Abstract
As advanced delivery techniques such as intensity‐modulated radiation therapy (IMRT) become conventional in veterinary radiotherapy, highly modulated radiation delivery helps to decrease dose to normal tissues. However, IMRT is only effective if patient setup and anatomy are accurately replicated for each treatment. Numerous techniques have been implemented to decrease patient setup error, however tumor shrinkage, variations in the patient's contour and weight loss continue to be hard to control and can result in clinically relevant dose deviation in radiotherapy plans. Adaptive radiotherapy (ART) is often the most effective means to account for gradual changes such as tumor shrinkage and weight loss, however it is often unclear when adaption is necessary. The goal of this retrospective, observational study was to review dose delivery in dogs and cats who received helical radiotherapy at University of Wisconsin, using detector dose data (D2%, D50%, D98%) and daily megavoltage computed tomography (MVCT) images, and to determine whether ART should be considered more frequently than it currently is. A total of 52 treatment plans were evaluated and included cancers of the head and neck, thorax, and abdomen. After evaluation, 6% of the radiotherapy plan delivered had clinically relevant dose deviations in dose delivery. Dose deviations were more common in thoracic and abdominal targets. While adaptation may have been considered in these cases, the decision to adapt can be complex and all factors, such as treatment delay, cost, and imaging modality, must be considered when adaptation is to be pursued.
Keywords: adaptive, IMRT, veterinary
Abbreviations
- ART
adaptive radiotherapy
- MVCT
megavoltage computed tomography
- D2%
minimum dose to 2% of the target
- D50%
minimum dose to 50% of the target
- D98%
minimum dose to 98% of the target
- IMRT
Intensity‐ modulated radiation therapy
- OAR
organ at risk
- GTV
gross tumor volume
- PTV
planning tumor volume
- CTV
clinical tumor volume
- CCC
collapsed cone convolution
- 4DCT
4‐dimensional computed tomography
- DVH
dose volume histogram
1. INTRODUCTION
As advanced delivery techniques such as intensity‐modulated radiation therapy (IMRT) become conventional in veterinary radiotherapy, highly modulated radiation delivery helps to decrease dose to normal tissues. As we become more precise in radiation delivery, identification, and management of variation in patient positioning and anatomy is of paramount importance. 1 Numerous techniques have been implemented to decrease patient setup error, however tumor shrinkage, variations in the patient's contour and weight loss continue to be hard to control and can result in relevant dose deviation in radiotherapy plans in humans. 2
Adaptive radiotherapy (ART) is often the most effective means to account for gradual changes such as tumor shrinkage and weight loss, however it is often unclear when adaption is necessary. 3 Ideally, ART implementation should include (1) treatment dose assessment, (2) treatment variation identification/evaluation, (3) treatment modification decisions, and (4) adaptive treatment modification. 4 This can be loosely translated to the process of (1) using one of many dose evaluation platforms to identify dose alterations, (2) identifying where in the plan dose alteration has occurred, (3) deciding whether adapting would improve this deviation and how to modify the radiotherapy plan to improve dose delivery, and (4) performing the adaptation. Many human radiotherapy institutions have investigated dosimetrically triggered ART, however this technology is likely not readily available in veterinary medicine. 5
The objective of this study was to evaluate treatment dose delivery in dogs and cats using detector data and daily megavoltage computed tomography (MVCT) images so that we may identify relevant deviation in dose delivery and assess whether adaptation should be considered more frequently than it currently is at our institution. Our null hypothesis was that none of the evaluated plans would display dose deviations of more than 10%.
2. MATERIALS AND METHODS
2.1. Selection and description of subjects
This was a retrospective observational study design. Data from radiation plans for dogs and cats that completed radiotherapy protocols on a helical radiotherapy device at the University of Wisconsin‐Madison School of Veterinary Medicine (RadixactTM Treatment Delivery System, Accuray Inc., Sunnyvale, CA, USA) between July 2020 to July 2021 were evaluated by a veterinary radiation oncology specialist and radiation oncology resident. To be included in the study, patients had to have available data within the adaptive dose calculation software (MIM Software, MIM Maestro / PreciseART, MIM Software Inc, Cleveland, OH, USA), which patients are automatically enrolled in when treatment plans are approved. The nature of this study did not require approval by an Institutional Animal Care and Use Committee. All patients were treated with approval from owners. Data were evaluated for dose deviation by an American College of Veteirnary Radiology (Radiation Oncology) [ACVR (RO)] certified radiation oncologist and a radiation oncology resident. This was done in agreement between the two observers. In regard to the data, no information about the patient was censored from the observers evaluating the data. Dogs and cats were separated according to the location of the target (head and neck, chest, abdomen) by the same observers listed above. There were no dogs or cats with two target locations.
