Abstract
Background and Aim
Metabolic syndrome (MetS) increases the risk of colorectal cancer (CRC), and the impact of MetS on CRC prognosis remains controversial after the diagnosis of CRC has been established. This study aimed to explore the impact of the individual components and synergies of MetS on the prognosis of patients with CRC.
Methods
We searched articles published before August 3, 2022, in four databases, including PubMed, Embase, Cochrane Library, and ScienceDirect. The random‐effects model inverse variance method was used to estimate the summarized effect size.
Results
Patients with CRC with MetS were 1.342 times more likely to experience all‐cause mortality than those without MetS, and the 95% confidence interval (CI) of hazard ratio (HR) was 1.107–1.627 (P = 0.003). CRC‐specific mortality in patients with CRC with MetS was 2.122 times higher than in those without MetS, and the 95% CI of HR was 1.080–4.173 (P = 0.029). CRC‐specific mortality exhibited an increasing trend of risk with increased metabolic risk factors. The HR of CRC‐specific mortality for one, two, and three metabolic risk factors was 1.206 (95% CI, 1.034–1.407; P = 0.017), 1.881 (95% CI, 1.253–2.824; P = 0.002), and 2.327 (95% CI, 1.262–4.291; P = 0.007), respectively.
Conclusions
Metabolic syndrome increased all‐cause and CRC‐specific mortality in patients with CRC. As a single component of MetS, diabetes mellitus increased overall mortality in patients with CRC, while obesity increased CRC‐specific mortality in patients with CRC, with a significant difference from non‐MetS. Moreover, the risk of CRC‐specific mortality increased with increasing number of metabolic risk factors.
Keywords: colorectal cancer, diabetes, glucose intolerance, metabolic syndrome, prognostic
Introduction
Metabolic syndrome (MetS) manifests itself as a group of clinical syndromes, including diabetes or glucose intolerance, hypertension (HTN), obesity, and dyslipidemia, with multiple metabolic diseases occurring simultaneously. 1 , 2 A sedentary lifestyle, chronic stress, an imbalanced diet, and lipodystrophy may increase the risk of MetS. 3 MetS is associated with a higher risk of metabolic and cardiovascular disorders, including chronic kidney disease, peripheral vascular disease, coronary artery disease, and stroke. The incidence of MetS has increased dramatically worldwide and has become a major public health problem due to aging, urbanization, and lifestyle changes.
In recent years, many lines of evidence have indicated that MetS has hormonal and systemic effects that increase susceptibility to various cancers. 4 , 5 , 6 Epidemiological studies have shown that MetS and its components are associated with an elevated risk of cancer, including colorectal cancer (CRC). CRC is the third most common neoplasm and the fourth most lethal malignancy worldwide, accounting for 10.2% 7 of all cancers. Although MetS increases the risk of CRC, its impact on CRC prognosis remains controversial after the diagnosis of CRC has been established. Shen et al. examined 503 Chinese patients with various stages of CRC and found that overall survival and disease‐free survival (DFS) were significantly reduced in patients with combined MetS. 8 Pathophysiological reasons for the association between diabetes mellitus (DM) and a poor prognosis of CRC may be related to insulin resistance, glucose utilization, angiogenesis, adipokine production, and oxidative stress. However, several studies have reached different conclusions. Goulart et al. 2 assessed the 30‐day prognosis of 134 patients who underwent CRC surgery and found that 46 were eligible for MetS. The authors found no association between MetS or its components and operative complications. A study of 1236 patients showed that MetS had no significant effect on postoperative complications or mortality. 9 To date, the value of MetS as a prognostic indicator has remained controversial, although there may be a link between MetS and CRC prognosis.
The prognostic analysis of CRC is based primarily on clinical factors, such as completeness of surgery, TNM stage, and number of lymph nodes procured, and secondarily on relevant pathologic features, such as microsatellite instability and grade. Despite these prognostic factors, the prognostic and predictive factors that guide treatment strategies are still lacking in many clinical situations. Therefore, these factors must be clinically recognized to improve treatment and outcomes. To address this issue, we carried out a meta‐analysis and systematic study to explore whether MetS affects the prognosis of patients with CRC. In this study, we separately investigated the impact of individual components and synergies of MetS on the prognosis of patients with CRC, which will help identify high‐risk components and provide ideas for clinical treatment and management.
Materials and methods
Protocol and guidance
This systematic review and meta‐analysis followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 10 The study protocol was published in the INPLASY database under registration number INPLASY202280050 (https://inplasy.com/inplasy‐2022‐8‐0050/).
Eligibility criteria
This study evaluated MetS and its components as prognostic factors for CRC. The inclusion criteria were as follows: (i) patients with CRC; (ii) intervention: MetS; (iii) control: without MetS; (iv) outcome: hazard ratio (HR) of survival or odds ratio (OR) of postoperative complications; and (v) study design: prospective or retrospective observational cohort studies.
Information sources and search strategy
We searched articles published before August 3, 2022, in four electronic databases, including PubMed, Embase, Cochrane Library, and ScienceDirect, using the following search terms: metabolic syndrome, colorectal cancer, colon cancer, rectal cancer, bowel cancer, rectal carcinoma, mortality, complication, prognosis, postoperative, death, prognosis, survival, readmission, length of stay, metastases, and recurrence.
Study selection
Two well‐trained independent reviewers screened all abstracts. By applying inclusion/exclusion criteria to the information contained in the abstracts, we reduced the number of potentially eligible articles. The full‐text articles retrieved were evaluated using the same inclusion and exclusion criteria as the abstracts. Any disagreements that arose during the selection process were discussed among the reviewers until a consensus was reached.
