Abstract
Objective
This study was undertaken to evaluate the long‐term safety and effectiveness of fenfluramine in patients with Lennox–Gastaut syndrome (LGS).
Methods
Eligible patients with LGS who completed a 14‐week phase 3 randomized clinical trial enrolled in an open‐label extension (OLE; NCT03355209). All patients were initially started on .2 mg/kg/day fenfluramine and after 1 month were titrated by effectiveness and tolerability, which were assessed at 3‐month intervals. The protocol‐specified treatment duration was 12 months, but COVID‐19‐related delays resulted in 142 patients completing their final visit after 12 months.
Results
As of October 19, 2020, 247 patients were enrolled in the OLE. Mean age was 14.3 ± 7.6 years (79 [32%] adults) and median fenfluramine treatment duration was 364 days; 88.3% of patients received 2–4 concomitant antiseizure medications. Median percentage change in monthly drop seizure frequency was −28.6% over the entire OLE (n = 241) and −50.5% at Month 15 (n = 142, p < .0001); 75 of 241 patients (31.1%) experienced ≥50% reduction in drop seizure frequency. Median percentage change in nondrop seizure frequency was −45.9% (n = 192, p = .0038). Generalized tonic–clonic seizures (GTCS) and tonic seizures were most responsive to treatment, with median reductions over the entire OLE of 48.8% (p < .0001, n = 106) and 35.8% (p < .0001, n = 186), respectively. A total of 37.6% (95% confidence interval [CI] = 31.4%–44.1%, n = 237) of investigators and 35.2% of caregivers (95% CI = 29.1%–41.8%, n = 230) rated patients as Much Improved/Very Much Improved on the Clinical Global Impression of Improvement scale. The most frequent treatment‐emergent adverse events were decreased appetite (16.2%) and fatigue (13.4%). No cases of valvular heart disease (VHD) or pulmonary arterial hypertension (PAH) were observed.
Significance
Patients with LGS experienced sustained reductions in drop seizure frequency on fenfluramine treatment, with a particularly robust reduction in frequency of GTCS, the key risk factor for sudden unexpected death in epilepsy. Fenfluramine was generally well tolerated; VHD or PAH was not observed long‐term. Fenfluramine may provide an important long‐term treatment option for LGS.
Keywords: developmental and epileptic encephalopathies, fenfluramine, Lennox–Gastaut syndrome, long‐term open‐label extension
Key Points.
-
Fenfluramine (median, 364 days) in 247 patients with LGS:
-
◦
Reduced drop and nondrop seizure frequencies (median = 28.6% and 45.9%).
-
◦
Reduced frequency of generalized tonic–clonic seizures by a median of 48.8%.
-
◦
Resulted in 75 of 241 patients (31.1%) with ≥50% reduction in drop seizure frequency.
-
◦
Was equally tolerable and effective in adult and pediatric patients.
-
◦
Was well tolerated, with no pulmonary arterial hypertension or valvular heart disease.
-
◦
At ~1 year (n = 170), reduction in drop seizure frequency was 51.8%.
At ~1 year, 51.2% of patients had ≥50% reduction in drop seizure frequency.
Fenfluramine may be a safe, effective long‐term option for patients with LGS.
1. INTRODUCTION
Lennox–Gastaut syndrome (LGS) is a developmental and epileptic encephalopathy characterized by multiple seizure types (most notably, tonic seizures, ~56%), accompanied by an abnormal electroencephalogram with diffuse slow spike‐and‐wave complexes at <3 Hz, and cognitive impairment, leading to life‐long disability. 1 , 2 , 3 Despite multiple available US Food and Drug Administration‐ and/or European Medicines Agency‐approved antiseizure medications (ASMs)—including clobazam, rufinamide, cannabidiol, topiramate, felbamate, and lamotrigine—and frequent use of polypharmacy to treat LGS, most patients have treatment‐resistant epilepsy. 4
Patients treated with fenfluramine .7 mg/kg/day experienced a 26.5% median reduction in drop seizures versus 14.2% in the .2‐mg/kg/day fenfluramine group and 7.6% in the placebo group, with an estimated median difference of −19.9% (p = .001) during a randomized, double‐blind, placebo‐controlled clinical trial (RCT) in 263 patients with LGS. 5 In addition, children and young adults with LGS treated with fenfluramine were more likely to show clinically meaningful improvement in the ability to regulate behavior and cognition, 6 , 7 , 8 which are commonly reported by families to be among the most difficult aspects of LGS to manage and accept. 9 The mechanism of action of fenfluramine is unique among ASMs and involves activity at both serotonergic and sigma‐1 receptors (sigma1Rs), 10 possibly contributing to antiseizure effects and improvement in everyday executive functioning. In a rational pharmacy approach, fenfluramine would be mechanistically complementary to the existing pharmacological options for treating LGS.
The objective of this study was to evaluate the long‐term safety and efficacy of fenfluramine in patients with LGS who participated in a phase 3, open‐label extension (OLE) study.
2. MATERIALS AND METHODS
2.1. Study participants and study drug
The study complied with current International Conference on Harmonization–Good Clinical Practice guidelines and was approved by the applicable regulatory authorities and independent ethics committee/institutional review board at each institution. All patients or their legal representatives provided signed informed consent before enrollment, and assent where applicable.
Patients with a confirmed LGS diagnosis who completed the phase 3 randomized clinical trial (aged 2–35 years at entry into the core study) were eligible to enroll in the OLE study (NCT03355209). In accordance with the International League Against Epilepsy criteria, confirmed LGS diagnosis included abnormal background activity accompanied by a slow spike‐and‐wave pattern on the electroencephalogram (<2.5 Hz) and seizures of multiple types, including tonic and tonic or atonic seizures as described for the core study as well as onset of seizures at <11 years and abnormal cognitive development. 5 To enroll in the OLE, patients must have continued to meet the eligibility criteria for the core study, 5 and all patients must have satisfactorily completed the core study. Patients were eligible to enroll in the OLE as long as they had not experienced any of the following at the end of the core study: clinically meaningful worsening of seizures, clinically significant laboratory findings (e.g., elevated alanine transaminase levels, decreased platelet count), or weight loss > 15% during the titration and maintenance period of the core study that failed to stabilize.
Per study inclusion criteria, all patients were maintained on a stable ASM regimen of concomitant ASMs during the entire OLE treatment period (in the core study, the median number of prior ASMs across all treatment groups was 7 [range = 1–20]; additionally, 82 [31%] patients in the core study were on vagus nerve stimulation and 11 [4%] were on ketogenic diets).
