Abstract
Background
Coronary artery bypass grafting (CABG) in the setting of an acute coronary syndrome is a high‐risk procedure, and the best strategy for myocardial revascularisation remains debated. This study compares the 30‐day mortality benefit of on‐pump CABG (ONCAB), off‐pump CABG (OPCAB), and on‐pump beating heart CABG (OnBHCAB) strategies.
Methods
A systematic search of three electronic databases was conducted for studies comparing ONCAB with OPCAB or OnBHCAB in patients with acute coronary syndrome (ACS). The primary outcome, 30‐day mortality, was compared using a Bayesian hierarchical network meta‐analysis (NMA). A random effects consistency model was applied, and direct and indirect comparisons were made to determine the relative effectiveness of each strategy on postoperative outcomes.
Results
One randomised controlled trial and eighteen observational studies fulfilling the inclusion criteria were identified. A total of 4320, 5559, and 1962 patients underwent ONCAB, OPCAB, and OnBHCAB respectively. NMA showed that OPCAB had the highest probability of ranking as the most effective treatment in terms of 30‐day mortality (odds ratio [OR], 0.50; 95% credible interval [CrI], 0.23−1.00), followed by OnBHCAB (OR, 0.62; 95% CrI, 0.20−1.57), however the 95% CrI crossed or included unity. A subgroup NMA of nine studies assessing only acute myocardial infarction (AMI) patients demonstrated a 72% reduction in likelihood of 30‐day mortality after OPCAB (CrI, 0.07−0.83). No significant increase in rate of stroke, renal dysfunction or length of intensive care unit stay was found for either strategy.
Conclusions
Although no single best surgical revascularisation approach in ACS patients was identified, the significant mortality benefit with OPCAB seen with AMI suggests high acuity patients may benefit most from avoiding further myocardial injury associated with cardiopulmonary bypass and cardioplegic arrest.
Keywords: acute coronary syndrome, coronary artery bypass grafting, OnBHCAB, ONCAB, OPCAB
1. INTRODUCTION
Coronary artery bypass grafting (CABG) surgery in the setting of an acute coronary syndrome (ACS), defined as a continuum from unstable angina to non‐ST‐segment elevation myocardial infarction to ST‐segment elevation myocardial infarction, is a high‐risk procedure given the high mortality compared to stable angina patients. 1 Though the majority of ACS patients are now treated first line with percutaneous coronary intervention (PCI), or thrombolytic therapy when PCI is not available, there remains a role for surgical revascularization in select patients. Such patients include those with symptoms refractory to medical therapy, haemodynamic instability, left main or triple vessel disease, complex coronary anatomy not suitable for PCI, ongoing ischemia despite attempts at PCI or failed PCI. 1 , 2
Following the emergence of alternative techniques to the conventional use of cardiopulmonary bypass (CPB) and cardioplegic arrest in the last 30 years, the best surgical strategy for CABG remains debated. 1 , 3 It is believed that keeping the heart beating during surgery provides myocardial protection by preserving coronary flow, hence reducing global myocardial ischemic injury, avoiding reperfusion injury, allowing earlier revascularisation of the culprit lesion and reducing myocardial oedema. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Off‐pump CABG (OPCAB) also avoids the well‐known deleterious effects of CPB and cardioplegic arrest, such as aortic manipulation and cross clamping, haemodilution and hypothermia, and allows for a more physiologic way of maintainin the functional integrity of the cardiovascular system. 2 On‐pump beating heart CABG (OnBHCAB), on the other hand, may reduce CPB time compared to conventional on‐pump CABG (ONCAB) and avoids the haemodynamic effects of cardiac manipulation associated with OPCACB. 6
On the other hand, the main drawbacks of beating heart surgery highlighted in the literature revolve around the more technically demanding nature of this approach, which has raised doubts over the completeness of revascularisation and long‐term patency of grafts achieved with OPCAB. 4 , 5 , 6 In the setting of ACS, some also believe that beating heart surgery may not be feasible or tolerated in haemodynamically compromised patients, especially if there is a need for extreme upward retraction of the heart to revascularise the left circumflex territory. 8 , 11 The high mortality and morbidity associated with emergent conversion of off‐pump onto on‐pump has also discouraged some surgeons from pursuing this approach in already high‐risk patients. 12 , 13 , 14 Nevertheless, it is largely accepted that with continuous experience and skill, both early and late outcomes of OPCAB are similar to ONCAB, as seen in results from dedicated high‐volume centres. 2 , 15 , 16 , 17
The primary aim of this study is to compare the 30‐day mortality benefit of OPCAB and OnBHCAB with ONCAB in patients presenting with ACS requiring emergent or urgent CABG.
2. MATERIAL AND METHODS
This study has been conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines for network meta‐analysis (NMA). 18
2.1. Literature search
Three electronic databases, MEDLINE via PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL), were systematically searched from their date of inception to October 2021. Search terms for the patient population included “acute coronary syndrome” OR “unstable angina pectoris” OR “myocardial infarction”; for the control intervention search terms included “coronary artery bypass” OR “heart muscle revascularisation”; and for the experimental intervention included “on pump” OR “cardiopulmonary bypass” OR “off pump” OR “on pump beating heart” (Supporting Information: Appendix A). Reference lists from previous reviews, meta‐analyses and included articles were also reviewed for suitable articles. The process of study selection is depicted in the flow chart seen in Supporting Information: Figure S1. 19
2.2. Study selection
Only studies with ACS patients whom underwent emergent or urgent CABG via ONCAB, OPCAB or OnBHCAB approach were included. Included studies compared two or more of these approaches in reporting 30‐day or in‐hospital mortality, which was the primary outcome for this study. All comparative study designs were included regardless of randomisation of the groups. Studies were excluded if the study population was non‐ACS patients (i.e., elective surgery), and if the CABG was a redo operation, combined with other cardiothoracic surgery or performed with robotic or minimally invasive techniques. Where the same cohort of patients was included in a later updated study, only the latest or most complete cohort data was included.
