Abstract
Recent studies have shown that in the preclinical phase of Alzheimer's disease (AD), subtle cognitive changes can be detected using sensitive neuropsychological measures, and have proposed the concept of objectively‐defined subtle cognitive decline (Obj‐SCD). We aimed to assess the functional alteration of hippocampal subfields in individuals with Obj‐SCD and its association with cognition and pathological biomarkers. Forty‐two participants with cognitively normal (CN), 29 with Obj‐SCD, and 55 with mild cognitive impairment (MCI) were retrospectively collected from the ADNI database. Neuropsychological performance, functional MRI, and cerebrospinal fluid (CSF) data were obtained. We calculated the seed‐based functional connectivity (FC) of hippocampal subfields (cornu ammonis1 [CA1], CA2/3/dentate gyrus [DG], and subiculum) with whole‐brain voxels. Additionally, we analyzed the correlation between FC values of significantly altered regions and neuropsychological performance and CSF biomarkers. The Obj‐SCD group showed lower FC between left CA1‐CA2/3/DG and right thalamus and higher FC between right subiculum and right superior parietal gyrus (SPG) compared with the CN and MCI groups. In the Obj‐SCD group, FC values between left CA2/3/DG and right thalamus were positively associated with Auditory Verbal Learning Test (AVLT) recognition (r = 0.395, p = 0.046) and CSF Aβ1–42 levels (r = 0.466, p = 0.019), and FC values between left CA1 and right thalamus were positively correlated with CSF Aβ1–42 levels (r = 0.530, p = 0.006). Taken together, dysfunction in CA1‐CA2/3/DG subregions suggests subtle cognitive impairment and AD‐specific pathological changes in individuals with Obj‐SCD. Additionally, increased subiculum connectivity may indicate early functional compensation for subtle cognitive changes.
Keywords: Alzheimer's disease, early detection, functional connectivity, hippocampal subfields, subtle cognitive decline
Obj‐SCD is considered to be the preclinical phase of AD. Different hippocampal subfields show specific functions. Hippocampal dysfunction in Obj‐SCD reflects subtle cognitive impairment and AD‐specific pathological changes.
1. INTRODUCTION
Alzheimer's disease (AD) is the leading cause of dementia in the elderly (Masters et al., 2015). It is an ongoing process that leads to alterations in cognitive function and results in status ranging from the preclinical phase, mild cognitive impairment (MCI), to the final dementia status (Veitch et al., 2019; Vermunt et al., 2019). Given the irreversible nature of AD, early intervention in its preclinical phase has the potential to prevent or delay cognitive decline due to AD (Sperling et al., 2011). In addition to subjective cognitive decline, objectively‐defined subtle cognitive decline (Obj‐SCD) is another type of slight cognitive change that can be captured using sensitive neuropsychological measures in the preclinical phase of AD and has recently gained widespread recognition (Edmonds, Delano‐Wood, Galasko, et al., 2015).
According to Edmonds/Thomas criteria (Edmonds, Delano‐Wood, Galasko, et al., 2015; Thomas, Edmonds, et al., 2018; Thomas, Eppig, et al., 2018), individuals with Obj‐SCD can be detected by six sensitive neuropsychological measures in three different cognitive domains (i.e., memory, language, and attention/executive function) and three process scores from Auditory Verbal Learning Test (AVLT). Specifically, the Obj‐SCD is defined by performance >1 standard deviation below the demographically adjusted (age, sex, and education) mean on (1) two neuropsychological measures in two different cognitive domains; (2) two process scores; or (3) one neuropsychological measure and one process score. Notably, in a follow‐up study over 10 years, individuals with Obj‐SCD progressed to MCI at a rate of 2.46–3.17 times faster than the cognitively normal subjects (Thomas, Edmonds, et al., 2018). In addition, Obj‐SCD was associated with faster amyloid accumulation, increased plasma phosphorylated‐tau181 levels, and neurodegeneration in a 4‐year follow‐up study (Thomas et al., 2020; Thomas, Bangen, et al., 2021).
Memory loss is considered to be an early and major clinical manifestation of AD. It is well known that the hippocampus plays a key role in cognitive function, especially in episodic memory (El Haj et al., 2016; Squire et al., 1992; Tulving & Markowitsch, 1998). Alterations in hippocampal structure and function (e.g., atrophy, disconnection, or decreased activation) have been widely reported to be associated with cognitive decline in patients with MCI and AD (Berron et al., 2020; Hampel et al., 2008; Jaroudi et al., 2017). The heterogeneous formation of the hippocampus is also well recognized, and it can be cytoarchitectonically divided into different subfields, including the four fields of the cornu ammonis (CA1–4), dentate gyrus (DG), and subiculum (Duvernoy et al., 2013). The most pronounced neuronal loss associated with AD occurs mainly in the CA1 region (West et al., 1994). Neuropathological studies have also shown that early tau pathology appears in the CA1 and subiculum before affecting the entire hippocampus (Braak & Braak, 1991; Lace et al., 2009). In addition, most of the hippocampal output originates from the subiculum, which is mainly connected to the medial temporal lobe, precuneus/posterior cingulate, and frontal regions (Aggleton & Christiansen, 2015; de Flores et al., 2017). Although the distinct functions of hippocampal subfields have been well described, little is still known about the alterations of intrinsic neural activity in the preclinical phase of AD.
Many functional magnetic resonance imaging (fMRI) studies have provided consistent evidence that disruption of functional connectivity (FC) precedes structural atrophy, reflecting alterations in underlying neural activity (Pievani et al., 2011). Based on resting‐state fMRI (rsfMRI), altered FC in hippocampal subfields has been widely reported in MCI and AD patients. For example, the intrinsic FC between the subiculum and frontal and posterior cingulate regions was significantly reduced in MCI patients compared with healthy controls (HC) (de Flores et al., 2017). In addition, patients with MCI also had decreased FC between right CA1 and right middle temporal gyrus as well as between the left CA2 and bilateral cuneal cortex compared with HC (Li et al., 2018). Furthermore, Yan et al. (2020) found that the FC in the CA1, CA2/3/DG, and subiculum tended to decrease with disease progression (AD < MCI < HC) and that disruption of these functions was positively correlated with cognitive impairment. And exploring the functional changes in hippocampal subfields of individuals with Obj‐SCD will deepen our understanding of the early physiological mechanisms.
In the present study, we aimed to evaluate FC changes in hippocampal subfields in individuals with Obj‐SCD. The hippocampus was divided into three subfields using in vivo MRI (Copara et al., 2014; Yan et al., 2020), including the CA1, CA2/3/DG, and subiculum. In addition, we explored the association between FC changes in hippocampal subfields and cognition and AD‐specific pathological biomarkers. Because cognitive impairment is milder in Obj‐SCD individuals than in MCI patients, we hypothesized that some functional compensation in addition to functional disruption could be detected in Obj‐SCD.