2.2. Study design
Over a 14‐day transition period from the core RCT into the OLE, all patients were titrated to .2 mg/kg/day fenfluramine and remained at this dose for 1 month regardless of their randomized treatment arm (fenfluramine .2 mg/kg/day, fenfluramine .7 mg/kg/day, or placebo) in the RCT. After Month 1, patients were flexibly titrated by effectiveness and tolerability, up to a maximum of .7 mg/kg/day (maximum daily dose = 26 mg/day; Figure 1A). Effectiveness and safety/tolerability were assessed at Months 1, 2, 3, 6, 9, and 12, with safety follow‐up visits at 3 and 6 months after the last dose; in some countries, an additional follow‐up occurred at 24 months after the last dose. Originally, the open label extension study was designed to be a 12‐month study. Due to the coronavirus disease (COVID)‐19‐related precautions imposed on monitoring visits and the challenges for some subjects with scheduling in‐person end‐of‐study visits, the completion of the study was delayed. Specifically, many subjects who received study drug under the protocol were unable to attend a final in‐person study visit within the protocol‐specified time period (365 ± 4 days), and their treatment was extended until the visit could take place and suitable transition of care could be arranged. Therefore, an interim analysis was performed based on a snapshot of the clinical database taken on October 19, 2020, a date chosen to ensure that the analysis included at least 365 ± 4 days of exposure in the OLE for all but one of the patients who remained in the trial. As a result, 142 patients in the OLE had data captured at Month 13–15, which was included in this article for completeness. Additionally, six patients in the OLE had data captured at Month 16–18 and one patient had data at Month 19–21. Effectiveness (reduction in seizure frequency) was calculated in 3‐month increments over time and from Month 1 to End of Study (i.e., last treatment dose) in the modified intent‐to‐treat population.
FIGURE 1.
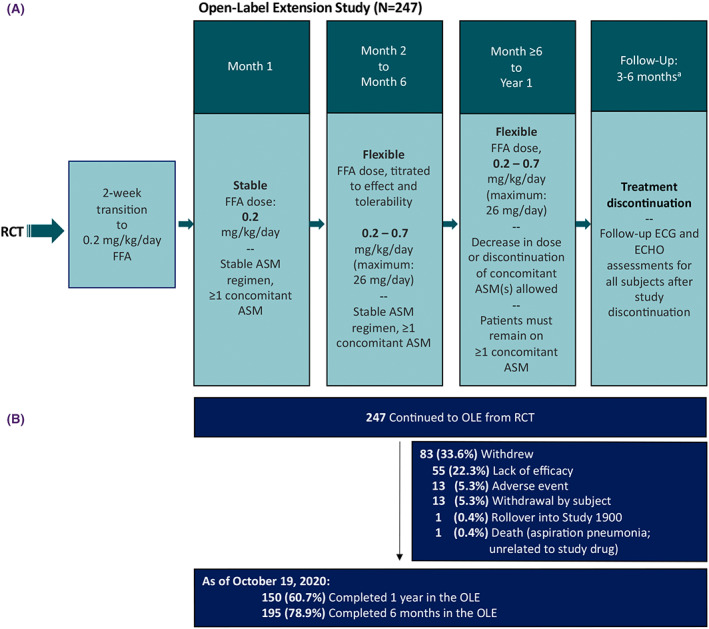
Study design and patient disposition. aPatients in Germany, the Netherlands, or France had an additional follow‐up at 24 months. ASM, antiseizure medication; ECG, electrocardiogram; ECHO, echocardiogram; FFA, fenfluramine; OLE, open‐label extension; RCT, randomized clinical trial.
2.3. Study endpoints
2.3.1. Effectiveness
Changes in seizure frequencies were compared to prerandomization baseline in the core study. Drop seizures were defined as seizures associated with a drop or fall: generalized tonic–clonic seizures (GTCS), secondary GTCS (SGTC; focal to bilateral tonic–clonic), tonic seizures (TS), atonic seizures (AS), and tonic–atonic seizures (TA). Seizures that do not result in drops (nondrop seizures) were defined per protocol as “any countable seizure types that did not meet the criteria of drop seizures (i.e., are classified as clonic seizures, hemiclonic seizures, focal with or without observable signs, myoclonic seizures, absence/atypical absence, infantile spasms, epileptic spasms, or other; or are seizures in the following classifications that were approved for each subject as nondrop seizure types by the Epilepsy Study Consortium: GTCS, SGTC, TS, AS, or TA).” Seizure subtype analysis assessed the median percentage reduction in GTCS, TS, AS, or TA in the subset of patients who experienced these seizure types at prerandomization baseline in the core study.
Responder rates were expressed as percentages of patients who achieved seizure frequency reduction at the ≥25%, ≥50%, and ≥75% levels. Seizure freedom or near seizure freedom was defined as patients who had ≤1 seizure during the treatment period. Median percentage increase in days free of drop seizures and median longest interval between drop seizures were assessed. Clinical Global Impression of Improvement (CGI‐I) was rated by investigators and caregivers on a 7‐point Likert scale, with scores of 1 (Very Much Improved) and 2 (Much Improved) considered to be clinically meaningful changes.
2.3.2. Safety and tolerability
Standardized two‐dimensional color Doppler echocardiogram (ECHO) assessed cardiac valve function/structure and any evidence of pulmonary arterial hypertension (PAH) every 3 months as described in a previous cardiovascular safety study 11 and the core study. 5 Treatment‐emergent adverse effects (TEAEs) were recorded during the treatment period, coded using the Medical Dictionary for Regulatory Activities version 20.1.
2.4. Statistical analyses
The OLE safety population was defined as all patients who received ≥1 dose of fenfluramine during the OLE. Effectiveness analyses were performed on the modified intent‐to‐treat population, defined as all patients with ≥1 dose of fenfluramine and ≥1 month of valid drop seizure data during the OLE. Continuous variables were summarized by mean, SD, median, and range. Categorical variables were summarized by frequency and percentages. For effectiveness endpoints, Wilcoxon signed‐rank tests assessed the statistical significance of median percentage change from prerandomized baseline, where statistical significance was defined a priori as p < .05. No imputation was made for missing data or dropouts in any of the efficacy endpoints. Standardized z‐scores were calculated for patients <20 years of age based on the most recent publicly available weight data from the US Centers for Disease Control and Prevention.
3. RESULTS
3.1. Patients
A total of 263 patients were randomized in the RCT. A total of 247 patients had enrolled in the OLE as of October 19, 2020 and had at least one dose of study drug and 1 month of diary data in the OLE (Figure 1A and Table 1). Eighty‐three (33.6%) patients withdrew; the most common reason for withdrawal was lack of efficacy (n = 55, 22.3%; Figures 1B and S1). Per protocol, concomitant ASM regimens were to remain stable for the first 6 months of the study, after which investigators were permitted to adjust the doses of one or more of the other concomitant ASMs as per routine clinical practice. Deviations from the protocol during the first 6 months included dosage changes (n = 23 patients), discontinuation of an ASM (n = 2), and new ASM added (n = 1); the most frequent ASM changes were valproate (n = 12), rufinamide (n = 4), and clobazam (n = 3). After 6 months, the most frequent changes (dosage changes, additions, or subtractions) were valproate (n = 19; 10 dosage decreases, six dosage increases, one addition, two added and then subtracted [i.e., single dosage event]), clobazam (n = 20; seven dosage decreases, two dosage increases, three additions, four subtractions, one indeterminate change, three modifications with no net change), lamotrigine (n = 11; five dosage decreases, two dosage increases, one addition, one subtraction, two modifications with no net change), cannabidiol (n = 10 additions), rufinamide (n = 8; three dosage decreases, one dosage increase, two subtractions, two dosage modifications with no net change), levetiracetam (n = 8; two dosage decreases, one dosage increase, two modifications with no net change, one subtraction, two added and then subtracted [i.e., single dosage event]), topiramate (n = 8; five dosage decreases, one addition, two subtractions), felbamate (n = 5; two dosage decreases, one dosage increase, one dose addition, one dosage modification with no net change), and lacosamide (n = 5; one dosage increase, one addition, one subtraction, one modification with no net change, one added and then subtracted [i.e., single dosage event]).
