Abstract
Hypertrophic cardiomyopathy (HCM) is the most prevalent cardiac disease in cats and lacks efficacious preclinical pharmacologic intervention, prompting investigation of novel therapies. Genetic mutations encoding sarcomeric proteins are implicated in the development of HCM and small molecule myosin inhibitors are an emerging class of therapeutics designed to target the interaction of actin and myosin to alleviate the detrimental effects of inappropriate contractile protein interactions. The purpose of this study was to characterize the pharmacodynamic effects of a single oral dose of the novel cardiac myosin inhibitor aficamten (CK‐274) on cardiac function in purpose bred cats with naturally occurring A31P MYBPC3 mutation and a clinical diagnosis of HCM with left ventricular outflow tract obstruction (LVOTO). Five purpose bred cats were treated with aficamten (2 mg/kg) or vehicle and echocardiographic evaluations were performed at 0, 6, 24, and 48 h post‐dosing. High dose aficamten (2 mg/kg) reduced left ventricular fractional shortening (LVFS%) by increasing the LV systolic internal dimension (LVIDs) and reduced isovolumic relaxation time (IVRT) compared with baseline without significant adverse effects. The marked reduction in systolic function and reduced IVRT coupled with an increased heart rate in treated cats, suggest a lower dose may be optimal. Further studies to determine optimal dosing of aficamten are indicated.
Keywords: feline, left ventricle, myosin binding protein C, pharmacodynamic, sarcomere
1. INTRODUCTION
Small molecule inhibitors are an emerging class of novel therapeutic agents that inhibit the function of specific proteins with the potential for neutralizing deleterious downstream effects (Bond et al., 2013). One class of these compounds include cardiac myosin inhibitors, which are being investigated for their potential therapeutic uses in a vast array of disease processes including hypertrophic cardiomyopathy (HCM) (Alsulami & Marston, 2020; Argirò et al., 2021).
Hypertrophic cardiomyopathy is the most common heart disease affecting cats and occurs in approximately 15% of subclinical domestic cats (Payne et al., 2015) with potential complications including congestive heart failure (CHF), arterial thromboembolism (ATE), development of arrythmias, and sudden cardiac death. While many cats may remain asymptomatic for their lifespan, the potential clinical sequelae of disease progression are detrimental and therapies to improve survival are lacking.
Hypertrophic cardiomyopathy is a disease of cardiac sarcomeric proteins with identified mutations in cats affecting the myosin heavy chain (MYH7) and myosin binding protein‐C (MyBPC3) genes, which alter the development of the motor protein β‐myosin heavy chain and the structural and regulatory protein cardiac myosin binding protein‐C, respectively (Kittleson et al., 2015). Sarcomeric proteins are responsible for muscle contraction and relaxation (Opie, 2004) and mutations in these proteins lead to histopathologic changes in the myocardium including myocardial fiber disarray, interstitial fibrosis, and intramural coronary arteriosclerosis (Kittleson et al., 1999, 2015). These changes result in left ventricular (LV) wall thickening in the absence of another pathogenic explanation (i.e., hyperthyroidism and systemic hypertension) and predominate hemodynamic alterations include hypercontractility of the LV and diastolic dysfunction which fuels progression of disease.
Evidence is currently lacking for therapies with proven efficacy in delaying progression of disease or conferring survival benefits in subclinical feline HCM patients (Luis Fuentes et al., 2020). The lack of therapeutic options for delaying or preventing harmful outcomes in the preclinical period of disease has prompted investigation into novel therapies targeted at the underlying molecular pathophysiology. A recent study demonstrated the efficacy of another novel small molecule inhibitor in relieving left ventricular outflow tract obstruction (LVOTO) in a feline model of hypertrophic obstructive cardiomyopathy (HOCM) (Stern et al., 2016). This small molecule inhibitor also demonstrated efficacy in suppressing histopathologic and gross pathologic changes in a mouse model of HCM (Green et al., 2016) substantiating the promise of these novel therapeutic agents. While mouse models are frequently employed in the study of HCM, their physiology limits applicability to other species. Ragdoll and Maine Coon cats with MYBPC3 mutations represent an established spontaneously occurring large animal model for this disease (Freeman et al., 2017; Maron & Fox, 2015) and serve as a valuable resource for the evaluation of novel therapies that may benefit both cats and their human counterparts.
The novel small molecule, aficamten (CK‐3773274, CK‐274), is a cardiac myosin inhibitor that decreases cardiac myosin ATPase activity in vitro and reduces cardiac fractional shortening in vivo (Chuang et al., 2021). The selected starting dose of this study was informed by prior work in the dog and rat (Chuang et al., 2021). The aim of this study was to characterize the pharmacodynamic effects of a single oral dose of aficamten on cardiac function in purpose bred cats with naturally occurring A31P MYBPC3 mutation and a clinical diagnosis of HCM with LVOT obstruction.
2. MATERIALS AND METHODS
2.1. Animals
Research was conducted in accordance with the University of California, Davis, IACUC (Protocol number 20565). A sample size of at least four cats in each dose group was calculated to have an 80% power to identify a 20% reduction in key parameters of echocardiographically assessed systolic function. This a‐priori sample size was estimated based upon unpublished, colony‐specific, preliminary data in HCM‐affected feline subjects. Five purpose bred mixed‐breed Maine Coon cats with naturally occurring A31P MYBPC3 mutations and a diagnosis of subclinical hypertrophic obstructive cardiomyopathy were used in this study. The cats were group housed in the feline research colony at the University of California, Davis, throughout the study period and were maintained on the same dietary regime without supplementation or medication beyond the study protocol. Cats were transported to the laboratory facilities and individually housed for the time of the study procedures and until full recovery from any sedation procedure was observed prior to return to the colony. Physical examinations were performed on all animals and no clinical signs of their known HCM were appreciated. The cats have experienced similar examination, diagnostic and transport procedures in the past, such that the acclimation to the facilities and approach are assumed. Table 1 describes the demographics of cats used in this study.
TABLE 1.
Cat demographics and maximal left ventricular diastolic wall thickness
| Subject | Sex | Age | Date of birth | A31P MYBPC3 genotype | Max IVSDd (mm) | Max LVPWd (mm) |
|---|---|---|---|---|---|---|
| 1 | Male | 4.9 | 3/1/15 | Heterozygous | 6.6 | 5.3 |
| 2 | Male | 9.0 | 2/15/11 | Heterozygous | 6.2 | 5.6 |
| 3 | Male | 2.5 | 8/8/17 | Heterozygous | 7 | 5.3 |
| 4 | Male | 1.9 | 4/3/18 | Heterozygous | 6.2 | 5.6 |
| 5 | Male | 2.5 | 8/8/17 | Heterozygous | 7 | 5.3 |
Abbreviations: IVSDd, interventricular septal diameter in diastole; LVPWd, left ventricular posterior wall diameter in diastole; MYBPC3, myosin binding protein C3.