2.3. Data collection
Two authors (B. H. and M. W.) independently performed the study selection and data extraction using a preformed template. Data items pertaining to study characteristics, patient characteristics, operative details, primary outcome, secondary outcomes and late outcomes were collected (Supporting Information: Appendix B). Any discrepancies between the two reviewers were resolved by means of discussion and consensus.
2.4. Quality analysis
Risk of bias in included studies was assessed using the Risk Of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) tool. 20 This tool appraises bias in seven domains: bias due to confounding, selection of participants into study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Using this tool, each study was classified as either low, moderate, serious or critical risk.
2.5. Statistical methods
Baseline demographic data was pooled, with continuous data presented as a mean and standard deviation and all discrete data displayed as a raw value and a percentage of total number of available data points. Where values were reported as a median and range, mean and standard deviation estimates were calculated using methods described by Hozo et al. 21 Pairwise meta‐analyses using the Mantel−Haenszel method in a random effects model was firstly performed in Review Manager (version 5.4.1, The Cochrane Collaboration, 2020). Heterogeneity was assessed using the I2 statistic obtained in each pair‐wise meta‐analysis; a value of more than 50% was deemed to be a significant level of heterogeneity between studies. A Bayesian hierarchical NMA was then performed using the “gemtc” package 22 in R (version 4.1.2, R Foundation for Statistical Computing). A network plot of all treatments arms was constructed to visually represent direct comparisons, with the size of the node and thickness of each connecting line representing the number of participants and number of studies assessed, respectively. A random effects model was used for all outcomes and subgroup analyses. Analysis was performed using the Markov‐chain Monte Carlo methods, based on 100,000 iterations with a burn‐in of 5000. Model convergence was evaluated using the trace plots and the Gelman−Rubin statistic. Network inconsistency was evaluated using a node‐splitting analysis, with a p value less than .05 considered significant for inconsistency between included studies. Dichotomous and continuous outcomes were reported as an odds ratios (OR) and mean difference, respectively, with a 95% credible interval (CrI). Findings were taken as significant where the 95% CrI did not include unity. Relative treatment effects were displayed as ranking probability graphs. Meta‐regression was conducted to evaluate the potential influence of study‐level covariates on the magnitude of treatment effect sizes in the NMA model. Meta‐regression for patient‐level covariates were not performed due to the risk of ecological bias. 23
3. RESULTS
The literature search identified a total of 1797 studies, of which 19 were identified to meet the inclusion criteria and included in the NMA (Table 1). Thirteen of these studies compared ONCAB and OPCAB, five studies compared ONCAB and OnBHCAB and one study compared all three strategies (Figure 1). One of these studies is a randomised controlled trial (RCT), 5 whereas the remaining eighteen are observational studies. Of the observational studies, five performed propensity score analyses to correct for the imbalance between groups (Table 1).
Table 1.
Characteristics of included studies
| Author, year published | Country | Study period | Study design | Type of ACS | Study arms | Patients | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ben‐Gal et al. (2011) a , 4 | USA | 2003−2005 | NR‐R | UA, NSTEMI | ONCAB | OPCAB | ‐ | 660 | 220 | ‐ |
| Biancari et al. (2008) a , 17 | Finland | 2003−2007 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 94 | 94 | ‐ |
| Darwazah et al. (2009) 6 | Israel | 1999−2005 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OPCAB | ‐ | 34 | 45 | ‐ |
| Emerson et al. (2015) 2 | USA | 2000−2011 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 111 | 160 | ‐ |
| Fattouch et al. (2011) 5 | Italy | 2002−2007 | RCT | AMI (STEMI) | ONCAB | OPCAB | ‐ | 145 | 62 | ‐ |
| Hirose et al. (2002) 24 | Japan | 1991−2001 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OPCAB | ‐ | 129 | 30 | ‐ |
| Kawamoto et al. (2018) 8 | Japan | 2008−2012 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | OnBHCAB | 1756 | 4464 | 1647 |
| Kaya et al. (2010) 43 | Turkey | 2006−2008 | NR‐P | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 56 | 142 | ‐ |
| Kerendi et al. (2005) 25 | USA | 1996−2003 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OPCAB | ‐ | 570 | 44 | ‐ |
| Locker et al. (2000) 26 | Israel | 1992−1998 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OPCAB | ‐ | 37 | 40 | ‐ |
| Martinez et al. (2010) 27 | Singapore | 2002−2007 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 68 | 68 | ‐ |
| Neumann et al. (2020) 28 | Germany | 2015−2016 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 109 | 96 | ‐ |
| Ochi et al. (2003) 29 | Japan | 1998−2001 | NR‐R | UA, NSTEMI, STEMI | ONCAB | OPCAB | ‐ | 47 | 25 | ‐ |
| Onorati et al. (2005) 30 | Italy | 2002−2004 | NR‐P | UA, NSTEMI | ONCAB | OPCAB | ‐ | 126 | 69 | ‐ |
| Afasiabirad et al. (2015) a , 7 | Iran | 2003−2011 | NR‐P | UA, NSTEMI, STEMI | ONCAB | OnBHCAB | ‐ | 157 | 157 | ‐ |
| Izumi et al. (2006) 9 | Japan | 1998−2004 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OnBHCAB | ‐ | 16 | 15 | ‐ |
| Miyahara et al. (2008) 10 | Japan | 1999−2005 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OnBHCAB | ‐ | 23 | 38 | ‐ |
| Tsutsumi et al. (2019) a , 31 | Japan | 1998−2017 | NR‐R | AMI (NSTEMI, STEMI) | ONCAB | OnBHCAB | ‐ | 28 | 28 | ‐ |
| Zhu et al. (2019) a , 32 | Australia | 2001−2015 | NR‐P | AMI (NSTEMI, STEMI) | ONCAB | OnBHCAB | ‐ | 154 | 77 | ‐ |
Abbreviations: ACS, Acute coronary syndrome; AMI; Acute myocardial infarction; NR‐P, Non‐randomised prospective; NR‐R, Non‐randomised retrospective; NSTEMI, Non‐ST elevated myocardial infarction; RCT, Randomised control trial; STEMI, ST elevated myocardial infarction; UA, Unstable angina.