2. MATERIALS AND METHODS
2.1. Cognitive groups
Data used in the preparation of this study were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/). We included 1380 participants without dementia, including cognitively normal (CN) and MCI participants from ADNI1, ADNI‐GO, and ADNI‐2, who completed baseline neuropsychological assessments. Notably, recent studies (Bondi et al., 2014; Edmonds, Delano‐Wood, Clark, et al., 2015) have noted that the conventional Petersen/Winblad ADNI criteria for MCI are prone to false‐positive diagnostic errors. Here, we reclassified 1380 participants according to the following criteria.
The cognitive status of MCI was determined using Jak/Bondi actuarial neuropsychological criteria (Bondi et al., 2014; Jak et al., 2009): (1) two impaired neuropsychological measure scores (>1 SD below demographically adjusted [age, sex, and education] mean) within the same cognitive domain or (2) at least one impaired measure score across all three cognitive domains. Specifically, there were two neuropsychological measures in three different cognitive domains (memory, language, and attention/executive function). The memory domain included the AVLT delayed free recall correct responses and AVLT recognition (hits minus false‐positives). The language domain included the 30‐item Boston Naming Test (BNT) total correct and Semantic Verbal Fluency (SVF) total score. The attention/executive function domain included Trail‐Making Test (TMT) parts A and B times to completion.
Participants without dementia who did not meet the Jak/Bondi MCI criteria were considered to be in the CN group or Obj‐SCD group. According to the Edmonds/Thomas actuarial criteria (Thomas, Edmonds, et al., 2018), the Obj‐SCD status was determined by the following criteria: (1) one impaired neuropsychological measure score (>1 SD below demographically adjusted mean) in two different cognitive domains or (2) two impaired process scores (>1 SD below demographically adjusted mean) in AVLT or (3) one impaired neuropsychological measure score and one impaired process score. The three process scores from AVLT used in the Obj‐SCD criteria included learning slope ([list A trial 5–list A trial 1]/5), retroactive interference (list A trial 6/list A trial 5), and total intrusion errors (total number of extra‐list intrusion errors across all recall trials).
The following are our steps in determining the regression weights and dividing the Obj‐SCD and MCI groups. First, we identified a robust normal control group with a sample of 239 CN participants who had not developed MCI (Petersen et al., 2010) for at least 4 years of follow‐up (range 4–15 years; mean 7.18 years). Detailed demographics and neuropsychological data for the 239 robust controls were summarized in Table S1. Second, we ran the regressions on just this robust group. For each neuropsychological test (including the neuropsychological and process score), we regressed test scores on age, sex, and education to obtain the regression weights. Third, we used these regression b‐weights derived from the robust group to calculate predicted scores for each test for all participants. Participant z‐scores were then calculated based on the discrepancy between the observed and predicted scores and divided by the estimated standard error of the regression model for the test‐specific control group. Finally, a threshold of >1 SD (i.e., z‐score <−1) below the mean was used to identify impairment on a given test. The z‐scores for the TMT tests were reverse encoded, so it is interpreted in the same way as other z‐scores.
Finally, the 1380 participants were reclassified into 452 CN, 275 Obj‐SCD, and 653 MCI participants based on the diagnostic criteria described above. Detailed demographic and neuropsychological data for the three groups were summarized in Table S2. To analyze the FC of hippocampal subfields, we only included 132 participants with complete neuropsychological measures and rsfMRI data from the original 1380 participants. After screening, five participants (including two MCI and three CN) were excluded due to excessive head motion (details later), and one CN participant was excluded due to poor image quality. Thus, a total of 42 CN participants, 29 Obj‐SCD participants, and 55 MCI participants were finally enrolled (Figure 1).
FIGURE 1.
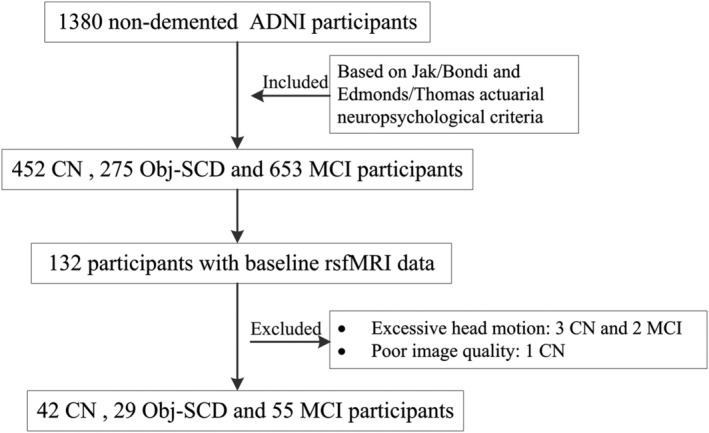
The flow diagram shows the inclusion process from the initial ADNI participants without dementia.
2.2. Biomarkers in cerebrospinal fluid
Cerebrospinal fluid (CSF) biomarkers included: amyloid‐beta 1–42 (Aβ1–42), total tau (t‐tau), and phosphorylated tau at position 181 (p‐tau181), which were all measured using the multiplex xMAPLuminex platform (Luminex) with Innogenetics (INNOBIA AlzBio3) immunoassay kit‐based reagents (Shaw et al., 2009). It is important to note that CSF samples were not available for all participants in this study, as the lumbar puncture is an invasive procedure that is not mandatory for healthy participants. Finally, the samples used for CSF analysis included 37 of 42 CN participants, 28 of 29 Obj‐SCD participants, and 50 of 55 MCI participants. In addition, detailed CSF data for 1380 participants were also summarized in Table S2.
2.3. Structural MRI and rsfMRI data
All participants were scanned using a 3.0‐Tesla Philips MRI scanner. Structural images were acquired using a 3D MPRAGE T1‐weighted sequence with the following parameters: repetition time (TR) = 2300 ms; echo time (TE) = 2.98 ms; inversion time (TI) = 900 ms; 170 sagittal slices; within plane FOV = 256 × 240 mm2; voxel size = 1.1 × 1.1 × 1.2 mm3; flip angle = 9°; bandwidth = 240 Hz/pix. The rsfMRI images were obtained using an echo‐planar imaging sequence with the following parameters: 140 time points; TR = 3000 ms; TE = 30 ms; flip angle = 80°; number of slices = 48; slice thickness = 3.3 mm; spatial resolution = 3.31 × 3.31 × 3.31 mm3; matrix = 64 × 64.
2.4. Imaging preprocessing
The rsfMRI data was processed using the Data Processing & Analysis for Brain Imaging (DPABI, http://rfmri.org/dpabi) (Yan et al., 2016) with Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm/) on the MATLAB platform (MathWorks, Natick, MA, USA). Briefly, the preprocessing steps were as follows: (1) removal of the first 10 timepoints; (2) slice timing; (3) realignment; (4) spatial normalization to the EPI template and resampling into 3 × 3 × 3 mm3; (5) nuisance covariate regression including Friston‐24 head motion parameters, white matter signal, and cerebrospinal fluid signal; (6) temporally filter (0.01–0.08 Hz). Subjects with more than 2.5 mm maximum displacement in any of the x, y, or z directions or 2.5° of any angular motion were discarded (including three CN and two MCI participants). In addition, the framewise displacement (FD) Jenkinson value of each subject was calculated to correct for the head motion artifacts.
