Abstract
Background
Radiation-induced xerostomia and oral mucositis are serious complications of radiation therapy for head and neck cancers. Current treatment options have limited efficacy. Mesenchymal stem cell (MSC) therapy has shown promising results in supporting the restoration of glandular secretion function and the regeneration of damaged tissues. This study aim to (1) assess the quality of evidence for MSCs treatment in rodent models of radiation-induced oral complications and (2) determine whether MSCs can improve the therapeutic effect of radiation-induced oral mucositis.
Methods
Intervention studies using MSCs in rodent models were comprehensively retrieved in the Pubmed, Medline, Embase, Web of Science, and Cochrane library databases on June 1, 2022. The quality of all in vivo experiments was assessed using SYRCLE, and this article is written following the PRISMA guidelines.
Results
A total of 12 studies were included in this systematic review. The study found that in animal models of radiation-induced xerostomia, MSCs could increase salivary protein secretion, improve SFR, shorten the salivary lag time, anti-apoptosis, etc. In animal models of radiation-induced oral mucositis, MSCs improve the micromorphology and macromorphology of RIOM. Moreover, the effect of MSCs on the modification of ulcer duration and latency may be related to the time of MSCs transplantation but further studies are needed.
Conclusion
The results of our systematic review suggest that MSCs appeared to be effective in the treatment of radiation-induced xerostomia and oral mucositis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-023-03301-y.
Keywords: Mesenchymal stem cells, Xerostomia, Oral mucositis, Ionizing radiation, Systematic review
Introduction
Ionizing radiation used as external radiotherapy, brachytherapy, and targeted radionuclide therapy plays a pivotal role in the treatment of patients with head and neck cancer (HNC). Approximately 80% (range 73.9–84.4%) of all HNC patients receive radiotherapy at least once during their disease [1]. Despite the advantages of radiotherapy in preserving tissue architecture, and the modern radiotherapy techniques that have been developed using advanced, highly conformal RT (e.g. IMRT) delivery methods. It is not always possible to completely avoid toxicity [2]. No technique can completely protect normal tissue from radiation. So patients will always experience some degree of radiation-related toxicity [3]. Many patients with head and neck cancer receive high-dose radiotherapy to large areas of the mouth, maxilla, mandible, and salivary glands [4], causing many acute and long-term oral adverse effects, such as mucositis, xerostomia, taste disturbances, vascular damage, increased risk of dental caries and periodontal disease, and the most severe radiation-induced osteonecrosis [5]. Oral complications due to radiotherapy can lead to high morbidity and reduced quality of life, increasing the cost of treatment and management. Among these, severe hypofunction of the salivary glands and early radiation reactions to the oral mucosa is particularly common and serious adverse effects of radiotherapy for advanced head and neck tumors [6]. The prevalence of oral dryness after radiotherapy for head and neck cancers ranges from 74 to 85% [7], and the prevalence of radiation-induced oral mucositis (RIOM) in head and neck radiotherapy approaches 100% [8, 9]. RIOM is a radiotherapy-induced condition affecting the inflammation of the oral mucosa, characterized by erythema, a painful ulcerative lesion affecting the oral lining. Due to oral mucosal damage, patients complain about burning sensations, oral pain, and ulcers, which lead to increased pain whenever the patients try to eat or drink [4], requiring the use of pain medication during treatment. In addition, there may even be bleeding, dysphagia, and dysarthria, which in turn interfere with the radiation treatment process and alter the local control of the tumor and ultimately affect the patient's survival. Radiation-induced xerostomia is a combination of irradiation-induced changes in salivary gland function or saliva quantification and quality. It combines subjective complaints of dry mouth and objective reduction in saliva production. Patients present with difficulty chewing and swallowing dry food, impaired vocalization, persistent dryness and burning sensation in the mouth, and tasting disturbances, which can be severe and may result in loss of taste, loss of appetite, and weight loss [10–12]. This can be very distressing for the patients and even hinder treatment or prompt abandonment. The two radiation-induced oral complications, oral mucositis, and xerostomia present formidable challenges to healthcare providers. Safer and more effective strategies need to be found to reduce their severity and deleterious effects on basic life functions and quality of life. Several recent studies suggest that mesenchymal stem cell therapy may be a viable treatment option for radiation oral mucositis and xerostomia [13–15].
Mesenchymal stem cells are cells that originate from the mesoderm of embryonic development and are widely found in a variety of tissues such as the umbilical cord, bone marrow, and adipose and have a strong capacity for self-renewal and differentiation [16] and have been used to treat various diseases such as neurodegenerative diseases [17, 18], spinal cord injuries [19], hematologic disorders [20], graft-versus-host disease GVHD [21, 22], and reproductive system disorders [23]. Special attention is paid to the fact that in recent years, MSCs have been widely used in various inflammatory diseases and salivary gland injury diseases. Since MSCs have functions such as directed differentiation [24], regulation of immunity [25], antioxidant [26], anti-apoptosis [27], promotion of cell regeneration [28], and angiogenesis [29], they have good therapeutic effects in various inflammatory diseases and salivary gland injury diseases.
These findings have led researchers to further think about the possibility of applying MSCs in the treatment of radiation injury to solve the key and difficult problems of the oral gland and mucosa repair after radiation injury. Jae-Yol Lim et al. have proposed that bone marrow-derived clonal mesenchymal stem cells (BM-MSCs) can differentiate into salivary epithelial cells and preserve salivary gland function through transplantation and transdifferentiation to improve salivary injury after radiation. BM-MSCs also can be used as a cell-based therapeutic source to restore radiation-induced hyposalivation [30]. Lee et al. have shown that adipose-derived mesenchymal stem cells (AdMSCs) secretion can reduce tissue damage by inhibiting apoptosis, inflammation, and fibrotic remodeling through a variety of trophic factors with various properties produced by paracrine activity. And AdMSCs promote angiogenesis and endogenous stem cells recruitment to repair and remodel salivary gland (SG) structures [31]. AdMSCs synthesize and release a variety of paracrine factors that can also contribute to mucosal damage by enhancing cell proliferation or inhibiting epithelial cell apoptosis, or by a combination of both to promote mucosal repair [18, 32–34]. The implanted MSCs also downregulate pro-inflammatory cytokines and significantly reduce cellular reactive oxygen species (ROS) levels to relieve oral mucositis [35].
Although MSC-based approaches have proven beneficial in models of xerostomia and oral mucositis after radiation injury, the optimal protocol, manipulation, or cellular product remains controversial. Therefore, the purpose of this review is to evaluate the efficacy and safety of MSCs in preclinical studies for radiation-induced xerostomia and oral mucositis and to assess the potential use of MSCs in future human trials.
Method and materials
Search strategy
Five databases (PubMed, Embase, the Web of Science, the Cochrane Library, and Medline) were searched from their inception dates to June 1st, 2022, The queries and study strings applied using Boolean operators are listed in Additional file 1. Database queries were performed on two separate topics, and differences were resolved after discussions with a third party. All citations were managed using Zotero software. In addition, the reference lists of all publications included in this review were hand-searched for additional studies. Retrieval strategies, screening, and data selection are carried out following PRISMA standards [36]. The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022299487.
Inclusion and exclusion criteria
Inclusion criteria for the screened publications were (1) studies involving animal models of radiation-induced oral disease (all species and sexes); (2) all animal models of radiation-induced oral disease were treated with MSCs; (3) received any delivery route and included multiple dosing regimens; (4) include efficacy outcome studies; (5) studies have a control group and the control group is a placebo control using PBS. Applied exclusion criteria were (1) all inclusion criteria were not fulfilled; (2) meeting abstracts, case reports, and case series; (3) reviews or meta-analysis; (4) the study was duplicated; (5) studies published in a non-English language.
