Abstract
Low‐dose aspirin has been hypothesized to prevent cancer risk by inhibiting platelet aggregation. However, the anti‐cancer effect of low‐dose aspirin has recently been questioned and its effect on breast cancer development remains unclear. The impact of other antiplatelet drugs on breast cancer risk has rarely been evaluated. Thus, this study aimed to investigate the associations between breast cancer risk and antiplatelet drug use in a nationwide nested case‐control study. From the Danish healthcare registries, we identified as cases all women with invasive breast cancer diagnosis between 2001 and 2018 (n = 68 852). The date of diagnosis corresponded to the index date. We matched cases to 10 population controls on age and calendar time, using risk set sampling. Controls were assigned the same index date as their matched case. We used the prescription registry to identify exposure to low‐dose aspirin, clopidogrel and dipyridamole. We defined ever use of antiplatelet drugs as at least two prescriptions filled up to 1 year before the index date. We applied conditional logistic regression to calculate odds ratios (ORs) and 95% confidence intervals for breast cancer associated with the use of antiplatelet drugs, overall, by breast cancer subtype and by cumulative dose. Twelve percent of women had ever been exposed to low‐dose aspirin, 2% to clopidogrel and 2% to dipyridamole. In multivariable models, breast cancer risk was not associated with ever use of low‐dose aspirin (OR = 1.00 [0.97‐1.03]), clopidogrel (OR = 0.93 [0.87‐1.00]), and dipyridamole (OR = 1.02 [0.94‐1.10]), compared with never use, and there was no evidence of a dose‐response relation. However, we found an inverse association between dipyridamole use and breast cancer risk among women aged <55 years old, with suggestion of a dose‐response relationship (OR per 1000 Defined Daily Doses = 0.72 [0.54‐0.95]). Associations did not differ by breast cancer histological type, estrogen receptor status or clinical stage at diagnosis. Overall, the findings from this study do not support the use of antiplatelet drugs for breast cancer prevention.
Keywords: antiplatelet drugs, breast cancer, low‐dose aspirin, registries
What's new?
Low‐dose aspirin has been hypothesized to prevent cancer risk by inhibiting platelet aggregation while the impact of other antiplatelet drugs on breast cancer risk has rarely been evaluated. The findings from this large nationwide nested case‐control study add to the growing evidence from randomized controls trials that low‐dose aspirin does not appear to be a suitable pharmacological candidate for breast cancer prevention. Further, our results do not provide strong support to the use of other antiplatelet drugs for breast cancer prevention.

Abbreviations
- BMI
body mass index
- CI
confidence interval
- DDD
defined daily dose
- ER
estrogen receptor
- OR
odd ratio
1. INTRODUCTION
It has been suggested that low‐dose aspirin may prevent several cancers, including breast cancer, by inhibiting platelet aggregation. 1 In a recent meta‐analysis of 22 cohort and 16 case‐control studies, aspirin use was associated with a 4% decreased risk of breast cancer in cohorts and a 17% decreased risk in case‐control studies. 2 However, there was substantial heterogeneity in the results between studies, which may be attributable to differences in the doses or durations of aspirin use that were evaluated or in the populations studied. The potential preventive effect of low‐dose aspirin on breast cancer incidence has not been confirmed in two randomized double‐blind placebo‐controlled trials. 3 , 4 The overall cancer preventive effect of low‐dose aspirin has even been questioned with emerging evidence from one of these trials, published in 2020, which reported a positive association between low‐dose aspirin and the risk of any stage 4 cancer among participants aged ≥70 years. 3 A meta‐analysis of randomized controlled trials, published in 2018, found that aspirin's effects on cancer might differ by body size, age, dose and timing of aspirin use. 5 Among participants aged ≥70 years and weighing <70 kg, aspirin exposure was associated with an increased risk of any cancer in the first 3 years of follow‐up, with a subsequent reduced risk after 5 years of follow‐up. 5 A recent study among women from the French E3N cohort (median age at follow‐up start: 63 years old), performed by our group, found the same pattern between low‐dose aspirin use and breast cancer incidence, with a transient higher breast cancer risk a few years after starting low‐dose aspirin use, followed by a lower risk after 4 years of use. 6
If low‐dose aspirin impacts breast cancer through its antiplatelet properties, other antiplatelet drugs are likely to elicit similar effects on breast cancer incidence. However, our analysis based on the E3N cohort was the only epidemiological study to consider the use of antiplatelet drugs other than aspirin in relation to breast cancer risk. We found some evidence that clopidogrel use was associated with a higher estrogen receptor (ER) negative breast cancer risk, 6 however this was based on a limited number of ER‐negative breast cancer cases (never exposed = 23).
Thus, we aimed to further evaluate this putative association using data from the nationwide Danish registries. In particular, we evaluated the associations between breast cancer incidence and antiplatelet drug use, overall and by breast cancer subtypes, types of antiplatelet drugs and cumulative dose (as a proxy for duration of use).
2. MATERIALS AND METHODS
We performed a nested case‐control analysis based on data from Danish nationwide registries. We compared the use of antiplatelet drugs among women diagnosed with invasive breast cancer (cases) with use among cancer‐free women (controls), estimating odds ratios (ORs) for breast cancer associated with antiplatelet drug use.
2.1. Nationwide registry sources
We used data from six nationwide registry sources: the Danish Cancer Registry, 7 the National Prescription Registry, 8 the National Patient Registry, 9 the Population Education Registry, 10 the Danish Pathology Register 11 and the Civil Registration System. 12 , 13 We described these registries in Data S1 (Additional File 1).
Almost all medical care in Denmark is funded by the Danish National Health Service, allowing population‐based register linkage studies covering all residents of Denmark. 14 Data sources were linked by a unique personal identification number, assigned to all residents since 1968. 13 All linkages were performed by Statistics Denmark, a government institution that collects and processes information for a variety of statistical and scientific purposes.
