Abstract
Purpose
To investigate the correlation between the baseline axial length (AL) and axial elongation in myopes undergoing orthokeratology (ortho‐k).
Methods
This was a retrospective study. During the 1‐year follow‐up, 1176 children (aged 8–14 years) were included and divided into an ortho‐k group (n = 588) and a single‐vision spectacle group (n = 588). The ortho‐k group participants (8–11 years of age) who completed the 3‐year follow‐up (n = 150) were further divided into three subgroups stratified by their baseline AL: subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm). AL was measured at baseline and during the annual visit.
Results
The ortho‐k group exhibited slower 1‐year axial elongation (39% reduction) than the spectacle group. The 1‐year axial elongation was negatively correlated with initial age in both groups. A negative association between 1‐year axial elongation and baseline AL was observed in the ortho‐k group but not in the spectacle group. However, this relationship only existed in ortho‐k participants 8–11 years of age. For the younger ortho‐k participants who completed the 3‐year follow‐up, the annual axial elongation was significantly higher in subgroup 1 for the first and second years but not in the third year compared with subgroups 2 and 3.
Conclusion
Axial elongation was negatively correlated with baseline AL in the ortho‐k group. Children aged 8–11 years with longer baseline AL (≥24.5 mm) demonstrated slower annual axial elongation during the first 2 years of ortho‐k treatment, which may provide insight into establishing individual guidelines for controlling myopia using ortho‐k in children with different baseline characteristics.
Keywords: axial elongation, axial length, myopia control, orthokeratology
Key points.
One‐year axial elongation was negatively correlated with baseline axial length in the orthokeratology group, but not in the single‐vision spectacle group.
Children aged 8–11 with a longer baseline axial length (≥24.5 mm) demonstrated slower axial elongation during the first 2 years of orthokeratology treatment than those with baseline axial length <24.5 mm.
Clinicians should make valid individual adjustments to achieve a better prognosis for young children with a baseline axial length <24.5 mm undergoing orthokeratology due to their predicted faster myopia progression.
INTRODUCTION
Myopia, a common eye disease globally, often manifests in children and adolescents. 1 The prevalence of myopia is estimated to rise to 49.8% of the global population by 2050, most dramatically among younger people in East and Southeast Asia. 2 Myopia is attributable to the interaction of genetic and environmental factors, leading to excessive axial elongation. Furthermore, the increasing rates of early onset and rapid progression of myopia may result in a dramatic increase in the number of people with high myopia, ultimately increasing the risks of complications such as myopic maculopathy, retinal detachment and glaucoma, possibly leading to incurable visual impairment or blindness. 3 , 4 Thus, delaying myopia onset and retarding its progression are recognised as priorities for myopia‐related public health concerns.
Orthokeratology (ortho‐k) is widely used worldwide as a reliable and effective method to control myopia, 5 , 6 with over 1.5 million users in China reported in 2016. 7 Previous studies have demonstrated that ortho‐k retarded axial elongation in myopic children by 32%–63% during a 2‐year follow‐up compared with traditional treatment with spectacles. 8 , 9 , 10 , 11 , 12 , 13 , 14 However, variations in ethnicity, age of onset and some baseline ocular parameters between studies affect the rates of myopic progression. Hence, identifying which children would most benefit from ortho‐k treatment is required. This would allow clinicians to make individual adjustments to achieve better outcomes.
Combining data from previous studies, the age of onset was a strong factor independently associated with myopic progression in children wearing ortho‐k lenses. 15 , 16 , 17 While the results of some studies are controversial, other baseline factors that may be associated with myopia control include refractive power, corneal curvature and stiffness and pupil size. 18 , 19 , 20 To date, few studies have focused on the role of baseline axial length (AL) on the myopia control effect of ortho‐k lenses in children. Recently, an 18‐month Danish randomised controlled trial, including 19 Scandinavian children (6–12 years) treated with ortho‐k, reported that of the three covariates included in their mixed model, i.e., baseline AL, initial age and baseline spherical equivalent refractive error (SER), baseline AL was significantly associated with myopia progression. 21 In addition, Kim et al. identified that greater baseline differences in AL (Central–Nasal 30°) and higher baseline manifest SER were significantly associated with lower AL elongation after ortho‐k treatment. 22 These studies indicated that initial AL might be a predictive factor associated with myopia progression in ortho‐k lenses.
Considering individual variability regarding the myopic control effects of ortho‐k lenses and the controversies regarding the possible factors influencing axial elongation, this study aimed to assess the control effect of ortho‐k lenses in myopic children with different baseline AL and to investigate the association between baseline AL and myopic progression in children wearing ortho‐k lenses during a 3‐year follow‐up period.
METHODS
Subjects
This retrospective study adhered to the tenets of the Declaration of Helsinki, and ethical approval was obtained from the Ethics Committee of Tianjin Medical University Eye Hospital. Data were obtained through an evaluation of the institution's medical records database in Tianjin Medical University Eye Hospital Optometric Center between 2014 and 2018 to identify children using spectacles or ortho‐k for 1–3 years. The inclusion criteria were cycloplegic spherical power between −0.75 and −6.00 D, astigmatism ≤1.50 D and monocular best‐corrected distance visual acuity not worse than 0.0 LogMAR (6/6). Individuals with ocular or systemic conditions other than ametropia, a history of ocular surgery or other anti‐myopia therapy were excluded. The children selected ortho‐k or spectacles as their preferred myopia control strategy. In the first cohort, 588 children aged 8–14 years wearing ortho‐k lenses and having completed a 1‐year visit were included, and 588 children with distance single‐vision spectacles were in the control group. The children in the control group preferred to use spectacles rather than ortho‐k for their myopia correction. Ortho‐k‐wearing children aged 8–11 years were screened from the first cohort, and those who completed the 3‐year follow‐up (n = 150) made up the second cohort. The baseline demographics and biometric data of children enrolled in this study are presented in Table 1. According to the medical records, all the children and their parents were fully informed of the risks and benefits of the treatment and signed written informed consent.