Studies that performed propensity score analysis.
Figure 1.
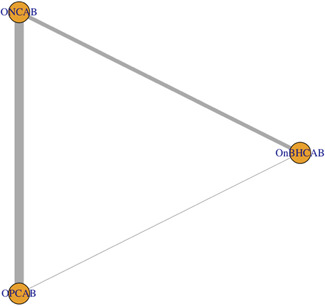
Network diagram of direct comparisons. The thickness of each arm and size of each node represents the number of data points in that comparison and number of included studies, respectively. OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
3.1. Baseline demographics
A total of 11,841 patients were included in this analysis, of which 4320 underwent ONCAB, 5559 underwent OPCAB and 1962 underwent OnBHCAB. The mean age of all three cohorts was 64 years and approximately three‐quarters were male. The mean additive EuroSCORE I was lowest in the ONCAB group (8.6 ± 3.4) and highest in the OPCAB group (11.3 ± 10.9). The number of diseased vessels and ejection fraction, however, were comparable across groups. The OnBHCAB group had relatively higher rates of patients in cardiogenic shock and requiring preoperative intra‐aortic balloon pump (IABP) (Table 2). Primary outcome data for each individual study is listed in Supporting Information: Figure S2. The mean rate of complete revascularisation, defined as the number of distal anastomoses divided by the number of diseased vessels, in the ONCAB group was 91.6% (7/19 studies), 80.5% in OPCAB group (5/14 studies) and 89.5% in the OnBHCAB group (2/5 studies).
Table 2.
Pooled available patient baseline data by revascularisation strategy
| Characteristic | ONCAB | OPCAB | OnBHCAB |
|---|---|---|---|
| Total patients, n | 4320 | 5559 | 1962 |
| Male, n (%) | 3274 (75.8%) | 4225 (76%) | 1471 (75.0%) |
| Age, mean ± SD (n/N) | 64.4 ± 3.9 (4320/4320) | 64.8 ± 5.1 (5559/5559) | 65.9 ± 4.8 (1962/1962) |
| No. of diseased vessels, mean ± SD (n/N) | 2.8 ± 0.3 (1053/4320) | 2.6 ± 0.2 (412/5559) | 2.6 ± 1.2 (38/1962) |
| EuroSCORE I, mean ± SD (n/N) | 8.6 ± 3.4 (487/4320) | 11.3 ± 10.9 (350/5559) | 9.75 ± 0.2 (115/1962) |
| Renal failure, n (%) | 386/3453 (11.2%) | 765/5043 (15.2%) | 295/1647 (17.9%) |
| Ejection fraction, % mean ± SD (n/N) | 43.8 ± 6.0 (1718/4320) | 43.2 ± 8.5 (581/5559) | 44 ± 8.5 (185/1962) |
| Cardiogenic shock, n (%) | 567/2991 (19.0%) | 783/4822 (16.2%) | 537/1790 (30%) |
| Preoperative IABP, n (%) | 396/1478 (26.8%) | 160/710 (22.5%) | 69/158 (43.7%) |
| Prior PCI, n (%) | 431/1957 (22.0%) | 1099/4489 (24.5%) | 437/1724 (25.3%) |
Abbreviations: IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention; SD, standard deviation.
3.2. Risk of bias
The assessment of risk of bias for each individual study using the ROBINS‐I tool is displayed in Supporting Information: Figure S3. Bias due to confounding and bias in classification of interventions were the two domains contributing most to risk of bias, owing to the inherent differences between groups, and lack of defining criteria for selection into either intervention group. Potential confounders contributing to the differences between groups were with regard to patient risk and severity and location of disease. Studies which performed propensity score analyses to attempt correction of any imbalances scored lower in assessment of risk of bias. Overall, one study 5 was considered low risk of bias due to randomisation, five studies 4 , 7 , 17 , 31 , 32 were considered moderate risk of bias and the remaining were considered serious risk of bias due to factors discussed above. No articles were deemed to be at a critical risk of bias.
3.3. Pairwise meta‐analysis
Pairwise meta‐analysis of two arms of the NMA favoured the OPCAB or OnBHCAB method in comparison to conventional ONCAB, and this bordered on statistical significance for OPCAB versus ONCAB (Figure 2). There was low heterogeneity present in the comparison between ONCAB and OPCAB (I 2 = 19%), however high heterogeneity was present in the direct comparison between OnBHCAB and ONCAB (I 2 = 81%) (Figure 3).
Figure 2.