2.2. Selection of breast cancer cases and population controls
We described the selection of breast cancer cases in Figure 1 and codes for cancer diagnoses in Data S2 (Additional File 1). From the Danish Cancer Registry, we identified cases as all women with a primary diagnosis of invasive breast cancer between January 1, 2001 and December 31, 2018 (n = 82 031). First, we excluded cases that were diagnosed at death/by death certificate (n = 158). The date of diagnosis corresponded to the index date. We excluded cases aged ≤18 and ≥85 years at the index date (n = 4403) and cases that were not histologically verified (n = 467). We further excluded women with any cancer diagnosis (except non‐melanoma skin cancer, n = 4422) or mastectomy before the index date (n = 220). Cases with any residency outside Denmark within 10 years prior to the index date were also excluded (n = 2051), thus ensuring at least 10 years of follow‐up for all study subjects and a minimum of 5 years of drug prescription data (the prescription registry opened in 1995). We restricted the study sample to women with no prescription of antiplatelet drugs between January 1, 1995 and December 31, 1995 in order to exclude women who had probably begun these drugs before the availability of prescription data. We ended‐up with 68 816 histologically verified invasive breast cancer cases. For each case, we selected 10 controls among Danish women matched by exact birth year and calendar time, and the selection criteria listed above was applied to both cases and controls. Controls were selected using risk set sampling and were assigned the same index date as the case to whom they were matched. Subjects were eligible for sampling as controls before they became cases, thereby the calculated ORs provide unbiased estimates of the incidence rate ratios that would be estimated from a cohort study utilizing the source population. 15 The final study population included 68 816 cases matched with 688 160 controls.
FIGURE 1.
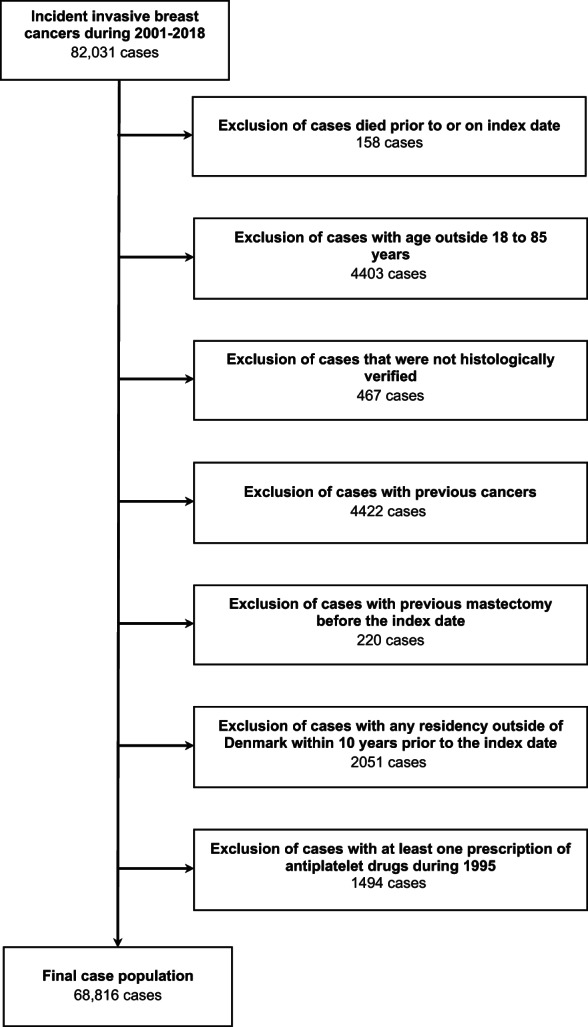
Flow‐chart of the selection of cases
2.3. Exposure
Low‐dose aspirin (≤150 mg), clopidogrel and dipyridamole are the most frequently prescribed antiplatelet drugs in Denmark. As they act through distinct pharmacological mechanisms, 16 they were analyzed separately. We described codes for drug exposure in Data S2 (additional file 1). We defined ever users of the drug of interest as women with at least two prescriptions between January 1, 1996 and 1 year prior to the index date. Women with 0 to 1 prescription were considered never users of the drug of interest (reference category). We also classified exposure according to cumulative number of defined daily doses (DDD). Long‐term use of antiplatelet drugs was defined as filled prescriptions equivalent to ≥1000 DDDs of antiplatelet drugs, corresponding to approximately 3 years of cumulative use. For all analyses, prescriptions filled in the year prior to the index date were disregarded as to allow for a minimum latency period and to account for potential reverse causality. 17
2.4. Covariates
Potential confounders were selected a priori based on the literature and availability in the registries. From the Prescription Registry, we retrieved prescriptions of drugs suspected to modify breast cancer risk and likely to be associated with the use of antiplatelet drugs including antidiabetics, statins, spironolactone, loop diuretics, β‐blockers, vascular calcium channel blockers and selective serotonin reuptake inhibitors. We defined ever users as women with at least two prescriptions of the drug of interest from 1995 to 1 year prior to the index date. For oral contraceptives or hormone replacement therapy, recent users were defined as women with at least two prescriptions in the penultimate year preceding the index date, and former users were defined as women with at least two prescriptions from 1995 to the penultimate year preceding the index date but who were not recent users.
From the Danish National Patient Registry, we retrieved information on diagnoses of chronic obstructive pulmonary disease and alcohol‐related diseases as proxies for heavy smoking and heavy alcohol consumption. We also considered comorbidities including hypertension, hypercholesterolemia, type 1 or 2 diabetes, acute myocardial infarction, other ischemic heart disease, angina pectoris, heart failure, stroke, other cerebrovascular diseases, atrial fibrillation or atrial flutter, pulmonary embolism and infarction, phlebitis and thrombophlebitis, portal vein thrombosis, and other venous embolism and thrombosis. Comorbidities were defined as a primary or secondary discharge or outpatient diagnosis or by related medications. The Charlson comorbidity index score (0 [low], 1‐2 [medium], or ≥ 3 [high]) was defined based on the prevalence of 19 chronic conditions. 18 , 19 Information within 1 year prior to the index date was also disregarded for comorbidities. From registries at Statistics Denmark and the Civil Registration System, we retrieved information on educational level as a crude measure of socioeconomic status (basic, medium, higher or unknown). Codes for all covariates are listed in Data S2 (Additional File 1).