2.2. Experimental design
This was a randomized, controlled, cross‐over study. Cats received single oral doses of vehicle (Veh) or aficamten at a dose of 2 mg/kg via oral gavage in 0.5% (w/v) Hydroxypropylmethylcellulose (HPMC) and 0.1% TWEEN® 80 in Ultra Pure water at a dose volume of 1 mg/kg. Subsequent echocardiographic evaluations were performed over a 48 hour period. Cats were randomized and crossed over to the other treatment group so that all cats received both treatments with a minimum washout period of 2 weeks.
2.3. Echocardiographic evaluations
Baseline echocardiographic evaluations were performed 24 hours prior to each treatment dosing. On the morning of the examination, cats were dosed with gabapentin (100 mg PO) approximately 1 hour prior to the study. The cats were sedated with butorphanol (0.3 mg/kg IM) and acepromazine (0.05 mg/kg IM) to facilitate echocardiographic evaluation.
All echocardiograms were performed by a single board‐certified cardiologist (MSO) using a 12–4 mHz sector array transducer with harmonics (Philips EPIC CVx, Philips Healthcare). Two‐dimensional (2D), M‐mode, color Doppler, and spectral Doppler echocardiographic images were obtained in standard imaging planes from right and left lateral recumbency (Boon, 2011). A concurrent lead II ECG was monitored for arrhythmias.
On the day of echocardiographic evaluation with vehicle or aficamten treatment, cats were sedated with alfaxalone (2 mg/kg IM) to facilitate oral gavage of one dose of specified treatment (vehicle or 2 mg/kg aficamten) followed by a 3 hour recovery period. To facilitate echocardiographic evaluation, cats were sedated with butorphanol (0.3 mg/kg IM) and acepromazine (0.05 mg/kg IM) at the 6, 24, and 48 h post‐dose echocardiography time points. All appropriate cardiac views were obtained for 2‐D, M‐Mode, color, and spectral Doppler assessment of LV hypertrophy, LVOT obstruction, LV FS%, indices of diastolic function and other parameters. Following echocardiographic evaluation, blood samples were obtained for plasma concentration analysis. Cats were observed and allowed to fully recover before returning to colony group housing.
Images were stored, and all measurements were performed by a single blinded observer (MSO) using a commercially available offline workstation (Syngo Dynamic Workplace, Version 10.0.01_HF04_Rev5 [Build 2884], Siemens Medical Solutions, Malvern Pennsylvania). Two‐dimensional right parasternal long axis or short axis imaging planes were measured to obtain the maximum 2D thickness of the interventricular septum (IVSd) and left ventricular posterior wall (LVPWd) in diastole using an inner‐edge to inner‐edge measuring technique. Segmental or diffuse IVS or LVPW thickness exceeding 6 mm in the absence of systemic hypertension or hyperthyroidism on at least two serial examinations >1 month apart was considered consistent with HCM (Luis Fuentes et al., 2020). Left ventricular outflow tract obstruction was identified from the left parasternal 5‐chamber view and was defined as the presence of color Doppler flow aliasing in the LVOT and a late‐peaking spectral CW Doppler signal with a velocity of >1.9 m/s (Chetboul et al., 2006; Oldach et al., 2019). The cursor was aligned with the color Doppler aliasing flow, and the maximal modal velocity obtained was recorded.
M‐mode and two‐dimensional right parasternal short axis imaging plane were used to measure the left ventricular internal dimensions at end‐diastole (LVIDd) and end‐systole (LVIDs). Fractional shortening (FS%) was calculated using the equation [(LVIDd‐LVIDs)/LVIDd] × 100.
Left atrial size was measured in 2D on the right parasternal short‐axis basilar view to determine the LA/Ao as previously described (Abbott & Maclean, 2006). The maximal left atrial diameter in long axis was measured from the right parasternal long axis view (Schober & Chetboul, 2015). The left auricular flow velocity was obtained in an oblique left apical parasternal long axis view with the pulsed wave Doppler sample volume positioned at the entrance to the left auricle (Schober & Maerz, 2005).
Spectral Doppler and pulsed wave tissue Doppler imaging (PW TDI) from the left apical 4‐chamber view were used to evaluate diastolic functional parameters including transmitral inflow patterns, lateral E' and A', and LV isovolumic relaxation time (IVRT) (Schober & Chetboul, 2015). Lateral mitral annulus S′ was measured from this view with PW TDI and MAPSE was measured with M‐mode. Transmitral spectral Doppler and diastolic tissue Doppler were frequently fused with tachycardia and precludes measurements of these values.
2.4. Blood sample collection and plasma analysis
Following each echocardiography assessment (at baseline, 6, 24, and 48 h post dose), 2 ml of whole blood was collected via direct venipuncture. Blood samples were collected into EDTA microtainer tubes and centrifuged for 5 min at 2655 g to obtain the plasma component. Plasma was stored frozen at −80°C until shipment to Cytokinetics for concentration determination by LC/MS/MS analysis.
Aficamten plasma concentration was determined using the LC/MS/MS method. A 25 μl aliquot of each plasma sample was diluted with 25 μl blank cat plasma. The plasma sample was then mixed with 100 μl of acetonitrile that contained N1‐(butylcarbamoyl)‐sulfanamide (0.3 μM) as the internal standard. The mixture was then vortexed and centrifuged. The resulting supernatant was transferred and filtered through a membrane (Whatman, Inc. Unifilter 96‐well Filter Plate, 0.45 μm hydrophilic PVDF membrane). 10 μl of the resulting solution was injected onto a reverse‐phase C18 column and the resultant peaks detected on a SCIEX API 4000 LC/MS/MS equipped with a turbo ion spray ionization source.
