Abstract
Background and Objective
Propofol is the most commonly used sedative in gastrointestinal endoscopic procedures, but is associated with cardiorespiratory suppression, particularly in elderly patients. Remimazolam is a new short‐acting GABA(A) receptor agonist with minimal impact on cardiorespiratory suppression, and may be a viable alternative in elderly patients undergoing endoscopic procedures.
Methods
This multicenter, randomized controlled trial was conducted between September 2020 and September 2021. Elderly patients (65–85 years of age) scheduled to undergo upper gastrointestinal endoscopy were randomized in 1:1 ratio to receive remimazolam tosilate (300 mg/h) or propofol (3 g/h) in addition to 50‐μg fentanyl, until the Modified Observer's Assessment of Alertness/Sedation Scale (MOAA/S) reached ≤1. MOAA/S was maintained at 0 or 1 throughout the procedure using 2.5 mg remimazolam or 0.5 mg/kg propofol boluses in the two groups, respectively. The primary outcome was the rate of hypotension (defined as systolic blood pressure at ≤90 mmHg or > 30% decline vs. the baseline). Bradycardia was defined as heart rate ≤50 per minute; respiratory depression was defined as respiratory rate <8 per minute and/or SpO2 < 90%.
Results
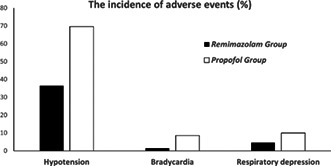
A total of 400 patients (161 men and 239 women; 70.4 ± 4.6 years of age) were enrolled (200 patients per group). Average body mass index was 22.2 ± 2.4 kg/m2. The rate of hypotension was 36.5% in the remimazolam group and 69.6% in the propofol group (p < 0.001). The remimazolam group also had a lower rate of bradycardia (1.5% vs. 8.5%, p < 0.001), respiratory depression (4.5% vs. 10.0%, p < 0.05) and pain at the injection site (0% vs. 12.0%, p < 0.001).
Conclusion
Remimazolam was associated with a lower rate of hypotension in elderly patients undergoing upper gastrointestinal endoscopy under deep sedation/anaesthesia than propofol.
Keywords: elderly patients, propofol, remimazolam, sedation, upper gastrointestinal endoscopy
The rate of hypotension was 36.5% in the remimazolam group and 69.6% in the propofol group (p < 0.001). The remimazolam group also had a lower rate of bradycardia (1.5% vs. 8.5%, p < 0.001) and respiratory depression (4.5% vs. 10.0%, p < 0.05).
1. INTRODUCTION
Propofol is the most commonly used sedative in patients undergoing upper gastrointestinal endoscopy, 1 , 2 , 3 but is associated with circulatory and respiratory suppression, 4 , 5 , 6 particularly in elderly patients. 7 , 8 , 9 , 10 Remimazolam is a short‐acting GABA(A) receptor agonist. 11 It has been shown to be safe and effective for procedural sedation in several clinical trials, 12 , 13 , 14 especially in upper gastrointestinal endoscopy. 15
Metabolism of remimazolam is independent of liver and kidney function, 16 and thus is not prone to accumulation and respiratory and circulatory inhibition. In a phase III trial in adult patients (18–60 years of age) that compared remimazolam with propofol, the incidence of hypotension was lower with remimazolam than propofol. 15 Few studies compared remimazolam and propofol in elderly patients undergoing endoscopic procedures. We speculated that remimazolam may be particularly useful in elderly patients receiving endoscopic procedures, and conducted a randomized controlled trial to test this hypothesis.
2. METHODS
2.1. Patients eligibility
This multicenter, randomized controlled trial was conducted between September 2020 and September 2021 at the Third Affiliated Hospital of Guangxi Medical University, Hechi Third People's Hospital and Liuzhou Municipal Liutie Central Hospital. Elderly patients (65–85 years of age) scheduled to undergo upper gastrointestinal endoscopy were eligible. Exclusion criteria included: (1) American Society of Anesthesiologists (ASA) physical status IV or higher; (2) a body mass index (BMI) below 18 or over 30 kg/m2; (3) requirement for tracheal intubation or difficult airways (Mallampati score of 3 or 4); (4) acute respiratory infection, asthma attack, uncontrolled hypertension (systolic blood pressures [SBP] ≥160 mmHg or diastolic blood pressure (DBP) ≥100 mmHg despite medical treatment) or hypotension (SBP ≤90 mmHg or DBP ≤60 mmHg); (5) haemoglobin <80 g/L; (6) suspected acute upper gastrointestinal bleeding, acute gastrointestinal perforation, gastrointestinal obstruction, or gastric retention; (7) a history of drug abuse and/or alcoholism within 2 years before screening; (8) a history of psychiatric disorders; (9) a known allergy to benzodiazepines, opioids, propofol, soy or a contraindication to receiving these medications; (10) participation in other clinical trials within the past 3 months; (11) expected procedure time at >30 min; (12) any other reason deemed not appropriate for this trial by the investigator (e.g., expected difficulty to physically attend the scheduled follow‐up).