TABLE 1.
Baseline demographics and biometric data of children enrolled in the study.
| One‐year follow‐up time | Three‐year follow‐up time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | 8–11 years old | Ortho‐k (n = 150) | ||||||||
| Ortho‐k (n = 588) | Spectacle (n = 588) | p Value | Ortho‐k (n = 341) | Spectacle (n = 384) | p Value | Subgroup 1 (n = 50) | Subgroup 2 (n = 50) | Subgroup 3 (n = 50) | p Value | |
| Age (year) | 10.88 ± 1.88 | 10.94 ± 1.92 | 0.99 a | 10.02 ± 1.09 | 10.17 ± 1.07 | 0.23 a | 9.56 ± 1.09 | 9.74 ± 1.07 | 9.94 ± 1.20 | 0.24 b |
| Sex (M/F) | 300/288 | 298/290 | 0.91 c | 181/160 | 195/189 | 0.54 c | 26/24 | 26/24 | 23/27 | 0.79 c |
| SER (D) | −2.83 ± 1.11 | −2.68 ± 1.09 | 0.16 a | −2.46 ± 1.16 | −2.39 ± 1.05 | 0.57 a | −2.32 ± 0.86 | −3.56 ± 1.12 | −4.91 ± 0.72 | <0.01 b |
| AL (mm) | 24.87 ± 0.96 | 24.73 ± 0.94 | 0.14 a | 24.58 ± 1.00 | 24.49 ± 0.90 | 0.39 a | 23.92 ± 0.47 | 25.07 ± 0.43 | 26.49 ± 0.40 | <0.01 b |
Abbreviations: AL, axial length; M/F, male/female; SER, spherical equivalent refraction.
2‐sample t‐test.
One‐way ANOVA.
Chi‐square test.
Examinations and follow‐up
A team of one ophthalmologist and three optometrists performed the examinations throughout the follow‐up period, and the same ophthalmologist performed the ortho‐k lens fitting. Three optometrists performed refractions, corneal topography and AL measurements. According to the medical records, all the children in this study underwent a comprehensive baseline eye examination, including cycloplegic refraction, visual acuity testing (standard LogMAR visual acuity chart), slit‐lamp examination, corneal topography and AL measurement. At baseline, cycloplegia was induced using four drops of tropicamide (5 mg/ml) at 5‐min intervals. Refractive examinations began with autorefraction. Subsequently, subjective refraction was performed by the same optometrist. SER was calculated as the spherical power plus one‐half cylindrical power.
The ortho‐k lenses used in this study were spherical 4‐zone lenses (Euclid Systems Corporation, euclidsys.com) made of oprifocon A (Boston EQUALENS II) with an oxygen permeability (Dk) of 127 × 10−11 (cm2/s) (ml O2/ml mm Hg). A certified ophthalmic technician fitted the participants with the lenses per the manufacturer's guidelines. The final parameters of these ortho‐k lenses were determined based on good centration, lens movement, the results of the fluorescein staining examination and a typical bull's eye pattern observed using corneal topography (Medmont E300; Medmont International Pty., Ltd, medmont.com.au). After lens dispensing, participants were required to wear their ortho‐k lenses for at least 8 h every night. In addition, the results of follow‐up examinations were recorded 1 day, 1 week and 1 month after the initial lens wear and at least once every 3 months afterwards. The lens prescription was modified only when the unaided monocular visual acuity was worse than 0.2 LogMAR (6/9) or significant lens decentration was observed.
The control group wore single‐vision spectacles, modified based on any changes in visual acuity, refractive error or interpupillary distance as appropriate throughout the 1‐year follow‐up period. In both the ortho‐k and control groups, AL was measured using the same noncontact optical biometer (Lenstar LS900; Haag‐Streit AG, haag‐streit.com) at baseline and during the annual visit. At each visit, three consecutive measurements were collected by an examiner, with the average being calculated and recorded.
Statistical analysis
Data from the right eyes were retrospectively reviewed and used for statistical analysis. The normality of the data was tested using the Kolmogorov–Smirnov test. Chi‐square tests compared the male/female ratio (M/F ratio) among groups. Other baseline data between two or three groups were compared using the 2‐sample t‐test or the one‐way analysis of variance (ANOVA) as appropriate. Univariate linear regression was used to correlate the 1‐year axial elongation with baseline age, AL and SER, and to evaluate the relationship between baseline age and AL. Two‐way ANOVA analysed the primary effect of age and treatment method (use of ortho‐k) on AL growth. The predictive effects of baseline age, baseline AL and treatment on axial elongation were further examined using stepwise multiple linear regression analysis. An independent t‐test was used to compare AL between the ortho‐k and spectacle subgroups in the younger children. For analysis of 3‐year follow‐up data, the changes in AL at different visits (first, second and third years) among the three subgroups stratified by baseline AL, 23 subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm), were compared using repeated‐measures ANOVA. For significant outcomes, post‐hoc comparisons using Bonferroni corrections were performed. All statistical analyses were performed using SPSS 25.0 software (ibm.com). A p‐value < 0.05 was considered significant.
