Abstract
Obesity is very common in patients with heart failure with preserved ejection fraction (HFpEF) and it has been suggested that obesity plays an important role in the pathophysiology of this disease. While body mass index defines the presence of obesity, this measure provides limited information on visceral adiposity, which is probably more relevant in the pathophysiology of HFpEF. Epicardial adipose tissue is the visceral fat situated directly adjacent to the heart and recent data demonstrate that accumulation of epicardial adipose tissue is associated with the onset, symptomatology and outcome of HFpEF. However, the mechanisms by which epicardial adipose tissue may be involved in HFpEF remain unclear. It is also questioned whether epicardial adipose tissue may be a specific target for therapy for this disease. In the present review, we describe the physiology of epicardial adipose tissue and the pathophysiological transformation of epicardial adipose tissue in response to chronic inflammatory diseases, and we postulate conceptual mechanisms on how epicardial adipose tissue may be involved in HFpEF pathophysiology. Lastly, we outline potential treatment strategies, knowledge gaps and directions for further research.
Keywords: HFpEF, Epicardial adipose tissue, Pathophysiology, Inflammation
Introduction
Obesity is rapidly becoming one of the most important risk factors for cardiovascular diseases, especially heart failure with preserved ejection fraction (HFpEF). 1 Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health and according to the World Health Organization, the prevalence of obesity has nearly tripled worldwide since 1975. 2 Although body mass index (BMI) is typically used to diagnose overall obesity, this measurement provides limited information on the actual burden and distribution of adipose tissue. This is important, because especially visceral adiposity (i.e. fat around the internal organs) imposes a risk for the development of HFpEF. 3 In particular epicardial adipose tissue (EAT), which is the fat tissue surrounding the heart, has gained increasing attention for its potential role in the pathophysiology of HFpEF. 4 , 5 For instance, increased EAT was associated with the incidence of HFpEF, but not with incident heart failure with reduced ejection fraction. 6 Furthermore, EAT was associated with structural and functional myocardial abnormalities typically seen in HFpEF, such as myocardial hypertrophy, diastolic dysfunction and increased filling pressures. Moreover, abundance of EAT in patients with HFpEF predicted a poor prognosis, independent of BMI. 7
While EAT may indeed be involved in the pathophysiology of HFpEF, the exact mechanisms remain poorly understood. In addition, it remains unclear whether EAT may be a viable target for treatment. Better insight into the mechanisms by which EAT may be involved in HFpEF pathophysiology may lead to (i) improved understanding of this complex disease, and (ii) the development of novel treatments that improve outcomes for patients with HFpEF. In this review we describe the physiology, pathophysiology, potential treatment strategies, knowledge gaps and future directions regarding the role of EAT in HFpEF.
Epicardial adipose tissue in health
Epicardial adipose tissue is the fat tissue situated directly between the myocardium and the visceral layer of the pericardium (Figure 1 ). Branches of the coronary arteries are responsible for the vascularization of EAT. 8 EAT is unevenly distributed around the heart, with the majority of fat being situated around the coronary arteries and the right ventricle. 9 In a healthy population without cardiovascular disease, men on average have more EAT compared to women. 6 Corradi et al. 9 showed that ventricular EAT accounts for approximately 20% of total ventricular weight in individuals who died of non‐cardiovascular causes. Since there is no basal layer between EAT and the myocardium, EAT may directly interact with, or infiltrate into the underlying myocardium. 8
Figure 1.
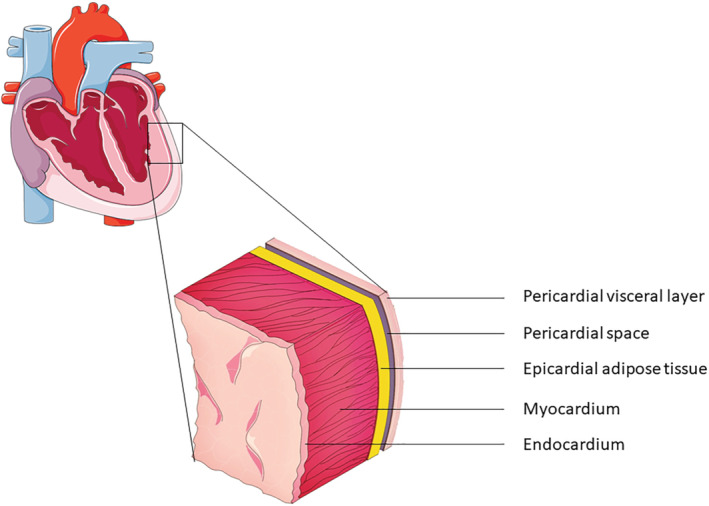
Schematic cross‐section view of the myocardial and pericardial layers, with epicardial adipose tissue being directly adjacent to the myocardium.
Several physiological mechanisms have been attributed to EAT. However, it is important to note that most assumptions are based on experimental studies and the majority have not been proven in humans. 10 , 11 For instance, it was previously shown in guinea pigs that EAT had a higher capacity of free fatty acid uptake and breakdown compared to other fat depots. 10 Because free fatty acids are the major energy source for the heart, it is possible that EAT may function as a local energy supplier to the myocardium. 10 Mechanistically, free fatty acids are thought to be both passively diffused and actively transported from EAT into the coronary circulation, and from there into the myocardial cells. 12 In addition to fatty acids being transported from EAT to the myocardium through the coronary circulation, it has been suggested that fatty acids may also directly diffuse from EAT into the myocardial cells, as these tissues are positioned directly adjacent to each other. 8 Conversely, EAT may also function as a buffer for harmful excess of energy from the coronary circulation. Free fatty acids would then be extracted and incorporated into the EAT.
Other than functioning as an energy source and buffer, EAT could function as a shock absorber for the coronary arteries from arterial pulse wave and cardiac contraction. In addition, EAT may regulate coronary arterial tone via the release of cytokines and chemokines into the bloodstream. 8 Lastly, EAT may function as an anatomical site for cardiac neurons. 11
Although studies have focused in particular on the effect that EAT may have on the heart, it is important to note that the possibility exists that the heart may also interact with EAT. Whether the interaction between EAT and the myocardium is unidirectional, bidirectional or whether there is no interaction at all between these tissues remains topic of debate, and should be further investigated.