Study selection
After obtaining the results of the electronic database search, the two authors first examined the titles, abstracts, and full texts of the studies separately. For eligible articles, which would be read further in full to clarify whether to include in the final study, the two authors negotiated divergentially at any stage of review and consulted third parties if necessary. Each original study was included only once, and we excluded studies without raw data.
Data extraction
We used Excel software to create a table to extract information. Two authors independently extracted key information from each study. The extracted information in the table includes first author, year of publication, study country, study type, type of ionizing radiation, disease model, experimental subjects, type of MSCs, MSCs implantation method, dose, follow-up time, and outcome indicators. If data were missing, the author of the article would be contacted by email for specific data information. If the author did not reply to the message, a second email contact was sent. If there was still no reply, the data were considered unavailable.
Quality assessment
Two authors independently assessed the methodological quality of the included studies. A third author was required in case of any dispute during data extraction and quality assessment. The quality of all in vivo experiments was assessed using the Risk of Bias in Animal Studies tool of the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) [37]. The quality of the studies was judged as “high risk,” “low risk,” or “unclear.” The SYRCLE Risk of Bias tool for animal studies has 10 items: They fall into 6 areas, including selection bias (Random Sequence Generation, Baseline Characterization, Allocation Concealment), performance bias (Randomized Housing, Blinding), detection bias (Randomized Outcome Assessment, Blinding), attrition bias (Incomplete Outcome Data), reporting bias (Selective Outcome Reporting) and other (Other Sources of Bias).
Results
Study selection
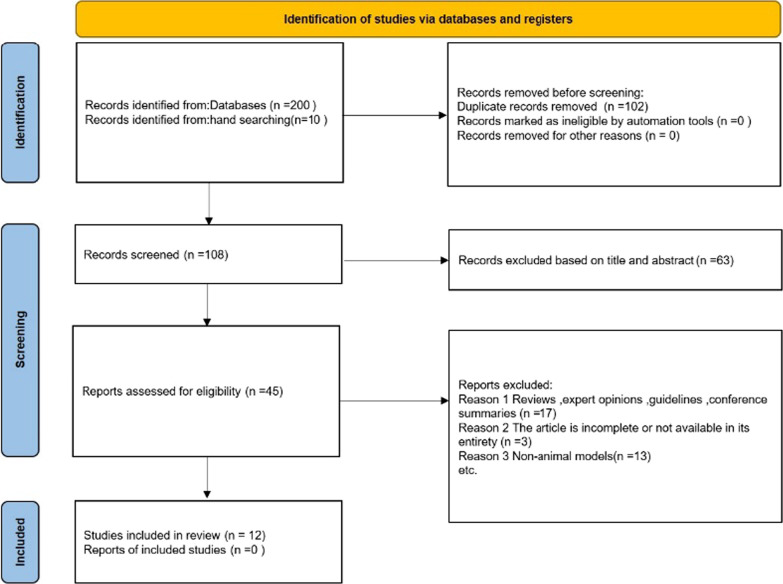
Based on the search strategy, we included 200 studies related to MSCs for the treatment of radiation and oral mucositis, and 102 duplicate articles were removed using Zotero software. Sixty-three articles were removed by reading the titles and abstracts, and the remaining 45 were reviewed in full text. Seventeen reviews, expert opinions, guidelines, or conference summaries were excluded. Because articles were incomplete or not available in their entirety, 3 articles were excluded. Furthermore, 13 articles were not included because of non-animal models. Finally, after study selection, 12 articles were included in this systematic review (Fig. 1) (If multiple interventions were offered in a single study, each intervention was considered independent).
Fig. 1.
Flow diagram of the study selection
Study characteristics
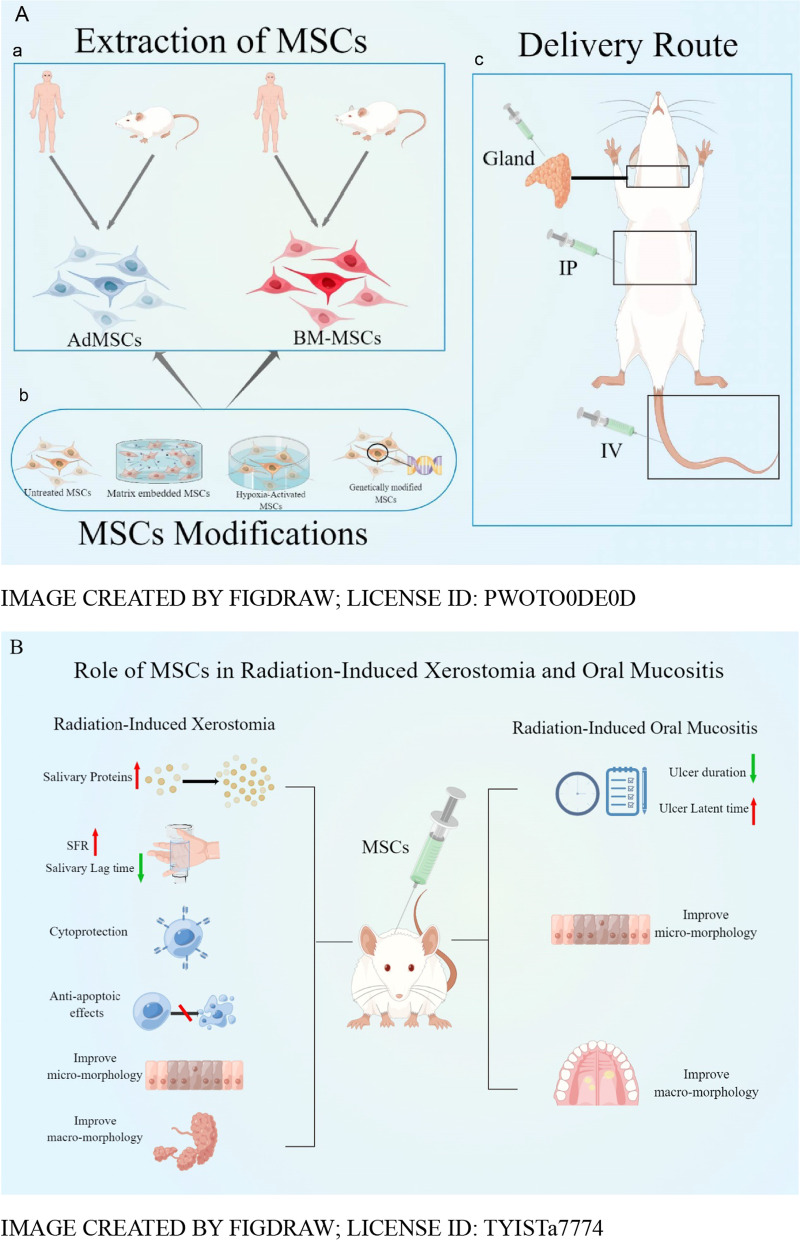
The basic characteristics of twelve articles are listed in Tables 1, 2, and Fig. 2 [13–15, 30, 35, 38–44]. The articles were published between 2013 and 2019. The studies conducted in different countries including Korea (n = 6) [15, 30, 39–42], Canada(n = 1) [38], Egypt (n = 1) [13],German(n = 1) [14], China(n = 1) [35],Turkey(n = 1) [43] and Indonesia(n = 1) [44]. The studies were conducted in rodents (rats [13, 43, 44] and mice [14, 15, 30, 38–40, 42, 45]). The animal models in all studies were induced by radiation. Stem cell types included BM-MSCs (n = 5) [13, 14, 30, 35, 44] and AdMSCs (n = 7) [15, 38–43]. The doses of interventions ranged from 105 to 107. MSCs were injected into a vein in five animal studies [13, 14, 35, 39, 42], injected into the abdominal cavity in two studies [38, 43], and injected into a gland in the rest of five studies [15, 30, 40, 41, 44]. The timing of cell administration ranged from 0 h to 28 days after induction of the model. The duration of the follow-up period ranged from 7 days to 6 months. For the outcome indicators involved in the study, we also summarized and concluded in Tables 3 and 4. Therefore, the systematic review included a total of twelve animal studies involving 659 animals.