RESULTS
Association between one‐year axial elongation and initial age
There were no significant differences in baseline values between the ortho‐k (n = 588) and spectacle groups (n = 588) in terms of age (range, 8–14 years), sex, baseline SER and baseline AL (p > 0.05, 2‐sample t‐test and Chi‐square test). Both groups of children displayed increases in AL during the 1‐year follow‐up. Univariate regression revealed that AL growth at the 1‐year visit was negatively associated with initial age in the ortho‐k and spectacle groups (Figure 1a, ortho‐k group: r = −0.43, p < 0.01; spectacle group: r = −0.40, p < 0.01). Figure 1b presents the 1‐year axial elongation (ΔAL) of children of different ages in the ortho‐k and spectacle groups. The relative myopia control efficacy was expressed using the percentage reduction calculated using the formula: | (ΔAL in ortho‐k‐ ΔAL in spectacle)/ΔAL in spectacle*100% |. Compared with the spectacle‐wearing children, axial elongation was reduced by 25% at age 8, 28% at age 9, 33% at age 10, 35% at age 11, 52% at age 12, 63% at age 13 and 74% at age 14 in the ortho‐k group. On average, AL growth in the ortho‐k group (0.19 ± 0.21 mm) was significantly slowed by 39% compared with the spectacle group (0.31 ± 0.19 mm) (p < 0.01, 2‐sample t‐test) after the 1‐year follow‐up. Furthermore, the two‐way ANOVA results revealed that axial elongation depended significantly on age (F = 151.36, p < 0.001) and treatment method (use of ortho‐k) (F = 53.05, p < 0.001). Meanwhile, a significant interaction between age and the treatment method (F = 2.59, p < 0.05) was identified.
FIGURE 1.
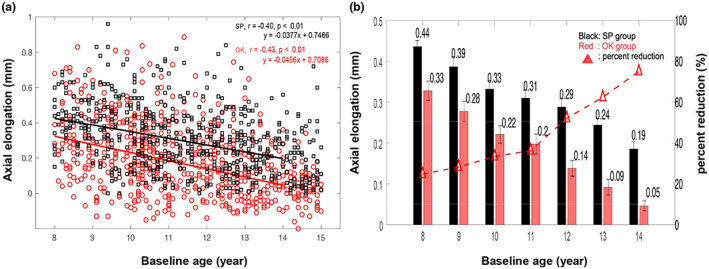
(a) Scatterplots showing correlations of axial elongation at the 1‐year visit with baseline age in the orthokeratology (OK) and spectacle groups (SP). (b) Histogram showing axial elongation of children of different ages in the ortho‐k (OK) and spectacle (SP) groups (data are expressed as the mean ± SD), with percent reduction in axial growth for the ortho‐k group versus the spectacle group.
Association between one‐year axial elongation and baseline AL
Univariate linear regression revealed that AL growth at the 1‐year visit was significantly associated with baseline AL in the ortho‐k group but not in the spectacle group (Figure 2a, ortho‐k group: r = −0.30, p < 0.01; spectacle group: r = −0.04, p = 0.31). During the 1‐year ortho‐k‐wearing period, AL increased less in children with a longer baseline AL. In correlation analysis, initial age revealed significant positive correlations with baseline AL both in the ortho‐k and spectacle groups (Figure 2b, ortho‐k group: r = 0.30, p < 0.01; spectacle group: r = 0.31, p < 0.01). Given the significant associations between age and axial growth as well as age and baseline AL, the potential for age to confound the relationship between axial growth and baseline AL was present. In order to control for age, the children in the ortho‐k and spectacle groups were divided into seven well‐matched subgroups stratified by age, from 8–14 years. Figure 3 revealed that the negative association between 1‐year axial elongation and baseline AL only existed in ortho‐k‐wearing children 8–11 years of age. No association between the 1‐year axial elongation and baseline AL was observed in any age group wearing spectacles.
FIGURE 2.
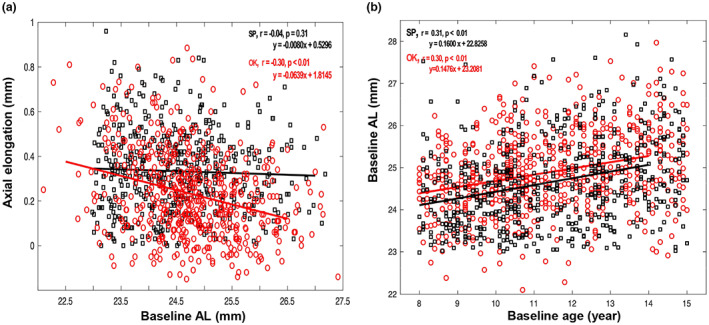
(a) Scatterplots showing the correlation of axial elongation over 1 year with baseline axial length (AL) in the orthokeratology (OK) and spectacle (SP) groups. (b) Scatterplots demonstrating the relationship between baseline AL and baseline age in the ortho‐k (OK) and spectacle (SP) groups.
FIGURE 3.