The study protocol was approved by the institutional review board of all three participating centers. Written informed consent was obtained from all participants before the start of any protocol‐specified procedures. This trial was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization of Good Clinical Practice and is registered with www.chictr.org.cn (18/08/2020, #ChiCTR2000035824).
2.2. Randomization and masking
Randomization was conducted with a block design using a centralized service (www.medresman.org.cn). Patients were randomized in a 1:1 ratio to receive remimazolam or propofol prior to gastrointestinal endoscopy. Investigational drugs were prepared by the attending anesthesiologists, and covered with opaque bags to achieve blinding (both the patients and outcome assessors).
2.3. Procedures
After an overnight fast and 2‐h water restriction, patients received 50‐μg fentanyl citrate by intravenous infusion. All patients received Ringer's lactate solution (2 ml/kg/h) throughout the procedure. Patients received remimazolam tosilate (HengRui Medicine Co., Ltd., China) at a rate of 300 mg/h or propofol (Aspen) at a rate of 3.0 g/h using a syringe pump until the Modified Observer's Assessment of Alertness/Sedation (MOAA/S) 17 score reached 1. Vita signs (including respiration, heart rate, blood pressure and SpO2) were monitored immediately prior to drug infusion, at 2 min after the initiation of drug infusion, and then at 3‐min interval. MOAA/S was determined immediately prior to drug infusion, every 30 s during the first 3 min, and then every 60 s until the patients regained consciousness (MOAA/S of 5). MOAA/S score was maintained at ≤1 throughout the procedure by bolus injection of either remimazolam tosilate (2.5 mg) or propofol (0.5 mg/kg) with at least 1‐min interval between the boluses; there was no limitation on the total dosage.
Supplemental oxygen (2–4 L/min) was provided via a nasal tube until the patient was fully awake and resumed normal breathing. Patients were observed in the post‐anaesthesia care unit (PACU) for at least half an hour after the completion of the procedure. Patients achieving a total Post Anaesthetic Discharge Scoring System (PADSS) score of 9 or 10 were considered fit for transfer or discharge to the next phase of recovery. 18 Hypotension was managed with rapid infusion of Ringer's lactate solution and/or vasopressors as appropriate by the attending anesthesiologists. Hypoxemia (SpO2 < 90%) was managed by jaw thrust manoeuvre and/or increase of oxygen flow, as appropriate. All patient management was decided at the discretion of the attending anesthesiologist (not blinded to group allocation).
2.4. Outcomes
The primary outcome was the rate of hypotension, defined as SBP ≤90 mmHg or a greater than 30% decline from the baseline. Baseline vital signs were collected when the patients entered the endoscopy room and before fentanyl injection. Secondary outcomes included bradycardia (heart rate ≤ 50 per minute), respiratory depression (respiratory rate <8 per minute and/or SpO2 < 90%), time to adequate sedation (MOAA/S score ≤1), procedure time (from the start of the procedure to endoscope removal), recovery time (from discontinuation of sedative use to the first of three consecutive MOAA/S scores of 5), and sedation time (from the start of intravenous infusion of sedative agent to fully alert). All outcomes were assessed by an anesthesiologist not otherwise involved in the study.
2.5. Safety
Adverse events (AEs) were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, and included pain at the injection site, nausea, vomiting, dizziness, inability to ambulate and delirium.
2.6. Efficacy
Sedation success was defined as no rescue sedation with a sedative agent other than the assigned treatment to maintain MOAA/S ≤ 1 throughout the procedure. Procedure success was defined as completion of the scheduled endoscopy procedure.
