Abstract
Aim
To evaluate the cardiovascular outcomes of glucagon‐like peptide‐1 receptor agonists (GLP1‐RA) in patients with type 2 diabetes (T2DM) and chronic kidney disease (CKD).
Materials and Methods
We searched PubMed, Ovid MEDLINE, CINAHL, and Web of Science databases for randomized controlled trials reporting event rates for a composite cardiovascular outcome of cardiovascular death, myocardial infarction, and stroke in patients with T2DM and CKD receiving GLP1‐RA or placebo. Studies were restricted to those reporting specific event rates for patients with CKD separately from the overall population. We conducted a meta‐analysis using a random‐effects model. This meta‐analysis was registered on PROSPERO (CRD42022320157).
Results
A total of four studies comprising 7130 patients was included in our analysis. Four different GLP1‐RA were assessed in a population with CKD defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. Treatment with GLP1‐RA was not associated with a significant reduction in the composite cardiovascular end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (odds ratio (OR) 0.80; 95% confidence interval (CI), 0.59–1.07; p = 0.13) among patients with T2DM and CKD. Individual components of the composite cardiovascular end point were assessed in two trials and did not show evidence of an effect of GLP1‐RA in reducing cardiovascular end points.
Conclusions
Pooled analysis of clinical trials reporting separate cardiovascular events rates in patients with T2DM and CKD did not find GLP1‐RA to be associated with a reduction in composite cardiovascular event rates. Select GLP1‐RA may offer cardiovascular event reduction in patients with T2DM and CKD, but this does not appear to be a class effect. Use of GLP1‐RA with demonstrated cardiovascular benefits should be preferred in patients with CKD and T2DM to further reduce cardiovascular risk.
Keywords: cardiovascular disease, chronic kidney disease, diabetes, GLP‐1 receptor agonist
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death and comorbidity among patients with chronic kidney disease (CKD). 1 Patients with type 2 diabetes mellitus (T2DM) and CKD have an even higher risk of developing CVD compared to the general population. 2 , 3 Patients with CKD are more likely to die of CVD than reach end‐stage renal disease (ESRD). 4 Appropriately managing blood glucose, treating cardiovascular risk factors, and preserving renal function is key to lowering cardiovascular risk in these patients. 5
Certain medications used to treat T2DM, such as sodium‐glucose cotransporter 2 (SGLT2) inhibitors and glucagon‐like peptide‐1 receptor agonists (GLP1‐RA), have shown to be cardioprotective and/or renoprotective in addition to lowering glucose. 6 Proposed mechanisms of cardiovascular risk reduction seen with GLP1‐RA can be attributed to non‐glycemic benefits, such as weight and blood pressure reduction. 7 In addition to that, their anti‐inflammatory effects and ability to reduce oxidative stress are other plausible mechanisms for reduction of kidney damage and thus, cardiovascular risk. 1 Current recommendations from the American Diabetes Association (ADA) and Kidney Disease Improving Global Outcomes (KDIGO) Diabetes Work Group recommend SGLT2 inhibitors with evidence of reducing CKD progression for patients with CKD and elevated albuminuria. 5 , 8 However, in patients with CKD without albuminuria or if an SGLT2 inhibitor cannot be used due to significant renal dysfunction (estimated glomerular filtration rate (eGFR) < 25 ml/min/1.73 m2) or an intolerance, a GLP1‐RA with demonstrated renal benefit is recommended. 5 , 8 A previous meta‐analysis reported that GLP1‐RA were associated with a 12% reduction in major adverse cardiovascular events (MACE) relative to placebo among patients with T2DM. 9 More recently, a meta‐analysis used pooled data from eight GLP1‐RA cardiovascular outcome trials (CVOT) with 60,080 patients with T2DM. 10 They reported a similar 14% reduction in composite cardiovascular events with GLP1‐RA use compared to placebo. However, an assessment of the cardiovascular benefits of GLP1‐RA in patients with T2DM and CKD is more uncertain as most of the CVOT predominantly included patients without CKD. Considering that a diagnosis of CKD in addition to T2DM amplifies CVD risk, our systematic review and meta‐analysis aims to assess the cardiovascular benefits of GLP1‐RA in patients with concomitant T2DM and CKD.