Data extraction
For all studies, two investigators extracted the study design, sample size, publication year, study country, participant characteristics (age and sex), follow‐up time, and results of interest. In the case of multiple publications, we included the latest or comprehensive information. If there was no HR value in numeric format, we obtained it from the Kaplan–Meier plots in the original study using the methods suggested by Tierney et al. 11
Definition of outcomes
Survival outcomes were HRs of overall mortality, CRC‐specific mortality, and DFS. The HR for survival between patients with and without MetS was calculated.
Postoperative outcomes were ORs of postoperative complications and postoperative mortality.
We also meta‐analyzed the effects of any single component of MetS, including DM, HTN, dyslipidemia, and obesity.
We also meta‐analyzed all‐cause mortality, CRC‐specific mortality, and DFS of each component to determine which of the aforementioned prognostic outcomes was associated with each component. However, we pooled only those studies that had been included to assess MetS and provided single‐component prognostic results and did not search separately for studies that assessed only the impact of a single component on CRC.
Survival outcomes were pooled based on the number of metabolic risk factors to assess whether the risk of death increased with the addition of metabolic risk factors.
Statistical analysis
A random‐effects model inverse variance method was used to estimate the summarized effect size, assuming that heterogeneity always exists. We reported the pooled estimates as the weighted mean difference and their respective 95% confidence interval (CI). Heterogeneity between studies was assessed using the Cochran Q test and a P‐value < 0.10 was considered significant. We also calculated the I 2 statistic as a measure of inconsistency between studies. Heterogeneity was considered significant if I 2 values were > 50%. Publication bias was examined using the Begg 12 and Egger 13 regression tests. STATA version 15.0 (College Station, TX, USA) was used for all analyses.
Quality assessment
To assess the risk of bias in observational studies, we used the Newcastle–Ottawa Quality Assessment Scale. The scale assigns stars (up to nine stars) based on the quality of selection, comparability, exposure, and outcome of the study participants.
Results
Study characteristics
We initially screened 944 studies and, after eliminating duplicate studies, 296 studies were obtained for the next step of title and abstract screening. Ninety‐three articles were evaluated in full text for eligibility, and 70 were excluded for the following reasons: 52 had no outcome measure of interest, 4 were duplicate trials, 9 reported only relative risk for a single component, and 5 were healthy controls. The final number of articles included in this meta‐analysis was 23 (Fig. 1). The total sample size was 399 773 participants, and the number of patients with MetS was 38 910; 39% of the studies were conducted in North America, 26% in Europe, and 35% in Asia. The 23 studies were observational, including 8 prospective, 14 retrospective, and 1 cross‐sectional study. Each included article was awarded at least six stars according to the Newcastle–Ottawa Scale (NOS). Table 1 summarizes the characteristics and NOS scores of the included studies.
Figure 1.
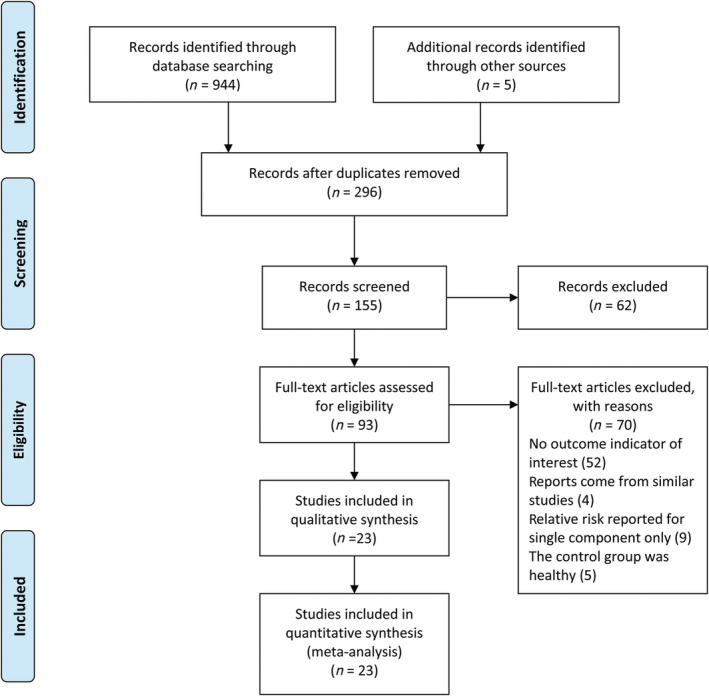
Flowchart of study selection.
Table 1.
