Abstract
Background
The immune response to infections could be largely driven by the individual’s genes, especially in the major histocompatibility complex (MHC) region. Varicella-zoster virus (VZV) is a highly communicable pathogen. In addition to infection, the reactivations of VZV can be a potential causal factor for multiple traits. Identification of VZV immune response-related health conditions can therefore help elucidate the aetiology of certain diseases.
Methods
A phenome-wide Mendelian randomization (MR) study of anti-VZV immunoglobulin G (IgG) levels with 1370 traits was conducted to explore the potential causal role of VZV-specific immune response on multiple traits using the UK Biobank cohort. For the robustness of the results, we performed MR analyses using five different methods. To investigate the impact of the MHC region on MR results, the analyses were conducted using instrumental variables (IVs) inside (IVmhc) and outside (IVno.mhc) the MHC region or all together (IVfull).
Results
Forty-nine single nucleotide polymorphisms (IVfull) were associated with anti-VZV IgG levels, of which five (IVmhc) were located in the MHC region and 44 (IVno.mhc) were not. Statistical evidence (false discovery rate < 0.05 in at least three of the five MR methods) for a causal effect of anti-VZV IgG levels was found on 22 traits using IVmhc, while no evidence was found when using IVno.mhc or IVfull. The reactivations of VZV increased the risk of Dupuytren disease, mononeuropathies of the upper limb, sarcoidosis, coeliac disease, teeth problems and earlier onset of allergic rhinitis, which evidence was concordant with the literature. Suggestive causal evidence (P < 0.05 in at least three of five MR methods) using IVfull, IVmhc and IVno.mhc was detected in 92, 194 and 56 traits, respectively. MR results from IVfull correlated with those from IVmhc or IVno.mhc. However, the results between IVmhc and IVno.mhc were noticeably different, as evidenced by causal associations in opposite directions between anti-VZV IgG and ten traits.
Conclusions
In this exploratory study, anti-VZV IgG was causally associated with multiple traits. IVs in the MHC region might have a substantial impact on MR, and therefore, could be potentially considered in future MR studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02843-5.
Keywords: Varicella- zoster virus, Anti-VZV IgG, Mendelian randomization, MR-PheWAS, MHC
Background
Varicella-zoster virus (VZV) is a highly communicable pathogen. It is widely present in the general population, with an estimated incidence of 4–4.5 per 1000 person-years [1]. Primary infections of VZV are commonly seen in children as chickenpox. The virus will then remain latent in the sensory dorsal root ganglion cells and can be reactivated throughout life. The reactivations of the virus may cause damage anywhere on the body, from the nerves to the skin [2]. The common manifestations are neurological symptoms, such as radicular pain, itching and unpleasant sensations. The virus can affect the cranial nerve and cause diseases (e.g. tooth exfoliation [3] and facial palsy [2]). Facial palsy could trigger other symptoms such as tinnitus, hearing loss and nausea. VZV reactivation may also result in retinal necrosis and severe ocular diseases [4]. The virus can directly enter the cerebrospinal fluid causing meningitis [5] or invade the spinal cord and produce myelopathy [6]. It is also possible that the virus travels to the arteries to induce vasculopathy like haemorrhagic stroke [7]. Recent studies have shown associations between herpes zoster and other diseases such as depression and anxiety [8]. Therefore, the infection of VZV can have a long-term impact on multiple health conditions.
Humoral immune responses to the same infectious agent vary greatly between individuals. Evidence shows that genetic factors may play a determining role in the individual antibody responses to a variety of viruses, especially the variants in the major histocompatibility complex (MHC) region [9]. Genes likely affect the humoral immune response by controlling the serum immunoglobulin levels, seroconversion rates and intensity of antigen-specific immune responses [10]. Therefore, once a host is exposed to the pathogen, their immune response will be affected by genetic factors. Studying infectious immunity-associated genes helps understand the pathologic mechanisms. Immunoglobulin G (IgG) antibody is the most common antibody in the blood and is persistent throughout a person’s life [11]. It can be used as a stable biomarker of lifetime exposure to viruses. Increased levels of anti-VZV IgG without the presence of herpes zosters implies that it correlates with VZV reactivations [12]. Previous genome-wide association studies (GWASs) investigated single nucleotide polymorphisms (SNPs) that influence the antibody responses against VZV infections. While Petars et.al found no SNPs significantly associated with anti-VZV IgG levels in 1000 healthy individuals [10], five SNPs (rs13197633, rs34073492, rs56401801, rs13204572, rs1048381) were identified in a larger cohort (N = 7595) from the UK Biobank [13].
Mendelian randomization (MR) is a well-established tool for inferring causal relationships. By using SNPs as instrumental variables (IVs), MR helps to avoid the issue of unobserved confounding in observational studies. The MHC region is a complex genomic region, in which genes are associated with the risks of many diseases [14]. We hypothesized that IVs in the MHC region may play an important role in MR analysis. However, using MHC IVs in MR has rarely been reported in the literature due to concerns about horizontal pleiotropy [15]. In fact, several robust MR approaches have emerged more recently with the aim to minimize the bias from horizontal pleiotropy and/or outliers. For example, MR-Egger estimation allows a genetic instrument to have a direct effect on the outcome, as long as the direct effect is independent of the instrument’s strength [16]. The weighted median method provides consistent causal estimates if at least 50% of the weights come from valid IVs [17]. MR-RAPS assumes that the pleiotropic effects are balanced and assigns low weights to outliers when estimating the causal effect [18]. The MR-PRESSO method detects the outliers which will then be removed to obtain robust estimations [19]. Nevertheless, each method has its own strengths and weaknesses and requires specific assumptions. It has been recommended that multiple MR methods can be performed to seek concordant evidence for a causal relationship [20].
This study aimed to explore the causal role that VZV-specific immune responses might play in the development of multiple traits, by performing phenome-wide MR studies (MR-PheWAS) between VZV titre measurement and 1370 independent traits using five different MR approaches. We also investigated the impact of the genetic instruments in the MHC region on MR analysis.
Methods
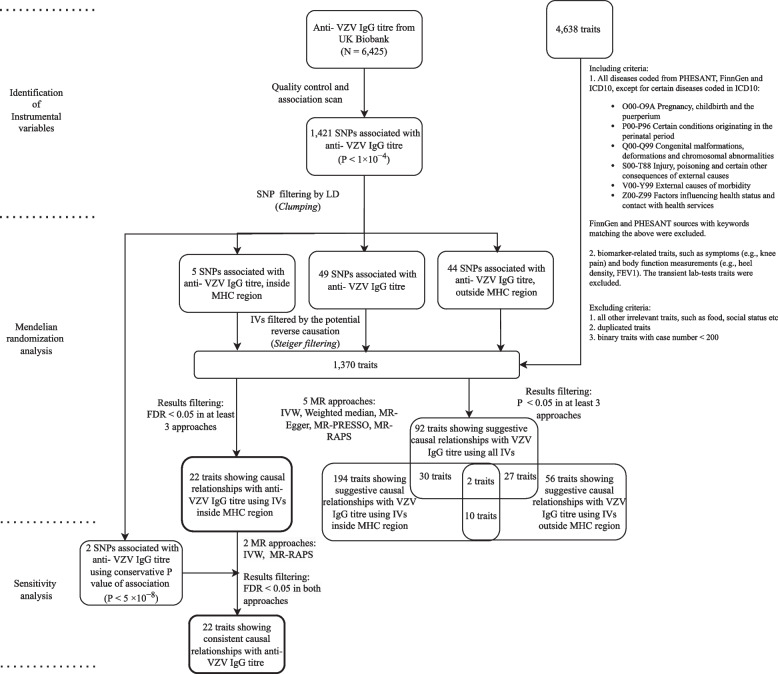
The outline of the study is shown in Fig. 1.
Fig. 1.
Flowchart of the phenome-wide Mendelian randomization study
Identification of instrumental variables
The UK Biobank [21] is a large-scale prospective database that has recruited more than 500,000 participants aged between 40 and 69. Within the cohort, 10,000 people were randomly selected to undertake the anti-VZV IgG tests [22]. We extracted genotype data and VZV IgG antibody measurement from the UK Biobank locked in March 2021. After excluding individuals with a high missing rate (missing rate > 0.02), outlying heterozygosity rate (± 3SD from the mean), unmatched sex, non-Caucasians and related individuals, 6425 individuals were included in the analysis. The anti-VZV IgG titres were measured using fluorescent bead-based multiplex serology technology at serum dilution 1:1000 [22]. The output data was median fluorescence intensity values. The titre data was log10-transformed due to skewness. We used linear regression to test for associations between anti-VZV IgG levels and each SNP. SNPs with low minor allele frequency (MAF < 0.01), high missing rate (missing rate > 0.02) and low Hardy–Weinberg equilibrium P-value (P < 1 × 10−6) were removed for downstream analysis. Anti-VZV IgG level-associated SNPs were then carried forward for MR analysis.