2.5. Statistical analyses
We computed the frequency and proportion of cases and controls within categories of exposure and covariates. We used conditional logistic regression to estimate ORs for the association of ever or long‐term antiplatelet drug use with breast cancer incidence. Secondary analyses examined potential dose‐response associations stratifying cumulative doses of antiplatelet drugs by predefined categories that is, <500, ≥500 to <1000, ≥1000 to <2000, ≥2000 to <3000 and ≥3000 DDDs. In all analyses, never use of antiplatelet drugs (defined as <2 prescriptions) served as the reference category. All models were adjusted for all potential confounders outlined previously and listed in the Data S3. In addition, analyses of low‐dose aspirin, clopidogrel and dipyridamole were simultaneously adjusted for each other.
We performed several subgroup and sensitivity analyses. First, we examined the association between antiplatelet drug exposure and breast cancer risk by histological type (ductal adenocarcinoma, lobular adenocarcinoma, and others), estrogen receptor (ER) status (ER‐positive, ER‐negative, and unknown), and clinical stage at diagnosis (localized, non‐localized and unknown). Then, we stratified our analyses by age at index date (<55, ≥55 to <70 and ≥70). It has been suggested that combination or concomitant use of low‐dose aspirin with other antiplatelet drugs might be associated with an increased cancer risk. 6 , 20 , 21 Therefore, we also defined exposure as follows: (i) use of low‐dose aspirin but never concomitantly with clopidogrel or dipyridamole, (ii) use of clopidogrel but never concomitantly with low‐dose aspirin, (iii) use of dipyridamole but never concomitantly with low‐dose aspirin, (iv) ever concomitant use of low‐dose aspirin and clopidogrel, and (v) ever concomitant use of low‐dose aspirin and dipyridamole. Women were considered concomitant users of two different antiplatelet drugs when they had a prescription of another drug on the same day or until ≤30 days after the prescription of a first drug. If antiplatelet drugs impacted breast cancer risk through their antithrombotic properties, other antithrombotic drugs such as Vitamin K antagonists would have similar effects on breast cancer incidence. We therefore examined the associations between Vitamin K antagonists (ATC code: B01AA) exposure and breast cancer risk (using the same primary exposure definition as previously outlined for antiplatelets). We repeated the main analyses varying the minimum latency period from 0 to 2 years. Finally, we restricted our analyses to women over the age of 55 years (ie, likely postmenopausal) who were never users of hormone replacement therapy and to those with a diagnosis of stroke or myocardial infarction. All statistical analyses were conducted using STATA version 17.0.
3. RESULTS
Among the 68 816 invasive breast cancer cancers, 75% were ductal adenocarcinomas, 13% lobular adenocarcinomas and 12% others. Among cases, 49 845 had information on ER status, of which 81% were ER‐positive and 19% ER‐negative. Among cases with information on stage (n = 55 651), 57% were localized and 43% non‐localized. The characteristics of the study population are presented in Table 1. The median age at index date was 62 years (interquartile range, 53‐70). Differences in characteristics at index date between cases and controls were generally minor, except for a higher use of hormone replacement therapy among cases compared to controls. At the index date, 12% women had ever used low‐dose aspirin, 2% clopidogrel and 2% dipyridamole, while 7% women were long‐term users of low‐dose aspirin, <1% of clopidogrel and <1% of dipyridamole.
TABLE 1.
Characteristics of breast cancer cases and matched controls
| Cases, n = 68 816 | Controls, n = 688 160 | |
|---|---|---|
| Age, median (IQR, years) | 62 (53‐70) | 62 (53‐70) |
| Use of low‐dose aspirin, n (%) | ||
| Never | 60 268 (88%) | 603 363 (88%) |
| Ever | 8548 (12%) | 84 797 (12%) |
| Long‐term | 5186 (7.5%) | 50 867 (7.4%) |
| Cumulative DDDs, median (IQR) | 1300 (500‐2500) | 1300 (500‐2470) |
| Use of clopidogrel, n (%) | ||
| Never | 67 737 (98%) | 676 397 (98%) |
| Ever | 1079 (1.6%) | 11 763 (1.7%) |
| Long‐term | 287 (0.4%) | 2981 (0.4%) |
| Cumulative DDDs, median (IQR) | 500 (340‐1074) | 448 (330‐1000) |
| Use of dipyridamole, n (%) | ||
| Never | 67 792 (99%) | 677 946 (99%) |
| Ever | 1024 (1.5%) | 10 214 (1.5%) |
| Long‐term | 527 (0.8%) | 5205 (0.8%) |
| Cumulative DDDs, median (IQR) | 1050 (360‐2130) | 1034 (360‐2100) |
| Ever use of other drugs, n (%) | ||
| Antidiabetics | 3432 (5.0%) | 33 756 (4.9%) |
| Statins | 10 504 (15%) | 106 227 (15%) |
| Spironolactone | 1127 (1.6%) | 10 182 (1.5%) |
| Loop diuretics | 4701 (6.8%) | 43 627 (6.3%) |
| Beta‐blockers | 2480 (3.6%) | 22 602 (3.3%) |
| Vascular calcium‐channel blockers | 8238 (12%) | 79 809 (12%) |
| Selective serotonin reuptake inhibitors | 10 532 (15%) | 100 842 (15%) |
| Raloxifene | 126 (0.2%) | 1986 (0.3%) |
| Recent use of oral contraceptives | 3053 (4.4%) | 24 678 (3.6%) |
| Former use of oral contraceptives | 13 729 (20%) | 130 851 (19%) |
| Recent use of hormone replacement therapy | 8434 (12%) | 48 379 (7.0%) |
| Former use of hormone replacement therapy | 18 162 (26%) | 146 237 (21%) |
| Comorbidities, n (%) | ||
| Alcohol related diseases | 2251 (3.3%) | 20 032 (2.9%) |
| Chronic obstructive pulmonary disease | 3354 (4.9%) | 31 051 (4.5%) |
| Hypertension | 30 618 (44%) | 296 223 (43%) |
| Hypercholesterolemia | 12 278 (18%) | 125 082 (18%) |
| Diabetes | 4127 (6.0%) | 40 489 (5.9%) |
| Acute myocardial infarction | 879 (1.3%) | 9558 (1.4%) |
| Other ischemic heart disease | 68 (0.1%) | 789 (0.1%) |
| Angina pectoris | 2536 (3.7%) | 25 757 (3.7%) |
| Heart failure | 931 (1.4%) | 8507 (1.2%) |
| Stroke | 1689 (2.5%) | 16 982 (2.5%) |
| Other cerebrovascular diseases | 992 (1.4%) | 10 092 (1.5%) |
| Atrial fibrillation or atrial flutter | 1948 (2.8%) | 16 319 (2.4%) |
| Pulmonary embolism and infarction | 342 (0.5%) | 3010 (0.4%) |
| Phlebitis and thrombophlebitis | 785 (1.1%) | 7124 (1.0%) |
| Portal vein thrombosis | 10 (0.0%) | 89 (0.0%) |
| Other venous embolism and thrombosis | 130 (0.2%) | 1020 (0.1%) |
| Charlson Comorbidity Index, n (%) | ||
| None (Score = 0) | 54 398 (79%) | 547 371 (80%) |
| Low (Score = 1) | 8760 (13%) | 88 909 (13%) |
| Medium (Score = 2) | 3326 (4.8%) | 30 579 (4.4%) |
| High (Score ≥ 3) | 2332 (3.4%) | 21 301 (3.1%) |
| Highest achieved education, n (%) | ||
| Basic (7‐10 years) | 198 (0.3%) | 3236 (0.5%) |
| Medium (11‐12 years) | 48 315 (70%) | 495 434 (72%) |
| Higher (≥13 years) | 18 845 (27%) | 174 428 (25%) |
| Unknown | 1458 (2.1%) | 15 062 (2.2%) |
Abbreviation: IQR, InterQuartile Range.