Pathophysiological transformation of adipose tissue
While EAT probably has a function in cardiac homeostasis during health, it is thought that this function can be disrupted by accumulation and inflammation of EAT. 13 The factors responsible for the accumulation of EAT are not fully understood, but may in part be related to lifestyle factors including a chronic positive energy balance and smoking. 14 In addition, menopausal status and certain genetic polymorphisms linked to atherosclerosis have also been reported to be positively associated with the amount of EAT. 15 , 16 One important hypothesis to explain the transformation of EAT is built on the concept of adipose tissue hypoxia. 14 During adipose tissue accumulation, more oxygen is needed to support this tissue. However, previous studies suggest that although increased amounts of oxygen are needed in the growing tissue, the proportion of cardiac output towards the tissue, as well as the extent of the blood flow, are not increased. 17 Secondly, blood flow towards adipose tissue is postprandially impaired in obese individuals compared to lean individuals. 18 Thirdly, adipocyte sizes may increase up to a diameter of 200 μm during accumulation, whereas a normal diffusion distance of oxygen across tissues is 100‐200 μm. 19 Fourthly, angiogenesis in adipose tissue during obesity seems to be impaired. 20 All these data support the hypothesis that during adipose tissue accumulation, oxygen delivery to the adipose tissue is negatively affected, leading to adipose tissue hypoxia. During this (chronic) hypoxic state, adipocytes start releasing pro‐inflammatory cytokines, which collectively result into the migration of macrophages and other immune cells to the adipose tissue. 21 Indeed, histological assessment of obese mice has shown that there is infiltration of T‐cells and macrophages into the adipose tissue. 21
Adipose tissue hypoxia is also associated with a decline in insulin sensitivity of the adipocytes. 22 The mechanisms leading to insulin resistance in adipocytes are multitude and include both direct and indirect pathways. 22 For instance, adipose tissue hypoxia itself may directly influence insulin sensitivity, but it is also related to the down‐regulation of the adipokine adiponectin, that has been associated with insulin sensitization. 22
It should be noted that this pathophysiological transformation is not restricted to EAT, but applies to adipose tissue in general. Together, these associations suggest that adipose tissue, including EAT, can transform from a healthy tissue to a hypertrophic, hypoxic and inflamed tissue. It is thought that during this transformation, EAT may start infiltrating and compressing the underlying myocardium, which will be further discussed.
Epicardial adipose tissue and HFpEF
In recent studies, accumulation of EAT has consistently been associated with left ventricular hypertrophy, diastolic dysfunction and dilatation of the atria, which are considered typical hallmarks of HFpEF. 23 , 24 , 25 In addition, EAT has been related to increased cardiac filling pressures and exercise intolerance in HFpEF. 26 , 27 While it is assumed that EAT is the driving force behind these phenomena, it must be noted that it is still unclear whether EAT is indeed an active contributor to HFpEF, or an innocent bystander. This is illustrated by the observation that comorbidities that are thought to affect cardiac functioning in HFpEF, such as coronary artery disease and type II diabetes, are also associated with increased EAT. 23 , 28 Furthermore, because most studies have a cross‐sectional design, it is also possible that HFpEF‐related comorbidities may simultaneously lead to (a) adverse myocardial remodelling resulting in HFpEF, and (b) increased EAT; while there is no direct cause–effect relation between EAT and HFpEF. Lastly, HFpEF is thought to be a multifactorial disease, in which multiple comorbidities, including coronary artery disease, type II diabetes, obesity, systemic hypertension, and chronic obstructive pulmonary disease, all play its pathophysiological role. 29 Because these comorbidities are highly prevalent in patients with HFpEF and often co‐exist in the same patient, it remains difficult to determine to what part EAT is involved in the pathophysiology of HFpEF in the individual patient. However, some longitudinal studies have shown that higher EAT at baseline is associated with new‐onset HFpEF, 6 , 30 as well as a worse prognosis in those with established HFpEF, 7 independent of overall obesity and important comorbidities. An overview of the recent studies that investigated EAT in relation to HFpEF is shown in Table 1 . 6 , 7 , 23 , 25 , 26 , 27 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Of note, one study by Haykowsky et al. 32 found that patients with HFpEF have reduced EAT compared to controls. The discrepancy in findings between the study of Haykowsky et al. and the majority of other studies may in part be related to differences in the study population. For instance, the inclusion of HFpEF patients in the study by Haykowsky et al. was based on overall lenient criteria, including signs and symptoms of heart failure in combination with a preserved left ventricular ejection fraction and all patients needed to be obese, whereas the inclusion criteria for all other HFpEF studies were more stringent, based on specific echocardiographic, invasive haemodynamic, and/or biomarker criteria in addition to overall signs and symptoms of heart failure and a preserved left ventricular ejection fraction. Therefore, the study population that was investigated by Haykowsky et al. may be more representative of a more general obesity population, rather than a specific HFpEF population, and may potentially explain the difference in EAT volume between HFpEF patients and controls with the other studies. Two complementary hypotheses have been postulated on the potential mechanisms by which EAT may be involved in the pathophysiology of HFpEF.
Table 1.
Overview of recent clinical studies investigating epicardial adipose tissue in heart failure with preserved ejection fraction
| Study design | N | Method of epicardial adipose tissue quantification | Major conclusions | |
|---|---|---|---|---|
| Obokata et al.,31 2017 | Observational, retrospective analysis |
HFpEF: 195 Controls: 71 |
Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | HFpEF patients have increased epicardial adipose tissue thickness compared to controls |
| van Woerden et al.,23 2018 | Observational, retrospective analysis |
HFpEF: 64 Controls: 20 |
Cardiac MRI: epicardial adipose tissue volume measured on cine‐short axis | HFpEF patients have increased epicardial adipose tissue volume compared to controls, epicardial adipose tissue is associated with comorbidities and biomarkers of myocardial damage |