Table 1.
Characteristics of animal studies
| Author/years | Country | Study designs | Species | Strain | Sex | Total number of animals | IR Types | Irradiation dose | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|
| Radiation-induced xerostomia | |||||||||
| An et al. 2015 [39] | Korea | RCT | Mice | C3H | Female | 105 | X-ray | 15 Gy | 16W |
| Kim et al. 2019 [40] | Korea | RCT | Mice | C57BL/6 | Female | 45 | Radioiodine | 0.01 mCi/g | 16W |
| Choi et al. 2018 [15] | Korea | RCT | Mice | C3H | Female | 140 | X-ray | 15 Gy | 16W |
| Shin et al. 2018 [41] | Korea | RCT | Mice | C3H | Female | 12 | X-ray | 15 Gy | 12W |
| Lim,et al. 2013 [30] | Korea | RCT | Mice | C57BL/6 | Unkown | 24 | X-ray | 15 Gy | 12W |
| Saylam et al. 2017 [43] | Turkey | RCT | Rats | Wistar albino | Female | 60 | Radioiodine | 2 mCi | 6 M |
| Lim et al. 2013 [42] | Korea | RCT | Mice | C3H | Female | 60 | X-ray | 15 Gy | 12W |
| Mulyani et al. 2019 [44] | Indonesia | RCT | Rats | Wistar | Male | 40 | X-ray | 15 Gy | 4W |
| Radiation-induced oral mucositis | |||||||||
| Maria et al. 2016 [38] | Canada | RCT | Mice | BALB/c | Male | 45 | X-ray | 18 Gy | 21D |
| Elsaadany et.al.2017 [13] | Egypt | RCT | Rats | Albino | Male | 60 | X-ray | 13 Gy | 7D |
| Schmidt et al. 2014 [14] | German | RCT | Mice | C3H/Neu | Male | 50 | X-ray | 15 Gy | 3W |
| Shen et al. 2018 [35] | China | RCT | Mice | C57 | Male | 18 | X-ray | 16 Gy | 10D |
D Days; M Months, RCT Randomized controlled trial, W Weeks
Table 2.
Mesenchymal stem cell characteristics
| Author/Years | Sources | Control group | Manipulation | Dose | Volume | Delivery route |
|---|---|---|---|---|---|---|
| Radiation-induced xerostomia | ||||||
| An et al. 2015 [39] | hAdMSCs | PBS | Hypoxia | 1 × 105 | 500μl | Intravenous injection |
| Kim et al. 2019 [40] | hAdMSCs | PBS | Non | 1 × 105 | Unkown | Intraglandular injection |
| Choi et al. 2018 [15] | hAdMSCs | PBS | Matrix | 1 × 105 | 20 μl | Intraglandular injection |
| Shin et al. 2018 [41] | hAdMSCs | PBS | Hypoxia | 2 × 105 | 10 μl | Intraglandular injection |
| Lim et al. 2013 [30] | BM-MSCs | PBS | Non | 1 × 105 | 15 μl | Intraglandular injection |
| Saylam et al. 2017 [43] | AdMSCs | PBS | Non | 2 × 106 | Unkown | Intraperitoneal injection |
| Lim et al. 2013 [42] | AdMSCs | PBS | Non | 1 × 106 | Unkown | Intravenous injection |
| Mulyani et al. 2019 [44] | BM-MSCs | PBS | Hypoxia | Unkown | Unkown | Unkown |
| Radiation-induced oral mucositis | ||||||
| Maria et al. 2016 [38] | AdMSCs | PBS | Non | 2.5 × 106 | 500 μl | Intraperitoneal injection |
| Elsaadany et al. 2017 [13] | BM-MSCs | PBS | Non | 1 × 107 | 0.2 ml | Intravenous injection |
| Schmidt et al. 2014 [14] | BM-MSCs | PBS | Non | 6 × 106 | Unkown | Intravenous injection |
| Shen et al. 2018 [35] | hBM-MSCs | PBS | Genetic | Unknown | Unknown | Intravenous injection |
AdMSCs Adipose tissue-derived mesenchymal stem cells; BM-MSCs Bone marrow-derived mesenchymal stem cells; PBS Phosphate buffered saline
Fig. 2.
Overall characteristics of the 12 studies. A Number of publications per year. B Number of papers published per country. C MSC source. E Route of delivery
Table 3.
Evaluation of the efficacy of mesenchymal stem cells in the treatment of radiation-induced Xerostomia
| Author/years | Salivary proteins | Salivary gland flow rate | Salivary lag time | Cytoprotection | Anti-apoptotic effects | Micromorphology | Macromorphology |
|---|---|---|---|---|---|---|---|
| An et al. 2015 [39] | + | + | + | + | + | + | + |
| Kim et al. 2019 [40] | + | + | + | + | + | + | + |
| Choi et al. 2018 [15] | + | + | + | + | + | + | + |
| Shin et al. 2018 [41] | + | + | + | + | – | + | + |
| Lim et al. 2013 [30] | + | + | + | – | + | + | + |
| Saylam et al. 2017 [43] | – | – | – | – | – | – | – |
| Lim et al. 2013 [42] | + | + | + | – | + | + | + |
| Mulyani et al. 2019 [44] | + | – | – | – | + | + | – |
Mention: +; Not Mention: –
Table 4.
Evaluation of the efficacy of mesenchymal stem cells in the treatment of radiation-induced Oral mucositis
| Author/years | Ulcer latency time | Duration of ulcers | Micromorphology | Macrosmorphology | The side effects of RIOM | BCL-2 | The tolerance of the oral mucosa | Pro-inflammatory cytokines |
|---|---|---|---|---|---|---|---|---|
| Maria et al. 2016 [38] | + | + | + | + | + | – | – | – |
| Elsaadany et al. 2017 [13] | – | – | + | – | – | + | – | – |
| Schmidtet al. 2014 [14] | + | + | – | – | – | – | + | – |
| Shen et al. 2018 [35] | – | – | + | + | – | – | – | + |
Mention: + ; Not Mention: –
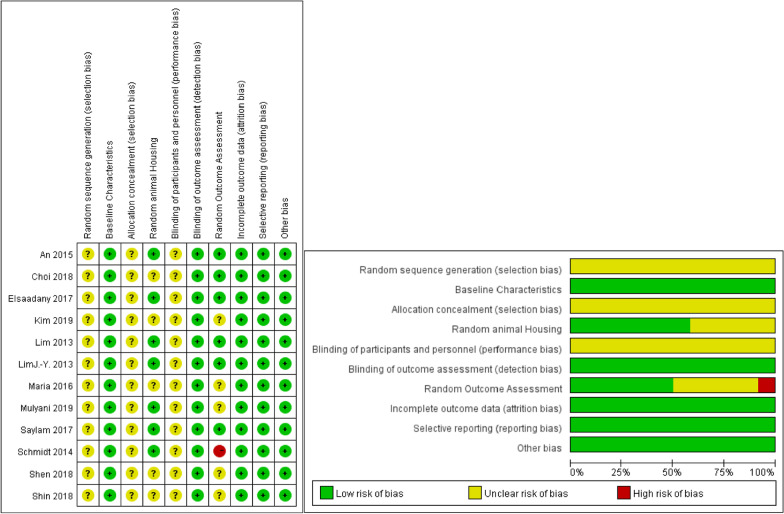
Risk of bias
Table 5 and Fig. 3 provide a detailed assessment of the quality of each study. All studies had selection bias secondary to baseline characteristics, detection bias secondary to randomized outcome assessment, attrition bias, reporting bias, and other biases [13–15, 30, 35, 38–44]. Among them, blinding is the main weakness of the study design. We think this may be due to the fact that the implementation of blinding in animal experiments is usually not feasible.
