Abstract
Background and Purpose
Reperfusion therapy is the standard of care for ischaemic stroke; however, there is a need to identify new therapeutic targets able to ameliorate cerebral damage. Neutrophil β1 adrenoceptors (β1AR) have been linked to neutrophil migration during exacerbated inflammation. Given the central role of neutrophils in cerebral damage during stroke, we hypothesize that β1AR blockade will improve stroke outcomes.
Experimental Approach
Rats were subjected to middle cerebral artery occlusion–reperfusion to evaluate the effect on stroke of the selective β1AR blocker metoprolol (12.5 mg·kg−1) when injected i.v. 10 min before reperfusion.
Key Results
Magnetic resonance imaging and histopathology analysis showed that pre‐reperfusion i.v. metoprolol reduced infarct size. This effect was accompanied by reduced cytotoxic oedema at 24 h and vasogenic oedema at 7 days. Metoprolol‐treated rats showed reduced brain neutrophil infiltration and those which infiltrated displayed a high proportion of anti‐inflammatory phenotype (N2, YM1+). Additional inflammatory models demonstrated that metoprolol specifically blocked neutrophil migration via β1AR and excluded a significant effect on the glia compartment. Consistently, metoprolol did not protect the brain in neutrophil‐depleted rats upon stroke. In patients suffering an ischaemic stroke, β1AR blockade by metoprolol reduced circulating neutrophil–platelet co‐aggregates.
Conclusions and Implications
Our findings describe that β1AR blockade ameliorates cerebral damage by targeting neutrophils, identifying a novel therapeutic target to improve outcomes in patients with stroke. This therapeutic strategy is in the earliest stages of the translational pathway and should be further explored.
Keywords: Ischemic Stroke, Neutrophils, I/R, β1AR, Neuroinflammation
Abbreviations
- AAR
area‐at‐risk
- ADC
apparent diffusion coefficient
- AMI
acute myocardial infarction
- CSPGs
chondroitin sulfate proteoglycans
- DSC
dynamic susceptibility contrast
- HT
haemorrhagic transformation
- I/R injury
ischaemia/reperfusion injury
- Iba1
ionized calcium binding adaptor molecule 1
- IS
infarct size
- MBP
myelin basic protein
- MVO
microvascular obstruction
- NG2
neuronal/glial antigen
- OGD
oxygen–glucose deprivation
- PDM
perfusion–diffusion mismatch
- RGMa
repulsive guidance molecule a
- rt‐PA
recombinant tissue‐plasminogen activator
- T2W
T2 weighted
- YM1/Chil3
chitinase 3‐like 3
What is already known
Cerebral ischaemia/reperfusion injury is a clinical challenge because few therapeutic interventions are available.
What does this study adds
β1AR blockade during ongoing stroke reduces brain injury and neuroinflammation, and improves neurological outcomes.
β1AR blockade decreases neutrophil migration and boosts pro‐resolving circuits which favour tissue homeostasis.
What is the clinical significance
Pharmacological β1AR blockade may represent a potential candidate in clinical trials for ischaemic stroke.
1. INTRODUCTION
Despite improved diagnostic imaging techniques and numerous attempts to establish neuroprotective strategies, stroke treatment is currently restricted to reperfusion without coadjuvant therapy (Powers et al., 2019). The only clinically available interventions are intravenous (i.v.) administration of the fibrinolytic agent recombinant tissue‐plasminogen activator (rt‐PA) and, more recently, endovascular thrombectomy, alone or in combination with rt‐PA (Powers et al., 2019).
Experimental evidence shows that reperfusion after prolonged brain ischaemia can trigger a set of potentially deleterious events, known as reperfusion injury. Tissue reperfusion was recently shown to exacerbate the inflammatory response, resulting in massive leukocyte infiltration (Lin et al., 2016). Among the first cells infiltrating the brain are neutrophils (Jickling et al., 2015; Strecker et al., 2017), and neutrophil pro‐inflammatory activation after stroke has been linked to increased infarct size (IS), blood–brain barrier (BBB) disruption, haemorrhagic transformation (HT), and worse neurological outcomes (Jickling et al., 2015; Strecker et al., 2017). Moreover, neutrophils form aggregates with erythrocytes and platelets, and after reperfusion, these aggregates form embolisms that impair tissue perfusion, despite the restoration of blood flow in the large cerebral arteries. This phenomenon is known as microvascular obstruction (MVO) and is a major contributor to ischaemia–reperfusion (I/R) injury and final IS (Ibanez et al., 2015).
Neutrophils have an extensive repertoire of biological functions and remain essential contributors for the resolution of inflammation and tissue repair (Horckmans et al., 2017; Yang et al., 2019). Cell plasticity allows neutrophils to take on different characteristics (Ballesteros et al., 2020; Mantovani et al., 2011; Silvestre‐Roig et al., 2016) under certain tissue environments or stimuli (Chakravarti et al., 2009; Fridlender et al., 2009; Ohms et al., 2020; Puellmann et al., 2006). Different neutrophil populations with disparate effects have been recently described in brain after stroke (Cuartero et al., 2013; Garcia‐Culebras et al., 2019), and thus it is vital to evaluate their phenotype in the context of therapies.
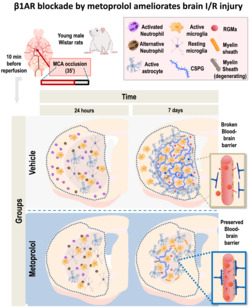
We recently showed that in the context of myocardial infarction, acute blockade of neutrophil β1‐adrenoceptors (β1AR) by metoprolol—but not by other β‐blockers—ameliorates myocardial I/R injury (Clemente‐Moragon et al., 2020; Garcia‐Prieto et al., 2017). These experimental studies were followed by a successful randomized clinical trial showing that injection of the selective β1AR blocker metoprolol before reperfusion resulted in clinical benefits in patients suffering an acute myocardial infarction (AMI) (Garcia‐Ruiz et al., 2016; Pizarro et al., 2014). These data prompted us to test this pharmacological strategy in ischaemic stroke. In the present study, we tested the effects of pre‐reperfusion intravenous (i.v.) administration of metoprolol in a rat model of brain I/R. We show that a single injection of metoprolol before reperfusion dampened oedema formation and reduced neuronal loss. This protective effect was associated with significantly reduced neuroinflammation, better preservation of BBB (blood‐brain barrier) integrity and lessened glial scar formation. Moreover, by attenuating the inflammatory environment, metoprolol reduced circulating neutrophil activation and boosted pro‐resolving circuits which favour the re‐establishment of tissue homeostasis. Consistent with this, the benefits of metoprolol were lost when the ischaemic stroke was induced in rats previously depleted of neutrophils.
2. METHODS
Full details of materials and experimental procedures are available in Supporting Information. The data that support the findings of this study are available from the corresponding author on reasonable request.
All experimental and other scientific procedures with animals conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC, enforced in Spanish law under Real Decreto 53/2013. Animal protocols were approved by the local ethics committees and the Animal Protection Area of the Comunidad Autónoma de Madrid (PROEX 056_17 and PROEX 176.3/20). Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020).
In summary, 8‐ to 10‐week‐old young male Wistar rats (200–250 g) were subjected to middle cerebral artery occlusion–reperfusion (MCAO/R) for 35 min and randomized to receive either a 12.5 mg·kg−1 i.v. bolus of metoprolol‐tartrate or vehicle 10 min before reperfusion. IS and brain oedema were evaluated by MRI at 24 h and 7 days post‐reperfusion and by histopathology at 7 days post‐reperfusion. Neutrophil infiltration and phenotype were assessed by immunohistochemistry (IHC) at 24 h post‐reperfusion, and the effect of metoprolol was also evaluated at 24 h and 7 days in rats depleted of circulating neutrophils. Specific neutrophil Adrb1 knock‐out mice were used for mechanistic experiments exploring the effect of metoprolol on neutrophil biology.
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
2.1. Statistics
Data are presented as mean ± standard error of the mean (SEM). Differences were deemed statistically significant at P values below 0.05. Further details are provided in Supporting Information.
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.2. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021).
3. RESULTS
3.1. Selective β1AR blockade during ongoing stroke reduces brain injury, prevents neuronal loss and improves neurological outcomes
Rats subjected to 35 min of middle cerebral artery occlusion (MCAO) received a single i.v. injection of the selective β1AR blocker metoprolol (12.5 mg·kg−1) (Garcia‐Prieto et al., 2017) or saline (0.9% NaCl, vehicle) 10 min before reperfusion (Figure 1a). The selected i.v. metoprolol dose was based on a dose–response study showing a moderate haemodynamic effect on arterial pressure and heart rate (<20%) (Figure S1A). Rats underwent three MRI studies: during ongoing cerebral ischaemia and 24 h and 7 days after reperfusion. The extent of cerebral ischaemia provoked by MCAO (area at risk, AAR) was determined from MRI dynamic susceptibility contrast (DSC) images obtained during occlusion, and did not differ between groups (275.0 ± 12.5 mm3 and 279.1 ± 8.85 mm3 for vehicle‐ and metoprolol‐injected rats, respectively, Figure 1c). Brain IS was measured as the AAR‐normalized extent of oedema on T2‐weighted (T2W) MRI images at 24 h post‐reperfusion (pre‐specified primary outcome) and at 7 days post‐reperfusion (Figure 1b). MRI‐quantified IS was significantly smaller in metoprolol‐treated rats both at 24 h (17.0 ± 1.06% of the AAR in vehicle‐treated rats vs. 11.6 ± 0.72% in metoprolol‐treated rats, P < 0.05, Figure 1c) and on day 7 (vehicle, 7.07 ± 1.42%; metoprolol, 2.46 ± 0.56%; P < 0.05, Figure 1c). A tendency towards IS reduction was observed when using a lower dose of metoprolol (6.25 mg·kg−1) (Figure S1B).
FIGURE 1.
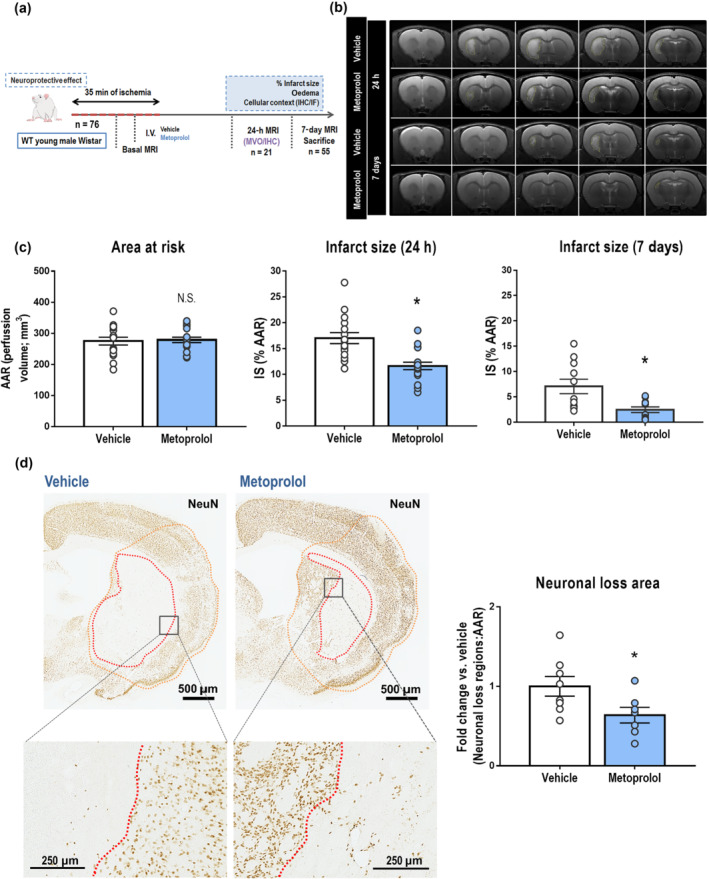
β1AR blockade by metoprolol reduces infarct size and neuronal loss. (a) Rat model of middle cerebral artery occlusion–reperfusion (MCAO/R), with euthanasia at 7 days post‐reperfusion. WT, wild‐type, MRI, magnetic resonance imaging, IF, immunofluorescence; IHC, immunohistochemistry; MVO, microvascular obstruction. (b) Comparative coronal T2‐weighted (T2W) MRI at 24 h (top rows) and 7 days (bottom rows) post‐reperfusion. Infarcted regions correspond to hyperintense areas. (c) Infarct size (IS) evaluated by coronal T2W MRI at 24 h (vehicle, n = 16; metoprolol, n = 18) and 7 days post‐reperfusion (vehicle, n = 11; metoprolol, n = 11). Final IS was calculated as the ratio of infarct volume to the area‐at‐risk (AAR) and expressed as %. (d) IHC of coronal sections with anti‐NeuN antibody. Higher magnifications of infarcted areas show territories of complete neuronal loss (delineated in red). Neuronal loss analysis in the middle cerebral artery territory (MCA, delineated in orange) of infarcted hemispheres shows neuronal preservation in metoprolol‐treated rats at 7 days post‐reperfusion (vehicle, n = 8; metoprolol, n = 7). Graphs show mean ± S.E.M. *P < 0.05
The exclusion of rats with hemispheric infarcts (IS > 40%) was an a priori exclusion criterion defined before experimentation. To rule out unintentional selection bias, we performed a post hoc analysis of all rats, including those meeting the exclusion criterion, at 24 h post‐reperfusion and metoprolol maintained its association with significantly smaller IS (Figure S2).
IS was also quantified as a percentage of perfusion‐diffusion (PDM), another validated normalization method. The results were consistent with IS/AAR data, with metoprolol‐treated rats having significantly smaller infarcts at 24 h and 7 days post‐reperfusion (Figure S3A). Furthermore, comparison of infarct volumes on T2‐weighted (T2W) MRI without normalization also showed that metoprolol‐infused rats had significantly smaller infarcts at 24 h and 7 days post‐reperfusion (Figure S3B).