Associations between antiplatelet drug use and breast cancer diagnosis are presented in Table 2. In age and calendar time‐adjusted models, ever use of low‐dose aspirin or dipyridamole, compared with never use, was not associated with breast cancer risk (low‐dose aspirin: OR = 1.01 [95% CI, 0.99 to 1.04] and dipyridamole: OR = 1.00 [95% CI, 0.94 to 1.07]). Ever use of clopidogrel was associated with lower breast cancer risk (OR = 0.91 [95% CI, 0.86 to 0.97]). Adjustment for measured potential confounders had little influence on the magnitude of the estimates (low‐dose aspirin: OR = 1.00 [95% CI, 0.97 to 1.03], clopidogrel: OR = 0.93 [95% CI, 0.86 to 1.00]; dipyridamole: OR = 1.02 [95% CI, 0.94 to 1.10]).
TABLE 2.
Associations between antiplatelet drug use and invasive breast cancer risk
| n cases | n controls | OR (95% CI) a | OR (95% CI) b | |
|---|---|---|---|---|
| Low‐dose aspirin | ||||
| Use categories | ||||
| Never use | 60 268 | 603 363 | 1.00 (ref.) | 1.00 (ref.) |
| Ever use | 8548 | 84 797 | 1.01 (0.99‐1.04) | 1.00 (0.97‐1.03) |
| Long‐term use | 5186 | 50 867 | 1.02 (0.99‐1.06) | 1.00 (0.97‐1.04) |
| Cumulative DDDs | ||||
| Never use | 60 268 | 603 363 | 1.00 (ref.) | 1.00 (ref.) |
| <500 | 1822 | 17 895 | 1.02 (0.97‐1.07) | 1.00 (0.95‐1.06) |
| ≥500 to <1000 | 1540 | 16 035 | 0.96 (0.91‐1.02) | 0.95 (0.90‐1.00) |
| ≥1000 to <2000 | 2197 | 21 785 | 1.01 (0.97‐1.06) | 1.00 (0.95‐1.05) |
| ≥2000 to <3000 | 1437 | 14 046 | 1.03 (0.97‐1.09) | 1.02 (0.96‐1.08) |
| ≥3000 | 1552 | 15 036 | 1.04 (0.98‐1.09) | 1.03 (0.97‐1.09) |
| OR per 1000 DDDs | 8548 | 84 797 | 1.01 (1.00‐1.02) | 1.01 (0.99‐1.02) |
| Clopidogrel | ||||
| Use categories | ||||
| Never use | 67 737 | 676 397 | 1.00 (ref.) | 1.00 (ref.) |
| Ever use | 1079 | 11 763 | 0.91 (0.86‐0.97) | 0.93 (0.87–1.00) |
| Long‐term use | 287 | 2981 | 0.96 (0.85‐1.08) | 0.96 (0.85‐1.09) |
| Cumulative DDDs | ||||
| Never use | 67 737 | 676 397 | 1.00 (ref.) | 1.00 (ref.) |
| <500 | 533 | 6226 | 0.85 (0.78‐0.93) | 0.87 (0.79–0.96) |
| ≥500 to <1000 | 259 | 2556 | 1.01 (0.89‐1.15) | 1.02 (0.89‐1.16) |
| ≥1000 to <2000 | 196 | 1947 | 1.00 (0.87‐1.16) | 1.01 (0.87‐1.17) |
| ≥2000 to <3000 | 60 | 599 | 1.00 (0.76‐1.30) | 1.00 (0.77‐1.31) |
| ≥3000 | 31 | 435 | 0.71 (0.49‐1.02) | 0.71 (0.49‐1.02) |
| OR per 1000 DDDs | 1079 | 11 763 | 0.96 (0.91‐1.01) | 0.97 (0.91‐1.03) |
| Dipyridamole | ||||
| Use categories | ||||
| Never use | 67 792 | 677 946 | 1.00 (ref.) | 1.00 (ref.) |
| Ever use | 1024 | 10 214 | 1.00 (0.94‐1.07) | 1.02 (0.94–1.10) |
| Long‐term use | 527 | 5205 | 1.01 (0.92‐1.11) | 1.03 (0.93‐1.14) |
| Cumulative DDDs | ||||
| Never use | 67 792 | 677 946 | 1.00 (ref.) | 1.00 (ref.) |
| <500 | 316 | 3204 | 0.99 (0.88‐1.11) | 0.99 (0.87‐1.11) |
| ≥500 to <1000 | 181 | 1805 | 1.00 (0.86‐1.17) | 1.02 (0.87‐1.20) |
| ≥1000 to <2000 | 250 | 2455 | 1.02 (0.89–1.16) | 1.04 (0.91‐1.19) |
| ≥2000 to <3000 | 145 | 1531 | 0.95 (0.80‐1.12) | 0.96 (0.81‐1.15) |
| ≥3000 | 132 | 1219 | 1.08 (0.90‐1.30) | 1.12 (0.93‐1.35) |
| OR per 1000 DDDs | 1024 | 10 214 | 1.01 (0.98‐1.05) | 1.02 (0.98‐1.06) |
Abbreviations: CI, confidence interval; DDD, defined daily dose; OR, Odds ratio.