Table 5.
SYRCLE’s RoB tool for each experimental animal study
| Random Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding (study Team) | Random Outcome Assessment | Blinding (Outcome Assessors) | Incomplete Outcome Data | Selective Outcome Reporting | Other sources of bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other | |||||
| An | ? | + | ? | + | ? | + | + | + | + | + |
| Maria | ? | + | ? | ? | ? | + | ? | + | + | + |
| Kim | ? | + | ? | ? | ? | + | ? | + | + | + |
| Elsaadany | ? | + | ? | + | ? | + | + | + | + | + |
| Schmidt | ? | + | ? | + | ? | + | – | + | + | + |
| Choi | ? | + | ? | ? | ? | + | + | + | + | + |
| Shen | ? | + | ? | ? | ? | + | ? | + | + | + |
| Shin | ? | + | ? | ? | ? | + | ? | + | + | + |
| Lim | ? | + | ? | + | ? | + | + | + | + | + |
| Saylam | ? | + | ? | + | ? | + | + | + | + | + |
| Lim J.-Y | ? | + | ? | + | ? | + | + | + | + | + |
| Mulyani | ? | + | ? | + | ? | + | ? | + | + | + |
Fig. 3.
SYRCLE’s RoB tool for each experimental animal study
Radiation-induced xerostomia
Salivary proteins
A total of seven studies [15, 30, 39–42, 44] reported the secretion of salivary proteins (salivary amylase, epidermal growth factor, mucin) after MSCs treatment. Four of them [15, 39–41] reported changes in salivary amylase and epidermal growth factor (EGF), with significantly higher levels of salivary amylase expression after MSCs infusion relative to control and mean EGF levels also significantly increased relative to the control group. Two studies [30, 42] reported changes in salivary amylase and mucin. The group treated with MSCs showed more amylase and mucin content in the tissue compared to the control group. One study [44] reported changes in salivary amylase only. Compared to the control group, the experimental group increased amylase activity after the use of MSCs and the difference was statistically significant (p < 0.05). Also, Mulyani et al. [44] found that MSCs activated under hypoxic conditions resulted in a further increase in amylase activity secreted by salivary glands compared to MSCs under normoxic conditions (p < 0.05).
Salivary gland flow rate and lag time
A total of six studies [15, 30, 39–42] recorded changes in salivary gland flow rate (SFR) and salivary lag time. SFR was calculated as the ratio of post-IR SFR to pre-IR SFR (Mean ± SEM). Salivary lag time was also calculated as the ratio of post-IR salivary lag time to pre-IR salivary lag time (Mean ± SEM). The six studies did not choose the same follow-up time. Three studies [15, 39, 40] chose to measure SFR and salivary lag time at 16 weeks after irradiation. And three studies [30, 41, 42] chose to measure SFR and salivary lag time at 12 weeks after irradiation. These six studies came to the consistent conclusion that the mean SFR was significantly higher in the MSCs group than in the control group, and the mean salivary lag time in the MSCs group time was significantly lower than that of the control group.
Cytoprotection
Four studies [15, 39–41] examined immunohistochemical markers to investigate the cytoprotective effects of MSCs on salivary epithelial, endothelial, myoepithelial, and progenitor cells. Two studies [15, 39] performed immunohistochemistry for CD31 (endothelial cells), AQP5 (a marker of salivary epithelial cells), α-SMA (myoepithelial cells), and c-Kit (progenitor cells) and showed that the MSCs group represented a significantly higher expression of CD31, AQP5, α-SMA, and c-Kit than the control group (p < 0.05). Kim et al. [40] also discussed the changes in AQP-5, CD31, and the marker AQP-5, CD31 were significantly increased in the treated group compared to the control group (p < < 0.05). The study by Shin et al. [41] similarly reported that compared to the control, the MSCs group with enhanced expression of salivary epithelial cell markers (AMY1A, AQP5, KRT7, and KRT18).
Anti-apoptotic effects
Six studies [15, 30, 39, 40, 42, 44] reported the anti-apoptotic effect of MSCs on salivary gland cells. Five studies [15, 30, 39, 40, 42] performed terminal dUTP node end labeling (TUNEL) to determine the number of apoptotic cells in the MSCs and control groups. These studies showed that the number of TUNEL-positive apoptotic cells was significantly lower in the MSCs group than in the control group. One of the studies by An et al. [39] also confirmed that the MSCs group under hypoxic conditions led to an increased anti-apoptotic effect of cells compared with the MSCs group under normoxic conditions (p < 0.01 at 1 or 2 weeks after treatment and p < 0.001 at 4 weeks after treatment). The expression of BCL-2, an anti-apoptotic protein secreted by cells, was upregulated and indicated a decrease in apoptotic activity. The study of Mulyani et al. [44] of BCL-2 expression was measured in the MSCs group and control group. The results showed that the expression of Bcl-2 was significantly stronger in the MSCs group than in the control group. In addition, Bcl-2 expression was stronger under hypoxic conditions compared to MSCs under normoxic conditions.
Micromorphology
Seven studies [15, 30, 39–42, 44] evaluated the improvement of micromorphology after treatment with MSCs. Three studies [15, 39, 40] found that 16 weeks after IR, the structure, fibrosis density, and degree of inflammatory cell infiltration of salivary gland ducts and periducts were well protected in rats or mice treated with MSCs compared to controls. Three studies [30, 41, 42] found that 12 weeks after IR, compared to control MSCs treatment resulted in a reduction in glandular follicular cell loss, cytoplasmic vacuolation, abnormal nuclei, and periductal and perivascular fibrosis. In addition, a study by Saylam et al. [43] compared the recovery of salivary gland microscopic morphology at different times. At 1 month after IR, periductal fibrosis and sclerosis of salivary gland ducts were significantly better after MSCs treatment compared with controls (p < 0.05). At 6 months after IR, edema, vacuolization, necrosis, ectasia, sclerosis, periductal fibrosis, and periductal sclerosis were better after MSCs treatment compared with controls (p < 0.05).
Macromorphology
Six studies [15, 30, 39–42] assessed macroscopic morphological improvement after treatment with MSCs. Three studies [15, 39, 40] assessed weight changes at 16 weeks after IR and found a significant increase in weight in the MSCs group compared to the control group. In addition, the study by An and Kim et al. [39, 40] also measured changes in salivary gland weight. It showed that gland weight was also significantly increased in the MSCs group compared to the control group. Three studies [30, 41, 42] assessed changes in body weight at 12 weeks after IR. The study by Shin et al. [41] showed a significant increase in body weight and gland weight in the MSCs treated mice compared to the control group. However, the other two studies [30, 42] showed an upward trend in body weight and salivary gland in the MSCs-treated mice/rats compared to the control group. But it lacked statistical significance.