The T1‐weighted images were preprocessed and analyzed using the Computational Anatomy Toolbox (CAT12, http://dbm.neuro.uni-jena.de/cat/) and SPM12. The images were bias‐corrected, tissue‐classified (grey matter [GM], WM, and CSF), and registered using linear (12 parameter affine) and non‐linear transformations (warping) within the CAT12 default preprocessing pipeline.
2.5. Seed‐based FC analysis
The masks for the region of interests (ROIs) (bilateral CA1, CA2/3/DG, and subiculum) were extracted respectively from probabilistic cytoarchitectonic maps of the SPM Anatomy Toolbox (Eickhoff et al., 2005). Seed‐to‐voxel FC map for each subject was constructed by the Dynamic brain connectome (DynamicBC) toolbox (http://restfmri.net/forum/DynamicBC) (Liao et al., 2014). The FC maps were generated by calculating the Pearson coefficients between the time course of seed ROIs and remaining voxels. After that, the FC maps were transformed to zFC maps by Fisher's Z transformation. Finally, the zFC maps were smoothed with a Gaussian kernel of 4 × 4 × 4 mm3 full widths at half maximum.
2.6. Statistical analysis
Statistical analyses of demographic, neuropsychological, and CSF data were performed using IBM SPSS 26.0 statistical software. The Kolmogorov–Smirnov test was used to test for normal distribution. One‐way analysis of variance (ANOVA) and Kruskal–Wallis test was used for continuous variables. Bonferroni correction was performed for pairwise comparisons of the three groups. Binary data, such as sex, were compared using the chi‐square test.
Statistical analyses of hippocampal subfields connectivity were performed using the DPABI toolbox (Yan et al., 2016). An analysis of covariance (ANCOVA) was performed with age, sex, education, and head motion (FD value) as covariates to determine FC alterations in hippocampal subfields between the three groups. To control for the effect of cortical atrophy on functional analysis, normalized modulated (with the volumetric information encoded) GM maps were used as covariate images. After Gaussian random field (GRF) correction, the threshold was set to p < 0.005 at the voxel level and p < 0.05 at the cluster level. In addition, we extracted mean FC values from regions showing significant group effects by ANCOVA and further performed post‐hoc pairwise comparisons (p < 0.05, Bonferroni correction). Finally, we performed partial correlation analyses to examine the correlation between mean FC values from regions showing significant group effects and neuropsychological performance and CSF biomarkers, with age, sex, and education level as covariates.
3. RESULTS
3.1. Demographics, neuropsychological, and CSF data
Demographics, neuropsychological performance, and CSF biomarkers are summarized in Table 1. There were no significant differences between the three groups in terms of age, sex, and education level. As expected, the MCI group performed the worst on almost all neuropsychological measures, followed by the Obj‐SCD and then the CN group. However, there were no significant differences among the three groups in CSF biomarkers, but such differences were found in all participants (Table S2).
TABLE 1.
Demographic, neuropsychological, and CSF data
| Variables | CN | Obj‐SCD | MCI | F/χ 2 | p |
|---|---|---|---|---|---|
| (N = 42) | (N = 29) | (N = 55) | |||
| Demographic factors | |||||
| Age | 71.29 (6.85) | 71.97 (6.47) | 73.58 (6.66) | 1.49 | 0.230 |
| Sex (F:M) | 24:18 | 14:15 | 27:28 | 0.78 | 0.676 |
| Education | 16.36 (2.55) | 16.59 (2.73) | 15.76 (2.49) | 1.18 | 0.311 |
| GDS | 1.26 (1.36) | 1.14 (0.95) | 1.55 (1.45) | 1.07 | 0.345 |
| Neuropsychological measures | |||||
| MMSE | 28.74 (1.21) | 28.59 (1.59) | 27.85 (1.79) | 4.31 | 0.015 b |
| Memory | |||||
| AVLT delayed recall | 8.36 (2.79) | 5.21 (3.08) | 3.15 (3.34) | 33.55 | <0.001 a , b , c |
| AVLT recognition | 12.76 (1.85) | 11.79 (2.11) | 8.36 (3.59) | 32.87 | <0.001 b , c |
| Language | |||||
| SVF (animal) | 22.05 (4.03) | 20.59 (4.08) | 17.29 (4.45) | 15.98 | <0.001 b , c |
| BNT | 28.52 (1.44) | 27.72 (1.81) | 26.60 (3.66) | 6.14 | 0.003 b |
| Attention/executive function | |||||
| TMT part A (s) | 29.48 (7.71) | 31.86 (6.59) | 44.91 (17.26) | 20.49 | <0.001bc |
| TMT part B (s) | 67.00 (17.75) | 89.07 (40.75) | 125.96 (68.46) | 16.86 | <0.001 a , b , c |
| Process scores | |||||
| AVLT learning slope | 1.33 (0.39) | 0.91 (0.45) | 0.74 (0.46) | 22.39 | <0.001 a , b |
| AVLT retroactive interference | 0.80 (0.17) | 0.69 (0.27) | 0.59 (0.24) | 10.96 | <0.001 b |
| AVLT intrusion errors | 2.24 (2.38) | 3.83 (2.90) | 3.51 (3.49) | 3.02 | 0.052 |
| CSF biomarkers * | |||||
| Aβ1–42 (pg/ml) | 195.84 (54.64) | 170.81 (48.35) | 182.35 (56.57) | 1.74 | 0.180 |
| p‐tau181 (pg/ml) | 37.71 (21.99) | 42.98 (18.05) | 41.80 (26.97) | 0.49 | 0.617 |
| t‐tau (pg/ml) | 77.37 (50.06) | 86.88 (47.00) | 81.16 (43.81) | 0.33 | 0.719 |
| Head motion (FD value) | 0.16 (0.07) | 0.14 (0.07) | 0.16 (0.08) | 0.41 | 0.664 |
Note: Values are expressed as mean (standard deviation), number of participants.
Abbreviations: CN, Cognitively normal; Obj‐SCD, Objectively‐defined Subtle Cognitive Decline; MCI, Mild Cognitive Impairment; GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; AVLT, Auditory Verbal Learning Test; SVF, Semantic Verbal Fluency; BNT, Boston Naming Test; TMT, Trail‐Making Test; CSF: Cerebrospinal fluid; FD, framewise displacement.
115 participants (CN = 37, Obj‐SCD = 28, MCI = 50) have CSF biomarkers.
Post hoc analysis further revealed the source of ANOVA difference (CN vs. Obj‐SCD) (p < 0.05, significant difference between the two groups).
Post hoc analysis further revealed the source of ANOVA difference (CN vs. MCI) (p < 0.05, significant difference between the two groups).
Post hoc analysis further revealed the source of ANOVA difference (Obj‐SCD vs. MCI) (p < 0.05, significant difference between the two groups).
