Abstract
Background
Autologous anti‐CD19 chimeric antigen receptor (CAR) T‐cell therapy is an effective treatment for approximately 40% of relapsed/refractory large B cell lymphomas (LBCL), and early identification of patients at risk for relapse or progression after CAR T‐cell therapy represents a clinical need.
Methods
The authors conducted a single‐center prospective study on 47 relapsed/refractory LBCL receiving CAR T‐cell therapy to evaluate the prognostic value of baseline and after infusion 18F‐fluorodeoxyglucose positron emission tomography (PET)‐computed tomography. Qualitative and quantitative metabolic parameters were evaluated before lymphodepletion, at day 30 and 90 post‐infusion.
Results
Deep variation of standardized uptake value (SUV)mean between baseline and day 30 correlated with response at day 90 (hazard ratio [HR], 1.49; 95% confidence interval [CI], 1.01–2.2); p = .04) and better progression‐free survival (PFS) (HR, 0.63; 95% CI, 0.41–0.97); p = .04). In the overall population, 1‐year PFS was 63% for Deauville score (DS)1–3 and 39% for DS4–5 patients, respectively (p = .02), however, the prognostic role of DS was lost when survivals are analyzed by considering 38 patients not progressing at 30 days. In these patients, in partial response or stable disease, the combination of DS and variation of SUVmean allowed identification of three groups with different prognosis: patients with DS1–3 and those with DS4–5 and decreased SUVmean had similar 1‐year PFS of 62% and 61%, whereas patients with DS4–5 and increased SUVmean had a poorer 1‐year PFS of 33% (p = .04).
Conclusions
PET parameters and association of DS and variation of SUVmean at 30 days could help in identify patients at high risk of CAR T‐cell failure.
Lay summary
This is a single‐center prospective study on 47 lymphoma patients receiving commercial chimeric antigen receptor T‐cell therapy aimed to evaluate the prognostic value of baseline and after infusion 18F‐fluorodeoxyglucose positron emission tomography.
Among patients in partial remission or stable disease at day 30, the authors observed two subgroups with significantly different prognosis; patients with Deauville score (DS)4–5 and a concomitant reduction of standardized uptake value (SUV)mean had higher probability of long‐lasting response than those with DS4–5 and an increase of SUVmean.
Keywords: CAR T‐cell, Deauville score, lymphoma, PET, SUV
Short abstract
The combination of qualitative and quantitative positron emission tomography parameters assessed at day 30 after chimeric antigen receptor T‐cell treatment could help identify prognostically different subgroups among patients with partial response or stable disease. At day 30, patients in partial remission (PR)/stable disease (SD) and a concomitant reduction of standardized uptake value (SUV)mean had higher probability of long‐lasting response than those with PR/SD and an increase of SUVmean.
INTRODUCTION
CD19‐directed chimeric antigen receptor (CAR) T‐cells represent a novel treatment paradigm for patients with relapsed and refractory diffuse large B cell lymphoma (DLBCL) and primary mediastinal B cell lymphoma (PMBCL). However, despite the encouraging results reported in clinical trials and in real‐world setting, 1 , 2 , 3 , 4 durable responses are observed in approximately 35%–40% of patients then the identification of easy and reliable biomarkers for early prediction of outcome represents a clinical need.
Factors influencing the CAR T cell outcome are related to pre‐infusion and post‐infusion variables. Among all clinical and laboratory data considered for prediction of response, tumor burden is the most consolidated prognostic factor as described in recent studies showing that a high tumor burden correlates with an Eastern Cooperative Oncology Group (ECOG) > 1, the need for bridging therapy and poor clinical outcome. 5 , 6
In lymphoma patients, the evaluation of baseline tumor burden and clinical response is formally done by positron emission tomography (PET) that allows a visual assessment through the 5‐point Deauville score (DS) and a quantitative assessment of the metabolic activity of the tumor lesions. It is well consolidated that baseline evaluation of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) correlates with clinical response and progression‐free survival (PFS) in patients receiving standard immunochemotherapy. 7 , 8 , 9
Preliminary data suggest that PET parameters could also play a role as prognostic factors in large B cell lymphoma (LBCL) receiving anti‐CD19 CAR T‐cells. Dean et al. 10 observed that relapsed and refractory lymphoma patients treated with axicabtagene ciloleucel (axi‐cel) with low MTV at baseline had significantly superior overall survival and PFS in comparison to patients with high MTV. Similarly, Iacoboni et al. 11 reported that high baseline TMTV was associated with lower PFS in patients receiving anti‐CD19 CAR T‐cells.
Early disease evaluation at day 30 is a clinically relevant time‐point to predict long‐term response or failure to CAR T‐cells. However, the interpretation of the day‐30 PET can be problematic due to the presence of confounding factors related to inflammation induced by the CAR T‐cells. Although patients with progression of disease at day 30 must be considered not responding to CAR T‐cells and candidates to new treatments, prognosis of patients with DS4 or DS5 without clinical progression is not clear. Recently, Al Zaki et al. 12 observed that patients in partial remission (PR)/stable disease (SD) (DS4–5) at day 30‐PET following CAR T‐cells with standardized uptake value (SUV)max >10 experienced a higher risk of progression than patients with lower SUV. Generally, the analysis of day‐30 PET is typically confined to DS assessment, whereas no data exist about the analysis of PET parameters variations between baseline and day 30. We suppose that a combined analysis of DS and quantitative PET parameters at day 30 could be helpful in establishing long‐term efficacy of CAR T‐cells and in identifying patients that require new treatments.
