Abstract
Simple Summary
The genus Dila Fischer von Waldheim of 1844 belongs to the tribe Blaptini Leach of 1815 within the subfamily Blaptinae. Comprising 22 species, this genus is broadly distributed in Southeastern Turkey, Iraq, Iran, and Central Asia. However, Dila is poorly known in China; only three species have been described from Jomda of Xizang, Dêgê, Sichuan and Xinjiang, China. In this study, the larva was associated with the adults using a combined molecular dataset. The larva and adult males and females of the new species are described and illustrated from the southwestern Himalayas. To date, the phylogenetic relationships within Blaptini is not well supported by molecular evidence. A preliminary phylogeney was hypothesized and discussed based on a molecular dataset with seven related genera of the tribe Blaptini. The monophyly of the subtribe Dilina and the taxonomic status of D. bomina are briefly discussed.
Abstract
In this study, a new species of the genus Dila Fischer von Waldheim, 1844, D. ngaria Li and Ren sp. n., was described from the southwestern Himalayas. The adult and larva were associated using molecular phylogenetic analyses based on fragments of three mitochondrial and one nuclear gene fragment (COI, Cytb, 16S and 28S-D2). Additionally, a preliminary phylogenetic tree was reconstructed and discussed based on a molecular dataset with seven related genera and 24 species of the tribe Blaptini. Meanwhile, the monophyly of the subtribe Dilina and the taxonomic status of D. bomina Ren and Li, 2001 are discussed. This work provides new molecular data for phylogenetic studies on the tribe Blaptini in the future.
Keywords: Dila, new species, morphological description, DNA sequence
1. Introduction
The genus Dila Fischer von Waldheim of 1844 belongs to the tribe Blaptini Leach of 1815 within the subfamily Blaptinae. The type species Dila laevicollis Gebler, found in 1841, is broadly distributed in Afghanistan, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Turkmenistan and Xinjiang, China [1]. Chigray (2019) revised the genus, considered Caenoblaps as a junior synonym of Dila, transferred four species from Caenoblaps to Dila, and described three new species from Turkey and Iran [2]. To date, the genus Dila comprises 22 species distributed in the mountain regions of Southeastern Turkey, Iraq, Iran, and Central Asia (Tien-Shan, Pamir-Alay, Western Himalaya, and Tibet) [2,3,4]. These species were described by Baudi di Selve (one species), Blair (one species), Chigray (three species), Gebler (one species), Kaszab (three species), König (one species), Reitter (five species), Ren (two species), Schuster (four species), and Semenov and Bogatshev (one species) in 1841–2019 [5,6,7,8,9,10,11]. In addition, Chigray proposed that the Iranian species Blaps platythorax Gemminger, found in 1870 (described from Shiraz), probably belongs to the genus Dila [2]. Although Dila exhibits species richness, only three species are currently known from China (D. laevicollis Gebler, 1841, D. bomina Ren and Li, 2001, and D. rugelytra Ren, 2016). Skopin noted the close similarity of the larvae of Dila to the primitive larvae of Blaps based on similar habitats [12]. However, the morphological characters of larva of Dila have not been described yet.
In this study, the larva and adult male and female of a new species are described based on morphological and molecular evidence. In addition, we constructed a molecular phylogeny for seven related genera of the tribe Blaptini and four outgroup taxa, to verify the taxonomic status the new species. We also provided molecular data for the phylogenetic studies of the tribe Blaptini in the future.
2. Materials and Methods
2.1. Morphological Examination
In total, 99 specimens from three sampling localities were examined for this study and have been deposited at the Museum of Hebei University, Baoding, China (MHBU). The figures were taken with three distinct imaging systems: (a) a Canon EOS 5D Mark III (Canon Inc., Tokyo, Japan) connected to a Laowa FF 100 mm F2.8 CA-Dreamer Macro 2× or Laowa FF 25 mm F2.8 Ultra Macro 2.5–5× (Anhui Changgeng Optics Technology Co., Ltd., Hefei, China); (b) a Leica M205A stereomicroscope equipped with a Leica DFC450 camera (Leica Microsystems, Singapore, Singapore), which was controlled using the Leica application suite 4.3; (c) a JVC® KY–F75U (JVC Kenwood, Long Beach, CA, USA) digital camera attached to a Leica Z16 APO dissecting microscope (Leica Microsystems, Buffalo Grove, IL, USA) with an apochromatic zoom objective and motor focus drive, using a Syncroscopy® Auto-Montage System (Synoptics, Cambridge, UK) and software. Multiple images were used to construct the final figures. Images were illuminated with either an LED ring light attached to the end of the microscope column, with incidental light filtered to reduce glare, or by a gooseneck illuminator with bifurcating fiberoptics; image stacks were white-balance-corrected using the software of the imaging system (Synoptics, Cambridge, UK). Label data are presented verbatim. A double slash (//) separates text on different lines of label, authors’ remarks are enclosed in brackets “[]”.