TABLE 1.
Patient demographics and baseline characteristics
| Characteristic | Value |
|---|---|
| N (OLE safety population) | 247 |
| Age at OLE entry, years, mean (SD) | 14 (8) |
| Age group, years, n (%) | |
| 2 to < 18 | 168 (68.0) |
| 18–36 | 79 (32.0) |
| Sex, male, n (%) | 136 (55.1) |
| Race, n (%) | |
| White | 199 (80.6) |
| Black or African American | 12 (4.9) |
| Asian | 8 (3.2) |
| Other, unknown, or multiple | 8 (3.2) |
| Not reported a | 20 (8.1) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 48 (19.4) |
| Not Hispanic or Latino | 178 (72.1) |
| Not reported a | 21 (8.5) |
| Region, n (%) | |
| North America | 122 (49.4) |
| Europe | 117 (47.4) |
| Australia | 8 (3.2) |
| Baseline frequency of drop seizures per 28 days, median (minimum, maximum) b | 75 (4, 2943) |
| Prior medications, median (range) | 7 (1–20) |
| Concomitant medications, median (range) | 3 (1–7) |
| n (%) | |
| Valproate, all forms | 141 (57.1) |
| Clobazam | 112 (45.3) |
| Lamotrigine | 87 (35.2) |
| Levetiracetam | 57 (23.1) |
| Rufinamide | 52 (21.1) |
| Cannabidiol | 12 (4.9) |
Abbreviation: OLE, open‐label extension.
Privacy laws in some regions/countries preclude disclosure of certain personal information.
Determined during the core study.
3.2. Fenfluramine exposure
Median treatment duration was comparable between pediatric and adult patients grouped by age at entry into the core study at prerandomization (364 days; Table 2). Most patients (113/247, 45.7%) received a mean daily dose of .3–.5 mg/kg/day fenfluramine.
TABLE 2.
Fenfluramine exposure during the OLE
| Characteristic | Value |
|---|---|
| N | 247 |
| Duration of exposure by age group at entry into core study, days, median (IQR) | |
| Pediatric: 2 to < 18 years, n = 174 a | 364 (191–368) |
| Adult: 18–36 years, n = 73 | 364 (210–373) |
| Mean daily dose of fenfluramine, mg/kg/day, n (%) | |
| Up to .2 | 6 (2.4) |
| >.2 to <.3 | 67 (27.1) |
| .3 to .5 | 113 (45.7) |
| >.5 to .7 | 60 (24.3) |
Abbreviations: IQR, interquartile range; OLE, open‐label extension.
Six patients in the core study turned 18 years of age before the start of the OLE (n = 168 patients were 2 to <18 years old at the beginning of the OLE).
A total of 170 patients were treated until at least Month 10–12 (original, protocol‐specified duration of the OLE); 142 patients had additional fenfluramine exposure to at least Month 13–15; six patients had fenfluramine exposure to at least Month 16–18; and one patient was treated to at least Month 19–21.
3.3. Effectiveness
At the end of the RCT (14 weeks combined titration + maintenance periods; N = 263) and while maintaining their baseline standard‐of‐care regimen, patients in the .7‐mg/kg/day fenfluramine group had a −26.5% change in drop seizure frequency, compared to a −14.2% change in the .2‐mg/kg/day fenfluramine group and −7.6% change in the placebo group. 5 Fenfluramine provided significant improvement in median drop seizure frequency at Month 3 of the OLE study (n = 227, −39.4%), and significant response was maintained through the end of study (Figure 2A). The following time points and varying n values reflect staggered entry and staggered withdrawal. At 4–6 months (n = 218), 7–9 months (n = 187), 10–12 months (n = 170), and 13–15 months (n = 142), patients experienced a median change in drop seizure frequency of −37.1%, −42.7%, −51.8%, and −50.5%, respectively (p < .0001 at each time point). Over the entire OLE, the median change in drop seizure frequency was −28.6% (n = 241, p < .0001). Over the entire OLE, the median change in all seizures not associated with a drop was −45.9% (n = 192, p = .0038; Figure 2B). Changes in drop seizures and nondrop seizures were comparable over time among pediatric and adult patients. Over the entire OLE, median change in drop seizure frequency was −25.6% in the 2‐ to <18‐year‐old group (n = 171, p = .0037) and −39.0% in the ≥18‐ to 35‐year‐old group (n = 70, p < .0001; Figure S2A). Over the entire OLE, median change in nondrop seizure frequency was −43.8% in the 2‐ to <18‐year‐old group (n = 138, p = .0990) and −54.9% in the ≥18‐ to 35‐year‐old group (n = 54, p = .0029; Figure S2B).
FIGURE 2.
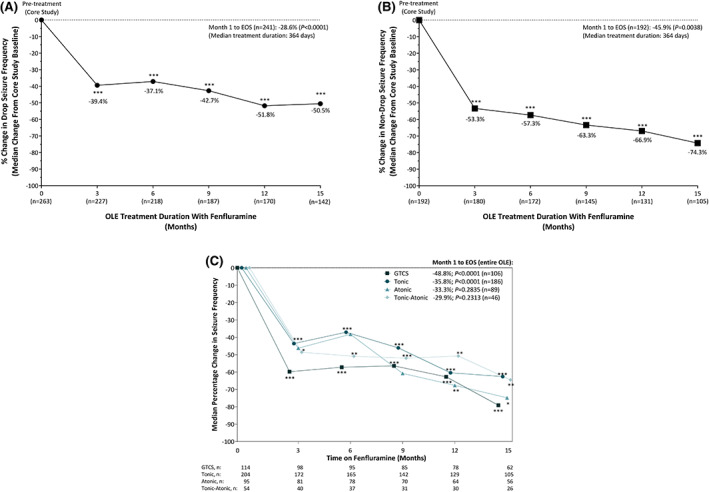
Sustained reduction in (A) drop seizure frequency and (B) nondrop seizure frequency after treatment for up to 15 months with fenfluramine in patients with Lennox–Gastaut syndrome. (C) Sustained reduction in frequency of seizure subtypes after up to 15 months of fenfluramine treatment. (A,B) ***p < .0001 by Wilcoxon signed‐rank test. (C) ***p < .001, **p ≤ .01, *p < .05 by Wilcoxon signed‐rank test. Decreasing n over time is due in part to staggered study entry. Months 6, 9, 12, and 15 represent median frequency for the previous 3 months versus randomized clinical trial baseline. Due to small n, percentage changes were not plotted for patients with data beyond 15 months. EOS, end of study (entire OLE duration); GCTS, generalized tonic–clonic seizures; OLE, open‐label extension.