2.2. Data recording and analysis
Two dose distribution datasets were obtained from each dog using the MIM dose evaluation software: the Planned and Delivered doses. The ‘Planned dose’ was the initial optimized plan calculated on the simulation CT. The ‘Delivered dose’ was calculated by creating a daily merged image consisting of the daily MVCT (augmented with the planning CT), deforming the planning contours onto the daily merged image, and calculating dose on the daily merged image. After this, the daily dose is deformed back onto the planning image (Figure 1). The D2%, D50%, and D98% doses were tabulated for targets contours and the D2% and D50% doses were tabulated for organs at risk (OAR). 6 The difference between the planned and delivered dose, or dose difference, was considered “clinically relevant” if the delivered dose was found to deviate more than 10% from the planned dose. The cutoff of 10% was an estimation by the authors as there is no current defined dose deviation cutoff for cases that would need to be adapted as the decision to adapt is often multifactorial. Gross tumor volume (GTV) and planning tumor volume (PTV) dose data were obtained for all patients. OAR data was obtained according to the tumor location. Each plan required available data for targets and OARs to be included. For head and neck tumors, data from the left and right eyes, brain and spinal cord were obtained. For tumors within the chest, data from the heart, lungs and spinal cord were obtained. For tumors within the abdomen, data from liver, kidney, bladder, colon, and rectum were obtained. If the delivered dose to the OAR was less than planned, this data was not considered clinically relevant and was not evaluated to determine if a plan should have been adapted.
FIGURE 1.
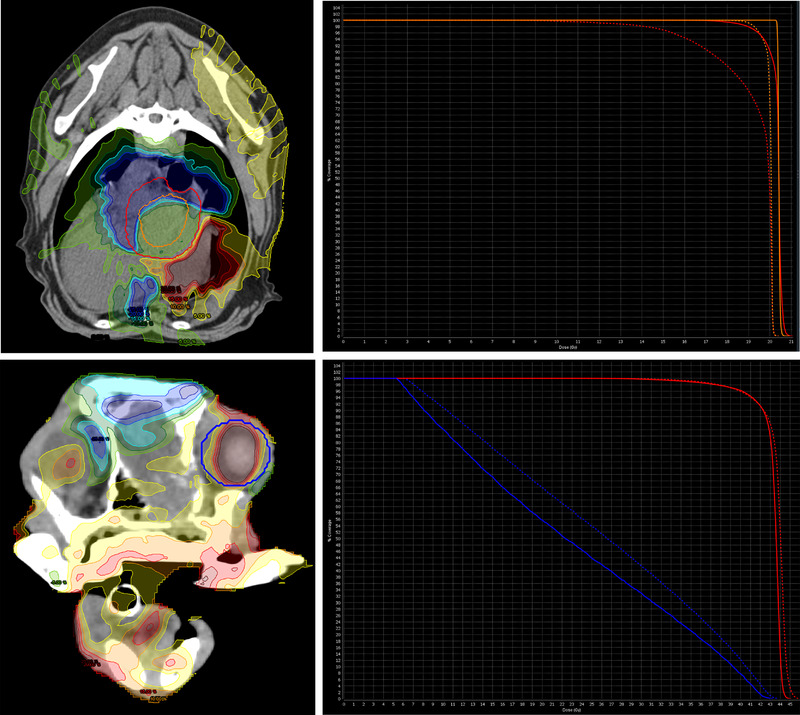
Isodose curves and dose volume histograms (DVH) of a dog with a heart‐based mass which displayed a significant decrease of D98% to its PTV (Above image) and a dog with a nasal tumor which displayed a significant increase in D50% to its right eye (Below image). In the above image, the dotted line and non‐dotted line correspond to the planned and delivered dose to the PTV (red line) and GTV (orange line) respectively. In the below image, the dotted line and non‐dotted line correspond to the planned and delivered dose to the PTV (red line) and the right eye (blue line) respectively. A table displaying representative isodose curves is located on the right side of the figure. [Colour figure can be viewed at wileyonlinelibrary.com]
2.2.1. Radiation setup and planning
As part of the inclusion criteria for head and neck tumors, all patients were set up in sternal recumbency with a bite block and vacuum mattress (Vac‐Lock). Occasionally, a stockinet was placed on the neck to better limit the motion of mobile skin folds. For abdominal and thoracic tumors, patients were setup in either sternal or lateral recumbency using a Vac‐Lock mattress. The immobilization techniques mentioned above were a requirement to be included in this study. Motion management techniques such as 4‐dimensional computed tomography (4DCT) were not used. For daily imaging, the Radixact has a single row xenon ion chamber array that is located opposite of the linear accelerator and stays within