Characteristics of included studies
| Study | Year | Study design | Age | Male% | Sample size | MetS | Country | Follow up (months) | Criteria for the definition of MetS | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | ||||||||||
| Akinyemiju 14 | 2018 | Cross‐sectional | — | — | 152 952 | 10 543 | USA | 0.0 | ATP III | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆ |
| Anderson 15 | 2016 | Retrospective | 64.1 (10.3) | 67.0% | 102 | 6 | USA | 0.0 | ATP III | ⋆⋆⋆ | ⋆ | ⋆ |
| Bhome 16 | 2021 | Prospective | 72.4 (25–93) | 59.0% | 1066 | 177 | UK | 50.0 | Self‐defined | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Cespedes Feliciano 17 | 2016 | Prospective | 64 (11) | 51.0% | 2446 | 897 | USA | 72.0 | ATP III | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Chen 18 | 2018 | Retrospective | 51.8 (12.6) | 100.0% | 838 | 215 | China | 60.0 | The Diabetes Society of Chinese Medical Association | ⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Colangelo 19 | 2002 | Retrospective | 40.0 (12.5) | 57.4% | 35 582 | — | USA | 314.4 | Self‐defined | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Croft 20 | 2019 | Retrospective | 68.9 | — | 142 | 53 | Canada | 59.6 | Self‐defined | ⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Feng 21 | 2021 | Retrospective | 59 | 55.3% | 2046 | 682 | China | 92.0 | IDF | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆ |
| Feng 22 | 2021 | Retrospective | 65 (60–74) | 60.7% | 1271 | 201 | China | 24 | Chinese Diabetes Society (CDS, 2004) | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Goulart 2 | 2017 | Prospective | 67.9 (12.9) | — | 134 | 46 | Portugal | 1.0 | ATP III | ⋆⋆⋆ | ⋆ | ⋆⋆ |
| Lohsiriwat 23 | 2010 | Prospective | 61 (29–91) | 56.1% | 114 | 42 | Thailand | 1.0 | 2005AHA/NHLBI | ⋆⋆⋆ | ⋆ | ⋆⋆ |
| Matthews 24 | 2010 | Prospective | 47.2 ± 9.8 | 100.0% | 33 230 | 9268 | USA | 172.8 | ATP III | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Ottaiano 25 | 2016 | Prospective | 31–78 | 47.1% | 102 | — | Italy | 46.0 | ATP III | ⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Peng 26 | 2016 | Prospective | 56 (45, 67) | 58.3% | 1318 | 213 | China | 58.6 | The Diabetes Society of Chinese Medical Association | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Reed 27 | 2019 | Retrospective | 70.1 | 66.4% | 122 | 43 | Canada | 72.0 | Self‐defined | ⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Shariq 28 | 2019 | Retrospective | 65.5 ± 13.5 | — | 91 566 | 7603 | USA | 1.0 | A modification of the NCEP‐ATP III | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆ |
| Shen 8 | 2010 | Retrospective | 64.13 | — | 507 | 179 | China | 45.1 | Self‐defined | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Silva 29 | 2021 | Retrospective | 70 (12) | 58.3% | 168 | 85 | Portugal | 60 | The Harmonized Criteria | ⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Trevisan 30 | 2001 | Retrospective | 20–69 | 57.1% | 37 302 | 1174 | Italy | 84.0 | Self‐defined | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| Yang 31 | 2013 | Retrospective | 77.1 (6.5) | 42.6% | 36 079 | 7024 | USA | 60.0 | ATP III | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ |
| You 32 | 2015 | Retrospective | 68.8 ± 10.8 | 56.6% | 1069 | 221 | China | 59.6 | The Diabetes Society of Chinese Medical Association | ⋆⋆⋆⋆ | ⋆ | ⋆⋆⋆ |
| Zarzavadjian Le Bian 9 | 2018 | Prospective | 70 (51–88) | 68.2% | 1236 | 85 | France | 3.0 | ATP III | ⋆⋆⋆ | ⋆ | ⋆⋆ |
| Zhou 33 | 2020 | Retrospective | 65 (16) | 61.4% | 381 | 153 | China | 1.0 | AHA/NHLBI | ⋆⋆⋆ | ⋆⋆ | ⋆⋆ |
| Total 23 studies | 399 773 | 38 910 | ||||||||||
MetS, metabolic syndrome; NOS, Newcastle–Ottawa Scale.
Synthesis of results
Association between metabolic syndrome and survival of patients with colorectal cancer
First, the pooled results of the 13 studies showed that patients with CRC with MetS were 1.342 times more likely to experience all‐cause mortality than patients with CRC without MetS, and the 95% CI of the HR was 1.107–1.627 (P = 0.003) (Fig. 2). Second, the pooled results of the two studies showed that CRC‐specific mortality in patients with CRC with MetS was 2.122 times higher than that of patients with CRC without MetS, and the 95% CI of HR was 1.080–4.173 (P = 0.029) (Fig. 3a). Third, the pooled results of the seven studies showed significant differences in DFS between patients with CRC with and without MetS (HR, 1.574; 95% CI, 1.086–2.281; P = 0.017) (Fig. 3b).
Figure 2.
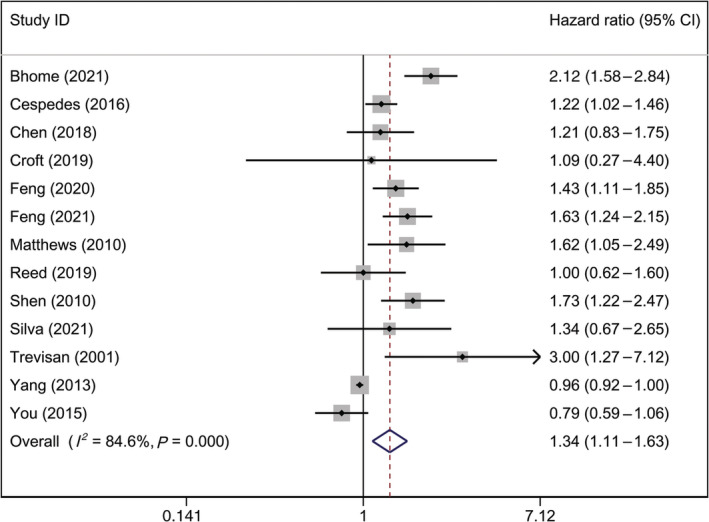
Forest plot of hazard ratio of all‐cause mortality among patients with CRC, MetS versus non‐MetS. Note: Weights are from random‐effects analysis. CI, confidence interval; CRC, colorectal cancer; MetS, metabolic syndrome.