2. MATERIALS AND METHODS
2.1. Data sources and search criteria
The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) Statement. 11 A systematic search strategy was developed according to PRISMA guidelines (Appendix S1). The search strategy and search were conducted by a health sciences librarian on the following databases: PubMed, Ovid MEDLINE, CINAHL, and Web of Science. Cited references were examined and journals dedicated to diabetes research were also reviewed. The initial search was performed in February 2022, with follow‐up searches performed on July 13, 2022. Search results were limited to the English language, human studies, and adult populations. No date restrictions were included as part of the search. Search terms included a combination of index terms and keywords for ‘composite renal events’, ‘cardiovascular events’, ‘chronic kidney disease’, ‘type 2 diabetes mellitus’, and ‘glucagon‐like peptide‐1 (GLP‐1) analog’. This meta‐analysis was registered on PROSPERO (CRD42022320157).
2.2. Study selection
Two authors (M.K. and H.R.) independently reviewed all retrieved articles for inclusion using the following criteria: (i) randomized controlled trial (RCT); (ii) evaluated a GLP1‐RA (treatment group) against either a placebo or other active medication (comparator group); (iii) study duration of 12 weeks or more; and (iv) reported cardiovascular event rates in treatment group and comparator group for patients with CKD. Reduced eGFR below 60 ml/min/1.73 m2 was used to define CKD in all articles. Articles were initially screened by reviewing abstracts; full‐text articles were reviewed for studies meeting all inclusion criteria or when all inclusion criteria could not be assessed by reviewing the abstract. Supplementary texts were evaluated for subgroup analyses when reported in the full text. Disagreements about study inclusion were resolved by a third author (J.L.). The selection process and results are outlined in Figure 1.
FIGURE 1.
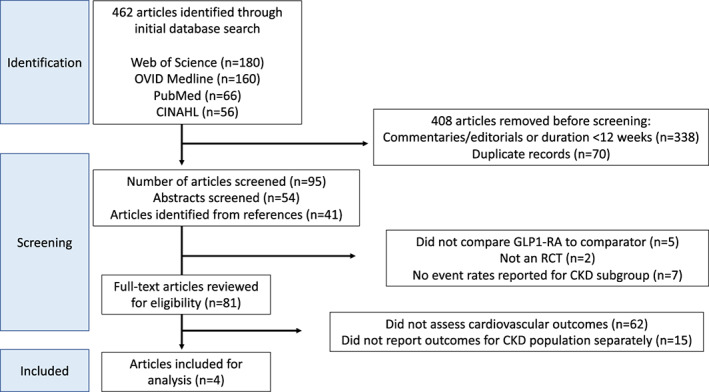
Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) flow diagram of identified studies. CKD, chronic kidney disease; CINAHL, Cumulative Index to Nursing and Allied Health Literature; GLP1‐RA, glucagon‐like peptide‐1 receptor agonists; OVID, Offshore Vessel Inspection Database; RCT, randomized controlled trial
2.3. Data extraction and quality assessment
Data from included studies was abstracted by one author (J.C.) and included study author and year, GLP1‐RA and comparator, definition of CKD as defined in the study, cardiovascular end points evaluated, number of patients with CKD in each group, and cardiovascular event rates for each group. Composite cardiovascular events, which included cardiovascular death, nonfatal myocardial infarction (MI) and stroke, as well as event rates for each individual event of the composite end point were collected. Study quality of included trials was evaluated independently by two authors (H.R. and J.L.) using the Cochrane risk‐of‐bias tool for randomized controlled trials version 2 (RoB2) 12 (Appendix S2).