Median percentage change in GTCS was −48.8% over the entire OLE (interquartile range [IQR] = −14.9% to −83.7%, n = 106, p < .0001) and, over time, ranged from −56.4% (Month 7–9, IQR = −91.1% to −6.7%, n = 85, p < .0001) to −79.1 (Month 13–15, IQR = −100.0% to −22.2%, n = 62, p < .0001; Figure 2C). Median percentage change in tonic seizures was −35.8% over the entire OLE (IQR = −65.9% to 13.4%, n = 186, p < .0001) and, over time, ranged from −37.1% (Month 4–6, IQR = −73.3% to 11.8%, n = 165, p < .0001) to −62.6% (Month 13–15, IQR = −100.0% to −18.8%, n = 105, p < .0001). Median percentage change in tonic–atonic seizures was −29.9% over the entire OLE (IQR = −66.8% to 25.5%, n = 46, p = .2313) and, over time, ranged from −48.6% (Month 3, IQR = −90.3% to 4.1%, n = 40, p = .0421) to −64.5% (Month 13–15, IQR = −100.0% to −1.7%, n = 26, p = .0131). For atonic seizures, median percentage change from prerandomization baseline was −33.3% over the entire OLE (IQR = −78.1% to 44.8%, n = 89, p = .2835) and did not reach statistical significance at Months 3, 4–6, or 7–9, but was significantly lower at Months 10–12 (−67.7%; IQR = −97.2% to 24.5%, n = 64, p = .005) and Months 13–15 (−74.8%; IQR = −100.0% to 31.6%, n = 56, p = .0484; Figure 2C).
In the overall treatment period, median percentage increase in days free of drop seizures was 41.6% compared to pretreatment baseline in the core RCT, with a median absolute change of 1.93 days per 28 days (range = −23.0 to 27.5 days; p < .0001). The longest interval between drop seizures increased from a median of 2.0 days (range = 1–17 days) to a median of 6.0 days (range = 1–365 days).
Over the entire OLE, 75 of 241 patients (31.1%, 95% confidence interval [CI] = 25.3%–37.4%) experienced a clinically meaningful (≥50%) reduction in percentage of drop seizures, including 28 of 241 patients (11.6%, 95% CI = 7.9%–16.4%) who demonstrated profound (≥75%) reduction in frequency of drop seizures (Figure 3A; see Figure S3A for response rates at ~1 year [n = 170]: ≥50% reduction, 51.2% [95% CI = 43.4%–58.9%]; ≥75% reduction, 25.3% [95% CI = 19.0%–32.5%]). Approximately one third of investigators (89/237, 37.6%, 95% CI = 31.4%–44.1%) and caregivers (81/230, 35.2%, 95% CI = 29.1%–41.8%) rated their patients as having clinically meaningful improvement on the CGI‐I (i.e., “Much Improved” or “Very Much Improved”; Figure 3B) at last assessment (see Figure S3B for CGI‐I data at ~1 year [n = 132 investigators, n = 129 caregivers]: “Much Improved” or “Very Much Improved” was reported by 49.2% of investigators [95% CI = 40.4%–58.1%] and 48.8% of caregivers [95% CI = 39.9%–57.8%]). There were no notable differences among pediatric and adult patients in either investigator‐rated or caregiver‐rated CGI‐I scores (Figure S3C).
FIGURE 3.
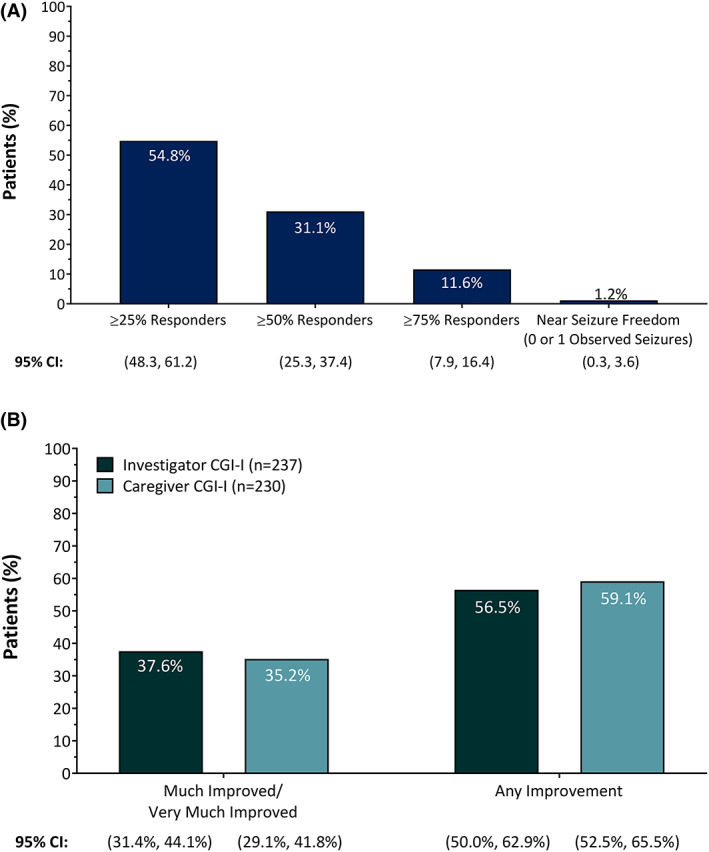
(A) Responder rates for drop seizures in patients with Lennox–Gastaut syndrome treated with fenfluramine over the entire open‐label extension (n = 241). (B) Clinical Global Impression of Improvement (CGI‐I) as rated by investigators (n = 237) or caregivers (n = 230) after treatment with fenfluramine at last assessment. CI, confidence interval.
3.4. Safety
The majority of patients (203/247, 82.2%) experienced a TEAE, most of which were mild or moderate in severity (188/247, 76.1%). The most common TEAEs were decreased appetite and fatigue (Table 3). Forty of 247 patients (16.2%) experienced a serious TEAE (SAE), including nine patients (3.6%) with changes in seizure presentation, eight patients (3.2%) with status epilepticus, and five patients (2.0%) with pneumonia. Comparable proportions of children and adults experienced TEAEs and SAEs (Table 3). Twelve patients (4.9%) experienced a TEAE that led to study discontinuation, most commonly fatigue or change in seizure presentation (n = 3, 1.2% each). A body weight loss of ≥7% (prespecified study protocol threshold) was reported at any visit for 42 of 247 (17.0%) of the patients (28 pediatric patients aged 2–18 years and 14 adult patients aged ≥18 years). Of the 42 patients who lost weight, 14 (33.3%) regained weight by end of study. In patients aged <20 years (n = 192), mean z‐score at last visit was −1.027, representing a decrease of .230 SD from prerandomization baseline in the core study. Weight loss was treated by standard‐of‐care clinical practice (e.g., dietary interventions); two pediatric patients (9 and 14 years) were hospitalized and had feeding tubes inserted to manage weight loss while maintaining a dose of .7 mg/kg/day fenfluramine.