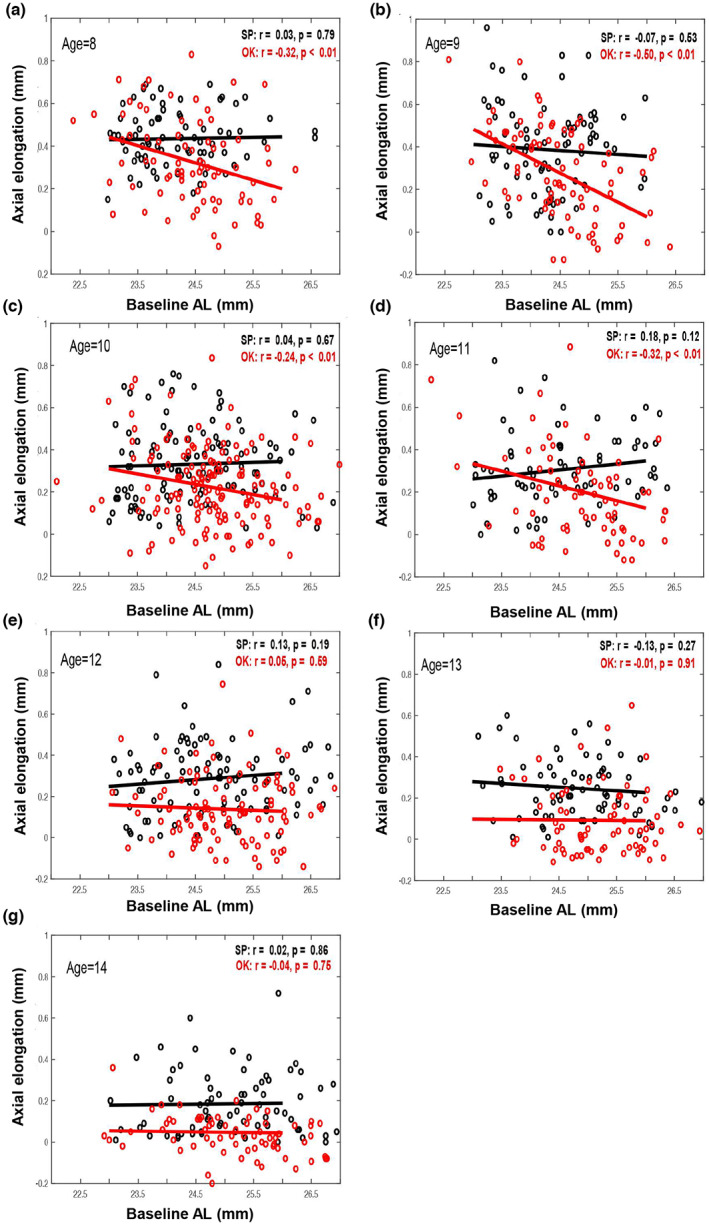
Scatterplots showing correlations of axial elongation over 1 year with baseline axial length (AL) in seven age (8, 9, 10, 11, 12, 13, 14) groups (a–g) divided into orthokeratology (OK) and spectacle (SP) groups.
Association between one‐year axial elongation and baseline SER
Simple linear regression revealed a significant association between baseline SER and axial elongation in the ortho‐k group but not in the spectacle group (Figure 4, ortho‐k group: r = 0.28, p < 0.01; spectacle group: r = 0.03, p = 0.56). Children in the ortho‐k group with greater myopia at baseline experienced a lesser change in AL during the 1‐year follow‐up.
FIGURE 4.
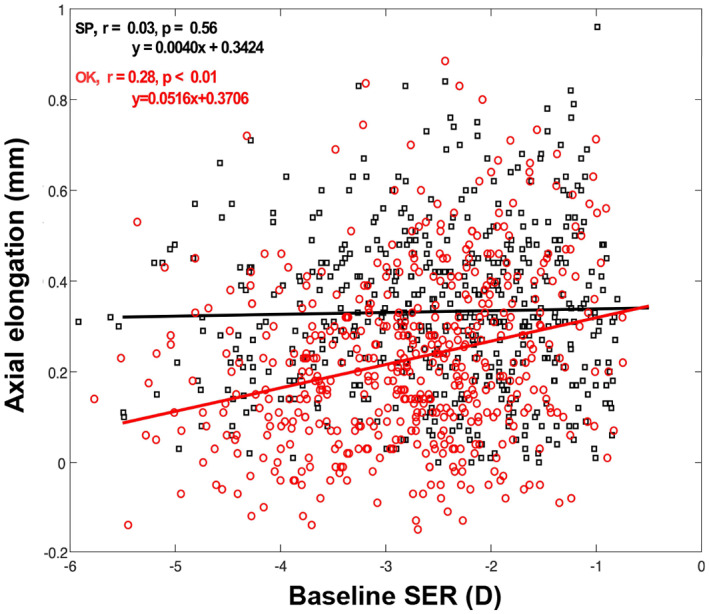
Scatter plots of axial elongation over 1 year relative to spherical equivalent refraction (SER) at baseline in the orthokeratology (OK) and spectacle (SP) groups.
Multiple regression analysis
Stepwise multiple regression analysis revealed that of the factors investigated, axial elongation was significantly associated with treatment (β = −0.11, p < 0.001), initial age (β = −0.03, p < 0.001) and baseline AL (β = −0.02, p < 0.05) but not with baseline SER. The regression model using treatment, initial age and baseline AL to predict axial elongation was fair (adjusted R 2 = 0.21) and significant (p < 0.001). Subsequently, multiple regression was conducted separately in the ortho‐k and spectacle groups. The regression equation using initial age and baseline AL to predict axial elongation was: axial elongation = 1.72 − (0.04*initial age) − (0.05*baseline AL) for the ortho‐k group (R 2 = 0.22, p < 0.001), and axial elongation = 0.43 – (0.03*initial age) for the spectacle group (R 2 = 0.09, p < 0.001).
Axial elongation over 1‐year in 8–11‐year‐old children with different baseline AL
Children (8–11 years) in the ortho‐k (n = 341) and spectacle groups (n = 384) screened from the first cohort were further divided into age‐matched subgroups according to their baseline AL, 23 i.e., subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm). Figure 5 reveals that when compared with spectacle subgroups 2 and 3, ortho‐k subgroups 2 and 3 exhibited noticeably slower axial elongation over 1 year (mean axial elongation in ortho‐k subgroup 2: 0.23 ± 0.19 mm; ortho‐k subgroup 3: 0.16 ± 0.17 mm versus mean axial elongation in spectacle subgroup 2: 0.38 ± 0.16 mm; spectacle subgroup 3: 0.33 ± 0.16 mm, both p < 0.01, 2‐sample t‐test). The axial elongation was 39% and 52% slower for ortho‐k subgroups 2 and 3, respectively. However, the mean axial elongation over 1 year in ortho‐k subgroup 1 was 0.35 ± 0.20 mm, similar to that (0.35 ± 0.20 mm) observed in spectacle subgroup 1 (p = 0.79, 2‐sample t‐test).