Previous MR studies have shown that a conservative P-value threshold (5 × 10−8) can lead to underpowered MR tests and paradoxical results [23]. In this study, we relaxed the P-value threshold to 1 × 10−4 to include more IVs because some of the MR methods we used are less prone to weak instrument bias. We then performed the sensitivity analysis using the conventional P-value threshold of 5 × 10−8. Clumping was carried out on all the neighbouring SNPs (within 10,000 kb) to filter out those in linkage disequilibrium (R2 ≥ 0.01) and only retained the one with the lowest P-value. SNPs selected after clumping were mutually independent and used as IVs for MR analysis. Association analysis between SNPs and anti-VZV IgG, as well as SNP clumping, was conducted using PLINK v1.9.
Data
We used anti-VZV IgG levels as the exposure. The summary statistics (estimated SNP effects and their standard errors) were extracted from our SNP-anti-VZV IgG association analysis. The Neale Lab GWASs included 361,194 European descendants from the UK Biobank for over 4000 traits, from which we only included diseases and biomarker-related traits as the outcomes. The summary statistics of SNP-outcome associations were obtained from the Neale Lab’s GWASs (round 2, http://www.nealelab.is/uk-biobank). We included traits coded from PHESANT, FinnGen and ICD10. Certain diseases such as injuries and chromosomal abnormalities were excluded (details of the criteria code are in Additional file 1: Text S1). We also included the biomarker-related traits, such as symptoms (e.g. knee pain) and body function measurements (e.g. heel density, forced expiratory volume in 1-s (FEV1)). The transient function measurements (e.g. microalbumin in urine, blood cell count, creatinine (quantile)) which fluctuate over time were removed. For continuous traits, we selected rank-normalized data. For binary traits, we filtered out those with a small number of cases (< 200) to increase the statistical power. For those traits measured in multiple ways, we only reported one of them following the criteria of (1) doctor-diagnosed rather than self-reported, (2) the one reflecting general condition rather than period-specific (e.g. general alcohol intake frequency vs alcohol consumption in last few days), and (3) for duplicated traits with the same phenotype descriptions, the one with a larger number of cases, less missingness or newer versions. We used single-gender data for gender-related diseases (e.g. female-only GWAS results for ovary-related traits). We used GWAS results of both sexes for all the remaining traits.
MR analysis
To investigate the impact of the MHC region on MR results, we partitioned the IVs by MHC region: in (IVmhc) and outside the region (IVno.mhc). For each trait, we performed three sets of MR tests—MHC (IVmhc), non-MHC (IVno.mhc) and combined IVs (IVfull). The MHC region was defined as chr6:28,477,797–33,448,354 (GRCh37) (the Genome Reference Consortium [24]). Harmonization was conducted for IVs between exposure and outcome datasets to ensure the same minor allele was used for each IV. Steiger filtering [25] was applied such that SNPs showing a stronger association with the outcome than with the exposure (PSteiger < 0.05) were excluded. MR analyses were conducted using five methods: random effect inverse-variance weighted (IVW) estimation [20] and four robust methods including weighted median [17], MR-Egger [16], MR-PRESSO [19] and MR-RAPS [18]. In the IVW analysis, the second-order term from the delta expansion was selected for the variance expression to better capture the uncertainty in the estimated causal effect from each SNP. Cochran’s Q tests were examined for heterogeneity across all instruments. The MR-Egger intercept test was performed to examine the direct pleiotropic effect of IVs on outcomes. The MR-PRESSO global and distortion tests were conducted to detect the effect of outliers on the results and adjust the estimated causal effects accordingly. The Benjamini-Hochberg’s false discovery rate (FDR) correction was applied to account for multiple testing [26]. We regarded the relationship between anti-VZV IgG levels and a trait as suggestive if there was statistical evidence (P < 0.05) in at least three out of the five methods. It was considered causal evidence if FDR < 0.05 in at least three MR methods. Sensitivity analyses were performed using P < 5 × 10−8 for IV filtering, where only two SNPs remained for IVW and MR-RAPS analyses as the other methods were not applicable. If FDR < 0.05 in both methods, it was regarded as consistent causal evidence. For those traits showing suggestive causal relationships with the anti-VZV IgG levels, Z-statistic from each MR analysis was used to compare the results from different methods and IVs. The Spearman correlation test was conducted to evaluate the correlations between the MR estimates from different sets of IVs. MR analyses were implemented using ‘MRPRESSO’, ‘TwoSampleMR’ and ‘MendelianRandomization’ packages in R. The R code for the analysis can be found in Additional file 1: Code S1.
Results
The mean anti-VZV IgG levels (log10-transformed) and standard deviation were 2.724 and 0.514, ranging from 0 to 4.021. As many as 1421 SNPs were found associated with anti-VZV IgG, of which 1205 (84.8%) were in the MHC region. The complete list of these SNPs was provided in Additional file 2: Table S1. Four of the SNPs (rs13197633, rs13204572, rs1048381 and rs56401801) were also associated with anti-VZV IgG in a previous UK biobank GWAS [13]. After clumping, 49 independent anti-VZV IgG-associated SNPs with F-statistic greater than 10 were selected as IVs (IVfull) for MR analysis, among which 5 SNPs were inside (IVmhc) and 44 were outside the MHC region (IVno.mhc) (Table 1). The average F-statistic for IVmhc, IVno.mhc and IVfull was 40, 19.1 and 16.7, respectively. The numbers of the IVs used in MR tests and the SNPs removed after Steiger filtering were listed in Additional file 2: Table S2. A total of 1370 traits (73 ordinal, 773 categorical, 459 binary and 65 continuous) were included in the MR analysis. Additional file 2: Table S2 listed all these results.
Table 1.
Instrumental variables identified from the genome-wide association scan
| SNP | CHR | Position | Minor allele | Beta | SE | MAF | P value | F-statistic | R2 | MHC region |
|---|---|---|---|---|---|---|---|---|---|---|
| rs749003 | 1 | 118,531,980 | C | 0.053 | 0.013 | 0.142 | 4.98E − 05 | 16.479 | 0.003 | No |