2.4. Statistical analysis
For each study included in the analysis, the number of patients with positive and negative cardiovascular end points was determined for both GLP1‐RA treatment and placebo. Binary outcomes were reported as odds ratios. Fixed (common) and random‐effects calculations were done using the Mantel–Haenszel method for pooling the studies. Between‐study variance (tau2) was computed using restricted maximum likelihood and the Q‐profile method was used to compute the confidence interval of tau2 and tau. 13 Heterogeneity was assessed using a Cochrane Q and quantified using the I2 statistic, which shows the variance attributable to heterogeneity as a percentage, with p‐values below 0.05 considered to indicate significant heterogeneity. Statistical analysis was done using The R Project for Scientific Computing version 4.1.1 on Rstudio with “meta” package (R Core Team 2021, Vienna, Austria).
3. RESULTS
A total of 81 full‐text articles were identified with our search criteria. Among those, four studies comprising 7130 patients met our study inclusion criteria and were included in the analysis. 14 , 15 , 16 , 17 The patients with CKD that were included in our analysis come from subgroup analyses of four CVOT. 16 , 17 , 18 , 19 Inclusion criteria for each CVOT varied but the proportion of patients with established CVD ranged from 73% to 85%. Each study included in our analysis evaluated a different GLP1‐RA, including once‐daily liraglutide, once‐weekly exenatide, once‐weekly subcutaneous semaglutide, and once‐daily oral semaglutide. 14 , 15 , 16 , 17 All studies evaluated patients with CKD defined as eGFR <60 ml/min/1.73 m2, although two studies 14 , 16 excluded patients with an eGFR <30 ml/min/1.73 m2. The percent of patients in the original four CVOT who had CKD ranged from 22.9% to 28.5%. 16 , 17 , 18 , 19 The composite cardiovascular end point assessed in each study included cardiovascular death, nonfatal MI, and nonfatal stroke. Two studies 14 , 15 also reported event rates for each of the individual outcomes. Each trial was considered to have an overall low risk of bias based on the assessment tool used (Appendix S2). 12 Information related to included studies and the original CVOT is shown in Table 1.
TABLE 1.
Summary of baseline demographic data of included studies
| CKD subgroup study | Original CVOT (n) | Inclusion criteria for CVOT | Number of patients w/CKD (%) | How CKD defined | GLP1‐RA vs comparator | Study duration | CV composite outcome |
|---|---|---|---|---|---|---|---|
| Bethel et al. (2020) 14 | EXSCEL (n = 14,752) | T2DM on ≤3 oral agents or insulin therapy alone or in combo with ≤2 oral agents | 3177 (22.9%) | eGFR 30–59 ml/min/1.73 m2 | Exenatide 2 mg weekly vs placebo | Median 3.2 years | 3‐point composite MACE |
| Mann et al. (2018) 15 | LEADER (n = 9340) | Age ≥ 50 years and ≥1 of the following:
|
2158 (23.1%) | eGFR <60 ml/min/1.73 m2 | Liraglutide 1.8 mg daily vs placebo | Median 3.8 years | 3‐point composite MACE |
| Husain et al. (2019) 16 | PIONEER‐6 (n = 3183) |
|
856 (26.9%) | eGFR 30–59 ml/min/1.73m2 | Semaglutide 14 mg daily vs Placebo | Median 15.9 months | 3‐point composite MACE |
| Marso et al. (2016) 17 | SUSTAIN‐6 (n = 3297) |
|
939 (28.5%) | eGFR<60 ml/min/1.73 m2 | Semaglutide 0.5 or 1 mg weekly vs Placebo | Median 2.1 years | 3‐point composite MACE |
Abbreviations: 3‐point composite MACE = major adverse cardiovascular events; CHD = coronary heart disease; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GLP1‐RA = glucagon‐like peptide‐1 receptor agonist; HF = heart failure; MI = myocardial infarction; NYHA = New York Heart Association; TIA = transient ischemic attack; T2DM = type 2 diabetes mellitus.