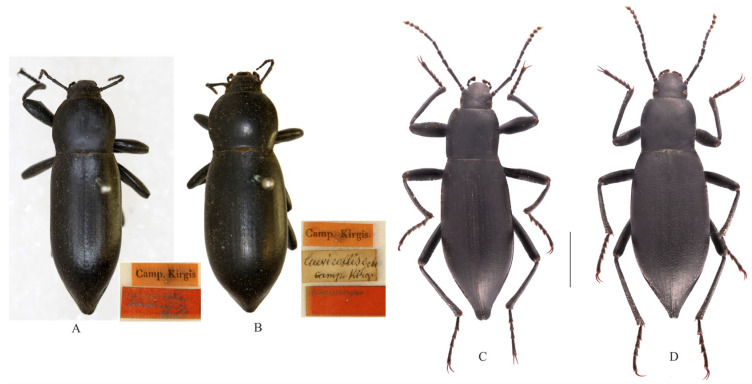
Type material: Dila laevicollis: lectotype: ♂, labelled: “Camp. Kirgis.”, “Lectotypus, Dila laevicollis Gebl”; paralectotype: 1 ♀: “Camp. Kirgis.”, “laevicollis Gebl, Camp. Kirg.”, “Hololectotypus”.
2.2. Taxon Sampling, DNA Extraction, PCR Amplification, and Sequencing
DNA was extracted from leg muscle tissue of adults using Insect DNA isolation Kit (BIOMIGA, Hangzhou, China) following the manufacturer’s protocols. Fragments of three mitochondrial molecular markers (cytochrome coxidase subunit I, COI; cytochrome b, Cytb; 16S ribosomal RNA, 16S), and of one nuclear molecular marker (28S ribosomal RNA domain D2, 28S–D2) were amplified and sequenced. The primers and annealing temperature are shown in Table 1.
Table 1.
Primer sequences for PCR.
| Gene | Primer (Forward/Reverse) |
Sequence (Forward and Reverse) 5′ → 3′ | PCR Conditions (Annealing) |
References |
|---|---|---|---|---|
| COI | F 2183 | CAACATTTATTTTGATTTTTTGG | 50 °C | Monteiro & Pierce, 2001 [13] |
| R 3014 | TCCAATGCACTAATCTGCCATATTA | |||
| Cytb | F revcb2h | TGAGGACAAATATCATTTTGAGGW | 50 °C | Simmons et al., 2001 [14] |
| R rebcbj | TCAGGTCGAGCTCCAATTCATGT | |||
| 16S | F 13398 | CGCCTGTTTATCAAAAACAT | 50 °C | Simon et al., 1994 [15] |
| R 12887 | CCGGTCTGAACTCAGATCAT | |||
| 28S-D2 | F 3665 | AGAGAGAGTTCAAGAGTACGTG | 58 °C | Belshaw et al., 1997 [16] |
| R 4068 | TTGGTCCGTGTTTCAAGACGGG |
The profile of the PCR amplification consisted of an initial denaturation step at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 1 min, annealing for 1 min, and extension at 72 °C for 1 min, and a final 8 min extension step at 72 °C. PCR was performed using TaKaRa ExTaq (TaKaRa, Dalian, China). PCR products were subsequently checked by 1% agarose gel electrophoresis, and sequencing was performed at GENERAL BIOL Co., Ltd. (Chuzhou, China).
2.3. Phylogenetic Analyses
Phylogenetic analyses were carried out on the concatenated dataset under maximum likelihood (ML). For ML analyses, we used IQ-TREE v1.6.6 [17] as implemented in the dedicated IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/, accessed on 1 June 2022). The ML tree was inferred under an edge-linked partition model for 5000 ultrafast bootstraps (1000 replicates) [18]. The phylogenetic tree was visualized in Figtree v.1.4.4. (http://tree.bio.ed.ac.uk/software/figtree, accessed on 1 June 2022). Altogether, 240 sequences from 62 individuals of 24 species of 7 genera (Ablaps: 1 species, Blaps: 6 species, Coelocnemodes: 2 species, Dila: 3 species, Gnaptor: 1 species, Nalepa: 7 species, and Prosodes: 4 species) of the tribe Blaptini were used in this phylogenetic analysis. To complete this sampling, we also used previously published sequences from one specimen of Blaps (B. rhynchoptera) and one specimen of Gnaptor (G. spinimanus). Meanwhile, we used sequences from 4 individuals of the tribe Platyscelidini as outgroup, which has been recovered as a close relative of Blaptini. Detailed information for all the samples used in this study are provided in Table S1.
3. Results
3.1. Morphological Study and Diagnosis
3.1.1. Key to Species of the Genus Dila Fischer von Waldheim, 1844, from China (Based on Males)
-
1.
The body is slender (Figure 2A) and elongated; the parameres are finger-shaped… 2
-
-
The body is robust and weakly elongated; the parameres are cone-shaped … … … 3
-
2.
The pronotum is wider than it is long and almost cordiform, with one tooth on the
ventral margin of the profemur … … … … … … … … D. laevicollis Gebler, 1841
-
-
The pronotum length and width are nearly equal, and the lateral margins show slight
arcuate narrowing to the anterior margin, with obtuse-angled prominence of the
profemur … … … … … … … … … … … … … … D. ngaria Li and Ren, sp. n.