In the present study, we investigated the day‐30 PET assessment by DS in relation to survivals and the relationship between baseline quantitative PET parameters or their variation over time with clinical response and survival outcomes. A secondary objective was to explore the combination of DS and quantitative PET parameters as prognostic tools in patients not progressing at 30 days after CAR T‐cells.
MATERIALS AND METHODS
Patients
This observational prospective study enrolled all consecutive patients treated at Fondazione IRCCS Istituto Nazionale dei Tumori in Milano with axicabtagene ciloleucel or tisagenlecleucel (axicel or tisacel) between March 2019 and July 2021. All patients had a baseline 18F‐fluorodeoxyglucose (FDG) PET performed before lymphodepletion (LD) and a PET reassessment at 30 (PET‐30) and 90 days (PET‐90) after infusion. Inclusion criteria included (1) diagnosis of DLBCL, PMBCL, or transformed‐follicular cell lymphomas (tFCL), (2) measurable nodal disease, and (3) at least 3 months of follow‐up.
All patients received LD with fludarabine and cyclophosphamide followed by infusion of axicel or tisacel. Evidence of progressive disease or relapse at any time was recorded as CAR T‐cell failure. Patients gave their written consent. The study was approved by ethical committee of Fondazione IRCCS Istituto Nazionale dei Tumori (INT178/19).
PET/computed tomography imaging and analysis
PET scans all have been performed at the same center, followed by the same technicians, and analyzed by the same physicians, allowing a large uniformity in execution and interpretation. All patients performed 18F‐FDG PET a few days before LD therapy, 30 days after CAR T‐cell infusion, and 90 days after CAR T‐cell infusion. At subsequent follow‐up time points, PET was used only in patients with persistent 18F‐FDG uptake or in patients with suspected relapse, otherwise a computed tomography (CT) scan was performed. All 18F‐FDG PET were evaluated by senior nuclear medicine physicians. Clinical response was assessed according to Lugano criteria, 13 and all PET examinations were evaluated based on DS.
18F‐FDG PET/CT image acquisition was performed according to the European Association of Nuclear Medicine guidelines. 14 Patient preparation with fasting at least 6 h before 18F‐FDG injection and blood glucose levels below 200 mg/dL were requested. PET/CT imaging was performed approximately 1 hour after intravenous administration of 3–4 MBq/kg of 18F‐FDG using either a Philips Gemini TF 64 (Philips Healthcare, Cleveland, Ohio) or General Electric Discovery 710 (General Electric Healthcare, Waukesha, Wisconsin) PET/CT scanner. All PET images were acquired from the base of the skull to mid‐thigh and corrected for attenuation using the acquired CT data. No intravenous contrast agents were administered. All images were reviewed on PET volume computer‐assisted reading (VCAR) software (GE Healthcare). PET/CT images were evaluated qualitatively for identified nodal or extra‐nodal FDG‐avid lesions. PET metabolic parameters (SUVmax, SUVmean, and SUVpeak) and volume‐based parameters (MTV and TLG) were calculated defining a volume of interest for each lesion by automatic delineation with the estimated threshold algorithm supplied with PET‐VCAR software. It was visually confirmed that only the intended structures were included. In this study, the lesion metabolic volume was automatically segmented using an adaptive iterative algorithm that separated the target volume from the background tissue by weighting the SUVmax and the SUVmean within the target volume with a weighting factor, represented as a Boolean variable. This segmentation strategy was previously used in different solid tumors 15 , 16 and can robustly, accurately, and precisely segment also small lesions in PET imaging. 17 To be representative of the entire lymphoma, SUVpeak and mean are reported as median value calculated across all lesions of each patient and SUVmax as maximum values of SUV between all lesions. Total MTV (TMTV) and total TLG (TTLG) were calculated as the sum of all individual lesions.
All PET parameters were calculated at baseline and at every time point. The variation of every single PET parameter between baseline and PET‐30 was calculated as Δ = (baseline value – PET‐30 value)/baseline value × 100.