FIGURE 5.
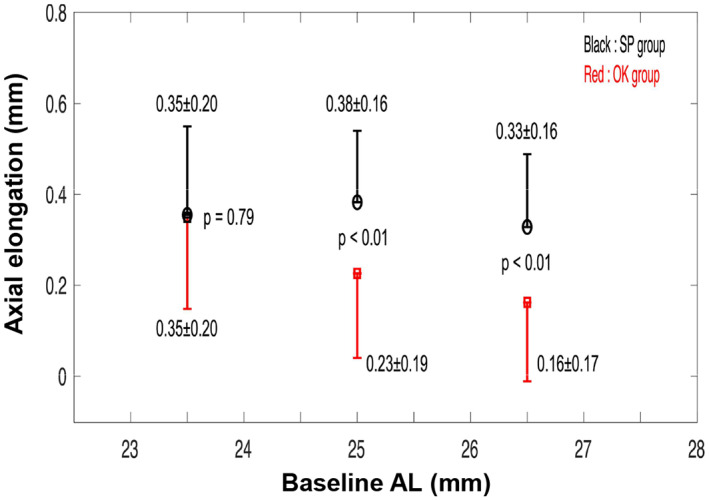
Graphs showing axial growth over the 1‐year treatment in younger children (8–11 years of age) by subgroups with different baseline axial lengths (AL): Subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm). Data are expressed as the mean ± SD. OK, orthokeratology, SP, spectacle wearers.
Annual axial elongation (ΔAL) in 8–11‐year‐old children wearing ortho‐k over the 3‐year follow‐up period
In order to observe the association between annual AL changes and baseline AL further, the ortho‐k wearing cohort aged 8–11 years who completed the 3‐year follow‐up were divided into subgroups based on baseline AL 23 i.e., subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm). Figure 6 presents the annual axial elongation (ΔAL) over the 3‐year study period. There were significant differences in axial elongation within subgroups over the study period (p < 0.01, repeated‐measures ANOVA). The following Bonferroni‐adjusted post‐hoc comparisons indicated that, compared with subgroups 2 and 3, axial elongation was significantly faster in subgroup 1 during the first (vs. subgroup 2, mean difference = 0.18 ± 0.03 mm, p < 0.01; vs. subgroup 3, mean difference = 0.25 ± 0.04 mm, p < 0.01) and second years (vs. subgroup 2, mean difference = 0.10 ± 0.03 mm, p < 0.01; vs. subgroup 3, mean difference = 0.09 ± 0.03 mm, p < 0.01) but not for the third year (vs. subgroup 2, mean difference = 0.06 ± 0.03 mm, p = 0.15; vs. subgroup 3, mean difference = 0.06 ± 0.03 mm, p = 0.19). There were no significant differences in ΔAL between subgroups 2 and 3 during the 3‐year follow‐up (p > 0.05).
FIGURE 6.
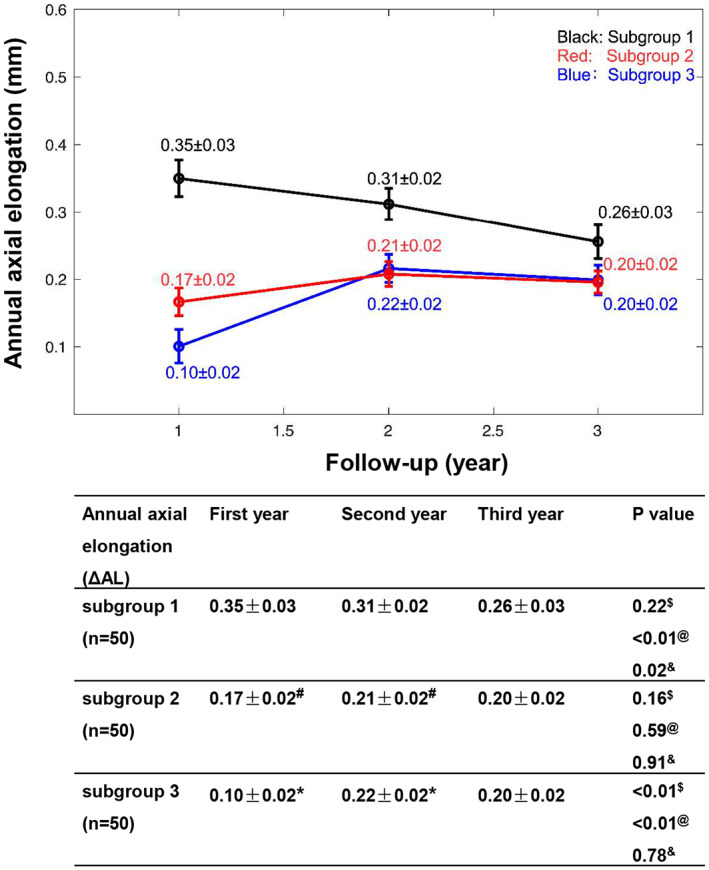
The time course of changes in axial length (AL) over the 3‐year study period for younger children (8–11 years of age) wearing orthokeratology (ortho‐k) lenses stratified by baseline AL: Subgroup 1 (AL < 24.5 mm), subgroup 2 (24.5 ≤ AL < 26 mm) and subgroup 3 (AL ≥ 26 mm). Data are expressed as the mean ± SEM. #Subgroup 1 versus subgroup 2, p < 0.01, *subgroup 1 versus subgroup 3, p < 0.01, $first year change in axial length (ΔAL) versus second year ΔAL, @first year ΔAL versus third year ΔAL, &second year ΔAL versus third year ΔAL.