| Haykowsky et al.,32 2018 | Observational, retrospective analysis |
HFpEF: 100 Controls: 61 |
Cardiac MRI: epicardial adipose tissue volume measured on axial images | HFpEF patients have decreased epicardial adipose tissue volume compared to controls, significant association with intra‐abdominal fat and greater peak oxygen uptake in HFpEF |
| Wu et al.,33 2020 | Observational, retrospective analysis |
HFpEF: 163 HFrEF: 34 Controls: 108 |
Cardiac MRI: epicardial adipose tissue mass | HFpEF patients have increased epicardial adipose tissue mass compared to controls, epicardial adipose tissue is associated with increased LV volume, LV mass, LA volume and extracellular volume |
| Gorter et al.,26 2020 | Observational, retrospective analysis | HFpEF: 75 | Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | Epicardial adipose tissue is associated with BMI, increased RV end‐diastolic pressure, lower peak oxygen uptake |
| Koepp et al.,27 2020 | Observational, retrospective analysis | HFpEF: 169 | Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | Epicardial adipose tissue is associated with BMI, higher LV eccentricity index, higher RA pressure, PA pressure and PCWP, and lower peak oxygen uptake |
| van Woerden et al.,25 2021 | Observational, retrospective analysis | HFpEF: 102 | Cardiac MRI: epicardial adipose tissue volume measured on cine‐short axis | Epicardial adipose tissue is predominantly distributed around the RV, regional epicardial adipose tissue is associated with local cardiac function and structure |
| Tromp et al.,34 2021 | Observational, retrospective analysis | HFpEF: 47 | Cardiac MRI: volume on cine‐short axis and echocardiography: epicardial adipose tissue thickness measured over the RV free wall | HFpEF patients have increased epicardial adipose tissue volume compared to controls, but reduced epicardial adipose tissue/LV mass ratio, epicardial adipose tissue volume is negatively associated with LV strain and positively with extracellular volume in HFpEF |
| Kenchaiah et al.,6 2021 | Observational, prospective analysis |
Participants with no HF during follow‐up: 6402 Participants who developed HF: 383 |
Cardiac CT: epi‐ and pericardial adipose tissue volume on tomographic slices | Epi‐ and pericardial adipose tissue accumulation is associated with incident HFpEF and HFmrEF, but not HFrEF |
| Ying et al.,35 2021 | Observational, retrospective analysis |
HFpEF: 55 Controls: 33 |
Cardiac MRI: epicardial adipose tissue thickness over the RV | HFpEF patients have increased epicardial adipose tissue thickness compared to controls, epicardial adipose tissue is associated with worse global well‐being score |
| Pugliese et al.,36 2021 | Observational, prospective analysis |
HFpEF: 188 HFrEF: 205 Controls: 44 |
Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | HFpEF patients have increased epicardial adipose tissue compared to HFrEF and controls. Epicardial adipose tissue is related to higher troponin T, CRP, lower peak oxygen uptake and adverse prognosis in HFpEF |
| van Woerden et al.,7 2021 | Observational, prospective analysis | HFpEF: 105 | Cardiac MRI: epicardial adipose tissue volume measured on cine‐short axis | Higher epicardial adipose tissue is associated with adverse prognosis in HFpEF |
| van Woerden et al.,37 2022 | Observational, retrospective analysis | HFpEF: 117 | Echocardiography, epicardial adipose tissue thickness measured over the RV free wall and cardiac MRI: epicardial adipose tissue volume measured on cine‐short axis | Modest correlation between epicardial adipose tissue volume on cardiac MRI and epicardial adipose tissue thickness on echocardiography. Epicardial adipose tissue thickness on echocardiography is limited in estimating epicardial adipose tissue volume on cardiac MRI |
| Mahabadi et al.,38 2022 | Observational, retrospective analysis |
Participants with no HF during follow‐up: 237 Participants who developed HF: 142 |
Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | Epicardial adipose tissue is associated with the development of HFpEF |
| He et al.,39 2022 | Observational, retrospective analysis |
HFpEF: 5 Non‐HF: 5 |
No quantification, biopsies of EAT were taken | Epicardial adipose tissue proteome in HFpEF is more strongly related to inflammation, lipid metabolic disorder and mitochondrial dysfunction |
| Venkateshvaran et al.,40 2022 | Observational, retrospective analysis | HFpEF: 182 | Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | Epicardial adipose tissue is related to cardiac structural alterations and proteins expressing adiposity, inflammation, lower insulin sensitivity and endothelial dysfunction |
| Jin et al.,41 2022 | Observational, retrospective analysis, 2 independent cohorts |
HFpEF: 99/243 HFrEF/HFmrEF: 366/180 Controls: 149/626 |
Echocardiography, epicardial adipose tissue thickness measured over the RV free wall | Epicardial adipose tissue is increased in HFpEF compared to HFrEF/HFmrEF and controls. In HFpEF, epicardial adipose tissue is associated with worse left‐sided function but with better left‐sided function in HFrEF/HFmrEF |
| Rao et al.,30 2021 | Observational, prospective analysis |
Participants with no HF during follow‐up: 1350 Participants who developed HF: 36 |
Cardiac CT: pericardial adipose tissue volume on tomographic slices | High pericardial adipose tissue, but not subcutaneous adipose tissue was associated with incident HFpEF. Pericardial adipose tissue was associated with mortality |
Abbreviation: BMI, body mass index; CRP, C‐reactive protein; CT, computed tomography; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrium; LV, left ventricle; MRI, magnetic resonance imaging; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; RA, right atrium; RV, right ventricle.
Infiltrative‐lipotoxic hypothesis
The infiltrative‐lipotoxic hypothesis describes that during its pathophysiological transformation, EAT increasingly penetrates the underlying myocardium, disrupting cardiac ultrastructure and electrophysiological properties and leading to functional impairment of the myocardium, including left ventricular hypertrophy and diastolic dysfunction (Figures 2A and 3 ). 42 Indeed, in patients undergoing coronary artery bypass grafting, it was shown that EAT infiltrated the atrial myocardium. 42 In that study it was also shown that the infiltration of EAT was associated with increased conduction heterogeneity of the atrial myocardium. 42
Figure 2.
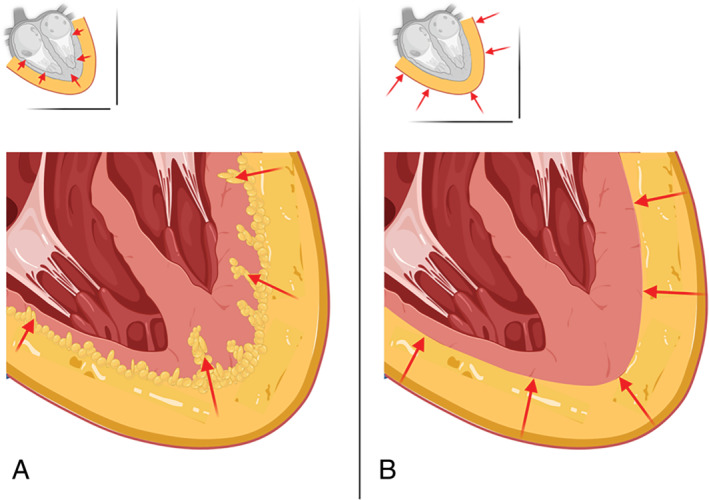
(A) Infiltrative‐lipotoxic hypothesis in which the epicardial adipose tissue increasingly starts infiltrating the underlying myocardial tissue and releasing pro‐inflammatory adipokines. (B) Pericardial restraint hypothesis in which the epicardial adipose tissue accumulates and mechanically obstructs the myocardium from dilating, therefore causing diastolic dysfunction.
Figure 3.