TABLE 3.
TEAEs occurring in ≥10% of patients during the interim analysis period of the open‐label extension
| Adverse event | 2 to <18 years, n = 174, n (%) | 18–36 years, n = 73, n (%) | Overall, n = 247, n (%) |
|---|---|---|---|
| Any TEAE | 138 (79.3%) | 65 (89.0%) | 203 (82.2%) |
| Any SAE | 29 (16.7%) | 11 (15.1%) | 40 (16.2%) |
| Decreased appetite | 28 (16.1%) | 12 (16.4%) | 40 (16.2%) |
| Fatigue | 23 (13.2%) | 10 (13.7%) | 33 (13.4%) |
| Nasopharyngitis | 23 (13.2%) | 8 (11.0%) | 31 (12.6%) |
| Seizure | 16 (9.2%) | 11 (15.1%) | 27 (10.9%) |
Note: Age groups are by entry into the core study.
Abbreviations: SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
One patient died. The cause was reported as severe convulsive status epilepticus and aspiration pneumonia and was considered unrelated to study drug. Echocardiography revealed that no patient had developed valvular heart disease (VHD) or PAH by the interim analysis cutoff date. Two patients in the LGS program demonstrated instances of mild aortic regurgitation (AR) without the presence of VHD. Neither patient exhibited valvular morphological (or structural) changes or restrictive valve motion, nor did findings progress to a higher grade of regurgitation. One of these patients had two diagnostic transesophageal ECHOs (a method with higher resolution than standard transthoracic ECHO) that demonstrated absent AR. Both patients demonstrated absent AR and normal valve structure. Both patients were examined by cardiologists, who concluded there was no clinical evidence of VHD in either patient. Both patients continue to be treated with fenfluramine without development of VHD.
Rates of mild AR observed in the study (n = 2/247, .8%) were consistent with those seen in the screening period prior to treatment with fenfluramine (n = 3/335, .9%).
4. DISCUSSION
The results of this OLE of fenfluramine for the treatment of LGS support and extend the observations reported in the RCT at 14 weeks. In our study, patients with LGS treated with fenfluramine for a median treatment duration of 364 days experienced a sustained reduction in the magnitude of drop seizure and nondrop seizure frequencies. GTCS were most sensitive to fenfluramine treatment, followed by tonic and atonic seizure subtypes. Reductions in seizure frequencies were comparable to the RCT, were sustained over the entire OLE treatment period, and were similar in pediatric (ages 2 to <18 years) and adult patients (ages ≥18 to 35 years). The TEAE profile was also comparable to the 14‐week RCT; no unexpected or new TEAEs emerged in the OLE. 5 Dropout rates (n = 83/247, 33.6%) were similar to recent OLEs for LGS: rufinamide (n = 13/54, 24.1%), 12 clobazam (79/267, 29.6%), 13 and cannabidiol (n = 122/366, 33.3%). 14 Combined with sustained effectiveness and tolerability over time, this relatively high retention rate supports a favorable risk:benefit ratio for fenfluramine treatment over the long term.
As noted in the RCT and the OLE, fenfluramine produced a robust reduction in the frequency of GTCS in the subset of patients with this seizure subtype at baseline. Patients who experienced a high burden of GTCS were at approximately a 10‐fold elevated risk of sudden unexpected death in epilepsy (SUDEP) in a recent registry study of 255 SUDEP cases and 1148 matched controls. 15 Although a direct association between SUDEP risk and fenfluramine treatment has yet to be established, a recent epidemiological study in patients with Dravet syndrome reported that SUDEP risk was lower after fenfluramine treatment (all cause and SUDEP mortality rate = 1.7 per 1000 person‐years) than historical controls (all cause: 15.8 per 1000 person‐years; SUDEP: 9.3 per 1000 person‐years). 16 , 17 Preclinical evidence also suggests that fenfluramine prevented seizure‐induced respiratory arrest in rodent models of SUDEP via 5‐HT4 receptor interaction. 18 These observations support further investigation into whether reduction in GTCS may make fenfluramine a particularly advantageous treatment option for patients with LGS who have a high burden of GTCS.
Most patients with LGS require polypharmacy to manage seizure multiplicity. 2 Clinicians may select ASMs with multimodal mechanisms of action to improve treatment effectiveness across a broad range of seizure types. 4 Fenfluramine has dual serotonergic and sigma1R activity, 10 , 19 , 20 a mechanism that is unique among ASMs approved to treat LGS or Dravet syndrome. 4 Preclinical models of Dravet syndrome suggest that serotonergic and positive modulatory sigma1R activity contributes to both seizure 19 and nonseizure outcomes, including improvements in learning and memory, 10 , 20 , 21 as well as lower SUDEP incidence. 18 Clinical studies support the preclinical evidence for improved nonseizure outcomes in Dravet syndrome after fenfluramine treatment, including neuropsychological aspects. Children and young adults with Dravet syndrome who were treated with fenfluramine for 14 weeks in RCTs or 1 year in OLEs experienced improvements in aspects of executive function as assessed by the Behavior Rating Inventory of Executive Function instrument. 22 , 23 Preliminary studies also support the positive impact of fenfluramine on executive function in children and adolescents with LGS aged 5–18 years 6 , 7 and in adults with LGS aged >18 years. 8 Taken together with the most recent LGS Cochrane review suggesting that treatment should ideally target both seizures and nonseizure outcomes such as cognition, behavior, and development, 9 these results suggest that fenfluramine's unique mechanism of action may make it an advantageous add‐on therapy in combination ASM regimens for LGS.
No treatment‐related deaths occurred in this study. The type and frequency of adverse events (AEs) observed in the OLE match previous RCT reports in both Dravet syndrome and LGS, 5 , 22 , 24 as well as long‐term safety data in patients with Dravet syndrome who were treated for a median treatment duration of 3 years. 25 The most common TEAEs were decreased appetite, fatigue, nasopharyngitis, and seizure (11%–16%).