Statistical analysis
Overall response rate (ORR) was calculated at 30 and 90 days as the percentage of patients achieving complete remission (CR) or PR. A univariate logistic regression model was implemented for each considered variable (i.e., PET parameter) to assess the association with response at 30 or 90 days. PFS was calculated as the time from infusion to the progression and overall survival (OS) as time until death. A landmark analysis was performed starting from the time of PET‐30 when were considered parameters at 30 days or in terms of Δ. The prognostic role of each PET and clinical variable was investigated in terms of PFS using a univariate Cox regression model. The relationship between continuous variables and the outcome was investigated by resorting to a regression model based on restricted cubic splines. 18 PET variables, retaining their significance after adjusting for significant clinical variables, were included in an initial multivariate model and a backward selection procedure was used to reach a final parsimonious model. According to the required number of event per variable, standard or penalized estimations were used to build multivariate model. 19 , 20 In addition, time‐dependent receiver operating characteristic (ROC) curve analyses were implemented and the corresponding area under the curve (AUC) were computed to evaluate the performance of the quantitative PET parameters to predicted patient progression at specific time points by using the inverse probability of censoring weighting method. 21 Quantitative PET parameters were dichotomized through the cutoff that better separates patients at high‐ or low‐risk of progression following Hothorn et al. 22 to make their clinical interpretation easier. The Kaplan–Meier method was used to estimate the pattern of PFS for dichotomous variables, and survival curves were compared using the log‐rank test.
All statistical analyses were performed with SAS (Version 9.4; SAS Institute) and R software by adopting a significance level of α = 0.05. Adjustments for multiple testing were not performed due to the exploratory nature of this study and coherently with its generating hypothesis purpose. 23
RESULTS
Patient characteristics
A total of 47 patients were included in this study, and all clinical data are detailed in Table 1. Patients were affected by DLBCL‐NOS (n = 21, 45%), high‐grade lymphomas (n = 10, 21%), PMBCL (n = 14, 30%), and tFCL (n = 2, 4%). Median age was 55 years (range, 22–70) and the majority of patients had an ECOG 0 (72%). Before LD, 41 (87%) patients received bridging therapy that included radiotherapy in 17 (36%) of them. At the time of LD, values of lactate dehydrogenase (LDH) and C‐reactive protein (CRP) above the upper normal value were observed in 38% and 21% of patients, respectively, and bulky disease intended as a single mass with a diameter ≥10 cm was present in 16 patients (34%).
TABLE 1.
Patient characteristics at starting lymphodepletion therapy
| Patient characteristics | N = 47 | % |
|---|---|---|
| Age, median (range), years | 55 (22–70) | |
| Sex | ||
| Female | 15 | 32 |
| Male | 32 | 68 |
| Histology | ||
| DLBCL NOS | 21 | 45 |
| DLBCL (tFCL) | 2 | 4 |
| DLBCL/high grade | 10 | 21 |
| PMBCL | 14 | 30 |
| ECOG | ||
| 0 | 34 | 72 |
| 1 | 13 | 28 |
| Bridging therapy | ||
| No | 6 | 13 |
| Yes | 41 | 87 |
| Radiotherapy as bridge | ||
| No | 30 | 64 |
| Yes | 17 | 36 |
| LDH pre LD | ||
| Normal value | 29 | 62 |
| High value (>ULN) | 18 | 38 |
| CRP pre LD | ||
| <5 mg/dL | 37 | 79 |
| >5 mg/dL | 10 | 21 |
| Bulky (lesion >10 cm) | ||
| No | 31 | 66 |
| Yes | 16 | 34 |
| Extranodal disease | ||
| No | 33 | 70 |
| Yes | 14 | 30 |
| Ferritin levels pre LD | ||
| Normal value | 13 | 28 |
| High value (>ULN) | 34 | 72 |
| CAR T‐cell | ||
| Axicabtagene ciloleucel | 31 | 66 |
| Tisagenlecleucel | 16 | 34 |
Abbreviations: CAR, chimeric antigen receptor; CRP, C‐reactive protein; DLBCL, diffuse large B cell lymphoma; ECOG, Eastern Cooperative Oncology Group; LD, lymphodepletive chemotherapy; LDH, lactate dehydrogenase; NOS, not otherwise specified; PMBCL, primary mediastinal B cell lymphoma; tFCL, transformed follicular cell lymphoma; ULN, upper limit of normal.
All patients performed PET/CT (PET‐baseline) at a median time of 9 days before infusion of CAR T‐cells, PET‐30 at a median of 32 days, and PET‐90 at a median of 98 days after infusion for a total of 136 examinations. Axicabtagene ciloleucel and tisagenlecleucel were infused to 31 (66%) and 16 (34%) patients, respectively.
Response to CAR T‐cells
At a median follow‐up time of 12 months (interquartile range, 8–20 months), 38 patients were alive and nine died of disease progression with an estimated 1‐year OS and PFS of 83% (95% CI, 66%–92%) and 46% (95% CI, 30%–60%), respectively (Figure 1). A total of 23 (49%) progressions were observed. No late serious toxic events were observed, and all events beyond day 90 were progressions or deaths related to lymphoma.
FIGURE 1.
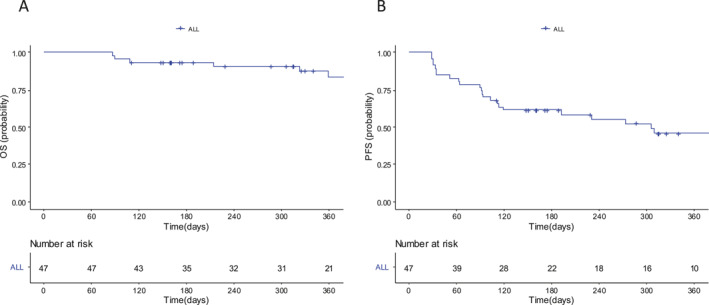
Kaplan–Meier curves for overall survival (A) and progression‐free survival (B) probability
ORR at 30 days was 68% (32 of 47): 21 patients achieved CR and 11 patients achieved PR. ORR at 90 days was 62% (26 of 41): 21 patients were in CR and five patients were in PR. At day 90, 6 patients were not evaluable for response. No toxic deaths were reported in this cohort.
