Google Scholar]
- National Audit Office . (2010). Major trauma care in England: Report by the comptroller and auditor general. National Audit Office, 1–30. http://www.nao.org.uk/wp‐content/uploads/2010/02/0910213.pdf [Google Scholar]
- National Highway Traffic Safety Administration . (2005). Traffic safety facts 2004: A compilation of motor vehicle crash data from the fatality analysis reporting system and the general estimates system. NHTSA. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/810631 [Google Scholar]
- Norton, R. , & Kobusingye, O. (2013). Injuries. New England Journal of Medicine, 368, 1723–1730. 10.1056/nejmra1109343 [DOI] [PubMed] [Google Scholar]
- O'Donnell, M. L. , Alkemade, N. , Nickerson, A. , Creamer, M. , McFarlane, A. C. , Silove, D. , Bryant, R. A. , & Forbes, D. (2014). Impact of the diagnostic changes to post‐traumatic stress disorder for DSM‐5 and the proposed changes to ICD‐11. British Journal of Psychiatry, 205, 230–235. 10.1192/bjp.bp.113.135285 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedler, A. , Kamper, S. J. , & Sterling, M. (2016). Addition of posttraumatic stress and sensory hypersensitivity more accurately estimates disability and pain than fear avoidance measures alone after whiplash injury. Pain, 157, 1645–1654. 10.1097/j.pain.0000000000000564 [DOI] [PubMed] [Google Scholar]
- Pedler, A. , & Sterling, M. (2013). Patients with chronic whiplash can be sub‐grouped on the basis of symptoms of sensory hypersensitivity and posttraumatic stress. Pain, 154, 1640–1648. 10.1016/j.pain.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Phifer, J. , Skelton, K. , Weiss, T. , Schwartz, A. C. , Wingo, A. , Gillespie, C. F. , Sands, L. A. , Sayyar, S. , Bradley, B. , Jovanovic, T. , & Ressler, K. J. (2011). Pain symptomatology and pain medication use in civilian PTSD. Pain, 152, 2233–2240. 10.1016/j.pain.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchand, R. , Marshall, G. N. , Schell, T. , & Jaycox, L. H. (2008). Posttraumatic distress and physical functioning: A longitudinal study of injured survivors of community violence. Journal of Consulting and Clinical Psychology, 76, 668–676. 10.1037/0022-006X.76.4.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn, S. L. , Hartvigsen, J. , Hansen, M. , Sterling, M. , & Andersen, T. E. (2018). Do post‐traumatic pain and post‐traumatic stress symptomatology mutually maintain each other? A systematic review of cross‐lagged studies. Pain., 159(11), 2159–2169. 10.1097/j.pain.0000000000001331 [DOI] [PubMed] [Google Scholar]
- Ravn, S. L. , Karstoft, K. I. , Sterling, M. , & Andersen, T. E. (2019). Trajectories of posttraumatic stress symptoms after whiplash: A prospective cohort study. European Journal of Pain, 23, 515–525. 10.1002/ejp.1325 [DOI] [PubMed] [Google Scholar]
- Ravn, S. L. , Sterling, M. , Lahav, Y. , & Andersen, T. E. (2018). Reciprocal associations of pain and post‐traumatic stress symptoms after whiplash injury: A longitudinal, cross‐lagged study. European Journal of Pain, 22, 926–934. 10.1002/ejp.1178 [DOI] [PubMed] [Google Scholar]
- Ravn, S. L. , Vaegter, H. B. , Cardel, T. , & Andersen, T. E. (2018). The role of posttraumatic stress symptoms on chronic pain outcomes in chronic pain patients referred to rehabilitation. Journal of Pain Research, 11, 527–536. 10.2147/JPR.S155241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier, D. A. , Kuhl, E. A. , & Kupfer, D. J. (2013). The DSM‐5: Classification and criteria changes. World Psychiatry, 12, 92–98. 10.1002/wps.20050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, R. D. , Moons, K. G. M. , Snell, K. I. E. , Ensor, J. , Hooft, L. , Altman, D. G. , Hayden, G. , Collins, G. S. , & Debray, T. P. A. (2019). A guide to systematic review and meta‐analysis of prognostic factor studies. The BMJ, 364, k4597. 10.1136/bmj.k4597 [DOI] [PubMed] [Google Scholar]