-
3.
The pronotum is wider than it is long and almost cordiform; with two teeth on the
ventral margin of the profemur, without one tooth on the ventral margin of the
mesofemur … … … … … … … … … … … … … D. bomina Ren and Li, 2001
-
-
The pronotum length and width are nearly equal, the lateral margins show
slight arcuate narrowing to the anterior margin, with one tooth on the ventral
margin of the profemur and without a tooth on the ventral margin of the
mesofemur … … … … … … … … … … … … … D. rugelytra Ren, 2016
Dila ngaria Li and Ren sp. n.
Type locality. China, Xizang, Tsada County, Xiangzi.
Type specimens (Adults). Holotype: ♂, with the following labels: “2015.VIII.24//Xiangzi Township, Tsada County, Xizang, China//Guo-Dong Ren et al.//Museum of Hebei University”//“31°32.038′N//79°59.020′E//Elev.4420 m//Museum of Hebei University”. Paratypes: 15 ♂ 7 ♀ [1 ♂ 1 ♀ in ethanol] (MHBU), same data as holotype; 2 ♀ in ethanol (MHBU), labeled “2018.VIII.11//Tuolin Township, Tsada County, Xizang, China//Xing-Long Bai et al.//Museum of Hebei University]”//“31°32.038′N//79°59.020′E//Elev. 4496 m//Museum of Hebei University”; 22♂30♀[9♂11♀ in ethanol] (MHBU), labeled “2022.VII.11//Burang Township, Burang County, Xizang, China//Guo-Dong Ren et al.//Museum of Hebei University]”//“30°16.5852′N//81°11.4735′E//Elev. 4006 m//Museum of Hebei University”.
Description (Figure 2C). Body length 20.0–22.0 mm, width 6.5–7.5 mm; black, nearly cylinder-shaped, elongated.
Head (Figure 1A,B). Anterior margin of epistome emarginated. Lateral margins of epistome straight; lateral margins of head with indistinct emargination between epistome and genae; head widest at eye level; mentum transverse, with elliptical lateral sides; coarsely punctate and slightly impressed in middle of anterior edge; antennae long, filiform, reaching beyond pronotal base when directed backwards, antennomeres Ⅲ–Ⅶ long and cylindrical, Ⅷ–Ⅹ oval, Ⅺ spindle (Figure 1C). Ratio of length/width of antennomeres Ⅱ–Ⅺ 4.0 (8.0): 40.0 (10.0): 22.0 (10.0): 24.0 (9.0): 23.0 (9.0): 26.0 (9.0): 12.0 (11.0): 13.0 (11.0): 13.0 (11.0): 16.0 (10.0).
Figure 1.
D. ngaria sp. n. (A). head, dorsal view; (B). head, ventral view; (C). antenna; (D). pronotum; (E). profemur; (F). protibia; (G). mesotibia; (H). protarsus (I). mesotarsus; (J). metatarsus; (K–M). aedeagus: (K). dorsal view; (L). ventral view; (M). lateral view. Scale bars: 1.0 mm.
Thorax (Figure 1D). Long and wide nearly equal, 1.6 times as wide as head; widest at middle, lateral margins more arcuately narrowing to anterior margin than to base; posterior margin straight; anterior angles obtuse, posterior ones weakly obtuse or rectangular; ratio of width at anterior margin to base 30: 42; disc strongly convex, smooth, surface with dense punctation.
Abdomen. Elytra elongate, 2.1 times as long as wide, widest at middle, weakly arcuated, 3.1 times as long and 1.35 times as wide as pronotum, 2.2 times as wide as head; strongly convex on disc, elytral surface smooth, with dense punctures, apex of elytra with attenuate sloping, obtuse; abdomen ventrites 1st–3rd with transverse/longitudinal wrinkles, abdominal ventrites 4th–5th with dense punctures and simple particles.
Legs (Figure 1E–J). Legs slender, profemur extension at middle, protibiae nearly straight, inner mesotibiae weakly curved, upper apical spur spinal; ventral surface of protarsomeres Ⅰ–Ⅳ, meso-, metatarsomeres Ⅰ–Ⅲ with hairy brush. Ratio of length of pro–, meso– and metatibiae 33: 31: 41, that of metatarsomeres I–Ⅳ 8.0 (4.0): 5.0 (3.0): 5.0 (3.0): 11.0 (3.0).
Male genitalia (Figure 1K–M). Aedeagus finger-shaped, length 4.5–4.6 mm, width 0.7–0.8 mm; parameres length 1.7–1.8 mm and width 0.5–0.6 mm, apex obtuse; parameres widest at base, evenly narrowed to acute apex in dorsal view; parameres almost in a straight line up to apex in lateral view; rods of spiculum gastrale merged at apex, forming long common stem (Figure 1L).