the primary beam during radiation delivery. Slice spacing ranges between 1 to 6 mm reconstruction intervals. Image resolution is 512×512 pixels (0.76 mm pixel size). Targets contoured (GTC, CTV, PTV) and intent (definitive vs palliative) varied between plans depending on the goals of treatment and this was not standardized within this study. MVCT images were obtained during each day of treatment and registration was confirmed by either a radiation oncology resident or board‐certified radiation oncologist. All plans underwent inverse computer planning using the Precision (Accuray Inc., Sunnyvale, CA, USA) treatment planning software using a collapsed cone convolution (CCC) algorithm with heterogeneity correction to create a plan where 95% of the PTV received 95% of the prescribed dose. This planning goal is our minimal acceptable institutional target dose that is routinely exceeded. All plans were delivered using a helical radiotherapy machine (Radixact, Accuray Inc., Sunnyvale, CA, USA).
2.2.2. Analyses
Quality analysis was performed by a medical physicist using a biplanar diode array dosimeter (Scandidos Delta4 System, Scandidos, Sweden). The agreement was calculated using gamma analysis, with a dose agreement of 3% and distance to agreement of 3 mm, with acceptance criteria of gamma less than 1 for more than 95% of the comparison points. In order to calculate the percentage change to the planned dose, the following equation was used: Planned Dose ÷ Delivered Dose × 100.
3. RESULTS
A total of 52 canine and feline patients were treated with radiotherapy plans during the included time and all these plans were included in this evaluation. Thirty‐seven of these plans were head and neck tumors, six were thoracic tumors and nine were abdominal tumors. Of the thirty‐seven head and neck tumors, six were cats. All the thoracic and abdominal tumors were dogs. PTV expansion in the majority of all head and neck targets was 2 mm, while an 8 mm expansion was used when the mandibular and/or retropharyngeal lymph nodes were included within the plan. Thoracic PTV expansions ranged from 5–8 mm while abdominal PTV expansions ranged from 8 mm to 1 cm. Dose statistics and prescriptions from plans that were found to have dose differences of more than 10% are provided in Tables 1 and 2. All plans that received more than a 10% deviation of dose within the GTV or PTV were individually evaluated using isodose distributions displaying the dose difference of the planned and delivered dose as well as dose volume histograms (DVH) displaying the planned/delivered PTV and GTV curves (Figure 1).
TABLE 1.
Dose data for GTV and PTV targets in the three radiotherapy plans that were found to have greater than 10% dose deviation between the planned and delivered dose within the thorax and abdomena
| D98 (Gy) | D50 (Gy) | D2 (Gy) | ||||||
|---|---|---|---|---|---|---|---|---|
| Planned | Delivered | Planned | Delivered | Planned | Delivered | |||
| Dog 1 (Thorax) | PTV | 4 Gy × 5 fx Daily | 18.96 | 14.14 | 20.42 | 19.96 | 20.7 | 20.17 |
| Dog 2 (Thorax) | PTV | 4 Gy × 5 fx Daily | 19.28 | 15.73 | 20.25 | 20.41 | 20.65 | 20.72 |
| Dog 1 (Abdomen) | GTV | 4 Gy × 5 fx Daily | 20.09 | 9.13 | 20.2 | 20.54 | 20.75 | 20.79 |
| PTV | 16.95 | 8.62 | 20.37 | 20.46 | 20.68 | 20.74 | ||
TABLE 2.