Immunohistochemical analysis of the neuronal marker NeuN at 7 days post‐reperfusion showed that early i.v. metoprolol administration was associated with significant smaller regions of neuronal loss than observed in the vehicle‐treated group (vehicle 1.00 ± 0.11; metoprolol, 0.65 ± 0.09; P < 0.05, Figure 1d).
To study whether the neuroprotective effect of metoprolol was consistent with better neurological outcomes, a longitudinal study was performed to evaluate long‐term effects of brain injury on sensory‐motor behaviours. The results obtained with the motor and behavioural scales (Figure S4) first showed a slow recovery of these neurological functions over the time in the groups subjected to ischaemia (metoprolol and vehicle groups), with no recovery in the sham group. With both scales, despite a better neurological function observed in the group treated with the drug in all time points, it was not until 21 days after the surgery when significant differences were observed, in particular, in the behavioural test. Our evaluation of the sensorimotor functions using the adhesive removal test again revealed an improvement in these neurological deficits in rats receiving i.v. metoprolol, with significant differences at 24 h and 7 days between these groups in the parameter of time for the first attempt to remove the adhesive.
3.2. β1AR blockade reduces brain cytotoxic and vasogenic oedema and preserves the BBB
The extent of cytotoxic and vasogenic oedema was detected as areas of reduced or increased diffusion, respectively, on MRI apparent diffusion coefficient (ADC) maps (Birenbaum et al., 2011). MRI during index ischaemia (before metoprolol or vehicle injection) showed no differences in brain cytotoxic oedema (vehicle, 98.1 ± 8.8 mm3; metoprolol, 105.2 ± 11.7 mm3). At 24 h after reperfusion, metoprolol‐treated rats had significantly less cytotoxic oedema than their vehicle‐treated counterparts (vehicle, 31.0 ± 2.54 mm3; metoprolol, 20.6 ± 1.62 mm3; P < 0.05) (Figure 2a). Besides, metoprolol‐treated rats had a significantly smaller extent of vasogenic oedema at 7 days post‐reperfusion (vehicle, 13.8 ± 1.87 mm3; metoprolol, 7.86 ± 1.97 mm3; P < 0.05) (Figure 2a). Vasogenic oedema was further assessed by comparing ipsilateral and contralateral tissue water content at 7 days post‐reperfusion (Figure 2b). MRI ADC maps revealed lower water content (ipsilateral:contralateral intensity ratio) in the brain tissue of metoprolol‐injected rats (vehicle, 1.18 ± 0.03; metoprolol, 1.07 ± 0.02; P < 0.05; Figure 2c). Similar results were obtained by T2W MRI analysis (vehicle, 1.24 ± 0.04; metoprolol, 1.08 ± 0.04; P < 0.05; Figure 2c).
FIGURE 2.
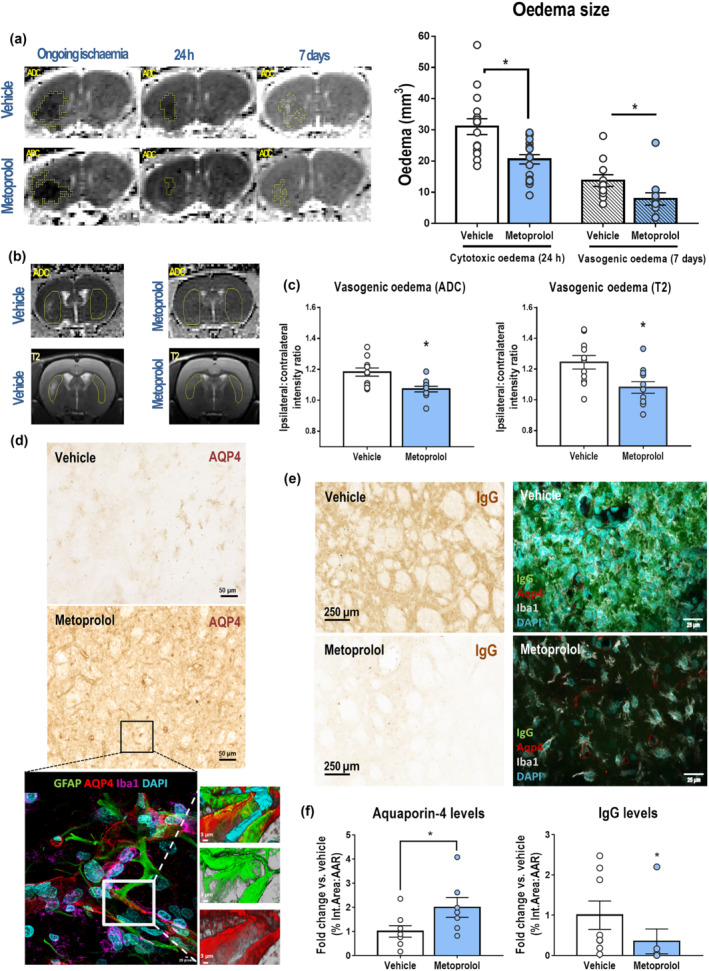
β1AR blockade by metoprolol reduces cerebral oedema and preserves the blood–brain barrier (BBB). (a) Coronal parametric MRI apparent diffusion coefficient (ADC) maps comparing rats receiving i.v. vehicle (top row) or metoprolol (bottom row) during ongoing ischaemia and 24 h and 7 days post‐reperfusion. Restricted diffusion was identified at 24 h as hypointense areas and increased diffusion at 7 days as hyperintense areas (outlined in yellow). Metoprolol reduces cytotoxic oedema at 24 h post‐reperfusion (vehicle, n = 16; metoprolol, n = 18) and vasogenic oedema at 7 days post‐reperfusion (vehicle, n = 11; metoprolol, n = 11). (b) Tissue water content analysed by comparison of ipsilateral (with infarct) and contralateral (without infarct) intensities in similar‐sized areas (yellow outlines), in a single slice per rat on ADC maps and T2W MRI. (c) Quantification of ipsilateral‐to‐contralateral intensity ratios from ADC maps and T2W images, showing reduced brain water content in metoprolol‐treated rats. (d) IHC of coronal sections at 7 days post‐reperfusion with anti‐aquaporin 4 (AQP4) antibody. The bottom panel shows representative triple GFAP+AQ4+Iba1+ immunofluorescence, illustrating AQP4 preservation (red) in astrocyte (GFAP; green) end‐feet and consequent preservation of BBB integrity. Microglia/macrophages (Iba1; purple) were found around vessels. Nuclei were revealed with DAPI (blue). (e) IHC of coronal sections at 7 days post‐reperfusion with anti‐immunoglobulin G (IgG) antibody (left panels). The right panels shows representative triple IgG+AQ4+Iba1+ immunofluorescence, illustrating IgG extravasation (green) in the absence of AQP4 (red). Microglia/macrophages (Iba1; grey) were found more reactive in the core lesion of vehicle‐treated rats and nuclei were revealed with DAPI (blue). (f) Quantification of AQP4 and IgG IHC in the MCA of infarcted hemispheres shows preservation of BBB integrity at 7 days post‐reperfusion in rats receiving i.v. metoprolol (vehicle, n = 8; metoprolol, n = 7). Graphs show mean ± S.E.M. *P < 0.05. Other abbreviations as in Figure 1
We assessed BBB integrity by immunohistochemical analysis of aquaporin‐4 (AQP4), whose expression on the end‐feet of astrocytes in the BBB prevents post‐stroke neuroinflammation‐related oedema (Fukuda & Badaut, 2012). AQP4 expression at day 7 post‐reperfusion was twofold higher in metoprolol‐treated rats (vehicle, 1.00 ± 0.24; metoprolol, 2.00 ± 0.41; P < 0.05; Figure 2d,f, Video S1). These results were consistent with an increase of IgG extravasation in the absence of AQP4 staining (Figures 2e,f and S5).
3.3. β1AR blockade during brain ischaemia prevents microglia/macrophage response and reduces subacute glial scar formation
In vehicle‐injected rats, reactive and hypertrophic astrocytes, detected by up‐regulated expression of glial fibrillary acidic protein (GFAP), accumulated in a barrier pattern surrounding the core lesion and expressed large amounts of chondroitin sulfate proteoglycans (CSPGs). Metoprolol injection reduced glial scar formation by fivefold (vehicle, 1.00 ± 0.18; metoprolol, 0.19 ± 0.07; P < 0.05; Figures 3a–c and S6A). In line with this result, proteoglycan production in the core lesion identified by CSPG deposition was reduced by 10‐fold in rats receiving i.v. metoprolol (vehicle, 1.00 ± 0.33; metoprolol, 0.09 ± 0.04; P < 0.05; Figures 3b,d and S6A,B), as well as microglia/macrophage response (vehicle, 1.00 ± 0.17; metoprolol, 0.52 ± 0.09; P < 0.05; Figure 3a,b,e). Confirming these results, double‐immunohistochemistry (IHC) and immunofluorescence (IF) for CSPG and GFAP in the vehicle group revealed astrocytes surrounding a scar containing a matrix of proteoglycans, whereas this phenomenon was not seen in metoprolol‐treated rats (Figure S6D). Moreover, quadruple IF showed that the absence of a glial scar and proteoglycan matrix in the core lesion of metoprolol‐treated rats was accompanied by a reduced microglia/macrophage response in the same area (Figure 3a), as observed with Iba1 immunohistochemistry (Figure 3b,e). IHC results at 24 h post‐reperfusion indicated that glial scarring was not present in any of the treatment groups. The latter suggests that metoprolol did not have a direct effect on glia (Figure S7).
FIGURE 3.
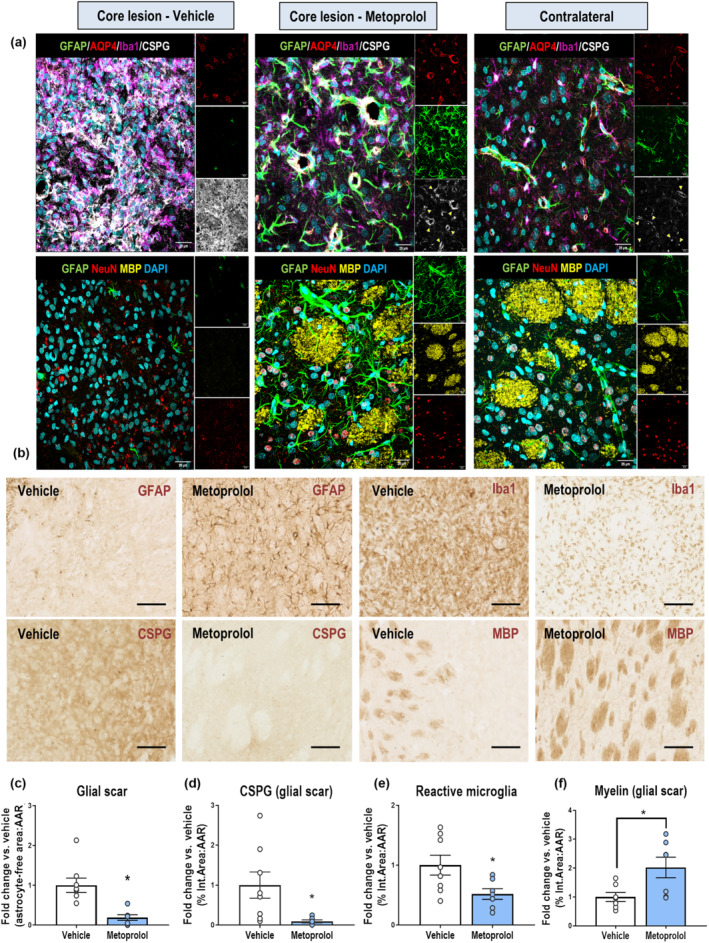
β1AR blockade by metoprolol prevents microglia/macrophage response and reduces subacute glial scar formation. (a) Single and merged channels of quadruple GFAP+AQP4+Iba1+CSPG+ (top row) and triple GFAP+MBP+NeuN (bottom row) IF. The quadruple IF shows glial scar formation (GFAP; green), proteoglycan deposition (CSPG; grey), microglia/macrophage response (Iba1; purple), and BBB disruption (AQP4; red) in a vehicle‐treated rat (left panels), contrasting the absence of glial scar in a metoprolol‐treated rat (central panels). Yellow arrowheads show a suggesting neural/glial antigen 2 expression by CSPG+ perivascular pericytes. The triple IF shows representative degeneration of neurons (NeuN; red) and the myelin sheath (MBP; yellow) in a vehicle‐treated rat (left panels) and their preservation in a metoprolol‐treated rat (central panels), where the lesion resembles the contralateral hemisphere (right panels) except for the astrocyte hypertrophy (GFAP; green). Nuclei were revealed with DAPI (blue). (b) Representative IHC (vehicle, left panels; metoprolol, right panels) of GFAP (glial fibrillary acidic protein), CSPGs (chondroitin sulfate proteoglycans), Iba1 (ionized calcium‐binding adapter molecule 1) and myelin basic protein (MBP) on coronal sections at 7 days post‐reperfusion, showing a reduction in glial scar formation (c), proteoglycans deposition (d), reactive microglia response and myelin sheath deterioration in metoprolol‐treated rats. Graphs show mean ± S.E.M. *P < 0.05. n = 6–8 rats per group. Other abbreviations as in Figure 1
Increased inflammation leads to secondary damage to other cell populations, such as neurons and oligodendrocytes (Hackett & Lee, 2016). Myelin basic protein (MBP) IHC, a marker of the state of axonal myelination, showed good preservation of myelin sheaths in metoprolol treated rats (vehicle, 1.00 ± 0.16; metoprolol, 2.02 ± 0.36; P < 0.05; Figures 3a,b,f and S6C). Myelin sheaths in the vehicle group showed evidence of damage, and there were large amounts of debris accumulated in the core lesion, coinciding with CSPG deposition (Figure S6E). Triple IF confirmed these results, illustrating how metoprolol injection was associated with better preserved myelin sheaths that resembled those on the contralateral side (Figure 3a). Moreover, the core lesions of vehicle‐treated rats were positive for release of repulsive guidance molecule a (RGMa), indicating a context inhibitory to axonal and neural regeneration, whereas RGMa was absent from the core lesions of metoprolol‐infused rats (Figure S8).