Sexual dimorphism (Figure 2D). Body nearly cylinder-shaped, elongated. Length 21.0–23.0 mm, width 7.5–8.0 mm; body wider than male; head 1.4 times as wide as interocular distance, pronotum 1.1 times as wide as long, elytra twice as long as wide. Antennae shorter than male, antennomeres Ⅲ–Ⅶ long and cylindrical, Ⅷ–Ⅹ oval, Ⅺ spindle; pronotum and elytra convex; protibiae nearly straight, inner mestibiae weakly curved, upper apical spur spinal.
Figure 2.
Habitus of the type species and a new Dila species. (A,B). Dila laevicollis. (A). male, lectotype, (B). female, paralectotype; (C,D). D. ngaria sp. n. (C). male, holotype, (D). female, paratype. Scale bars: 5.0 mm.
Etymology. This species is named from the type locality (located in Ngari).
Distribution. China, Xizang.
Diagnosis. The new species is morphologically very similar to Dila laevicollis, but can be distinguished from the latter by the following male character states: (1) the body length is 20.0–22.0 mm, elongated, nearly cylinder-shaped, (the body length is 29.0–32.0 mm, elongated, cylinder-shaped in D. laevicollis); (2) the pronotum length and width are nearly equal, and the lateral margins show slight arcuate narrowing to the anterior margin (the pronotum is wider than it is long and is almost cordiform in D. laevicollis); (3) there is one tooth-like process on the ventral margin of the profemur (with obtuse-angled prominence of the profemur in D. laevicollis).
3.1.2. Larva
Body (Figure 3A–C). Mature larvae length 20.0–22.0 mm, width 2.5–3.0 mm. Body orthosomatic with sides subparallel, subcylindrical with sharp tail-end; moderately sclerotized; yellowish brown; body vestiture consisting of short to moderately elongate; vestiture smooth; median line is obvious at the first 3 segments, Y–shaped; covered with pairs long setae on each tergite (besides the last segment); posterior border of each segment with dark brown longitudinal stripes.
Figure 3.
Larva of Dila ngaria sp. n. A–C. Habitus: (A). dorsal view; (B). ventral view; (C). lateral view. (D). head, dorsal view; (E). head and prothoracic leg, ventral view; (F). pygopods, dorsal view; (G). pygopods, ventral view. Scale bars: 5 mm (A–C), 1 mm (D–G).
Head (Figure 3D,E). Prognathous, slightly narrower than width of prothorax, slightly convex dorsally, sides rounded (Figure 3D); labrum transverse, weakly emarginate, apical margin with sparse of setae; mandibles well developed, left and right sides symmetrical, with 2 pairs short setae, elongate anterior extension; clypeus transverse, trapezoid-shaped; lateral margins arcuately narrowing to middle, then arcuately narrowing to anterior margin; disc feebly convex, anterior margin slightly linear, and with 4 long erect clypeal setae; epicranial stem Y-shaped (Figure 3E); frons and epicranial plate slightly convex, smooth, with sparse and fine punctures, lateral margins with two row dense long hairs, the center of anterior rim of frons with 4 pairs hairs (Figure 3E); maxillary palps 3-segmented, subcylindrical, with cone-shaped at peak; Ⅰ widest, Ⅱ and Ⅲ with same length, twice as long as Ⅰ (Figure 3E); labial palps 2-segmented, short, Ⅱ cone-shaped (Figure 3E); mentum convex, U-shaped, base of mentum straight, prementum with 2 long hairs; submentum trapezoid-shaped, anterior margin nearly straight, posterior margin curved; antennae well developed, 3-segmented with dome-like at peak, approximate cylindrical, shorter than length of head, segment Ⅰ noticeably wider; Ⅲ is longest, about 1.5 times as long as the Ⅱ, and 0.9 times as wide as Ⅱ.
Thorax (Figure 3D,E). Thoracic segmentation C-shaped in dorsal view, sides parallel, widest in middle, with transverses plicae; each thoracic tergum with long slender setae on sides of anterior and posterior margins; pronotum longest, about 0.8 times as long as sum of meso- and metanotum; mesonotum shortest.
Legs (Figure 3E). Legs well developed; prothoracic leg noticeably stronger, much thicker than meso- and metathoracic ones; profemur, femora and tibia with sparse long setae; pro- and mesotarsungulus strongly sclerotized sharp claw-like, profemora and protibia gradually narrowing towards apex, profemora about 0.8 times length of protibia; metatarsungulus highly ossified hook-like.
Abdomen (Figure 3A–C,F,G). Approximately 3.6 times as long as thorax; segments Ⅰ–Ⅷ subcylindrical with transverses plicae, faintly rugose, with sparse elongate setae ventrally; tergum nearly same long and segments Ⅶ–Ⅷ narrower than others, pygopods subconical in dorsal view, marginal with two rows of Ⅶ–Ⅸ short spines, urogomphi with 2 short spines at about middle; urogomphi suddenly upturned to apex in lateral view; surface of convex disc with sparse long setae in ventral view (Figure 3F,G).