Most patients with LGS require polypharmacy. Clinical practice requires qualitative and quantitative adjustments in concomitant ASMs to balance optimal seizure control with AEs. In our study, recurrent ASM dose adjustments were mostly dosage decreases in the ASMs valproate, clobazam, lamotrigine, rufinamide, topiramate, levetiracetam, and felbamate; cannabidiol was the most common addition after 6 months. In clinical practice, adjustments are made in these medications to reduce side effects, minimize drug–drug interactions, and optimize efficacy. 26 , 27 , 28 , 29 , 30
Fenfluramine was originally developed as an anorexigenic agent, making weight loss a TEAE of interest in long‐term studies. As in the OLEs with Dravet syndrome, 31 weight loss stabilized over time in our study. Furthermore, weight loss was not different between adult and pediatric patients. Patients with developmental and epileptic encephalopathies often experience growth abnormalities, with a developmental delay in height and weight compared to normative neurotypical populations. 32 Armeno et al. reported a median z‐score weight change of −.24 SD over 1 year in a population of 45 children with intractable epilepsies (including LGS). 33 This change is comparable to the change in z‐score reported in our study (cf. −.230 SD at last postbaseline assessment). Taken together, these data suggest that the growth curves of patients with LGS who were ≤20 years of age in our study were below the average neurotypical reference population yet were comparable to historical data in patients with refractory epilepsy on fenfluramine‐free regimens. In patients with Dravet syndrome, Gil‐Nagel and colleagues reported a weight z‐score change of −.4 SD over 1 year of fenfluramine. These reductions in weight were comparable to a historical control group comprised of patients with Dravet syndrome on fenfluramine‐free regimens. 32 , 34 Over time, changes in weight may stabilize in many patients with Dravet syndrome 31 ; similarly, we report that approximately one third of the patients with LGS who experienced weight loss of ≥7% regained weight by end of study. Our study results also suggest that the dynamics of weight loss are discontinuous in some patients and may stabilize over time. Managing short‐term (discontinuous, stabilizing) and long‐term (continuous) weight loss and decreased appetite can be accomplished with standard approaches used in clinical practice (e.g., dietary interventions, adjustments in concomitant ASM dose). 27 , 34 Furthermore, children with LGS require continuous caregiver support, thereby allowing for identification and timely intervention to manage weight should issues arise.
VHD and PAH were TEAEs of special interest in this study, because fenfluramine was withdrawn from worldwide markets in 1997 due to an association with cardiac valve abnormalities reported in obese adults taking fenfluramine for weight loss. 35 , 36 Because of this history, the fenfluramine US label includes a black box warning and is available only under restricted access. Patients are required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program with periodic cardiovascular monitoring in the United States. 37 Results of a long‐term cardiovascular safety study demonstrate no evidence of VHD or PAH in patients with Dravet syndrome who were treated for a median duration of 256 days 11 and up to 3 years. 38 Additionally, 19 patients with Dravet syndrome were treated up to approximately 30 years with no evidence of VHD/PAH documented. 38 Taken together, results from the REMS program, the experience of fenfluramine used long‐term in the treatment of Dravet syndrome, and the data presented in this OLE continue to support the cardiovascular safety of fenfluramine (Zogenix, data on file).
4.1. Limitations
This study has some limitations. Inherent in the OLE design is the lack of a placebo control group. Furthermore, 22.3% of the patient population withdrew from the OLE for lack of efficacy, leading to a bias toward enrichment for effectiveness over time. Withdrawal of patients from the RCT prior to entry in the OLE due to AEs may lead to an underreporting of AEs over time. Delays due to COVID‐19 resulted in 142 patients with data captured beyond the protocol‐specified 12‐month study duration, leading to fewer patients (smaller n) at later time points.
5. CONCLUSIONS
In this OLE study, patients with LGS experienced sustained (39.4%–51.8%, 28.6% overall), clinically meaningful reduction in frequency of drop seizures during treatment with fenfluramine at a median treatment duration of 364 days. Similar to observations in the RCT, those patients whose phenotype included GTCS at baseline experienced a sustained robust reduction in frequency of GTCS throughout the entire OLE. Fenfluramine was generally well tolerated, with no observations of VHD or PAH during the OLE. Fenfluramine may be an important new treatment option with a novel mechanism of action for patients with LGS.
AUTHOR CONTRIBUTIONS
Gail M. Farfel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Kelly G. Knupp, Berten Ceulemans, Joseph Sullivan, Lieven Lagae, Sameer M. Zuberi, Anupam Agarwal, Michael Lock, Gail M. Farfel, Bradley S. Galer, Antonio Gil‐Nagel. Acquisition, analysis, or interpretation of data: Kelly G. Knupp, Ingrid E. Scheffer, Berten Ceulemans, Katherine C. Nickels, Renzo Guerrini, Sameer M. Zuberi, Rima Nabbout, Kate Riney, Anupam Agarwal, Michael Lock, David Dai, Gail M. Farfel, Bradley S. Galer, Arnold R. Gammaitoni, Shikha Polega, Ronald Davis, Antonio Gil‐Nagel. Drafting of the manuscript: Kelly G. Knupp, Joseph Sullivan, Bradley S. Galer, Arnold R. Gammaitoni, Shikha Polega, Antonio Gil‐Nagel. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Kelly G. Knupp, Michael Lock, David Dai, Gail M. Farfel, Bradley S. Galer, Arnold R. Gammaitoni. Obtained funding: Kelly G. Knupp, Gail M. Farfel, Bradley S. Galer. Administrative, technical, or material support: Kate Riney, Anupam Agarwal, Bradley S. Galer, Arnold R. Gammaitoni, Ronald Davis. Supervision: Kelly G. Knupp, Berten Ceulemans, Joseph Sullivan, Lieven Lagae, Renzo Guerrini, Anupam Agarwal, Gail M. Farfel, Bradley S. Galer, Arnold R. Gammaitoni, Antonio Gil‐Nagel.