3.2. The resting‐state FC of hippocampal subfields
By ANCOVA analyses, significant differences were detected (1) between left CA1 and right thalamus, (2) between left CA2/3/DG and right thalamus, and (3) between right subiculum and right superior parietal gyrus (SPG) (Figure 2, Table 2). Specifically, the Obj‐SCD group showed decreased FC between left CA1‐CA2/3/DG and right thalamus and increased FC between right subiculum and right SPG compared with the CN and MCI groups (Figure 3). Additionally, the MCI group showed increased FC between left CA2/3/DG and right thalamus compared with the CN group.
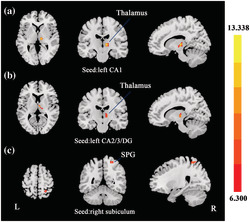
FIGURE 2.
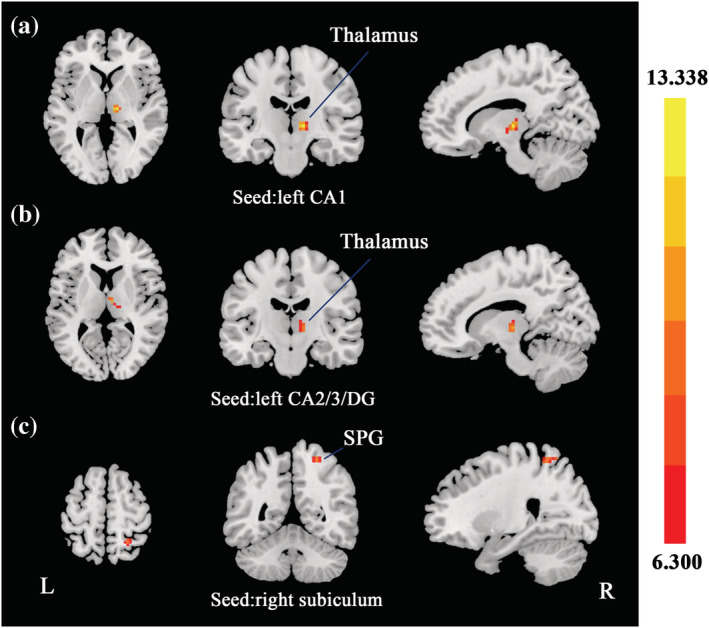
Group differences of FC in hippocampal subfields. Based on the three ROIs of hippocampal subfields, regions that showed significant FC differences were located in right thalamus (seed: left CA1; a), right thalamus (seed: left CA2/3/DG; b), and right SPG (seed: right subiculum; c). SPG, superior parietal gyrus. The statistical threshold was set at p < 0.005 with a cluster level of p < 0.05 (two‐tailed, GRF corrected).
TABLE 2.
Group differences in FC of hippocampal subfields
| Seeds | Regions | Peak MNI coordinate | Peak intensity | Cluster voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| L CA1 | R thalamus | 12 | −18 | 3 | 13.3378 | 26 |
| L CA2/3/DG | R thalamus | 6 | −12 | 9 | 10.1862 | 25 |
| R subiculum | R SPG | 24 | −51 | 60 | 9.4404 | 25 |
Note: The statistical threshold was set at p < 0.005 with a cluster‐level of p < 0.05 (two‐tailed, GRF corrected).
Abbreviations: FC, functional connectivity; CA, cornu ammonis; DG, dentate gyrus; SPG, superior parietal gyrus; L, left; R, right.
FIGURE 3.
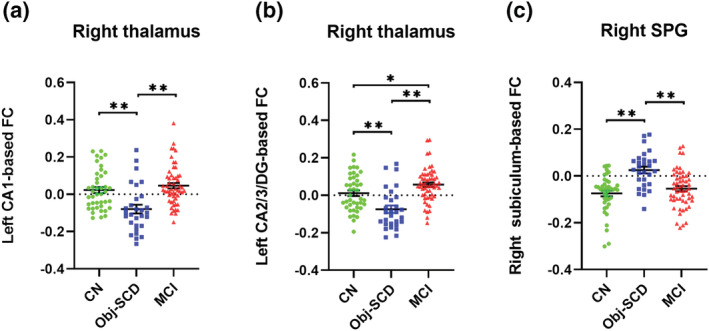
The post hoc analysis of FC in hippocampal subfields. The Obj‐SCD group showed decreased FC between left CA1 and right thalamus (a), and between left CA2/3/DG and right thalamus (b), as well as increased FC between right subiculum and right SPG (c) compared with the CN and MCI groups. The MCI group showed increased FC between left CA2/3/DG and right thalamus compared to the CN group (b). SPG, superior parietal gyrus. *p < 0.05, ** p < 0.001
3.3. Correlation of FC with cognition and CSF biomarkers
In the Obj‐SCD group, FC values between left CA2/3/DG and right thalamus were positively associated with AVLT recognition (r = 0.395, p = 0.046) and CSF Aβ1–42 levels (r = 0.466, p = 0.019), and FC values between left CA1 and right thalamus were positively correlated with CSF Aβ1–42 levels (r = 0.530, p = 0.006) (Figure 4). We did not find significant correlations of FC values with cognition and CSF biomarkers in the MCI group.
FIGURE 4.
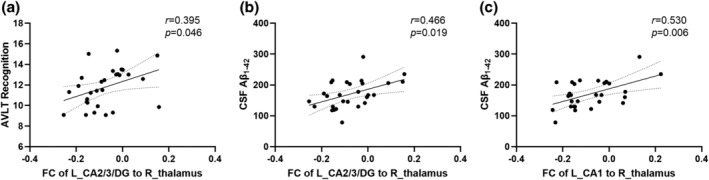
Scatter plots graphs of relationship between: FC of left CA2/3/DG to right thalamus and AVLT recognition (a); FC of left CA2/3/DG to right thalamus and CSF Aβ1–42 levels (b); FC of left CA1 to right thalamus and CSF Aβ1–42 levels (c) in the Obj‐SCD group. AVLT, Auditory Verbal Learning Test, SPG, superior parietal gyrus
4. DISCUSSION
To our knowledge, this is the first study to investigate FC alterations of hippocampal subfields in individuals with Obj‐SCD. Specifically, compared with the CN and MCI groups, the Obj‐SCD group showed decreased FC between left CA1‐CA2/3/DG and right thalamus and increased FC between right subiculum and right SPG. In addition, functional alterations of hippocampal subfields in the Obj‐SCD group were associated with cognitive decline and pathological biomarkers. Our study extends prior work and demonstrates the functional abnormalities in individuals with Obj‐SCD by examining the FC of hippocampal subfields.