| rs843971 | 1 | 153,277,423 | T | − 0.037 | 0.009 | 0.367 | 7.71E − 05 | 15.647 | 0.002 | No |
| rs114925113 | 1 | 170,714,714 | A | − 0.139 | 0.033 | 0.017 | 2.63E − 05 | 17.679 | 0.003 | No |
| rs2642449 | 1 | 220,985,677 | C | − 0.060 | 0.013 | 0.139 | 3.06E − 06 | 21.819 | 0.003 | No |
| rs708108 | 1 | 228,189,855 | T | − 0.037 | 0.009 | 0.402 | 8.41E − 05 | 15.482 | 0.002 | No |
| rs74482711 | 2 | 127,357,322 | G | − 0.067 | 0.016 | 0.092 | 3.74E − 05 | 17.013 | 0.003 | No |
| rs34499171 | 2 | 129,591,736 | T | 0.045 | 0.011 | 0.192 | 7.62E − 05 | 15.667 | 0.002 | No |
| rs61731470 | 2 | 211,085,491 | A | 0.112 | 0.028 | 0.028 | 5.96E − 05 | 16.132 | 0.003 | No |
| rs12471761 | 2 | 241,310,093 | G | − 0.110 | 0.028 | 0.028 | 7.47E − 05 | 15.702 | 0.002 | No |
| rs17495909 | 3 | 1,323,130 | C | − 0.097 | 0.022 | 0.042 | 1.22E − 05 | 19.169 | 0.003 | No |
| rs809764 | 3 | 61,958,023 | G | − 0.039 | 0.010 | 0.327 | 5.27E − 05 | 16.372 | 0.003 | No |
| rs116664692 | 3 | 62,271,345 | G | 0.180 | 0.046 | 0.010 | 8.90E − 05 | 15.380 | 0.002 | No |
| rs11706859 | 3 | 100,713,091 | C | − 0.137 | 0.034 | 0.019 | 7.03E − 05 | 15.819 | 0.002 | No |
| rs35573656 | 3 | 126,612,552 | C | − 0.073 | 0.018 | 0.072 | 3.00E − 05 | 17.442 | 0.003 | No |
| rs73112967 | 4 | 27,778,474 | C | − 0.083 | 0.020 | 0.055 | 5.44E − 05 | 16.302 | 0.003 | No |
| rs156572 | 4 | 155,739,138 | T | − 0.109 | 0.025 | 0.036 | 1.23E − 05 | 19.141 | 0.003 | No |
| rs72696744 | 4 | 163,745,634 | T | 0.117 | 0.029 | 0.025 | 4.75E − 05 | 16.577 | 0.003 | No |
| rs1395479 | 4 | 178,318,191 | A | − 0.043 | 0.010 | 0.264 | 2.68E − 05 | 17.650 | 0.003 | No |
| rs78827810 | 4 | 186,683,778 | G | − 0.115 | 0.029 | 0.026 | 8.06E − 05 | 15.553 | 0.002 | No |
| rs56244403 | 6 | 24,017,867 | T | − 0.038 | 0.010 | 0.383 | 8.13E − 05 | 15.546 | 0.002 | No |
| rs12193110 | 6 | 29,937,104 | T | − 0.065 | 0.010 | 0.299 | 8.83E − 11 | 42.179 | 0.007 | Yes |
| rs17200698 | 6 | 31,483,700 | T | − 0.087 | 0.022 | 0.049 | 5.14E − 05 | 16.425 | 0.003 | Yes |
| rs116462901 | 6 | 31,488,532 | C | − 0.138 | 0.032 | 0.020 | 2.17E − 05 | 18.059 | 0.003 | Yes |
| rs3129888 | 6 | 32,411,726 | G | − 0.058 | 0.011 | 0.194 | 2.66E − 07 | 26.544 | 0.004 | Yes |
| rs1048381 | 6 | 32,610,445 | A | 0.116 | 0.012 | 0.171 | 9.28E − 23 | 97.288 | 0.015 | Yes |
| rs80018618 | 6 | 37,337,516 | A | 0.161 | 0.039 | 0.013 | 4.04E − 05 | 16.864 | 0.003 | No |
| rs41265357 | 6 | 71,238,178 | G | − 0.186 | 0.047 | 0.010 | 7.06E − 05 | 15.816 | 0.002 | No |
| rs2180346 | 6 | 168,944,598 | C | 0.072 | 0.015 | 0.106 | 9.25E − 07 | 24.110 | 0.004 | No |
| rs72695580 | 9 | 6,632,950 | G | 0.170 | 0.042 | 0.014 | 6.02E − 05 | 16.118 | 0.003 | No |
| rs11139344 | 9 | 84,239,037 | C | 0.042 | 0.010 | 0.268 | 3.49E − 05 | 17.164 | 0.003 | No |
| rs116919233 | 9 | 119,991,655 | T | − 0.123 | 0.031 | 0.021 | 8.77E − 05 | 15.398 | 0.002 | No |
| rs1072523 | 10 | 28,819,406 | A | 0.036 | 0.009 | 0.492 | 8.63E − 05 | 15.436 | 0.002 | No |
| rs3759012 | 11 | 118,901,262 | T | 0.036 | 0.009 | 0.386 | 8.99E − 05 | 15.356 | 0.002 | No |
| rs11609865 | 12 | 105,952,417 | C | − 0.073 | 0.018 | 0.068 | 5.83E − 05 | 16.173 | 0.003 | No |
| rs116950913 | 12 | 132,889,076 | A | − 0.101 | 0.025 | 0.038 | 4.66E − 05 | 16.605 | 0.003 | No |
| rs75782087 | 13 | 38,822,878 | T | − 0.096 | 0.023 | 0.037 | 3.94E − 05 | 16.932 | 0.003 | No |
| rs111259346 | 13 | 39,377,730 | G | − 0.092 | 0.024 | 0.034 | 9.86E − 05 | 15.181 | 0.002 | No |
| rs73241676 | 13 | 82,454,247 | G | 0.055 | 0.014 | 0.131 | 5.36E − 05 | 16.332 | 0.003 | No |
| rs72740509 | 15 | 56,533,519 | T | − 0.055 | 0.014 | 0.123 | 6.59E − 05 | 15.954 | 0.002 | No |
| rs9747501 | 17 | 2,739,061 | A | 0.043 | 0.010 | 0.260 | 3.62E − 05 | 17.085 | 0.003 | No |
| rs12948236 | 17 | 65,633,567 | C | 0.037 | 0.009 | 0.426 | 6.97E − 05 | 15.841 | 0.002 | No |
| rs6507201 | 18 | 35,016,217 | A | 0.038 | 0.009 | 0.359 | 6.26E − 05 | 16.042 | 0.002 | No |
| rs319445 | 18 | 55,360,524 | A | 0.055 | 0.014 | 0.122 | 8.86E − 05 | 15.390 | 0.002 | No |
| rs622989 | 19 | 7,574,308 | C | 0.127 | 0.032 | 0.020 | 6.79E − 05 | 15.875 | 0.002 | No |
| rs34335847 | 19 | 14,705,499 | A | − 0.061 | 0.015 | 0.102 | 4.25E − 05 | 16.780 | 0.003 | No |
| rs45465702 | 20 | 33,320,571 | C | − 0.050 | 0.012 | 0.184 | 2.04E − 05 | 18.189 | 0.003 | No |
| rs78492969 | 20 | 49,248,213 | T | − 0.101 | 0.024 | 0.037 | 2.39E − 05 | 17.868 | 0.003 | No |
| rs62222450 | 21 | 40,471,448 | T | 0.073 | 0.018 | 0.063 | 6.07E − 05 | 16.110 | 0.003 | No |
| rs117884976 | 22 | 28,107,887 | T | − 0.112 | 0.029 | 0.024 | 9.00E − 05 | 15.359 | 0.002 | No |
F-statistic, F-statistic of the SNP-exposure association test, R2, the proportion of variance in the anti-VZV IgG level explained by a given SNP, MAF minor allele frequency
MR results using IVs in the MHC region (IVmhc)
Suggestive causal relationships (P < 0.05 in at least three of the five MR methods) were shown between anti-VZV IgG and 194 traits, among which the results of 86, 104 and 4 traits passed the nominal P value threshold 0.05 in three, four and five MR methods, respectively. Elevated anti-VZV IgG levels increased the risks of cellulitis, disease of the blood and blood-forming organs and certain disorders involving the immune mechanism, mouth/teeth dental problems: dentures and other serious medical condition/disability diagnosed by a doctor (P < 0.05 in all five MR methods).
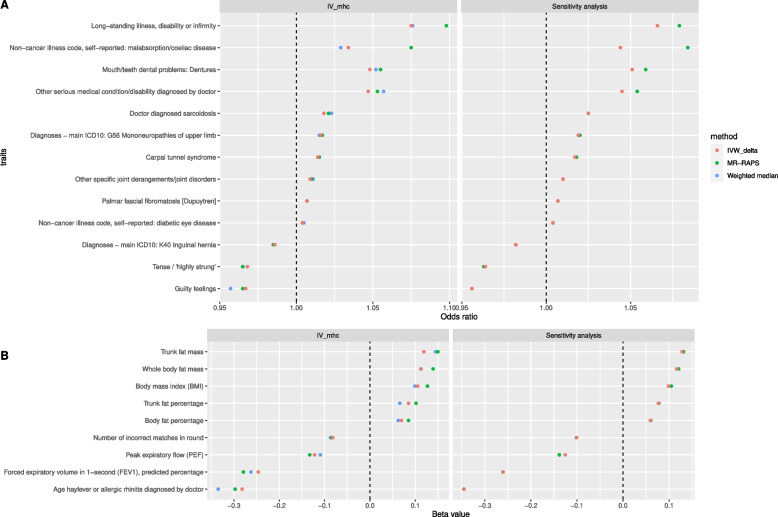
Causal evidence (FDR < 5% in at least three of the five MR methods) was found between anti-VZV IgG and 22 traits, which appeared to be consistent causal evidence because all the results had FDR < 5% in both the MR methods in the sensitivity analysis (Table 2, Fig. 2). Increased level of anti-VZV IgG led to a higher risk of 10 health conditions, including diabetic eye disease, palmar fascial fibromatosis/Dupuytren disease, other specific joint derangements/joint disorders, carpal tunnel syndrome, mononeuropathies of the upper limb, sarcoidosis, malabsorption/coeliac disease, other serious medical conditions/disabilities, mouth/teeth dental problems: dentures and long-standing illness, disability, or infirmity. Anti-VZV IgG also showed a positive causal effect on body fat mass and percentage, trunk fat mass and percentage and body mass index (BMI), which suggested that VZV reactivations increased the chance of being obese.
Table 2.