Radiation oral mucositis
Ulcer time
Two studies [14, 38] involved the exploration of ulcer latency times and the duration of ulcers. Maria et al. [38] showed that the latent duration of ulcers in control group was 9.3 ± 0.3 days. However, with using of MSCs, the latent duration of ulcers extended to 11.3 ± 0.9 days. Meanwhile, compared to the control group, the duration of ulceration was also reduced by 72% in the MSCs group. But, experiments by Schmidt et al. [14] showed that although the experimental group using MSCs could shorten the duration of ulcers compared to the control group, the ulcer incubation time was prolonged. And we can suppose that under the conditions of MSC transplantation, the regulation of RIOM in RT mouse models depends on the transplant time relative to the RT exposure time.
Micromorphology
Three studies [13, 35, 38] evaluated microscopic morphological improvements after treatment with MSCs. The study by Maria et al. [38] and the study by Elsaadany et al. [13] reported changes in epithelial height. And the mean values of epithelial height were significantly higher after MSCs treatment compared with the control group. In addition, the study by Elsaadany et al. [13] reported a significant decrease in the number of blood vessels, and better preservation of keratin and basal cell structure after MSCs treatment compared to controls after 7 days of irradiation [35].The study by Shen et al. [35] confirmed that, compared to control, after treatment with MSCs, radiation-induced loss of filopodiaulceration, disruption of the mucosal epithelial layer, and the degree of inflammatory cell infiltration were reduced. Furthermore, the mucosal thickness was better maintained.
Macromorphology
Two studies [35, 38] reported changes in ulcer size after treatment with MSCs. The results showed that the MSCs-treated group showed a smaller RIOM ulcer size compared to the control group at all time points tested after 15 consecutive days of observation. The study by Maria et al. [38] showed that the therapeutic benefit of MSCs depends on dose size and frequency, the number of doses, and the time of treatment initiation relative to the duration of RT exposure.
Other indicators
The study by Maria et al. [38] confirmed that MSCs treatment improved RIOM side effects. Compared to controls, the MSCs-treated animals had a significant improvement in hydration status, a significant reduction in weight loss, and a significant increase in the rate and extent of weight gain.
Elsaadany et al. [13] determined apoptosis, using the expression level of BCL-2 as an indicator for determination. The results showed that at 3 days and 7 days after IR, the expression level of BCL-2 was significantly increased in the group of the systemic use of stem cells compared to the normal control, and almost reached normal levels at 7 days after irradiation, with significant decrease in the apoptotic effect.
Schmidt et al. [14] studied the tolerance of the oral mucosa and expressed it as ED50 (the amount of radiation tested that would be expected to cause ulcers in 50% of the animals). The results showed that both split irradiation (3 Gy/day × 5 days) and single irradiation (15 Gy/dose) significantly increased the ED 50 value and significantly increased the residual tolerance of the oral mucosa in the MSCs treatment group compared to the normal control.
A study by Shen et al. [35] confirmed that mRNA expression of pro-inflammatory cytokines in the mucosa of mice after MSC treatment was significantly downregulated in MSCs. Also, MSCs enhanced the removal of ROS, reduced the production of radioactive ROS (p < 0.05), accelerated the recovery from mucositis, and protected tongue cells from cell death.
Safety
The studies included in this review did not test the safety of MSCs for the treatment of radiation-induced xerostomia and oral mucositis in rodent models [13–15, 30, 35, 38–44].
Discussion
This is the first systematic evaluation of the efficacy and safety of MSCs in radiation-induced oral complications. The results showed that MSCs exhibited good therapeutic efficacy in radiation-induced xerostomia and some improvement in radiation oral mucositis, despite differences in MSC source and graft type, the timing of administration, route of administration, dose, receptor type, and multiple models. Although previous reviews [45, 46] have explored the role of MSCs in oral dryness and oral mucositis, radiotherapy-induced xerostomia and oral mucositis have not been widely recognized, and whether MSCs have a therapeutic role in radiotherapy-induced oral dry mouth and oral mucositis and their therapeutic mechanisms have not been explored in detail. Therefore, we conducted a systematic evaluation to focus on this issue. Our review bridges the gap between previous experiments and confirms that the benefits of MSCs in radiation therapy-induced xerostomia and oral mucositis can ameliorate the discomfort they cause.
Radiation-induced xerostomia
From the systematic evaluation of the eight included studies, it is evident that MSCs can demonstrate their therapeutic effects by increasing salivary protein secretion with SFR, shortening salivary retention time, anti-apoptosis, enhancing tissue cytoprotective effects, and effects on the outcomes of micro- and macro-morphological changes [15, 30, 39–44]. Paracrine action has long been one of the key mechanisms investigated for the ameliorative effects of MSCs on radiation-induced xerostomia.MSCs can prevent SFR decline and improve the symptoms of oral dryness through paracrine induction of anti-inflammatory and tissue regeneration genes (EGF, TGF-α,) [47]. Previous studies have shown that MSCs secrete TGFβ1 to regulate lymphocyte proliferation, differentiation, and survival to maintain tolerance and control the initiation and regression of the inflammatory response by regulating the chemotaxis, activation, and survival of lymphocytes, natural killer cells, dendritic cells, macrophages, mast cells, and granulocytes [48]. MSCs also secrete EGF, TGF-α, HGF, and VEGF to exert nutritional effects on salivary glandular vesicles, ducts, and mucosal epithelial cells, maintain normal salivary gland secretion and excretion [49], promote neovascularization, stimulate salivary gland cells proliferation, inhibit salivary gland cells apoptosis and improve the symptoms of oral dryness [50]. Also, paracrine-mediated immunomodulatory and trophic effects of stem cells may prevent the development of autoimmune activities by inhibiting immune cells from investigating tissue damage, establishing a regenerative microenvironment containing nutrient-active molecules, and promoting the proliferation of tissue cells and blood vessels [51] to restore damaged tissue and salivary organ functions after radiation injury [52]. Our included studies also showed that MSCs can paracrine EGF [40], FGF10 [41], HGF, and VEGF [44] leading to activation of PI3K and phosphorylation of downstream targets AKT and MDM2, inhibiting p53-mediated radiation-induced apoptotic pathway and protect salivary gland epithelial cells from radiation injury. Thus, MSCs promote the structural integrity of salivary glands in vitro and in vivo, and the preservation of endocrine function [41, 53, 54].
In addition, cellular transdifferentiation has been a controversial mechanism by which MSCs improve oral dryness. Previous studies have shown that MSCs can undergo a mesenchymal-to-epithelial transformation when induced by natural SG-specific extracellular matrix and then differentiate into corresponding salivary gland cells to repair damaged glands by replacing damaged salivary gland cells [55]. Furthermore, when MSCs are co-cultured with SGEC, MSCs can transdifferentiate into SGEC and promote amylase and mucopolysaccharide secretion from salivary gland epithelial cells by inducing salivary gland gene expression [45], promoting salivary secretion and alleviating oral dryness. However, recently Bhartiya et al. [23] made a strong generalization after reading and summarizing a large body of literature on the subject, suggesting that MSCs may not be able to differentiate into multiple adult cell types They are more like "paracrine providers" of tissue-resident stem cells. This is because similar beneficial effects are observed when their secretome (microvesicles or exosomes) are transplanted. Similarly, our included studies showed that only a minority of infused hAdMSCs transdifferentiated into salivary gland mast cells when treated with MSCs alone and produced amylase in vivo mainly through paracrine effects rather than transdifferentiation [30, 42]. This suggests that in the context of treatment with MSCs alone, MSCs may indirectly promote salivary cell survival, endogenous progenitor cell mobilization, or neointima formation. Ultimately, MSCs ameliorate radiation-induced xerostomia, mainly through paracrine effects rather than transdifferentiation [30, 42].