Dose data for organs at risk within the head and neck, thorax, and abdomen that showed a >10% dose deviation between the planned and delivered dose.
| D50 | D2 | |||||
|---|---|---|---|---|---|---|
| Planned | Delivered | Planned | Delivered | |||
| Head and Neck | Right Eye | 4.2 Gy × 10 fx Daily | 22.33 | 26.56 | 41.77 | 42.64 |
| 2.5 Gy × 20 fx Daily | 2.37 | 3.41 | 23.37 | 22.01 | ||
| 8 Gy × 4 fx Weekly | 2.23 | 2.47 | 5.99 | 6.26 | ||
| 4 Gy × 5 fx Daily | 3.63 | 4.04 | 7.76 | 8.1 | ||
| 8 Gy × 3 fx Daily | 0.17 | 0.19 | 0.58 | 0.65 | ||
| Left Eye | 2.5 Gy × 20 fx Daily | 1.75 | 2.98 | 14.75 | 18.24 | |
| 8 Gy × 4 fx Weekly | 3.8 | 4.1 | 15.55 | 13.5 | ||
| 8 Gy × 3 fx Daily | 0.09 | 0.1 | 0.02 | 0.19 | ||
| 8 Gy × 3 fx Daily | 0.15 | 0.15 | 0.29 | 0.32 | ||
| 8 gy × 1 fx Daily | 0.23 | 0.32 | 1.31 | 1.32 | ||
| Spinal Cord | 4.2 Gy × 10 fx Daily | 5.02 | 5.56 | 24 | 28.27 | |
| 4 Gy × 5 fx Daily | 1.63 | 2.2 | 10.43 | 9.66 | ||
| 8 gy × 1 fx Daily | 0.01 | 0.02 | 0.02 | 0.03 | ||
| Brain | 4.2 Gy × 10 fx Daily | 2.91 | 3.23 | 43.71 | 43.12 | |
| 4.2 Gy × 10 fx Daily | 2.09 | 2.59 | 39.99 | 40.01 | ||
| Thorax | Spinal Cord | 6 Gy × 5 fx MThMThM | 10.27 | 10.53 | 17.58 | 21.24 |
| Abdomen | Rectum | 4 Gy × 5 fx Daily | 9.19 | 12.97 | 19.98 | 19.96 |
| 4 Gy × 5 fx Daily | 11.15 | 13.25 | 19.98 | 19.96 | ||
| 4 Gy × 5 fx Daily | 0.39 | 0.39 | 0.76 | 0.84 | ||
| 4 Gy × 5 fx Daily | 1.34 | 4.29 | 20.68 | 20.69 | ||
For head and neck targets, none of the plans with >10% dose deviation were found to have relevant alterations in dose coverage. However, three of the thoracic and abdominal targets that were found to have >10% change in dose delivery displayed potentially clinically relevant loss of dose coverage to the target on their respective DVH, as seen in the two examples of abdominal and thoracic radiotherapy plans within Figure 1. There were three plans in total whose DVH showed a drop in dose coverage below the planned objective of 95% dose to 95% of the PTV. These three plans were considered to have relevant clinical deviations in dose.
Although many dose alterations to OARs within the head and neck, abdomen, and thorax were identified, many of these changes in dose delivery were considered clinically irrelevant, especially when the OAR was found to have received less radiation than originally planned. Only one increase in planned dose was observed within the evaluated OARs. This was in the right eye of a dog receiving definitive radiation for a nasal tumor (4.2 Gy × 10 fractions). In this case, the planned D50% and D2% were 22.33 and 41.77 Gy respectively, while the delivered D50% and D2% were 26.56 and 42.64 Gy respectively. The isodose lines as well as DVH of the planned and delivered doses are included in Figure 1. There were no reported adverse effects to the right eye within the medical record of this patient.
In total, three patients were found to have clinically relevant dose deviations that would have warranted a discussion on whether to adapt (Table 1). This accounts for approximately 6% of the total plans delivered. When considering location, 2 of the thoracic plans and 1 abdominal plan were found to have potentially clinically relevant deviation in dose delivery while none of the head and neck plans were found to have clinically relevant deviation in dose delivery.