MR results of traits causally affected by anti-VZV IgG levels
| Phenotype description | Sample size | Case | Control | Source | IV | IVW | MR-Egger | MR-PRESSO | MR-RAPS | Weighted median | |||||
| Beta | FDR | Beta | FDR | Beta | FDR | Beta | FDR | Beta | FDR | ||||||
| Age hay fever or allergic rhinitis diagnosed by a doctor | 20,904 | NA | NA | phesant | IVmhc | − 0.282 | 0.045 | − 0.461 | 0.979 | − 0.29 | 0.329 | − 0.297 | 0.002 | − 0.335 | 0.01 |
| Sensitivity analysis | − 0.345 | 0.004 | NA | NA | NA | NA | − 0.346 | 0.004 | NA | NA | |||||
| Body fat percentage | 354,628 | NA | NA | phesant | IVmhc | 0.07 | 0.043 | 0.023 | 0.994 | 0.08 | 0.359 | 0.085 | 0 | 0.062 | 0.017 |
| Sensitivity analysis | 0.059 | 0.011 | NA | NA | NA | NA | 0.06 | 0.01 | NA | NA | |||||
| Body mass index (BMI) | 359,983 | NA | NA | phesant | IVmhc | 0.105 | 0.027 | 0.197 | 0.979 | 0.119 | 0.332 | 0.127 | 0 | 0.099 | 0.002 |
| Sensitivity analysis | 0.099 | 0.001 | NA | NA | NA | NA | 0.105 | 0 | NA | NA | |||||
| Forced expiratory volume in 1-s (FEV1), predicted percentage | 117,241 | NA | NA | phesant | IVmhc | − 0.246 | 0.019 | − 0.197 | 0.979 | − 0.264 | 0.326 | − 0.279 | 0 | − 0.262 | 0 |
| Sensitivity analysis | − 0.26 | 0 | NA | NA | NA | NA | − 0.261 | 0 | NA | NA | |||||
| Number of incorrect matches in round | 360,686 | NA | NA | phesant | IVmhc | − 0.082 | 0.01 | − 0.057 | 0.979 | − 0.084 | 0.326 | − 0.085 | 0 | − 0.087 | 0.002 |
| Sensitivity analysis | − 0.101 | 0 | NA | NA | NA | NA | − 0.101 | 0 | NA | NA | |||||
| Peak expiratory flow (PEF) | 329,404 | NA | NA | phesant | IVmhc | − 0.122 | 0 | − 0.06 | 0.979 | − 0.129 | 0.301 | − 0.133 | 0 | − 0.109 | 0 |
| Sensitivity analysis | − 0.126 | 0 | NA | NA | NA | NA | − 0.138 | 0 | NA | NA | |||||
| Trunk fat mass | 354,597 | NA | NA | phesant | IVmhc | 0.119 | 0.043 | 0.27 | 0.979 | 0.132 | 0.329 | 0.15 | 0 | 0.144 | 0 |
| Sensitivity analysis | 0.128 | 0 | NA | NA | NA | NA | 0.131 | 0 | NA | NA | |||||
| Trunk fat percentage | 354,619 | NA | NA | phesant | IVmhc | 0.085 | 0.019 | 0.053 | 0.979 | 0.096 | 0.335 | 0.102 | 0 | 0.066 | 0.031 |
| Sensitivity analysis | 0.077 | 0.005 | NA | NA | NA | NA | 0.078 | 0.004 | NA | NA | |||||
| Whole body fat mass | 354,244 | NA | NA | phesant | IVmhc | 0.112 | 0.043 | 0.257 | 0.979 | 0.108 | 0.329 | 0.139 | 0 | 0.113 | 0.001 |
| Sensitivity analysis | 0.116 | 0 | NA | NA | NA | NA | 0.121 | 0 | NA | NA | |||||
| Odds ratio | Odds ratio | Odds ratio | Odds ratio | Odds ratio | |||||||||||
| Carpal tunnel syndrome | 361,194 | 7973 | 353,221 | finngen | IVmhc | 1.014 | 0.045 | 1.022 | 0.979 | 1.014 | 0.329 | 1.015 | 0 | 1.015 | 0.007 |
| Sensitivity analysis | 1.017 | 0 | NA | NA | 1.018 | 0 | NA | ||||||||
| Diagnoses—main ICD10: G56 mononeuropathies of upper limb | 361,194 | 8130 | 353,064 | icd10 | IVmhc | 1.016 | 0.011 | 1.026 | 0.979 | 1.016 | 0.326 | 1.017 | 0 | 1.015 | 0.008 |
| Sensitivity analysis | 1.019 | 0 | NA | NA | 1.02 | 0 | NA | ||||||||
| Diagnoses—main ICD10: K40 Inguinal hernia | 361,194 | 13,147 | 348,047 | icd10 | IVmhc | 0.986 | 0.047 | 0.965 | 0.979 | 0.985 | 0.329 | 0.985 | 0 | 0.985 | 0.025 |
| Sensitivity analysis | 0.982 | 0.001 | NA | NA | 0.982 | 0.001 | NA | ||||||||
| Doctor diagnosed sarcoidosis | 91,787 | 395 | 91,392 | phesant | IVmhc | 1.018 | 0.043 | 1.04 | 0.979 | 1.02 | 0.328 | 1.021 | 0 | 1.023 | 0 |
| Sensitivity analysis | 1.025 | 0 | NA | NA | 1.025 | 0 | NA | ||||||||
| Guilty feelings | 351,907 | 100,128 | 251,779 | phesant | IVmhc | 0.967 | 0.045 | 0.966 | 0.979 | 0.966 | 0.329 | 0.965 | 0.001 | 0.957 | 0.002 |
| Sensitivity analysis | 0.956 | 0.001 | NA | NA | 0.956 | 0.001 | NA | ||||||||
| Long-standing illness, disability or infirmity | 352,798 | 114,798 | 238,000 | phesant | IVmhc | 1.075 | 0.043 | 1.099 | 0.979 | 1.094 | 0.326 | 1.098 | 0 | 1.076 | 0 |
| Sensitivity analysis | 1.066 | 0 | NA | NA | 1.079 | 0 | NA | ||||||||
| Mouth/teeth dental problems: dentures | 359,841 | 60,977 | 298,864 | phesant | IVmhc | 1.048 | 0.01 | 1.118 | 0.979 | 1.052 | 0.326 | 1.055 | 0 | 1.052 | 0.001 |
| Sensitivity analysis | 1.051 | 0 | NA | NA | 1.059 | 0 | NA | ||||||||
| Non-cancer illness code, self-reported: diabetic eye disease | 361,141 | 699 | 360,442 | phesant | IVmhc | 1.004 | 0.001 | 1.006 | 0.979 | 1.004 | 0.301 | 1.004 | 0 | 1.005 | 0 |
| Sensitivity analysis | 1.004 | 0.001 | NA | NA | 1.004 | 0.001 | NA | ||||||||
| Non-cancer illness code, self-reported: malabsorption/coeliac disease | 361,141 | 1587 | 359,554 | phesant | IVmhc | 1.034 | 0.045 | 1.12 | 0.979 | 1.036 | 0.484 | 1.075 | 0 | 1.029 | 0 |
| Sensitivity analysis | 1.044 | 0 | NA | NA | 1.084 | 0 | NA | ||||||||
| Other serious medical condition/disability diagnosed by a doctor | 354,588 | 72,965 | 281,623 | phesant | IVmhc | 1.047 | 0.043 | 1.119 | 0.979 | 1.051 | 0.329 | 1.053 | 0 | 1.057 | 0 |
| Sensitivity analysis | 1.045 | 0 | NA | NA | 1.054 | 0 | NA | ||||||||
| Other specific joint derangements/joint disorders | 361,194 | 7943 | 353,251 | finngen | IVmhc | 1.009 | 0.05 | 1.008 | 0.979 | 1.009 | 0.326 | 1.01 | 0.011 | 1.011 | 0.022 |
| Sensitivity analysis | 1.01 | 0.042 | NA | NA | 1.01 | 0.04 | NA | ||||||||
| Palmar fascial fibromatosis [Dupuytren] | 361,194 | 2948 | 358,246 | finngen | IVmhc | 1.007 | 0.01 | 1.006 | 0.979 | 1.007 | 0.325 | 1.007 | 0 | 1.007 | 0.027 |
| Sensitivity analysis | 1.007 | 0.02 | NA | NA | 1.007 | 0.022 | NA | ||||||||
| Tense/ ‘highly strung’ | 350,159 | 60,513 | 289,646 | phesant | IVmhc | 0.968 | 0.043 | 0.956 | 0.979 | 0.966 | 0.329 | 0.965 | 0 | 0.965 | 0.005 |
| Sensitivity analysis | 0.964 | 0.001 | NA | NA | 0.963 | 0.001 | NA | ||||||||
FDR Benjamini-Hochberg’s false discovery rate
NA not applicable
IVW inverse-variance weighted method, IVmhc instrumental variables using SNPs in the MHC region
Fig. 2.
Traits causally affected by anti-VZV IgG levels. Listed are traits with statistical evidence (FDR < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables inside the MHC region (IVmhc) (left panel). The results are consistent in the sensitivity analyses (right panel). Odds ratio (A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times
Negative causal associations with anti-VZV IgG levels were found in seven traits. A higher level of anti-VZV IgG resulted in a diagnosis of hay fever or allergic rhinitis at a younger age and worse performance in the expiratory test including predicted FEV1 percentage and peak expiratory flow (PEF), meaning that VZV reactivation might have a detrimental effect on respiratory functions. However, anti-VZV IgG appeared to be a protective factor of four traits because those with higher anti-VZV IgG had a smaller number of incorrect matches in intelligence tests and a lower chance of having hernia, guilty feelings and tension.
MR results using IVs not in the MHC region (IVno.mhc)
By using IVno.mhc, we found suggestive causal evidence between anti-VZV IgG levels and 56 traits, among which the results of 42, 12 and 2 traits passed the nominal P value threshold in three, four and five MR methods, respectively. The suggestive causal associations of two traits (body fat percentage and mouth/teeth dental problems: dentures) with anti-VZV IgG were also found when using IVmhc (Table 2) and with the estimated causal effects on the contrary direction. None of these suggestive causal signals remained after FDR correction.
MR results using IVs from all regions (IVfull)
Suggestive evidence for a causal relationship with anti-VZV IgG levels was found in 92 traits, among which 33 passed the nominal P value threshold in 4 MR methods and 9 traits in all five methods. Three of the traits (other specific joint derangements/joint disorders, malabsorption/coeliac disease and tension) showing suggestive causal associations with anti-VZV IgG also showed the same evidence when using IVmhc (Table 2) and with the estimated causal effects in the same directions. Again, no causal evidence passed FDR correction.