3.4. β1AR blockade reduces brain microvascular obstruction (MVO) and blunts neutrophil infiltration into brain parenchyma
Upon reperfusion, MVO induced by circulating cells contributes significantly to the final extent of injury (Ibanez et al., 2015). We quantified trapped erythrocytes as a surrogate for MVO by treating brain tissues from rats killed 24 h post‐reperfusion (Figure 4a) with NaBH4, which renders haemoglobin fluorescent. Pre‐reperfusion metoprolol injection was associated with a twofold reduction in the density of erythrocytes trapped in capillary networks in penumbra areas (vehicle, 1.00 ± 0.16; metoprolol, 0.50 ± 0.11; P < 0.05) (Figure 4b). Neutrophil pro‐inflammatory activation after stroke is associated with increased IS and BBB breakdown (Strecker et al., 2017). Brain neutrophil infiltration at 24 h (Figure 4a) was significantly twofold less in metoprolol‐treated rats 24 h after reperfusion (vehicle, 1.00 ± 0.25; metoprolol, 0.46 ± 0.10; P < 0.05, Figure 4c).
FIGURE 4.
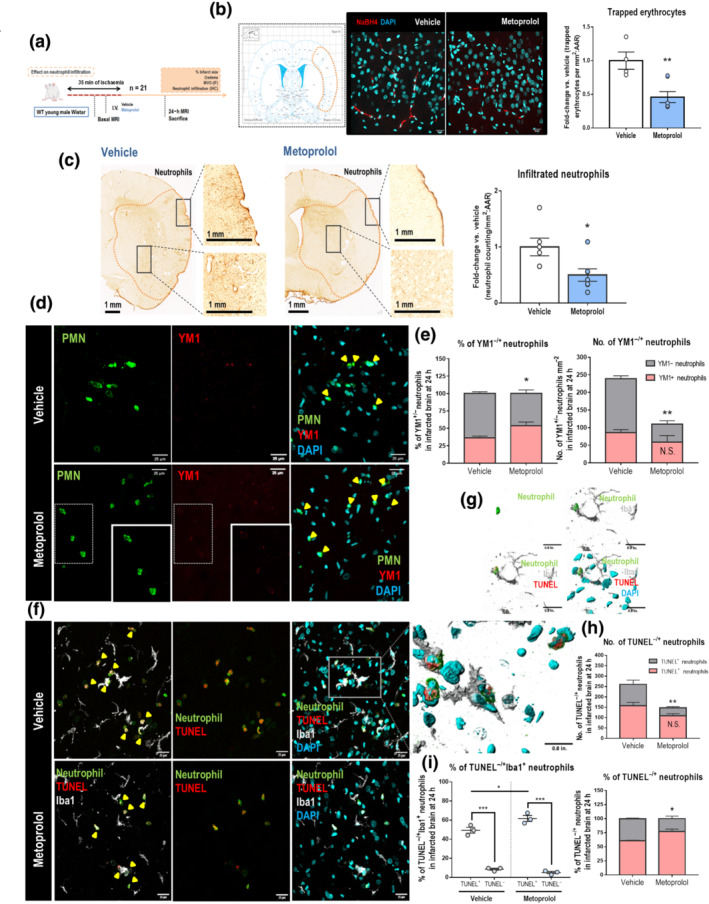
Metoprolol reduces MVO and neutrophil infiltration into brain parenchyma via β1AR. (a) Rat model of MCAO/R. Rats were killed at 24 h post‐reperfusion. (b) Representative confocal imaging of NaBH4‐treated tissues, showing trapped erythrocytes (red) in penumbra areas of rats receiving pre‐reperfusion vehicle (left) or metoprolol (right). Penumbra areas correspond to the orange‐bordered brain cortex region in the rat brain atlas. Nuclei were revealed with DAPI (blue). The relative numbers of trapped erythrocytes mm−2 in penumbra areas show reduced MVO in metoprolol‐treated rats. (c) Representative anti‐neutrophil IHC on coronal sections at 24 h post‐reperfusion. Quantification of neutrophil infiltration shows lower numbers of neutrophils mm−2 in brain parenchyma of metoprolol‐treated rats at 24 h post‐reperfusion (vehicle, n = 5; metoprolol, n = 7). (d) Evaluation of infiltrated neutrophils in brain (green) expressing YM1 (Chitinase 3‐like 3 protein, red) by IF at 24 h post‐reperfusion in vehicle‐ (top row) and metoprolol‐treated rats (bottom row). Nuclei were revealed with DAPI (blue). (e) The number of YM1− neutrophils mm−2, but not of YM1+, is significantly reduced by metoprolol and, as a consequence, the % of alternative neutrophils (N2, YM1+) was then higher in brain parenchyma. (f) Evaluation by IF of apoptotic (TUNEL+, red) neutrophils (green) in brain being engulfed by microglia (Iba1, grey) at 24 h post‐reperfusion in vehicle‐ (top row) and metoprolol‐treated rats (bottom row). Nuclei were revealed with DAPI (blue). (g) 3D reconstructions of confocal images show how neutrophils undergoing apoptosis are preferably phagocytized by microglia. (h) % of apoptotic neutrophils was increased in brain parenchyma, as the number of TUNEL− neutrophils mm−2, but not of TUNEL+, was significantly reduced by metoprolol. (i) Apoptotic neutrophils were preferentially engulfed by microglia. n = 3 rats per group. Graphs show mean ± S.E.M. *P < 0.05. Other abbreviations as in Figure 1
3.5. β1AR blockade specifically blunts pro‐inflammatory neutrophils, which results in a shift towards an anti‐inflammatory environment in brain
Neutrophils have been shown to be plastic cells with distinct modifiable phenotypes and specific functional‐derived properties (Ballesteros et al., 2020; Mantovani et al., 2011; Silvestre‐Roig et al., 2016). Because neutrophil plasticity makes them sensitive to changeable environmental stimuli (Chakravarti et al., 2009; Fridlender et al., 2009; Ohms et al., 2020; Puellmann et al., 2006), a heterogeneous population of these cells could be found in brain after stroke (Cuartero et al., 2013; Garcia‐Culebras et al., 2019). After documenting that metoprolol reduced neutrophil presence in the brain 24 h after reperfused stroke, we phenotypically characterized the infiltrated neutrophils by IF on the basis of Chitinase 3‐like 3 (YM1/Chil3) staining, which is as a marker of alternative anti‐inflammatory N2 neutrophils. Surprisingly, whereas the number of YM1+ neutrophils was not significantly different between metoprolol‐treated and control rats (vehicle, 85.6 ± 8.85 neutrophils mm−2; metoprolol, 59.2 ± 18.4 neutrophils mm−2), there was a strong reduction in YM1‐ neutrophil population (vehicle, 153 ± 8.42 neutrophils mm−2; metoprolol, 50.7 ± 9.62 neutrophils mm−2; P < 0.05). Consequently, the relative density of N2 anti‐inflammatory neutrophil subset was overtly higher in metoprolol‐injected rats (vehicle, 36.2 ± 2.55%; metoprolol, 53.9 ± 5.13%; P < 0.05). These results suggest that metoprolol selectively inhibit pro‐inflammatory neutrophil extravasation without affecting the dynamics of anti‐inflammatory neutrophils (Figure 4d,e).
Since N2 polarization has been shown to be associated with an increased neutrophil clearance and inflammation resolution (Cuartero et al., 2013), we performed a TUNEL assay on brain sections that allows us to assess neutrophil apoptosis by immunofluorescence. The density of apoptotic neutrophils was significantly higher in metoprolol‐treated rats (vehicle, 60.7 ± 1.11%; metoprolol, 76.9 ± 4.51%; P < 0.05). Apoptotic neutrophils clearance in brain is mainly carried out by phagocytic microglia via efferocytosis, in which therefore an immunosuppressive, pro‐resolving and tissue‐repair response is activated (Doran et al., 2020; Jones et al., 2016). We observed microglia preferential engulfment of TUNEL+ neutrophils (vehicle, 49.4 ± 3.06%; metoprolol, 61.7 ± 3.15%; P < 0.05) (Figure 4f–i, Video S2).
Overall, these data show that β1AR blockade results in a selective inhibition of pro‐inflammatory N1 neutrophils infiltration and a faster resolution of inflammation in the post‐stroke brain.
3.6. Metoprolol exerts a specific disruptive effect on neutrophils in a β1AR‐dependent manner
To evaluate whether metoprolol blunts neutrophil migratory capacities through β1AR, we exposed isolated neutrophils from WT and Adrb1 KO mice across the transwell filter to the chemoattractant CXCL1 in the presence or absence of metoprolol (10 μM). The number of cells migrating across the transwell membrane was quantified by flow cytometry after 90 min. Metoprolol inhibited baseline WT neutrophil migration across the CXCL1 gradient (vehicle, 1.83 ± 0.41 × 103 neutrophils; metoprolol, 1.04 ± 0.23 × 103 neutrophils; P < 0.05), whereas no effect was seen in the absence of neutrophil β1AR expression (vehicle, 1.23 ± 0.32 × 103 neutrophils; metoprolol, 1.20 ± 0.23 × 103 neutrophils) (Figure 5a–c). Next, we explored whether the effect of metoprolol on neutrophil crawling dynamics was abolished in neutrophil specific Adrb1 KO mice. To this end, we used 2D intravital microscopy (IVM) to image migration in the cremaster muscle vessels of mice injected with TNFα, which triggers massive neutrophil recruitment (Figure 5d) (Sreeramkumar et al., 2014). Consistent with the in vitro results, metoprolol disrupted in vivo neutrophil kinetics (velocity, accumulated and Euclidean distance, and directionality), as well as neutrophil–platelet interactions only in WT but not in neutrophil specific Adrb1 KO mice (Figure 5e–h, Video S3A–D). Altogether, these data demonstrate that metoprolol regulates neutrophil dynamics through β1AR modulation.
FIGURE 5.
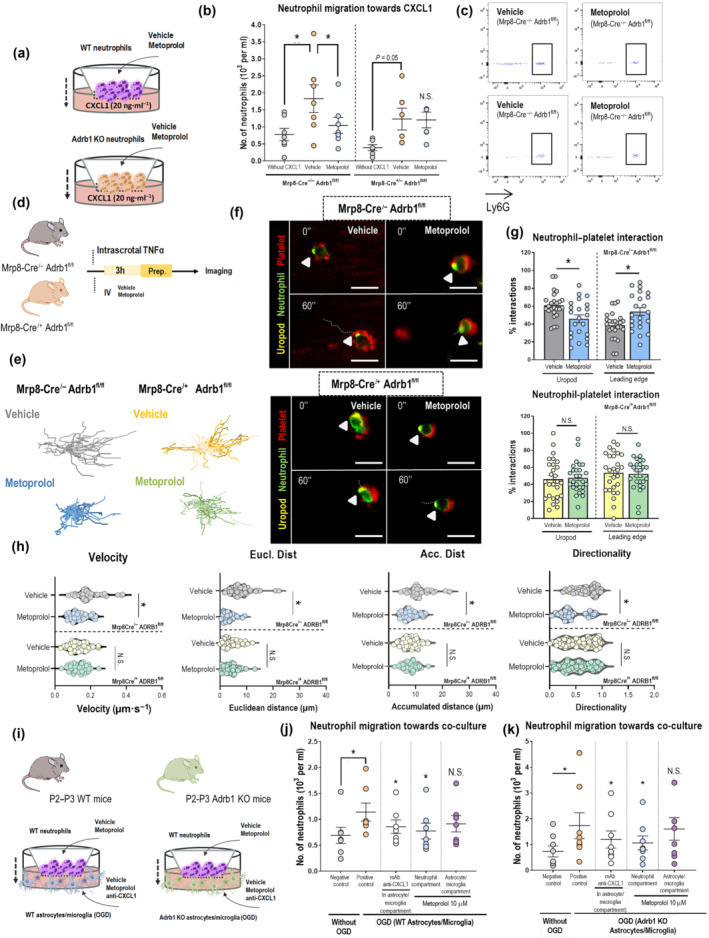
β1AR blockade by metoprolol blunts pro‐inflammatory neutrophils and favours an anti‐inflammatory environment in brain. (a) Experimental scheme for CXCL1‐induced transwell migration analysis. (b) Limiting effect of metoprolol on chemokine‐induced neutrophil migration in WT but not in conditional Adrb1 KO mice. (c) Flow cytometry plots illustrating reduced migration of neutrophils (Ly6G+ cells) upon treatment with metoprolol only in the presence of β1AR. Each independent experiment was conducted with leukocytes pooled from five to six animals, and each condition was run with two technical replicates (n = 8 for WT; n = 7 for Adrb1 KO). (d) Experimental scheme for 2D intravital microscopy. (e) Representative tracks of crawling neutrophils within inflamed vessels. (f) Representative time‐lapse images of platelets (CD41+ cells, red) with the polarized neutrophil uropod (CD62L+ domain, yellow) or leading edge (Ly6G+ domain, green). Arrowheads indicate interactions with the uropod domain, and dotted lines indicate displacement of the neutrophil over 60 s. (g) Percentage of platelet interactions with the neutrophil uropod or leading edge; n = 22–25 cells from three mice per condition. (h) 2D intravascular motility parameters: velocity (μm·s−1), accumulated and Euclidean distance (μm), and directionality; n = 35–54 cells from three mice per condition. WT mice: Mrp8‐Cre/− Adrb1fl/fl; neutrophil specific Adrb1 KO mice: Mrp8‐Cre/+ Adrb1fl/fl. (i) Experimental schemes of neutrophil migration towards WT (n = 7 per condition) or Adrb1 KO (n = 8 per condition) glia‐based co‐cultures. (j, k) The limiting effect of metoprolol on neutrophil migration was directly exerted on these cells, as it did not alter astrocyte/microglia chemoattractant properties. Graphs show mean ± S.E.M. *P < 0.05. Other abbreviations as in Figure 1
The role of βAR in astrocytes and microglia in the context of stroke has been a matter of controversy (Follesa & Mocchetti, 1993; Goyagi et al., 2006; Han et al., 2009; Junker et al., 2002; Lechtenberg et al., 2019; Monai et al., 2019; Semkova et al., 1996). We wanted to explore whether β1AR blocked in these cells had an impact on neutrophil recruitment. Astrocyte/microglia neonatal co‐cultures (Figure S8A) were subjected to 1 h oxygen–glucose deprivation (OGD) followed by 24 h of reperfusion (Figure 5i). Neutrophils isolated from WT mice were then placed on a transwell filter having the post‐OGD astrocyte/microglia as the attractant stimuli. WT astrocyte/microglia subjected to 1 h OGD triggered a significant increase in neutrophil migration across the transwell compared to astrocyte/microglia not undergoing OGD (control) (control, 0.69 ± 0.16 × 103 neutrophils; vehicle, 1.14 ± 0.18 × 103 neutrophils; P < 0.05). This effect was consistent with an increase in CXCL1 levels in the supernatants of the co‐culture (control, 2.40 ± 0.56 ng·ml−1; vehicle, 5.38 ± 0.73 ng·ml−1; P < 0.05, Figure S6B) and disruption of neutrophil migration after CXCL1 antibody‐mediated neutralization (0.86 ± 0.13 × 103 neutrophils; P < 0.05, Figure 5j). When metoprolol (10 μM) was added on neutrophil (0.78 ± 0.15 × 103 neutrophils; P < 0.05) chamber migration was significantly disrupted; however, this effect was not seen when added on astrocyte/microglia chamber (before reperfusion). Consistent with this, CXCL1 levels in supernatant of metoprolol‐treated co‐cultures were not altered. Moreover, CXCL1 levels in supernatants of co‐cultures treated with metoprolol after reperfusion indicate that metoprolol does not have any effect at that point (Figure S8B). These results were replicated in astrocyte/microglia co‐cultures from whole body Adrb1 KO littermate mice, but no additional effects were observed in the absence of astrocyte/microglia β1AR expression (Figures 5i,k and S8C).