Compared with the CN group, the Obj‐SCD group showed reduced FC between the left CA1‐CA2/3/DG and the right thalamus, reflecting early dysfunction of hippocampal subfields in the preclinical phase of AD. Our findings are consistent with previous work that has found reduced connectivity or activity in hippocampal subfields in individuals at high risk for AD who have not yet been considered cognitively impaired. For example, young APOE ε4 carriers had reduced pattern separation in CA3 compared with non‐carriers, suggesting that genetic AD risk may influence the neural mechanisms underlying pattern separation in hippocampal subfields (Lee et al., 2020). Weaker FC strength between the body of hippocampus and the default mode network (DMN) was observed in individuals with subjective cognitive decline (Zajac et al., 2020). Also, the subjective cognitive decline individuals exhibited reduced connectivity strength in structural covariance networks seeding from the anterior and posterior hippocampus compared with normal controls (Fu et al., 2021). Therefore, combining our findings with previous results, we speculate that decreased connectivity in hippocampal subfields may be an early biological marker of cognitive deficits due to AD. In addition, inconsistent with previous studies on reduced FC in the CA1 and CA2/3/DG in MCI (de Flores et al., 2017; Yan et al., 2020), we found increased FC between the CA1‐CA2/3/DG and the thalamus in the MCI group relative to the Obj‐SCD group. A possible explanation is that the increased FC in the MCI group could be compensation mechanism for the brain functional disruption/cognitive decline (Clément & Belleville, 2010). Similar results of increased brain activity, FC and glucose metabolism have also been widely reported in patients with MCI (Ashraf et al., 2015; Gardini et al., 2015; Pan et al., 2017).
We also found increased FC between the right subiculum and right SPG in the Obj‐SCD group compared with the CN and MCI groups. Anatomically, the subiculum is the primary source of extrinsic projections that modulates neuronal activation from the hippocampus to cortical or subcortical areas (O'Mara, 2006). Previous studies reported decreased FC from the subiculum to frontal, occipital, and posterior cingulate regions in patients with MCI and AD (de Flores et al., 2017; Yan et al., 2020). In contrast, our results of increased FC in the subiculum in the Obj‐SCD group may indicate early functional compensation for pathologic processes, whereas higher FC is required to maintain relatively intact cognitive function. Previous studies also support this finding with regional hyperperfusion (Thomas, Osuna, et al., 2021) and increased local functional activity (Cui et al., 2021) in the Obj‐SCD group compared with the CN group.
In terms of correlation analysis between hippocampal subfields connectivity and cognition, we found that left CA2/3/DG and right thalamus connectivity was positively correlated to AVLT recognition in the Obj‐SCD group. The CA2/3/DG regions have been reported to be associated with encoding, retrieval, and discrimination of memories (Hainmueller & Bartos, 2020; Meira et al., 2018; Rebola et al., 2017). The thalamus, as the key structure of the ‘extended hippocampal system’, is directly and interconnected with hippocampal formation and plays an important role in episodic memory (Aggleton & Brown, 1999; Frost et al., 2021). Therefore, disrupted connectivity in the CA2/3/DG subfield may reflect subtle memory deficits in the preclinical phase of AD. Furthermore, the FC between the left CA1‐CA2/3/DG and right thalamus in the Obj‐SCD group was also positively correlated with CSF Aβ1–42 levels. The accumulation of cerebral Aβ is considered to be a characteristic pathological change in AD and can be detected at a very early stage (Hardy & Higgins, 1992). The CSF biomarkers for all participants (Table S2) showed lower Aβ1–42 levels, but not tau levels in the Obj‐SCD group than in the CN group, which is consistent with the fact that the accumulation of Aβ in the brain occurs earlier than tau‐induced neurofibrillary tangles (Jack et al., 2018). Therefore, we hypothesized that decreased FC between the left CA1‐CA2/3/DG and right thalamus in the Obj‐SCD group could be caused by increased amyloid accumulation in the brain. Similarly, previous human and animal studies have reported that hippocampal disconnection is associated with increased cerebral amyloid deposition in AD (Franzmeier et al., 2021; Vyas et al., 2020; Wu et al., 2019), which supports our results. Unexpectedly, no significant differences in CSF biomarkers were found between the three groups for the FC analysis, but such differences were found in all participants. A possible explanation is that the sample containing rsfMRI data is relatively small and may not be representative of all participants.
It is well known that neuroimaging asymmetric features are common in structural and functional imaging of neurodegenerative diseases, especially in AD. Specifically, left‐lateralized brain degeneration, such as cortical atrophy (Janke et al., 2001), hypometabolism (Weise et al., 2018), and Aβ deposition (Frings et al., 2015) may dominate in the progression of AD. In disagreement with previous studies, we observed that regions showing significant alterations in FC with various hippocampal subfields between the three groups were located in the right hemisphere. These findings align very well with the cognitive reserve hypothesis. Based on the critical role of noradrenergic (NA) activity in mediating the protective effects of cognitive reserve (Robertson, 2013), Robertson further highlighted the close relationship between the cognitive processes with the right hemisphere, fronto‐parietal localization, which is strongly regulated by NA (Robertson, 2014). Several studies have shown that alterations in the cerebral hemispheres associated with AD/cognitive impairment involve the right hemisphere more than the left, which supports our findings. For example, in APOE ε4 carriers who have not yet developed dementia symptoms, greater right‐lateralized activity was observed than in non‐ε4 carriers, which may be a compensation for APOE ε4‐related deficits (Han et al., 2007). Additionally, in an implicit memory task, Tyrer et al. (Tyrer et al., 2020)suggested that compensatory mechanisms in the right hemisphere might be employed to alleviate left‐lateralized network deficits in AD.
This study has several limitations that need to be addressed. First, the ADNI was a predominantly white, highly educated, and healthy sample, which may limit the generalizability of the study results. Second, the sample size used for FC analysis was relatively small, especially for the Obj‐SCD group. Future studies will require larger sample sizes to validate our findings. Third, not all participants in this study had access to CSF samples because a lumbar puncture is an invasive operation, which may affect statistical power. Finally, the cross‐sectional design of the current study does not imply causality, and further longitudinal studies combining rsfMRI data and AD pathological biomarkers are needed to delineate the sequence of potentially pathogenic events.
Overall, the disrupted FC of the CA1 and CA2/3/DG subfields in individuals with Obj‐SCD patients may reflect subtle cognitive impairment and AD‐specific pathological changes. Also, increased FC of the subiculum subfield may imply early functional compensation for subtle cognitive changes. The results of this study complement existing papers showing that Edmonds/Thomas actuarial Obj‐SCD definitions, including neuropsychological measures and AVLT process scores, are sensitive markers for future progression to MCI/AD as well as for individuals with increased amyloid accumulation, elevated plasma p‐tau181 levels, and increased risk of functional and structural degeneration (Cui et al., 2021; Thomas et al., 2020; Thomas, Bangen, et al., 2021; Thomas, Osuna, et al., 2021). Finally, the use of multi‐model functional MRI to assess cerebral functional network alterations or the integrity of white matter microstructure in Obj‐SCD will be particularly significant in future studies.
AUTHOR CONTRIBUTIONS
Author contributions included conception and study design (TTQ, FX, and SPD), data collection or acquisition (TTQ, QZZ, and YSZ), statistical analysis (TTQ), interpretation of results (TTQ, FX, and XPX), drafting the manuscript work or revising it critically for important intellectual content (XDL, XL, ZJS, KCL, PYH, and MMZ) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
CONSENT TO PARTICIPATE
Written informed consent was obtained from all participants and/or authorized representatives and the study partners before any protocol‐specific procedures were carried out in the ADNI study.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15860.