Spiracles (Figure 3B). A well-developed pair, round thoracic spiracles, situated ventrolaterally on anterolateral margins of terga Ⅰ–Ⅷ.
3.2. Phylogenetic Relationships
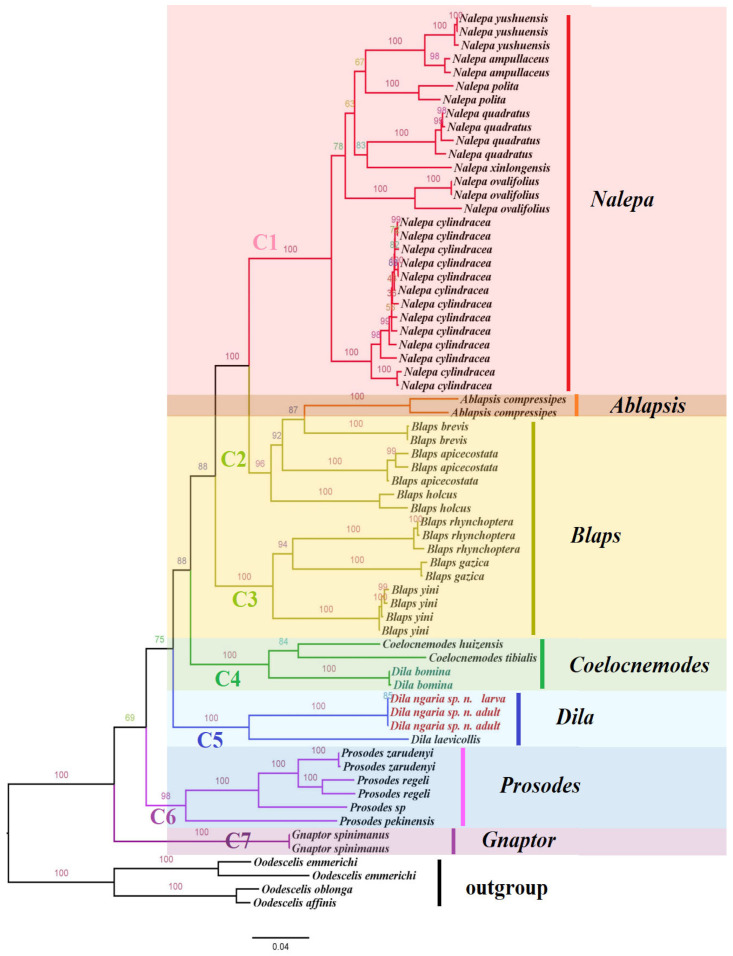
The final, concatenated dataset was 2289 bp long, including sequences of seven genera from Blaptini (COI, 744 bp; Cytb, 579 bp; 16S, 503 bp; 28S-D2, 463 bp). IQ-TREE analyses yielded a topology, and the preliminary phylogenetic relationship was hypothesized for the seven genera of Blaptini (Figure 4).
Figure 4.
Maximum likelihood phylogenetic tree based on 2289 bp of mitochondrial and nuclear DNA sequences (COI, Cytb, 16S rDNA, and 28S rDNA) within the tribe Blaptini. Support for each node is represented by ultrafast bootstrap values (uBV).
The ML tree revealed that there was a reasonable correlation of membership of each of these major clades to the currently most circumscribed genera. The intergeneric relationships were well supported overall. The individuals of Blaptini were grouped into seven well-supported clades: clade C1 (uBV = 100%), clade C2 (uBV = 96%), clade C3 (uBV = 100%), clade C4 (uBV = 100%), clade C5 (uBV = 100%), clade C6 (uBV = 98%), and clade C7 (uBV = 100%). The clade C1 (Nalepa), clade C6 (Prosodes), and clade C7 (Gnaptor) corresponded to the currently circumscribed genera and were strongly supported (uBV = 100%). The clade C2 contained three species of Blaps, as well as the monotypic genus Ablapsis (A. compressipes Reitter); the clade C3 was also well supported (uBV = 100%) and included the remaining species of the genus Blaps.
However, the clade C4 included two samples of D. bomina Ren and Li, 2001, and two samples of Coelocnemodes (C. huizensis Ren and Li, 2001; C. tibialis Ren, 2016). Thus, taxonomic status of D. bomina Ren and Li, 2001, may need to be re-evaluated. The clade C5 includes the larva sample and two adult samples of D. ngaria sp. n. with D. laevicollis (uBV = 100%). Based on the above results, the larva sample was confirmed to be conspecific with the adults of D. ngaria sp. n. The new species from the western Himalaya is very similar with the type species of Dila (D. laevicollis) in morphological character (body nearly cylinder-shaped, elongated; antennomeres Ⅲ–Ⅶ long and cylindrical; legs slender, parameres finger-shaped, strongly elongated). Therefore, its taxonomic status was confirmed by both molecular and morphological evidence.
3.3. Bionomics
The new species was found in the southwestern Himalayas of the Ngari Prefecture of China. Interestingly, this species is well adapted to semi-arid and arid environments. The adults were found below big stones or a shield in the grass, and were probably feeding on decaying plant roots or leaves (Figure 5).