2.7. Sample size and statistical analysis
Sample size calculation was based on the following assumptions: (1) hypotension in 13/20 (65%) of the patients receiving propofol versus 8/20 (40%) in patients receiving remimazolam tosilate (based on our preliminary study that 40 cases in total, 20 cases in each group); (2) 2‐sided α of 0.05 and a power of 0.8; (3) a dropout rate of 20%. The calculation yielded 200 subjects in each group.
All statistical analyses were conducted using SPSS version 22.0. Normally distributed continuous variables were presented as mean ± standard deviation and analysed using Student's t test. Non‐normally distributed continuous variables were expressed as median (interquartile range) and analysed using Mann–Whitney U test. Categorical variables were analysed using chi‐square test. Analysis of the primary outcome included all patients who underwent randomization and received a dose of the study drug, underwent the endoscopy procedure, and had at least one efficacy assessment. Statistically significant difference was defined as p < 0.05 (2‐sided).
3. RESULTS
3.1. Patient demographic and baseline characteristics
Patient flow through the trial is shown in Figure 1. A total of 461 patients were screened for eligibility and 400 patients were randomized (200 patients in each group). Demographic and baseline variables are shown in Table 1.
FIGURE 1.
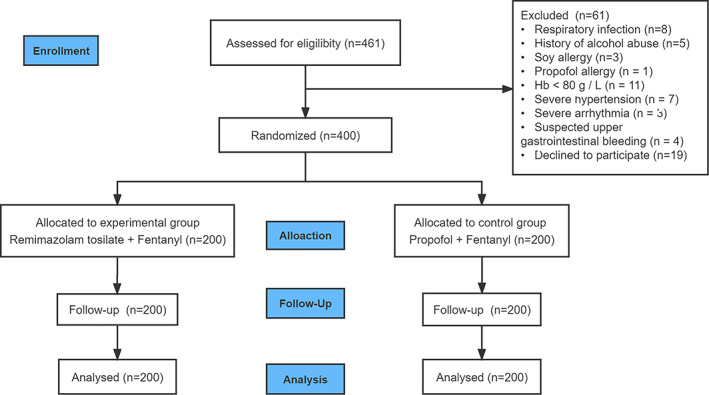
Flow diagram of the study. A total of 461 patients were screened for eligibility, 42 patients were excluded because they did not meet the inclusion criteria, 19 patients refused to participate in the trial and 400 patients were randomized (200 patients in each group).
TABLE 1.
Patient demographics and baseline characteristics
| Variables | Remimazolam (n = 200) | Propofol (n = 200) |
|---|---|---|
| Male sex, n (%) | 78 (39.0) | 83 (46.5) |
| Age (years), mean (SD) | 70.6(4.7) | 70.1(4.5) |
| Weight (kg), mean (SD) | 56.2(8.7) | 56.4(8.8) |
| Body mass index (kg/m2), mean (SD) | 22.2(2.5) | 22.2(2.3) |
| ASA class, n (%) | ||
| I | 17 (8.5) | 6 (3.0) |
| II | 181 (90.5) | 192 (96.0) |
| III | 2 (1.0) | 2 (1.0) |
| Co‐morbidities, n (%) | ||
| Hypertension | 62 (31.0) | 68 (34.0) |
| Diabetes | 14 (7.0) | 16 (8.0) |
| Haemoglobin (g/dl), mean (SD) | 12.2(1.7) | 12.6(1.6) |
| Systolic blood pressure (mmHg), mean (SD) | 133.9(13.7) | 134.8(12.7) |
| Diastolic blood pressure (mmHg), mean (SD) | 78.3(7.8) | 78.4(7.5) |
| Heart rate (beats/min), mean (SD) | 74.8(9.8) | 75.4(10.7) |
| Respiratory rate (breaths/min), mean (SD) | 19.8(0.8) | 19.8(0.6) |
| SPO2 (%), mean (SD) | 99.1(1.3) | 99.2(1.2) |
Abbreviations: ASA, American Society of Anesthesiologists; SD, standard deviation.
The rate of hypotension was 36.5% in the remimazolam group versus 69.6% the propofol group (p < 0.001) (Table 2). The rate of vasoactive drug use was 12.0% in remimazolam group versus 38.5% in propofol group (p < 0.001). The remimazolam group also had lower rate of bradycardia (1.5% vs. 8.5%; p = 0.001), respiratory depression (4.5% vs. 10.0%; p = 0.034), and lower rate of any AEs (41.0% vs. 70.5%; p < 0.001).