2.5. Statistics
Data were tested for normality by visual inspection and the D'Agostino and Pearson Omnibus normality test. Descriptive statistics were performed to provide both the mean and standard deviation (for parametric data) and the median and interquartile range (non‐parametric data). Data were compared between vehicle and treatment (aficamten 2.0 mg/kg) across each of four time points (0, 6, 24, and 48 h postdose). When all data sets being compared were normally distributed, a repeated measures one‐way ANOVA was performed to identify differences between time points. When the repeated measures one‐way ANOVA returned a p < .05, the post hoc Tukey's multiple comparisons test was performed to identify which, if any, time points were statistically different from each other. When all data sets being compared were not normally distributed, a Friedman test was performed to identify differences between time points. When the Friedman test returned a p < .05, the post hoc Dunn's multiple comparisons test was performed to identify which, if any, time points were statistically different from each other. Normally distributed data are presented as mean ± standard deviation and non‐parametric data are presented as median (Interquartile range, IQR). For all statistical testing, a p < .05 was considered significant.
3. RESULTS
Five intact male purpose bred mixed breed Maine Coon cats with documented A31P MyBPC3 mutations (five heterozygous/0 homozygous positive) and a clinical diagnosis of occult hypertrophic obstructive cardiomyopathy were enrolled in the study. The mean age of cats was 4.2 ± 2.9 years. The mean IVS thickness was 6.6 ± 0.40 and the mean LVPW thickness was 5.42 ± 0.16 mm (mean ± SD).
All five cats received vehicle and aficamten at a dose of 2 mg/kg. In the cats receiving aficamten at 2 mg/kg, the LV fractional shortening (LVFS%) was reduced 45.2 and 38.5% from baseline at 6 and 24 h post‐treatment (p = .015, p = .006, respectively; Figure 1a). The reduction in LVFS% was due to increased LV systolic internal dimension (LVIDs) at 6 and 24‐h post‐treatment (p = .01, p = .008; Figure 1b), and aficamten did not affect LV diastolic internal dimension (LVIDd). Aficamten also reduced right heart function as assessed by TAPSE from baseline at 6 h (p = .012, Figure 1c). Left ventricular outflow tract Vmax, peak pressure gradient, and the number of obstructed cats were lower after aficamten (2 mg/kg), but these parameters did not reach statistical significance. Heart rate (HR) was significantly increased to 176 ± 9 bpm 6 h after aficamten administration (2 mg/kg; p = .014, Figure 1d). This finding was also noted in the vehicle treated cats with a significant increase in heart rate to 214 ± 26.94 at 6 h compared with baseline (p = .0434, Figure 2d). Lastly, isovolumic relaxation time (IVRT) was significantly reduced to 42.6 ± 6.8 at the 48 h timepoint after administration of aficamten (p = .014; Figure 1e). There were no additional statistically significant differences of vehicle treated patients from baseline echocardiographic values, supporting a pharmacologic effect (Figure 2a–c). Plasma concentrations of aficamten are found in Table 2. Complete echocardiographic data and comparisons are found in Tables 3 and 4, respectively, for vehicle and the 2 mg/kg treatment group.
FIGURE 1.
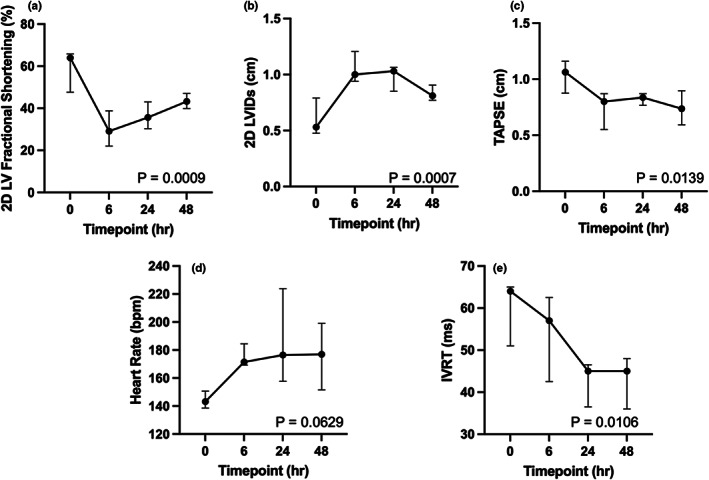
Selected parameters following administration of aficamten (2 mg/kg). (a) Left ventricular fractional shortening % (LVFS%); (b) Left ventricular systolic internal dimension (LVIDs); (c) Tricuspid annular plane systolic excursion (TAPSE); (d) Heart rate (HR); and (e) Isovolumic relaxation time (IVRT) are all shown at time 0, 6, 24, and 48 h after administration of aficamten (2 mg/kg). The median is denoted at each time point and the whiskers represent the interquartile range. The p values represent overall significance for the repeated measures ANOVA or Friedman's test.
FIGURE 2.
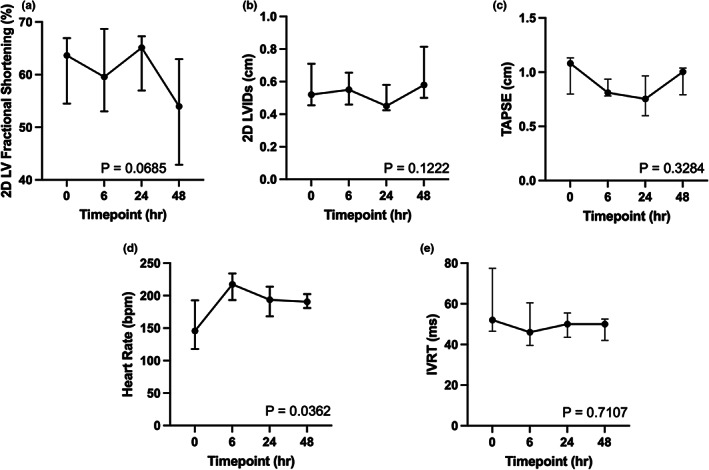
Selected parameters following administration of vehicle. (a) Left ventricular fractional shortening % (LVFS%); (b) Left ventricular systolic internal dimension (LVIDs); (c) Tricuspid annular plane systolic excursion (TAPSE); (d) Heart rate (HR); and (e) Isovolumic relaxation time (IVRT) are all shown at time 0, 6, 24, and 48 h after administration of vehicle. The median is denoted at each time point and the whiskers represent the interquartile range. The p values represent overall significance for the repeated measures ANOVA or Friedman's test
TABLE 2.
Plasma concentration of aficamten
|
6 h Post‐dose |
24 h Post‐dose |
48 h Post‐dose |
|
|---|---|---|---|
| 2 mg/kg (n = 5) | 0.408 ± 0.046 μM | 0.32 ± 0.015 μM | 0.233 ± 0.015 μM |
TABLE 3.