Supporting information
Table S1. The demographic and neuropsychological data of the robust controls.
Table S2. The demographic, neuropsychological and CSF data.
ACKNOWLEDGEMENTS
This study was funded by the Linyi Science and Technology Development Program (Grant No. 202020024), Shandong Medicine and Health Science and Technology Program (Grant No. 202009010844), and National Natural Science Foundation of China (Grant No. 81901707, 82001766 and 81901721). The data collection and sharing for this project were funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense Award No. W81XWH‐12‐2‐0012).
Qiu, T. , Zeng, Q. , Zhang, Y. , Luo, X. , Xu, X. , Li, X. , Shen, Z. , Li, K. , Wang, C. , Huang, P. , Zhang, M. , Dai, S. , Xie, F. , & for the Alzheimer's Disease Neuroimaging Initiative (2022). Altered functional connectivity pattern of hippocampal subfields in individuals with objectively‐defined subtle cognitive decline and its association with cognition and cerebrospinal fluid biomarkers. European Journal of Neuroscience, 56(12), 6227–6238. 10.1111/ejn.15860
Edited by: Tara Spires‐Jones.
Tiantian Qiu and Qingze Zeng have contributed equally to this work and share first authorship.
Shouping Dai and Fei Xie have contributed equally to this work and share senior authorship.
Funding information Linyi Science and Technology Development Program, Grant/Award Number: 202020024; Shandong Medicine and Health Science and Technology Program, Grant/Award Number: 202009010844; National Natural Science Foundation of China, Grant/Award Numbers: 81901707, 81901721, 82001766; ADNI, Grant/Award Number: U01 AG024904; DOD ADNI, Grant/Award Number: W81XWH‐12‐2‐0012
DATA AVAILABILITY STATEMENT
Data used in preparation of this article were obtained from the Alzheimer's disease Neuroimaging Initiative (ADNI) database (http://www.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
REFERENCES
- Aggleton, J. P. , & Brown, M. W. (1999). Episodic memory, amnesia, and the hippocampal‐anterior thalamic axis. The Behavioral and Brain Sciences, 22(3), 425–444. 10.1017/S0140525X99002034 [DOI] [PubMed] [Google Scholar]
- Aggleton, J. P. , & Christiansen, K. (2015). The subiculum: The heart of the extended hippocampal system. Progress in Brain Research, 219, 65–82. 10.1016/bs.pbr.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Ashraf, A. , Fan, Z. , Brooks, D. J. , & Edison, P. (2015). Cortical hypermetabolism in MCI subjects: A compensatory mechanism? European Journal of Nuclear Medicine and Molecular Imaging, 42(3), 447–458. 10.1007/s00259-014-2919-z [DOI] [PubMed] [Google Scholar]
- Berron, D. , van Westen, D. , Ossenkoppele, R. , Strandberg, O. , & Hansson, O. (2020). Medial temporal lobe connectivity and its associations with cognition in early Alzheimer's disease. Brain, 143(4), 1233–1248. 10.1093/brain/awaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi, M. W. , Edmonds, E. C. , Jak, A. J. , Clark, L. R. , Delano‐Wood, L. , McDonald, C. R. , Nation, D. A. , Libon, D. J. , Au, R. , Galasko, D. , & Salmon, D. P. (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer's Disease, 42(1), 275–289. 10.3233/jad-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak, H. , & Braak, E. (1991). Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathologica, 82(4), 239–259. 10.1007/bf00308809 [DOI] [PubMed] [Google Scholar]
- Clément, F. , & Belleville, S. (2010). Compensation and disease severity on the memory‐related activations in mild cognitive impairment. Biological Psychiatry, 68(10), 894–902. 10.1016/j.biopsych.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Copara, M. S. , Hassan, A. S. , Kyle, C. T. , Libby, L. A. , Ranganath, C. , & Ekstrom, A. D. (2014). Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. The Journal of Neuroscience, 34(20), 6834–6842. 10.1523/jneurosci.5341-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, L. , Zhang, Z. , Zac Lo, C. Y. , & Guo, Q. (2021). Local functional MR change pattern and its association with cognitive function in objectively‐defined subtle cognitive decline. Frontiers in Aging Neuroscience, 13, 684918. 10.3389/fnagi.2021.684918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Flores, R. , Mutlu, J. , Bejanin, A. , Gonneaud, J. , Landeau, B. , Tomadesso, C. , Mezenge, F. , de La Sayette, V. , Eustache, F. , & Chetelat, G. (2017). Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Human Brain Mapping, 38(10), 4922–4932. 10.1002/hbm.23704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy, H. M. , Cattin, F. , Risold, P. Y. , Vannson, J. L. , & Gaudron, M. (2013). The human hippocampus: Functional anatomy, vascularization and serial sections with MRI (fourth ed.). Springer. 10.1007/978-3-642-33603-4 [DOI] [Google Scholar]
- Edmonds, E. C. , Delano‐Wood, L. , Clark, L. R. , Jak, A. J. , Nation, D. A. , McDonald, C. R. , Libon, D. J. , Au, R. , Galasko, D. , Salmon, D. P. , & Bondi, M. W. (2015). Susceptibility of the conventional criteria for mild cognitive impairment to false‐positive diagnostic errors. Alzheimer's & Dementia, 11(4), 415–424. 10.1016/j.jalz.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds, E. C. , Delano‐Wood, L. , Galasko, D. R. , Salmon, D. P. , & Bondi, M. W. (2015). Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. Journal of Alzheimer's Disease, 47(1), 231–242. 10.3233/jad-150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- El Haj, M. , Antoine, P. , Amouyel, P. , Lambert, J. C. , Pasquier, F. , & Kapogiannis, D. (2016). Apolipoprotein E (APOE) ε4 and episodic memory decline in Alzheimer's disease: A review. Ageing Research Reviews, 27, 15–22. 10.1016/j.arr.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier, N. , Ren, J. , Damm, A. , Monté‐Rubio, G. , Boada, M. , Ruiz, A. , Ramirez, A. , Jessen, F. , Düzel, E. , Rodríguez Gómez, O. , Benzinger, T. , Goate, A. , Karch, C. M. , Fagan, A. M. , McDade, E. , Buerger, K. , Levin, J. , Duering, M. , Dichgans, M. , … Ewers, M. (2021). The BDNF (Val66Met) SNP modulates the association between beta‐amyloid and hippocampal disconnection in Alzheimer's disease. Molecular Psychiatry, 26(2), 614–628. 10.1038/s41380-019-0404-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings, L. , Hellwig, S. , Spehl, T. S. , Bormann, T. , Buchert, R. , Vach, W. , Minkova, L. , Heimbach, B. , Klöppel, S. , & Meyer, P. T. (2015). Asymmetries of amyloid‐β burden and neuronal dysfunction are positively correlated in Alzheimer's disease. Brain, 138(Pt 10), 3089–3099. 10.1093/brain/awv229 [DOI] [PubMed] [Google Scholar]