In addition, subgroup 1 presented a noticeable decrease in ΔAL over time. Axial elongation in children from subgroup 1 was significantly faster in the first‐ and second years than in the third year (first‐third year period, mean difference = 0.09 ± 0.03 mm, p < 0.01; second‐third year period, mean difference = 0.05 ± 0.03 mm, p = 0.02). No significant difference in ΔAL was observed in subgroup 2 during the 3‐year follow‐up. The annual increase in AL in subgroup 3 was significantly lower in the first year than in the second and third years (first‐second year period, mean difference = −0.12 ± 0.02 mm, p < 0.01; first‐third year period, mean difference = −0.10 ± 0.02 mm, p < 0.01). However, this stabilised in the third year.
DISCUSSION
The current study demonstrated that the axial elongation in children after 1 year of ortho‐k treatment was negatively correlated with the baseline AL, and 8–11 year‐old children with a longer baseline AL (≥24.5 mm) presented slower axial elongation during the first 2 years of ortho‐k treatment. Considering the inter‐individual variation in myopia progression after wearing ortho‐k lenses, our results may offer a new perspective on myopia control using ortho‐k, specifically for establishing individual guidelines for myopia management in children with different baseline characteristics.
Many studies have suggested that myopic progression in children wearing ortho‐k lenses slows with age. 9 , 11 , 16 , 24 , 25 In accordance with this, our results demonstrated that initial age was negatively correlated with axial elongation during ortho‐k treatment. Combining data from the ROMIO and TO‐SEE studies showed a stronger negative association between initial age (6–12 years) and axial elongation in the control group than in the ortho‐k group during the 2‐year follow‐up. 15 However, our results revealed that the negative relationship was similar in the ortho‐k and spectacle groups for children aged 8–14 at the 1‐year visit. The differences in participant age, follow‐up time and sample size may account for this discrepancy. In addition, we observed that, in comparison with spectacle‐wearing children, axial elongation was reduced by over 50% in children wearing ortho‐k lenses with an initial age of 12–14 years, but only by 25%, 28%, 33% and 35% in 8, 9, 10 and 11 year olds, respectively, which was below the overall percentage reduction (39%) of axial elongation for the ortho‐k group versus the spectacle group. This finding indicated that children over 11 years of age had a more positive myopic control effect than children aged 8–11 years old after 1‐year of ortho‐k treatment. This may partly be because the children experienced a natural reduction in the rate of axial elongation with age, regardless of treatment. 26 , 27 , 28 Nevertheless, children with myopia onset prior to 10 years of age were at risk for high myopia. 29 From another point of view, the use of ortho‐k in these younger children helped slow myopia progression earlier, thus reducing the risk of developing high myopia.
Regarding the association between baseline AL and myopia progression in children undergoing treatment with ortho‐k lenses, there are conflicting results among previous studies. A retrospective investigation involving 184 ortho‐k‐wearing children (6–14 years old) assessed myopia progression and reported that the change in SER over time was significantly correlated with baseline AL. 30 In addition, one prospective observational study enrolled 32 eyes wearing ortho‐k lenses and observed that higher baseline differences between central and peripheral AL were significantly associated with slower axial elongation. 22 Consistent with the results of a Danish randomised controlled trial using ortho‐k lenses, 21 our data revealed that AL growth at the 1‐year visit was significantly associated with baseline AL in the ortho‐k group; however, this was not observed in the spectacle wearing group. Initial age was positively correlated with baseline AL both in the present study and that of Lee et al. 31 After adjusting for age, we observed that the negative association between the 1‐year axial elongation and baseline AL only existed in 8‐, 9‐, 10‐ and 11‐year‐olds wearing ortho‐k lenses. Further analysis revealed that younger children (aged 8–11) whose baseline AL was <24.5 mm in the ortho‐k group had a 1‐year axial growth similar to that of the spectacle group. In contrast, the younger ortho‐k wearers with a longer baseline AL (≥24.5 mm) presented significantly slower axial elongation than spectacle wearers. Therefore, baseline AL may be a predictive factor influencing myopia progression in younger children (8–11 years) using ortho‐k lenses. In contrast, several clinical trials observed no significant association between AL growth and baseline AL in children undergoing treatment with ortho‐k lenses. 16 , 32 Considering that the different studies investigated different sets of variables, as well as using different instruments, sample sizes and types of ortho‐k lenses, the same factor may exert alternative effects across studies.
Until now, it has been unclear whether baseline AL is a noteworthy predictor of the long‐term control effect of ortho‐k lenses on myopic AL elongation. Hiraoka et al. proposed that the effect of ortho‐k was limited to the first 3 years compared with spectacles. 13 Thus, inspired by Xu et al., 32 we conducted external validation using 150 ortho‐k‐wearing children (8–11 years of age) who completed the 3‐year follow‐up. We observed a significantly faster annual increase in AL in the subgroup with a shorter baseline AL (<24.5 mm) in the first 2 years, but not in the third year when compared to the subgroup with a longer baseline AL (≥24.5 mm). The children with baseline AL <24.5 mm started treatment at a mean age of 9.5 years, and most of them would be over 11 years of age after 2 years of treatment, explaining their slower myopic progression in the third year. In addition, the annual axial elongation in children with baseline AL between 24.5 and 26 mm was consistent (0.17–0.20 mm) during the 3 years, indicating that the children benefited greatly from ortho‐k treatment. However, the annual axial elongation in children with baseline AL > 26 mm increased from 0.10 mm to 0.22 mm in the first 2 years of treatment; this stabilised in the third year (0.20 mm). Children with AL ≥ 26–26.5 mm may develop high myopia and show pathological retinal signs. 3 , 33 Charm et al. 14 and Zhu et al. 16 also supported the notion that ortho‐k treatment demonstrated better myopia control in highly myopic patients in the first year of the study compared to the second year. A possible explanation may be the adaptation of these high myopic children to the signal that slows myopic progression after 1 year of ortho‐k treatment. 9 As discussed above, the AL at baseline may be a nonnegligible factor associated with subsequent axial elongation in ortho‐k treatment. Therefore, for ortho‐k‐wearing children with baseline AL 24.5 mm at age 8–11, clinicians should develop individualised strategies, such as combined application with low‐concentration atropine, 34 to achieve a better prognosis due to their predicted faster myopic progression.