Treatment group: Vehicle alone
| Echocardiographic parameter | Hour 0 | Hour 6 | Hour 24 | Hour 48 | p‐Value |
|---|---|---|---|---|---|
| LA (long axis) | |||||
| Median (IQR) | 1.38 (1.33–1.40) | 1.29 (1.24–1.30) | 1.29 (1.27–1.32) | 1.34 (1.32–1.35) | |
| Mean ± SD | 1.36 ± 0.08 | 1.27 ± 0.04 | 1.29 ± 0.08 | 1.33 ± 0.05 | .311 |
| LVIDd (M mode) | 1.54 (1.53–1.56) | 1.32 (1.31–1.41) | 1.31 (1.30–1.31) | 1.36 (1.34–1.54) | |
| 1.52 ± 0.12 | 1.37 ± 0.14 | 1.32 ± 0.04 | 1.42 ± 0.17 | .0980 | |
| LVIDs (M mode) | 0.60 (0.57–0.62) | 0.57 (0.43–0.59) | 0.51 (0.47–0.52) | 0.61 (0.51–0.71) | |
| 0.59 ± 0.05 | 0.52 ± 0.12 | 0.50 ± 0.08 | 0.63 ± 0.20 | .2183 | |
| LV FS (M mode) | 61.04 (59.62–62.20) | 63.52 (55.30–69.50) | 60.77 (60.31–63.57) | 54.48 (47.79–57.85) | |
| 61.27 ± 3.63 | 62.07 ± 8.58 | 62.89 ± 5.88 | 55.29 ± 11.65 | .2907 | |
| LVIDd (2D) | 1.43 (1.43–1.56) | 1.40 (1.36–1.45) | 1.32 (1.29–1.33) | 1.36 (1.31–1.40) | |
| 1.46 ± 0.10 | 1.42 ± 0.08 | 1.32 ± 0.04 | 1.36 ± 0.09 | .060 | |
| LVIDs (2D) | 0.52 (0.48–0.68) | 0.55 (0.51–0.64) | 0.45 (0.43–0.54) | 0.58 (0.55–0.7) | |
| 0.57 ± 0.13 | 0.56 ± 0.10 | 0.49V0.09 | 0.6 ± 0.17 | .122 | |
| LV FS (2D) | 63.64 (56.41–66.43) | 59.56 (53.79–66.67) | 65.12 (60.58–66.41) | 53.97 (44.85–58.02) | |
| 61.29 ± 6.51 | 60.59 ± 8.01 | 62.73 ± 5.93 | 53.13 ± 10.71 | .069 | |
| LA:Ao | 1.25 (1.23–1.26) | 1.23 (1.18–1.35) | 1.19 (1.08–1.26) | 1.24 (1.20–1.30) | |
| 1.23 ± 0.11 | 1.25 ± 0.10 | 1.17 ± 0.10 | 1.25 ± 0.07 | .603 | |
| LVOT Vmax | 2.53 (2.24–2.84) | 2.33 (2.14–3.28) | 2.46 (2.21–2.78) | 2.37 (2.24–2.67) | |
| 2.50 ± 0.39 | 2.71 ± 0.71 | 2.50 ± 0.53 | 2.57 ± 0.53 | .924 | |
| LVOT PG | 25.60 (20.07–32.26) | 21.72 (18.32–43.03) | 24.21 (19.54–30.91) | 22.47 (20.07–28.52) | |
| 25.52 ± 7.61 | 30.93 ± 16.37 | 25.80 ± 10.63 | 27.27 ± 11.89 | .658 | |
| HR at peak LVOT | 145.63 (119.58–180.32) | 217.39 (214.29–221.40) | 193.55 (169.59–202.02) | 190.48 (190.39–198.15) | |
| 153.41 ± 38.85 | 214.42 ± 26.94 | 191.59 ± 24.31 | 191.40 ± 13.00 | .0362 | |
| Transmitral E | 0.71 (0.61–0.73) | 0.61 (0.59–0.64) | 0.56 (0.56–0.56) | 0.72 (0.69–0.75) | |
| 0.66 ± 0.13 | 0.62 ± 0.05 | 0.56 ± 0.00 | 0.72 ± 0.08 | ||
| Transmitral A | 0.54 (0.45–0.59) | 0.53 (0.46–0.60) | 0.44 (0.42–0.46) | 0.51 (0.40–0.61) | |
| 0.51 ± 0.14 | 0.53 ± 0.14 | 0.44 ± 0.05 | 0.51 ± 0.30 | ||
| E Deceleration | 64.00 (53.00–70.00) | 60.00 (59.50–67.50) | 71.50 (68.75–74.25) | 62.50 (60.25–64.75) | |
| 60.67 ± 17.24 | 64.67 ± 8.96 | 71.50 ± 7.78 | 62.50 ± 6.36 | ||
| IVRT | 52.00 (49.00–62.00) | 46.00 (42.00–48.00) | 50.00 (47.00–51.00) | 50.00 (44.00–50.00) | |
| 60.00 ± 19.58 | 49.20 ± 13.95 | 49.60 ± 7.23 | 47.80 ± 5.85 | .440 | |
| TD E' | 0.09 (0.08–0.09) | 0.04 (0.04–0.05) | 0.05 (0.05–0.05) | 0.07 (0.06–0.09) | |
| 0.09 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.00 | 0.07 ± 0.03 | ||
| TD A' | 0.06 (0.05–0.07) | 0.05 (0.05–0.06) | 0.05 (0.04–0.05) | 0.05 (0.05–0.06) | |
| 0.06 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | ||
| TD S′ | 0.08 (0.07–0.08) | 0.09 (0.08–0.09) | 0.07 (0.07–0.08) | 0.08 (0.08–0.09) | |
| 0.08 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.01 | .587 | |
| MAPSE | 0.48 (0.42–0.48) | 0.38 (0.33–0.45) | 0.38 (0.33–0.45) | 0.45 (0.39–0.46) | .210 |
| 0.46 ± 0.05 | 0.42 ± 0.12 | 0.42 ± 0.12 | 0.36 ± 0.18 | ||
| TAPSE | 1.08 (1.03–1.10) | 0.81 (0.80–0.88) | 0.75 (0.68–0.90) | 1.00 (0.85–1.03) | .372 |
| 0.99 ± 0.24 | 0.85 ± 0.09 | 0.78 ± 0.20 | 0.93 ± 0.14 | ||
| Auricular flow | 75.46 (65.80–78.30) | 58.30 (46.00–68.40) | 59.67 (56.00–76.30) | 59.30 (54.84–70.64) | |
| 68.77 ± 18.58 | 59.18 ± 14.75 | 67.45 ± 16.73 | 60.74 ± 14.44 | .678 | |
Note: Echocardiographic variables for the vehicle treatment group with reported mean ± standard deviation and median and interquartile range. The p value for variables that were statistically evaluated by either repeated measures one‐way ANOVA or Friedman's test are shown.