- Frost, B. E. , Martin, S. K. , Cafalchio, M. , Islam, M. N. , Aggleton, J. P. , & O'Mara, S. M. (2021). Anterior thalamic inputs are required for subiculum spatial coding, with associated consequences for hippocampal spatial memory. The Journal of Neuroscience, 41(30), 6511–6525. 10.1523/jneurosci.2868-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Z. , Zhao, M. , He, Y. , Wang, X. , Lu, J. , Li, S. , Li, X. , Kang, G. , Han, Y. , & Li, S. (2021). Divergent connectivity changes in gray matter structural covariance networks in subjective cognitive decline, amnestic mild cognitive impairment, and Alzheimer's disease. Frontiers in Aging Neuroscience, 13, 686598. 10.3389/fnagi.2021.686598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini, S. , Venneri, A. , Sambataro, F. , Cuetos, F. , Fasano, F. , Marchi, M. , Crisi, G. , & Caffarra, P. (2015). Increased functional connectivity in the default mode network in mild cognitive impairment: A maladaptive compensatory mechanism associated with poor semantic memory performance. Journal of Alzheimer's Disease, 45(2), 457–470. 10.3233/jad-142547 [DOI] [PubMed] [Google Scholar]
- Hainmueller, T. , & Bartos, M. (2020). Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nature Reviews. Neuroscience, 21(3), 153–168. 10.1038/s41583-019-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel, H. , Bürger, K. , Teipel, S. J. , Bokde, A. L. , Zetterberg, H. , & Blennow, K. (2008). Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimer's & Dementia, 4(1), 38–48. 10.1016/j.jalz.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Han, S. D. , Houston, W. S. , Jak, A. J. , Eyler, L. T. , Nagel, B. J. , Fleisher, A. S. , Brown, G. G. , Corey‐Bloom, J. , Salmon, D. P. , Thal, L. J. , & Bondi, M. W. (2007). Verbal paired‐associate learning by APOE genotype in non‐demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging, 28(2), 238–247. 10.1016/j.neurobiolaging.2005.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J. A. , & Higgins, G. A. (1992). Alzheimer's disease: The amyloid cascade hypothesis. Science, 256(5054), 184–185. 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- Jack, C. R. Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Dunn, B. , Haeberlein, S. B. , Holtzman, D. M. , Jagust, W. , Jessen, F. , Karlawish, J. , Liu, E. , Molinuevo, J. L. , Montine, T. , Phelps, C. , Rankin, K. P. , Rowe, C. C. , Scheltens, P. , Siemers, E. , Snyder, H. M. , & Sperling, R. (2018). NIA‐AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia, 14(4), 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak, A. J. , Bondi, M. W. , Delano‐Wood, L. , Wierenga, C. , Corey‐Bloom, J. , Salmon, D. P. , & Delis, D. C. (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American Journal of Geriatric Psychiatry, 17(5), 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke, A. L. , de Zubicaray, G. , Rose, S. E. , Griffin, M. , Chalk, J. B. , & Galloway, G. J. (2001). 4D deformation modeling of cortical disease progression in Alzheimer's dementia. Magnetic Resonance in Medicine, 46(4), 661–666. 10.1002/mrm.1243 [DOI] [PubMed] [Google Scholar]
- Jaroudi, W. , Garami, J. , Garrido, S. , Hornberger, M. , Keri, S. , & Moustafa, A. A. (2017). Factors underlying cognitive decline in old age and Alzheimer's disease: The role of the hippocampus. Reviews in the Neurosciences, 28(7), 705–714. 10.1515/revneuro-2016-0086 [DOI] [PubMed] [Google Scholar]
- Lace, G. , Savva, G. M. , Forster, G. , de Silva, R. , Brayne, C. , Matthews, F. E. , Barclay, J. J. , Dakin, L. , Ince, P. G. , & Wharton, S. B. (2009). Hippocampal tau pathology is related to neuroanatomical connections: An ageing population‐based study. Brain, 132(Pt 5), 1324–1334. 10.1093/brain/awp059 [DOI] [PubMed] [Google Scholar]
- Lee, H. , Stirnberg, R. , Wu, S. , Wang, X. , Stöcker, T. , Jung, S. , Montag, C. , & Axmacher, N. (2020). Genetic Alzheimer's disease risk affects the neural mechanisms of pattern separation in hippocampal subfields. Current Biology, 30(21), 4201–4212.e3. 10.1016/j.cub.2020.08.042 [DOI] [PubMed] [Google Scholar]
- Li, H. , Jia, X. , Qi, Z. , Fan, X. , Ma, T. , Pang, R. , Ni, H. , Li, C. R. , Lu, J. , & Li, K. (2018). Disrupted functional connectivity of Cornu Ammonis subregions in amnestic mild cognitive impairment: A longitudinal resting‐state fMRI study. Frontiers in Human Neuroscience, 12, 413. 10.3389/fnhum.2018.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, W. , Wu, G. R. , Xu, Q. , Ji, G. J. , Zhang, Z. , Zang, Y. F. , & Lu, G. (2014). DynamicBC: A MATLAB toolbox for dynamic brain connectome analysis. Brain Connectivity, 4(10), 780–790. 10.1089/brain.2014.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, C. L. , Bateman, R. , Blennow, K. , Rowe, C. C. , Sperling, R. A. , & Cummings, J. L. (2015). Alzheimer's disease. Nature Reviews. Disease Primers, 1, 15056. 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- Meira, T. , Leroy, F. , Buss, E. W. , Oliva, A. , Park, J. , & Siegelbaum, S. A. (2018). A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nature Communications, 9(1), 4163. 10.1038/s41467-018-06501-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara, S. (2006). Controlling hippocampal output: The central role of subiculum in hippocampal information processing. Behavioural Brain Research, 174(2), 304–312. 10.1016/j.bbr.2006.08.018 [DOI] [PubMed] [Google Scholar]
- Pan, P. , Zhu, L. , Yu, T. , Shi, H. , Zhang, B. , Qin, R. , Zhu, X. , Qian, L. , Zhao, H. , Zhou, H. , & Xu, Y. (2017). Aberrant spontaneous low‐frequency brain activity in amnestic mild cognitive impairment: A meta‐analysis of resting‐state fMRI studies. Ageing Research Reviews, 35, 12–21. 10.1016/j.arr.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Petersen, R. C. , Aisen, P. S. , Beckett, L. A. , Donohue, M. C. , Gamst, A. C. , Harvey, D. J. , Jack, C. R. Jr. , Jagust, W. J. , Shaw, L. M. , Toga, A. W. , Trojanowski, J. Q. , & Weiner, M. W. (2010). Alzheimer's disease neuroimaging initiative (ADNI): Clinical characterization. Neurology, 74(3), 201–209. 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani, M. , de Haan, W. , Wu, T. , Seeley, W. W. , & Frisoni, G. B. (2011). Functional network disruption in the degenerative dementias. Lancet Neurology, 10(9), 829–843. 10.1016/s1474-4422(11)70158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola, N. , Carta, M. , & Mulle, C. (2017). Operation and plasticity of hippocampal CA3 circuits: Implications for memory encoding. Nature Reviews. Neuroscience, 18(4), 208–220. 10.1038/nrn.2017.10 [DOI] [PubMed] [Google Scholar]