TABLE 2.
Summary of adverse events (AEs)
| AEs | Remimazolam (n = 200) | Propofol (n = 200) | p value |
|---|---|---|---|
| All AEs, n | 87 | 204 | / |
| Patients with AEs | 82 (41.0) | 141 (70.5) | <0.001 a |
| Specific AEs | / | ||
| Hypotension | 73 (36.5) | 139 (69.6) | <0.001 a |
| Bradycardia | 3 (1.5) | 17 (8.5) | 0.001 a |
| Respiratory depression | 9 (4.5) | 20 (10.0) | 0.034 a |
| Hypoxemia | 2 (1.0) | 4 (2.0) | 0.411 |
| Pain at injection site | 0 | 24 (12.0) | <0.001 a |
| Inability to ambulate | 0 | 0 | / |
| Nausea | 0 | 0 | / |
| Vomiting | 0 | 0 | / |
| Dizziness | 0 | 0 | / |
| Delirium | 0 | 0 | / |
| Vasoactive drug use | 24 (12.0) | 77 (38.5) | <0.001 a |
Note: Data are expressed as n(%). Hypotension was defined as systolic blood pressure ≤ 90 mmHg or greater than 30% decline from baseline; bradycardia was defined as a heart rate ≤ 50 per minute; respiratory depression is defined as a respiratory rate less than eight breaths per minute and/or SpO2 < 90%; hypoxemia was defined as SpO2 < 90%.
p < 0.05.
The induction and total doses of remimazolam were 10.7 ± 1.9 mg (range, 7.4–20.3 mg) and 13.9 ± 3.7 mg (range, 7.6–28.5 mg), respectively. The induction and total doses of propofol was 102.4 ± 13.9 mg (range, 58.3–170.0 mg) and 120.2 ± 31.5 mg (range, 58.3–301.6 mg), respectively. In a correlation analysis, higher body weight correlated with higher dosage of remimazolam and propofol (r = 0.249, p < 0.001 for remimazolam; r = 0.432, p < 0.001 for propofol). The rate of sedation and procedure success was 100% in both groups (Table 3).
TABLE 3.
Other outcomes
| Remimazolam (n = 200) | Propofol (n = 200) | p value | |
|---|---|---|---|
| Sedation success, n (%) | 200 (100) | 200 (100) | / |
| Procedure success, n (%) | 200 (100) | 200 (100) | / |
| Time to adequate sedation, min | 0.131 | ||
| Mean (SD) | 2.1 ± 0.4 | 2.1 ± 0.4 | |
| Range | (1.0, 4.0) | (1.5, 4.0) | |
| Procedure time, min | 0.663 | ||
| Mean (SD) | 10.8 ± 5.1 | 10.6 ± 4.7 | |
| Range | (2.0, 28.0) | (3.0, 29.0) | |
| Sedation time, min | 0.103 | ||
| Mean (SD) | 16.5 ± 5.2 | 15.7 ± 4.6 | |
| Range | (8.0, 32.0) | (8.0, 37.0) | |
| Time to fully alert, min | 0.143 | ||
| Mean (SD) | 9.3 ± 3.7 | 9.8 ± 3.7 | |
| Range | (2.0, 19.0) | (2.0, 28.0) |
Note: Sedation success was defined as no rescue sedation with a sedative agent other than the assigned treatment to maintain Modified Observer's Assessment of Alertness/Sedation (MOAA/S) ≤1 throughout the procedure. Procedure success was defined as completion of the scheduled endoscopy procedure.
Abbreviation: SD, standard deviation.
The time to adequate sedation was 2.1 ± 0.4 min (range, 1.0–4.0 min) and 2.1 ± 0.4 min (range, 1.5–4.0 min) in the remimazolam and propofol groups, respectively (p = 0.131). The two groups did not differ in procedural time (10.8 ± 5.1 vs. 10.6 ± 4.7 min; p = 0.663), sedation time (16.5 ± 5.2 vs. 15.7 ± 4.6 min; p = 0.103), and recovery time (9.3 ± 3.7 vs. 9.8 ± 3.7 min; p = 0.143).