Abbreviations: 2D, two dimensional; E deceleration, deceleration time of the transmitral early filling wave; FS, fractional shortening; HR, heartrate; IVRT, isovolumetric relaxation time; LA, left atrium; LA; Ao, left atrium to aortic root ratio; LV, left ventricle; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVOT, left ventricular outflow tract; M mode, motion mode; MAPSE, mitral annular plane systolic excursion; PG, pressure gradient; TAPSE, tricuspid annular plane systolic excursion.TD A', tissue Doppler velocity of the active filling motion; TD E', tissue Doppler velocity of the early passive filling motion; TD S′ '’, tissue Doppler velocity of the systolic wave; Transmitral A, transmitral Doppler velocity of the active filling wave; Transmitral E, transmitral Doppler velocity of the early passive filling wave; Vmax, peak velocity.
TABLE 4.
Treatment group: 2 mg/kg aficamten
| Echocardiographic parameter | Hour 0 | Hour 6 | Hour 24 | Hour 48 | p‐Value |
|---|---|---|---|---|---|
| LA (long axis) | |||||
| Median (IQR) | 1.32 (1.31–1.33) | 1.33 (1.26–1.34) | 1.38 (1.36–1.39) | 1.19 (1.18–1.21) | |
| Mean ± SD | 1.30 ± 0.06 | 1.31 ± 0.06 | 1.38 ± 0.08 | 1.23 ± 0.09 | |
| LVIDd (M mode) | 1.52 (1.41–1.56) | 1.62 (1.58–1.68) | 1.46 (1.45–1.51) | 1.58 (1.54–1.66) | |
| 1.47 ± 0.11 | 1.61 ± 0.08 | 1.48 ± 0.10 | 1.55 ± 0.14 | .0816 | |
| LVIDs (M mode) | 0.56 (0.41–0.66) | 0.97 (0.96–1.23) | 0.93 (0.90–1.07) | 0.98 (0.78–1.00) | |
| 0.56 ± 0.18 | 1.06 ± 0.17 | 0.91 ± 0.20 | 0.92 ± 0.13 | <.0001 | |
| LV FS (M mode) | 64.10 (56.58–70.92) | 35.57 (25.60–40.12) | 40.40 (26.71–42.94) | 39.76 (37.97–41.35) | |
| 62.42 ± 9.80 | 34.09 ± 10.25 | 38.72 ± 12.93 | 41.04 ± 4.97 | .0006 | |
| LVIDd (2D) | 1.45 (1.42–1.47) | 1.51 (1.48–1.58) | 1.56 (1.50–1.60) | 1.55 (1.37–1.60) | |
| 1.45 ± 0.07 | 1.52 ± 0.08 | 1.53 ± 0.09 | 1.48 ± 0.19 | .443 | |
| LVIDs (2D) | 0.53 (0.50–0.70) | 1.00 (0.98–1.16) | 1.03 (0.90–1.04) | 0.81 (0.79–0.88) | |
| 0.61 ± 0.18 | 1.06 ± 0.14 | 0.97 ± 0.12 | 0.83 ± 0.07 | .0007 | |
| LV FS (2D) | 63.95 (51.72–64.79) | 29.08 (23.18–33.78) | 35.63 (33.33–42.03) | 43.23 (42.34–44.64) | |
| 58.19 ± 10.10 | 30.14 ± 9.13 | 36.48 ± 6.77 | 43.42 ± 4.28 | .0009 | |
| LA:Ao | 1.23 (1.20–1.31) | 1.34 (1.22–1.38) | 1.29 (1.23–1.36) | 1.34 (1.24–1.36) | |
| 1.25 ± 0.08 | 1.32 ± 0.11 | 1.31 ± 0.10 | 1.27 ± 0.14 | .476 | |
| LVOT Vmax | 2.11 (0.86–2.13) | 0.60 (0.57–0.63) | 0.72 (0.68–0.91) | 0.86 (0.77–0.89) | .123 |
| 2.02 ± 1.51 | 0.63 ± 0.15 | 0.88 ± 0.38 | 1.03 ± 0.62 | ||
| LVOT PG | 17.81 (2.96–18.15) | 1.44 (1.30–1.59) | 2.07 (1.85–3.31) | 2.96 (2.37–3.17) | .123 |
| 23.70 ± 31.45 | 1.65 ± 0.81 | 3.57 ± 3.32 | 5.49 ± 6.93 | ||
| HR at peak LVOT | 143.20 (140.19–147.06) | 171.43 (170.45–179.64) | 176.47 (167.13–202.70) | 176.99 (153.06–190.48) | |
| 144.33 ± 6.67 | 175.77 ± 8.71 | 187.87 ± 37.45 | 175.63 ± 24.55 | .0629 | |
| Transmitral E | 0.59 (0.55–0.59) | 0.79 | 0.61 | 0.45 | |
| 0.57 ± 0.04 | 0.79 | 0.61 | 0.45 | ||
| Transmitral A | 0.41 (0.41–0.43) | 0.58 | 0.48 | 0.50 | |
| 0.42 ± 0.02 | 0.58 | 0.48 | 0.50 | ||
| E Deceleration | 64.00 (63.00–66.00) | 50.00 | 77.00 | 61.00 | |
| 64.67 ± 3.06 | 50.00 | 77.00 | 61.00 | ||
| IVRT | 64.00 (56.00–64.00) | 57.00 (47.00–60.00) | 45.00 (38.00–46.00) | 45.00 (40.00–48.00) | |
| 59.20 ± 8.32 | 53.40 ± 10.83 | 42.20 ± 5.36 | 42.60 ± 6.77 | .011 | |
| TD E' | 0.06 (0.06–0.06) | 0.08 (0.07–0.09) | 0.09 (0.08–0.10) | 0.12 (0.11–0.13) | |
| 0.06 ± 0.01 | 0.08 ± 0.03 | 0.09 ± 0.01 | 0.12 ± 0.02 | ||
| TD A' | 0.05 (0.04–0.06) | 0.06 (0.06–0.06) | 0.06 (0.05–0.07) | 0.26 (0.17–0.35) | |
| 0.05 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.26 ± 0.26 | ||
| TD S′ | 0.07 (0.06–0.08) | 0.05 (0.05–0.05) | 0.06 (0.05–0.06) | 0.07 (0.06–0.07) | |
| 0.08 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.01 | .0614 | |
| MAPSE | 0.44 (0.43–0.49) | 0.37 (0.33–0.38) | 0.40 (0.34–0.41) | 0.33 (0.29–0.34) | |
| 0.45 ± 0.04 | 0.34 ± 0.07 | 0.36 ± 0.08 | 0.33 ± 0.05 | .0956 | |
| TAPSE | 1.06 (0.99–1.08) | 0.80 (0.59–0.82) | 0.84 (0.77–0.85) | 0.74 (0.62–0.83) | |
| 1.03 ± 0.18 | 0.73 ± 0.17 | 0.82 ± 0.05 | 0.74 ± 0.16 | .0139 | |
| Auricular flow | 64.06 (58.46–74.00) | 55.72 (48.70–57.00) | 54.40 (41.00–56.16) | 61.40 (60.00–68.80) | |
| 64.74 ± 17.09 | 55.81 ± 10.22 | 50.79 ± 12.25 | 63.74 ± 5.85 | .282 | |
Note: Echocardiographic variables for the 2 mg/kg aficamten treatment group with reported mean ± standard deviation and median and interquartile range. The p‐value for variables that were statistically evaluated by either repeated measures one‐way ANOVA or Friedman's test are shown.