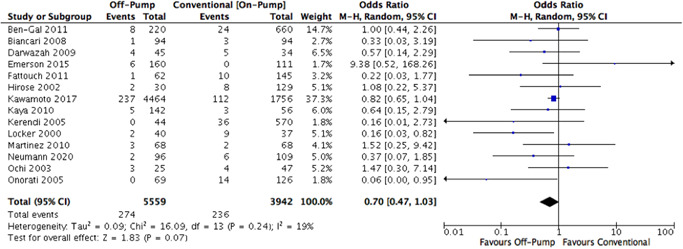
ONCAB versus OPCAB pairwise meta‐analysis forest plot. ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
Figure 3.
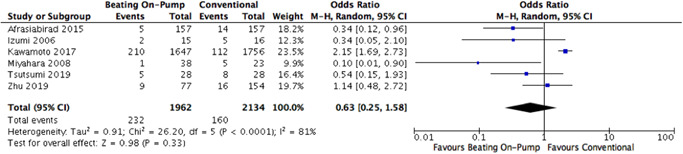
ONCAB versus OnBHCAB pairwise meta‐analysis forest plot. OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump.
Furthermore, six studies comparing OPCAB and ONCAB reported mortality data at 1‐year follow‐up. Though survival beyond 1 year favoured ONCAB (OR, 1.16; 95% confidence interval [CI], 0.80−1.68), this was not statistically significant (Supporting Information: Figure S4).
3.4. Network meta‐analysis
3.4.1. Primary outcome
For the primary outcome, the NMA random effects model demonstrated that the odds of 30‐day mortality after OnBHCAB is 0.50 (95% CrI, 0.23−1.00) compared to ONCAB. Similarly, the odds after OPCAB is 0.62 (95% CrI, 0.20−1.57) compared to ONCAB. The odds of early mortality after OnBHCAB compared to OPCAB is 1.23 (95% CrI, 0.35−3.84). However, the CrI crossed or included unity for all relative effect measures in this analysis (Table 3). The ranking probability graph obtained demonstrated OPCAB had the highest probability (~65%) of being ranked the most effective revascularisation strategy with respect to 30‐day mortality, followed by OnBHCAB (Figure 4). Node splitting analysis did not suggest any inconsistency within the network (p = .315) (Supporting Information: Figure S5).
Table 3.
Relative effects table for 30‐day mortality in all ACS patients
| Control | Experiment | ||
|---|---|---|---|
| OnBHCAB | ONCAB | OPCAB | |
| OnBHCAB | ‐ | 1.62 (0.64−4.88) | 0.81 (0.26−2.83) |
| ONCAB | 0.62 (0.20−1.57) | ‐ | 0.50 (0.23‐1.00) |
| OPCAB | 1.23 (0.35−3.84) | 2.00 (1.00−4.28) | ‐ |
Note: Relative effects given as odds ratio with 95% credible interval.
Abbreviations: OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
Figure 4.
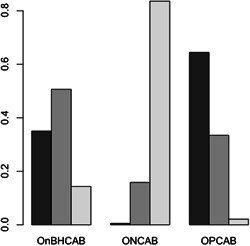
Ranking probability graph for 30‐day mortality. OPCAB has ~65% likelihood of being ranked the most effective revascularisation strategy, followed by OnBHCAB with a ~35% likelihood, then ONCAB with ~1% likelihood. OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
A subgroup NMA looking at a higher acuity patient population was also performed. Nine studies 5 , 6 , 9 , 10 , 24 , 25 , 26 , 31 , 32 assessing only patients with acute myocardial infarction (AMI) requiring emergency coronary revascularisation were identified. This subgroup NMA demonstrated a 72% reduced likelihood (OR, 0.28; CrI, 0.07‐0.83) of 30‐day mortality after OPCAB compared to ONCAB (Table 4). The CrI for this result did not cross unity. There was moderate heterogeneity in the ONCAB and OPCAB comparison (I 2 = 48.9%) and low heterogeneity in the ONCAB and OnBHCAB comparison (I 2 = 23%) (Supporting Information: Figure S6). Node splitting analysis could not be performed as there were no indirect evidence available in this network.
Table 4.
Relative effects table for 30‐day mortality in AMI patients requiring emergent revascularisation
| Control | Experiment | ||
|---|---|---|---|
| OnBHCAB | ONCAB | OPCAB | |
| OnBHCAB | ‐ | 2.06 (0.72−7.94) | 0.57 (0.11−3.29) |
| ONCAB | 0.49 (0.13−1.38) | ‐ | 0.28 (0.07−0.83) |
| OPCAB | 1.74 (0.30−9.09) | 3.58 (1.21−13.42) | ‐ |
Note: Relative effects given as odds ratio with 95% credible interval.
Abbreviations: OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
3.4.2. Secondary outcomes
The evaluation of secondary outcomes was limited by inconsistent reporting of outcomes across studies. Secondary outcomes evaluated via NMA were those which greater than 50% of studies across all direct comparisons had reported. These included stroke, renal dysfunction and length of intensive care unit (ICU) stay (Table 5). No increase in rate of stroke, renal dysfunction or length of ICU stay was found for either of these strategies in comparison to ONCAB. Furthermore, pairwise meta‐analysis of secondary outcomes was conducted where greater than 50% of studies in a single direct comparison reported this outcome (Table 6). In the OPCAB group, reoperation for bleeding was 46% less likely (OR, 0.54; 95% CI, 0.37−0.80; p = .002), and 40% less postoperative myocardial infarctions occurred (OR, 0.60; 95% CI, 0.38−0.93, p = .02) compared to ONCAB (Supporting Information: Figures S7 and S8).