The qualitative evaluation of PET/CT at 30 days identifies 21 patients with DS1–3 (one patient with DS1, 12 patients with DS2, and eight patients with DS3), 14 patients with DS4, and 12 patients with DS5. In the DS5 group, eight patients presented a progressive disease whereas four patients showed a SD. In one patient, DS at 30 days was not evaluable but at 90 days reached a CR. The disease evaluation at day 30 and at day 90 is shown in the consort diagram (Figure 2), three patients in CR at day 30 progressed at day 90. No specific clinical or imaging characteristics were identified as prognostic in these patients.
FIGURE 2.
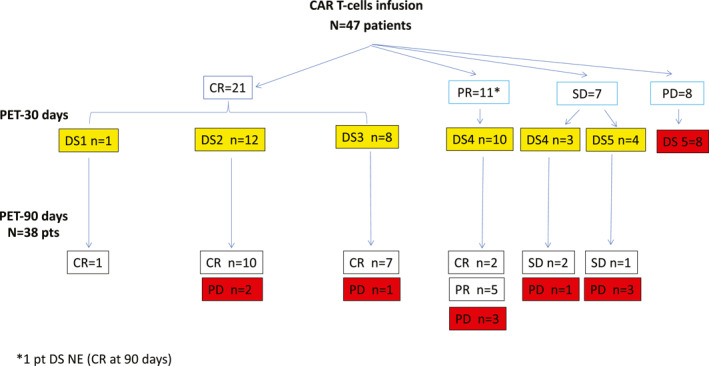
CONSORT diagram showing response of patients at 30 days after chimeric antigen receptor T‐cells according to Lugano criteria and detailed by DS assessment. In the bottom section of the diagram: disease response at 90 days according to Lugano criteria. Patients highlighted in yellow are the patients considered for study analysis. DS indicates Deauville score; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable
One year PFS was 63% (95% CI, 34%–82%) for DS1‐3 and 39% (95% CI, 19%–58%) for DS4‐5 patients, respectively (p = .02). To better evaluate the prognostic role of DS we analyzed survivals by considering patients not in progression at 30 days (n = 38) and we observed 1‐year PFS of 62% (95% CI, 33%–82%) in D1‐3 and 51% (95% CI, 26%–72%) in D4/5 group, respectively (HR, 1.96 [95% CI, 0.68–5.67]; p = .2) (Figure 3A).
FIGURE 3.
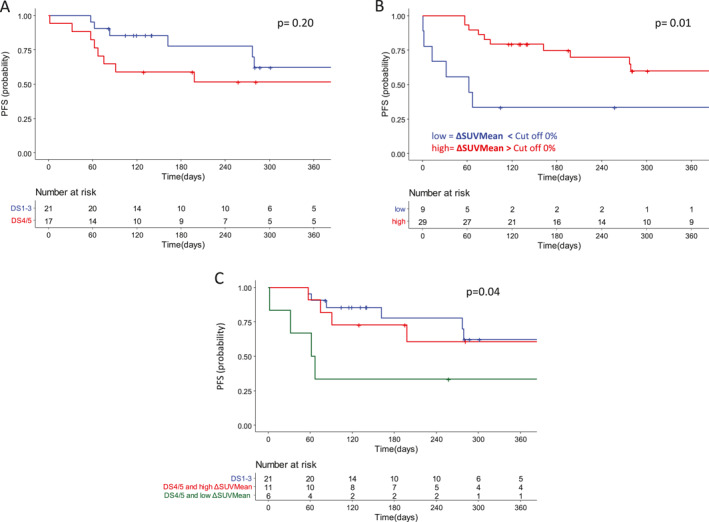
Kaplan–Meier curves for progression‐free survival probability in patients not progressing at day 30 according to (A) Deauville score, (B) ΔSUVmean, and (C) their combination
As concerns the considered clinical variables, only high LDH values and the presence of extranodal involvement at CAR T‐cells infusion were associated with a worst PFS whereas high levels of ferritin, CRP, specific extranodal sites, and the presence of bulky disease by CT scan did not have that association (Table S1).
Seventeen patients received radiotherapy as bridging therapy. At day 30, nine were in complete remission whereas eight showed DS4 or DS5. The uptake was reported in sites of previous radiotherapy in only five cases (30%). Following tisagenlecleucel, we observed eight patients with complete remissions (DS1–3), six patients with progressive disease, and two patients with DS4. The 30 days ORR was similar to response observed with axi‐cel.
Quantitative PET parameters and response to CAR T‐cells
Quantitative PET parameters of all patients were calculated at baseline, at PET‐30 and at PET‐90 and descriptive statistics for each considered PET parameter at baseline and in terms of Δ are reported in Table S2.