Comparisons of MR results using different sets of IVs (IVmhc, IVno.mhc, IVfull)
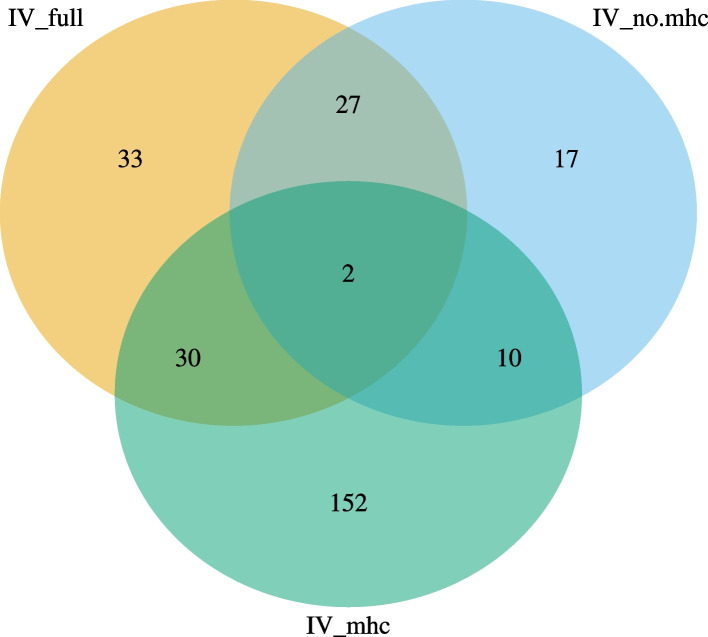
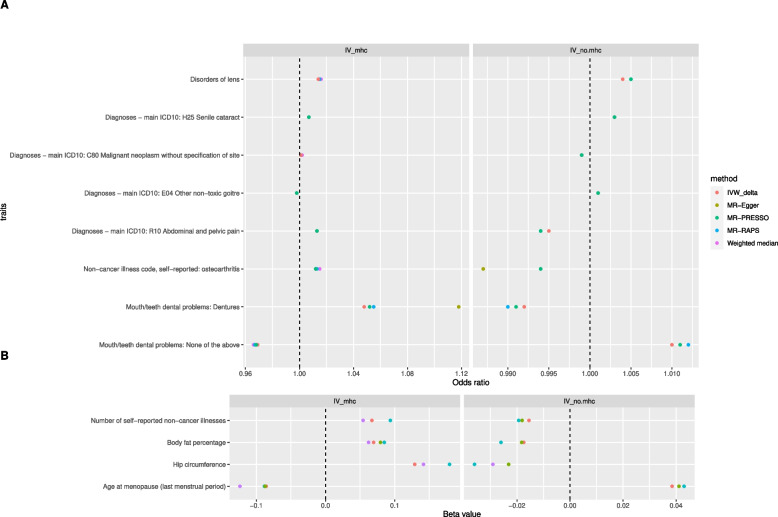
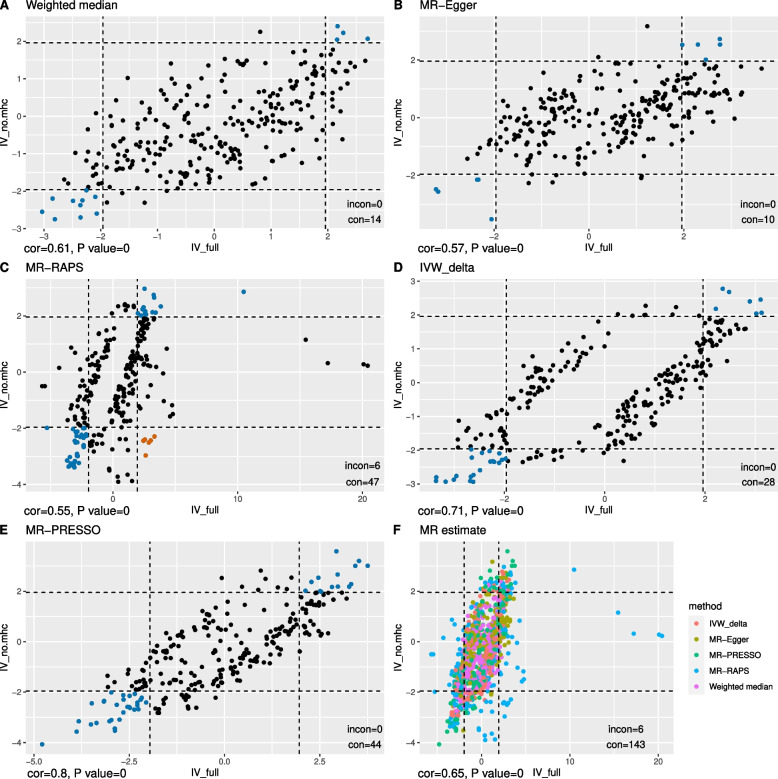
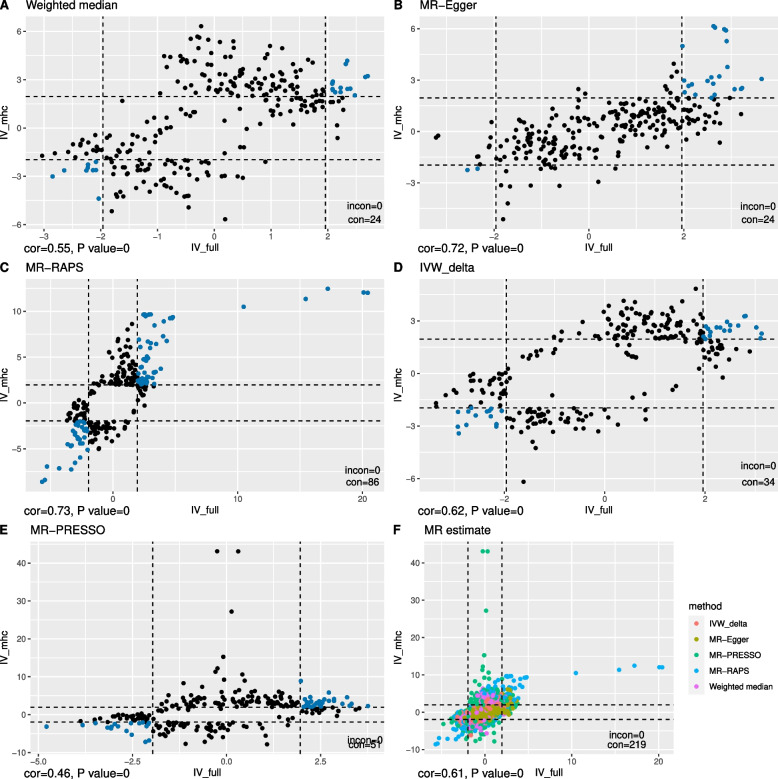
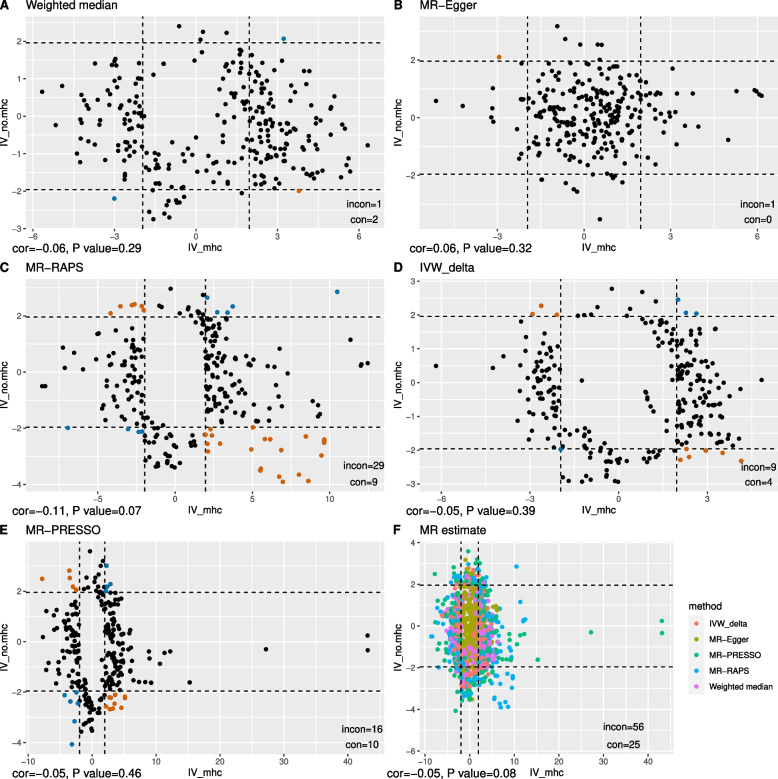
In total, 271 traits had suggestive causal relationships with anti-VZV IgG (Fig. 3, Additional file 2: Table S3). Increased risks of senile cataract and disease of lens were attributed to a high level of anti-VZV IgG, using any one of the three sets of IVs. Besides, suggestive causal evidence was shown in 29 traits whether using IVfull or IVno.mhc (Additional file 3: Figure S1), and in 32 traits whether using IVfull or IVmhc (Additional file 3: Figure S2). Interestingly, the estimated causal effects of anti-VZV on ten traits from IVmhc and from IVno.mhc were in the opposite direction (Fig. 4). Overall, there was a correlation between MR estimates from IVfull and those from IVno.mhc (r = 0.65, P = 3.7 × 10−165) or between those from IVfull and from IVmhc (r = 0.61, P = 5.9 × 10−139). The causal associations of 6 traits estimated from MR-RAPs were in the opposite directions between IVfull and IVno.mhc (Fig. 5C) while 143 MR estimates between IVfull and IVno.mhc were in the same directions (Fig. 5F). The concordance of the MR estimates between IVfull and IVmhc was pronounced to a high degree, as all the 219 MR estimates had the same signs (Fig. 6), but less so between IVmhc and IVno.mhc with 56 estimated causal effects in the opposite directions and no statistically significant correlations (r = − 0.05, P = 0.08) (Fig. 7).