CONFLICT OF INTEREST
K.G.K. discloses research grants from Zogenix and Pediatric Epilepsy Research Foundation during the conduct of the study, as well as from the Colorado Department of Public Health and West Therapeutics, and received other support as a data safety monitoring board member and from Greenwich Pharmaceuticals outside the submitted work. I.E.S. discloses compensation from Zogenix during the conduct of the study, has received other support from Zynerba Pharmaceuticals, GW Pharmaceuticals, Ovid Therapeutics, Marinus, and Ultragenyx, has received personal fees and other compensation from UCB, has received personal fees from GlaxoSmithKline, Eisai, BioMarin, Nutricia, and Xenon Pharmaceuticals outside the submitted work, and has received research funding from the National Health and Medical Research Council, Health Research Council of New Zealand, and National Institutes of Health. B.C. discloses research funding from Brabant and Zogenix, has served as consultant for Brabant and Zogenix, is a patent‐holder for ZX008, and potentially benefits financially from a royalty arrangement that is related to this research if Zogenix is successful in marketing its product, fenfluramine, with the terms of this arrangement reviewed and approved by the cobeneficiary, KU Leuven University/Antwerp University Hospital. J.S. discloses research grants from Stoke, Marinus, Zogenix, and BioPharm, served as consultant/advisor for the Dravet Syndrome Foundation, Epygenix, Encoded, GW Pharmaceuticals, Asceneuron, Longboard Pharmaceuticals, Knopp Biosciences, and Neurocrine, owns stock options in Epygenix, has received travel support from Zogenix, and has served as a reviewer for the Epilepsy Study Consortium. L.L. discloses grants, personal fees, and other support from, and has served as consultant/speaker for, Zogenix during the conduct of the study; has received other support from and served as consultant/speaker for LivaNova; has received grants and other support from, and served as consultant/speaker for, UCB; has received other support from and served as consultant/speaker for Shire; has served as consultant/speaker for Eisai, Brabant, and Ovid, outside the submitted work; holds a patent for ZX008 for the treatment of Dravet syndrome and infantile epilepsies assigned to his institution and licensed to Zogenix; and potentially benefits financially from a royalty arrangement that is related to this research if Zogenix is successful in marketing its product, fenfluramine, with the terms of this arrangement reviewed and approved by the cobeneficiary, KU Leuven University/Antwerp University Hospital. R.G. discloses research grants from Zogenix during the conduct of the study, has served as speaker/consultant for Zogenix outside the submitted work, has served as an investigator for studies with Biocodex, UCB, Angelini, and Eisai, and has served as speaker and advisory board member for Biocodex, Novartis, BioMarin, and GW Pharma outside the submitted work. S.M.Z. discloses research support from Epilepsy Research UK, Dravet Syndrome UK, and Zogenix, and has served as consultant/speaker and advisory board member for GW Pharma, Encoded Therapeutics, Stoke Therapeutics, Eisai, UCB, Jaguar Gene Therapy, Arvelle, and Zogenix. R.N. discloses research funding from Eisai, GW Pharma, Novartis, Shire, and Zogenix, has served as consultant/advisor for Eisai, Biogen, GW Pharma, Novartis, Shire, and Zogenix, and has served as speaker for Advicenne, Eisai, BioMarin, GW Pharma, Novartis, and Zogenix. K.R. has received honoraria for educational symposia, advisory boards, and/or consultancy work from Eisai, LivaNova, Medlink Neurology, Novartis, and UCB Australia. Her institution has supported clinical trials for Biogen Idec Research, DSLP, Eisai, Epigenyx Therapeutics, GW Research, Janssen‐Cilag, Marinus Pharmaceuticals, Medicure International, LivaNova, Neurocrine Biosciences, Noema Pharma, Novartis, SK Lifesciences, UCB Australia, UCB Biopharma SRL, and Zogenix. A.A. discloses personal fees from, owns stock in, and was an employee of Zogenix, with patents pending. M.L. discloses personal fees from, owns stock in, and was an employee of Zogenix, with patents pending during the time the research was conducted (at time of publication, reported independent consultancy for Zogenix). G.M.F., B.S.G., A.R.G., and S.P. disclose personal fees from, own stock in, and/or are/were employees of Zogenix (now a part of UCB), with patents pending. R.D. has served as speaker for LivaNova, Eisai, and Lundbeck, and has served as an investigator for LivaNova, Eisai, Global Pharmaceuticals, Lundbeck, Pfizer, UCB, and Zogenix. A.G.‐N. discloses personal fees or research grants from Arvelle Therapeutics, Bial, Biocodex, Eisai, Esteve, GW Pharma, GW Research, PTC Therapeutics, Sanofi, Stoke, UCB, and Zogenix. K.C.N. has no conflict of interest to disclose. D.D. has served as consultant for Zogenix.
Supporting information
Figure S1
ACKNOWLEDGMENTS
The authors thank the patients who participated in the study and their families; and Dr Rachel Tham (UCB) for statistical support. This study was sponsored by Zogenix (Emeryville, CA, USA; now a part of UCB). Professional medical writing and editing were provided to the authors by Danielle Ippolito, PhD, CMPP, MWC, and Scott Bergfeld, PhD, of PharmaWrite (Princeton, NJ, USA), and were paid for by Zogenix.
Knupp KG, Scheffer IE, Ceulemans B, Sullivan J, Nickels KC, Lagae L, et al. Fenfluramine provides clinically meaningful reduction in frequency of drop seizures in patients with Lennox–Gastaut syndrome: Interim analysis of an open‐label extension study. Epilepsia. 2023;64:139–151. 10.1111/epi.17431
REFERENCES
- 1. Gastaut H, Roger J, Soulayrol R, Tassinari CA, Regis H, Dravet C, et al. Childhood epileptic encephalopathy with diffuse slow spike‐waves (otherwise known as “petit mal variant”) or Lennox syndrome. Epilepsia. 1966;7(2):139–79. [DOI] [PubMed] [Google Scholar]
- 2. Arzimanoglou A, French J, Blume WT, Cross JH, Ernst JP, Feucht M, et al. Lennox‐Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82–93. [DOI] [PubMed] [Google Scholar]
- 3. Cross JH, Auvin S, Falip M, Striano P, Arzimanoglou A. Expert opinion on the management of Lennox‐Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Löscher W, Klein P. The pharmacology and clinical efficacy of antiseizure medications: from bromide salts to cenobamate and beyond. CNS Drugs. 2021;35(9):935–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knupp K, Scheffer I, Ceulemans B, Sullivan J, Nickels K, Lagae L, et al. Efficacy and safety of fenfluramine for the treatment of seizures associated with Lennox‐Gastaut syndrome: a randomized clinical trial. JAMA Neurol. 2022;79(6):554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bishop KI, Isquith PK, Gioia GA, Knupp KG, Scheffer IE, Sullivan J, et al. FINTEPLA (fenfluramine) treatment improves everyday executive functioning in patients with Lennox‐Gastaut syndrome: analysis from a phase 3 clinical trial. Oral presentation at: American Academy of Neurology Annual Meeting; April 17–22, 2021; virtual meeting.
- 7. Bishop KI, Isquith PK, Gioia GA, Knupp KG, Sullivan J, Nabbout R, et al. Fenfluramine (Fintepla®) treatment improves everyday executive functioning in patients with Lennox‐Gastaut syndrome: analysis from a phase 3 clinical trial. Poster presented at: American Academy of Neurology Annual Meeting; April 2–7, 2022; Seattle, Washington.
- 8. Bishop KI, Isquith PK, Roth RM, Gioia GA, Knupp KG, Sullivan J, et al. Adults with Lennox‐Gastaut syndrome have improved everyday executive functioning with fenfluramine (Fintepla®). Poster presented at: European Epilepsy Congress; July 9–13, 2022; Geneva, Switzerland.