Figure 3.
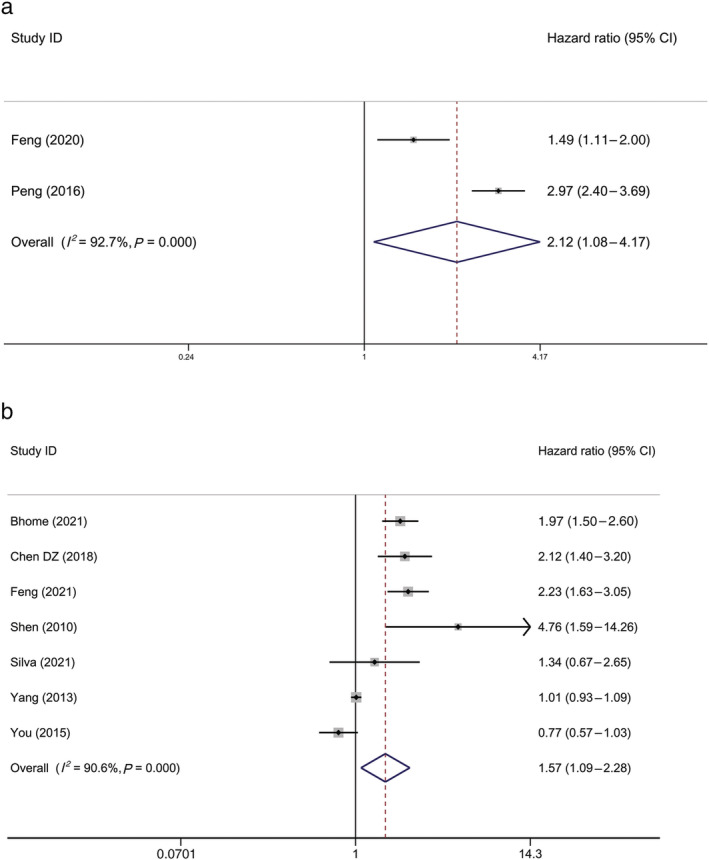
Forest plot of hazard ratio of (a) CRC‐specific mortality among patients with CRC, MetS versus non‐MetS, and (b) disease‐free survival among patients with CRC, MetS versus non‐MetS. Note: Weights are from random‐effects analysis. CI, confidence interval; CRC, colorectal cancer; MetS, metabolic syndrome.
Associations between metabolic syndrome and postoperative outcomes of patients with colorectal cancer
The pooled results of the six studies and three studies did not show significant differences in the incidence of postoperative complications and postoperative mortality between patients with CRC with and without MetS, with ORs of 1.138 (95% CI, 0.909–1.424; P = 0.259) and 0.809 (95% CI, 0.311–2.106; P = 0.664), respectively (Fig. 4).
Figure 4.
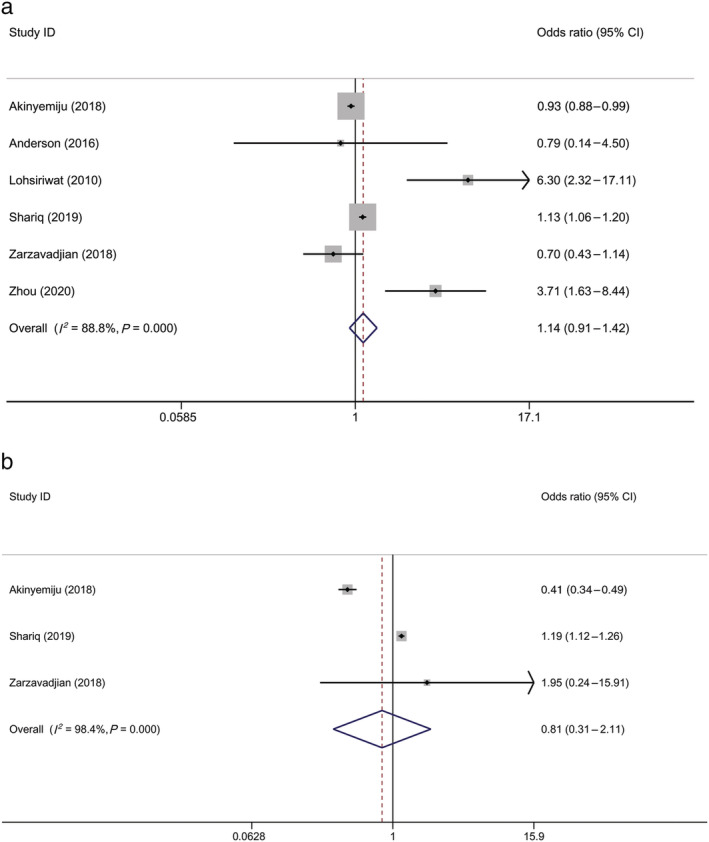
Forest plot of odds ratio of (a) postoperative complications among patients with CRC, MetS versus non‐MetS, and (b) postoperative mortality among patients with CRC, MetS versus non‐MetS. Note: Weights are from random‐effects analysis. CI, confidence interval; CRC, colorectal cancer; MetS, metabolic syndrome.
Association between a single component of metabolic syndrome and survival of patients with colorectal cancer
Associations between the four components of MetS (DM, HTN, dyslipidemia, and obesity) and the three types of survival (all‐cause mortality, CRC‐specific mortality, and DFS) were assessed. The results showed that the associations between DM and overall mortality, obesity, and CRC‐specific mortality were significantly different compared with patients without MetS, with HRs of 1.170 (95% CI, 1.127–1.214; P < 0.000) and 1.333 (95% CI, 1.157–1.537; P < 0.000), respectively (Table 2).