4. DISCUSSION
Hypotension is common in endoscopic procedures that require deep sedation. 6 , 19 , 20 In this trial, the rate of hypotension was significantly lower in the remimazolam group than in the propofol control (36.5% vs. 69.6%, p < 0.001). The rate of bradycardia was also significantly lower in the remimazolam group (1.5% vs. 8.5%, p < 0.001). These results are generally consistent with the safety profile reported by a previous trial by Liu et al. 21
The incidence of hypotension (69.6%) in the propofol arm in this trial was higher than reported in previous studies (e.g., 42.86% in a phase III trial that compared remimazolam with propofol). 15 Such a discrepancy mostly likely reflects the older age of the patients in this trial, and highlights the concern of hypotension in elderly patients.
The use of propofol is hampered by cardiorespiratory suppression, particularly in patients with compromised liver and/or kidney functions due to drug retention. 22 The incidence and case fatality of postoperative complications are higher in elderly patients with diminished physical function and possibly with multiple chronic diseases. 4 , 23 Remimazolam can be rapidly hydrolyzed in vivo by non‐specific esterases to the pharmacologically inactive metabolite zolam propionate. 11 , 24 Remimazolam has an onset time of sedation between 1.5 and 2.5 min at 0.1–0.2 mg/kg, with minimal impact on respiration and circulation. 14 Previous studies in younger adults showed significantly lower rate of hypotension and hypoxemia with remimazolam than propofol. 11 , 25 , 26 Our study extended such findings to elderly patients undergoing gastrointestinal endoscopy under deep sedation.
Pambianco et al. found that patients who underwent colonoscopy with remimazolam had better circulatory and respiratory stability, and hypoxemia could be relieved by jaw lift without the need of mechanical or artificial ventilation. 12 The rate of respiratory depression is also significantly lower in patients receiving remimazolam tosilate versus propofol in emergency settings. 27 Another advantage of remimazolam is the rapid reversal of severe respiratory depression with inadvertent overdose by flumazenil. 28
The recommended dose of remimazolam tosilate in Chinese patients is 5 mg for sedation induction in gastroscopy in adults, with 96% success rate in a phase III trial. 15 In clinical practice, however, such a dose may be inadequate for sufficient sedation in a subset of patients. In a study in women undergoing hysterectomy by Zhang et al., the success sedation rate was 100% for both remimazolam and propofol. 29 In the current trial, both remimazolam and propofol achieved 100% procedural success rate. The time metrics including time to adequate sedation, procedure time, sedation time and recovery time were comparable in patients receiving remimazolam and those receiving propofol. Jia et al. observed that the 95% effective dose (ED95%) of remimazolam tosilate was 0.22 mg/kg when combined with 5‐ug sufentanil for deep sedation during fiberoptic bronchoscopy. 30 A slightly higher dose of remimazolam (0.25 mg/kg) may be needed when used in combination with 0.1‐ug/kg sufentanil. 31 Because the induction dose of remimazolam was unknown for elderly patients, the mode of constant and slow administration by syringe pump was used in this study. The results suggested that induction dose of remimazolam at 0.2 mg/kg with background fentanyl is appropriate.
Chen et al. reported that the awakening time of remimazolam was longer than that of propofol. 15 In a previous trial comparing remimazolam with midazolam for sedation in bronchoscopy, remimazolam showed a faster onset of action and a faster recovery of consciousness than midazolam. 32 In a phase II trial in patients undergoing gastrointestinal endoscopy, remimazolam had an onset of action similar to midazolam but a shorter time to recovery. 16 Similar to these previous reports, the current trial showed similar recovery time with 0.2‐mg/kg remimazolam versus 1.5‐mg/kg propofol, thus supporting the advantage of remimazolam.
This trial has several limitations. The study drugs were designed to be administered at a single constant rate. Future studies are needed to determine the minimum effective dose (pump speed) for sedation of elderly patients undergoing upper gastrointestinal endoscopic procedures. More importantly, the generalizability of the results obtained in this trial is unknown. In most setting in Europe and US, conscious sedation is used for simple endoscopic procedures in relatively healthy subjects (ASA grade I or II). Deep sedation is typically only in patients with significant comorbidities (ASA grade III or IV), anticipated failure of sedation or complex procedures. Deep sedation was used in the current trial for several reasons. First, due to the relatively poor patient‐physician relationship and concerns of malpractice (for which there was no insurance protection), endoscopists tend to insist deep sedation for a more thorough examination. Second, patients also prefer and tend to choose deep sedation for such procedure. Third, major insurance plans cover deep sedation but not conscious sedation. Indeed, deep sedation is recommended by the Chinese Experts' Consensus. 33
5. CONCLUSION
In conclusion, the rates of hypotension, bradycardia and respiratory suppression were lower in elderly patients receiving remimazolam versus propofol on a fentanyl background for upper gastrointestinal endoscopic procedure.