Although MSCs have therapeutic efficacy in radiation xerostomia, several factors/variables have been identified that may influence the outcomes of MSCs intervention. These factors mainly include the culture conditions and dependency vectors of MSC exosomes. The culture conditions of MSCs have been reported to affect their dryness and paracrine function and influence the therapeutic outcomes. An et al. [39] and Shin et al. [41] demonstrated that hypoxic conditions enhanced the paracrine activity of MSCs, the protein levels of secretions released by MSCs, and expressed higher levels of anti-apoptotic and angiogenic genes, including EGF, FGF10, HGF, IGF, and VEGF. A study by Mulyani et al. [44] also confirmed that hypoxic pretreatment could better induce the cellular repair process by better promoting the migration of MSCs in the ductal basement membrane and glandular vesicle cells. The delivery vehicle of MSCs is another factor to be considered and ideally tested. A study by Choi et al. [17] showed that delivery of MSCs as a vehicle in SIS MSCs not only maintained SG-specific cell expansion capacity but also organized glandular structures by enhancing glandular constituent cells (i.e., salivary epithelial cells, myoepithelial cells, and endothelial cells).
Radiation-induced oral mucositis
We included four studies using MSCs in the treatment of radiation oral mucositis [13, 14, 35, 38] to assess the efficacy of MSCs in the treatment of radiation oral mucositis. Among the three components that we focused on assessing, two indicators (macroscopic and microscopic status of ulcers) were significantly improved and showed a positive effect of MSCs on RIOM (Table 4) [13, 14, 35, 38]. Although the current study failed to agree on the effect of MSCs on the improvement of ulcer latency and duration, previous studies confirmed that the latency and severity of ulcers due to RIOM correlate with the mouse strain chosen for the study [56] and the radiation dose [57]. This may be the reason why the effect of improvement in ulcer latency and duration in the included studies [14, 38] could not be agreed upon. We also observed that the concentration, mode of administration, and frequency of administration of MSCs in the included studies were not completely consistent. We can speculate that these factors also have an effect on ulcer latency and duration.
The current study found that MSCs are mainly involved in tissue mucosal repair in three ways. (1) Through direct differentiation [58], for example, bone marrow cells can be transplanted into the skin in response to wound stimulation, inducing the incorporation and differentiation of bone marrow-derived cells into non-hematopoietic skin structures and stimulating the regeneration of damaged skin tissue to promote skin wound healing. (2) Direct cellular interaction [59], such as direct interaction with cells of the innate and adaptive immune system, leading to the regulation of some effector functions. or regulating intracellular reactive oxygen species (ROS) levels by donating mitochondria to damaged cells, reducing the activation of ROS in damaged tissues, and ultimately improving the manifestation of tissue damage and inflammation by regulating cellular metabolism in damaged tissues. [60]. (3) Secretion of soluble factors [61], such as mesenchymal stem cells can secrete fibroblast growth factor [28], and one of its members, keratinocyte growth factor (KGF), can promote the proliferation of oral epithelial cells and enhance the radiation resistance of oral epithelial cells, thus improving radiation oral mucositis [62, 63].
Our included studies found that the paracrine effect of MSCs may be the most prominent mechanism for improving radiation oral mucositis. MSCs paracrine secrete anti-inflammatory and antioxidant cytokines and growth factors, which promote oral mucosal repair by promoting cell and tissue regeneration [13, 14, 35]. However, there is no definite conclusion on the specific cytokines secreted by MSCs. Further exploration is needed. As for the factors that promote cell and tissue regeneration, previous studies have suggested that the cytokines secreted by MSCs can promote mucosal repair by enhancing cell proliferation or inhibiting epithelial cell apoptosis, or a combination of both. For example, IL-11 secreted by MSCs can exert cytoprotective functions and reduce apoptosis by upregulating IL-11 receptor complex and heat shock protein 25 [32]. HGF secreted by MSCs induces proliferation of epithelial cells through phosphatidylinositol 3-kinase/Akt signaling pathway [33]. IGF-I secreted by MSCs can downregulate the expression of pro-apoptotic genes such as p53 or activate the expression of anti-apoptotic genes such as bc1-2 to inhibit radiation-induced apoptosis [34]. For anti-inflammatory factors, paracrine IL10 secreted by MSCs can down-regulate TNFα, INFβ, IL2, and other inflammatory factors to mediate the anti-inflammatory effects. In addition, some other unknown anti-inflammatory factors secreted by MSCs may promote anti-inflammatory effects by altering the inflammatory profile of Thelper1 cells toward a more anti-inflammatory Thelper2 profile and increasing the number of anti-inflammatory T regulatory cells [64, 65].
However, our included studies [13, 14, 35] also showed that the clonal characterization of stem cells in the tongue mucosa lining was identified by finding no clonal characterizations of transplanted stem cells or too few clonal characterizations of stem cells in the tongue mucosa lining, and the overall cell count in the oral mucosa was the same in the transplanted and irradiated groups. In addition, Shen et al. [35] explored the mechanism of action of MSCs in reducing cellular ROS levels, using transwell or direct co-culture systems of MSCs and tongue epithelial cells or fibroblasts to verify ROS levels, and found no significant difference in ROS levels between the two culture systems, which showed similar antioxidant effects. Therefore, these findings do not support for the time being the effects of MSCs on ameliorative effects of radiation oral mucositis were significantly correlated with transdifferentiation and direct cellular interaction.
Furthermore, we found that genetic modifications enhanced the role of MSCs in the treatment of radiation oral mucositis. Shen’s study [35] showed that MSCs-mediated CXCR2 overexpression by upregulating the expression of P-Akt and P-Erk1/2, which enhanced the migration ability and targeting ability of MSCs to the inflamed oral mucosa, prolonged the survival time and further improve the therapeutic effect of MSCs in radiation oral mucositis.
Limitations
Although our conclusions suggest that MSCs are promising therapeutic agents, the use of MSCs for the treatment of radiation-induced oral complications is still in its infancy. There are still some critical steps to be taken to get through the FDA approval process and eventually into the clinic. First, all of the studies included in this evaluation were conducted on small animals. Therefore, future efforts should be made to move to larger animal models as well as multi-animal models. Second, future preclinical studies need to refine the pharmacological and toxicological studies of MSCs to elucidate in detail the pharmacological phenomena, mechanism of action (MOA), toxicity profile, toxic target organs, drug absorption, distribution, metabolism and excretion (ADME), and adverse effect profile of MSCs in radiation-induced xerostomia and oral mucositis. Third, we need to supplement the context of investigation new drug (IND) to refine and evaluate the clinical study protocol, investigator information, and provide detailed information on clinical procedures and the qualifications of the investigators. We also need to provide information on the production of MSCs, such as components, impurities, stabilities, source, and qualities,. To ensure that the company can adequately produce and supply stable batches of the drug. Fourth, we should follow the FDA guidelines and conduct clinical trials after IND approval, especially conducting large phase III clinical trials to fully validate the safety and efficacy to obtain a Biologics License Application (BLA).