Table 2.
Associations between single component of the MetS and survival of patients with CRC
| Component | CRC‐specific mortality | DFS | Overall mortality |
|---|---|---|---|
| DM | 1.903 (0.632–5.728) | 1.263 (0.933–1.711) | 1.170 (1.127–1.214) |
| HTN | 1.242 (0.97–1.591) | 1.089 (0.803–1.477) | 1.315 (0.879–1.966) |
| Dyslipidemia | 1.381 (0.982–1.942) | 0.818 (0.624–1.073) | 1.029 (0.816–1.298) |
| Obesity | 1.333 (1.157–1.537) | 1.087 (0.812–1.455) | 1.041 (0.914–1.186) |
CRC, colorectal cancer; DFS, disease‐free survival; DM, diabetes mellitus; HTN, hypertension; MetS, metabolic syndrome.
Association between the number of metabolic risk factors and survival of patients with colorectal cancer
First, CRC‐specific mortality exhibited an increasing trend with increasing metabolic risk factors. The HR of CRC‐specific mortality for one, two, and three metabolic risk factors was 1.206 (95% CI, 1.034–1.407; P = 0.017), 1.881 (95% CI, 1.253–2.824; P = 0.002), and 2.327 (95% CI, 1.262–4.291; P = 0.007), respectively. Second, as the number of metabolic risk factors increased, no pattern was found for overall mortality risk. The HR of overall mortality for one, two, and three metabolic risk factors was 1.140 (95% CI, 1.08–1.204; P < 0.000), 1.558 (95% CI, 0.896–2.708; P = 0.116), and 1.384 (95% CI, 0.852–2.246; P = 0.189), respectively (Fig. 5).
Figure 5.
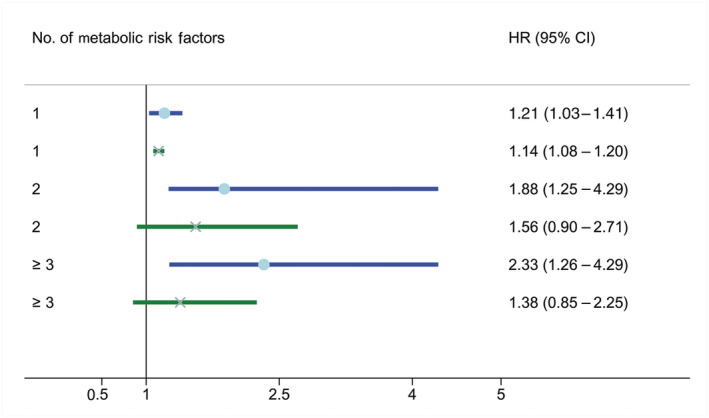
Plot of associations between number of metabolic risk factors and survival of patients with CRC.  , Overall mortality;
, Overall mortality;  , CRC mortality. CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio.
, CRC mortality. CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio.
Sensitivity analysis
Comparisons of postoperative mortality and postoperative complications between patients with CRC with and without MetS included a cross‐sectional study 14 ; therefore, we excluded this study from the sensitivity analysis.
The pooled results of postoperative mortality showed significant differences; the OR was 1.186 (95% CI, 1.118–1.258; P < 0.000), while when the study by Akinyemiju et al. was included, the original pooled result was 0.809 (95% CI, 0.311–2.106; P = 0.664). This suggests that the study by Akinyemiju et al. 14 is sensitive to this outcome; the results of postoperative mortality between patients with CRC with and without MetS should be interpreted with caution.
There was no substantial change in the pooled results of postoperative complications after excluding the study by Akinyemiju et al. 14
Publication bias
The Egger test was the best for synthesizing results with 10 or more datasets. Only data on overall mortality (MetS vs non‐MetS) were included. The Egger test showed P = 0.007, which was inconsistent with the Begg test (P = 0.714). Based on a visual inspection of the funnel plots (Fig. S1), we adopted the detection results of the Egger test and believed that publication bias existed.
Discussion
This meta‐analysis included 21 studies with a total sample size of 398 334 subjects. Our study revealed the following: (i) Patients with CRC with MetS were more significantly associated with all‐cause mortality and CRC‐specific mortality than those without MetS; (ii) the associations between DM and overall mortality, obesity, and CRC‐specific mortality were significantly different compared with patients without MetS, but the association between DM and obesity and DFS was not significant; (iii) CRC‐specific mortality increases with increased metabolic risk factors; and (iv) the incidence of postoperative complications and mortality in patients with CRC with MetS was not significantly different from those without MetS.