AUTHOR CONTRIBUTIONS
Shanshan Wei, Jun Jiang and Yanjuan Huang designed the study. Yanxia Wei, Xuelian Ran, Meixu Wang, Ning Wei, Yanying Liao, Zailing Qin, Wenwen Ling, Meitao Pan, Qimei Wei, Liuhui Fu, Boquan Xiong, Chendong Ma participated in patient recruitment. Kejian Lu performed statistical analyses and drafted the manuscript. Yanjuan Huang revised the manuscript. All authors are aware of and responsible for the research data. All authors read and approved the manuscript in its final version.
FUNDING INFORMATION
This study was supported in part by the Guangxi Health Commission Self‐funded Scientific Research Projects foundation (#Z20210001; Z‐A20221153).
CONFLICT OF INTEREST
HengRui Medicine Co., Ltd provide financial support to cover the cost of remimazolam and data management, but was not involved in trial design, conduct and data analysis.
ETHICS STATEMENT
This study was approved by the IRB of the Third Affiliated Hospital of Guangxi Medical University (#Y2020059), Hechi Third People's Hospital (#K2021001), Liuzhou Municipal Liutie Central Hospital (#2021037), and registered at http://www.chictr.org.cn (18/08/2020, #ChiCTR‐2,000,035,824). The study protocol followed the CONSORT guidelines. The trial was performed in compliance with all relevant guidelines. Written informed consent was obtained from all patients.
Lu K, Wei S, Ling W, et al. Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J Clin Pharm Ther. 2022;47(12):2230‐2236. doi: 10.1111/jcpt.13797
Kejian Lu, Shanshan Wei and Wenwen Ling contributed equally to this work.
Funding information Guangxi Health Commission Self‐funded Scientific Research Projects foundation, Grant/Award Numbers: Z20210001, Z‐A20221153
Contributor Information
Shanshan Wei, Email: 89596292@qq.com.
Jun Jiang, Email: jiangjun158800@126.com.
Yanjuan Huang, Email: huangyanjuan66@163.com.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are not publicly available due to institutional restrictions but are available from the corresponding author on request.
REFERENCES
- 1. Lin YJ, Wang YC, Huang HH, Huang CH, Liao MX, Lin PL. Target‐controlled propofol infusion with or without bispectral index monitoring of sedation during advanced gastrointestinal endoscopy. J Gastroenterol Hepatol. 2020;35:1189‐1195. [DOI] [PubMed] [Google Scholar]
- 2. Riphaus A, Wehrmann T, Weber B, et al. S3 guideline: sedation for gastrointestinal endoscopy 2008. Endoscopy. 2009;41:787‐815. [Google Scholar]
- 3. Heuss LT, Inauen W. The dawning of a new sedative: propofol in gastrointestinal endoscopy. Digestion. 2004;69:20‐26. [DOI] [PubMed] [Google Scholar]
- 4. Goudra B, Gouda G, Mohinder P. Recent developments in drugs for GI endoscopy sedation. Dig Dis Sci. 2020;65:2781‐2788. [DOI] [PubMed] [Google Scholar]
- 5. Uzman S, Gurbulak B, Gurbulak EK, Donmez T, Hut A, Yildirim D. A comparison of propofol and midazolam/meperidine sedation in upper gastrointestinal endoscopy. Wideochirurgia Tec M. 2016;11:178‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: an analysis of 5 Years' data from a tertiary center in the USA. Clin Endosc. 2017;50:161‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amornyotin S, Leelakusolvong S, Chalayonnawin W, Kongphlay S. Age‐dependent safety analysis of propofol‐based deep sedation for ERCP and EUS procedures at an endoscopy training center in a developing country. Clin Exp Gastroenterol. 2012;5:123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Conscious sedation with propofol in elderly patients: a prospective evaluation. Aliment Pharmacol Ther. 2003;1493‐1501:1493‐1501. [DOI] [PubMed] [Google Scholar]
- 9. Friedrich K, Stremmel W, Sieg A. Endoscopist‐administered propofol sedation is safe‐a prospective evaluation of 10,000 patients in an outpatient practice. J Gastrointestin Liver Dis. 2012;21:259‐263. [PubMed] [Google Scholar]
- 10. Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist‐directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229‐1237. [DOI] [PubMed] [Google Scholar]