TABLE 2.
Univariate logistic model
| Variable | Univariate logistic model | |||||||
|---|---|---|---|---|---|---|---|---|
| Response at 30 days | Response at 90 days | |||||||
| OR | 95% CI | p | OR | 95% CI | p | |||
| Baseline SUVmax | 0.61 | 0.30 | 1.23 | .17 | 0.80 | 0.36 | 1.78 | .58 |
| Baseline SUVmean | 0.57 | 0.28 | 1.19 | .14 | 1.11 | 0.69 | 1.79 | .68 |
| Baseline TLG | 1.15 | 0.75 | 1.77 | .53 | 1.17 | 0.80 | 1.71 | .42 |
| Baseline MTV | 1.27 | 0.82 | 1.98 | .29 | 1.22 | 0.84 | 1.78 | .30 |
| Baseline TTLG | 0.67 | 0.31 | 1.47 | .32 | 1.18 | 0.83 | 1.66 | .36 |
| Baseline TMTV | 0.85 | 0.39 | 1.82 | .67 | 1.17 | 0.68 | 2.01 | .56 |
| Δ SUVmax | — | — | — | — | 1.03 | 0.81 | 1.31 | .81 |
| ΔSUVmean | — | — | — | — | 3.49 | 1.25 | 9.77 | .02 |
| ΔTLG | — | — | — | — | 2.09 | 0.81 | 5.38 | .13 |
| ΔMTV | — | — | — | — | 0.96 | 0.83 | 1.11 | .62 |
| ΔTTLG | — | — | — | — | 5.71 | 0.52 | 62.58 | .15 |
| ΔTMTV | — | — | — | — | 4.57 | 0.64 | 32.66 | .13 |
Note: Δ = (baseline value – PET‐30 value)/baseline value × 100.
Abbreviations: MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesions glycolysis; TMTV, total MTV; TTLG, total TLG.
Possible association with response and PFS was investigated for all parameters. No statistically significant association was seen between baseline quantitative PET parameters and response to CAR T‐cells at 30 and 90 days (Figure S1). Differently, patients with a deeper variation of SUVmean between baseline and PET‐30 (high ΔSUVmean) had higher probability to be in PR or CR at day 90 (OR, 3.49 [95% CI, 1.25–9.77], p = .02) (Table 2). Median ΔSUVmean was 18% (range, −94% to 88%) for patients in SD/progressive disease (PD) at day 90 and 51% (range, −11% to 91%) for patients in CR/PR. On the contrary the variation of SUVmax, TLG, MTV, TTLG, and TMTV was not statistically associated with response (Figure S2).
In the bivariate Cox model, among baseline parameters, only baseline SUVmax resulted significantly associated with PFS (HR, 1.59; 95% CI, 1.12–2.24; p = .01) whereas baseline SUVmean, MTV, TLG, TMTV, and TTLG were not (Table 3). Conversely, analysis of variation of PET parameters between baseline and day 30 showed that ΔTMTV, ΔSUVmean, and ΔTLG were associated with PFS as illustrated in Figure S3.
TABLE 3.
Univariate Cox model for PFS of quantitative PET parameters
| PET parameters | HR | 95% CI | p | |
|---|---|---|---|---|
| SUVmax baseline | 1.59 | 1.12 | 2.24 | .01 |
| SUVmean baseline | 1.42 | 0.96 | 2.08 | .08 |
| MTV baseline | 0.82 | 0.49 | 1.37 | .44 |
| TLG baseline | 0.91 | 0.58 | 1.42 | .69 |
| TMTV baseline | 1.27 | 0.88 | 1.84 | .20 |
| TTLG baseline | 1.26 | 0.88 | 1.80 | .22 |
| Variables a | ||||
| ΔSUVmax | 1.07 | 0.68 | 1.70 | .76 |
| ΔSUVmean | 0.63 | 0.41 | 0.97 | .04 |
| ΔTTLG | 0.73 | 0.52 | 1.02 | .06 |
| ΔTMTV | 0.68 | 0.48 | 0.95 | .02 |
| ΔMTV | 1.12 | 0.66 | 1.91 | .67 |
| ΔTLG | 0.69 | 0.49 | 0.96 | .03 |
Note: Δ = (baseline value – PET‐30 value)/baseline value × 100.
Abbreviations: CI, confidence interval; HR, hazard ratio; MTV, metabolic tumor volume; PET, positron emission tomography; PFS, progression‐free survival; SUV, standardized uptake value; TLG, total lesions glycolysis; TMTV, total MTV; TTLG, total TLG.
Landmark analysis at PET‐30.