Adjusted for age and calendar time (by risk‐set matching and the conditional analysis).
Adjusted for age and calendar time (by risk‐set matching and the conditional analysis) and covariates listed in the Data S3.
Compared to never use, long‐term use (≥1000 DDDs) of each antiplatelet drug was not associated with breast cancer risk (low‐dose aspirin: OR = 1.00 [95% CI, 0.97 to 1.04], clopidogrel: OR = 0.96 [95% CI, 0.85 to 1.09]; dipyridamole: OR = 1.03 [95% CI, 0.93 to 1.14]).
Analyses according to number of DDDs revealed an inverse association between short‐term clopidogrel use (<500 DDDs) and breast cancer risk (OR = 0.87 [0.79‐0.96]). However, this was not apparent for other dose categories and there was no evidence of a dose response relationship (ORper1000DDDs = 0.97 [95% CI, 0.91 to 1.03]). Similarly, there was no evidence of a dose response trend for other antiplatelet drugs (low‐dose aspirin: ORper1000DDDs = 1.01 [95% CI, 0.99 to 1.02]; dipyridamole: ORper1000DDDs = 1.02 [95% CI, 0.98 to 1.06]).
The associations between long‐term use of dipyridamole and breast cancer risk differed by age (p homogeneity = <.01, Table 3) suggesting an inverse association among women aged <55 years (OR = 0.53 [95% CI, 0.30 to 0.95]) but not among women aged between 55 and 69 years old (OR = 1.08 [95% CI, 0.92 to 1.28]) or women aged ≥70 years old (OR = 1.05 [95% CI, 0.92 to 1.19]). Among women aged <55 years old, there was a dose‐response relationship between dipyridamole and breast cancer risk (ORper 1000 DDDs = 0.71 [0.53‐0.95]). Associations between breast cancer risk and low‐dose aspirin or clopidogrel did not differ by age (p homogeneity ≥ .05).
TABLE 3.
Associations of antiplatelet drug use with risk of invasive breast cancer, stratified by age at index date
| Age < 55 years | Age ≥ 55 and <70 years | Age ≥ 70 years | p heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n cases | n controls | OR (95% CI) a | n cases | n controls | OR (95% CI) a | n cases | n controls | OR (95% CI) a | ||
| Low‐dose aspirin | ||||||||||
| Use categories | ||||||||||
| Never use | 19 788 | 197 360 | 1.00 (ref.) | 27 448 | 274 983 | 1.00 (ref.) | 13 032 | 131 020 | 1.00 (ref.) | |
| Ever use | 401 | 4530 | 0.95 (0.85‐1.07) | 3228 | 31 777 | 0.98 (0.94‐1.03) | 4919 | 48 490 | 1.00 (0.96‐1.05) | .64 |
| Long‐term use | 130 | 1765 | 0.81 (0.66‐0.99) | 1816 | 17 714 | 0.99 (0.93‐1.05) | 3240 | 31 388 | 1.02 (0.97‐1.07) | .05 |
| Cumulative DDDs | ||||||||||
| Never use | 19 788 | 197 360 | 1.00 (ref.) | 27 448 | 274 983 | 1.00 (ref.) | 13 032 | 131 020 | 1.00 (ref.) | |
| <500 | 181 | 1816 | 1.04 (0.89‐1.22) | 765 | 7600 | 0.97 (0.90‐1.05) | 876 | 8479 | 1.02 (0.94‐1.09) | |
| ≥500 to <1000 | 90 | 949 | 1.02 (0.82‐1.28) | 647 | 6463 | 0.97 (0.89‐1.05) | 803 | 8623 | 0.92 (0.85‐0.99) | |
| ≥1000 to <2000 | 80 | 1029 | 0.84 (0.66‐1.07) | 849 | 8311 | 0.99 (0.92‐1.07) | 1268 | 12 445 | 1.01 (0.95‐1.08) | |
| ≥2000 to <3000 | 29 | 471 | 0.67 (0.46‐0.99) | 504 | 4979 | 0.98 (0.89‐1.08) | 904 | 8596 | 1.05 (0.97‐1.13) | |
| ≥3000 | 21 | 265 | 0.85 (0.54‐1.33) | 463 | 4424 | 1.01 (0.91‐1.12) | 1068 | 10 347 | 1.04 (0.97‐1.12) | |
| OR per 1000 DDDs | 401 | 4530 | 0.92 (0.84‐1.00) | 3228 | 31 777 | 1.00 (0.98‐1.02) | 4919 | 48 490 | 1.01 (0.99‐1.03) | |
| Clopidogrel | ||||||||||
| Use categories | ||||||||||
| Never use | 20 120 | 201 096 | 1.00 (ref.) | 30 285 | 302 584 | 1.00 (ref.) | 17 332 | 172 717 | 1.00 (ref.) | |
| Ever use | 69 | 794 | 1.00 (0.75‐1.32) | 391 | 4176 | 0.95 (0.85–1.07) | 619 | 6793 | 0.92 (0.83‐1.01) | .80 |
| Long‐term use | 18 | 166 | 1.28 (0.77‐2.12) | 107 | 982 | 1.07 (0.87‐1.32) | 162 | 1833 | 0.88 (0.75‐1.05) | .24 |
| Cumulative DDDs | ||||||||||
| Never use | 20 120 | 201 096 | 1.00 (ref.) | 30 285 | 302 584 | 1.00 (ref.) | 17 332 | 172 717 | 1.00 (ref.) | |
| <500 | 42 | 459 | 1.07 (0.75‐1.51) | 191 | 2321 | 0.85 (0.73‐1.00) | 300 | 3446 | 0.87 (0.77‐0.99) | |
| ≥500 to <1000 | 9 | 169 | 0.60 (0.30‐1.18) | 93 | 873 | 1.06 (0.85‐1.32) | 157 | 1514 | 1.04 (0.88‐1.24) | |
| ≥1000 to <2000 | 17 | 110 | 1.74 (1.03‐2.96) | 72 | 683 | 1.03 (0.80–1.32) | 107 | 1154 | 0.94 (0.76‐1.15) | |
| ≥2000 to <3000 | < 5 | 39 | (−) | 23 | 166 | 1.37 (0.88‐2.13) | 36 | 394 | 0.92 (0.65‐1.31) | |
| ≥3000 | < 5 | 17 | (−) | 12 | 133 | 0.89 (0.49‐1.61) | 19 | 285 | 0.67 (0.42‐1.06) | |
| OR per 1000 DDDs | 69 | 794 | 0.89 (0.66‐1.18) | 391 | 4176 | 1.03 (0.94‐1.13) | 619 | 6793 | 0.95 (0.88‐1.02) | |
| Dipyridamole | ||||||||||
| Use categories | ||||||||||
| Never use | 20 141 | 201 316 | 1.00 (ref.) | 30 287 | 303 094 | 1.00 (ref.) | 17 364 | 173 536 | 1.00 (ref.) | |
| Ever use | 48 | 574 | 0.86 (0.62‐1.19) | 389 | 3666 | 1.03 (0.91‐1.17) | 587 | 5974 | 1.02 (0.93‐1.13) | .52 |
| Long‐term use | 12 | 226 | 0.51 (0.28‐0.94) | 209 | 1891 | 1.08 (0.92‐1.28) | 306 | 3088 | 1.05 (0.92‐1.19) | .005 |