Table 5.
Odds ratio (95% credible interval) of secondary outcomes for OPCAB and OnBHCAB in comparison to ONCAB
| OPCAB | OnBHCAB | |
|---|---|---|
| Stroke | 0.50 (0.11−1.30) | 1.10 (0.20−4.90) |
| Renal dysfunction | 0.77 (0.40−1.70) | 0.82 (0.27−1.70) |
| Length of ICU stay (days)a | −0.60 (−1.40 to 0.26) | −0.21 (−1.60 to 1.10) |
Abbreviations: OnBHCAB, On‐pump beating heart CABG; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG.
Treatment effect measured as mean difference for continuous variables.
Table 6.
Pairwise meta‐analysis of secondary outcomes for OPCAB in comparison to ONCAB
| OR (95% CI) | p Value | |
|---|---|---|
| Reoperation for bleeding | 0.54 (0.37−0.80) | .002 |
| Postoperative MI | 0.60 (0.38−0.93) | .02 |
| Mediastinitis | 0.87 (0.61−1.23) | .42 |
Abbreviations: CI, confidence interval; MI, myocardial infarction; ONCAB, on‐pump CABG; OPCAB, Off‐pump CABG; OR, odd ratio.
3.4.3. Meta‐regression
Meta‐regression showed that whether or not the study groups were randomised or performed propensity score matching, as opposed to studies that did not, this had no influence on the results of the NMA model. Similarly, whether the study was deemed low‐to‐moderate risk of bias or high risk of bias had no influence on the results. In both meta‐regression models, the estimate of effect of covariate had a CrI that crossed unity and a deviance information criterion that was higher than the model which did not adjust for the covariate.
4. DISCUSSION
It is difficult to justify a single surgical strategy to treat ACS, which encompasses a broad and heterogeneous group of patient conditions at presentation. 8 Despite the theoretical benefits of avoiding CPB and cardioplegic arrest, there remains speculation over the true benefit of OPCAB and OnBHCAB, especially in the lesser studied setting of ACS. This study which evaluates three surgical myocardial revascularisation strategies through a NMA demonstrates comparable 30‐day mortality in patients across the spectrum of ACS, and a significant mortality benefit for OPCAB in high‐risk patients with AMI.
There is a mixed consensus in the literature regarding the benefit of OPCAB compared to conventional ONCAB for ACS, in part due to significant challenges in conducting RCTs in this urgent setting. Previous RCTs in elective patients 33 , 34 , 35 have found comparable 30‐day mortality between these two techniques, whilst other studies have associated OPCAB with a significantly increased early mortality, 36 or an increased incidence of late complications and reintervention. 26 , 37 This may be attributable to the higher rates of incomplete revascularisation with beating heart surgery, a frequent criticism of this technique, 11 , 38 though recent studies suggest that this rate decreases with not only surgical experience, but familiarity of the anaesthetic team in managing expected haemodynamic fluctuations. 2 , 15 , 16 , 17 Other factors which could contribute to the lack of mortality benefit provided by off‐pump surgery include technical difficulty increasing operative time, lower number of grafts per patient and that the avoidance of CPB may not prevent the already pro‐inflammatory state induced by the ACS. 11 Moreover, conversion from off‐pump to on‐pump surgery intraoperatively is associated with high mortality and morbidity. 12 , 13 , 14 In saying this, the main criticism by proponents of off‐pump surgery towards these RCTs showing equivocal results is that their low risk and highly selected patient populations are not reflective of the “real‐life” setting, which is especially true in the emergency context of ACS.
Furthermore, there are several well demonstrated benefits of off‐pump surgery in the literature, including reduced risk of stroke, renal failure, prolonged ventilation, pulmonary complications, inotropic support, transfusion requirement, and length of hospital and ICU stay. 1 , 39 , 40 Rastan et al has previously shown that beating heart surgery is associated with better in‐hospital and long‐term outcomes, including lower hospital mortality. 1 This study has likewise identified OPCAB to significantly reduce the likelihood of 30‐day mortality in AMI patients, a subset of high‐risk patients such as those that were haemodynamically unstable. The notable reduction in early mortality for these patients after OPCAB may suggest that those who benefit most from avoiding CPB and cardioplegic arrest are sicker patients, in whom the systemic inflammatory response and multiorgan damage caused by ischemia‐reperfusion may be more pronounced. 25 , 26 This is supported by results from Stamou and colleagues whom reported that coronary revascularisation without CPB was associated with an improved 2‐year survival rate. 41 This all lends support to the use of OPCAB as the optimal approach in this subgroup of at‐risk patients, including those in cardiogenic shock, with poor ventricular function, ongoing myocardial ischemia, risk of perioperative stroke and elderly patients with multiple comorbidities. 42 Locker and colleagues have found that the use of OPCAB is especially favourable in patients with cardiogenic shock operated on within the first six hours following symptom onset, with statistical significance even after adjusting for confounding variables. 26 This challenges the general belief that patients who are haemodynamically unstable despite medical therapy are not suitable for beating heart surgery, given the patient may not tolerate further haemodynamic fluctuations with manipulation of the heart. 25 , 29 , 43 In such high‐risk cases, OnBHCAB may provide an acceptable trade‐off whereby it can provide haemodynamic stability during bypass grafting, whilst avoiding further intraoperative global myocardial ischemia generated by cardioplegic arrest. 44 , 45 The results of Mizutani et al. suggest that the avoidance of cardioplegic arrest may be preferential to the avoidance of CPB in these emergent cases. 44
This study has also demonstrated significant reduction in rates of reoperation for bleeding in the OPCAB group compared to ONCAB. Reduced bleeding and need for transfusion or reoperation observed in the OPCAB group may be attributable to less total heparin being required, as well as avoidance of derangements in platelet count and function associated with CPB. 40 , 46 However, this study did not demonstrate a reduction in risk of stroke with beating heart surgery, which is often proclaimed as a major advantage of avoiding aortic cross‐clamping. This advantage, however, depends on the strategy used for proximal anastomosis and the degree of aortic insults inflicted, with the anaortic or “no touch” technique being the least aggressive option, followed by clampless facilitating devices such as the HeartString, then aortic cannulation and lastly the use of side‐biting or cross clamps. 47 Out of the 10 included studies which reported their approach to proximal anastomosis, 7 , 9 , 17 , 25 , 26 , 28 , 29 , 30 , 31 only one study used a primarily anaortic technique. 29 Most studies primarily performed proximal aortic anastomoses with a side‐biting clamp, with avoidance of the aorta only in cases where it was substantially calcified.