4. DISCUSSION
The data refuted our null hypothesis that there would be minimal dose deviation from the planned dose, as we did find three plans within the thorax and abdomen that showed clinically relevant dose deviation. However, there were no radiotherapy plans within our head and neck patients that showed relevant divergence of dose. These data suggest that our radiation oncology service could benefit from closely monitoring our thoracic and abdominal targets. Frequent dose evaluation using dose assessment software such as MiM throughout the treatment may identify cases in the future in need of adaptation. There were a select few proactively adapted cases throughout July 2020 to July 2021 year that were not included in this analysis. These were cases that had large changes in tumor size before the start of or during treatment (feline nasal lymphoma). There are previous publications that investigate the positive impacts of adaptation for tumors that are suspected to have a rapid and robust response to radiation. 7 The goal of this study was focused more on identification of plans where adaptation should have been considered but was not pursued in real time.
It is unclear why there was such discrepancy between thoracic and abdominal cases compared to head and neck cases. It is likely there is more physiologic variation within the thorax and abdomen compared to the head and neck. Changes in position of the contents within the chest and abdomen (heart, lungs, diaphragm, and bowel loops) as a result of breathing and physiologic function (bladder filling) may result in alterations in the deposition of radiation. Additionally, there may be inherent error within the collapsed cone convolution (CCC) algorithm that is used within the Precision treatment planning software. Although CCC is considered very accurate compared to the gold standard of Monte Carlo, 8 there is evidence that lung‐tissue interfaces can result in significant error in dose calculation. 9 Regardless, the results of this study can be used to inform on the need for more frequent imaging and dose deviation analysis.
There were no clear cases where OARs received more radiation than was tolerable. However, one case of a potentially significant overdose to a right eye in a dog with a nasal tumor. There are no clear normal tissue constraints for canine ocular structures, however this dog may have been more at risk for acute or chronic ocular side effects such as keratoconjunctivitis sicca or lenticular cataracts. We generally attempt to keep the eyes under the constraint of 15 Gy to no more than 60% of the eye at University of Wisconsin, 10 however it is clear in Figure 1 that the tumor was immediately adjacent to the right eye, so we were unable to satisfy this constraint in the original plan and side effects were expected. It is unclear whether adaptation would have benefited this dog. This is especially true because the eyes are sometimes hard to define on MVCT images. Discrepancies of radiation delivery to OARs may become more important to monitor when delivering stereotactic protocols, where the Dmax of a stereotactic plan may be located very close to sensitive organs like the spinal cord.
Alterations in dose delivery resulting in reduced dose coverage to targets may increase the risk of tumor progression. However, adaptation is associated with inherent risk and increased cost. At University of Wisconsin, patients are adapted using the MVCT that was obtained during positioning verification. MVCT is obtained using a variation of the high energy treatment beam which means that contouring accuracy may be hindered by poor soft tissue resolution, specifically when it comes to intra‐abdominal or intra‐thoracic targets. 11 If we were unable to use the MVCT for adaption, a repeat CT simulation would need to be obtained, which carries increased cost, can delay treatment, and increases time under general anesthesia. For this reason, although this investigation has identified radiotherapy plans with significant dose alterations within the chest and abdomen, the decision to adapt cannot be made without consideration of all these factors.
The authors do not recommend using 10% as distinct criteria for adaptation. Numerous publications have debated how accurate we should aim to be when delivering radiation, and although there is yet to be a consensus, many authors have brought forth an intended accuracy of ±5%. 12 A more conservative value of 10% was chosen in this study as there has never been a clearly defined threshold for adaptation in veterinary medicine. Our goal in this publication was not to define a threshold for adaptation. Instead, our aim was to identify distinct and explicit trends and patterns and to begin a discussion of criteria for adaptation. Additionally, we do not know the accuracy of the MIM software used in this study, as discussed below.
The main limitation within this study was the unknown accuracy of the software that was used for this analysis. The gold standard for confirmation of dose would be in‐vivo dosimetry, which is logistically difficult. This software provides a middle ground where daily detector data can be back‐projected onto the daily MVCT. Finally, an additional limitation is that our dosimetry data is based on contours that are deformed by MiM for each MVCT that is obtained throughout the treatment. Errors in deformation of these contours may result in altered dose data.
In conclusion, this investigation identified the potential alteration of dose to targets within a small fraction of radiotherapy plans, specifically within the thorax and abdomen. This will result in an increased frequency of dose evaluation during radiotherapy protocols for all thoracic and abdominal tumors that will be treated in the future. However, the decision to adapt can be complex and all factors, such as treatment delay, cost, and imaging modality, should be considered when adaptation is to be pursued. Future studies are needed to investigate the clinical significance of these dose deviations as well as the accuracy of the software used to calculate the dose deviation reported in this study.