There were several limitations to this study. First, the study was based on retrospective data. Furthermore, factors that may affect myopia progression, such as baseline AL in nasal and temporal gaze, 22 corneal biomechanics, 35 pupil size 36 or its relative size to the treatment zone, 37 parental myopia, rate of myopia progression before baseline, 38 outdoor activity and near work 39 were not recorded in any group. Second, two cohorts were included in this study, and the sample size of the second cohort was relatively small. Hence, a longitudinal study with a larger sample size and longer duration is warranted to verify the association between baseline AL and axial elongation during ortho‐k treatment. Third, simply analysing the influence of baseline characteristics on the efficacy of ortho‐k lenses is insufficient. The mechanism of ortho‐k in controlling myopia progression is complex, and modification of ortho‐k lens parameters, such as reducing the back optic zone diameter, may influence myopia control efficacy. 37 Factors that induce asymmetric optical changes during ortho‐k treatment, such as increased higher‐order aberrations, 40 should be considered in future studies.
In conclusion, children with an older baseline age and a longer baseline AL experienced slower axial elongation after ortho‐k treatment. However, children 8–11 years of age with baseline AL <24.5 mm presented relatively faster axial growth during the first 2 years of ortho‐k treatment. These findings suggest that for younger children with baseline AL <24.5 mm undergoing ortho‐k treatment, clinicians should make appropriate individual adjustments, such as optimising the lens design or using combination therapy to achieve better outcomes. Future studies are needed to elucidate these issues and explore the underlying mechanisms.
AUTHOR CONTRIBUTIONS
Weiping Lin: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); project administration (equal); software (lead); supervision (equal); validation (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Na Li: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (supporting); supervision (equal); writing – original draft (lead); writing – review and editing (equal). Kunpeng Lu: Data curation (equal); methodology (equal); software (equal). Zhaochun Li: Methodology (equal); writing – review and editing (supporting). Xiaohua Zhuo: Methodology (equal); writing – review and editing (supporting). Ruihua Wei: Formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (supporting); project administration (equal); resources (lead); supervision (equal); validation (equal); writing – original draft (supporting); writing – review and editing (lead).
CONFLICT OF INTEREST
The authors declare no competing interests.
ACKNOWLEDGEMENTS
We thank the National Natural Science Foundation of China for their support, Grant/Award Numbers: 82070929.
Lin W, Li N, Lu K, Li Z, Zhuo X, Wei R. The relationship between baseline axial length and axial elongation in myopic children undergoing orthokeratology. Ophthalmic Physiol Opt. 2023;43:122–131. 10.1111/opo.13070
Weiping Lin and Na Li have contributed equally to this work and share first authorship.
REFERENCES
- 1. Resnikoff S, Jonas J, Friedman D, He M, Jong M, Nichols J, et al. Myopia ‐ a 21st century public health issue. Invest Ophthalmol Vis Sci. 2019;60:Mi–Mii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holden B, Fricke T, Wilson D, Jong M, Naidoo K, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. [DOI] [PubMed] [Google Scholar]
- 3. Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith EL III, Zhou X, et al. Myopia. Nat Rev Dis Primers. 2020;6:99. 10.1038/s41572-020-00231-4 [DOI] [PubMed] [Google Scholar]
- 4. Modjtahedi B, Abbott R, Fong D, Lum F, Tan D. Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children: the Academy's task force on myopia. Ophthalmology. 2021;128:816–26. [DOI] [PubMed] [Google Scholar]
- 5. Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62:6. 10.1167/iovs.62.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wildsoet C, Chia A, Cho P, Guggenheim J, Polling J, Read S, et al. Interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019;60:M106–31. [DOI] [PubMed] [Google Scholar]
- 7. Xie P, Guo X. Chinese experiences on orthokeratology. Eye Contact Lens. 2016;42:43–7. [DOI] [PubMed] [Google Scholar]
- 8. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80. [DOI] [PubMed] [Google Scholar]
- 9. Cho P, Cheung SW. Retardation of myopia in orthokeratology (ROMIO) study: a 2‐year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–85. [DOI] [PubMed] [Google Scholar]
- 10. Santodomingo‐Rubido J, Villa‐Collar C, Gilmartin B, Gutiérrez‐Ortega RJ. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012;53:5060–5. [DOI] [PubMed] [Google Scholar]
- 11. Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO‐SEE study). Invest Ophthalmol Vis Sci. 2013;54:6510–7. [DOI] [PubMed] [Google Scholar]
- 12. Paune J, Morales H, Armengol J, Quevedo L, Faria‐Ribeiro M, Gonzalez‐Meijome JM. Myopia Control with a novel peripheral gradient soft lens and orthokeratology: a 2‐year clinical trial. Biomed Res Int. 2015;2015:507572. 10.1155/2015/507572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long‐term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5‐year follow‐up study. Invest Ophthalmol Vis Sci. 2012;53:3913–9. [DOI] [PubMed] [Google Scholar]
- 14. Charm J, Cho P. High myopia‐partial reduction ortho‐k: a 2‐year randomized study. Optom Vis Sci. 2013;90:530–9. [DOI] [PubMed] [Google Scholar]