| Cumulative DDDs | ||||||||||
| Never use | 20 141 | 201 316 | 1.00 (ref.) | 30 287 | 303 094 | 1.00 (ref.) | 17 364 | 173 536 | 1.00 (ref.) | |
| <500 | 26 | 222 | 1.20 (0.79‐1.84) | 115 | 1132 | 0.98 (0.80‐1.19) | 175 | 1850 | 0.97 (0.82‐1.14) | |
| ≥500 to <1000 | 10 | 126 | 0.82 (0.42‐1.58) | 65 | 643 | 1.00 (0.77–1.31) | 106 | 1036 | 1.07 (0.87‐1.31) | |
| ≥1000 to <2000 | 9 | 125 | 0.72 (0.36‐1.45) | 105 | 897 | 1.15 (0.93‐1.42) | 136 | 1433 | 1.00 (0.83‐1.20) | |
| ≥2000 to <3000 | < 5 | 73 | (−) | 55 | 566 | 0.94 (0.71‐1.25) | 87 | 892 | 1.03 (0.82‐1.29) | |
| ≥3000 | < 5 | 28 | (−) | 49 | 428 | 1.13 (0.83‐1.53) | 83 | 763 | 1.17 (0.92‐1.48) | |
| OR per 1000 DDDs | 48 | 574 | 0.71 (0.53–0.95) | 389 | 3666 | 1.03 (0.97‐1.10) | 587 | 5974 | 1.03 (0.98‐1.08) | |
Abbreviations: CI, confidence interval; DDD, defined daily dose; OR, Odds ratio.
Adjusted for age, calendar time (by risk‐set matching and the conditional analysis) and and covariates listed in the Data S3.
Overall, there was little evidence that associations between antiplatelet drugs and breast cancer differed by ER status (p homogeneity ≥ .08, Table S1). There was no evidence of associations with ever use of antiplatelets and the risk of breast cancer with unknown ER status (low‐dose aspirin: OR = 0.99 [95% CI, 0.93 to 1.05], clopidogrel: OR = 1.00 [95% CI, 0.89 to 1.13], dipyridamole: OR = 1.13 [95% CI, 0.98 to 1.31]; data not shown). In addition, the associations between antiplatelet drugs and breast cancer risk did not differ by breast cancer histological type (p homogeneity ≥ .10, Table S2), and stage (p homogeneity ≥ .06, Table S3). Changing the minimum latency period to 0 or 2 years instead of 1 year (main analysis) only marginally altered the estimates (Table S4). Our findings remained unchanged after restricting the study sample to women with a diagnosis of stroke or myocardial infarction (Table S5) or to women over the age of 55 years who were never users of hormone replacement therapy (Table S6). Overall, analyses of antiplatelets alone or of clopidogrel or dipyridamole used concomitantly with low‐dose aspirin revealed similar results (Table S7).
Weak positive associations were observed between ever and long‐term use of Vitamin K antagonists and breast cancer risk with no evidence of a dose‐response relation (Table S8).
4. DISCUSSION
In this large registry‐based case‐control study, overall we did not observe strong evidence of association between antiplatelet drugs and breast cancer risk. While we did observe a lower breast cancer risk with short‐term use of clopidogrel (<500 DDD, that is, approximately <1.5 year), there was no evidence of a dose response relationship. There was no association between long‐term use of any antiplatelet drug and breast cancer risk. In sub‐group analyses, we found that dipyridamole was associated with a lower breast cancer risk only among women aged <55 years old, with some evidence for a dose‐response relationship.
In our study, we found no association between low‐dose aspirin and breast cancer risk, overall and by subgroups. Our results on low‐dose aspirin are consistent with two randomized controlled trials suggesting that its use (at a 100 mg daily dose) had no effect on breast cancer risk compared to placebo (observational follow‐up of the Women's Health Study: HR = 1.02 95%CI 0.89‐1.18, 385 exposed cases 4 ; and the ASPREE trial: HR = 1.03 95%CI 0.80‐1.32, 127 exposed cases 3 ). In contrast to our results, two recent meta‐analyses of observational studies suggested that a long duration of any aspirin use was associated with a lower breast cancer risk. 2 , 22 However, interpretation of these results was difficult because of high heterogeneity in terms of exposure definition and study design. The protective effect of aspirin on breast cancer was supported mostly in studies with selection and recall biases due to their retrospective designs. 2 , 22 Furthermore, these meta‐analyses did not distinguish between low‐dose and high‐dose aspirin. Among those prospective studies which have examined low‐dose aspirin, 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 four noted no association with breast cancer risk, 26 , 27 , 28 , 29 three noted a lower breast cancer risk with long‐term exposure 24 , 25 , 26 and one reported an higher breast cancer risk. 30 A recent study performed by our group using data from the E3N cohort suggested that use of low‐dose aspirin was associated with a transient higher risk of postmenopausal breast cancer few years after treatment start, followed by a lower risk after 4 years of treatment. 6 However, the E3N cohort includes women insured by a health insurance scheme that covers mainly teachers and results from this population cannot be directly extrapolated to other populations.