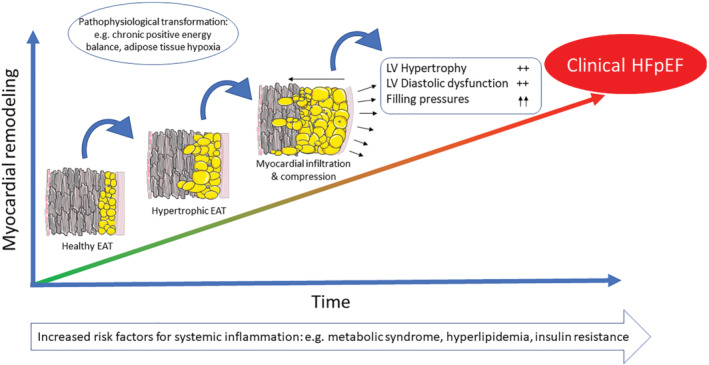
Schematic overview of the pathways by which epicardial adipose tissue (EAT) may accumulate and eventually affect the underlying myocardium, leading to left ventricular (LV) hypertrophy, LV diastolic dysfunction and increased cardiac filling pressures and ultimately resulting in clinical heart failure with preserved ejection fraction (HFpEF).
In addition to EAT infiltrating the adjacent cardiac structure, EAT may secrete pro‐inflammatory adipokines to the underlying tissues. It has been suggested that the secretion of these adipokines works through two pathways. 8 Via the paracrine pathway, EAT may release adipokines directly onto the myocardium and coronary arteries by means of diffusion. This hypothesis is supported by two studies. 42 , 43 In the study by Shimokawa et al., 43 the coronary arteries of pigs were exposed to the inflammatory adipokine interleukin (IL)‐1β, which is abundant in EAT. The authors found that intimal thickening was greater at the IL‐1β treated sites compared to the sites without IL‐1β, suggesting diffusion of adipokines is possible and may potentially even aggravate coronary atherosclerotic lesions. Nalliah et al. 42 observed a paracrine effect of EAT on cardiomyocytes, demonstrated by a longer field potential duration when cardiomyocytes were subjected to EAT. A recent clinical study utilizing magnetic resonance spectroscopy found a positive relation between EAT volume and intramyocardial triglyceride content. 44 In another study by Wu et al., 33 it was found that HFpEF patients have higher intramyocardial triglyceride content compared to controls. These findings also support the notion of a potential infiltrative effect of EAT.
The vasocrine signalling pathway has been suggested in a study by Yudkin et al., 45 and states that adipokines are not diffused directly onto the adjacent tissues, but are released into the vasa vasorum where they are carried downstream and may cause local harm.
Pericardial restraint hypothesis
Epicardial adipose tissue may also exert direct mechanical compression of the myocardium when it is accumulating within the pericardium leading to a constrictive pericarditis‐like situation. 26 Because the pericardium has limited pliability, enlargement of EAT would cause the encased myocardium to be less able to dilate, thereby leading to imposed diastolic dysfunction and increased cardiac filling pressures (Figures 2B and 3 ). Indeed, two studies examined the haemodynamic effects of increased EAT in patients with HFpEF and found that EAT accumulation was associated with higher cardiac filling pressures and with increased left ventricular eccentricity index, further supporting the hypothesis that EAT may lead to increased ventricular interdependence by pericardial constraint. 26 , 27
Epicardial adipose tissue and cardiac death
Whereas increasing data suggest that EAT may be involved in HFpEF pathophysiology by means of infiltration or mechanical compression, it remains largely unknown whether these same mechanisms may lead to cardiac death. A recently published review article suggests that EAT may, via infiltration of EAT into the myocardium and the release of pro‐inflammatory cytokines, ultimately lead to cardiac arrhythmias, including ventricular tachyarrhythmias. 46 EAT accumulation may therefore be related to sudden cardiac death, however, this has not been investigated yet. On the other hand, EAT has been related to increased cardiac filling pressures, 26 , 27 lower peak oxygen uptake, 26 , 27 and higher New York Heart Association functional class, 7 all indicating worsening heart failure, also a common mode of death in patients with HFpEF. 47 Therefore, EAT accumulation, whether by infiltration or by mechanical compression, may also be involved in worsening heart failure as a cause of death in patients with HFpEF. Lastly, the possibility exists that EAT is not directly related to mortality, but rather a risk marker that coincides with other risk factors for mortality including type II diabetes mellitus and coronary artery disease.
Epicardial adipose tissue accumulation and obesity in HFpEF
Both increased EAT and obesity are associated with myocardial impairments typical for HFpEF, including left ventricular hypertrophy, diastolic dysfunction and left atrial dilatation. 23 , 25 , 48 , 49 In addition, both EAT and obesity are associated with incident HFpEF. 3 , 6 However, it is important to consider here that obesity and epicardial adiposity are not always mutually related. A recent study observed that patients with HFpEF have increased EAT volume compared to controls without heart failure, whereas BMI between these groups was similar. 23 In addition, in another HFpEF study, 22% of patients were classified as non‐obese, whereas these patients actually had a high EAT volume. 7 Conversely, in the same study, 18% of patients who were classified as obese, had low EAT volume. In Table 2 , the putative differential effects of EAT and obesity on the heart, as well as their relation to natriuretic peptides, atrial fibrillation and mortality, are summarized. EAT is thought to exert local mechanical and inflammatory effects on the myocardium, 25 , 33 , 50 whereas obesity is thought to affect the heart in systemic ways, including increased cardiac loading caused by obesity‐related volume overload. 31 Furthermore, increased EAT is related to higher natriuretic peptide levels, 51 whereas conversely, obesity is related to lower natriuretic peptide levels. 52 Lastly, conflicting evidence exists regarding the association between obesity and mortality in patients with HFpEF. Some studies suggest that obesity is associated with increased risk of death in patients with HFpEF 53 ; however, other studies show a beneficial effect of obesity on survival. 54 Furthermore, the discrepancy between the association of obesity with incident HFpEF in the general population on the one hand, and the beneficial association of obesity on survival in those with established HFpEF on the other is called the ‘obesity paradox’. 54 However, recent studies have been challenging the obesity paradox. 55 BMI does not discriminate between weight by muscle or by fat and it does not provide insight into where the fat is located. Indeed, in a recent study it was shown that in those with established heart failure, BMI was associated with improved survival. 55 However, when the authors used waist‐to‐hip ratio, a measure that more accurately reflects visceral adiposity than BMI, they found that increased waist‐to‐hip ratio was associated with adverse survival in women. Although less widely investigated, EAT was consistently associated with a higher rate of hospitalizations for heart failure and death in HFpEF in two independent studies. 7 , 36
Table 2.