Fig. 3.
Venn diagram of suggestive causal results using IVs inside, outside MHC region or all IVs. The Venn diagram showed the number of traits that were suggestively casually associated (P < 0.05 in at least three Mendelian randomization methods) with anti-VZV IgG levels using instrumental variables inside MHC region (IVmhc) (green circle), outside MHC region (IVno.mhc) (blue circle) and combined IVs (IVfull) (yellow circle). Numbers in the overlapping region represent the causal traits identified by both or all IV sets
Fig. 4.
Traits suggestively causally affected by anti-VZV IgG levels using IVs inside or outside the MHC region. Listed are traits with suggestive statistical evidence (P < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables inside the MHC region (IVmhc) (left panel) or outside the MHC region (IVno.mhc) (right panel). Odds ratio (A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times. Except for disorders of lens and senile cataract, the causal estimates of other traits with the exposure are in the opposite directions between IVmhc and IVno.mhc
Fig. 5.
Comparisons between MR estimates inferred from all IVs and IVs outside the MHC region. Scatter plot of the Z statistics of MR estimates of traits showing suggestive causal evidence with anti-VZV IgG levels using IVs outside MHC region (IVno.mhc, vertical axis) or all IVs (IVfull, horizontal axis). A–E Results inferred from one MR method, with blue and orange dots indicating consistent (in the same causal directions) or inconsistent (in the opposite causal directions) MR results separately. F The combined MR results inferred from different methods, which are suggested by different colours. The dashed lines are the 95% confidence interval (CI) of Z statistics. Dots outside the CI are statistically significant (P < 0.05) MR results. The annotation on the bottom-left of each figure shows the Spearman correlation estimate (cor) and P-value of the correlation test. The legend on the bottom-right of each figure represents the number of the results that are statistically consistent (con) and statistically inconsistent (incon)
Fig. 6.
Comparisons between MR estimates inferred from all IVs and IVs inside the MHC region. Scatter plot of the Z statistics of MR estimates of traits showing suggestive causal evidence with anti-VZV IgG levels using IVs outside MHC region (IVmhc, vertical axis) or all IVs (IVfull, horizontal axis). A–E The results inferred from one MR method, with blue and orange dots indicating consistent (in the same causal directions) or inconsistent (in the opposite causal directions) MR results separately. F The combined MR results inferred from different methods, which are suggested by different colours. The dashed lines are the 95% confidence interval (CI) of Z statistics. Dots outside the CI are statistically significant (P < 0.05) MR results. The annotation on the bottom-left of each figure shows the Spearman correlation estimate (cor) and P-value of the correlation test. The legend on the bottom-right of each figure represents the number of the results that are statistically consistent (con) and statistically inconsistent (incon)
Fig. 7.
Comparisons between MR estimates inferred from IVs inside and outside the MHC region. Scatter plot of the Z statistics of MR estimates of traits showing suggestive causal evidence with anti-VZV IgG levels using IVs inside (IVmhc, horizontal axis) or outside the MHC region (IVno.mhc, vertical axis). A–E The results inferred from one MR method, with blue and orange dots indicating consistent (in the same causal directions) or inconsistent (in the opposite causal directions) MR results separately. F The combined MR results inferred from different methods, which are suggested by different colours. The dashed lines are the 95% confidence interval (CI) of Z statistics. Dots outside the CI are statistically significant (P < 0.05) MR results. The annotation on the bottom-left of each figure shows the Spearman correlation estimate (cor) and P-value of the correlation test. The legend on the bottom-right of each figure represents the number of the results that are statistically consistent (con) and statistically inconsistent (incon)
Discussion
Among the traits that showed a positive causal association with anti-VZV IgG, six (Dupuytren disease, mononeuropathies of upper limb, sarcoidosis, coeliac disease, teeth problems and led to earlier onset of allergic rhinitis) were reported in the literature. In particular, the onset of herpes leading to tooth exfoliation was reported in many patients, as the virus could spread to the trigeminal nerve and cause damage to the tooth nerve [27, 28]. When the anti-VZV level was high, the increased risks of upper limp mononeuropathy could be explained by an uncommon complication of herpes zoster infections, zoster-associated mononeuropathy (ZAM) [29]. Although uncommon, herpes zoster directly leading to mononeuropathy was found in a few clinical cases. Patients having ZAM suffered from viral spreading to the peripheral nerves [30]. Dupuytren disease is a condition that affects the fascia and causes deformity to the hand, though the causes of itself remain unknown [31]. A positive association between the risk of herpes zoster and plantar fascial fibromatosis incidence was identified in the literature [32], but no causal evidence was ever reported. Coeliac disease, sarcoidosis and allergic rhinitis are the consequences of dysregulation of the immune system. Coeliac disease and sarcoidosis are caused by the immune system attacking the intestine and multiple systems, respectively. Allergic rhinitis often refers to the overreacts of the immune system in the upper respiratory tract. There has been extensive discussion about the correlation between the incidence of herpes zoster reactivations and autoimmune diseases [33, 34]. VZV reactivation can induce different types of sarcoidosis, such as scar sarcoidosis [35] and central nervous system sarcoidosis [36]. The incidence of herpes zoster was positively correlated with coeliac disease [37] and allergic rhinitis [38]. All the evidence from these studies supported our causal findings. However, the relationships between herpes infections and these autoimmune diseases might not be simply unidirectional. For example, the virus itself might increase the risk of flares in autoimmune diseases like systemic lupus erythematosus [39]. In addition, herpes zosters are more likely to reactivate in these patients as they might take immunosuppressive medications [40]. However, the immunosuppressive treatment also decreases the antibody levels in one’s body [41]. Therefore, interactions between immune responses to infections, autoimmune diseases and immunosuppressive treatments could be potentially considered in future studies.
Causal associations between VZV infections and eight traits (number of incorrect matches in pairs matching [42, 43], guilty/tense feeling [8, 44, 45], BMI [46, 47] and body/trunk fat mass and percentage) were inconclusive from previous studies. The direct causal relationships of four traits (carpal tunnel syndrome, FEV1, PEF, other specific joint disorders) and VZV were not reported before, but they may have plausible biological links [48–51]. In addition, we discovered that inguinal hernia and diabetic eye disorder may be causally associated with VZV immune response. The definitions of other serious medical conditions/disabilities diagnosed by a doctor and long-standing illness, disability or infirmity were unclear and require further explorations. Our MR results provided new insight into the causal relationships between VZV immune response and these traits, which however need to be replicated in other independent studies.
The role of the MHC region plays in MR studies had largely been overlooked [52]. In some studies, IVs in the MHC region were simply removed to minimize the risk of bias due to the pleiotropy introduced by these SNPs [15, 53]. However, some MHC SNPs may be strongly associated with exposure, especially for immunity-related traits [13]. Here, we explored the impact of MHC instruments by performing three separate MR analyses using IVfull, IVmhc and IVno.mhc. For the traits showing a suggestive causal relationship with anti-VZV IgG levels, there were substantial differences in the results among the three IV sets. Evidence for a correlation of the estimated causal effects was shown between IVfull and IVno.mhc and also between IVfull and IVmhc, with approximately one-third of the traits showing statistically significant (P < 0.05) causal relationships with anti-VZV IgG and in the same directions. However, using the two subsets (IVno.mhc and IVmhc) cannot fully identify all the causal relationships found using IVfull. In fact, each subset had its specific causal findings. The number of traits that showed suggestive causal evidence with VZV immune response was almost four times larger using IVs from the MHC region than from non-MHC regions. As the IVs in the MHC regions have stronger associations with anti-VZV IgG, it was not unexpected that causal relationships were detected in a higher number of traits when using IVmhc. Interestingly, there was a discrepancy in causal estimates from IVmhc and IVno.mhc with opposite causal directions shown in ten traits. This might indicate that IVs in and outside the MHC region drive different mechanisms or intermediaries behind anti-VZV IgG levels. For example, MHC SNPs might be more involved in the immune responses in one’s body [54] while non-MHC SNPs be more associated with general health conditions that also affect the antibody levels [55]. Taken together, MHC instruments might play a different but important role in affecting the antibody levels and subsequently the clinical outcomes. Partitioning the IVs by MHC region might lead to conclusions that are different from the MR results using all the IVs. It would be advisable to investigate this separately in future MR studies. Moreover, as MHC IVs can potentially have an impact on MR analysis, imputing the region using specialist software would enhance accuracy and resolution for causal findings [56].