Additionally, proliferation assays based on EdU staining were performed to study astrocyte behaviour in response to ischaemia, as an indirect marker of reactivity and glial scar formation (Li et al., 2015; Ou‐Yang et al., 2018; Wang et al., 2012). EdU+/DAPI+ nuclei were quantified and related to proliferative astrocytes due to their large numbers in co‐cultures (~85%) compared to microglia. Astrocytes subjected to OGD prior to reperfusion exhibit a greater proliferation compared to controls; however, metoprolol treatment did not rescue significantly astrocytes from proliferation. Neither GFAP intensity nor GFAP area/nuclei significantly changes among conditions (Figure S8D,E). Immunoblot analyses of M2 markers (YM1, ARG1, and CD243) were performed to assess whether metoprolol exerts an effect on glia polarization; nevertheless, no differences were observed among conditions (Figure S8F,G).
3.7. The neuroprotective effect of β1AR blockade by metoprolol is abolished in the absence of circulating neutrophils
To further explore whether the benefits of metoprolol in stroke are mediated by an effect on neutrophils, as it has been previously described for myocardial infarction (Garcia‐Prieto et al., 2017), we evaluated the impact of β1AR blockade in the absence of circulating neutrophils (Figure 6a). Neutrophils were selectively depleted (~75%) by i.p. administration of anti‐polymorphonuclear (PMN) cell serum 24 h and 48 h before stroke induction (Figure 6a,b). Reduction in lymphocytes and platelets counts was much lower in comparison (~ 20%) (Figure S7A). Metoprolol injection before reperfusion produced no additional benefit in rats previously depleted of neutrophils at 24 h post‐reperfusion (vehicle, 12.1 ± 0.73%; metoprolol, 13.9 ± 2.87%, Figures 6c,d and S10B). Consistent with previous literature (Frieler et al., 2017; Herz et al., 2015; Wang et al., 2019), pre‐stroke neutrophil depletion was associated with a significant reduction in IS in rats subjected to MCAO/R (non‐depleted vehicle, 17.0 ± 1.06%; depleted vehicle, 12.1 ± 0.73%; P < 0.05, Figures 6e and S10C), as well as in cytotoxic oedema (non‐depleted vehicle, 31.0 ± 2.54 mm3; depleted vehicle, 12.4 ± 1.16%; P < 0.05, Figure 6g), at 24 h post‐reperfusion. As expected, in the case of metoprolol treatment, there were not differences in IS or cytotoxic oedema at 24 h post‐reperfusion (Figures 6f,g and S10C).
FIGURE 6.
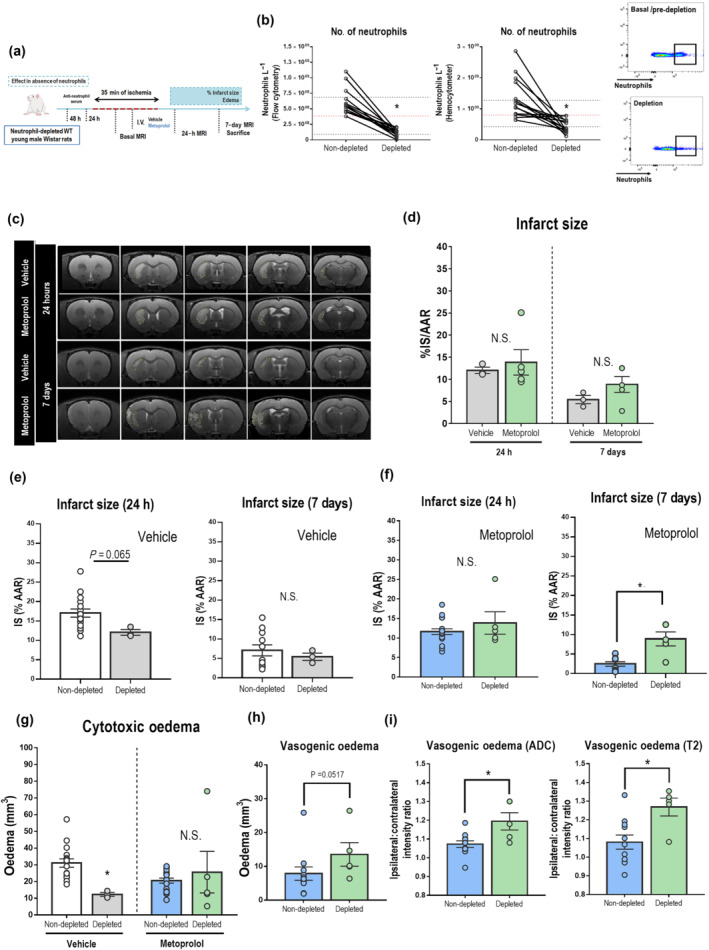
The neuroprotective effect of β1AR blockade by metoprolol is abolished in neutrophil‐depleted rats. (a) Rat model of neutrophil depletion before stroke induction by MCAO/R. Rats were killed at 7 days post‐reperfusion. (b) Depletion of circulating neutrophils with anti‐PMN serum assessed by flow cytometry and haemocytometry. Upper and lower dotted black lines represent peripheral blood neutrophil counting means (baseline and after depletion). Red dotted lines represent minimal neutrophil threshold of depletion. Flow cytometry plots illustrate the reduction in peripheral blood neutrophils after depletion. The black square outlines the anti‐neutrophil+ population. (c) Comparative coronal T2W MRI at 24 h (top rows) and 7 days (bottom rows) post‐reperfusion. Infarcted regions correspond to hyperintense areas. (d) IS evaluated by coronal T2W MRI at 24 h and 7 days post‐reperfusion. Final IS was calculated as the ratio of infarct volume to the AAR and expressed as %. (e) Neutrophil‐depletion was associated with smaller IS at 24 h but not at 7 days post‐reperfusion. (f) Metoprolol treatment in the absence of neutrophils did not provide any additional benefit at 24 h (f, g); however, its effect is completely abolished at 7 days post‐reperfusion, as determined by IS and oedema quantification (h, i). Non‐depleted vehicle, n = 16; non‐depleted metoprolol, n = 18; depleted vehicle, n = 3; depleted metoprolol, n = 5. Graphs show mean ± S.E.M. *P < 0.05. Abbreviations as in Figure 1.
As our previous results pointed, neutrophil stunning by metoprolol creates thereafter a distinct tissue environment, which follows a positive feedback in favour of resolution in which neutrophils feature a key role. Because of that, neutrophil‐depleted rats were followed‐up until 7 days in order to assess whether the absence of neutrophil determines the final stroke outcome. At 7 days there was not any difference between treatment conditions (vehicle, 5.44 ± 0.93%; metoprolol, 8.88 ± 1.80%, Figures 6c,d and S10B); however, the absence of neutrophils did not protect for long (Figures 6e and S10D–F). The neuroprotection exerted by metoprolol at 7 days post‐reperfusion was surprisingly eradicated in the absence of neutrophils (non‐depleted metoprolol, 2.46 ± 0.56%; depleted metoprolol, 8.88 ± 1.80%; P < 0.05; Figure 6f). Moreover, vasogenic oedema also was increased compared to non‐depleted rats under metoprolol treatment (Figure 6h,i). These results highlight the relevance of neutrophil contribution to counteract and balance the inflammatory response and promote tissue recovery.
3.8. β1AR blockade by metoprolol inhibits neutrophil–platelet interactions in stroke patients
To investigate whether β1AR blockade alters neutrophil dynamics and inhibits neutrophil–platelet interactions in humans, whole blood drawn from patients with reperfused acute ischaemic stroke was incubated ex‐vivo with metoprolol (10 μM) or vehicle. Platelet (CD61+ cells) and neutrophils (CD45+ /CD66+ cells) were detected by flow cytometry, and neutrophils positive for CD61 staining were identified as neutrophil–platelet co‐aggregates. Metoprolol significantly inhibited neutrophil–platelet co‐aggregates in ischaemic stroke patients receiving reperfusion therapy (vehicle, 14.0 ± 3.90%; metoprolol, 12.3 ± 3.46%; P < 0.05), whereas it did not exert any effect in healthy volunteers (Figure 7a,b).
FIGURE 7.
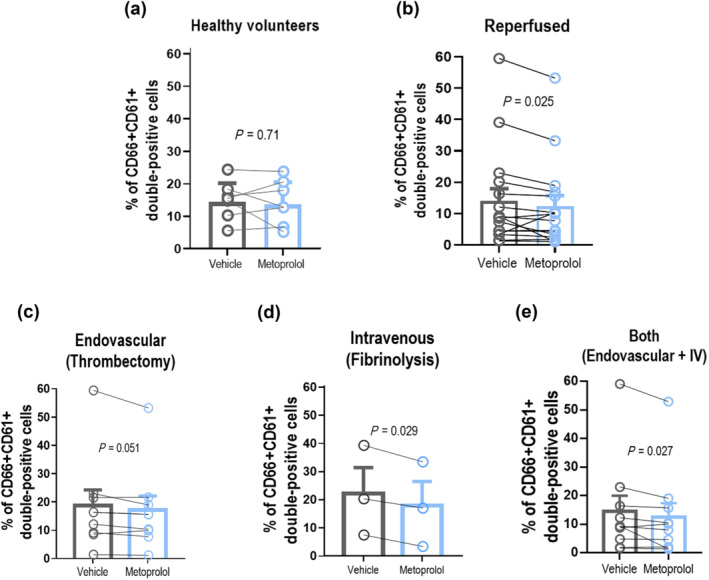
β1AR blockade blocks neutrophil–platelet aggregates in ischaemic stroke patients. (a, b) Platelet (CD61+ cells) and neutrophils (CD45+/CD66+ cells) were detected by flow cytometry, and neutrophils positive for CD61 staining were identified as neutrophil–platelet co‐aggregates. Neutrophil–platelet interactions were inhibited by metoprolol in ischaemic stroke patients who received reperfusion therapy (therapy, n = 16; non‐therapy, n = 8). These interactions were also determined in healthy volunteers (n = 7), who did not respond to metoprolol treatment. A subanalysis was performed based on reperfusion therapy (endovascular thrombectomy, n = 9; fibrinolysis, n = 3; or both, n = 10). Likewise, metoprolol limited co‐aggregates in all groups of treatment (c–e). Graphs show mean ± S.E.M. *P < 0.05
A sub‐analysis was performed based on the reperfusion therapy. Likewise, metoprolol limited neutrophil–platelet interactions in all groups of reperfusion treatment: thrombectomy plus thrombolysis (vehicle, 14.6 ± 5.41%; metoprolol, 12.5 ± 4.92; P < 0.05), thrombectomy (vehicle, 18.7 ± 5.57%; metoprolol, 17.2 ± 4.96%; P = 0.05) and thrombolysis (vehicle, 22.3 ± 9.20%; metoprolol, 17.9 ± 8.64%; P < 0.05) (Figure 7c–e).
4. DISCUSSION
Timely reperfusion is the standard of care for ischaemic stroke; however, increasing evidence supports the notion that reperfusion of an ischaemic arterial bed induces a secondary form of brain damage, known as reperfusion injury, which leads to BBB dysfunction and HT of the ischaemic tissue. Moreover, with the recent advent of endovascular therapy (extending the time window for stroke treatment; Nogueira et al., 2018), I/R injury has become an increasing clinical challenge because there are few interventions able to limit the damage. Neutrophils feature as key protagonists in the proposed mechanisms of cerebral I/R injury, related to their capacity to exacerbate local vascular and tissue injury (Jickling et al., 2015; Kolaczkowska & Kubes, 2013; Lin et al., 2016; Strecker et al., 2017).