Differences between epicardial adipose tissue and obesity with respect to heart failure
| Epicardial adipose tissue | Obesity | |
|---|---|---|
| Local mechanical and inflammatory effect on the heart | + | − |
| Systemic volume loading and inflammatory effect on the heart | − | + |
| Association with natriuretic peptides (NPs) | NPs ↑ | NPs ↓ |
| Related to atrial fibrillation in heart failure and preserved ejection fraction | + | + |
| Related to mortality in heart failure and preserved ejection fraction | + | +/− |
It is therefore important to not only assess adiposity as measured by BMI, but to specifically take into account whether a patient has an increased amount of EAT, at least for prognostic purposes. As a starting point, one could use anthropometric measurements that better reflect visceral fat status than BMI, such as relative fat mass or waist‐to‐hip ratio to estimate EAT. 56 In those patients with a high relative fat mass or waist‐to‐hip ratio, it may be considered to precisely quantify the amount of EAT with cardiac magnetic resonance (CMR) or computed tomography (CT) imaging. 6 , 25
Epicardial adipose tissue in HFrEF versus HFpEF
Recent data suggest that EAT may play a different role in the pathophysiology of HFrEF compared to HFpEF. In two studies, it was reported that patients with HFrEF have less EAT compared to healthy controls and patients with HFpEF. 36 , 57 However, contrastingly, in two other studies EAT was increased in HFrEF compared to controls and HFpEF. 33 , 34 Nonetheless, in those with HFrEF, increased EAT was associated with a lower risk of cardiovascular death and heart failure hospitalizations. 36 This finding that EAT is protective of cardiovascular events in HFrEF is in contrast with HFpEF, where EAT is associated with an adverse prognosis. 7 , 36 This difference in association of EAT and outcome between HFrEF and HFpEF may be related to the association between EAT and overall cardiometabolic profile. In HFrEF, higher EAT is associated with lower C‐reactive protein, lower troponin T and lower IL‐6 levels, suggesting that increased EAT in HFrEF is associated with a beneficial cardiometabolic profile. 36 Contrastingly, in HFpEF higher EAT is associated with increased C‐reactive protein, increased troponin T and creatine‐kinase MB, suggesting an adverse cardiometabolic profile. 7 , 36 On the other hand, it is also possible that the quality of EAT itself may differ between HFrEF and HFpEF, where EAT in HFrEF has a more anti‐inflammatory profile and in HFpEF a more pro‐inflammatory profile, with a direct adverse effect on the myocardium in HFpEF and a beneficial effect in HFrEF. However, this has not yet been investigated.
Quantification and qualification of epicardial adipose tissue
Non‐invasive quantification of EAT is generally done using CMR, CT, or transthoracic echocardiography. 6 , 25 , 33 , 37 Figure 4 depicts typical examples of EAT assessed with each of these imaging techniques. Both CMR and CT allow for EAT volume quantification and localization. An advantage of CT is the option to assess EAT density, which is associated with inflammation of EAT. 58 The density of EAT was associated with the presence of coronary artery disease, even after adjustment for overall EAT volume, which suggests that not only the quantity of EAT is of importance, but also the quality of EAT in terms of inflammation. 58 Moreover, one study even found that in patients with a high‐risk coronary plaque, assessed by positron emission tomography (PET), surrounding EAT density around the plaque was higher compared to EAT surrounding low‐risk coronary plaques in patients with low‐risk coronary plaques, indicating very local inflammation. 59 In addition, the possibility exists that EAT may also impair coronary vascular function in those with non‐obstructive coronary artery disease on PET, potentially leading to diastolic dysfunction and HFpEF. 29 , 60 , 61
Figure 4.
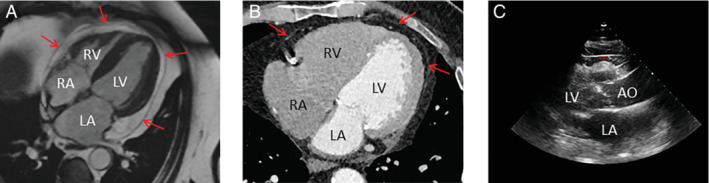
Three different imaging methods for assessment of epicardial adipose tissue. Red arrows indicate the pericardium. (A) Cardiac magnetic resonance imaging. (B) Computed tomography imaging. (C) Transthoracic echocardiography. AO, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
On the other hand, an advantage of CMR imaging is that it also allows for the assessment of intramyocardial adiposity if magnetic resonance spectroscopy techniques are added. 33 Furthermore, with CMR imaging, functional analyses of the myocardium can be performed in addition to EAT quantification. 25
Transthoracic echocardiography has also been used to quantify the amount of EAT. 37 However, contrary to CMR and CT, only the thickness of EAT can be measured on transthoracic echocardiography. 37 The chances of over‐ or underestimation of the actual EAT volume are therefore higher with transthoracic echocardiography, making this measurement less reliable for EAT quantification in individual patients. 37
Potential sex differences in epicardial adipose tissue in HFpEF
Clinical studies show conflicting data regarding the amount of EAT in men compared to women. 15 , 62 Although some studies observed higher amounts of EAT in women, 15 other studies reported a higher amount in men. 62 This discrepancy may in part be related to the post‐menopausal status of women, as older women (>60 years) have higher EAT volumes compared to younger women. 15 Interestingly, regardless of the amount of fat, EAT seems to be associated with worse systolic and diastolic left ventricular function in women, but not in men. 15 Similarly, a recent study in women with HFpEF demonstrated that visceral adipose tissue accumulation was associated with increased cardiac filling pressures, whereas this association was not observed in men. Because EAT and visceral abdominal fat are highly correlated, 63 it may be speculated that this difference in cardiac filling pressures between men and women could also be due to local effects of EAT.
Potential treatment options for epicardial adipose tissue accumulation
If EAT indeed contributes to the pathophysiology of HFpEF, it may be a promising therapeutic target. However, to date there are no proven treatment options specifically targeting EAT. Here we discuss four potential strategies for directly targeting EAT as well as interventions that are approved in the treatment of obesity and type II diabetes, which have been shown to reduce the volume and inflammatory nature of EAT.
Surgical resection of epicardial adipose tissue
A few experimental studies have investigated the surgical resection of EAT. In one study, miniature swine were subjected to either surgical resection of EAT around the left anterior descending coronary artery, or sham surgery. 64 The authors found that resection of EAT was associated with a halted progression of coronary artery disease. In one other study, obese rats subjected to acute myocardial infarction, and the resection of the fat adjacent to the heart was associated with better left ventricular function compared to rats with myocardial infarction and no resection of fat. 65 Although it is difficult to distinguish between pericardial adipose tissue and EAT in rodent models, the result suggests that removal of cardiac fat may have a positive effect on coronary artery disease and potentially also on myocardial function.
One case report described the specific removal of the epicardial fat pad in a clinical patient with excessive EAT volume. 66 In this patient, resection of EAT improved left ventricular diastolic function. Combined, these data suggest that resection of EAT may have favourable effects on the heart. However, whether this is a viable treatment option in patients with HFpEF remains to be elucidated.
Pericardiotomy for pericardial restraint relief
Recent studies have shown that incision of the parietal pericardium in animals with features of HFpEF is associated with a less stark increase in cardiac filling pressures after saline infusion compared to animals without this procedure. 67 , 68 Although this procedure does not specifically target EAT, in theory it may be particularly beneficial in patients with high intra‐pericardial pressures possibly as the result of an abundance of EAT volume, since increased EAT volume inside the stiff pericardial sac is believed to constrain the myocardium which in turn may lead to higher cardiac filling pressures, 26 , 27 as described earlier. However, whether pericardiotomy is effective in reducing cardiac filling pressures, particularly in HFpEF patients with high EAT volume, is currently unknown and should be investigated.