3.1. Composite cardiovascular outcome
In the pooled analysis using a random‐effects model, treatment with GLP1‐RA was not associated with a significant reduction in the composite cardiovascular end point in patients with CKD (odds ratio (OR) 0.80; 95% confidence interval (CI), 0.59–1.07; p = 0.13) (Figure 2). Three GLP1‐RA (liraglutide, oral and subcutaneous semaglutide) were associated with a lower risk of the composite end point 15 , 16 , 17 but only one of these agents, liraglutide, 15 demonstrated a statistically significant result for the composite cardiovascular outcome. The fourth GLP1‐RA, exenatide, was associated with a non‐significant increase in OR for the composite outcome. 14
FIGURE 2.
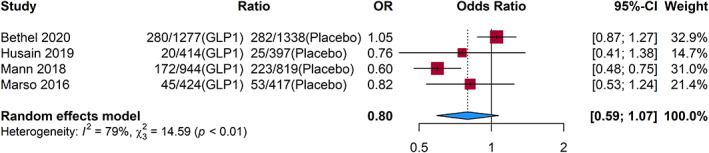
Composite cardiovascular outcomes. CI, confidence interval; GLP1, glucagon‐like peptide‐1; OR, odds ratio
3.2. Individual cardiovascular outcomes
Only two studies 14 , 15 reported event rates for individual components of the composite end point for the CKD population (Figure 3). Similar to the OR for the composite cardiovascular outcome, there was no evidence of benefit in lowering risk of cardiovascular death (OR 0.83; 95% CI, 0.5–1.36; p = 0.46), nonfatal MI (OR 0.85; 95% CI, 0.63–1.15; p = 0.30), and nonfatal stroke (OR 0.78; 95% CI, 0.33–1.86; p = 0.58) with GLP1‐RA compared to placebo.
FIGURE 3.
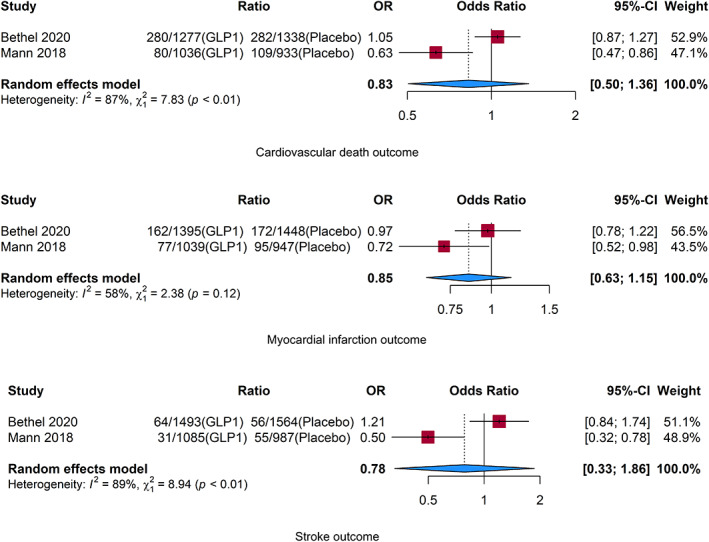
Cardiovascular death, myocardial infarction, and stroke individual outcomes. CI, confidence interval; GLP1, glucagon‐like peptide‐1; OR = odds ratio
4. DISCUSSION
Our meta‐analysis is the first to assess the effects of GLP1‐RA on cardiovascular events in patients with T2DM and CKD by evaluating specific event rates from CVOT and did not observe a benefit of GLP1‐RA in reducing composite cardiovascular outcomes. In previous meta‐analyses, which utilized pooled hazard ratios, 9 , 10 subgroup analyses by CKD status showed GLP1‐RA were associated with a lower risk of composite cardiovascular events in patients with and without CKD, defined as eGFR below or greater than 60 ml/min/1.73 m2, respectively, at baseline. In the meta‐analysis by Kristensen et al., 9 GLP1‐RA was associated with a reduced hazard ratio (HR) for the 3‐point MACE outcome in patients with CKD (HR 0.88; 95% CI, 0.76–1.03), which was similar to the study group without CKD (HR 0.85; 95% CI, 0.76–0.96) p = 0.72. Likewise, in the more recent meta‐analysis by Sattar et al., 10 the 3‐point MACE outcome was lower in patients with CKD (HR 0.88; 95% CI, 0.77–1.01) and without CKD (HR 0.83; 95% CI, 0.74–0.93) treated with GLP1‐RA. Interestingly, both analyses reported identical composite cardiovascular event rates for eGFR subgroups. In patients with baseline eGFR of 60 ml/min/1.73 m2 or higher, composite cardiovascular event rates for GLP1‐RA versus placebo were 9% versus 10%, while composite event rates in patients with baseline eGFR below 60 ml/min/1.73 m2 were 14% versus 16%, respectively. Based on the reported event rates, patients with CKD have higher cardiovascular event rates and treatment with GLP1‐RA is associated with a greater absolute risk reduction (2%) compared to non‐CKD patients (1%). However, our analysis evaluating specific reported event rates in patients with CKD did not find GLP1‐RA to be associated with reduced composite cardiovascular event rates.