Studies have shown that MetS is associated with CRC prognosis. A meta‐analysis indicated that MetS is associated with reduced survival in patients with CRC. 34 To determine the relationship between MetS and CRC prognosis, we examined the relationship between MetS and the survival of patients with CRC. We found that patients with CRC with MetS had significantly higher all‐cause mortality and CRC‐specific mortality. Currently, it is widely accepted that the pathogenesis of MetS and CRC may be associated with certain endocrine hormone abnormalities such as hyperinsulinemia and insulin resistance. MetS causes hyperinsulinemia and insulin resistance, leading to elevated levels of insulin‐like growth factor‐1 (IGF‐1). The activation of the insulin/IGF‐1 system by increased levels of circulating insulin through the ligation of the insulin receptor A (IR‐A) expressed in CRC cells can promote the activity of the MEK/Raf/Ras and PI3K/Akt pathways. When these axes are dysregulated, they induce cancer‐promoting effects, such as the promotion of angiogenesis, inhibition of apoptosis, and proliferation. Many patients with MetS have visceral obesity. As visceral fat is metabolically more active than subcutaneous fat, visceral adipocytes can release potentially harmful levels of tumor necrosis factor‐α and interleukin 6, leading to a chronic proinflammatory state and insulin resistance. 28 Moreover, recent studies have shown that MetS is associated with gut microbiota dysfunction. 35 , 36 , 37 Disorders in the gut microbiota can affect fat intake and lead to weight gain, which, in turn, is a source of cytokines that induce carcinogenesis and low‐grade, chronic inflammation. 38 , 39 , 40 Obesity itself can also affect the ecology of the intestinal flora. 41 Several studies have revealed that MetS increases the risk of mortality in many diseases. Ju et al. found that MetS was associated with an increased risk of all‐cause and cardiovascular disease mortality in adults aged ≥ 60 years. They observed a 24% increase in the risk of cardiovascular disease mortality and a 23% increase in the risk of all‐cause mortality among older adults with MetS compared with those without MetS. 42 The meta‐analysis included 19 studies that revealed that MetS was significantly associated with a higher risk of prostate cancer‐specific death (relative risk [RR], 1.12; 95% CI, 1.02–1.23). 43 Pre‐existing MetS among patients with coronavirus disease was significantly associated with a higher risk of short‐term mortality (OR, 2.30; 95% CI, 1.52–3.45). 44 MetS was associated with an increased risk of all‐cause death in patients with breast cancer. 45 End‐stage renal disease patients with MetS had a significantly increased risk of all‐cause mortality (RR, 1.92; 95% CI, 1.15–3.21) compared with those without MetS. 46 These findings are consistent with those of the present study. Our study showed that patients with CRC with MetS had significantly higher all‐cause mortality (HR, 1.342; 95% CI, 1.107–1.627) and CRC‐specific mortality (HR, 2.122; 95% CI, 1.080–4.173). However, there is a lack of comparison between the effects of MetS on mortality from various diseases.
It is essential to guide treatment decisions in many clinical CRC scenarios. Currently, CRC prognosis is mainly based on clinical factors. Despite these prognostic factors, there is still a lack of readily available, replicable, and inexpensive prognostic factors that can guide patients or therapeutic decisions. MetS data are usually available or easily accessible and do not require additional molecular pathology studies that may be specialized and expensive. They are easily obtainable, reusable, inexpensive, and well suited for guidance and adjuvant therapy. We expected a significant increase in all‐cause and CRC‐specific mortality in patients with CRC with MetS. This finding may provide ancillary information to guide treatment decisions, discuss the prognosis with patients, and guide lifestyle changes after cancer diagnosis.
This study not only focused on the prognostic impact of MetS as a syndrome but also examined the impact of obesity, DM, and other components of MetS (e.g. HTN and dyslipidemia) on survival outcomes in patients with CRC separately. Our goal was to further discuss whether individual factors can provide stronger predictions than the combined effects of these factors. The measures of MetS components are available for all patients, and if each component validates its prognostic significance, it will facilitate more precise treatment guidance and help guide lifestyle changes after cancer diagnosis. Most current findings agree that DM may be a major prognostic factor for progression‐free survival in CRC. A meta‐analysis of 36 studies and approximately 2.3 million participants on the association between DM and CRC revealed a moderate adverse effect of DM on overall survival. 47 This might be because patients with DM often have diabetic gastrointestinal motility dysmotility. End products of metabolism remain in the intestine for a long time, resulting in a prolonged action of toxins and carcinogens in colorectal mucosal cells, which are prone to malignant transformation and have a high degree of infiltration. One study found that in patients with CRC who received adjuvant chemotherapy with capecitabine and oxaliplatin, obesity promoted chronic neurotoxicity and stimulated the development of micro‐metastases. 25 The mechanism by which obesity affects the prognosis of CRC may involve an imbalance in the adipokine spectrum. Adipose tissue is a highly active participant in the innate immune system, and adipokines are responsible for the paracrine cycle between macrophages and adipocytes. This interaction leads to low‐level chronic inflammation throughout the body, providing an enabling environment for the development of tumors. Several studies have revealed that serum adiponectin levels are negatively correlated with CRC. 48 , 49 , 50 Interestingly, MetS, as a whole, affects CRC outcomes, but HTN or dyslipidemia as a single component does not. We speculate that these components may work synergistically. Although individual factors may not work, they can produce synergies when combined. For example, when obesity is combined with insulin resistance, it promotes chronic inflammation, leading to more malignant tumors and a poorer prognosis. 25 , 51 Our study also found that the risk of CRC‐specific mortality increased with the number of metabolic risk factors, confirming a synergistic effect between them.
Many patients with CRC require surgical treatment, and a significant number of them have MetS. DM is generally believed to increase perioperative morbidity and mortality. Patients with DM who undergo surgery are more prone to poor wound healing, hematoma, wound infection, admission to the intensive care unit, and prolonged hospitalization. Due to its effects on immunity and blood vessels, DM has been identified as a risk factor for poor healing. DM is a major component of MetS and has increased perioperative complications. However, the effect of MetS on the postoperative outcome of CRC still lacks a description. We also analyzed the incidence of postoperative complications and mortality in this group of patients. Our pooled results from seven studies showed that the incidence of postoperative complications and mortality in patients with CRC with MetS were not significantly different from those in patients with CRC without MetS. Although our findings suggest that MetS does not affect postoperative complications, more in‐depth research is needed due to the paucity of studies.