- 15. Cho P, Cheung SW. Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO‐SEE studies. Invest Ophthalmol Vis Sci. 2017;58:1411–6. [DOI] [PubMed] [Google Scholar]
- 16. Zhu M, Feng H, He X, Zou H, Zhu J. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol. 2014;14:141. 10.1186/1471-2415-14-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Queiros A, Lopes‐Ferreira D, Yeoh B, Issacs S, Amorim‐De‐Sousa A, Villa‐Collar C, et al. Refractive, biometric and corneal topographic parameter changes during 12 months of orthokeratology. Clin Exp Optom. 2020;103:454–62. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Li Z, Zeng J. A review of the potential factors influencing myopia progression in children using orthokeratology. Asia Pac J Ophthalmol. 2016;5:429–33. [DOI] [PubMed] [Google Scholar]
- 19. Lam AKC, Hon Y, Leung SYY, Shu‐Ho L, Chong J, Lam DCC. Association between long‐term orthokeratology responses and corneal biomechanics. Sci Rep. 2019;9:12566. 10.1038/s41598-019-49041-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vincent SJ, Cho P, Chan KY, Fadel D, Ghorbani‐Mojarrad N, Gonzalez‐Meijome JM, et al. CLEAR ‐ orthokeratology. Cont Lens Anterior Eye. 2021;44:240–69. [DOI] [PubMed] [Google Scholar]
- 21. Jakobsen TM, Moller F. Control of myopia using orthokeratology lenses in Scandinavian children aged 6 to 12 years. Eighteen‐month data from the Danish randomized study: clinical study of near‐sightedness: treatment with orthokeratology lenses (CONTROL study). Acta Ophthalmol. 2021;100:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Lim DH, Han SH, Chung TY. Predictive factors associated with axial length growth and myopia progression in orthokeratology. PLoS One. 2019;14:e0218140. 10.1371/journal.pone.0218140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee MW, Lee SE, Lim HB, Kim JY. Longitudinal changes in axial length in high myopia: a 4‐year prospective study. Br J Ophthalmol. 2020;104:600–3. [DOI] [PubMed] [Google Scholar]
- 24. Lin W, Li N, Gu T, Tang C, Liu G, Du B, et al. The treatment zone size and its decentration influence axial elongation in children with orthokeratology treatment. BMC Ophthalmol. 2021;21:362. 10.1186/s12886-021-02123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang B, Naidu RK, Qu X. Factors related to axial length elongation and myopia progression in orthokeratology practice. PLoS One. 2017;12:e0175913. 10.1371/journal.pone.0175913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saw S, Nieto F, Katz J, Schein O, Levy B, Chew S. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000;77:549–54. [DOI] [PubMed] [Google Scholar]
- 27. Hyman L, Gwiazda J, Hussein M, Norton T, Wang Y, Marsh‐Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–87. [DOI] [PubMed] [Google Scholar]
- 28. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL 3rd, Holden BA. Myopia progression rates in urban children wearing single‐vision spectacles. Optom Vis Sci. 2012;89:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin Z, Peng T, Zhang Z, Lou J, Wang C, Deng R, et al. Myopia progression and stabilization in school‐aged children with single‐vision lenses. Acta Ophthalmol. 2021;100:e950–6. [DOI] [PubMed] [Google Scholar]
- 30. Chen Z, Zhang Z, Xue F, Zhou J, Zeng L, Qu X, et al. The relationship between myopia progression and axial elongation in children wearing orthokeratology contact lenses. Cont Lens Anterior Eye. 2021;101517. 10.1016/j.clae.2021.101517 [DOI] [PubMed] [Google Scholar]
- 31. Lee EJ, Lim DH, Chung T‐Y, Hyun J, Han J. Association of axial length growth and topographic change in orthokeratology. Eye Contact Lens. 2018;44:292–8. [DOI] [PubMed] [Google Scholar]
- 32. Xu S, Li Z, Hu Y, Zhao W, Jiang J, Feng Z, et al. Development and validation of a prediction model for axial length elongation in myopic children treated with overnight orthokeratology. Acta Ophthalmol. 2021;99:e686–93. [DOI] [PubMed] [Google Scholar]
- 33. Ruiz‐Medrano J, Montero JA, Flores‐Moreno I, Arias L, Garcia‐Layana A, Ruiz‐Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115. [DOI] [PubMed] [Google Scholar]
- 34. Zhou H, Zhao G, Li Y. Adjunctive effects of orthokeratology and atropine 0.01% eye drops on slowing the progression of myopia. Clin Exp Optom. 2022;105:520–6. [DOI] [PubMed] [Google Scholar]
- 35. Liu G, Rong H, Zhang P, Xue Y, Du B, Wang B, et al. The effect of axial length elongation on corneal biomechanical property. Front Bioeng Biotechnol. 2021;9:777239. 10.3389/fbioe.2021.777239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Z, Niu L, Xue F, Qu X, Zhou Z, Zhou X, et al. Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci. 2012;89:1636–40. [DOI] [PubMed] [Google Scholar]
- 37. Paune J, Fonts S, Rodriguez L, Queiros A. The role of back optic zone diameter in myopia control with orthokeratology lenses. J Clin Med. 2021;10:336. 10.3390/jcm10020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santodomingo‐Rubido J, Villa‐Collar C, Gilmartin B, Gutiérrez‐Ortega R. Factors preventing myopia progression with orthokeratology correction. Optom Vis Sci. 2013;90:1225–36. [DOI] [PubMed] [Google Scholar]
- 39. Wen L, Cao Y, Cheng Q, Li X, Pan L, Li L, et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol. 2020;104:1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau JK, Vincent SJ, Cheung SW, Cho P. Higher‐order aberrations and axial elongation in myopic children treated with orthokeratology. Invest Ophthalmol Vis Sci. 2020;61:22. 10.1167/iovs.61.2.22 [DOI] [PMC free article] [PubMed] [Google Scholar]