4.1. Limitations and strengths
The largest limitation for this study is the lack of randomised data. The use of almost entirely retrospective observational cohorts introduces variability between studies and between groups, and therefore bias. Although all included studies had patients with ACS as the population of interest, this is a heterogeneous group of pathologies and hence there was variability on the type of ACS that constituted the study cohort. Furthermore, high heterogeneity in terms of operative risk between the intervention groups resulting from selection bias was expected given that choice of surgical technique was based on surgeon preference rather than a strict criterion. Interestingly, the majority of studies reported no significant difference between preoperative risk (determined by EuroSCORE, cardiogenic shock, preoperative IABP, ejection fraction, renal function), whereas four studies reported higher preoperative risk in the control group 17 , 24 , 25 , 43 and three studies reported vice versa. 6 , 8 , 10 However, in these studies in which there was a higher preoperative risk in the OPCAB or OnBHCAB group, there were marginally better or similar short and intermediate term results compared to ONCAB.
Further limitations of this study include lack of mid‐ to long‐term survival data, as 30 days is rarely sufficient for a true evaluation of the mortality benefit of each CABG technique. A low postoperative mortality event rate also does not facilitate comparative analysis of the techniques, with meta‐analytic methods potentially giving misleading results 40 , 48 Few included studies reported on surgeon expertise and predominance of OPCAB use, hence this could not be accounted for in the analysis. Results from experienced high volume centres may not necessarily be applicable to low volume centres still on the learning curve of these difficult techniques.
Despite their limitations, there still exists a role for the evaluation of non‐randomised data in meta‐analytic studies as it can address certain limitations of RCTs, such as small sample size and highly selected populations. 49 This study had a large combined cohort of 10,194 patients and is the first of its kind to compare the three CABG strategies in ACS patients through a NMA.
4.2. Future suggestions
Other important outcomes of interest which could not be evaluated in this study due to limited reporting include impact of type of grafts used, long‐term graft patency and rate of reintervention. Future NMA studies should evaluate completeness of revascularisation and graft patency outcomes which are the focus of current criticism towards off‐pump approaches, as well as evaluate longer term data to more accurately assess the effectiveness of each intervention. Furthermore, more RCTs comparing these surgical strategies in the context of ACS is required to reduce the selection bias associated with observational studies and allow for more conclusive meta‐analytic results.
5. CONCLUSION
This present NMA aimed to compare ONCAB, OPCAB, and OnBHCAB myocardial revascularisation strategies performed in patients presenting with ACS. Whilst the results suggest that OPCAB and OnBHCAB may confer greater 30‐day mortality benefit compared to conventional ONCAB, this was not significant. Hence, no single best surgical technique in this ACS population was identified. However, in patients with AMI, there was a significant mortality benefit seen for OPCAB, suggesting that these high‐risk patients may benefit most from avoiding further myocardial ischemic and inflammatory injury associated with CPB and cardioplegic arrest.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENT
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Hwang B, Williams ML, Tian DH, Yan TD, Misfeld M. Coronary artery bypass surgery for acute coronary syndrome: a network meta‐analysis of on‐pump cardioplegic arrest, off‐pump, and on‐pump beating heart strategies. J Card Surg. 2022;37:5290‐5299. 10.1111/jocs.17149
This paper was presented at the STS Coronary Conference, 4‐6 June 2022, Ottawa, Canada.