This study has some limitations. First, we included MetS cases defined by different organizations in this meta‐analysis, which may have led to the heterogeneity of the study. Second, because all studies we included were related to MetS, the pooled effect size of the relationship between the MetS single component and prognosis outcomes did not incorporate the results of those studies that examined a single component (such as diabetes) of MetS, although there are more than enough of such studies. Third, although adjustments were made for known risk factors and potential confounding variables (age, sex, smoking, alcohol consumption, family history, year of CRC diagnosis, tumor site, and TNM stage) in almost all included studies, the adjustment varied with each study. This adjustment did not occur in one study because they believed that there were no statistical differences between the two groups of patients at baseline. Therefore, the findings of this study should be interpreted with caution.
Conclusions
Overall, our findings suggest that MetS increases all‐cause and CRC‐specific mortality in patients with CRC. As a single component of MetS, DM increased overall mortality in patients with CRC, while obesity increased CRC‐specific mortality in patients with CRC, with a significant difference from non‐MetS. Moreover, the risk of CRC‐specific mortality increased with increasing number of metabolic risk factors. This study might provide physicians with useful information to help guide lifestyle changes or treatment strategies after cancer diagnosis.
Supporting information
Figure S1 Funnel plot of hazard ratio of all‐cause mortality among patients with CRC, MetS vs non‐MetS.
Lu, B. , Qian, J.‐M. , and Li, J.‐N. (2023) The metabolic syndrome and its components as prognostic factors in colorectal cancer: A meta‐analysis and systematic review. Journal of Gastroenterology and Hepatology, 38: 187–196. 10.1111/jgh.16042.
Declaration of conflict of interest: The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions: Bo Lu, Jia‐Ming Qian, and Jing‐Nan Li conceived and designed the study. Bo Lu and Jia‐Ming Qian screened and extracted data. Bo Lu and Jing‐Nan Li performed the statistical analyses. All the authors contributed to the interpretation of data. Bo Lu drafted the manuscript; all the authors revised it critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Financial support: This work was supported by the National Natural Science Foundation of China (No. 81770559 and No. 81370500). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Jia‐Ming Qian, Email: qianjm@pumch.cn.
Jing‐Nan Li, Email: lijn@pumch.cn.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
- 1. He Q, Zhang H, Yao S et al. A study on relationship between metabolic syndrome and colorectal cancer. J. BUON 2018; 23: 1362–1368. [PubMed] [Google Scholar]
- 2. Goulart A, Varejão A, Nogueira F, Martins S, Mesquita‐Rodrigues A, Sousa N, Leão P. The influence of metabolic syndrome in the outcomes of colorectal cancer patients. Diabetes Metab. Syndr. 2017; 11: S867–s871. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group . The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 4. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta‐analysis. Diabetes Care 2012; 35: 2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishino K, Mutoh M, Totsuka Y, Nakagama H. Metabolic syndrome: a novel high‐risk state for colorectal cancer. Cancer Lett. 2013; 334: 56–61. [DOI] [PubMed] [Google Scholar]
- 6. Stocks T, Van Hemelrijck M, Manjer J et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012; 59: 802–810. [DOI] [PubMed] [Google Scholar]
- 7. Lee J, Lee KS, Kim H et al. The relationship between metabolic syndrome and the incidence of colorectal cancer. Environ Health Prev Med. 2020; 25: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen Z, Ye Y, Bin L, Yin M, Yang X, Jiang K, Wang S. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am. J. Surg. 2010; 200: 59–63. [DOI] [PubMed] [Google Scholar]
- 9. Zarzavadjian Le Bian A, Denet C, Tabchouri N et al. The effect of metabolic syndrome on postoperative outcomes following laparoscopic colectomy. Tech. Coloproctol. 2018; 22: 215–221. [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akinyemiju T, Sakhuja S, Vin‐Raviv N. In‐hospital mortality and post‐surgical complications among cancer patients with metabolic syndrome. Obes. Surg. 2018; 28: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson BJ, Wahlquist AE, Hill EG, Marshall DT, Kimchi ET, Staveley O'Carroll KF, Camp ER. The impact of metabolic syndrome on outcome and response to neoadjuvant chemoradiation in locally advanced rectal cancer patients. Int. J. Surg. 2016; 33: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhome R, Peppa N, Karar S, McDonnell D, Mirnezami A, Hamady Z. Metabolic syndrome is a predictor of all site and liver‐specific recurrence following primary resection of colorectal cancer: prospective cohort study of 1006 patients. Eur. J. Surg. Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA et al. Metabolic dysfunction, obesity, and survival among patients with early‐stage colorectal cancer. J. Clin. Oncol. 2016; 34: 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen DZ, Ji FY, Xu QM, Wu XX, Cai C, Zhang LJ, Li LJ. Interaction of smoking and metabolic syndrome in increasing the recurrence risk of colorectal cancer in a Chinese male cohort: a retrospective study. Sci. Rep. 2018; 8: 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol. Biomarkers Prev. 2002; 11: 385–391. [PubMed] [Google Scholar]
- 20. Croft B, Reed M, Patrick C, Kovacevich N, Voutsadakis IA. Diabetes, obesity, and the metabolic syndrome as prognostic factors in stages I to III colorectal cancer patients. J. Gastrointest. Cancer 2019; 50: 221–229. [DOI] [PubMed] [Google Scholar]