- Rosenbloom, B. N. , Khan, S. , McCartney, C. , & Katz, J. (2013). Systematic review of persistent pain and psychological outcomes following traumatic musculoskeletal injury. Journal of Pain Research, 6, 39–51. 10.2147/JPR.S38878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Párraga, G. T. , & López‐Martínez, A. E. (2014). The contribution of posttraumatic stress symptoms to chronic pain adjustment. Health Psychology, 33, 958–967. 10.1037/hea0000040 [DOI] [PubMed] [Google Scholar]
- Sareen, J. , Cox, B. J. , Stein, M. B. , Afifi, T. O. , Fleet, C. , & Asmundson, G. (2007). Physical and mental comorbidity, disability, and suicidal behavior associated with post traumatic stress disorder in a large community sample. Psychosomatic Medicine, 26, 242–248. 10.1097/PSY.0b013e31803146d8 [DOI] [PubMed] [Google Scholar]
- Schellong, J. , Hanschmidt, F. , Ehring, T. , Knaevelsrud, C. , Schäfer, I. , Rau, H. , Dyer, A. , & Krüger‐Gottschalk, A. (2019). Diagnostik der PTBS im Spannungsfeld von DSM‐5 und ICD‐11. Nervenarzt, 90, 733–739. 10.1007/s00115-018-0668-0 [DOI] [PubMed] [Google Scholar]
- Sharp, T. J. , & Harvey, A. G. (2001). Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clinical Psychology Review, 21, 857–877. 10.1016/S0272-7358(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Shevlin, M. , Hyland, P. , Vallières, F. , Bisson, J. , Makhashvili, N. , Javakhishvili, J. , Shipker, M. , & Roberts, B. (2018). A comparison of DSM‐5 and ICD‐11 PTSD prevalence, comorbidity and disability: An analysis of the Ukrainian internally displaced person's mental health survey. Acta Psychiatrica. Scandinavica, 137, 138–147. 10.1111/acps.12840 [DOI] [PubMed] [Google Scholar]
- Shipherd, J. C. , Keyes, M. , Jovanovic, T. , Ready, D. J. , Baltzell, D. , Worley, V. , Gordon‐Brown, V. , Hayslett, C. , & Duncan, E. (2007). Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? Journal of Rehabilitation Research & Development, 44, 153–165. 10.1682/jrrd.2006.06.0065 [DOI] [PubMed] [Google Scholar]
- Siqveland, J. , Hussain, A. , Lindstrøm, J. C. , Ruud, T. , & Hauff, E. (2017). Prevalence of posttraumatic stress disorder in persons with chronic pain: A meta‐analysis. Frontiers in Psychiatry, 8, 164. 10.3389/fpsyt.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålnacke, B. (2009). Relationship between symptoms and psychological factors five years after whiplash injury. Journal of Rehabilitation Medicine, 41, 353–359. 10.2340/16501977-0349 [DOI] [PubMed] [Google Scholar]
- Tesarz, J. , Baumesiter, D. , Andersen, T. E. , & Vaegter, H. G. (2020). Pain perception and processing in individuals with posttraumatic stress disorder: A systematic review with meta‐analysis. Pain Reports, 5, e849. 10.1097/PR9.0000000000000849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk, D. C. (2002). A diathesis‐stress model of chronic pain and disability following traumatic injury. Pain Research and Management, 7, 9–19. 10.1155/2002/252904 [DOI] [PubMed] [Google Scholar]
- Turk, D. C. , Dworkin, R. H. , Allen, R. R. , Bellamy, N. , Brandenburg, N. , Carr, D. B. , & Witter, J. (2003). Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain, 106, 337–345. 10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (1992). The ICD‐10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/37958/9241544228_eng.pdf?sequence=8&isAllowed=y [Google Scholar]
- World Health Organization . (2018). Global status report on road safety 2018. World Health Organization. https://www.who.int/violence_injury_prevention/road_safety_status/2018/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6