To our knowledge, the only previous epidemiological study evaluating the association between clopidogrel and breast cancer was performed by our group. 6 In the E3N study, clopidogrel use was associated with a higher ER− breast cancer risk, with no clear trend according to duration of use. This result was based on a limited number of ER− breast cancer cases (ever exposed n = 23) and, to the best of our knowledge, we are not aware of any biological mechanism that may explain this association. Our current study, which included 132 ER− breast cancer cases exposed to clopidogrel, found that women exposed to clopidogrel were not at higher risk for ER− breast cancer than nonexposed women. However, while there was some evidence that use of clopidogrel may be associated with small reductions in the odds of breast cancer, this was observed for very short‐term use and no evidence of a dose‐response was observed.
To our knowledge, this is the first study to evaluate the association between dipyridamole use and breast cancer risk. Overall results suggest null associations; however, subgroup analysis by age did suggest an inverse association only among women aged <55 years old, with evidence of a dose response. However, because our findings were based on relatively small numbers of exposed cases (n = 48) and since we performed a relatively large number of tests, these results may be due to chance and should be interpreted carefully before replication in other settings.
This was the largest study to evaluate the antiplatelet drugs‐breast cancer associations to date. The principal strength of the present study is the use of nationwide registries of high validity, 7 , 31 with complete coverage of an entire nation, that allowed us to capture histologically verified breast cancer cases and risk‐set sampling of controls with low risk of selection bias. In addition, the prospective design with the use of information from a drug prescription database to identify antiplatelet drug exposure for up to 23 years allowed us to minimize differential recall bias between cases and noncases and to consider precise information on exposure. We were also able to adjust our models for socioeconomic parameters, use of other drugs and comorbidities.
Despite these strengths, this study also had a number of limitations. Firstly, there is the potential for exposure misclassification for those considered unexposed to aspirin due to a lack of information available on over‐the‐counter low‐dose aspirin purchases. However, misclassification is likely to be minimal as, in Denmark, the prescribed proportion of low‐dose aspirin varied between 60% and 87% from 1999 to 2007 and remained around 90% from 2006, with evidence suggesting the influence on study estimates to be negligible. 32 In addition, we assumed that the long‐term use of low‐dose aspirin is primarily managed through prescriptions due to the need for medical surveillance and possibility of financial reimbursement. Misclassification for clopidogrel or dipyridamole treatments are unlikely because these are only available upon prescription in Denmark. While we had no data regarding compliance and adherence to dispensed antiplatelets, it is likely that this may be less of a concern among long‐term users of antiplatelets. Then, we were not able to consider medical follow‐up in our analyses which might mask any decreased breast cancer risk associated with antiplatelet drug use. Further, we used chronic obstructive pulmonary disease and alcohol‐related diseases as proxies for heavy smoking and alcohol consumption, but residual confounding may remain due to lack of information on these factors. We also had no data on other risk factors for breast cancer, including obesity, physical activity and parity. These factors might also be associated with use of antiplatelet drugs either positively or inversely, and uncontrolled confounding from these factors might bias our findings towards the null. Finally, the latest meta‐analysis of randomized controlled trials on aspirin and cancer risk reported that aspirin's effects on cancer might differ by body size. 5 However, as data was not available, we were unable to stratify our analyses by that factor.
5. CONCLUSION
The findings from this large nationwide nested case‐control study add to the growing evidence from randomized controls trials that low‐dose aspirin does not appear to be a suitable pharmacological candidate for breast cancer prevention. Further, our results do not provide strong support to the use of other antiplatelet drugs for breast cancer prevention.
AUTHOR CONTRIBUTIONS
Anton Pottegård and Morten Olesen analyzed the data. Manon Cairat and Blánaid Hicks were responsible for drafting the manuscript. Anton Pottegård, Agnès Fournier and Laure Dossus provided advice on the analysis and interpretation of the results. All authors read and approved the final manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
CONFLICT OF INTEREST
Anton Pottegård reports participation in research projects funded by Alcon, Almirall, Astellas, Astra‐Zeneca, Boehringer‐Ingelheim, Novo Nordisk, Servier and LEO Pharma, all regulator‐mandated phase IV‐studies, all with funds paid to the institution where he was employed (no personal fees) and with no relation to the work reported in this paper. The other authors declare no conflict of interest.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Supporting information
DATA S1 Danish Nationwide Registries.
DATA S2 Codes and definitions of outcome, exposure and exclusion criteria.
DATA S3 Codes and definitions of covariates.
TABLE S1 Associations of antiplatelet drug use with risk of invasive breast cancer, stratified by estrogen receptor status.
TABLE S2 Associations of antiplatelet drug use with risk of invasive breast cancer by histological type.
TABLE S3 Associations of antiplatelet drug use with risk of invasive breast cancer by tumor stage.
TABLE S4 Association between antiplatelet drug use and invasive breast cancer risk with exposure with no lag period, 1‐year lag and 2‐year lag.
TABLE S5 Associations between antiplatelet drug use and invasive breast cancer risk restricted to women with a diagnosis of stroke or myocardial infarction.
TABLE S6 Associations between antiplatelet drug use and breast cancer risk among women who are >55 years and never users of hormone replacement therapy.
TABLE S7 Association between breast cancer risk and antiplatelet drugs taken alone or in combination with low‐dose aspirin.
TABLE S8 Associations between Vitamin K antagonists use and invasive breast cancer risk.
ACKNOWLEDGEMENT
The work reported in this paper was performed during Agnès Fournier's term as a Visiting Scientist at the International Agency for Research on Cancer.