- 9. Brigo F, Jones K, Eltze C, Matricardi S. Anti‐seizure medications for Lennox‐Gastaut syndrome. Cochrane Database Syst Rev. 2021;4:CD003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin P, Reeder T, Sourbron J, de Witte PAM, Gammaitoni AR, Galer BS. An emerging role for sigma‐1 receptors in the treatment of developmental and epileptic encephalopathies. Int J Mol Sci. 2021;22(16):8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai WW, Galer BS, Wong PC, Farfel G, Pringsheim M, Keane MG, et al. Cardiovascular safety of fenfluramine in the treatment of Dravet syndrome: analysis of an ongoing long‐term open‐label safety extension study. Epilepsia. 2020;61(11):2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Long‐term safety and seizure outcome in Japanese patients with Lennox‐Gastaut syndrome receiving adjunctive rufinamide therapy: an open‐label study following a randomized clinical trial. Epilepsy Res. 2016;121:1–7. [DOI] [PubMed] [Google Scholar]
- 13. Ng YT, Conry J, Mitchell WG, Buchhalter J, Isojarvi J, Lee D, et al. Clobazam is equally safe and efficacious for seizures associated with Lennox‐Gastaut syndrome across different age groups: post hoc analyses of short‐ and long‐term clinical trial results. Epilepsy Behav. 2015;46:221–6. [DOI] [PubMed] [Google Scholar]
- 14. Patel AD, Mazurkiewicz‐Beldzinska M, Chin RF, Gil‐Nagel A, Gunning B, Halford JJ, et al. Long‐term safety and efficacy of add‐on cannabidiol in patients with Lennox‐Gastaut syndrome: results of a long‐term open‐label extension trial. Epilepsia. 2021;62(9):2228–39. [DOI] [PubMed] [Google Scholar]
- 15. Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: a nationwide population‐based case‐control study. Neurology. 2020;94(4):e419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cross JH, Galer BS, Gil‐Nagel A, Devinsky O, Ceulemans B, Lagae L, et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure. 2021;93:154–9. [DOI] [PubMed] [Google Scholar]
- 17. Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–7. [DOI] [PubMed] [Google Scholar]
- 18. Tupal S, Faingold CL. Serotonin 5‐HT4 receptors play a critical role in the action of fenfluramine to block seizure‐induced sudden death in a mouse model of SUDEP. Epilepsy Res. 2021;177:106777. [DOI] [PubMed] [Google Scholar]
- 19. Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti‐epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front Pharmacol. 2017;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma‐1 receptors. Epilepsy Behav. 2020;105:106989. [DOI] [PubMed] [Google Scholar]
- 21. Martin P, Maurice T, Gammaitoni A, Farfel G, Boyd B, Galer B. Fenfluramine modulates the anti‐amnesic effects induced by sigma‐1 receptor agonists and neuro(active)steroids in vivo. Epilepsy Behav. 2022;127:108526. [DOI] [PubMed] [Google Scholar]
- 22. Lagae L, Sullivan J, Knupp K, Laux L, Polster T, Nikanorova M, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2019;394(10216):2243–54. [DOI] [PubMed] [Google Scholar]
- 23. Bishop KI, Isquith PK, Gioia GA, Gammaitoni AR, Farfel G, Galer BS, et al. Improved everyday executive functioning following profound reduction in seizure frequency with fenfluramine: analysis from a phase 3 long‐term extension study in children/young adults with Dravet syndrome. Epilepsy Behav. 2021;121(Pt A):108024. [DOI] [PubMed] [Google Scholar]
- 24. Nabbout R, Mistry A, Zuberi S, Villeneuve N, Gil‐Nagel A, Sanchez‐Carpintero R, et al. Fenfluramine for treatment‐resistant seizures in patients with Dravet syndrome receiving stiripentol‐inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77(3):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scheffer IE, Devinsky O, Perry MS, Wheless J, Thiele EA, Wirrell E, et al. Efficacy and tolerability of adjunctive FINTEPLA (fenfluramine HCl) in an open‐label extension study of Dravet syndrome patients treated for up to 3 years. Poster presented at: Virtual American Epilepsy Society Annual Meeting; December; 4–8, 2020.
- 26. Besag FMC, Vasey MJ. Neurocognitive effects of antiseizure medications in children and adolescents with epilepsy. Paediatr Drugs. 2021;23(3):253–86. [DOI] [PubMed] [Google Scholar]
- 27. Buraniqi E, Dabaja H, Wirrell EC. Impact of antiseizure medications on appetite and weight in children. Paediatr Drugs. 2022;24(4):335–63. [DOI] [PubMed] [Google Scholar]
- 28. Martin P, Czerwiński M, Limaye PB, Ogilvie BW, Smith S, Boyd B. In vitro evaluation suggests fenfluramine and norfenfluramine are unlikely to act as perpetrators of drug interactions. Pharmacol Res Perspect. 2022;10(3):e00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin P, Czerwiński M, Limaye PB, Muranjan S, Ogilvie BW, Smith S, et al. In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions. Pharmacol Res Perspect. 2022;10(3):e00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyd B, Smith S, Gammaitoni A, Galer BS, Farfel GM. A phase I, randomized, open‐label, single‐dose, 3‐period crossover study to evaluate the drug‐drug interaction between ZX008 (fenfluramine HCl oral solution) and a regimen of stiripentol, clobazam, and valproate in healthy subjects. Int J Clin Pharmacol Ther. 2019;57(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan J, Scheffer IE, Lagae L, Nabbout R, Pringsheim M, Talwar D, et al. Fenfluramine HCl (Fintepla®) provides long‐term clinically meaningful reduction in seizure frequency: analysis of an ongoing open‐label extension study. Epilepsia. 2020;61(11):2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eschbach K, Scarbro S, Juarez‐Colunga E, Allen V, Hsu S, Knupp K. Growth and endocrine function in children with Dravet syndrome. Seizure. 2017;52:117–22. [DOI] [PubMed] [Google Scholar]
- 33. Armeno M, Verini A, Del Pino M, Araujo MB, Mestre G, Reyes G, et al. A prospective study on changes in nutritional status and growth following two years of ketogenic diet (KD) therapy in children with refractory epilepsy. Nutrients. 2019;11(7):1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gil‐Nagel A, Sullivan J, Ceulemans B, Wirrell E, Devinsky O, Nabbout R, et al. Treatment with fenfluramine in patients with Dravet syndrome has no long‐term effects on weight and growth. Epilepsy Behav. 2021;122:108212. [DOI] [PubMed] [Google Scholar]
- 35. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med. 1997;337(9):581–8. [DOI] [PubMed] [Google Scholar]
- 36. Wong J, Reddy SS, Klein AL. Anorectic drugs and valvular heart disease: a biological and clinical perspective. Cleve Clin J Med. 1998;65(1):35–41. [DOI] [PubMed] [Google Scholar]
- 37. FINTEPLA® (fenfluramine) oral solution, CIV [prescribing information]. Emeryville, CA: Zogenix; 2022. [Google Scholar]
- 38. Agarwal A, Farfel GM, Gammaitoni AR, Wong PC, Pinto FJ, Galer BS. Long‐term cardiovascular safety of fenfluramine in patients with Dravet syndrome treated for up to 3 years: findings from serial echocardiographic assessments. Eur J Paediatr Neurol. 2022;39:35–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