Abbreviations: 2D, two dimensional; E deceleration, deceleration time of the transmitral early filling wave; FS, fractional shortening; M mode, motion mode; HR, heartrate; IVRT, isovolumetric relaxation time; LA, left atrium; LA; Ao, left atrium to aortic root ratio; LV, left ventricle; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVOT, left ventricular outflow tract; MAPSE, mitral annular plane systolic excursion; PG, pressure gradient; TAPSE, tricuspid annular plane systolic excursion; TD A', tissue Doppler velocity of the active filling motion; TD E', tissue Doppler velocity of the early passive filling motion; TD S′, tissue Doppler velocity of the systolic wave; Transmitral A, transmitral Doppler velocity of the active filling wave; Transmitral E, transmitral Doppler velocity of the early passive filling wave; Vmax, peak velocity.
4. DISCUSSION
A research colony of mixed breed Maine Coon cats with A31P MBPC mutations and a clinical diagnosis of HOCM was used to demonstrate effects of a single oral dose of a novel cardiac myosin inhibitor, aficamten. In healthy rats and dogs, aficamten has been shown to reduce cardiac fractional shortening in a dose‐dependent manner (Chuang et al., 2021). Treatment with a single dose of aficamten in HOCM cats resulted in significantly reduced LVFS% secondary to increased LVIDs and preserved LVIDd as well as a significantly reduced IVRT, suggesting improvement in diastolic function. Following drug administration, LVOT Vmax, pressure gradient, and number of cats with LVOT were reduced, but these parameters did not reach statistical significance. This is thought to be due to the small number of cats in the study. At 6 h following the administration of aficamten, HR was significantly increased which is thought to represent a reflex tachycardia from reduced systolic function. This finding supports that the optimal therapeutic dose is likely less than 2 mg/kg and concurrent studies demonstrate similar beneficial effects without significant changes in heart rate at a lower doses (Oldach et al., 2020). In the five cats evaluated in this study, a significant increase in heart rate was also noted in the vehicle at 6 h compared with baseline, suggesting the possibility of a non‐pharmacologic effect on heart rate.
Hypertrophic cardiomyopathy is the most common disease in feline patients as well as the most prevalent inherited cardiac disease in human beings with an estimated prevalence of up to 1:500 (0.2%) in the general population (Zamorano et al., 2014). The identification and treatment of LVOT obstruction are important in humans with HCM as it likely contributes to symptoms and is an independent predictor of progression to severe symptoms of heart failure and death (Maron et al., 2003). Current therapies including beta‐blockers and calcium channels blockers have not shown clear benefits in randomized clinical trials in the human population (Olivotto et al., 2013) and have also failed to demonstrate a clinical benefit in cats with HCM (Coleman et al., 2020). In patients that continue to experience shortness of breath, angina, or syncope despite current medical options, surgery to relieve obstruction can be considered and may relieve associated symptoms and normalize life expectancy (Howell & Bradlow, 2015). However, all of the aforementioned therapies are focused on relieving symptoms rather than targeting the underlying pathophysiology of HCM. Although worse outcomes are not proven in cats with LVOT obstructions, the identification of symptoms in cats prior to heart failure or thromboembolic events remains challenging and this may represent limitations in the definition (Luis Fuentes et al., 2020) of LVOTO or identification of subtle signs.
The development of novel cardiac myosin inhibitors is a unique and promising new frontier for targeted therapies in affected individuals and may improve clinical signs as well as prevent deleterious effects of abnormal actin and myosin interactions, leading to reduced histopathologic changes and delayed progression of disease. A study in mice with HCM demonstrated the ability of another small molecule inhibitor, mavacamten, to significantly reduce the hallmark histopathologic features of fibrosis and myocardial disarray when administered prior to the development of significant hypertrophy (Green et al., 2016). A previous study of mavacamten in this feline research colony also demonstrated reduction of LVOTO using a provokable model of isoproterenol induced LVOTO under anesthesia (Stern et al., 2016). These novel myosin inhibitors are currently being evaluated in human HCM cohorts. Safety and tolerability of aficamten was demonstrated in a Phase 1 study in healthy adults, allowing advancement to a Phase 2 study (REDWOOD‐HCM) evaluating various doses of aficamten in patients with HOCM. The REDWOOD‐HCM clinical trial demonstrated statistically significant reductions from baseline compared with placebo in both the mean resting and provoked left ventricular (LV) outflow tract pressure gradients without generating safety concerns (adverse events were balanced between the treatment arms). There were also associated significant improvements in serum NT‐proBNP levels and a numeric and dose‐dependent improvement in heart failure functional class (Maron, 2021). A Phase 3 trial evaluating the impact of aficamten on exercise capacity in patients with symptomatic obstructive HCM (SEQUOIA‐HCM) is currently underway. EXPLORER‐HCM, the Phase 3 clinical trial using mavacamten, demonstrated improved exercise capacity, symptom burden, and functional class in parallel with significant improvements in LVOTO (Olivotto et al., 2020). A second Phase 3 study (VALOR‐HCM) has completed enrollment and will report on the treatment effect of mavacamten in patients referred for septal reduction procedures. These activities and study results highlight the intense interest and the ongoing clinical need for continued investigation of these and additional small molecule inhibitors, while demonstrating a unique opportunity for valuable therapeutic potential in the preclinical and clinical stages.