Cairat M, Pottegård A, Olesen M, Dossus L, Fournier A, Hicks B. Antiplatelet drugs and breast cancer risk in a large nationwide Danish case‐control study. Int J Cancer. 2023;152(7):1337‐1347. doi: 10.1002/ijc.34343
DATA AVAILABILITY STATEMENT
This study is based on anonymized registry data located on a secure platform at Statistics Denmark, which can be accessed given the relevant data permits. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Patrignani P, Patrono C. Aspirin, platelet inhibition and cancer prevention. Platelets. 2018;29(8):779‐785. [DOI] [PubMed] [Google Scholar]
- 2. Cao Y, Tan A. Aspirin might reduce the incidence of breast cancer: an updated meta‐analysis of 38 observational studies. Medicine (Baltimore). 2020;99(38):e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNeil JJ, Gibbs P, Orchard SG, et al. Effect of aspirin on cancer incidence and mortality in older adults. J Natl Cancer Inst. 2021;113(3):258‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate‐day, low‐dose aspirin and cancer risk: long‐term observational follow‐up of a randomized trial. Ann Intern Med. 2013;159(2):77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet Lond Engl. 2018;392(10145):387‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cairat M, Al Rahmoun M, Gunter MJ, Severi G, Dossus L, Fournier A. Antiplatelet drug use and breast cancer risk in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2021;30(4):643‐652. [DOI] [PubMed] [Google Scholar]
- 7. Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42‐45. [DOI] [PubMed] [Google Scholar]
- 8. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7_suppl):38‐41. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health. 2011;39(7 Suppl):91‐94. [DOI] [PubMed] [Google Scholar]
- 11. Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72‐74. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541‐549. [DOI] [PubMed] [Google Scholar]
- 13. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22‐25. [DOI] [PubMed] [Google Scholar]
- 14. Thygesen LC, Daasnes C, Thaulow I, Brønnum‐Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7_suppl):12‐16. [DOI] [PubMed] [Google Scholar]
- 15. Rothman KJ, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins. 3rd ed. Philadelphia: Wolters Kluwer Health; 2008. [Google Scholar]
- 16. Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet Lond Engl. 2015;386(9990):281‐291. [DOI] [PubMed] [Google Scholar]
- 17. Pottegård A, Hallas J. New use of prescription drugs prior to a cancer diagnosis. Pharmacoepidemiol Drug Saf. 2017;26(2):223‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of patients. BMC Med Res Methodol. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serebruany VL, Cherepanov V, Golukhova EZ, Kim MH. The dual antiplatelet therapy trial after the FDA update: noncardiovascular deaths, cancer and optimal treatment duration. Cardiology. 2015;132(2):74‐80. [DOI] [PubMed] [Google Scholar]
- 21. Smeda M, Kieronska A, Proniewski B, et al. Dual antiplatelet therapy with clopidogrel and aspirin increases mortality in 4T1 metastatic breast cancer‐bearing mice by inducing vascular mimicry in primary tumour. Oncotarget. 2018;9(25):17810‐17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santucci C, Gallus S, Martinetti M, La Vecchia C, Bosetti C. Aspirin and the risk of nondigestive tract cancers: an updated meta‐analysis to 2019. Int J Cancer. 2021;148(6):1372‐1382. [DOI] [PubMed] [Google Scholar]
- 23. Ready A, Velicer CM, McTiernan A, White E. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat. 2008;109(3):533‐543. [DOI] [PubMed] [Google Scholar]
- 24. Clarke CA, Canchola AJ, Moy LM, et al. Regular and low‐dose aspirin, other non‐steroidal anti‐inflammatory medications and prospective risk of HER2‐defined breast cancer: the California Teachers Study. Breast Cancer Res. 2017;19(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang YS, Kornelius E, Chiou JY, et al. Low‐dose aspirin reduces breast cancer risk in women with diabetes: a nationwide retrospective cohort study in Taiwan. J Womens Health. 2002;26(12):1278‐1284. [DOI] [PubMed] [Google Scholar]
- 26. Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti‐inflammatory drugs: prospective results from the Women's health initiative. Cancer Res. 2003;63(18):6096‐6101. [PubMed] [Google Scholar]
- 27. Ajrouche A, De Rycke Y, Dalichampt M, et al. Reduced risk of cancer among low‐dose aspirin users: data from French health care databases. Pharmacoepidemiol Drug Saf. 2019;28(9):1258‐1266. [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Shore DL, Wilson LE, et al. Lifetime use of nonsteroidal anti‐inflammatory drugs and breast cancer risk: results from a prospective study of women with a sister with breast cancer. BMC Cancer. 2015;15:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollestein LM, van Herk‐Sukel MPP, Ruiter R, et al. Incident cancer risk after the start of aspirin use: results from a Dutch population‐based cohort study of low dose aspirin users. Int J Cancer. 2014;135(1):157‐165. [DOI] [PubMed] [Google Scholar]
- 30. Tsoi KKF, Ho JMW, Chan FCH, Sung JJY. Long‐term use of low‐dose aspirin for cancer prevention: a 10‐year population cohort study in Hong Kong. Int J Cancer. 2019;145(1):267‐273. [DOI] [PubMed] [Google Scholar]
- 31. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaster N, Hallas J, Pottegård A, Friis S, Schmidt M. The validity of Danish prescription data to measure use of aspirin and other non‐steroidal anti‐inflammatory drugs and quantification of bias due to non‐prescription drug use. Clin Epidemiol. 2021;13:569‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1 Danish Nationwide Registries.
DATA S2 Codes and definitions of outcome, exposure and exclusion criteria.
DATA S3 Codes and definitions of covariates.
TABLE S1 Associations of antiplatelet drug use with risk of invasive breast cancer, stratified by estrogen receptor status.
TABLE S2 Associations of antiplatelet drug use with risk of invasive breast cancer by histological type.
TABLE S3 Associations of antiplatelet drug use with risk of invasive breast cancer by tumor stage.
TABLE S4 Association between antiplatelet drug use and invasive breast cancer risk with exposure with no lag period, 1‐year lag and 2‐year lag.
TABLE S5 Associations between antiplatelet drug use and invasive breast cancer risk restricted to women with a diagnosis of stroke or myocardial infarction.
TABLE S6 Associations between antiplatelet drug use and breast cancer risk among women who are >55 years and never users of hormone replacement therapy.
TABLE S7 Association between breast cancer risk and antiplatelet drugs taken alone or in combination with low‐dose aspirin.
TABLE S8 Associations between Vitamin K antagonists use and invasive breast cancer risk.
Data Availability Statement
This study is based on anonymized registry data located on a secure platform at Statistics Denmark, which can be accessed given the relevant data permits. Further information is available from the corresponding author upon request.