Recently, we have reported that selective blockade of β1AR in neutrophils by metoprolol has a unique and pronounced effect on their activity that results in an amelioration of I/R injury and, consequently, smaller myocardial infarctions (Clemente‐Moragon et al., 2020; Garcia‐Prieto et al., 2017). We then speculated that a single injection of the selective β1AR blocker metoprolol might protect the brain during stroke. Even though some studies attributed neuroprotective properties to β‐blocker drugs in stroke (Ajmo et al., 2009; Goyagi et al., 2006; Goyagi et al., 2010, 2011; Gui et al., 2013; Hertz et al., 2014; Iwata et al., 2010; Little et al., 1982; Savitz et al., 2000), their role has always been controversial and raised debatable discussion. The diversity of β‐blocker profiles, routes, and timings of drug administration, as well as the different stroke models and methods for IS and oedema evaluation, has made it difficult to come to a conclusion regarding the use of these agents in stroke outcome. In addition, few mechanisms have been ascribed to this neuroprotection.
Herein, our findings provide strong evidence that the modulating effect of metoprolol on neutrophil dynamics (Garcia‐Prieto et al., 2017) significantly reduces brain injury and promotes inflammatory resolution and tissue repair in a validated model of MCAO/R (Cai et al., 2016) (Graphical Abstract, in Supporting Information). By attenuating acute neutrophil infiltration, metoprolol reduced brain injury and prevented neuronal loss and cerebral oedema progression. This outcome resulted in better preservation of BBB integrity and reduced subacute neuroinflammation. Owing to the initial neutrophil blockade, metoprolol disrupted the self‐amplifying pro‐inflammatory loop and created a pro‐resolving environment that led to a tissue condition favourable to recovery. Furthermore, the ability of metoprolol to decrease IS was abolished in the absence of neutrophils, strongly suggesting that β1AR modulation by metoprolol in these cells is mechanistically involved in these benefits.
Since reperfusion therapy is clearly associated with clinical benefits, and given that endovascular thrombectomy has recently extended the reperfusion time window (Nogueira et al., 2018), we decided to test a therapy with the potential to reduce reperfusion‐related injury in a rodent model of MCAO/R. The non‐invasive nature of MRI allowed us to perform a longitudinal analysis of IS at early (day 1) and late (day 7) after reperfusion. This was important because there is uncertainty about the optimal time for IS quantification (Neumann‐Haefelin et al., 2000). Additionally, MRI performed during brain ischaemia allowed us to accurately estimate the size of the hypoperfused brain region (AAR) and exclude any influence of anatomical variation on recorded differences between groups (Figures 1 and S2).
Cerebral oedema is a severe complication of ischaemic stroke, and medical strategies to reduce its impact are limited (Thoren et al., 2017). Our results indicate that systemic administration of the selective β1AR blocker metoprolol before reperfusion reduces acute cytotoxic and subacute vasogenic oedema (Figure 2). Acute excessive neutrophil infiltration is directly linked to neuronal damage and BBB breakdown. Preservation of BBB integrity is associated with better clearance of toxic cellular by‐products, less subsequent permeability and vasogenic oedema, and less secondary neuroinflammation and neuronal death (Abdullahi et al., 2018). Our results show that early pre‐reperfusion i.v. metoprolol prevents MVO and neutrophil infiltration, stemming progression of the infarct to distant areas and preserving BBB structural integrity, evaluated by the presence of AQP4 water channels at astrocyte end feet and a suggesting neural/glial antigen 2 expression by CSPG+ perivascular pericytes (Sharif et al., 2018). BBB integrity preservation by metoprolol was also confirmed by the absence of IgG extravasation (Figures 2, 3, 4).
Neutrophils' capacities expand further from first‐line defence functions, and their plastic nature makes them able to reprogram and adapt their functions in different inflammatory contexts (Ballesteros et al., 2020; Mantovani et al., 2011; Silvestre‐Roig et al., 2016). In the case of stroke, an excessive neutrophil accumulation is deleterious, while some degree of neutrophil infiltration into brain might be needed to promote infarct resolution. In line with this, metoprolol first protects the brain from an excessive and harmful neutrophil migration by disrupting their dynamics via β1AR, as demonstrated by 2D IVM when exposed to TNFα. This β1AR‐mediated modulatory action results highly specific for neutrophils as metoprolol did not show any significant effect on astrocyte and microglia functionality (Figure 5). Besides, the lack of benefit from β1AR blockade in the absence of neutrophils strongly confirms that the mechanism responsible for this protection is by modulating the deleterious effect of neutrophil accumulation in brain (Figure 6).
Even though neutrophil infiltration is acutely blocked by metoprolol, there still remain a number of neutrophils which do infiltrate, and could regulate the inflammatory reaction and play a key role in the eventual outcome of brain infarct. Under pro‐inflammatory stimuli neutrophils become activated and prolong their lifespan by delaying apoptosis, which favour their accumulation at sites of inflammation (Brostjan & Oehler, 2020). However, by attenuating the acute neutrophil inflow, metoprolol completely counteracts the pro‐inflammatory state, while conserving a population mostly of anti‐inflammatory neutrophils (N2, YM1 or CD206+), more prone to apoptosis. The latter favours neutrophil clearance by microglia and triggers anti‐inflammatory and pro‐resolving responses (Figure 4) (Doran et al., 2020; Jones et al., 2016).
Consistent with all these data, the fact that the rats receiving pre‐reperfusion i.v. metoprolol had better long‐term behavioural and motor capacities, through a wide range of neurological tests, reinforce and consolidate the translational potential of this therapy as a neuroprotective strategy (Figure S4). Although there is abundant experimental evidence that neutrophils play a central role in initiating brain I/R injury, translation to the clinic has so far not yielded clear benefits. The ROS scavenger edaravone was found to improve functional outcomes in several clinical trials when administrated within 24 h of stroke onset (Enomoto et al., 2019). However, trials testing the ability of agents to block neutrophil–endothelium adhesion failed to improve outcomes (e.g., natalizumab in the ACTION trial (Elkins et al., 2017) and the recombinant protein UK‐279,276 in the ASTIN trial (Krams et al., 2003). The strategy used in the present study differs from those tested in these earlier trials. β1AR blockade by metoprolol has an anti‐migratory effect in neutrophils and significantly reduces neutrophil—erythrocyte and neutrophil–platelet (Garcia‐Prieto et al., 2017) interactions, as illustrated here in our cohort of ischaemic stroke patients undergoing reperfusion therapy (Figure 7). Moreover, the anti‐neutrophil–endothelium agents in the ACTION and ASTIN trials were initiated after reperfusion, whereas in our study metoprolol was injected during ongoing cerebral ischaemia. The timing of administration seems critical, since MVO occurs immediately upon reperfusion. Intravenous administration of the selective β1AR blocker used in this study has been shown to be safe in several clinical trials enrolling acutely ill patients (Chatterjee et al., 2013), including a recent study showing that its use in COVID‐19 patients with acute respiratory distress results in a massive reduction of lung neutrophil infiltration and this translates into less days on mechanical ventilation (Clemente‐Moragon et al., 2021). In addition, metoprolol is easy to administer and inexpensive. Therefore, this study constitutes a largely proof of principle which proposes metoprolol as a strong and promising candidate to be further explored for ischaemic stroke treatment.
5. STUDY LIMITATIONS
This study is at the very earliest stages of the translational pathway and therefore presents some limitations that should be addressed in order to translate the potential impact of our findings to the clinics. One of the main limitations is that the study was carried out in young male rats, without including females or any comorbidity such as ageing, hypertension, or diabetes. Moreover, although the MCAO/R model in rodents is one of the most aggressive of ischaemic stroke, 35 min of ischaemia is known to cause mild but not severe strokes. To better establish the therapeutic time window of metoprolol, the neuroprotective effect of its administration should be explored at different times of cerebral ischaemia and upon (or early after) reperfusion. In addition, testing pre‐reperfusion i.v. metoprolol in a stroke model of longer time of ischaemia (≈90 min) could be also interesting to be considered before translation to clinics.
CONFLICT OF INTEREST
Javier Sanchez‐Gonzalez is an employee of Philips Healthcare (Madrid, Spain). All other authors have declared no conflict of interest.
AUTHOR CONTRIBUTIONS
B.I, E.O., M.D. and A.C‐M. are responsible for the design of the entire study. Animal maintenance, MCAO/R surgery and followed‐up by MRI was performed by D.C. and L.C. Haemodynamic dose–response study was carried out by M.G., A.C‐M. and E.O. Brain Magnetic Resonance Imaging Protocol was designed by J.G‐S., L. C. and M.D. Quantification of IS and oedema by MRI was done by A.C‐M. Analyses of MRI results were carried out by A.C‐M., E.O. and J.S‐G. Sensory‐motor assessment was performed by J.M‐P. and MA.M. Neutrophil depletion and evaluation of cell population levels was carried out by A.C‐M. Tissue processing and immunostaining experiments were done by A.C‐M. Immunohistochemical analyses were done by A.C‐M., E.O. and M.C‐C. Inflammatory in vitro and in vivo models in mice were carried out by A.C‐M. Evaluation of human neutrophil–platelet co‐aggregates was done by R.C., N.R. and J.M.B. Analyses of neutrophil–platelet interactions were done by R.C., N.R., J.M.B., J.C.F‐F, J.C‐R, A.C‐M. and E.O. Statistical analyses were done by A.C‐M. and E.O. All results were interpreted by A.C‐M, M.C‐C., J.S‐G., E.O. and B.I. Manuscript was drafted by A.C‐M., and critically revised by E.O., M.C‐C, J.S‐G., V.F. and B.I. B.I., M.D., E.O. and A.C‐M are responsible for the final version of the manuscript, which was approved by all authors.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1. (A) The haemodynamic study of 8 different doses of metoprolol (3.13, 6.25, 12.5 (the formerly chosen dose), 25, 50, 100, 150 and 200 mg/Kg). Intravenous 12.5 mg/kg dose of metoprolol induced a moderate haemodynamic effect, as indicated by decrease in heart rate (bpm) and arterial pressure (mmHg) of less than 20%. These data support the selection of 12.5 mg/Kg i.v. injection for the experimental model of ischaemic stroke. n=5 animals. (B) Infarct size (IS) evaluated by coronal T2W MRI at 24 h (vehicle, n=5; metoprolol, n=5; 1 rat excluded upon confirmation of the absence of artery occlusion). Final IS was calculated as the ratio of infarct volume to the area‐at‐risk (AAR) and expressed as %. A lower dose of metoprolol (6.25 mg/Kg) tended to decrease IS, although no significant differences were observed. Data are presented as mean±SEM.
Figure S2. (A) Area at risk (AAR), determined by dynamic susceptibility contrast perfusion imaging, and infarct size assessed by coronal T2‐weighted (T2W) MRI at 24 h post‐reperfusion (vehicle, n=19; metoprolol, n=23). Infarct size was calculated as the ratio of infarct volume to the AAR and expressed as a percentage. After inclusion of all rats in the statistical analysis (including those with IS > 40%), the significant difference (P<0.05) was maintained between rats receiving pre‐reperfusion vehicle or metoprolol. Dots correspond to individual rats, column heights to median values, and whiskers to interquartile range. *P<0.05; unpaired Mann‐Whitney test. (B) T2W MRI at 24 h post‐reperfusion (top row; coronal plane) and 7 d post‐reperfusion (middle and bottom rows; coronal and axial planes, respectively) in vehicle‐treated rats (left) and metoprolol‐treated rats (right) with IS >40%. Infarct regions correspond to hyperintense areas. Rats with IS >40% also showed a midline shift (yellow arrowheads) or haemorrhage (red arrowheads) at 7 d post‐reperfusion.
Figure S3. (A) Infarct size (IS) normalized to at‐risk‐to‐infarction tissue assessed as perfusion‐diffusion mismatch (PDM). The results were similar to those obtained by normalization to area‐at‐risk (AAR) (see Figure 1) at 24 h post‐reperfusion (vehicle, 29.9 ± 3.39%; metoprolol, 21.4 ± 2.79%; P<0.05) and 7 d post‐reperfusion (vehicle, 11.5 ± 2.78%; metoprolol, 3.55 ± 0.78%; P<0.05) (b) Non‐normalized T2‐weighted MRI infarct volumes; the benefit of metoprolol is still evident at 24 h post‐reperfusion (vehicle, 46.7 ± 3.11 mm3; metoprolol, 32.0 ± 1.77 mm3; P<0.05) and 7 d post‐reperfusion (vehicle, 19.6 ± 3.84 mm3; metoprolol, 7.29 ± 1.83 mm3; P<0.05), indicating that IS was not conditioned by either AAR or PDM. Data are means ± S.E.M. *P<0.05; unpaired Mann‐Whitney or Student t‐test.
Figure S4. A longitudinal study was performed to evaluate long‐term effects of brain injury on sensory‐motor behaviours. The results obtained with the behavioural (A) and motor (B) scales showed a better neurological function in the metoprolol‐treated group. Our evaluation of the sensorimotor functions using the adhesive removal test also revealed an improvement in these neurological deficits (C, D) in the parameter of time for the first attempt to remove the adhesive (C). Sham, n=5; vehicle, n=5; metoprolol, n=10; 1 rat excluded upon confirmation of the absence of artery occlusion. Data are means ± S.E.M. *P<0.05; unpaired Mann‐Whitney or Student t‐test.