- 11. Rogers WK, McDowell TS. Remimazolam, a short‐acting GABA (a) receptor agonist for intravenous sedation and/or anesthesia in day‐case surgical and non‐surgical procedures. IDrugs. 2010;13:929‐937. [PubMed] [Google Scholar]
- 12. Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83:984‐992. [DOI] [PubMed] [Google Scholar]
- 13. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427‐437. [DOI] [PubMed] [Google Scholar]
- 14. Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double‐blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771‐780. [DOI] [PubMed] [Google Scholar]
- 15. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non‐inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36:474‐481. [DOI] [PubMed] [Google Scholar]
- 16. Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra–short‐acting benzodiazepine. Anesthesiology. 2007;107:60‐66. [DOI] [PubMed] [Google Scholar]
- 17. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244‐251. [PubMed] [Google Scholar]
- 18. Ead H. From Aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs. 2006;21:259‐267. [DOI] [PubMed] [Google Scholar]
- 19. Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Digest Liver Dis. 2021;53:94‐101. [DOI] [PubMed] [Google Scholar]
- 20. Verschoore T, Vandecandelaere S, Vandecandelaere P, Vanderplancke T, Bergs J. Risk factors for complications and mortality related to endoscopic procedures in adults. Acta Gastro‐ENT Belg. 2016;79:39‐46. [PubMed] [Google Scholar]
- 21. Liu X, Ding B, Shi F, et al. The efficacy and safety of Remimazolam Tosilate versus etomidate‐Propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single‐blind. Non‐Inferiority Trial Drug des Dev Ther. 2021;15:4675‐4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goudra BG, Singh PM. SEDASYS, sedation, and the unknown. J Clin Anesth. 2014;26:334‐336. [DOI] [PubMed] [Google Scholar]
- 23. Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. Jama. 2014;311:2110‐2120. [DOI] [PubMed] [Google Scholar]
- 24. Piehl E. Remimazolam. GABAA benzodiazepine site receptor agonist, anesthetic/sedative. Drug Future. 2013;38:625‐630. [Google Scholar]
- 25. Aggarwal S, Goyal VK, Chaturvedi SK, Mathur V, Baj B, Kumar A. A comparative study between propofol and etomidate in patients under general anesthesia. Rev Bras Anestesiol. 2016;66:237‐241. [DOI] [PubMed] [Google Scholar]
- 26. Probst S, Bevilacqua C, Eibel S, et al. Difference in vasopressor use and usage patterns in patients undergoing cardiac surgery with remimazolam versus propofol/sevoflurane for general anesthesia. Anesthesiology A. 2015;4025:1‐2. [Google Scholar]
- 27. Miller MA, Levy P, Patel MM. Procedural sedation and analgesia in the emergency department: what are the risks? Emerg Med Clin N Am. 2005;23:551‐572. [DOI] [PubMed] [Google Scholar]
- 28. Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose‐finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093‐1100. [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Wang J, Ran R, et al. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single‐blind, parallel controlled trial. J Clin Pharm Ther. 2021;00:1‐6. [DOI] [PubMed] [Google Scholar]
- 30. Jia Z, Ren L, Fan Y, Tan Z. Observation of effective dosage of remimazolam tosilate used for moderate‐to‐deep sedation in fiberoptic bronchoscopy. Chin Med J (Engl). 2021;101:813‐816. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single‐Centre randomized controlled trial. BMC Anesthesiol. 2021;21:156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137‐146. [DOI] [PubMed] [Google Scholar]
- 33. Li ZS, Deng XM, Sun T, Du YQ, Li JB. Chinese experts' consensus on the diagnosis and treatment of sedation and anaesthesia in digestive endoscopy. Chin J Pract Intern Med. 2014;34:756‐764. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to institutional restrictions but are available from the corresponding author on request.