Conclusion
In this review, we comprehensively evaluated the efficacy and safety of MSCs for the treatment of radiation-induced xerostomia and oral mucositis based on available preclinical animal studies. Our study demonstrates that MSCs are effective in ameliorating radiation-induced xerostomia, and our study also suggests that MSCs may provide some minor therapeutic benefits for radiation-induced oral mucositis. In the future, we expect more researchers to carry out further evaluations of the effectiveness and safety of MSCs in improving radiation-induced xerostomia and oral mucositis to promote faster and better development of the stem cell field.
Supplementary Information
Acknowledgements
The authors appreciate the Second Hospital of Jilin University and the School of Nursing of Jilin University for providing full support to the study.
Abbreviations
- SG
Salivary gland
- RT
Radiation therapy
- HNC
Head and neck cancer
- IMRT
Intensity-modulated radiotherapy
- RIOM
Radiation-induced oral mucositis
- EV
Extracellular vesicle
- SYRCLE
Systematic Review Centre for Laboratory Animal Experimentation
- ROS
Reactive oxygen species
- SFR
Salivary gland flow rate
- KGF
Keratinocyte growth factor
- EGF
Epidermal growth factor
- MSC
Mesenchymal stem cell
- AdMSC
Adipose tissue-derived mesenchymal stem cell
- BM-MSC
Bone marrow-derived mesenchymal stem cell
- PBS
Phosphate buffered saline
- TUNEL
Terminal dUTP node end labeling
- MOA
Mechanism of action
- ADME
Absorption, distribution, metabolism and excretion
- IND
Investigation new drug
- BLA
Biologics License Application
- FDA
Food and Drug Administration
Author contributions
ZRG conceived the study, participated in the design and coordination of the study, and drafted the manuscript. JXZ participated in data extraction and guided the methodology. NJ participated in data extraction and investigation. MYT participated in the design of the study. HYW supervised the study, and participated in the coordination of the study. BL supervised the study, participated in the revision of the manuscript, and secured financial support for the study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 31700738), Jilin Province Health Science and Technology Capability Improvement Project (Grant number 2021JC041), and Jilin Province Science and Technology Development Plan Project (Grant number 20180520127JH).
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongyong Wang and Bing Liang contributed equally to this work and should be considered as co-corresponding authors
Contributor Information
Zirui Guan, Email: rethsguan@163.com.
Jiaxin Zhang, Email: 1019750436@qq.com.
Nan Jiang, Email: 540519893@qq.com.
Mingyan Tian, Email: 2497119775@qq.com.
Hongyong Wang, Email: wanghongyong@jlu.edu.cn.
Bing Liang, Email: liangbing716@jlu.edu.cn.
References
- 1.Borras JM, Barton M, Grau C, Corral J, Verhoeven R, Lemmens V, et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: Methodology of a population based analysis by the ESTRO-HERO project. Radiother Oncol. 2015;116(1):45–50. doi: 10.1016/j.radonc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 2.van der Veen J, Nuyts S. Can Intensity-modulated-radiotherapy reduce toxicity in head and neck squamous cell carcinoma? Cancers. 2017;9(10):135. doi: 10.3390/cancers9100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett GC, West CML, Dunning AM, Elliott RM, Coles CE, Pharoah PDP, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9(2):134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jham BC, da Silva Freire AR. Oral complications of radiotherapy in the head and neck. Braz J Otorhinolaryngol. 2006;72(5):704–708. doi: 10.1016/S1808-8694(15)31029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung EM, Sung EC. Dental management of chemoradiation patients. J Calif Dent Assoc. 2006;34(9):735–742. doi: 10.1080/19424396.2006.12222243. [DOI] [PubMed] [Google Scholar]
- 6.Skiba-Tatarska M, Kusa-Podkańska M, Surtel A, Wysokińska-Miszczuk J. The side-effects of head and neck tumors radiotherapy. Pol Merkur Lekarski. 2016;41(241):47–49. [PubMed] [Google Scholar]
- 7.Jensen SB. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010 doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 8.Muanza TM, Cotrim AP, McAuliffe M, Sowers AL, Baum BJ, Cook JA, et al. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin Cancer Res. 2005;11(14):5121–5127. doi: 10.1158/1078-0432.CCR-05-0403. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima N, Watanabe S, Kiyoi T, Tanaka A, Suemaru K, Araki H. Evaluation of edaravone against radiation-induced oral mucositis in mice. J Pharmacol Sci. 2015;127(3):339–343. doi: 10.1016/j.jphs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Bruce SD. Radiation-induced xerostomia: how dry is your patient? Clin J Oncol Nurs. 2004;8(1):61–67. doi: 10.1188/04.CJON.61-67. [DOI] [PubMed] [Google Scholar]
- 11.Peterson DE. Oral problems in supportive care: no longer an orphan topic? Support Care Cancer. 2000;8(5):347–348. doi: 10.1007/s005200050001. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096–1101. doi: 10.1002/j.0022-0337.2001.65.10.tb03455.x. [DOI] [PubMed] [Google Scholar]
- 13.Elsaadany B, El Kholy S, El Rouby D, Rashed L, Shouman T. Effect of transplantation of bone marrow derived mesenchymal stem cells and platelets rich plasma on experimental model of radiation induced oral mucosal injury in albino rats. Int J Dent. 2017;2017:8634540. doi: 10.1155/2017/8634540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M, Haagen J, Noack R, Siegemund A, Gabriel P, Dörr W. Effects of bone marrow or mesenchymal stem cell transplantation on oral mucositis (mouse) induced by fractionated irradiation. Strahlenther Onkol. 2014;190(4):399–404. doi: 10.1007/s00066-013-0510-3. [DOI] [PubMed] [Google Scholar]
- 15.Choi JS, An HY, Shin HS, Kim YM, Lim JY. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J Tissue Eng Regen Med. 2018;12(2):e695–706. doi: 10.1002/term.2352. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293–1306. doi: 10.1517/14712598.2015.1051528. [DOI] [PubMed] [Google Scholar]
- 18.Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang A-J, Hong S-Q, Xu Q, Wang H-Y, Yang Y, Wang Z-F, et al. NT-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011;1391:102–113. doi: 10.1016/j.brainres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. 2017;21(2):241–255.e6. doi: 10.1016/j.stem.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 23.Bhartiya D, Singh P, Sharma D, Kaushik A. Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Rev and Rep. 2022;18:1718–1727. doi: 10.1007/s12015-021-10243-6. [DOI] [PubMed] [Google Scholar]
- 24.Ryu J-S, Jung Y-H, Cho M-Y, Yeo JE, Choi Y-J, Kim YI, et al. Co-culture with human synovium-derived mesenchymal stem cells inhibits inflammatory activity and increases cell proliferation of sodium nitroprusside-stimulated chondrocytes. Biochem Biophys Res Commun. 2014;447:715–720. doi: 10.1016/j.bbrc.2014.04.077. [DOI] [PubMed] [Google Scholar]
- 25.Chihaby N, Orliaguet M, Le Pottier L, Pers J-O, Boisramé S. Treatment of Sjögren’s syndrome with mesenchymal stem cells: a systematic review. Int J Mol Sci. 2021;22:10474. doi: 10.3390/ijms221910474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HH, Hsu SP, Chien CT. Intrarenal transplantation of hypoxic preconditioned mesenchymal stem cells improves glomerulonephritis through anti-oxidation, anti-ER stress, anti-inflammation, anti-apoptosis, and anti-autophagy. Antioxidants. 2019 doi: 10.3390/antiox9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almansoori AA, Khentii N, Kim B, Kim S-M, Lee J-H. Mesenchymal stem cell therapy in submandibular salivary gland allotransplantation: experimental study. Transplantation. 2019;103:1111–1120. doi: 10.1097/TP.0000000000002612. [DOI] [PubMed] [Google Scholar]