Figure S5. Representative IgG immunohistochemistry (IHC) on coronal sections at 7 d post‐reperfusion, showing IgG extravasation in the core lesion of a vehicle‐treated rat (upper panels) and its absence in a metoprolol‐treated one (lower panels). IgG extravasation in the basal ganglia (outlined in purple) at 7 d post‐reperfusion was attenuated in metoprolol‐treated rats.
Figure S6. (A) Representative GFAP immunohistochemistry (IHC) on coronal sections at 7 d post‐reperfusion, showing a glial scar (red outline) in the core lesion of a vehicle‐treated rat (left) and the absence of scarring in a metoprolol‐treated rat (right). Glial scar size was measured as the area lacking astrocytes in the middle cerebral artery (MCA) territory (outlined in orange) at 7 d post‐reperfusion, showing abolition of scarring in metoprolol‐treated rats. (B) Representative IHC of chondroitin sulfate proteoglycans (CSPGs) on coronal sections at 7 d post‐reperfusion, showing CSPG deposition (red outline) in the core lesion of a vehicle‐treated rat (left) and its absence in a metoprolol‐treated rat (right). CSPG deposition in the basal ganglia (outlined in purple) at 7 d post‐reperfusion was attenuated in metoprolol‐treated rats. (C) Representative myelin basic protein (MBP) IHC on coronal sections at 7 d post‐reperfusion, showing myelin sheath degeneration in the core lesion (red outline) of a vehicle‐treated rat (left) and preserved myelination in a metoprolol‐treated rat (right). Quantification of basal ganglia (outlined in purple) myelin sheath degeneration at 7 d post‐reperfusion shows preserved myelination in metoprolol‐treated rats. High magnification views illustrate this process. (D) Representative double immunostaining for CSPG (blue) and GFAP (brown), showing a glial scar filled with a proteoglycan matrix in a vehicle‐treated rat (left) and the absence of scarring in a metoprolol‐treated rat (right). (E) Representative double immunostaining for CSPG (blue) and MBP (brown), showing CSPG deposition and the accumulation of myelin sheath debris in the core lesion of a vehicle‐treated rat, creating an inhibitory context for axonal and neuronal regeneration. In the metoprolol‐injected rat, CSPG deposition is inhibited and myelin sheaths are preserved.
Figure S7. (A) Representative IHC of NeuN, GFAP and Iba1 on coronal sections at 24 h post‐reperfusion, showing no changes in the core lesion between vehicle (left) and metoprolol (right). Neuronal nuclei lost staining and microglia started to acquire a reactive morphology; however, early after reperfusion, there is not glial scarring yet, nor microglia/macrophages pro‐inflammatory response and accumulation. (B) Representative IHC of aquaporin 4 (AQP4) on coronal sections at 24 h postreperfusion, which show no changes in the core lesion between vehicle (top row) and metoprolol (bottom row). Quantification of AQP4 IHC in the MCA of infarcted hemispheres shows no differences between both treatments. Vehicle, n=5; metoprolol, n=7. Graphs show mean ± S.E.M. Abbreviations as in Supplemental Figure 3.
Figure S8. Single and merged channels of triple GFAP+CSPG+RGMa IF, showing Repulsive Guidance Molecule A expression (RGMa; yellow) and proteoglycan deposition (CSPG; grey) in the core lesion of a vehicle‐treated rat (top row) and their absence in a metoprolol‐treated rat (bottom row). Nuclei were revealed with DAPI (blue).
Figure S9. (A) Flow cytometry plots illustrating the purity of glia‐based co‐cultures. (B, C) CXCL1 levels in glia‐based co‐culture supernatants. (D) Fluorescence images of proliferative (EdU, red) astrocytes Clemente‐Moragón, A., et al: β1AR blockade blunts stroke neuroinflammation (GFAP, green) subjected or not to OGD, and treated or not with vehicle or metoprolol before or after reperfusion. Nuclei were revealed with DAPI (blue). (E) Graphs show quantification of % of EdU+nuclei over total nuclei (DAPI+), GFAP mean intensity and GFAP area/nuclei (n=4 per condition) (F, G) Immunoblot analyses of Arginase‐1 (37 kDa), YM1 (44 kDa), CD243 (55kDa) and vinculin (120 kDa) protein expression in co‐cultures subjected or not to OGD, and treated or not with vehicle or metoprolol before or after reperfusion (n=4 per condition). Graphs show mean ± S.E.M.
Figure S9.
Figure S10. (A) White blood cell counts after treatment with anti‐PMN serum assessed by hemocytometry. Upper and lower dotted black lines represent white blood cells counting means (baseline and after depletion). (B, C) Neither between distinct treatment conditions in the absence of neutrophils, nor between same treatment conditions in the presence or absence of neutrophils, differences were observed in AAR. (D) The absence of neutrophils did not provide neuroprotection as metoprolol did in wild‐type rats at 7 d post‐reperfusion (depleted vehicle, n=3; depleted metoprolol, n=18). (E, F) The absence of neutrophils did not reduce vasogenic oedema (non‐depleted vehicle, n=16; depleted vehicle, n=3). Graphs show mean ± S.E.M. *P<0.05.
Video S1. 3D confocal reconstruction of triple GFAP+AQ4+Iba1+ immunofluorescence on a coronal brain section of a rat receiving a pre‐reperfusion i.v. bolus of metoprolol at 7 d postreperfusion, illustrating preservation of aquaporin‐4 expression (AQP4; red) in the end feet of astrocytes (GFAP; green), indicating preserved blood‐brain barrier integrity. Microglia/macrophages (Iba1; purple) were found around vessels. Nuclei were revealed with DAPI (blue).
Video S2. 3D confocal reconstruction of triple Neutrophil+TUNEL+Iba1+ immunofluorescence on a coronal brain section of a rat receiving a pre‐reperfusion i.v. bolus of vehicle at 7 d post‐reperfusion, illustrating how apoptotic (positive for TUNEL [red]) neutrophils (green) are preferably engulfed by microglia (positive for Iba1 [grey]). Nuclei were revealed with DAPI (blue).
Video S3 (A‐D). In vivo 2D IVM recordings, showing representative crawling neutrophils (positive for Ly6G [green] and CD62L [yellow]), interacting with platelets (positive for CD41 [red]) in inflamed cremaster muscle vessels, of WT or neutrophil conditional Adrb1 KO mice receiving i.v. vehicle (saline) or metoprolol. Metoprolol did not provide any further effect on neutrophil behaviour in the absence of β1AR.
ACKNOWLEDGEMENTS
We particularly thank Alexandra de Francisco, Yolanda Sierra and María de la Jara‐Felipe for their project involvement and great skills with surgical procedures in rats. We also thank Lucía López Palomar for her help collecting and fixing brains, Ana Marcos for sharing her expertise with brain tissue processing, Verónica Labrador for her command with microscopy image capture and video editing and Bahia El Maimouni for her assistance as colony manager. We thank Antonio de Molina for his support with the histopathological analysis and Antonio José Sánchez for his help with human neutrophils manipulation. We wish to thank the donors, and the Biobank Hospital Universitario Puerta de Hierro Majadahonda/Instituto de Investigación Sanitaria Puerta de Hierro‐Segovia de Arana (IDIPHISA) (PT17/0015/0020 in the Spanish National Biobanks Network) for the human specimens used in this study. We thank Carlos Galán for showing once again his magnificent skills creating the graphical abstract. We thank the CNIC Cellomics, Microscopy, Comparative Medicine, Histopathology and Advanced Imaging Units. Simon Bartlett (CNIC) provided English editing.
Clemente‐Moragón, A. , Oliver, E. , Calle, D. , Cussó, L. , Gómez, M. , Pradillo, J. M. , Castejón, R. , Rallón, N. , Benito, J. M. , Fernández‐Ferro, J. C. , Carneado‐Ruíz, J. , Moro, M. A. , Sánchez‐González, J. , Fuster, V. , Cortés‐Canteli, M. , Desco, M. , & Ibáñez, B. (2023). Neutrophil β1 adrenoceptor blockade blunts stroke‐associated neuroinflammation. British Journal of Pharmacology, 180(4), 459–478. 10.1111/bph.15963
Funding Information This study received funding from the Instituto de Salud Carlos III (ISCIII; PI16/02110 to B.I. and PT20/00044 to M.D.), the European Regional Development Fund (ERDF) “A way of making Europe,” the Comunidad de Madrid (S2017/BMD‐3867 RENIM‐CM to M.D. and B.I.), cofunding from European structural, and investment funds, and by Agencia Estatal de Investigación (PID2019‐110369RB‐I00 to B.I.). B.I. is a recipient of funding from the European Research Council (ERC) under the European Union Horizon 2020 Research and Innovation Programme (ERC‐Consolidator Grant agreement No. 819775). E.O. is a recipient of funds from the Comunidad de Madrid Programa de Atracción de Talento (2017‐T1/BMD‐5185) and from a Ramón y Cajal grant (RYC2020‐028884‐I) funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future.” A.C.M. is the beneficiary of an FPU fellowship from the Ministerio de Ciencia e Innovación (FPU2017/01932). M.C.C. is the beneficiary of a Miguel Servet contract (MS16/00174). The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Innovación and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (CEX2020‐001041‐S).
Agustín Clemente‐Moragón and Eduardo Oliver contributed equally to this work.
Contributor Information
Manuel Desco, Email: desco@hggm.es.
Borja Ibáñez, Email: bibanez@cnic.es.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- Abdullahi, W. , Tripathi, D. , & Ronaldson, P. T. (2018). Blood‐brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. American Journal of Physiology Cell Physiology, 315(3), C343–C356. 10.1152/ajpcell.00095.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo, C. T. Jr. , Collier, L. A. , Leonardo, C. C. , Hall, A. A. , Green, S. M. , Womble, T. A. , Cuevas, J. , Willing, A. E. , & Pennypacker, K. R. (2009). Blockade of adrenoreceptors inhibits the splenic response to stroke. Experimental Neurology, 218(1), 47–55. 10.1016/j.expneurol.2009.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). The concise guide to pharmacology 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros, I. , Rubio‐Ponce, A. , Genua, M. , Lusito, E. , Kwok, I. , Fernandez‐Calvo, G. , Khoyratty, T. E. , van Grinsven, E. , González‐Hernández, S. , Nicolás‐Ávila, J. Á. , Vicanolo, T. , Maccataio, A. , Benguría, A. , Li, J. L. , Adrover, J. M. , Aroca‐Crevillen, A. , Quintana, J. A. , Martín‐Salamanca, S. , Mayo, F. , … Hidalgo, A. (2020). Co‐option of neutrophil fates by tissue environments. Cell, 183(5), 1282–1297e1218. 10.1016/j.cell.2020.10.003 [DOI] [PubMed] [Google Scholar]
- Birenbaum, D. , Bancroft, L. W. , & Felsberg, G. J. (2011). Imaging in acute stroke. The Western Journal of Emergency Medicine, 12(1), 67–76. [PMC free article] [PubMed] [Google Scholar]
- Brostjan, C. , & Oehler, R. (2020). The role of neutrophil death in chronic inflammation and cancer. Cell Death Discovery, 6, 26. 10.1038/s41420-020-0255-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. , Xu, G. , Liu, J. , Wang, L. , Deng, G. , Liu, J. , & Chen, Z. (2016). A modification of intraluminal middle cerebral artery occlusion/reperfusion model for ischemic stroke with laser Doppler flowmetry guidance in mice. Neuropsychiatric Disease and Treatment, 12, 2851–2858. 10.2147/NDT.S118531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti, A. , Rusu, D. , Flamand, N. , Borgeat, P. , & Poubelle, P. E. (2009). Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Laboratory Investigation; a Journal of Technical Methods and Pathology, 89(10), 1084–1099. 10.1038/labinvest.2009.74 [DOI] [PubMed] [Google Scholar]
- Chatterjee, S. , Chaudhuri, D. , Vedanthan, R. , Fuster, V. , Ibanez, B. , Bangalore, S. , & Mukherjee, D. (2013). Early intravenous beta‐blockers in patients with acute coronary syndrome—A meta‐analysis of randomized trials. International Journal of Cardiology, 168(2), 915–921. 10.1016/j.ijcard.2012.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente‐Moragon, A. , Gomez, M. , Villena‐Gutierrez, R. , Lalama, D. V. , Garcia‐Prieto, J. , Martinez, F. , Sánchez‐Cabo, F. , Fuster, V. , Oliver, E. , & Ibáñez, B. (2020). Metoprolol exerts a non‐class effect against ischaemia‐reperfusion injury by abrogating exacerbated inflammation. European Heart Journal, 41(46), 4425–4440. 10.1093/eurheartj/ehaa733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente‐Moragon, A. , Martinez‐Milla, J. , Oliver, E. , Santos, A. , Flandes, J. , Fernandez, I. , Rodríguez‐González, L. , Serrano Del Castillo, C. , Ioan, A. M. , López‐Álvarez, M. , Gómez‐Talavera, S. , Galán‐Arriola, C. , Fuster, V. , Pérez‐Calvo, C. , & Ibáñez, B. (2021). Metoprolol in critically ill patients with COVID‐19. Journal of the American College of Cardiology, 78(10), 1001–1011. 10.1016/j.jacc.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero, M. I. , Ballesteros, I. , Moraga, A. , Nombela, F. , Vivancos, J. , Hamilton, J. A. , Corbí, Á. L. , Lizasoain, I. , & Moro, M. A. (2013). N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the PPARgamma agonist rosiglitazone. Stroke, 44(12), 3498–3508. 10.1161/STROKEAHA.113.002470 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran, A. C. , Yurdagul, A. Jr. , & Tabas, I. (2020). Efferocytosis in health and disease. Nature Reviews Immunology, 20(4), 254–267. 10.1038/s41577-019-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins, J. , Veltkamp, R. , Montaner, J. , Johnston, S. C. , Singhal, A. B. , Becker, K. , Lansberg, M. G. , Tang, W. , Chang, I. , Muralidharan, K. , Gheuens, S. , Mehta, L. , & Elkind, M. S. V. (2017). Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo‐controlled, double‐blind phase 2 trial. The Lancet Neurology, 16(3), 217–226. 10.1016/S1474-4422(16)30357-X [DOI] [PubMed] [Google Scholar]