Figure 5.
Habitat for D. ngaria sp. n. Photo by Xinglong Bai at Zanda of Ngari Prefecture, Xizang, China, on August 2018.
4. Discussion
4.1. Taxonomic Remarks
The larvae and adults of Dila ngaria sp. n. were collected in the field; hence, it was rather difficult to judge the larve stage. Skopin noted that the larvae of Dila were similar to the primitive larvae of Blaps from a similar habitat [12]. The larval description above was inferred to be in its final instar stage based on previous descriptions of Blaps and Nalepa. Larval morphology has been found to offer numerous characters for species recognition and has been used to support the close relationships of genera or subtribes [19,20,21,22]. Blaptini is mostly distributed in centraleastern Asia, and comprise about 500 species [23]. However, for the description of larval morphology within Blaptini, available data are mostly from Blaps and several species of Nalepa, Gnaptorina, and Agnaptoria [24,25,26,27,28,29]. The characteristics of larve for most species are unknown due to the lack of specimens. So, we hope to continue collecting more larvae and associating them with their respective adults by rearing and/or sequencing data. We will then solve the relationships of genera or subtribes of the tribe Blaptini through the addition of larval morphological characters.
4.2. Phylogeny and Classification of Species
Blaptini is currently divided into five or six subtribes: Blaptina Leach, Gnaptorina Medvedev, Gnaptorinina Medvedev, Prosodina Skopin, Remipedellina Semenow, and Dilina Ren [10,29]. The subtribe Dilina Ren, 2016 (type genus Dila), was erected by Ren in 2016. The position of Dila within Blaptini and the classification of Dilina are debatable because of the presence or absence of apical and ventral teeth on the inner side of the profemora, the structure of the male genitalia, and the close similarity of the larvae of Dila with some Blaps and Nalepa of Blaptina [2,12,30]. Nearly all past contributions focusing on the Blaptini have been based on morphological characters, which did not strongly support the monophyly of the subtribe Dilina [2]. The present work is the first molecular phylogenetic analysis within the Blaptini. The result showed that the classification system of Dila and Dilina are not supported by available phylogenetic data.
The genus Blaps was erected by Fabricius in 1775. All species are flightless and well adapted to semi-arid and arid environments because of several specific behavioral and morphological adaptations [31]. In this study, the phylogenetic relationships of seven species indicated that the genus Blaps is likely paraphyletic. The genus comprises about 250 species, and it would be premature to comment on monophyletic of the genus based on a molecular analysis of only seven species. In order to better understand the genus, subgeneric, and species relationships in Blaptini, we are undertaking additional phylogenetic analyses that will include more species.
In the last revision of Dila by Chigray, four species were transferred from Caenoblaps to Dila with their holotype or lectotype photos provided. Meanwhile, three new species were described from the Hakkary Province of Turkey and West Azerbaijan Province of Iran [2]. Most species of Dila are distributed in medium-high to high elevation mountain habitats. The type species D. laevicollis Gebler, 1841, inhabit stony low-elevation mountains and foothills (including the Betpak-Dala desert) and are widespread in Afghanistan, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Turkmenistan, and Xinjiang, China [6,7]. Coelocnemodes is an endemic genus of the Tibetan Plateau that is distributed in Pakistan and Yunnan, China. All species of Coelocnemodes have two teeth on the inner side of the profemora and differ from Dila due to a wider body. In contrast, most species of the genus Dila have only one apical tooth on the profemora and slender, elongate bodies. The preliminary phylogenetic results show that the two samples of D. bomina Ren and Li, 2001, with C. huizensis Ren and Li, 2001, and C. tibialis Ren, 2016, from Coelocnemodes comprise a single well-supported clade C4 (uBV = 100%); the larva sample and two adult samples of D. ngaria sp. n. and D. laevicollis are clustered in a single well-supported clade C5 (uBV = 100%). The specimen type and topotypes of D. bomina were re-examined: the body is wider; antennomere VII is slightly longer than antennomere VIII; one or two apical tooth exist on the profemora; the male genitalia is C-shaped, the basal piece of the aedeagus is weakly bent, and the apical piece has parameres that are not bent, strongly elongate, and acute. These characters are distinct with the type species D. laevicollis and the new species D. ngaria sp. n. (body nearly cylinder-shaped; antennomere VII distinctly longer than antennomere VIII; legs slender, profemora with obtuse tooth; male genitalia finger-shaped, apex obtuse, widest at base, evenly narrowed to acute apex), but the morphological characters of D. bomina are very similar with some species of Dila, such as D. difformis König, 1906, D. nitida Schuster, 1920, D. baeckmanni Schuster, 1928, D. kulzeri Schuster, 1928, D. hakkarica Chigray, 2019, and D. svetlanae Chigray, 2019 (body larger or robust, slender; profemur with tooth; basal piece of parameres is wider, evenly narrowed to acute apex, elongated). In addition, D. bomina was found from Bowo, Xizang (Eastern Himalayas), with Coelocnemodes being found from a similar habitat. We suspect that the genus Dila is probably non-monophyletic, and the taxonomic status of D. bomina is in question upon combining these morphological characters and the results of molecular phylogenetic analysis. However, the molecular data of more Dila species are needed to further explore the above questions.