Several limitations to this study exist. There is the possibility of type II error given the small sample size of five cats. The temperament of the colony cats and drug administration by oral gavage require significant sedation which may impact functional parameters. To minimize these effects, a 3 h recovery period was allowed following sedation with alfaxalone for oral gavage and a standardized sedation protocol (butorphanol and acepromazine) that previously demonstrated minimal effects on echocardiographic variables (Ribas et al., 2015; Ward et al., 2012) was used for each evaluation. The tachycardia noted 6 h following the administration of aficamten, coupled with significant decreases in systolic function, may represent reflex tachycardia; however, the cats were not instrumented with blood pressure monitoring equipment, limiting this assessment. The significant increase in heart rate in vehicle treated cats at this time point supports the possibility that this increase was not a pharmacologic effect of aficamten. A period effect of drug administration was also not assessed in this cross over study.
The development of novel small molecule inhibitors is an exciting frontier for the advancement of therapeutic options in the management of preclinical HCM and the current study further highlights the utility of this feline model in evaluation of novel therapies that may benefit the human population. Additional studies are indicated to determine the optimal dose of aficamten and to evaluate long‐term treatment effects in a clinical population.
5. CONCLUSION
Aficamten reduces LV systolic function via increased LVIDs with preserved LVIDd. Aficamten successfully reduced LV pressure gradient and reduced incidence of LVOTO. At the highest dose (2 mg/kg) an increased HR, reduced IVRT, and reduced right ventricular systolic function (TAPSE) were observed. These findings suggest that the target dose for future chronic dosing studies in cats with HCM could be within the 0.3–1 mg/kg dose range. Studies investigating the chronic effects of target dosing are warranted and this translational model of hypertrophic cardiomyopathy may provide valuable insight to the investigation of this novel therapy.
AUTHOR CONTRIBUTIONS
JAS, SPH, and DTH conceived of the study. JAS, MSO, ANS, JLK, VR, and SF carried out the experiments and data analysis. ANS, VR, JAS, and DTH wrote the manuscript. SPH, FIM, and BPM critically reviewed and revised the manuscript.
FUNDING INFORMATION
This study was funded by Cytokinetics, Inc.
CONFLICT OF INTEREST
D.T.H., B.P.M., and F.I.M. are the employees of Cytokinetics and were financially compensated for their work.
ETHICAL APPROVAL
This research involved animal subjects and it conforms with ARRIVE guidelines. Research was conducted in accordance with the University of California, Davis, IACUC (Protocol number 20565).
ACKNOWLEDGEMENT
We would like to acknowledge the expert technical assistance of Carina Gonzalez and Maria Montano for their role in completing this study.
Sharpe, A. N. , Oldach, M. S. , Kaplan, J. L. , Rivas, V. , Kovacs, S. L. , Hwee, D. T. , Morgan, B. P. , Malik, F. I. , Harris, S. P. , & Stern, J. A. (2023). Pharmacokinetics of a single dose of Aficamten (CK‐274) on cardiac contractility in a A31P MYBPC3 hypertrophic cardiomyopathy cat model. Journal of Veterinary Pharmacology and Therapeutics, 46, 52–61. 10.1111/jvp.13103
DATA AVAILABILITY STATEMENT
All relevant data are supplied within the manuscript.
REFERENCES
- Abbott, J. A. , & Maclean, H. N. (2006). Two‐dimensional echocardiographic assessment of the feline left atrium. Journal of Veterinary Internal Medicine, 20, 111–119. [DOI] [PubMed] [Google Scholar]
- Alsulami, K. , & Marston, S. (2020). Small molecules acting on myofilaments as treatments for heart and skeletal muscle diseases. International Journal of Molecular Sciences, 21(24), 1–31. 10.3390/ijms21249599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argirò, A. , Zampieri, M. , Berteotti, M. , Marchi, A. , Tassetti, L. , Zocchi, C. , Iannone, L. , Bacchi, B. , Cappelli, F. , Stefàno, P. , Marchionni, N. , & Olivotto, I. (2021). Emerging medical treatment for hypertrophic cardiomyopathy. Journal of Clinical Medicine, 10(5), 951. 10.3390/jcm10050951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, L. M. , Tumbarello, D. A. , Kendrick‐Jones, J. , & Buss, F. (2013). Small‐molecule inhibitors of myosin proteins. Future Medicinal Chemistry, 5(1), 41–52. 10.4155/fmc.12.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon, J. A. (2011). Veterinary echocardiography (2nd ed.). Wiley‐Blackwell. [Google Scholar]
- Chetboul, V. , Carlos Sampedrano, C. , Tissier, R. , Gouni, V. , Saponaro, V. , & Nicolle, A. P. (2006). Quantitative assessment of velocities of the annulus of the left atrioventricular valve and left ventricular free wall in healthy cats by use of two‐dimensional color tissue Doppler imaging. American Journal of Veterinary Research, 67(2), 250–258. 10.2460/ajvr.67.2.250 [DOI] [PubMed] [Google Scholar]
- Chuang, C. , Collibee, S. , Ashcraft, L. , Wang, W. , Vander Wal, M. , Wang, X. , Hwee, D. T. , Wu, Y. , Wang, J. , Chin, E. R. , Cremin, P. , Zamora, J. , Hartman, J. , Schaletzky, J. , Wehri, E. , Robertson, L. A. , Malik, F. I. , & Morgan, B. P. (2021). Discovery of Aficamten (CK‐274), a next‐generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. Journal of Medicinal Chemistry, 64(19), 14142–14152. 10.1021/acs.jmedchem.1c01290 [DOI] [PubMed] [Google Scholar]