It is worth noting that suggestive causal relationships between some traits and anti-VZV IgG shown in this study were already reported previously (e.g. stroke [57] and systemic lupus erythematosus [39]) (Additional file 2: Table S3). However, the evidence we found was not strong enough possibly due to the complicated causal mechanisms that weakened the marginal causal effect size and therefore reduced the power to detect them in a phenome-wide MR study. Previous GWAS identified five SNPs associated with anti-VZV IgG levels [13], four (rs13197633, rs13204572, rs1048381 and rs56401801) of which were also found associated with anti-VZV IgG levels in our study. Rs34073492, also identified in previous GWAS, was however removed from analysis post-QC (MAF > 0.02).
The anti-VZV IgG levels in our study population were not likely driven by vaccination. This is because the age group enrolled in the UK national programme for shingles vaccination is between 70 and 79 years [58], while the participants for the UK biobank cohort were aged 40–69 when first enrolled.
Multiple testing decreases type I error but increases type II error [59]. It would be excessively conservative to use a stringent statistical significance threshold in MR studies which are in general of low power [60], with a risk of not detecting potentially important causal relationships. Therefore, in this exploratory study, we also reported MR results with a P-value less than 0.05 in the supplementary tables to minimize the reporting bias and to enable researchers to take forward our findings for further investigations in their studies.
Limitations
We used the anti-VZV IgG level to represent both seropositivity of VZV and the lifetime exposure to the virus. The anti-VZV IgG level was expected to represent the level of VZV reactivations or immune responses to VZV, which could, however, be affected by vaccination and other factors. In our study population, the assumption of anti-VZV IgG not driven by vaccination was plausible because the age group enrolled in the UK national programme for shingles vaccination was later than those first enrolled in the UK biobank. However, the fact that anti-VZV IgG levels can be affected by other factors in addition to vaccination makes the interpretation of the measurement difficult. Except for blocking infections, antibodies may have other meaningful functions to the host. They might work interactively with cells of the immune system for a more effective protest against pathogens or contribute to immunity with different unknown mechanisms [61, 62]. A high anti-VZV IgG level may also indicate a healthy immune function. Distinct causal effects could be inferred from IV clusters when the risk is not a single entity but a composite factor with multiple components [55]. In our study, the differences in causal results inferred from IVmhc and IVno.mhc may be explained by different causal pathways behind VZV immunity.
All consistent causal findings (FDR < 0.05 in at least three of the five MR methods and FDR < 0.05 in both the MR methods in sensitivity analysis) were detected using IVmhc but not from IVs outside the MHC region. One possible reason would be that the instruments in the MHC region had stronger associations with anti-VZV IgG. It might also be that the results were biassed due to pleiotropy.
Conclusions
Our phenome-wide MR study found anti-VZV IgG to be a causal risk factor of 22 traits, which helped gain new insights into the causal roles of VZV-specific immunity in diseases. While some of the causal relationships have priori biological explanations, others require further investigations. When selecting IVs for MR analysis, separating SNPs from the MHC region may potentially provide information on different causal pathways, and help better understand the specific role of the MHC region in future MR studies.
Supplementary Information
Additional file 1: Text S1. Traits inclusion and exclusion criteria for the phenome-wide Mendelian randomization study. Code S1. R code for the Mendelian Randomization analysis.
Additional file 2: Table S1. SNPs associated with anti-VZV IgG levels identified by the Genome-wide association scan. Table S2. Full results of the phenome-wide Mendelian randomization analyses of 1,370 traits. Table S3. Traits showed a suggestive causal relationship with anti-VZV IgG levels.
Additional file 3: Figure S1. Traits suggestively causally affected by anti-VZV IgG levels using IVs outside MHC region or all regions. Listed are traits with suggestive statistical evidence (P < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables from all regions (IVfull) (left panel) or outside MHC region (IVno.mhc) (right panel). Odds ratio (Panel A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (Panel B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times. Figure S2. Traits suggestively causally affected by anti-VZV IgG levels using IVs inside MHC region or all regions. Listed are traits with suggestive statistical evidence (P < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables from all regions (IVfull) (left panel) or inside MHC region (IVmhc) (right panel). Odds ratio (Panel A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (Panel B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times.
Acknowledgements
We would like to thank the UK Biobank participants and staff. This study used the UK Biobank application number 5864. We also thank the NHGRI-EBI and the Neale Lab for making GWAS results publicly accessible.
Abbreviations
- BMI
Body mass index
- FDR
False discovery rate
- FEV1
Forced expiratory volume in 1-s
- GWAS
Genome-wide association study
- IgG
Immunoglobulin G
- IV
Instrumental variable
- IVW
Inverse-variance weighted
- MAF
Minor allele frequency
- MHC
Histocompatibility complex
- MR
Mendelian randomization
- MR-PheWAS
Mendelian randomization phenome-wide association study
- PEF
Peak expiratory flow
- SNP
Single nucleotide polymorphism
- VZV
Varicella-zoster virus
- ZAM
Zoster-associated mononeuropathy
Authors’ contributions
XY, HG, AL and KRM conceived the study. HG supervised the statistical analysis. XY and KM performed the data analysis. All authors interpreted the results and wrote the first draft of the manuscript. XY, HG, AL and KRM reviewed and edited the manuscript. The authors read and approved the final manuscript.
Funding
XY, KRM, AL and KM were supported by the Advantage Foundation.
Availability of data and materials
Publicly available data from the UK Biobank study was analysed in this study. The datasets are available to researchers through an open application via https://www.ukbiobank.ac.uk/enable-your-research/register. Publicly available GWAS summary statistics used in this study is available at http://www.nealelab.is/uk-biobank. The R code used for Mendelian randomization analyses is in Additional file 1: Code S1.
Declarations
Ethics approval and consent to participate
All participants gave written informed consent prior to data collection. The UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kenneth R. Muir and Hui Guo contributed equally to this work.
References
- 1.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol. 2013;15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambade P, Lambade D, Saha TK, Dolas RS, Pandilwar PK. Maxillary osteonecrosis and spontaneous teeth exfoliation following herpes zoster. Oral Maxillofac Surg. 2012;16:369–372. doi: 10.1007/s10006-011-0303-8. [DOI] [PubMed] [Google Scholar]
- 4.Kurimoto T, Tonari M, Ishizaki N, Monta M, Hirata S, Oku H, et al. Orbital apex syndrome associated with herpes zoster ophthalmicus. Clin Ophthalmol. 2011;5:1603–1608. doi: 10.2147/OPTH.S25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind L, Eriksson K, Grahn A. Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by varicella-zoster virus. J Neuroinflammation. 2019;16:42. doi: 10.1186/s12974-019-1428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilden D, Nagel MA, Ransohoff RM, Cohrs RJ, Mahalingam R, Tanabe JL. Recurrent varicella zoster virus myelopathy. J Neurol Sci. 2009;276:196–198. doi: 10.1016/j.jns.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang J-H, Ho J-D, Chen Y-H, Lin H-C. Increased risk of stroke after a herpes zoster attack. Stroke. 2009;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 8.Chen M-H, Wei H-T, Su T-P, Li C-T, Lin W-C, Chang W-H, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76:285–291. doi: 10.1097/PSY.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 9.Hammer C, Begemann M, McLaren PJ, Bartha I, Michel A, Klose B, et al. Amino acid variation in HLA class II proteins is a major determinant of humoral response to common viruses. Am J Hum Genet. 2015;97:738–743. doi: 10.1016/j.ajhg.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scepanovic P, Alanio C, Hammer C, Hodel F, Bergstedt J, Patin E, et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10:59. doi: 10.1186/s13073-018-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kachuri L, Francis SS, Morrison ML, Wendt GA, Bossé Y, Cavazos TB, et al. The landscape of host genetic factors involved in immune response to common viral infections. Genome Med. 2020;12:93. doi: 10.1186/s13073-020-00790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing KJ, Ouwendijk WJD, Koelle DM, Verjans GMGM. Immunobiology of varicella-zoster virus infection. J Infect Dis. 2018;218 suppl_2:S68–74. [DOI] [PMC free article] [PubMed]
- 13.Butler-Laporte G, Kreuzer D, Nakanishi T, Harroud A, Forgetta V, Richards JB. Genetic determinants of antibody-mediated immune responses to infectious diseases agents: a genome-wide and HLA association study. Open Forum Infect Dis. 2020;7:ofaa450. [DOI] [PMC free article] [PubMed]
- 14.Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18:325–339. doi: 10.1038/nri.2017.143. [DOI] [PubMed] [Google Scholar]
- 15.Harroud A, Richards JB, Baranzini SE. Mendelian randomization study shows no causal effects of serum urate levels on the risk of MS. Neurol - Neuroimmunol Neuroinflammation. 2021;8. [DOI] [PMC free article] [PubMed]
- 16.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. 2020;48:1742–1769. doi: 10.1214/19-AOS1866. [DOI] [Google Scholar]
- 19.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44:313–329. doi: 10.1002/gepi.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed]
- 22.Brenner N, Waterboer T. Serological measurement of infectious agents in UK Biobank: a pilot study in 10 000 samples. 2019.
- 23.Julian TH, Boddy S, Islam M, Kurz J, Whittaker KJ, Moll T, et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain. 2022;145:832–842. doi: 10.1093/brain/awab420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Genome Reference Consortium. The Genome Reference Consortium. https://www-ncbi-nlm-nih-gov.manchester.idm.oclc.org/grc. Accessed 15 Dec 2022.