5. Conclusions
In this present study, the larva and adult males and females of a new Dila species were described and illustrated based on morphological and molecular evidence. Meanwhile, a preliminary phylogenetic tree of seven related genera of Blaptini suggests that Dila is probably a non-monophyletic genus, and the taxonomic status of D. bomina Ren and Li, 2001, needs to re-evaluated. The monophyly of the subtribe Dilina is not supported by available phylogenetic data. However, this work provides valuable data for further study on the molecular phylogeny of the tribe Blaptini. We suggest that these incongruences will be more fully resolved by phylogenetic analyses of Blaptini that include more genera, species, and genomic sequences.
Acknowledgments
We would like to thank Xinglong Bai for his kind help with field work, and for providing samples from Xizang; we also wish to thank Jigang Li and Junxia Zhang for additional comments and corrections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2075-4450/14/3/284/s1, Table S1: List of specimens used in this study with the corresponding accession number.
Author Contributions
Conceptualization, X.-M.L. and G.-D.R.; data curation, B.J. and J.T.; formal analysis, X.-M.L. and B.J.; writing—original draft, X.-M.L.; visualization, B.J. and J.T.; funding acquisition, X.-M.L. and G.-D.R.; writing—review and editing, X.-M.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data of the research were deposited at the College of Life Sciences, Hebei University, Baoding, China. The specimens associated with this paper, as well as the sequence data submitted to GenBank, conform to the 2014 Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (https://www.cbd.int/abs/, accessed on 1 June 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by the National Natural Science Foundation of China (32170477; 32070488; 31970452) and the National Natural Science Foundation of Hebei (C202101020).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Iwan D., Löbl I. Catalogue of Palaearctic Coleoptera. Volume 5. Koninklijke Brill NV; Leiden, The Netherlands: 2020. pp. 1–969. [Google Scholar]
- 2.Chigray I.A., Nabozhenko M., Abdurakhmanov G., Keskin B. A systematic review of the genus Dila Fischer von Waldheim, 1844 (=Caenoblaps, syn.n.) (Coleoptera: Tenebrionidae) from the Caucasus, Turkey and boundary territories of Iran. Insect Syst. Evol. 2019;99:914–923. [Google Scholar]
- 3.Ren G.D., Wang X.P. Eight new species of the genus Blaps Fabricius (Coleoptera: Tenebrionidae: Blaptini) of China. Entomotaxonomia. 2001;1:15–27. [Google Scholar]
- 4.Löbl I., Nabozhenko M.V., Merkl O. Tribe Blaptini Leach, 1815. In: Löbl I., Smetana A., editors. Catalogue of Palaearctic Coleoptera. Volume 5. Apollo Books; Stenstrup, Denmark: 2008. pp. 219–257. Tenebrionoidea. [Google Scholar]
- 5.Reitter E. Uebersicht der mir bekannten Arten der Coleopteren-Gattung Dila Fisch. Entomol. Nachr. 1900;26:295–296. [Google Scholar]
- 6.Blair K.G. Some new species of Indian Tenebrionidae. Ann. Mag. Nat. Hist. 1913;12:56–58. doi: 10.1080/00222931308693366. [DOI] [Google Scholar]
- 7.Kaszab Z. Die Tenebrioniden Afghanistans, auf Grand der Ergebnisse der Sammelreise des Herrn, J. Klapperich in den Jahren 1952/53 (Col.). 1. Fortsetzung und Schluss. Entomol. Arb. Dem Mus. G. Frey. 1960;11:1–179. [Google Scholar]
- 8.Kaszab Z. Beiträge zur Kenntnis der Fauna Afghanistans (Sammelergebniss von O. Jakeš 1963–64, D. Povolný & Fr. Tenora 1966, J. Šimek 1965–66, D, Povolný, J. Geiser, Z. Šebek & Fr. Tenora 1967). Tenebrionidae, Col. Časopis Morav. Mus. 1970;54:5–182. [Google Scholar]
- 9.Reitter E. Dila leptoscelis n. sp. Entomol. Blätter. 1909;5:239. [Google Scholar]
- 10.Ren G.D., Ba Y.B., Liu H.Y., Niu Y.P., Zhu X.C., Li Z., Shi A.M. Coleoptera: Tenebrionidae (I) Volume 63. Science Press; Beijing, China: 2016. p. 532. Fauna Sinica. Insecta. [Google Scholar]
- 11.Semenov-Tian-Schanskij A.P., Bogatshev A.V. Characterized additions to the fauna of the USSR on order Coleoptera, I. Byulleten’. Mosk. Obs. Ispyt. Prir. 1940;49:201–209. [Google Scholar]