- Coleman, A. E. , DeFrancesco, T. C. , Griffiths, E. H. , Lascelles, B. D. X. , Kleisch, D. J. , Atkins, C. E. , & Keene, B. W. (2020). Atenolol in cats with subclinical hypertrophic cardiomyopathy: A double–clinical trial of effect on quality of life, activity, and cardiac biomarkers. Journal of Veterinary Cardiology, 30, 77–91. 10.1016/j.jvc.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Freeman, L. M. , Rush, J. E. , Stern, J. A. , Huggins, G. S. , & Maron, M. S. (2017). Feline hypertrophic cardiomyopathy: A spontaneous large animal model of human HCM. Cardiology Research, 8(4), 139–142. 10.14740/cr578w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, E. M. , Wakimoto, H. , Anderson, R. L. , Evanchik, M. J. , Gorham, J. M. , Harrison, B. C. , Henze, M. , Kawas, R. , Oslob, J. D. , Rodriguez, H. M. , & Song, Y. (2016). A small‐molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science, 351(6273), 617–621. 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, N. , & Bradlow, W. (2015). Surgical management of left ventricular outflow obstruction in hypertrophic cardiomyopathy. Echo Research and Practice, 2(1), R37–R44. 10.1530/ERP-15-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson, M. D. , Meurs, K. M. , & Harris, S. P. (2015). The genetic basis of hypertrophic cardiomyopathy in cats and humans. Journal of Veterinary Cardiology, 17(Suppl 1), S53–S73. 10.1016/j.jvc.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson, M. D. , Meurs, K. M. , Munro, M. J. , Kittleson, J. A. , Liu, S. K. , Pion, P. D. , & Towbin, J. A. (1999). An animal model of human disease: Cardiomyopathy. Circulation, 99, 3172–3180. [DOI] [PubMed] [Google Scholar]
- Luis Fuentes, V. , Abbott, J. , Chetboul, V. , Côté, E. , Fox, P. R. , Häggström, J. , Kittleson, M. D. , Schober, K. , & Stern, J. A. (2020). ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats, 34, 1062–1077. 10.1111/jvim.15745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron, B. J. , & Fox, P. R. (2015). Hypertrophic cardiomyopathy in man and cats. Journal of Veterinary Cardiology, 17, S6–S9. 10.1016/j.jvc.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Maron, M. (2021). Heart Failure Society of America annual scientific meeting. Redwood HCM . [Google Scholar]
- Maron, M. S. , Olivotto, I. , Betocchi, S. , Casey, S. A. , Lesser, J. R. , Losi, M. A. , Cecchi, F. , & Maron, B. J. (2003). Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. The New England Journal of Medicine, 348(4), 295–303. 10.1155/2015/481245 [DOI] [PubMed] [Google Scholar]
- Oldach, M. S. , Hwee, D. T. , Ontiveros, E. S. , Fousse, S. L. , Gonzalez, C. , Morgan, B. P. , Malik, F. I. , Harris, S. P. , & Stern, J. A. (2020). Abstract 14254: Pharmacodynamic effects of a single dose of CK‐3773274 in cats with hypertrophic cardiomyopathy. Circulation, 142(Suppl_3), 17.32628549 [Google Scholar]
- Oldach, M. S. , Ueda, Y. , Ontiveros, E. S. , Fousse, S. L. , Harris, S. P. , & Stern, J. A. (2019). Cardiac effects of a single dose of pimobendan in cats with hypertrophic cardiomyopathy; a randomized, placebo‐controlled, crossover study. Frontiers in Veterinary Science, 6, 1–8. 10.3389/fvets.2019.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivotto, I. , Oreziak, A. , Barriales‐Villa, R. , Abraham, T. P. , Masri, A. , Garcia‐Pavia, P. , Saberi, S. , Lakdawala, N. K. , Wheeler, M. T. , Owens, A. , Kubanek, M. , Wojakowski, W. , Jensen, M. K. , Gimeno‐Blanes, J. , Afshar, K. , Myers, J. , Hegde, S. M. , Solomon, S. D. , Sehnert, A. J. , … Yamani, M. (2020). Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet, 396(10253), 759–769. 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- Olivotto, I. , Tomberli, B. , Spoladore, R. , Mugelli, A. , Cecchi, F. , & Camici, P. G. (2013). Hypertrophic cardiomyopathy: The need for randomized trials. Global Cardiology Science and Practice, 2013(3), 31–248. 10.5339/gcsp.2013.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie, L. H. (2004). Myocardial contraction and relaxation. In Wilkins L. W. (Ed.), Heart physiology: From cell to circulation (4th ed., pp. 221–245). Lippincott Williams & Wilkins. [Google Scholar]
- Payne, J. R. , Brodbelt, D. C. , & Luis, F. V. (2015). Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). Journal of Veterinary Cardiology, 17, S244–S257. 10.1016/j.jvc.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Ribas, T. , Bublot, I. , Junot, S. , Beaufrère, H. , Rannou, B. , Gagnière, P. , Cadoré, J. L. , & Pariaut, R. (2015). Effects of intramuscular sedation with alfaxalone and butorphanol on echocardiographic measurements in healthy cats. Journal of Feline Medicine and Surgery, 17(6), 530–536. 10.1177/1098612X14551187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober, K. E. , & Chetboul, V. (2015). Echocardiographic evaluation of left ventricular diastolic function in cats: Hemodynamic determinants and pattern recognition. Journal of Veterinary Cardiology, 17, S102–S133. 10.1016/j.jvc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Schober, K. E. , & Maerz, I. (2005). Doppler echocardiographic assessment of left atrial appendage flow velocities in normal cats. Journal of Veterinary Cardiology, 7(1), 15–25. 10.1016/j.jvc.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Stern, J. A. , Markova, S. , Ueda, Y. , Kim, J. B. , Pascoe, P. J. , Evanchik, M. J. , Green, E. M. , & Harris, S. P. (2016). A small molecule inhibitor of sarcomere contractility acutely relieves left ventricular outflow tract obstruction in feline hypertrophic cardiomyopathy. PLoS One, 11(12), e0168407. 10.1371/journal.pone.0168407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J. L. , Schober, K. E. , Fuentes, V. L. , & Bonagura, J. D. (2012). Effects of sedation on echocardiographic variables of left atrial and left ventricular function in healthy cats. Journal of Feline Medicine and Surgery, 14(10), 678–685. 10.1177/1098612X12447729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorano, J. L. , Anastasakis, A. , Borger, M. A. , Borggrefe, M. , Cecchi, F. , Charron, P. , Hagege, A. A. , Lafont, A. , Limongelli, G. , Mahrholdt, H. , McKenna, W. J. , Mogensen, J. , Nihoyannopoulos, P. , Nistri, S. , Pieper, P. G. , Pieske, B. , Rapezzi, C. , Rutten, F. H. , Tillmanns, C. , & Watkins, H. (2014). 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal, 35(39), 2733–2779. 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are supplied within the manuscript.