‘Browning’ of epicardial adipose tissue
Studies have not only distinguished fat on the basis of location, but also on the basis of function. The majority of fat in the human body is regarded white adipose tissue, which is primarily associated with the storage of energy. 69 On the other hand, there is brown adipose tissue, which accounts for a small percentage of all fat, and this adipose tissue is associated with thermoregulation. 69 Brown adipose tissue is characterized by an abundance of mitochondria that use energy to produce heat. Moreover, higher volume of brown adipose tissue is associated with a lower risk of cardiovascular diseases whereas, conversely, increased white adipose tissue is associated with a higher risk of cardiovascular diseases. 70 A recent study observed that certain key proteins associated with brown adipose tissue are expressed in EAT, suggesting EAT has brown adipose tissue‐like features. 71 However, the amount of brown adipose tissue seems to decrease with higher age and obesity, 71 , 72 both of which are highly prevalent in HFpEF. Interestingly, it has recently been shown that the function of fat can be redirected to a more brown adipose tissue‐like function with the administration of a β3‐adrenergic receptor agonist, a process called ‘browning’. 73
Because EAT highly expresses proteins associated with brown adipose tissue, it may be speculated that EAT could be particularly susceptible for the effects of a β3‐adrenergic receptor agonist. However, side effects of treatment with a β3‐adrenergic receptor agonist include higher heart rate and increased blood pressure, 73 and it remains debatable whether treatment with a β3‐adrenergic receptor agonist will lead to improved survival in older, fragile patients with HFpEF.
Epicardial adipose tissue as a target for gene therapy
In gene therapy, genetic material is deleted from, added to, or altered in specific cells, in order to stimulate better functioning of the cell. This is done using a vehicle that delivers the genetic material to the cells, typically a virus. These viruses are stripped from their own viral genes, so that they do not inflict an immune response. Although gene therapy has mainly focused on treating non‐cardiovascular diseases, it has recently been suggested that adipose tissue may also be a potential target for gene therapy. 74 One study directly targeted visceral adipose tissue in mice. 74 The gene therapy in this study focused on overexpressing a protein called fibroblast growth factor 21 (FGF21) in visceral adipose tissue. Higher expression of FGF21 was associated with improved insulin sensitivity and lower inflammatory cytokine release of the visceral adipose tissue. Although no studies have yet investigated whether specifically EAT is amenable for gene therapy, it may be a promising target. Local injection into the pericardial sac with a virus that carries the genetic code for FGF21 may specifically ameliorate the inflammatory character of EAT. However, such a procedure does introduce a significant risk for puncture of the heart or pericarditis, and should be further investigated.
Other treatment options
Therapies that are used for the management of obesity and type II diabetes, such as diet, exercise and bariatric surgery, but also therapies specifically for the management of type II diabetes, including glucagon‐like peptide 1 (GLP‐1) receptor agonists and sodium–glucose cotransporter 2 (SGLT‐2) inhibitors, appear to reduce EAT volume and seem to lower its pro‐inflammatory character. 75 In addition by reducing the amount of visceral adiposity, these therapies also have multiple systemic effects that may be beneficial for patients with HFpEF, such as weight loss, lowering of blood pressure, and better glycaemic control. It is therefore difficult to detangle whether these therapies have a beneficial effect on HFpEF specifically due to lowering of EAT, or due to their systemic effects. Future research should therefore investigate whether the reduction in EAT observed with diet and exercise, bariatric surgery, GLP‐1 receptor agonists and SGLT‐2 inhibitors is associated with better survival in patients with HFpEF, independent of the systemic effects of these therapies.
Knowledge gaps and future directions
Even though our understanding of EAT in the setting of HFpEF is increasing, there are still several important knowledge gaps. Further study is essential to advance our understanding of the pathogenesis, pathophysiological mechanisms and treatment of EAT accumulation and inflammation in HFpEF. In Table 3 these knowledge gaps are summarized and future areas of potential discovery are mentioned, such as determining the predominant mechanism of action of EAT, and identifying treatment regimens that specifically target EAT beyond overall obesity. To answer these questions, the use of both clinical and experimental studies is greatly supported.
Table 3.
Knowledge gaps and areas for future research of the pathophysiology and treatment of epicardial adipose tissue in heart failure with preserved ejection fraction
| Domain | Important knowledge gaps | Areas of potential discovery | Execution and limitations of potential study |
|---|---|---|---|
| Function and development | Physiological function of epicardial adipose tissue | Determining the scope of physiological functions of healthy epicardial adipose tissue | Animal model: healthy animal. Resection vs. no resection of epicardial adipose tissue, fed ad libitum vs. caloric restricted. Assessment of altered cardiomyocyte metabolism in animals with no epicardial adipose tissue. Limitation: Not feasible in humans |
| Development of epicardial adipose tissue over time | Assessing the clinical course of epicardial adipose tissue development during health and HFpEF | Animal model: biopsies of epicardial adipose tissue during normal ageing and during HFpEF development. Limitation: not ethical and not feasible to take epicardial adipose tissue biopsies in healthy humans, or HFpEF patients not undergoing open heart surgery | |
| Pathophysiological mechanism | Is epicardial adipose tissue a cause or a consequence of HFpEF? | Determining whether epicardial adipose tissue is a cause or a consequence of HFpEF, or neither | Animal model: healthy, normal weight animal. Randomized to treatment leading to high epicardial adipose tissue vs. low epicardial adipose tissue. Assessment of HFpEF development. Control vs. HFpEF, matched for age, sex, weight. Serial assessment of epicardial adipose tissue in both arms. Limitation: currently unknown how to specifically increase epicardial adipose tissue, not feasible in humans |
| Pathophysiological mechanism of epicardial adipose tissue in HFpEF | Assessing whether epicardial adipose tissue primarily infiltrates, or compresses the myocardium, or both | Animal model: controls vs. HFpEF. Assessment of epicardial adipose tissue infiltration using histological lipid staining. Assessment of epicardial adipose tissue compression using invasive haemodynamics. Humans: assessment of epicardial adipose tissue infiltration using autopsy studies. Assessment of local epicardial adipose tissue volume compression using invasive haemodynamics in HFpEF vs. controls. Limitation: potentially difficult to obtain IRB approval for performing invasive haemodynamics in otherwise healthy controls | |