- 25.Hemani G, Tilling K, Smith GD. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLOS Genet. 2017;13:e1007081. doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 27.Volvoikar P, Patil S, Dinkar A. Tooth exfoliation, osteonecrosis and neuralgia following herpes zoster of trigeminal nerve. Indian J Dent Res. 2002;13:11–14. [PubMed] [Google Scholar]
- 28.Mendieta C, Miranda J, Brunet L l., Gargallo J, Berini L. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontol. 2005;76:148–53. [DOI] [PubMed]
- 29.Alshekhlee A, Tay E, Buczek M, Shakir ZA, Katirji B. Herpes zoster with motor involvement: discordance between the distribution of skin rash and localization of peripheral nervous system dysfunction. J Clin Neuromuscul Dis. 2011;12:153–157. doi: 10.1097/CND.0b013e31820d4f31. [DOI] [PubMed] [Google Scholar]
- 30.Reda H, Watson JC, Jones LK., Jr Zoster-associated mononeuropathies (ZAMs): a retrospective series. Muscle Nerve. 2012;45:734–739. doi: 10.1002/mus.23342. [DOI] [PubMed] [Google Scholar]
- 31.Broekstra DC, Groen H, Molenkamp S, Werker PMN, van den Heuvel ER. A systematic review and meta-analysis on the strength and consistency of the associations between Dupuytren disease and diabetes mellitus, liver disease, and epilepsy. Plast Reconstr Surg. 2018;141:367–379. doi: 10.1097/PRS.0000000000004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu C-Y, Ke D-S, Lin C-L, Kao C-H. Plantar fascial fibromatosis and herpes zoster. PLoS ONE. 2021;16:e0259942. doi: 10.1371/journal.pone.0259942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gepner P, Piette AM, Patrat JF, Graveleau P, Chapman A. Does arthritis due to varicella zoster virus exist? Rev Rhum Mal Osteoartic. 1992;59:735–737. [PubMed] [Google Scholar]
- 34.Krasselt M, Baerwald C, Liebert UG, Seifert O. Humoral immunity to varicella zoster virus is altered in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38:2493–2500. doi: 10.1007/s10067-019-04563-9. [DOI] [PubMed] [Google Scholar]
- 35.Holzmann RD, Astner S, Forschner T, Sterry G. Scar sarcoidosis in a child: case report of successful treatment with the pulsed dye laser. Dermatol Surg. 2008;34:393–396. doi: 10.1111/j.1524-4725.2007.34077.x. [DOI] [PubMed] [Google Scholar]
- 36.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludvigsson JF, Choung RS, Marietta EV, Murray JA, Emilsson L. Increased risk of herpes zoster in patients with coeliac disease – nationwide cohort study. Scand J Public Health. 2018;46:859–866. doi: 10.1177/1403494817714713. [DOI] [PubMed] [Google Scholar]
- 38.Joesoef RM, Harpaz R, Leung J, Bialek SR. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc. 2012;87:961–967. doi: 10.1016/j.mayocp.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun F, Chen Y, Wu W, Guo L, Xu W, Chen J, et al. Varicella zoster virus infections increase the risk of disease flares in patients with SLE: a matched cohort study. Lupus Sci Med. 2019;6:e000339. doi: 10.1136/lupus-2019-000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarty EF. Incidence and prevention of herpes zoster reactivation in patients with autoimmune diseases. Rheum Dis Clin N Am. 2017;43:111–121. doi: 10.1016/j.rdc.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Kareva I, Zutshi A, Mateo CV, Papasouliotis O. Identifying safety thresholds for immunosuppressive drugs: applying insights from primary antibody deficiencies to mitigate adverse events in secondary antibody deficiencies using mathematical modeling of preclinical and early clinical data. Eur J Drug Metab Pharmacokinet. 2021;46:601–611. doi: 10.1007/s13318-021-00706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grahn A, Nilsson S, Nordlund A, Lindén T, Studahl M. Cognitive impairment 3 years after neurological Varicella-zoster virus infection: a long-term case control study. J Neurol. 2013;260:2761–2769. doi: 10.1007/s00415-013-7057-1. [DOI] [PubMed] [Google Scholar]
- 43.Eckerström M, Nilsson S, Zetterberg H, Blennow K, Grahn A. Cognitive impairment without altered levels of cerebrospinal fluid biomarkers in patients with encephalitis caused by varicella-zoster virus: a pilot study. Sci Rep. 2020;10:22400. doi: 10.1038/s41598-020-79800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. 2012;34:1–17. doi: 10.1007/BF03391675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun. 2011;25:759–766. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freuer D, Linseisen J, Waterboer T, Pessler F, Guzmán CA, Wawro N, et al. Seropositivity of selected chronic infections and different measures of obesity. PLoS ONE. 2020;15:e0231974. doi: 10.1371/journal.pone.0231974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt SAJ, Sørensen HT, Langan SM, Vestergaard M. Associations of lifestyle and anthropometric factors with the risk of herpes zoster: a nationwide population-based cohort study. Am J Epidemiol. 2021;190:1064–1074. doi: 10.1093/aje/kwab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell D. Presentation of varicella-zoster mimicking carpal tunnel syndrome. J Hand Surg Eur. 2007;32:233–234. doi: 10.1016/J.JHSB.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Davis AR, Sheppard J. Herpes zoster ophthalmicus review and prevention. Eye Contact Lens. 2019;45:286–291. doi: 10.1097/ICL.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 50.Mohsen AH, McKendrick M. Varicella pneumonia in adults. Eur Respir J. 2003;21:886–891. doi: 10.1183/09031936.03.00103202. [DOI] [PubMed] [Google Scholar]
- 51.Mirouse A, Vignon P, Piron P, Robert R, Papazian L, Géri G, et al. Severe varicella-zoster virus pneumonia: a multicenter cohort study. Crit Care. 2017;21:137. doi: 10.1186/s13054-017-1731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Guo Z, Zheng Y, Yu C, Hou H, Chen B. No casual relationship between T2DM and the risk of infectious diseases: a two-sample Mendelian randomization study. Front Genet. 2021;12:720874. [DOI] [PMC free article] [PubMed]
- 53.Binzer S, Jiang X, Hillert J, Manouchehrinia A. Depression and multiple sclerosis: a bidirectional Mendelian randomisation study. Mult Scler J. 2021;27:1799–1802. doi: 10.1177/1352458521996601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley CN, Mason AM, Kirk PDW, Burgess S. MR-Clust: clustering of genetic variants in Mendelian randomization with similar causal estimates. Bioinformatics. 2021;37:531–541. doi: 10.1093/bioinformatics/btaa778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naito T, Okada Y. HLA imputation and its application to genetic and molecular fine-mapping of the MHC region in autoimmune diseases. Semin Immunopathol. 2022;44:15–28. doi: 10.1007/s00281-021-00901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagel MA, Bubak AN. Varicella zoster virus vasculopathy. J Infect Dis. 2018;218 suppl_2:S107–12. [DOI] [PMC free article] [PubMed]
- 58.Green_book_of_immunisation_28a_Shingles.pdf. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1012943/Green_book_of_immunisation_28a_Shingles.pdf. Accessed 9 May 2022.
- 59.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186. doi: 10.12688/wellcomeopenres.15555.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125 2 0 2:S41–52. [DOI] [PMC free article] [PubMed]
- 62.Webb NE, Bernshtein B, Alter G. Tissues: the unexplored frontier of antibody mediated immunity. Curr Opin Virol. 2021;47:52–67. doi: 10.1016/j.coviro.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Text S1. Traits inclusion and exclusion criteria for the phenome-wide Mendelian randomization study. Code S1. R code for the Mendelian Randomization analysis.
Additional file 2: Table S1. SNPs associated with anti-VZV IgG levels identified by the Genome-wide association scan. Table S2. Full results of the phenome-wide Mendelian randomization analyses of 1,370 traits. Table S3. Traits showed a suggestive causal relationship with anti-VZV IgG levels.
Additional file 3: Figure S1. Traits suggestively causally affected by anti-VZV IgG levels using IVs outside MHC region or all regions. Listed are traits with suggestive statistical evidence (P < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables from all regions (IVfull) (left panel) or outside MHC region (IVno.mhc) (right panel). Odds ratio (Panel A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (Panel B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times. Figure S2. Traits suggestively causally affected by anti-VZV IgG levels using IVs inside MHC region or all regions. Listed are traits with suggestive statistical evidence (P < 0.05) in at least three Mendelian randomization (MR) methods, using instrumental variables from all regions (IVfull) (left panel) or inside MHC region (IVmhc) (right panel). Odds ratio (Panel A, horizontal axis), for a binary case/control trait, represents the estimated odds ratio when the anti-VZV IgG levels increase by 9 times. Beta value (Panel B, horizontal axis), for a continuous trait, represents the estimated change in the tested trait when the anti-VZV IgG levels increase by 9 times.
Data Availability Statement
Publicly available data from the UK Biobank study was analysed in this study. The datasets are available to researchers through an open application via https://www.ukbiobank.ac.uk/enable-your-research/register. Publicly available GWAS summary statistics used in this study is available at http://www.nealelab.is/uk-biobank. The R code used for Mendelian randomization analyses is in Additional file 1: Code S1.