REFERENCES
- 1. Rastan AJ, Eckenstein JI, Hentschel B, et al. Emergency coronary artery bypass graft surgery for acute coronary syndrome: beating heart versus conventional cardioplegic cardiac arrest strategies. Circulation. 2006;114(suppl 1):I477‐I485. [DOI] [PubMed] [Google Scholar]
- 2. Emerson DA, Hynes CF, Greenberg MD, Trachiotis GD. Coronary artery bypass grafting during acute coronary syndrome: outcomes and comparison of Off‐Pump to conventional coronary artery bypass grafting at a veteran affairs hospital. Innovations (Phila). 2015;10(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 3. Gaudino M, Angelini GD, Antoniades C, et al. Off‐Pump coronary artery bypass grafting: 30 years of debate. J Am Heart Assoc. 2018;7(16):e009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben‐Gal Y, Stone GW, Smith CR, et al. On‐pump versus off‐pump surgical revascularization in patients with acute coronary syndromes: analysis from the acute catheterization and urgent intervention triage strategy trial. J Thorac Cardiovasc Surg. 2011;142(2):e33‐e39. [DOI] [PubMed] [Google Scholar]
- 5. Fattouch K, Runza G, Moscarelli M, et al. Graft patency and late outcomes for patients with ST‐segment elevation myocardial infarction who underwent coronary surgery. Perfusion. 2011;26(5):401‐408. [DOI] [PubMed] [Google Scholar]
- 6. Darwazah AK, Sham'a RAA, Isleem I, Hanbali B, Jaber B. Off‐pump coronary artery bypass for emergency myocardial revascularization. Asian Cardiovasc Thorac Ann. 2009;17(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 7. Afrasiabirad A, Safaie N, Montazergaem H. On‐pump beating coronary artery bypass in high risk coronary patients. Iran J Med Sci. 2015;40(1):40‐44. [PMC free article] [PubMed] [Google Scholar]
- 8. Kawamoto S, Miyata H, Motomura N, Tanemoto K, Takamoto S, Saiki Y. Surgical outcomes of isolated coronary artery bypass grafting for acute coronary syndrome ‐ based on the Japan adult cardiovascular surgery database. Circ J. 2018;82(1):123‐130. [DOI] [PubMed] [Google Scholar]
- 9. Izumi Y, Magishi K, Ishikawa N, Kimura F. On‐pump beating‐heart coronary artery bypass grafting for acute myocardial infarction. Ann Thorac Surg. 2006;81(2):573‐576. [DOI] [PubMed] [Google Scholar]
- 10. Miyahara K, Matsuura A, Takemura H, et al. On‐pump beating‐heart coronary artery bypass grafting after acute myocardial infarction has lower mortality and morbidity. J Thorac Cardiovasc Surg. 2008;135(3):521‐526. [DOI] [PubMed] [Google Scholar]
- 11. Harling L, Moscarelli M, Kidher E, Fattouch K, Ashrafian H, Athanasiou T. The effect of off‐pump coronary artery bypass on mortality after acute coronary syndrome: a meta‐analysis. Int J Cardiol. 2013;169(5):339‐348. [DOI] [PubMed] [Google Scholar]
- 12. Patel NC, Patel NU, Loulmet DF, McCabe JC, Subramanian VA. Emergency conversion to cardiopulmonary bypass during attempted off‐pump revascularization results in increased morbidity and mortality. J Thorac Cardiovasc Surg. 2004;128(5):655‐661. [DOI] [PubMed] [Google Scholar]
- 13. Jin R, Hiratzka LF, Grunkemeier GL, Krause A, Page US, 3rd . Aborted off‐pump coronary artery bypass patients have much worse outcomes than on‐pump or successful off‐pump patients. Circulation. 2005;112(suppl 9):I332‐I337. [DOI] [PubMed] [Google Scholar]
- 14. Reeves BC, Ascione R, Caputo M, Angelini GD. Morbidity and mortality following acute conversion from off‐pump to on‐pump coronary surgery. Eur J Cardiothorac Surg. 2006;29(6):941‐947. [DOI] [PubMed] [Google Scholar]
- 15. Puskas JD, Williams WH, O'Donnell R, et al. Off‐pump and on‐pump coronary artery bypass grafting are associated with similar graft patency, myocardial ischemia, and freedom from reintervention: long‐term follow‐up of a randomized trial. Ann Thorac Surg. 2011;91(6):1836‐1843. [DOI] [PubMed] [Google Scholar]
- 16. Song H. Safe evolution towards routine off‐pump coronary artery bypass: negotiating the learning curve. Eur J Cardiothorac Surg. 2003;24(6):947‐952. [DOI] [PubMed] [Google Scholar]
- 17. Biancari F, Mahar MAA, Mosorin M, et al. Immediate and intermediate outcome after off‐pump and on‐pump coronary artery bypass surgery in patients with unstable angina pectoris. Ann Thorac Surg. 2008;86(4):1147‐1152. [DOI] [PubMed] [Google Scholar]
- 18. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777‐784. [DOI] [PubMed] [Google Scholar]
- 19. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and shiny app for producing PRISMA 2020‐compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022;18:e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Valkenhoef G, Kuiper J. 2021. Package “gemtc”: network meta‐analysis using bayesian methods. Accessed April, 2022. https://cran.r-project.org/web/packages/gemtc/gemtc.pdf
- 23. Geissbühler M, Hincapié CA, Aghlmandi S, Zwahlen M, Jüni P, da Costa BR. Most published meta‐regression analyses based on aggregate data suffer from methodological pitfalls: a meta‐epidemiological study. BMC Med Res Methodol. 2021;21(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirose H, Amano A, Takahashi A, Takanashi S. Urgent off‐pump coronary artery bypass grafting. Jpn J Thorac Cardiovasc Surg. 2002;50(8):330‐337. [DOI] [PubMed] [Google Scholar]
- 25. Kerendi F, Puskas JD, Craver JM, et al. Emergency coronary artery bypass grafting can be performed safely without cardiopulmonary bypass in selected patients. Ann Thorac Surg. 2005;79(3):801‐806. [DOI] [PubMed] [Google Scholar]