- Enomoto, M. , Endo, A. , Yatsushige, H. , Fushimi, K. , & Otomo, Y. (2019). Clinical effects of early edaravone use in acute ischemic stroke patients treated by endovascular reperfusion therapy. Stroke, 50(3), 652–658. 10.1161/STROKEAHA.118.023815 [DOI] [PubMed] [Google Scholar]
- Follesa, P. , & Mocchetti, I. (1993). Regulation of basic fibroblast growth factor and nerve growth factor mRNA by beta‐adrenergic receptor activation and adrenal steroids in rat central nervous system. Molecular Pharmacology, 43(2), 132–138. [PubMed] [Google Scholar]
- Fridlender, Z. G. , Sun, J. , Kim, S. , Kapoor, V. , Cheng, G. , Ling, L. , Worthen, G. S. , & Albelda, S. M. (2009). Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: “N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieler, R. A. , Chung, Y. , Ahlers, C. G. , Gheordunescu, G. , Song, J. , Vigil, T. M. , Shah, Y. M. , & Mortensen, R. M. (2017). Genetic neutrophil deficiency ameliorates cerebral ischemia‐reperfusion injury. Experimental Neurology, 298(Pt A), 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, A. M. , & Badaut, J. (2012). Aquaporin 4: A player in cerebral edema and neuroinflammation. Journal of Neuroinflammation, 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Culebras, A. , Duran‐Laforet, V. , Pena‐Martinez, C. , Moraga, A. , Ballesteros, I. , Cuartero, M. I. , de la Parra, J. , Palma‐Tortosa, S. , Hidalgo, A. , Corbí, A. L. , Moro, M. A. , & Lizasoain, I. (2019). Role of TLR4 (toll‐like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke, 50(10), 2922–2932. 10.1161/STROKEAHA.119.025085 [DOI] [PubMed] [Google Scholar]
- Garcia‐Prieto, J. , Villena‐Gutierrez, R. , Gomez, M. , Bernardo, E. , Pun‐Garcia, A. , Garcia‐Lunar, I. , Crainiciuc, G. , Fernández‐Jiménez, R. , Sreeramkumar, V. , Bourio‐Martínez, R. , García‐Ruiz, J. M. , Del Valle, A. S. , Sanz‐Rosa, D. , Pizarro, G. , Fernández‐Ortiz, A. , Hidalgo, A. , Fuster, V. , & Ibanez, B. (2017). Neutrophil stunning by metoprolol reduces infarct size. Nature Communications, 8, 14780. 10.1038/ncomms14780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Ruiz, J. M. , Fernandez‐Jimenez, R. , Garcia‐Alvarez, A. , Pizarro, G. , Galan‐Arriola, C. , Fernandez‐Friera, L. , Mateos, A. , Nuno‐Ayala, M. , Aguero, J. , Sánchez‐González, J. , García‐Prieto, J. , López‐Melgar, B. , Martínez‐Tenorio, P. , López‐Martín, G. J. , Macías, A. , Pérez‐Asenjo, B. , Cabrera, J. A. , Fernández‐Ortiz, A. , Fuster, V. , & Ibáñez, B. (2016). Impact of the timing of metoprolol administration during STEMI on infarct size and ventricular function. Journal of the American College of Cardiology, 67(18), 2093–2104. 10.1016/j.jacc.2016.02.050 [DOI] [PubMed] [Google Scholar]
- Goyagi, T. , Horiguchi, T. , Nishikawa, T. , & Tobe, Y. (2010). Post‐treatment with selective beta1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Research, 1343, 213–217. 10.1016/j.brainres.2010.04.079 [DOI] [PubMed] [Google Scholar]
- Goyagi, T. , Kimura, T. , Nishikawa, T. , Tobe, Y. , & Masaki, Y. (2006). Beta‐adrenoreceptor antagonists attenuate brain injury after transient focal ischemia in rats. Anesthesia and Analgesia, 103(3), 658–663. 10.1213/01.ane.0000228859.95126.69 [DOI] [PubMed] [Google Scholar]
- Goyagi, T. , Nishikawa, T. , & Tobe, Y. (2011). Neuroprotective effects and suppression of ischemia‐induced glutamate elevation by beta1‐adrenoreceptor antagonists administered before transient focal ischemia in rats. Journal of Neurosurgical Anesthesiology, 23(2), 131–137. 10.1097/ANA.0b013e31820369c1 [DOI] [PubMed] [Google Scholar]
- Gui, H. , Guo, Y. F. , Liu, X. , Zhang, J. M. , Yang, Y. L. , Huang, G. Z. , & Liu, J. G. (2013). Effects of combination therapy with levamlodipine and bisoprolol on stroke in rats. CNS Neuroscience & Therapeutics, 19(3), 178–182. 10.1111/cns.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, A. R. , & Lee, J. K. (2016). Understanding the NG2 glial scar after spinal cord injury. Frontiers in Neurology, 7, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, R. Q. , Ouyang, Y. B. , Xu, L. , Agrawal, R. , Patterson, A. J. , & Giffard, R. G. (2009). Postischemic brain injury is attenuated in mice lacking the beta2‐adrenergic receptor. Anesthesia and Analgesia, 108(1), 280–287. 10.1213/ane.0b013e318187ba6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz, L. , Xu, J. , Chen, Y. , Gibbs, M. E. , Du, T. , Hertz, L. , Xu, J. , Chen, Y. , Gibbs, M. E. , & Du, T. (2014). Antagonists of the vasopressin V1 receptor and of the beta(1)‐adrenoceptor inhibit cytotoxic brain edema in stroke by effects on astrocytes—But the mechanisms differ. Current Neuropharmacology, 12(4), 308–323. 10.2174/1570159X12666140828222723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz, J. , Sabellek, P. , Lane, T. E. , Gunzer, M. , Hermann, D. M. , & Doeppner, T. R. (2015). Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke, 46(10), 2916–2925. 10.1161/STROKEAHA.115.010620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horckmans, M. , Ring, L. , Duchene, J. , Santovito, D. , Schloss, M. J. , Drechsler, M. , Weber, C. , Soehnlein, O. , & Steffens, S. (2017). Neutrophils orchestrate post‐myocardial infarction healing by polarizing macrophages towards a reparative phenotype. European Heart Journal, 38(3), 187–197. 10.1093/eurheartj/ehw002 [DOI] [PubMed] [Google Scholar]
- Ibanez, B. , Heusch, G. , Ovize, M. , & Van de Werf, F. (2015). Evolving therapies for myocardial ischemia/reperfusion injury. Journal of the American College of Cardiology, 65(14), 1454–1471. 10.1016/j.jacc.2015.02.032 [DOI] [PubMed] [Google Scholar]
- Iwata, M. , Inoue, S. , Kawaguchi, M. , Nakamura, M. , Konishi, N. , & Furuya, H. (2010). Posttreatment but not pretreatment with selective beta‐adrenoreceptor 1 antagonists provides neuroprotection in the hippocampus in rats subjected to transient forebrain ischemia. Anesthesia and Analgesia, 110(4), 1126–1132. 10.1213/ANE.0b013e3181d278f7 [DOI] [PubMed] [Google Scholar]
- Jickling, G. C. , Liu, D. , Ander, B. P. , Stamova, B. , Zhan, X. , & Sharp, F. R. (2015). Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 35(6), 888–901. 10.1038/jcbfm.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, H. R. , Robb, C. T. , Perretti, M. , & Rossi, A. G. (2016). The role of neutrophils in inflammation resolution. Seminars in Immunology, 28(2), 137–145. 10.1016/j.smim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Junker, V. , Becker, A. , Huhne, R. , Zembatov, M. , Ravati, A. , Culmsee, C. , & Krieglstein, J. (2002). Stimulation of beta‐adrenoceptors activates astrocytes and provides neuroprotection. European Journal of Pharmacology, 446(1–3), 25–36. 10.1016/S0014-2999(02)01814-9 [DOI] [PubMed] [Google Scholar]
- Kolaczkowska, E. , & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13(3), 159–175. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- Krams, M. , Lees, K. R. , Hacke, W. , Grieve, A. P. , Orgogozo, J. M. , Ford, G. A. , & ASTIN Study Investigators . (2003). Acute stroke therapy by inhibition of neutrophils (ASTIN): An adaptive dose‐response study of UK‐279,276 in acute ischemic stroke. Stroke, 34(11), 2543–2548. 10.1161/01.STR.0000092527.33910.89 [DOI] [PubMed] [Google Scholar]
- Lechtenberg, K. J. , Meyer, S. T. , Doyle, J. B. , Peterson, T. C. , & Buckwalter, M. S. (2019). Augmented beta2‐adrenergic signaling dampens the neuroinflammatory response following ischemic stroke and increases stroke size. Journal of Neuroinflammation, 16(1), 112. 10.1186/s12974-019-1506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. Y. , Li, X. , Liu, S. F. , Qu, W. S. , Wang, W. , & Tian, D. S. (2015). Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen‐glucose deprivation and reoxygenation. Neurochemistry International, 83‐84, 9–18. 10.1016/j.neuint.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. , Cirino, G. , Docherty, J. R. , George, C. H. , Insel, P. A. , Izzo, A. A. , Ji, Y. , Panettieri, R. A. , Sobey, C. G. , Stefanska, B. , Stephens, G. , Teixeira, M. , & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177(16), 3611‐3616. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. , Wang, X. , & Yu, Z. (2016). Ischemia‐reperfusion injury in the brain: Mechanisms and potential therapeutic strategies. Biochemistry & Pharmacology: Open Access, 5(4), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, J. R. , Latchaw, J. P. Jr. , Slugg, R. M. , Lesser, R. P. , & Stowe, N. T. (1982). Treatment of acute focal cerebral ischemia with propranolol. Stroke, 13(3), 302–307. 10.1161/01.STR.13.3.302 [DOI] [PubMed] [Google Scholar]
- Mantovani, A. , Cassatella, M. A. , Costantini, C. , & Jaillon, S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology, 11(8), 519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Monai, H. , Wang, X. , Yahagi, K. , Lou, N. , Mestre, H. , Xu, Q. , Abe, Y. , Yasui, M. , Iwai, Y. , Nedergaard, M. , & Hirase, H. (2019). Adrenergic receptor antagonism induces neuroprotection and facilitates recovery from acute ischemic stroke. Proceedings of the National Academy of Sciences of the United States of America, 116(22), 11010–11019. 10.1073/pnas.1817347116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann‐Haefelin, T. , Kastrup, A. , de Crespigny, A. , Yenari, M. A. , Ringer, T. , Sun, G. H. , & Moseley, M. E. (2000). Serial MRI after transient focal cerebral ischemia in rats: Dynamics of tissue injury, blood‐brain barrier damage, and edema formation. Stroke, 31(8), 1965–1972discussion 1972‐1963. 10.1161/01.STR.31.8.1965 [DOI] [PubMed] [Google Scholar]
- Nogueira, R. G. , Jadhav, A. P. , Haussen, D. C. , Bonafe, A. , Budzik, R. F. , Bhuva, P. , Yavagal, D. R. , Ribo, M. , Cognard, C. , Hanel, R. A. , Sila, C. A. , Hassan, A. E. , Millan, M. , Levy, E. I. , Mitchell, P. , Chen, M. , English, J. D. , Shah, Q. A. , Silver, F. L. , … DAWN Trial Investigators . (2018). Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. The New England Journal of Medicine, 378(1), 11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- Ohms, M. , Moller, S. , & Laskay, T. (2020). An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Frontiers in Immunology, 11, 532. 10.3389/fimmu.2020.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou‐Yang, L. , Liu, Y. , Wang, B. Y. , Cao, P. , Zhang, J. J. , Huang, Y. Y. , Shen, Y. , & Lyu, J. X. (2018). Carnosine suppresses oxygen‐glucose deprivation/recovery‐induced proliferation and migration of reactive astrocytes of rats in vitro. Acta Pharmacologica Sinica, 39(1), 24–34. 10.1038/aps.2017.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , Browne, W. J. , Clark, A. , Cuthill, I. C. , Dirnagl, U. , Emerson, M. , Garner, P. , Holgate, S. T. , Howells, D. W. , Karp, N. A. , Lazic, S. E. , Lidster, K. , MacCallum, C. J. , Macleod, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro, G. , Fernandez‐Friera, L. , Fuster, V. , Fernandez‐Jimenez, R. , Garcia‐Ruiz, J. M. , Garcia‐Alvarez, A. , Mateos, A. , Barreiro, M. V. , Escalera, N. , Rodriguez, M. D. , de Miguel, A. , García‐Lunar, I. , Parra‐Fuertes, J. J. , Sánchez‐González, J. , Pardillos, L. , Nieto, B. , Jiménez, A. , Abejón, R. , Bastante, T. , … Ibanez, B. (2014). Long‐term benefit of early pre‐reperfusion metoprolol administration in patients with acute myocardial infarction: Results from the METOCARD‐CNIC trial (effect of metoprolol in cardioprotection during an acute myocardial infarction). Journal of the American College of Cardiology, 63(22), 2356–2362. 10.1016/j.jacc.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Powers, W. J. , Rabinstein, A. A. , Ackerson, T. , Adeoye, O. M. , Bambakidis, N. C. , Becker, K. , Biller, J. , Brown, M. , Demaerschalk, B. M. , Hoh, B. , Jauch, E. C. , Kidwell, C. S. , Leslie‐Mazwi, T. M. , Ovbiagele, B. , Scott, P. A. , Sheth, K. N. , Southerland, A. M. , Summers, D. V. , & Tirschwell, D. L. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 50(12), e344–e418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- Puellmann, K. , Kaminski, W. E. , Vogel, M. , Nebe, C. T. , Schroeder, J. , Wolf, H. , & Beham, A. W. (2006). A variable immunoreceptor in a subpopulation of human neutrophils. Proceedings of the National Academy of Sciences of the United States of America, 103(39), 14441–14446. 10.1073/pnas.0603406103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, S. I. , Erhardt, J. A. , Anthony, J. V. , Gupta, G. , Li, X. , Barone, F. C. , & Rosenbaum, D. M. (2000). The novel beta‐blocker, carvedilol, provides neuroprotection in transient focal stroke. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 20(8), 1197–1204. 10.1097/00004647-200008000-00005 [DOI] [PubMed] [Google Scholar]