The lack of evidence of supporting reduced cardiovascular events found in our meta‐analysis may be explained by a low percentage of patients with CKD included in CVOT evaluating patients with T2DM treated with GLP1‐RA, as well as a potential confounding effect of exenatide treatment. Our data were generated from CVOT reporting specific event rates in the study population with CKD, but none of these trials were powered to assess treatment differences in CKD subgroups. In previous analyses by Kristensen and Sattar, the proportion of patients with CKD was 19% and 20%, respectively. 9 , 10 Our analysis included four trials,” which reported cardiovascular event rates for the CKD subgroup separately from the overall population. The number of patients with CKD in these four trials ranged from 23% to 28.5% of the entire population studied in the original CVOT. Therefore, we believe the proportion of patients with CKD assessed in our analysis to be of adequate size for subgroup analysis.
Perhaps the most compelling explanation for the lack of statistical significance in our analysis may be due to the neutral cardiovascular effects of exenatide. The exenatide CVOT (EXSCEL) evaluated the effect of once‐weekly exenatide on major cardiovascular events among patients with T2DM and increased cardiovascular risk. 19 This study included 14,752 patients, which is the largest sample size among GLP1‐RA CVOT included in our analysis, and excluded patients with an eGFR <30 ml/min/1.73 m2. Over a study period of 3.2 years, exenatide did not significantly reduce the risk of MACE compared to placebo (HR 0.91; 95% CI, 0.83–1.00). In our analysis, the EXSCEL trial included the lowest percentage of patients with a reduced eGFR (22.9%), but accounted for the largest weighted effect of the pooled analysis (32.9%) due the large sample size of the overall trial. Subgroup analysis of the EXSCEL trial found no treatment effect by CKD status, yet composite cardiovascular event rates appeared to be higher in patients across all CKD subgroups who received exenatide versus placebo. For example, the composite cardiovascular event rate occurred at a higher rate in patients with eGFR below 60 ml/min/1.73 m2 treated with exenatide compared to placebo (18.1% exenatide vs 17.5% placebo). Conversely, other GLP1‐RA evaluated in our meta‐analysis were associated with reduced cardiovascular event rates among patients with CKD, with the largest absolute reductions reported with liraglutide and once‐weekly semaglutide. 15 , 17 Similarly, the meta‐analysis by Kristensen et al. 9 reported possible heterogeneity between GLP‐1 homology, although comparison did not reach statistical significance (p‐interaction = 0.06).