In the bivariate Cox model including significant clinical variables, only high LDH value resulted associated with a higher risk of CAR T‐cells failure (HR, 2.7 [95% CI, 1.1–6.7], p = .03). From bivariate Cox models including LDH and each of the significant PET variables, only ΔSUVmean and ΔTTMV retained their significance. From an initial model including the above variables, the backward selection procedure identifies a final model including ΔSUVmean and LDH. Specifically, high ΔSUVmean was associated with a better PFS (HR, 0.6 [95% CI, 0.4–0.9], p = .02) whereas high LDH values were associated with a worse PFS (HR, 3.3 [95% CI, 1.3–8.2]; p = .01) (Table S3). This result was confirmed by penalized Cox regression model as the number of events was reduced in landmark analysis. The time‐dependent ROC curve performed at 91, 182, 273, and 365 days showed that the best AUC values was observed at 91 days that represents the time at which we observed the best predictive performance of the ΔSUVmean (Figure S4).
We pursued analyses investigating the prognostic value of the combination between qualitative and quantitative PET parameters. For this purpose, by dichotomizing ΔSUVmean using as cutoff 0%, (that means classify patients with an increased or a decreased SUVmean value between baseline and PET‐30), we observed that patients with reduction of SUVmean presented a significant better PFS in comparison to the counterpart, being PFS of 58% (95% CI, 37%–76%) versus 33% (95% CI, 8%–62%), respectively (p = .01) (Figure 3B).
We further evaluated whether variation of SUVmean between baseline and day 30 could identify different prognostic subgroups in association with DS assessment. Interestingly, the combination of variations of SUVmean and DS at PET‐30 allowed the identification of three subgroups of patients. For this analysis, we excluded eight patients known to have a very poor prognosis because of a PD status at day 30 and one patient that was not evaluable at 30 days. For the groups of patients with DS1–3 and DS4–5 associated with reduction of SUVmean, we observed similar prognosis (1‐year PFS of 62% [95% CI, 33%–82%] and 61% [95% CI, 25%–83%]). In the group including patients with DS4–5 associated with increased SUVmean, we observed a poorer prognosis (1‐year PFS of 33% [95% CI, 5%–68%]) (p = .04) (Figure 3C).
DISCUSSION
Early identification of parameters associated with long‐lasting response or progression after CAR T‐cell infusion represents an unmet medical need. There is a great interest in understanding the prognostic value of the early disease assessment at day 30 to plan a salvage treatment as early as possible. This is even more important in the near future when we will have new immunotherapies such as bispecific antibodies or new cellular therapies.
By excluding patients progressing early after CAR T‐cell infusion for which a new treatment is mandatory, patients in PR or SD represent a clinical dilemma for clinicians. In fact, approximately 20%–30% of patients experienced PR or SD at day 30 but only a minority of them achieved a long‐lasting response. In the real‐life study by US Lymphoma CAR T Consortium, 1 only 30% of patients in PR and 7% of those in SD at day 30 achieved a subsequent CR. Recently, Kuhnl et al. 24 investigated the use of the visual assessment by DS score at day 30 following CAR T‐cell infusion. They confirmed the prognostic role of DS and that long‐term PFS (ranging from 63% to 77%) was achieved in patients with DS1–3 and DS4 previously exposed to radiotherapy whereas an inferior response was described in the remaining patients with DS4–5.
In our cohort we confirmed the prognostic value of DS, in fact patients with DS 4–5 represent a population with high probability to fail the CAR T‐cell treatment. However, when we excluded patients with PD at day 30 who are patients known to have a very poor prognosis, the DS was not able to distinguish long‐lasting responders from patients who will relapse. These data confirm that interpretation of a residual PET positivity early at day 30 can be difficult due to the presence of inflammation, tumor flare, and pseudoprogression. The hypothesis of our project is that a combination of DS and quantitative assessment could strengthen the PET evaluation and support the identification of patients with very poor prognosis.
We decided to include in our analyses the quantitative PET parameters as potential markers of CAR T activity. ΔSUV has been already explored as a prognostic factor in a cohort of patients affected by newly diagnosed DLBCL treated with R‐CHOP or R‐ACVBP. During first‐line therapy, the authors showed that ΔSUVmax >66% at interim PET was associated with a better PFS and OS. 25 However, few experiences are reported in patients treated with CAR T‐cells.
In our study, we observed that a deeper variation of SUVmean between baseline and day 30 is associated with a higher probability of response at day 90 and longer PFS. Variation of SUV is a parameter that is easy to assess, and it reflects well the reduction in metabolic activity inside the lymphoma mass. There is some debate about the most reliable quantitative approach by FDG‐PET for monitoring lymphoma patients. In this article, SUVmean are reported as median value calculated across all lesions of each patient, therefore it seems that SUVmean reflects well the burden of disease and the presence of multiple heterogenic lymphoma lesions. As a consequence, ΔSUVmean represents the variation of the SUV of the entire disease burden. We identified the cutoff of ΔSUVmean as 0% as the best value to dichotomize our cohort in subgroups with different prognosis. This results in an easy tool for PET interpretation because patients can be easily assigned to the group with increased or decreased SUVmean at day 30.
Based on the results observed with the analysis of SUVmean, we explored the prognostic value of a combination of ΔSUVmean and DS, and we identify three prognostically different groups of patients. Interestingly, among DS4–5 patients, we observed two subgroups with significantly different prognosis; patients with DS4–5 and a concomitant reduction of SUVmean at day 30 had a higher probability of long‐lasting response than those with DS4–5 and an increase of SUVmean.