- 28.Najafi S, Nosrati H, Faraji Z, Mohamadnia A, Shirian S, Mortazavi SM, et al. Reconstruction of necrotic submandibular salivary gland using mesenchymal stem cells. Heliyon. 2020;6:e05162. doi: 10.1016/j.heliyon.2020.e05162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21:E1306. doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim HJ, et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013;49(2):136–143. doi: 10.1016/j.oraloncology.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Lee RH, Oh JY, Choi H, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem. 2011;112(11):3073–3078. doi: 10.1002/jcb.23250. [DOI] [PubMed] [Google Scholar]
- 32.Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Can Res. 2007;67(19):9501–9506. doi: 10.1158/0008-5472.CAN-07-0810. [DOI] [PubMed] [Google Scholar]
- 33.Kanayama M, Takahara T, Yata Y, Xue F, Shinno E, Nonome K, et al. Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G230–G239. doi: 10.1152/ajpgi.00068.2007. [DOI] [PubMed] [Google Scholar]
- 34.Mylonas PG, Matsouka PT, Papandoniou EV, Vagianos C, Kalfarentzos F, Alexandrides TK. Growth hormone and insulin-like growth factor I protect intestinal cells from radiation induced apoptosis. Mol Cell Endocrinol. 2000;160(1):115–122. doi: 10.1016/S0303-7207(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 35.Shen Z, Wang J, Huang Q, Shi Y, Wei Z, Zhang X, et al. Genetic modification to induce CXCR2 overexpression in mesenchymal stem cells enhances treatment benefits in radiation-induced oral mucositis. Cell Death Dis. 2018;9(2):229. doi: 10.1038/s41419-018-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooijmans CR, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014 doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maria OM, Shalaby M, Syme A, Eliopoulos N, Muanza T. Adipose mesenchymal stromal cells minimize and repair radiation-induced oral mucositis. Cytotherapy. 2016;18(9):1129–1145. doi: 10.1016/j.jcyt.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 39.An HY, Shin HS, Choi JS, Kim HJ, Lim JY, Kim YM. Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation-induced salivary gland damage. PLoS ONE. 2015;10(11):e0141862. doi: 10.1371/journal.pone.0141862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, Kim JM, Choi ME, Kim SK, Kim YM, Choi JS. Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model. Sci Rep. 2019;9(1):15752. doi: 10.1038/s41598-019-51775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin HS, Lee S, Kim YM, Lim JY. Hypoxia-activated adipose mesenchymal stem cells prevents irradiation-induced salivary hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem CellS. 2018;36(7):1020–1032. doi: 10.1002/stem.2818. [DOI] [PubMed] [Google Scholar]
- 42.Lim JY, Ra JC, Shin IS, Jang YH, An HY, Choi JS, et al. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PLoS ONE. 2013;8(8):e71167. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saylam G, Bayir O, Gultekin SS, Pinarli FA, Han U, Korkmaz MH, et al. Protective/restorative role of the adipose tissue-derived mesenchymal stem cells on the radioiodine-induced salivary gland damage in rats. Radiol Oncol. 2017;51(3):307–316. doi: 10.1515/raon-2017-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulyani SWM, Astuti ER, Wahyuni OR, Ernawati DS, Ramadhani NF. Xerostomia therapy due to ionized radiation using preconditioned bone marrow-derived mesenchymal stem cells. Eur J Dent. 2019;13(2):238–242. doi: 10.1055/s-0039-1694697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong B, Zheng L, Huang W, Pu J, Pan S, Liang Y, et al. Murine embryonic mesenchymal stem cells attenuated xerostomia in Sjögren-like mice via improving salivary gland epithelial cell structure and secretory function. Int J Clin Exp Pathol. 2020;13(5):954–963. [PMC free article] [PubMed] [Google Scholar]
- 46.Tran SD, Sumita Y, Khalili S. Bone marrow-derived cells: a potential approach for the treatment of xerostomia. Int J Biochem Cell Biol. 2011;43(1):5–9. doi: 10.1016/j.biocel.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Khalili S, Faustman DL, Liu Y, Sumita Y, Blank D, Peterson A, et al. Treatment for salivary gland hypofunction at both initial and advanced stages of Sjögren-like disease: a comparative study of bone marrow therapy versus spleen cell therapy with a 1-year monitoring period. Cytotherapy. 2014;16(3):412–423. doi: 10.1016/j.jcyt.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. Transforming growth factor-β regulation of immune responses. Ann Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 49.Koski H, Konttinen YT, Hietanen J, Tervo T, Malmström M. Epidermal growth factor, transforming growth factor-α, and epidermal growth factor receptor in labial salivary glands in Sjogren’s syndrome. J Rheumatol. 1997;24:1930–1935. [PubMed] [Google Scholar]
- 50.Wang D, Xie Y, Peng H-Q, Wen Z-M, Ying Z-Y, Geng C, et al. LPS preconditioning of MSC-CM improves protection against hypoxia, reoxygenation-induced damage in H9c2 cells partly via HMGB1, Bach1 signalling. Clin Exp Pharmacol Physiol. 2022 doi: 10.1111/1440-1681.13714. [DOI] [PubMed] [Google Scholar]
- 51.Tran SD, Liu Y, Xia D, Maria OM, Khalili S, Wang RW, et al. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS ONE. 2013;8(4):e61632. doi: 10.1371/journal.pone.0061632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho JM, Yoon YJ, Lee S, Kim D, Choi D, Kim J, et al. Retroductal delivery of epidermal growth factor protects salivary progenitors after irradiation. J Dent Res. 2021;100:883–890. doi: 10.1177/0022034521999298. [DOI] [PubMed] [Google Scholar]
- 54.Yoon YJ, Shin H-S, Lim J-Y. A hepatocyte growth factor/MET-induced antiapoptotic pathway protects against radiation-induced salivary gland dysfunction. Radiother Oncol. 2019;138:9–16. doi: 10.1016/j.radonc.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Tran ON, Wang H, Li S, Malakhov A, Sun Y, Abdul Azees PA, et al. Organ-specific extracellular matrix directs trans-differentiation of mesenchymal stem cells and formation of salivary gland-like organoids in vivo. Stem Cell Res Ther. 2022 doi: 10.1186/s13287-022-02993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dörr W, Spekl K, Martin M. Radiation-induced oral mucositis in mice: strain differences. Cell Prolif. 2002;35(Suppl 1):60–67. doi: 10.1046/j.1365-2184.35.s1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Napenas JJ, Shetty KV, Streckfus CF. Oral mucositis: review of pathogenesis, diagnosis, prevention, and management. Gen Dent. 2007; 55(4):335–44; quiz 345–6, 376. [PubMed]
- 58.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196(2):245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 59.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial TRANSFER and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. 2020 doi: 10.3389/fcell.2020.603292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif. 2002;35(Suppl 1):78–85. doi: 10.1046/j.1365-2184.35.s1.8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doerr W, Lacmann A, Noack R, Spekl K, Rex K, Farrell CL. Modulation of radiation effects in tissues by keratinocyte growth factor (KGF); Modulation der Strahlenwirkung an Plattenepithelien durch Keratinozyten-Wachstumsfaktor (KGF). 2000; Available from: https://www.osti.gov/etdeweb/biblio/20222947.
- 64.Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18:101–115. doi: 10.1089/ten.teb.2011.0488. [DOI] [PubMed] [Google Scholar]
- 65.Miguel MPD, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.