The neutral effects of exenatide may be explained by the fact that it is dissimilar to other GLP1‐RA with regard to renal effects. Once‐weekly exenatide is not recommended in patients with eGFR <30 ml/min/1.73 m2 due to increased and unpredictable drug exposure. 20 Exenatide is an exendin‐4 analog, which is metabolized and eliminated by the kidneys, and could accumulate in patients with CKD. Alternatively, human GLP1‐RA analogs (such as liraglutide, semaglutide, and dulaglutide) are not eliminated by the kidneys and may be used in patients with CKD, including patients receiving hemodialysis, without dose adjustment. 21 , 22 , 23 , 24 Additionally, positive composite renal outcomes have been demonstrated with several GLP1‐RA (liraglutide, dulaglutide, and semaglutide) 10 whereas exenatide had no significant effect on the composite renal outcome among the population studied in the EXSCEL trial. 14 An ongoing phase 3 clinical trial, the FLOW trial (NCT03819153), will assess the effects of once‐weekly semaglutide compared to placebo on the composite renal and cardiovascular death end point among patients with T2DM and CKD receiving maximally tolerated renin‐angiotensin‐aldosterone system inhibiting medications.
The recently updated 2022 ADA Standards of Medical Care in Diabetes guidelines now includes a stand‐alone chapter on managing CKD and provides specific recommendations for preferred antihyperglycemic agents in patients with T2DM. 25 An SGLT2 inhibitor is recommended for patients with CKD and elevated albuminuria, but either a GLP1‐RA or SGLT2 inhibitor with demonstrated cardiovascular risk reduction is recommended for patients with CKD without albuminuria to lower cardiovascular risk. 8 In the event that patients are unable to take an SGLT2 inhibitor due to adverse effects or significant renal dysfunction (eGFR <25 ml/min/1.73 m2), a GLP1‐RA may be used in its place given the elevated cardiovascular risk associated with T2DM and CKD. Adverse effects of SGLT2 inhibitors are well characterized and mainly include genitourinary infections. 26 The risk of euglycemic diabetic ketoacidosis may also be of concern, especially in patients with advanced CKD, as SGLT2 inhibitors appear to promote ketogenesis and reduce renal elimination of ketones. 27 In the event that there is a concern for these adverse effects, a GLP1‐RA may be the preferred option.
Our study has a few limitations. First, only four studies met our inclusion criteria in providing individual event rates for the CKD subgroup, which may limit the generalizability of our findings to the four GLP1‐RA studied in those trials. Additionally, the proportion of patients with CKD included in the original CVOT was low, and none of the trials were powered to assess cardiovascular end points between patients with and without CKD. Furthermore, our I 2 statistic values appear high (79% for the composite cardiovascular end point), suggesting a high proportion of total variability due to between‐study heterogeneity. This may be due to differences in the GLP1‐RA studied, the proportion of patients with previous CVD, and potential differences in use of other medications associated with improved cardiovascular benefit. For example, the proportion of patients with established CVD was 73% in the EXSCEL trial and 85% in the PIONEER‐6 study. 14 , 16 Additionally, SGLT2‐inhibitor use was reported in 10% of patients from PIONEER‐6 compared to 1% in EXSCEL. Lastly, the patients with CKD who were included in our study may also have other risk factors contributing to high CVD risk. We did not account for additional CVD risk factors, other than CKD, which could have influenced cardiovascular event rates and the effects of the GLP1‐RA.
5. CONCLUSION
The presence of CKD concomitantly with T2DM increases CVD risk. Select GLP1‐RA have shown reduced cardiovascular event rates in patients with T2DM and elevated cardiovascular risk (including CKD); however, our meta‐analysis found that GLP1‐RA was not associated with reduced risk of the composite cardiovascular end point in a subgroup population with T2DM and CKD compared to placebo. The limited number of included trials or heterogeneity of evaluated GLP1‐RA may explain the lack of cardiovascular effect associated with GLP1‐RA in the CKD patient subgroup. Based on available clinical trials and subgroup analysis, use of GLP1‐RA with demonstrated renal and cardiovascular benefits (such as liraglutide or once‐weekly semaglutide) should be preferred in patients with CKD and T2DM to further reduce cardiovascular risk. Additional studies to evaluate the cardiovascular effects of GLP1‐RA in patients with T2DM and CKD would be useful to determine if this class does reduce composite cardiovascular event rates in this subgroup population.