To our knowledge, this is the first article exploring the prognostic value of the variation of PET parameters before and after CAR T‐cell infusion in association to DS. This is of great interest for the interpretation of PET‐30 and early identification of patients requiring further therapies.
The major limitation of this study is the limited sample size, however, the monocentric design of the study allowed homogeneity in PET execution and interpretation and also in baseline and follow‐up reassessment of patients. Another limit of this study concerns the segmentation/threshold method, the delineation on MTV is still considering challenging due to the low signal‐to‐noise ratio of PET images and limited spatial resolution associated with partial volume effects. No general agreement has been reached concerning which segmentation approach performs best. We used an adaptive iterative algorithm for segmentation based on our experience, and we did not observe clinical correlation when we tested the 41% threshold (data not showed). The findings of this study should be considered with caution and will need further investigation in larger studies.
Our results disagree with published observations of the impact of baseline disease parameters on response. Vercellino et al. 26 showed that a TMTV higher than 80 ml, but not tumor bulk defined by CT scan, was associated with a higher risk of early relapse and death. We observed only a significant association between baseline SUVmax and PFS, but no association was observed for baseline TMTV and TTLG. Whether baseline SUVmax indirectly represents the extension and proliferation index of single lymphoma lesion, TMTV and TTLG probably suffer by the large heterogeneity of our population in terms of histotype (inclusion of DLBCL and primary mediastinal B‐cell lymphomas and high‐grade lymphomas) and disease burden. Similarly, in univariate analysis, baseline high LDH levels and the presence of extranodal lesions were associated with a poorer prognosis, however, ferritin, CRP, and bulky disease were not. This can be due to the limited sample size. Interestingly, patients receiving tisa‐cel had a similar proportion of response at 30 days in comparison to those receiving axi‐cel, suggesting similar response kinetics after treatment with the two products.
Patients included in the present analyses are representative of a population treated with CAR T‐cells in a real‐life setting with a percentage of patients with high LDH and with bulky disease comparable with other published cohorts. Similarly, the observed clinical responses are superimposable with those observed in other groups of patients treated outside clinical studies.
In conclusion, we observed that a combination of qualitative and quantitative PET parameters could help in interpretation of early reassessment of CAR T‐cells patients. Patients with a reduction of SUVmean early after 30 days have an increased probability of response at 90 days and to survive free of progression.
Moreover, prognostic evaluation of patients with a response that is difficult to establish because of a 30‐day PET showing PR or SD could be supported by analysis of quantitative PET parameters. In fact, patients not progressing at day 30 with PET showing DS4–5 and concomitant reduction of SUVmean seems a subgroup with a good prognosis whereas those with an increased SUVmean are not. This observation could help in planning new therapeutic strategies for patients identified as non‐responders.
AUTHOR CONTRIBUTIONS
Anna Guidetti: Study design and manuscript writing. Anna Dodero: Study design and manuscript writing. Alice Lorenzoni: Analyzed and collected PET data. Giovanni Argiroffi: Analyzed and collected PET data. Alessandra Alessi: Analyzed and collected PET data. Ettore Seregni: Analyzed and collected PET data. Filippo Bagnoli: Clinical data collection. Vincenzo Marasco: Clinical data collection. Cristiana Carniti: Clinical data collection. Chiara Monfrini: Clinical data collection. Martina Pennisi: Clinical data collection. Sara Pizzamiglio: Statistical analysis. Paolo Verderio: Statistical analysis. Paolo Corradini: Manuscript writing. All authors provided final approval of the manuscript.
CONFLICTS OF INTEREST
Giovanni Argiroffi reports consulting fees from Fondazione IRCCS Istituto Nazionale Tumori. Filippo Bagnoli reports consulting fees from Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico. Annalisa Chiappella reports consulting fees from AstraZeneca, Celgene, Clinigen, Gilead Sciences, Incyte, Janssen Biotech, Novartis, Roche, Secura Bio, and Takeda Oncology. Paolo Corradini reports consulting fees from Gilead Sciences and Novartis. Anna Guidetti reports consulting fees from Gilead Sciences and GlaxoSmithKline and travel fees from F. Hoffmann‐La Roche, Janssen Biotech, and Novartis. Martina Pennisi reports consulting fees from Bristol‐Myers Squibb, Gilead Sciences, and Nektar Therapeutics and travel fees from Roche s.p.a. The other authors made no disclosures.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Authors received no financial support for the research, authorship, and/or publication of this article.
Open access funding provided by BIBLIOSAN.
Guidetti A, Dodero A, Lorenzoni A, et al. Combination of Deauville score and quantitative positron emission tomography parameters as a predictive tool of anti‐CD19 chimeric antigen receptor T‐cell efficacy. Cancer. 2023;129(2):255‐263. doi: 10.1002/cncr.34532
Anna Guidetti and Anna Dodero contributed equally to this article.