- 26. Locker C, Shapira I, Paz Y, et al. Emergency myocardial revascularization for acute myocardial infarction: survival benefits of avoiding cardiopulmonary bypass. Eur J Cardiothorac Surg. 2000;17(3):234‐238. [DOI] [PubMed] [Google Scholar]
- 27. Martinez EC, Emmert MY, Thomas GN, Emmert LS, Lee CN, Kofidis T. Off‐pump coronary artery bypass is a safe option in patients presenting as emergency. Ann Acad Med Singapore. 2010;39(8):607‐612. [PubMed] [Google Scholar]
- 28. Neumann A, Vöhringer L, Fischer J, et al. Off‐Pump coronary artery bypass grafting in acute coronary syndrome: focus on safety and completeness of revascularization. Thorac Cardiovasc Surg. 2020;68(8):679‐686. [DOI] [PubMed] [Google Scholar]
- 29. Ochi M, Hatori N, Saji Y, Sakamoto S, Nishina D, Tanaka S. Application of off‐pump coronary artery bypass grafting for patients with acute coronary syndrome requiring emergency surgery. Ann Thorac Cardiovasc Surg. 2003;9(1):29‐35. [PubMed] [Google Scholar]
- 30. Onorati F, FEO M, Mastroroberto P, et al. Unstable angina and non‐ST segment elevation: surgical revascularization with different strategies. Eur J Cardiothorac Surg. 2005;27(6):1043‐1050. [DOI] [PubMed] [Google Scholar]
- 31. Tsutsumi K, Ishida O, Hashizume K, Inoue Y. Emergency surgery for left main disease: with and without cardioplegic arrest. Asian Cardiovasc Thorac Ann. 2019;27(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 32. Zhu MZL, Huq MM, Billah BM, et al. On‐Pump beating heart versus conventional coronary artery bypass grafting early after myocardial infarction: a propensity‐score matched analysis from the ANZSCTS database. Heart, Lung Circ. 2019;28(8):1267‐1276. [DOI] [PubMed] [Google Scholar]
- 33. Lamy A, Devereaux PJ, Prabhakaran D, et al. Five‐Year outcomes after off‐pump or on‐pump coronary‐artery bypass grafting. N Engl J Med. 2016;375(24):2359‐2368. [DOI] [PubMed] [Google Scholar]
- 34. Shroyer AL, Hattler B, Wagner TH, et al. Five‐Year outcomes after on‐pump and off‐pump coronary‐artery bypassbypass. N Engl J Med. 2017;377(7):623‐632. [DOI] [PubMed] [Google Scholar]
- 35. Diegeler A, Börgermann J, Kappert U, et al. Off‐pump versus on‐pump coronary‐artery bypass grafting in elderly patients. N Engl J Med. 2013;368(13):1189‐1198. [DOI] [PubMed] [Google Scholar]
- 36. Møller CH, Perko MJ, Lund JT, et al. No major differences in 30‐day outcomes in high‐risk patients randomized to off‐pump versus on‐pump coronary bypass surgery: the best bypass surgery trial. Circulation. 2010;121(4):498‐504. [DOI] [PubMed] [Google Scholar]
- 37. Gundry SR, Romano MA, Shattuck OH, Razzouk AJ, Bailey LL. Seven‐year follow‐up of coronary artery bypasses performed with and without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115(6):1273‐1278. [DOI] [PubMed] [Google Scholar]
- 38. Davierwala PM. Current outcomes of off‐pump coronary artery bypass grafting: evidence from real world practice. J Thorac Dis. 2016;8(Suppl 10):S772‐S786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Livesay JJ. The benefits of off‐pump coronary bypass: a reality or an illusion? Tex Heart Inst J. 2003;30(4):258‐260. [PMC free article] [PubMed] [Google Scholar]
- 40. Lawton JS. Off‐pump coronary artery bypass grafting. Mo Med. 2012;109(4):277‐280. [PMC free article] [PubMed] [Google Scholar]
- 41. Stamou SC, Jablonski KA, Hill PC, Bafi AS, Boyce SW, Corso PJ. Coronary revascularization without cardiopulmonary bypass versus the conventional approach in high‐risk patients. Ann Thorac Surg. 2005;79(2):552‐557. [DOI] [PubMed] [Google Scholar]
- 42. García Fuster R, Montero JA, Gil O, et al. Advantages of off‐pump coronary bypass surgery in high‐risk patients. Rev Esp Cardiol. 2002;55(4):383‐390. [PubMed] [Google Scholar]
- 43. Kaya K, Cavolli R, Telli A, et al. Off‐pump versus on‐pump coronary artery bypass grafting in acute coronary syndrome: a clinical analysis. J Cardiothorac Surg. 2010;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizutani S, Matsuura A, Miyahara K, et al. On‐pump beating‐heart coronary artery bypass: a propensity matched analysis. Ann Thorac Surg. 2007;83(4):1368‐1373. [DOI] [PubMed] [Google Scholar]
- 45. Perrault LP, Menasché P, Peynet J, et al. On‐pump, beating‐heart coronary artery operations in high‐risk patients: an acceptable trade‐off. Ann Thorac Surg. 1997;64(5):1368‐1373. [DOI] [PubMed] [Google Scholar]
- 46. Raja SG, Dreyfus GD. Impact of off‐pump coronary artery bypass surgery on postoperative bleeding: current best available evidence. J Card Surg. 2006;21(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 47. Zhao DF, Edelman JJ, Seco M, et al. Coronary artery bypass grafting With and Without manipulation of the ascending aorta. J Am Coll Cardiol. 2017;69(8):924‐936. [DOI] [PubMed] [Google Scholar]
- 48. Deeks JJ, Higgins JPT, Altman DG. Chapter 10.4.4.3: validity of methods of meta‐analysis for rare events. Cochrane handbook for systematic reviews of interventions version 6.1. Cochrane, 2020. Available from https://training.cochrane.org/handbook/archive/v6.1/chapter-10
- 49. Cameron C, Fireman B, Hutton B, et al. Network meta‐analysis incorporating randomized controlled trials and non‐randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev. 2015;4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