| HFpEF vs. HFrEF differences in epicardial adipose tissue pathophysiology | Determining whether epicardial adipose tissue has different pathophysiological properties in HFpEF vs. HFrEF | Animal model: HFpEF vs. HFrEF. Biopsies of epicardial adipose tissue during HFpEF and HFrEF development. Humans: HFpEF vs. HFrEF. Biopsies of epicardial adipose tissue in HFpEF and HFrEF to determine biochemical differences. Limitation: biopsies only feasible in HFpEF and HFrEF patients undergoing open heart surgery | |
| Sex differences in epicardial adipose tissue pathophysiology | Investigating if epicardial adipose tissue relates differently to HFpEF pathophysiology in men compared to women | Animal model: HFpEF, male vs. female. Biopsies of epicardial adipose tissue during HFpEF development in both male and female species. Humans: HFpEF, men vs. women. Relating epicardial adipose tissue in men and women with HFpEF to outcome. Biopsies of epicardial adipose tissue in men and women with HFpEF to determine biochemical differences. Limitation: biopsies only feasible in HFpEF patients undergoing open heart surgery | |
| Genetic influence of epicardial adipose tissue accumulation | Determining the role of genetic factors in accumulation of epicardial adipose tissue in HFpEF | Animal model: HFpEF vs. controls. Biopsies of epicardial adipose tissue, determining genetic factors associated with epicardial adipose tissue accumulation and inflammation. Humans: HFpEF. biopsies of epicardial adipose tissue, determining genetic factors associated with epicardial adipose tissue accumulation and inflammation. Limitation: biopsies only feasible in HFpEF patients undergoing open heart surgery, and not feasible in healthy subjects | |
| Diagnosis | Diagnosis of hypertrophic epicardial adipose tissue | Defining a clinically relevant cut‐off value for increased epicardial adipose tissue volume, to enable physicians which patients to treat with epicardial adipose tissue lowering therapies | Humans: healthy subjects. Epicardial adipose tissue volume measurement, determining cut‐off value for when epicardial adipose tissue is increased by calculating the 95% percentile of mean epicardial adipose tissue volume. Limitations: great number of subjects that undergo cardiac magnetic resonance or computed tomography is needed |
| Treatment | Epicardial adipose tissue volume reduction | Investigation of the feasibility and effect of local surgical removal of epicardial adipose tissue in HFpEF and whether it is associated with better outcomes | Animal model: HFpEF. Investigate feasibility of epicardial adipose tissue resection. If feasible, randomize animals to either epicardial adipose tissue resection or sham surgery and relate to cardiac function and structure. Limitations: currently not feasible in humans |
| Epicardial adipose tissue ‘browning’ | Assessment whether epicardial adipose tissue inflammation is reduced in HFpEF after administration of a β3‐adrenergic receptor agonist | Animal model: HFpEF. Randomize animals to either treatment or no treatment, assess inflammation by taking biopsies of epicardial adipose tissue. Humans: HFpEF, biopsies of epicardial adipose tissue in patients with or without treatment to determine biomarkers of inflammation. Limitations: only feasible in patients undergoing open heart surgery |
Abbreviation: HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IRB, institutional review board.
Conclusions
Evidence is accumulating that EAT is involved in the pathophysiology, symptomatology and clinical course of HFpEF. However, many questions on the pathophysiology and potential treatment of EAT still remain unanswered. More studies are needed to tackle these questions and to identify therapeutic options that specifically reduce EAT accumulation and inflammation to improve outcomes for patients with HFpEF.
Conflict of interest: none declared.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2021;24:4–131. [DOI] [PubMed] [Google Scholar]
- 2.World Health Orgaizattion. World Health Organization Key Facts on Obesity and Overweight . https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight (last accessed 20 January 2022).
- 3. Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, et al. Adiposity and incident heart failure and its subtypes: MESA (Multi‐Ethnic Study of Atherosclerosis). JACC Heart Fail. 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory‐metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rao VN, Fudim M, Mentz RJ, Michos ED, Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021;77:2638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, et al. Epicardial adipose tissue and outcome in heart failure with mid‐range and preserved ejection fraction. Circ Heart Fail. 2021;15:e009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–17. [DOI] [PubMed] [Google Scholar]
- 9. Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–6. [DOI] [PubMed] [Google Scholar]
- 10. Marchington JM, Pond CM. Site‐specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high‐fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013–22. [PubMed] [Google Scholar]
- 11. Arora RC, Waldmann M, Hopkins DA, Armour JA. Porcine intrinsic cardiac ganglia. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:249–58. [DOI] [PubMed] [Google Scholar]
- 12. van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res. 2000;45:279–93. [DOI] [PubMed] [Google Scholar]
- 13. Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour A. Role of epicardial adipose tissue in health and disease: a matter of fat? Compr Physiol. 2017;7:1051–82. [DOI] [PubMed] [Google Scholar]
- 15. Kim SA, Kim MN, Shim WJ, Park SM. Epicardial adipose tissue is related to cardiac function in elderly women, but not in men. Nutr Metab Cardiovasc Dis. 2017;27:41–7. [DOI] [PubMed] [Google Scholar]
- 16. Sousa JA, Mendonca MI, Serrao M, Borges S, Henriques E, Freitas S, et al. Epicardial adipose tissue: the genetics behind an emerging cardiovascular risk marker. Clin Med Insights Cardiol. 2021;15:11795468211029244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virtanen KA, Lonnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–10. [DOI] [PubMed] [Google Scholar]
- 18. Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. [DOI] [PubMed] [Google Scholar]
- 19. Skurk T, Alberti‐Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. [DOI] [PubMed] [Google Scholar]
- 20. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. [DOI] [PubMed] [Google Scholar]
- 23. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail. 2018;20:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–51. [DOI] [PubMed] [Google Scholar]
- 25. van Woerden G, van Veldhuisen DJ, Gorter TM, van Empel VPM, Hemels MEW, Hazebroek EJ, et al. Importance of epicardial adipose tissue localization using cardiac magnetic resonance imaging in patients with heart failure with mid‐range and preserved ejection fraction. Clin Cardiol. 2021;44:987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, et al. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:667–76. [DOI] [PubMed] [Google Scholar]
- 27. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mancio J, Azevedo D, Saraiva F, Azevedo AI, Pires‐Morais G, Leite‐Moreira A, et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging. 2018;19:490–7. [DOI] [PubMed] [Google Scholar]
- 29. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 30. Rao VN, Bush CG, Mongraw‐Chaffin M, Hall ME, Clark D, Fudim M, et al. Regional adiposity and risk of heart failure and mortality: the Jackson Heart Study. J Am Heart Assoc. 2021;10:e020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, et al. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:445–54. [DOI] [PubMed] [Google Scholar]