REFERENCES
- 1. Nastoupil LJ, Jain MD, Feng L, et al. Standard‐of‐care axicabtagene ciloleucel for relapsed or refractory large B‐cell lymphoma: Results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119‐3128. doi: 10.1200/jco.19.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster SJ, Tam CS, Borchmann P, et al. Long‐term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B‐cell lymphomas (JULIET): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2021;22(10):1403‐1415. doi: 10.1016/s1470-2045(21)00375-2 [DOI] [PubMed] [Google Scholar]
- 3. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B‐cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839‐852. doi: 10.1016/s0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 4. Locke FL, Ghobadi A, Jacobson CA, et al. Articles Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1 – 2 trial. Lancet Oncol. 2019;20:31‐42. doi: 10.1016/s1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B‐cell lymphoma. Blood Advances. 2020;4(19):4898‐4911. doi: 10.1182/bloodadvances.2020002394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T‐cell therapy in relapsed/refractory diffuse large B‐cell lymphoma. Blood Advances. 2020;4(22):5607‐5615. doi: 10.1182/bloodadvances.2020003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kostakoglu L, Mattiello F, Martelli M, et al. Total metabolic tumor volume as a survival predictor for patients with diffuse large B‐cell lymphoma in the GOYA study. Haematologica. 2021;107(7):1633‐1642. doi: 10.3324/haematol.2021.278663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceriani L, Gritti G, Cascione L, et al. SAKK38/07 study: Integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Advances. 2020;4(6):1082‐1092. doi: 10.1182/bloodadvances.2019001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasanelli M, Meignan M, Haioun C, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B‐cell lymphoma. Eur J Nucl Med Mol Imag. 2014;41(11):2017‐2022. doi: 10.1007/s00259-014-2822-7 [DOI] [PubMed] [Google Scholar]
- 10. Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B‐cell lymphoma. Blood Advances. 2020;4(14):3268‐3276. doi: 10.1182/bloodadvances.2020001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iacoboni G, Simó M, Villacampa G, et al. Prognostic impact of total metabolic tumor volume in large B ‐ cell lymphoma patients receiving CAR T ‐ cell therapy. Ann Hematol. 2021;100(9):2303‐2310. doi: 10.1007/s00277-021-04560-6 [DOI] [PubMed] [Google Scholar]
- 12. Al Zaki A, Shpall Anderson E, Watson G, et al. Day 30 SUV max Predicts Progression in Lymphoma Patients Achieving PR/SD After CAR T‐cell Therapy. Blood Advances. 2022;6(9):2867‐2871. doi: 10.1182/bloodadvances.2021006715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non‐Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol. 2014;32(27):3059‐3067. doi: 10.1200/jco.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boellaard R, Delgado‐Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imag. 2015;42(2):328‐354. doi: 10.1007/s00259-014-2961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crivellaro C, Signorelli M, Guerra L, et al. 18F‐FDG PET/CT can predict nodal metastases but not recurrence in early stage uterine cervical cancer. Gynecol Oncol. 2012;127(1):131‐135. doi: 10.1016/j.ygyno.2012.06.041 [DOI] [PubMed] [Google Scholar]
- 16. Parkinson C, Foley K, Whybra P, et al. Evaluation of prognostic models developed using standardised image features from different PET automated segmentation methods. EJNMMI Res. 2018;8(1):29. doi: 10.1186/s13550-018-0379-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sebastian TB, Manjeshwar RM, Akhurst TJ, Miller JV. Objective PET Lesion Segmentation Using a Spherical Mean Shift Algorithm. Med Image Comput Comput Assist Interv. 2006;9(Pt 2):782‐789. [DOI] [PubMed] [Google Scholar]
- 18. Durrleman S, Simon F. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551‐561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 19. Peter P, Concato J, Feinstein AR, Holford TR. Importance of Events Per Independent in Proportional Hazards Regression Analysis and Precision of Regression Importance. J Clin Epidemiol. 1995;48(12):1503‐1510. [DOI] [PubMed] [Google Scholar]
- 20. Moons KGM, Donders ART, Steyerberg EW, Harrell FE. Penalized maximum likelihood estimation to directly adjust diagnostic and prognostic prediction models for overoptimism: A clinical example. J Clin Epidemiol. 2004;57(12):1262‐1270. doi: 10.1016/j.jclinepi.2004.01.020 [DOI] [PubMed] [Google Scholar]
- 21. Hung H, Chiang C. Estimation methods for time‐dependent AUC with survival data. Can J Stat. 2010;38(1):8‐26. [Google Scholar]
- 22. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121‐137. doi: 10.1016/S0167-9473(02)00225-6 [DOI] [Google Scholar]
- 23. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43‐46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 24. Kuhnl A, Roddie C, Kirkwood AA, et al. Early FDG‐PET response predicts CAR‐T failure in large B‐cell lymphoma. Blood Advances. 2022;6(1):321‐326. doi: 10.1182/bloodadvances.2021005807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casasnovas R‐O, Meignan M, Berriolo‐Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B‐cell lymphoma. Blood. 2011;118(1):37‐43. doi: 10.1182/blood-2010-12-327767 [DOI] [PubMed] [Google Scholar]
- 26. Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396‐1405. doi: 10.1182/blood.2019003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