- 12.Skopin N.G. Materials on the morphology and ecology of larvae of the tribe Blaptini (Coleoptera, Tenebrionidae) Tr. Inst. Zool. Akad. Nauk. Kazakhskoy SSR. 1960;11:36–71. [Google Scholar]
- 13.Monteiro A., Pierce N.E. Phylogeny of Bicyclus (Lepidoptera:Nymphalidae) inferred from COI, COII and EF-1α gene sequences. Mol. Phylogenet. Evol. 2001;18:264–281. doi: 10.1006/mpev.2000.0872. [DOI] [PubMed] [Google Scholar]
- 14.Simmons R.B., Weller S.J. Utility and evolution of cytochrome b in insects. Mol. Phylogenet. Evol. 2001;20:196–210. doi: 10.1006/mpev.2001.0958. [DOI] [PubMed] [Google Scholar]
- 15.Simon C., Frati F., Bekenbach A., Crespi B., Liu H., Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994;87:651–701. doi: 10.1093/aesa/87.6.651. [DOI] [Google Scholar]
- 16.Belshaw R., Quicke D.L.J. A molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae) Mol. Phylogenet. Evol. 1997;7:281–293. doi: 10.1006/mpev.1996.0400. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minh B.Q., Nguyen M.A.T., Von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chigray I.A. A new genus and species of darkling beetles of the tribe Blaptini (Coleoptera: Tenebrionidae) from Afghanistan and taxonomic changes in the tribe. Entomol. Rev. 2019;99:914–923. doi: 10.1134/S0013873819070054. [DOI] [Google Scholar]
- 20.Grebennikov V.V., Scholtz C.H. The basal phylogeny of Scarabaeoidea (Insecta: Coleoptera) inferred from larval morphology. Invertebr. Syst. 2004;18:321–348. doi: 10.1071/IS03013. [DOI] [Google Scholar]
- 21.Lawrence J.F., Seago A.E., Newton A.F., Thayer M.K., Marvaldi A.E., Slipinski A. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 2011;61:1–217. doi: 10.3161/000345411X576725. [DOI] [Google Scholar]
- 22.Soldati L., Condamine F.L., Clamens A.L., Kergoat G.J. Documenting tenebrionid diversity: Progress on Blaps Fabricius (Coleoptera, Tenebrionidae, Tenebrioninae, Blaptini) systematics, with the description of five new species. Eur. J. Taxon. 2017;282:1–29. doi: 10.5852/ejt.2017.282. [DOI] [Google Scholar]
- 23.Watt J. A revised subfamily classifcation of Tenebrionidae (Coleoptera) N. Z. J. Zool. 1974;11:381–452. doi: 10.1080/03014223.1974.9517846. [DOI] [Google Scholar]
- 24.Yu Y.Z., Ren G.D., Sun X.Q. Morphology and a key of common larvae of Blaptini in northern China (Coleoptera: Tenebrionidae) Entomol. Knowl. 1996;33:198–203. [Google Scholar]
- 25.Yu Y.Z., Zhang D.Z., Wang X.P. Morphology description of five larvae (Coleoptera: Tenebrionidae, Blaptini) J. Ningxia Agric. Coll. 1999;20:15–20. [Google Scholar]
- 26.Yu Y.Z., Zhang D.Z., Ren G.D. Systematic research of the genus Blaps Fabricius-larvae in China (Coleoptera: Tenebrionidae (Part I) J. Hebei Univ. 2000;20:94–101. [Google Scholar]
- 27.Zhao M., Feng Y., Chen X.M., Ji H.H. Morphological and biological characteristics of Blaps rhynchopetera (Coleoptera: Tennbrionidae) J. Environ. Entomol. 2009;31:348–355. [Google Scholar]
- 28.Zhu X.C., Ren G.D. The larvae of Gnaptorina felicitana and Agnaptoria amdoensis of the tribe Blaptini from China (Coleoptera: Tenebrionidae) Zool. Syst. 2014;39:275–282. [Google Scholar]
- 29.Medvedev G.S., Merkl O. Novye vidy zhukov-chernotelok triby Blaptini (Coleoptera, Tenebrionidae) iz yugo-zapadnogo Kitaya. Entomol Obozr. 2001;80:620–626. [Google Scholar]
- 30.Li X.M., Tian J., Fan J.J., Ren G.D. Systematic Review of the Genus Nalepa Reitter, 1887(Coleoptera, Tenebrionidae, Blaptinae, Blaptini) from the Tibetan Plateau, with Description of Six New Species and Two Larvae. Insects. 2022;13:598. doi: 10.3390/insects13070598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condamine F.L., Soldati L., Rasplus J.Y., Kergoat G.J. New insights on systematics and phylogenetics of Mediterranean Blaps species (Coleoptera: Tenebrionidae: Blaptini), assessed through morphology and dense taxon sampling. Syst. Entomol. 2011;36:340–361. doi: 10.1111/j.1365-3113.2010.00567.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the research were deposited at the College of Life Sciences, Hebei University, Baoding, China. The specimens associated with this paper, as well as the sequence data submitted to GenBank, conform to the 2014 Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (https://www.cbd.int/abs/, accessed on 1 June 2022).