- 34. Tromp J, Bryant JA, Jin X, van Woerden G, Asali S, Yiying H, et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail. 2021;23:835–8. [DOI] [PubMed] [Google Scholar]
- 35. Ying W, Sharma K, Yanek LR, Vaidya D, Schär M, Markl M, et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8:2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021;23:1858–71. [DOI] [PubMed] [Google Scholar]
- 37. van Woerden G, van Veldhuisen DJ, Gorter TM, Ophuis B, Saucedo‐Orozco H, van Empel VPM, et al. The value of echocardiographic measurement of epicardial adipose tissue in heart failure patients. ESC Heart Fail. 2022;9:953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahabadi AA, Anapliotis V, Dykun I, Hendricks S, Al‐Rashid F, Lüdike P, et al. Epicardial fat and incident heart failure with preserved ejection fraction in patients with coronary artery disease. Int J Cardiol. 2022;357:140–5. [DOI] [PubMed] [Google Scholar]
- 39. He S, Zhu H, Zhang J, Wu X, Zhao L, Yang X. Proteomic analysis of epicardial adipose tissue from heart disease patients with concomitant heart failure with preserved ejection fraction. Int J Cardiol. 2022;362:118–25. [DOI] [PubMed] [Google Scholar]
- 40. Venkateshvaran A, Faxen UL, Hage C, Michaëlsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: insights from the PROMIS‐HFpEF study. Eur J Heart Fail. 2022;24:2251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail. 2022;24:1346–56. [DOI] [PubMed] [Google Scholar]
- 42. Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76:1197–211. [DOI] [PubMed] [Google Scholar]
- 43. Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, et al. Chronic treatment with interleukin‐1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet‐derived growth factor. J Clin Invest. 1996;97:769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng ACT, Strudwick M, van der Geest RJ, Ng ACC, Gillinder L, Goo SY, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imaging. 2018;11:e007372. [DOI] [PubMed] [Google Scholar]
- 45. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–20. [DOI] [PubMed] [Google Scholar]
- 46. Ernault AC, Meijborg VMF, Coronel R. Modulation of cardiac arrhythmogenesis by epicardial adipose tissue: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:1730–45. [DOI] [PubMed] [Google Scholar]
- 47. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69:556–69. [DOI] [PubMed] [Google Scholar]
- 48. Aiad NN, Hearon CJ, Hieda M, Dias K, Levine BD, Sarma S. Mechanisms of left atrial enlargement in obesity. Am J Cardiol. 2019;124:442–7. [DOI] [PubMed] [Google Scholar]
- 49. Kossaify A, Nicolas N. Impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clin Med Insights Cardiol. 2013;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. [DOI] [PubMed] [Google Scholar]
- 51. Nyawo TA, Dludla PV, Mazibuko‐Mbeje SE, Mthembu SXH, Nyambuya TM, Nkambule BB, et al. A systematic review exploring the significance of measuring epicardial fat thickness in correlation to B‐type natriuretic peptide levels as prognostic and diagnostic markers in patients with or at risk of heart failure. Heart Fail Rev. 2022;27:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT‐proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I‐PRESERVE) trial. Circ Heart Fail. 2011;4:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all‐cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–49. [DOI] [PubMed] [Google Scholar]
- 55. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Waist‐to‐hip ratio and mortality in heart failure. Eur J Heart Fail. 2018;20:1269–77. [DOI] [PubMed] [Google Scholar]
- 56. Suthahar N, Meems LMG, Withaar C, Gorter TM, Kieneker LM, Gansevoort RT, et al. Relative fat mass, a new index of adiposity, is strongly associated with incident heart failure: data from PREVEND. Sci Rep. 2022;12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Wang S, Wang Y, Zhou N, Shu J, Stamm C, et al. Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis. 2019;284:230–6. [DOI] [PubMed] [Google Scholar]
- 59. Kitagawa T, Nakamoto Y, Fujii Y, Sasaki K, Tatsugami F, Awai K, et al. Relationship between coronary arterial 18F‐sodium fluoride uptake and epicardial adipose tissue analyzed using computed tomography. Eur J Nucl Med Mol Imaging. 2020;47:1746–56. [DOI] [PubMed] [Google Scholar]
- 60. Bakkum MJ, Danad I, Romijn MA, Stuijfzand WJ, Leonora RM, Tulevski II, et al. The impact of obesity on the relationship between epicardial adipose tissue, left ventricular mass and coronary microvascular function. Eur J Nucl Med Mol Imaging. 2015;42:1562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nappi C, Ponsiglione A, Acampa W, Gaudieri V, Zampella E, Assante R, et al. Relationship between epicardial adipose tissue and coronary vascular function in patients with suspected coronary artery disease and normal myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging. 2019;20:1379–87. [DOI] [PubMed] [Google Scholar]
- 62. Mancio J, Pinheiro M, Ferreira W, Carvalho M, Barros A, Ferreira N, et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int J Cardiol. 2017;249:419–25. [DOI] [PubMed] [Google Scholar]
- 63. Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10. [DOI] [PubMed] [Google Scholar]
- 64. McKenney‐Drake ML, Rodenbeck SD, Bruning RS, Kole A, Yancey KW, Alloosh M, et al. Epicardial adipose tissue removal potentiates outward remodeling and arrests coronary atherogenesis. Ann Thorac Surg. 2017;103:1622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang KW, Ko JY, Lee H, Shin SY, Lee WS, Hong J, et al. Surgically metabolic resection of pericardial fat to ameliorate myocardial mitochondrial dysfunction in acute myocardial infarction obese rats. J Korean Med Sci. 2022;37:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smail H, Baciu A, Dacher JN, Litzler PY. Surgical resection of circumferential epicardial adipose tissue hypertrophy: case report and systematic review of the literature. J Thorac Cardiovasc Surg. 2016;151:e27–30. [DOI] [PubMed] [Google Scholar]
- 67. Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jain CC, Pedrotty D, Araoz PA, Sugrue A, Vaidya VR, Padmanabhan D, et al. Sustained improvement in diastolic reserve following percutaneous pericardiotomy in a porcine model of heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14:e007530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saely CH, Geiger K, Heinz D. Brown versus white adipose tissue: a mini‐review. Gerontology. 2012;58:15–23. [DOI] [PubMed] [Google Scholar]
- 70. Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein‐1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–5. [DOI] [PubMed] [Google Scholar]
- 72. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–9. [PMC free article] [PubMed] [Google Scholar]
- 73. Cypess AM, Weiner LS, Roberts‐Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a beta3‐adrenergic receptor agonist. Cell Metab. 2015;21:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Queen NJ, Bates R, Huang W, Xiao R, Appana B, Cao L. Visceral adipose tissue‐directed FGF21 gene therapy improves metabolic and immune health in BTBR mice. Mol Ther Methods Clin Dev. 2020;20:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Launbo N, Zobel EH, von Scholten BJ, Faerch K, Jorgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: a systematic review and meta‐analysis. Obes Rev. 2021;22:e13136. [DOI] [PubMed] [Google Scholar]