LIST OF AUTHOR CONTRIBUTIONS
Category 1
-
(a)
Conception and Design: Randhawa, Van Asselt
-
(b)
Acquisition of Data: Randhawa, Van Asselt
-
(c)
Analysis and Interpretation of Data: Randhawa, Van Asselt, Kvasnica, Ferris, Christensen
Category 2
-
(a)
Drafting the Article: Randhawa, Van Asselt
-
(b)
Revising Article for Intellectual Content: Randhawa, Van Asselt, Kvasnica, Ferris, Christensen
Category 3
-
(a)
Final Approval of the Completed Article: Randhawa, Van Asselt, Kvasnica, Ferris, Christensen
Category 4
-
(a)
Agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Randhawa, Van Asselt, Kvasnica, Ferris, Christensen
PREVIOUS PUBLICATION OR PRESENTATION
This manuscript was not presented or published in any other media.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Van Asselt N, Randhawa K, Kvasnica K, Ferris W, Christensen N. Evaluation of mega‐voltage CT images for completed radiotherapy treatments for dogs and cats reveals uncommon but potentially consequential dose deviation in thoracic and abdominal tumors. Vet Radiol Ultrasound. 2023;64:149–154. 10.1111/vru.13176
DATA AVAILABILITY STATEMENT
Please contact University of Wisconsin Veterinary Care for more information regarding this study or patients within the study (608‐263‐7600).
REFERENCES
- 1. The Management of Respiratory Motion in Radiation Oncology Report of AAPM Task Group 76. 2006. [DOI] [PubMed]
- 2. Brock KK. Adaptive Radiotherapy: Moving Into the Future. Semin Radiat Oncol. W.B. Saunders; 2019;181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li A, Wang JZ, Stewart RD. Physics in Medicine & Biology To cite this article: Di Yan et al. Phys. Med. Biol. 1997. [Google Scholar]
- 4. Yan D. Adaptive Radiotherapy: Merging Principle Into Clinical Practice. Semin Radiat Oncol. 2010;79–83. [DOI] [PubMed] [Google Scholar]
- 5. Lim K, Stewart J, Kelly V, et al. Dosimetrically triggered adaptive intensity modulated radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. Elsevier Inc.; 2014. Sep 1;90:147–154. [DOI] [PubMed] [Google Scholar]
- 6. Dawson P, Deluca PM, Doi K, et al. Journal of the ICRU. 2010; Available from: http://jicru.oxfordjournals.org/ [Google Scholar]
- 7. Rohrer Bley C, Meier V, Schneider U. Dosimetric benefit of adaptive radiotherapy in the neoadjuvant management of canine and feline thymoma—An exploratory case series. Veterinary and Comparative Oncology. Blackwell Publishing Ltd; 2018. Sep 1;16:324–329. [DOI] [PubMed] [Google Scholar]
- 8. Carrasco P, Jornet N, Duch MA, et al. Comparison of dose calculation algorithms in phantoms with lung equivalent heterogeneities under conditions of lateral electronic disequilibrium. Medical Physics. John Wiley and Sons Ltd; 2004;31:2899–2911. [DOI] [PubMed] [Google Scholar]
- 9. Dobler B, Walter C, Knopf A, et al. Optimization of extracranial stereotactic radiation therapy of small lung lesions using accurate dose calculation algorithms. Radiat Oncol. 2006. Nov 29;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawrence JA, Forrest LJ, Turek MM, et al. Proof of Principle of Ocular sparing in dogs with sinonasal tumors treated with intensity‐modulated radiation therapy. Vet Radiol Ultrasound. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forrest LJ, Mackie TR, Ruchala K, et al. The utility of megavoltage computed tomography images from a helical tomotherapy system for setup verification purposes. Int J Radiat Oncol Biol Phys. 2004 Dec 1;60:1639–1644. [DOI] [PubMed] [Google Scholar]
- 12. van Dyk J, Battista JJ, Bauman GS. Introduction 362 11.2 Terminology 364 11.2.1 Measurement terminology 364.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact University of Wisconsin Veterinary Care for more information regarding this study or patients within the study (608‐263‐7600).