- Semkova, I. , Schilling, M. , Henrich‐Noack, P. , Rami, A. , & Krieglstein, J. (1996). Clenbuterol protects mouse cerebral cortex and rat hippocampus from ischemic damage and attenuates glutamate neurotoxicity in cultured hippocampal neurons by induction of NGF. Brain Research, 717(1–2), 44–54. 10.1016/0006-8993(95)01567-1 [DOI] [PubMed] [Google Scholar]
- Sharif, Y. , Jumah, F. , Coplan, L. , Krosser, A. , Sharif, K. , & Tubbs, R. S. (2018). Blood brain barrier: A review of its anatomy and physiology in health and disease. Clinical Anatomy, 31(6), 812–823. 10.1002/ca.23083 [DOI] [PubMed] [Google Scholar]
- Silvestre‐Roig, C. , Hidalgo, A. , & Soehnlein, O. (2016). Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood, 127(18), 2173–2181. 10.1182/blood-2016-01-688887 [DOI] [PubMed] [Google Scholar]
- Sreeramkumar, V. , Adrover, J. M. , Ballesteros, I. , Cuartero, M. I. , Rossaint, J. , Bilbao, I. , Nácher, M. , Pitaval, C. , Radovanovic, I. , Fukui, Y. , McEver, R. , Filippi, M. D. , Lizasoain, I. , Ruiz‐Cabello, J. , Zarbock, A. , Moro, M. A. , & Hidalgo, A. (2014). Neutrophils scan for activated platelets to initiate inflammation. Science, 346(6214), 1234–1238. 10.1126/science.1256478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker, J. K. , Schmidt, A. , Schabitz, W. R. , & Minnerup, J. (2017). Neutrophil granulocytes in cerebral ischemia—Evolution from killers to key players. Neurochemistry International, 107, 117–126. 10.1016/j.neuint.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Thoren, M. , Azevedo, E. , Dawson, J. , Egido, J. A. , Falcou, A. , Ford, G. A. , Holmin, S. , Mikulik, R. , Ollikainen, J. , Wahlgren, N. , & Ahmed, N. (2017). Predictors for cerebral edema in acute ischemic stroke treated with intravenous thrombolysis. Stroke, 48(9), 2464–2471. 10.1161/STROKEAHA.117.018223 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Zhang, X. , Zhang, J. , Fan, Y. , Shen, Y. , Hu, W. , & Chen, Z. (2012). Oxygen‐glucose deprivation induced glial scar‐like change in astrocytes. PLoS ONE, 7(5), e37574. 10.1371/journal.pone.0037574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Jin, H. , Wang, W. , Wang, F. , & Zhao, H. (2019). Myosin1f‐mediated neutrophil migration contributes to acute neuroinflammation and brain injury after stroke in mice. Journal of Neuroinflammation, 16(1), 77. 10.1186/s12974-019-1465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Tao, Y. , Wu, Y. , Zhao, X. , Ye, W. , Zhao, D. , Fu, L. , Tian, C. , Yang, J. , He, F. , & Tang, L. (2019). Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nature Communications, 10(1), 1076. 10.1038/s41467-019-09046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) The haemodynamic study of 8 different doses of metoprolol (3.13, 6.25, 12.5 (the formerly chosen dose), 25, 50, 100, 150 and 200 mg/Kg). Intravenous 12.5 mg/kg dose of metoprolol induced a moderate haemodynamic effect, as indicated by decrease in heart rate (bpm) and arterial pressure (mmHg) of less than 20%. These data support the selection of 12.5 mg/Kg i.v. injection for the experimental model of ischaemic stroke. n=5 animals. (B) Infarct size (IS) evaluated by coronal T2W MRI at 24 h (vehicle, n=5; metoprolol, n=5; 1 rat excluded upon confirmation of the absence of artery occlusion). Final IS was calculated as the ratio of infarct volume to the area‐at‐risk (AAR) and expressed as %. A lower dose of metoprolol (6.25 mg/Kg) tended to decrease IS, although no significant differences were observed. Data are presented as mean±SEM.
Figure S2. (A) Area at risk (AAR), determined by dynamic susceptibility contrast perfusion imaging, and infarct size assessed by coronal T2‐weighted (T2W) MRI at 24 h post‐reperfusion (vehicle, n=19; metoprolol, n=23). Infarct size was calculated as the ratio of infarct volume to the AAR and expressed as a percentage. After inclusion of all rats in the statistical analysis (including those with IS > 40%), the significant difference (P<0.05) was maintained between rats receiving pre‐reperfusion vehicle or metoprolol. Dots correspond to individual rats, column heights to median values, and whiskers to interquartile range. *P<0.05; unpaired Mann‐Whitney test. (B) T2W MRI at 24 h post‐reperfusion (top row; coronal plane) and 7 d post‐reperfusion (middle and bottom rows; coronal and axial planes, respectively) in vehicle‐treated rats (left) and metoprolol‐treated rats (right) with IS >40%. Infarct regions correspond to hyperintense areas. Rats with IS >40% also showed a midline shift (yellow arrowheads) or haemorrhage (red arrowheads) at 7 d post‐reperfusion.
Figure S3. (A) Infarct size (IS) normalized to at‐risk‐to‐infarction tissue assessed as perfusion‐diffusion mismatch (PDM). The results were similar to those obtained by normalization to area‐at‐risk (AAR) (see Figure 1) at 24 h post‐reperfusion (vehicle, 29.9 ± 3.39%; metoprolol, 21.4 ± 2.79%; P<0.05) and 7 d post‐reperfusion (vehicle, 11.5 ± 2.78%; metoprolol, 3.55 ± 0.78%; P<0.05) (b) Non‐normalized T2‐weighted MRI infarct volumes; the benefit of metoprolol is still evident at 24 h post‐reperfusion (vehicle, 46.7 ± 3.11 mm3; metoprolol, 32.0 ± 1.77 mm3; P<0.05) and 7 d post‐reperfusion (vehicle, 19.6 ± 3.84 mm3; metoprolol, 7.29 ± 1.83 mm3; P<0.05), indicating that IS was not conditioned by either AAR or PDM. Data are means ± S.E.M. *P<0.05; unpaired Mann‐Whitney or Student t‐test.
Figure S4. A longitudinal study was performed to evaluate long‐term effects of brain injury on sensory‐motor behaviours. The results obtained with the behavioural (A) and motor (B) scales showed a better neurological function in the metoprolol‐treated group. Our evaluation of the sensorimotor functions using the adhesive removal test also revealed an improvement in these neurological deficits (C, D) in the parameter of time for the first attempt to remove the adhesive (C). Sham, n=5; vehicle, n=5; metoprolol, n=10; 1 rat excluded upon confirmation of the absence of artery occlusion. Data are means ± S.E.M. *P<0.05; unpaired Mann‐Whitney or Student t‐test.
Figure S5. Representative IgG immunohistochemistry (IHC) on coronal sections at 7 d post‐reperfusion, showing IgG extravasation in the core lesion of a vehicle‐treated rat (upper panels) and its absence in a metoprolol‐treated one (lower panels). IgG extravasation in the basal ganglia (outlined in purple) at 7 d post‐reperfusion was attenuated in metoprolol‐treated rats.
Figure S6. (A) Representative GFAP immunohistochemistry (IHC) on coronal sections at 7 d post‐reperfusion, showing a glial scar (red outline) in the core lesion of a vehicle‐treated rat (left) and the absence of scarring in a metoprolol‐treated rat (right). Glial scar size was measured as the area lacking astrocytes in the middle cerebral artery (MCA) territory (outlined in orange) at 7 d post‐reperfusion, showing abolition of scarring in metoprolol‐treated rats. (B) Representative IHC of chondroitin sulfate proteoglycans (CSPGs) on coronal sections at 7 d post‐reperfusion, showing CSPG deposition (red outline) in the core lesion of a vehicle‐treated rat (left) and its absence in a metoprolol‐treated rat (right). CSPG deposition in the basal ganglia (outlined in purple) at 7 d post‐reperfusion was attenuated in metoprolol‐treated rats. (C) Representative myelin basic protein (MBP) IHC on coronal sections at 7 d post‐reperfusion, showing myelin sheath degeneration in the core lesion (red outline) of a vehicle‐treated rat (left) and preserved myelination in a metoprolol‐treated rat (right). Quantification of basal ganglia (outlined in purple) myelin sheath degeneration at 7 d post‐reperfusion shows preserved myelination in metoprolol‐treated rats. High magnification views illustrate this process. (D) Representative double immunostaining for CSPG (blue) and GFAP (brown), showing a glial scar filled with a proteoglycan matrix in a vehicle‐treated rat (left) and the absence of scarring in a metoprolol‐treated rat (right). (E) Representative double immunostaining for CSPG (blue) and MBP (brown), showing CSPG deposition and the accumulation of myelin sheath debris in the core lesion of a vehicle‐treated rat, creating an inhibitory context for axonal and neuronal regeneration. In the metoprolol‐injected rat, CSPG deposition is inhibited and myelin sheaths are preserved.
Figure S7. (A) Representative IHC of NeuN, GFAP and Iba1 on coronal sections at 24 h post‐reperfusion, showing no changes in the core lesion between vehicle (left) and metoprolol (right). Neuronal nuclei lost staining and microglia started to acquire a reactive morphology; however, early after reperfusion, there is not glial scarring yet, nor microglia/macrophages pro‐inflammatory response and accumulation. (B) Representative IHC of aquaporin 4 (AQP4) on coronal sections at 24 h postreperfusion, which show no changes in the core lesion between vehicle (top row) and metoprolol (bottom row). Quantification of AQP4 IHC in the MCA of infarcted hemispheres shows no differences between both treatments. Vehicle, n=5; metoprolol, n=7. Graphs show mean ± S.E.M. Abbreviations as in Supplemental Figure 3.
Figure S8. Single and merged channels of triple GFAP+CSPG+RGMa IF, showing Repulsive Guidance Molecule A expression (RGMa; yellow) and proteoglycan deposition (CSPG; grey) in the core lesion of a vehicle‐treated rat (top row) and their absence in a metoprolol‐treated rat (bottom row). Nuclei were revealed with DAPI (blue).
Figure S9. (A) Flow cytometry plots illustrating the purity of glia‐based co‐cultures. (B, C) CXCL1 levels in glia‐based co‐culture supernatants. (D) Fluorescence images of proliferative (EdU, red) astrocytes Clemente‐Moragón, A., et al: β1AR blockade blunts stroke neuroinflammation (GFAP, green) subjected or not to OGD, and treated or not with vehicle or metoprolol before or after reperfusion. Nuclei were revealed with DAPI (blue). (E) Graphs show quantification of % of EdU+nuclei over total nuclei (DAPI+), GFAP mean intensity and GFAP area/nuclei (n=4 per condition) (F, G) Immunoblot analyses of Arginase‐1 (37 kDa), YM1 (44 kDa), CD243 (55kDa) and vinculin (120 kDa) protein expression in co‐cultures subjected or not to OGD, and treated or not with vehicle or metoprolol before or after reperfusion (n=4 per condition). Graphs show mean ± S.E.M.
Figure S9.
Figure S10. (A) White blood cell counts after treatment with anti‐PMN serum assessed by hemocytometry. Upper and lower dotted black lines represent white blood cells counting means (baseline and after depletion). (B, C) Neither between distinct treatment conditions in the absence of neutrophils, nor between same treatment conditions in the presence or absence of neutrophils, differences were observed in AAR. (D) The absence of neutrophils did not provide neuroprotection as metoprolol did in wild‐type rats at 7 d post‐reperfusion (depleted vehicle, n=3; depleted metoprolol, n=18). (E, F) The absence of neutrophils did not reduce vasogenic oedema (non‐depleted vehicle, n=16; depleted vehicle, n=3). Graphs show mean ± S.E.M. *P<0.05.
Video S1. 3D confocal reconstruction of triple GFAP+AQ4+Iba1+ immunofluorescence on a coronal brain section of a rat receiving a pre‐reperfusion i.v. bolus of metoprolol at 7 d postreperfusion, illustrating preservation of aquaporin‐4 expression (AQP4; red) in the end feet of astrocytes (GFAP; green), indicating preserved blood‐brain barrier integrity. Microglia/macrophages (Iba1; purple) were found around vessels. Nuclei were revealed with DAPI (blue).
Video S2. 3D confocal reconstruction of triple Neutrophil+TUNEL+Iba1+ immunofluorescence on a coronal brain section of a rat receiving a pre‐reperfusion i.v. bolus of vehicle at 7 d post‐reperfusion, illustrating how apoptotic (positive for TUNEL [red]) neutrophils (green) are preferably engulfed by microglia (positive for Iba1 [grey]). Nuclei were revealed with DAPI (blue).
Video S3 (A‐D). In vivo 2D IVM recordings, showing representative crawling neutrophils (positive for Ly6G [green] and CD62L [yellow]), interacting with platelets (positive for CD41 [red]) in inflamed cremaster muscle vessels, of WT or neutrophil conditional Adrb1 KO mice receiving i.v. vehicle (saline) or metoprolol. Metoprolol did not provide any further effect on neutrophil behaviour in the absence of β1AR.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