- 21. Feng Q, Xu L, Li L et al. Risk of death in colorectal cancer patients with multi‐morbidities of metabolic syndrome: a retrospective multicohort analysis. Cancer Res Treat. 2021; 53: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feng Q, Tang L‐J, Luo D‐H et al. Metabolic syndrome‐related hyperuricemia is associated with a poorer prognosis in patients with colorectal cancer: a multicenter retrospective study. Cancer Manag. Res. 2021; 13: 8809–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, Lohsiriwat D. Impact of metabolic syndrome on the short‐term outcomes of colorectal cancer surgery. Dis. Colon Rectum 2010; 53: 186–191. [DOI] [PubMed] [Google Scholar]
- 24. Matthews CE, Sui X, LaMonte MJ, Adams SA, Hébert JR, Blair SN. Metabolic syndrome and risk of death from cancers of the digestive system. Metabolism 2010; 59: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ottaiano A, Nappi A, Tafuto S et al. Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology 2016; 90: 36–42. [DOI] [PubMed] [Google Scholar]
- 26. Peng F, Hu D, Lin X et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Int. J. Cancer 2016; 139: 2705–2713. [DOI] [PubMed] [Google Scholar]
- 27. Reed M, Patrick C, Croft B, Walde N, Voutsadakis IA. The metabolic syndrome and its components as prognostic factors in metastatic colorectal cancer. Indian J. Gastroenterol. 2019; 38: 15–22. [DOI] [PubMed] [Google Scholar]
- 28. Shariq OA, Hanson KT, McKenna NP et al. Does metabolic syndrome increase the risk of postoperative complications in patients undergoing colorectal cancer surgery? Dis. Colon Rectum 2019; 62: 849–858. [DOI] [PubMed] [Google Scholar]
- 29. Silva A, Pereira SS, Monteiro MP, Araújo A, Faria G. Effect of metabolic syndrome and individual components on colon cancer characteristics and prognosis. Front. Oncol. 2021: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2001; 10: 937–941. [PubMed] [Google Scholar]
- 31. Yang Y, Mauldin PD, Ebeling M et al. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer 2013; 119: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 32. You J, Liu WY, Zhu GQ et al. Metabolic syndrome contributes to an increased recurrence risk of non‐metastatic colorectal cancer. Oncotarget 2015; 6: 19880–19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou CJ, Cheng YF, Xie LZ et al. Metabolic syndrome, as defined based on parameters including visceral fat area, predicts complications after surgery for rectal cancer. Obes. Surg. 2020; 30: 319–326. [DOI] [PubMed] [Google Scholar]
- 34. Esposito K, Chiodini P, Capuano A et al. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta‐analysis. Endocrine 2013; 44: 634–647. [DOI] [PubMed] [Google Scholar]
- 35. Saetang J, Sangkhathat S. Diets link metabolic syndrome and colorectal cancer development (Review). Oncol. Rep. 2017; 37: 1312–1320. [DOI] [PubMed] [Google Scholar]
- 36. Parekh PJ, Balart LA, Johnson DA. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin. Transl. Gastroenterol. 2015; 6: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marchesi JR, Adams DH, Fava F et al. The gut microbiota and host health: a new clinical frontier. Gut 2016; 65: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pigrau M, Rodiño‐Janeiro BK, Casado‐Bedmar M, Lobo B, Vicario M, Santos J, Alonso‐Cotoner C. The joint power of sex and stress to modulate brain‐gut‐microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol. Motil. 2016; 28: 463–486. [DOI] [PubMed] [Google Scholar]
- 39. Aballay LR, Eynard AR, Díaz Mdel P, Navarro A, Muñoz SE. Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr. Rev. 2013; 71: 168–179. [DOI] [PubMed] [Google Scholar]
- 40. Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl. Res. 2016; 167: 257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2005; 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all‐cause and cardiovascular mortality in the elderly: a meta‐analysis of prospective cohort studies. Medicine (Baltimore). 2017; 96: e8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiang YZ, Xiong H, Cui ZL et al. The association between metabolic syndrome and the risk of prostate cancer, high‐grade prostate cancer, advanced prostate cancer, prostate cancer‐specific mortality and biochemical recurrence. J. Exp. Clin. Cancer Res. 2013; 32: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zuin M, Rigatelli G, Bilato C, Cervellati C, Zuliani G, Roncon L. Prognostic role of metabolic syndrome in COVID‐19 patients: a systematic review meta‐analysis. Viruses 2021; 13: 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li P, Wang T, Zeng C, Yang M, Li G, Han J, Wu W. Association between metabolic syndrome and prognosis of breast cancer: a meta‐analysis of follow‐up studies. Diabetol. Metab. Syndr. 2020; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanguankeo A, Upala S. Metabolic syndrome increases mortality risk in dialysis patients: a systematic review and meta‐analysis. Int J Endocrinol Metab. 2018; 16: e61201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: a meta‐analysis based on the cohort studies. PLoS One 2017; 12: e0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Otake S, Takeda H, Suzuki Y et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin. Cancer Res. 2005; 11: 3642–3646. [DOI] [PubMed] [Google Scholar]
- 49. Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig. Dis. Sci. 2009; 54: 862–868. [DOI] [PubMed] [Google Scholar]
- 50. Otake S, Takeda H, Fujishima S et al. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J. Gastroenterol. 2010; 16: 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Amptoulach S, Gross G, Kalaitzakis E. Differential impact of obesity and diabetes mellitus on survival after liver resection for colorectal cancer metastases. J. Surg. Res. 2015; 199: 378–385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Funnel plot of hazard ratio of all‐cause mortality among patients with CRC, MetS vs non‐MetS.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.