AUTHOR CONTRIBUTIONS
Authors MK, HR, JL were responsible for the conception and design. IP performed the initial literature search; RB conducted the statistical analysis; JC contributed to literature review and data abstraction for statistical analysis. All authors contributed to writing of the manuscript. All authors have reviewed and approved the submitted version and ensure the accuracy and integrity of this work.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1
Appendix S2
Kelly M, Lewis J, Rao H, Carter J, Portillo I, Beuttler R. Effects of GLP‐1 receptor agonists on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease: A systematic review and meta‐analysis. Pharmacotherapy. 2022;42:921‐928. doi: 10.1002/phar.2737
REFERENCES
- 1. Sarnak MJ, Amann K, Bangalore S, et al. The present and future chronic kidney disease and coronary artery disease JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;74(14):1823‐1838. doi: 10.1016/j.jacc.2019.08.1017 [DOI] [PubMed] [Google Scholar]
- 2. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157‐1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007‐2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lovre D, Shah S, Sihota A, Fonseca VA. Managing diabetes and cardiovascular risk in chronic kidney disease patients. Endocrinol Metab Clin N Am. 2018;47(1):237‐257. doi: 10.1016/j.ecl.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4 S):S1‐S115. doi: 10.1016/j.kint.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 6. Brown JM, Everett BM. Cardioprotective diabetes drugs: what cardiologists need to know. Cardiovasc Endocrinol Metab. 2019;8(4):96‐105. doi: 10.1097/XCE.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cox EJ, Alicic RZ, Neumiller JJ, Tuttle KR. Clinical evidence and proposed mechanisms for cardiovascular and kidney benefits from glucagon‐like Peptide‐1 receptor agonists. US Endocrinol. 2020;16(2):80‐87. doi: 10.17925/USE.2020.16.2.80 [DOI] [Google Scholar]
- 8. American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical Care in Diabetes—2022. Diabetes Care. 2021;45(Suppl_1):S125‐S143. doi: 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 9. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776‐785. doi: 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 10. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653‐662. doi: 10.1016/S2213-8587(21)00203-5 [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JAC, Savović J, Page MJ, et al. RoB2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 13. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153‐160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bethel MA, Mentz RJ, Merrill P, et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once‐weekly exenatide: insights from the EXSCEL trial. Diabetes Care. 2020;43(2):446‐452. doi: 10.2337/dc19-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138(25):2908‐2918. doi: 10.1161/CIRCULATIONAHA.118.036418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841‐851. doi: 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 17. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 18. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuttle KR, Heilmann C, Hoogwerf BJ, Brown C, Anderson PW. Effects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetes. Am J Kidney Dis off J Natl Kidney Found. 2013;62(2):396‐398. doi: 10.1053/j.ajkd.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 21. Górriz JL, Soler MJ, Navarro‐González JF, et al. GLP‐1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947. doi: 10.3390/jcm9040947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trulicity (Dulaglutide) [Package Insert]. Eli Lilly and Company; 2021. [Google Scholar]
- 23. Victoza (Liraglutide) [Package Insert]. Novo Nordisk Inc.; 2021. [Google Scholar]
- 24. Ozempic (Semaglutide) [package insert]. Novo Nordisk Inc; 2022. [Google Scholar]
- 25. American Diabetes Association . Chronic kidney disease and risk management: standards of medical Care in Diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S175‐S184. doi: 10.2337/dc22-S011 [DOI] [PubMed] [Google Scholar]
- 26. Qiu R, Balis D, Xie J, Davies MJ, Desai M, Meininger G. Longer‐term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin. 2017;33(3):553‐562. doi: 10.1080/03007995.2016.1271780 [DOI] [PubMed] [Google Scholar]
- 27. Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33(5):e2886. doi: 10.1002/dmrr.2886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