- Robertson, I. H. (2013). A noradrenergic theory of cognitive reserve: Implications for Alzheimer's disease. Neurobiology of Aging, 34(1), 298–308. 10.1016/j.neurobiolaging.2012.05.019 [DOI] [PubMed] [Google Scholar]
- Robertson, I. H. (2014). Right hemisphere role in cognitive reserve. Neurobiology of Aging, 35(6), 1375–1385. 10.1016/j.neurobiolaging.2013.11.028 [DOI] [PubMed] [Google Scholar]
- Shaw, L. M. , Vanderstichele, H. , Knapik‐Czajka, M. , Clark, C. M. , Aisen, P. S. , Petersen, R. C. , Blennow, K. , Soares, H. , Simon, A. , Lewczuk, P. , Dean, R. , Siemers, E. , Potter, W. , Lee, V. M. , & Trojanowski, J. Q. (2009). Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of Neurology, 65(4), 403–413. 10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling, R. A. , Aisen, P. S. , Beckett, L. A. , Bennett, D. A. , Craft, S. , Fagan, A. M. , Iwatsubo, T. , Jack, C. R. Jr. , Kaye, J. , Montine, T. J. , Park, D. C. , Reiman, E. M. , Rowe, C. C. , Siemers, E. , Stern, Y. , Yaffe, K. , Carrillo, M. C. , Thies, B. , Morrison‐Bogorad, M. , … Phelps, C. H. (2011). Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L. R. , Ojemann, J. G. , Miezin, F. M. , Petersen, S. E. , Videen, T. O. , & Raichle, M. E. (1992). Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proceedings of the National Academy of Sciences of the United States of America, 89(5), 1837–1841. 10.1073/pnas.89.5.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R. , Bangen, K. J. , Edmonds, E. C. , Weigand, A. J. , Walker, K. S. , Bondi, M. W. , Galasko, D. R. , & Alzheimer's Disease Neuroimaging Initiative . (2021). Objective subtle cognitive decline and plasma phosphorylated tau181: Early markers of Alzheimer's disease‐related declines. Alzheimers Dement, 13, e12238. 10.1002/dad2.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R. , Bangen, K. J. , Weigand, A. J. , Edmonds, E. C. , Wong, C. G. , Cooper, S. , Delano‐Wood, L. , & Bondi, M. W. (2020). Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology, 94(4), e397–e406. 10.1212/wnl.0000000000008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R. , Edmonds, E. C. , Eppig, J. , Salmon, D. P. , & Bondi, M. W. (2018). Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. Journal of Alzheimer's Disease, 64(1), 195–204. 10.3233/jad-180229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R. , Eppig, J. , Edmonds, E. C. , Jacobs, D. M. , Libon, D. J. , Au, R. , Salmon, D. P. , & Bondi, M. W. (2018). Word‐list intrusion errors predict progression to mild cognitive impairment. Neuropsychology, 32(2), 235–245. 10.1037/neu0000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K. R. , Osuna, J. R. , Weigand, A. J. , Edmonds, E. C. , Clark, A. L. , Holmqvist, S. , Cota, I. H. , Wierenga, C. E. , Bondi, M. W. , & Bangen, K. J. (2021). Regional hyperperfusion in older adults with objectively‐defined subtle cognitive decline. Journal of Cerebral Blood Flow and Metabolism, 41(5), 1001–1012. 10.1177/0271678x20935171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving, E. , & Markowitsch, H. J. (1998). Episodic and declarative memory: Role of the hippocampus. Hippocampus, 8(3), 198–204. [DOI] [PubMed] [Google Scholar]
- Tyrer, A. , Gilbert, J. R. , Adams, S. , Stiles, A. B. , Bankole, A. O. , Gilchrist, I. D. , & Moran, R. J. (2020). Lateralized memory circuit dropout in Alzheimer's disease patients. Brain Communications, 2(2), fcaa212. 10.1093/braincomms/fcaa212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch, D. P. , Weiner, M. W. , Aisen, P. S. , Beckett, L. A. , Cairns, N. J. , Green, R. C. , Harvey, D. , Jack, C. R. Jr. , Jagust, W. , Morris, J. C. , Petersen, R. C. , Saykin, A. J. , Shaw, L. M. , Toga, A. W. , & Trojanowski, J. Q. (2019). Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's disease neuroimaging initiative. Alzheimer's & Dementia, 15(1), 106–152. 10.1016/j.jalz.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Vermunt, L. , Sikkes, S. A. M. , van den Hout, A. , Handels, R. , Bos, I. , van der Flier, W. M. , Kern, S. , Ousset, P. J. , Maruff, P. , Skoog, I. , Verhey, F. R. J. , Freund‐Levi, Y. , Tsolaki, M. , Wallin, Å. , Olde Rikkert, M. , Soininen, H. , Spiru, L. , Zetterberg, H. , Blennow, K. , … ICTUS/DSA study groups . (2019). Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimer's & Dementia, 15(7), 888–898. 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas, Y. , Montgomery, J. M. , & Cheyne, J. E. (2020). Hippocampal deficits in amyloid‐β‐related rodent models of Alzheimer's disease. Frontiers in Neuroscience, 14, 266. 10.3389/fnins.2020.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise, C. M. , Chen, K. , Chen, Y. , Kuang, X. , Savage, C. R. , Reiman, E. M. , & Alzheimer's Disease Neuroimaging Initiative . (2018). Left lateralized cerebral glucose metabolism declines in amyloid‐β positive persons with mild cognitive impairment. NeuroImage: Clinical, 20, 286–296. 10.1016/j.nicl.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M. J. , Coleman, P. D. , Flood, D. G. , & Troncoso, J. C. (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet, 344(8925), 769–772. 10.1016/s0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- Wu, M. , Thurston, R. C. , Tudorascu, D. L. , Karim, H. T. , Mathis, C. A. , Lopresti, B. J. , Kamboh, M. I. , Cohen, A. D. , Snitz, B. E. , Klunk, W. E. , & Aizenstein, H. J. (2019). Amyloid deposition is associated with different patterns of hippocampal connectivity in men versus women. Neurobiology of Aging, 76, 141–150. 10.1016/j.neurobiolaging.2018.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , Wang, X. D. , Zuo, X. N. , & Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Yan, S. , Zheng, C. , Cui, B. , Qi, Z. , Zhao, Z. , An, Y. , Qiao, L. , Han, Y. , Zhou, Y. , & Lu, J. (2020). Multiparametric imaging hippocampal neurodegeneration and functional connectivity with simultaneous PET/MRI in Alzheimer's disease. European Journal of Nuclear Medicine and Molecular Imaging, 47(10), 2440–2452. 10.1007/s00259-020-04752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac, L. , Koo, B. B. , Tripodis, Y. , Mian, A. , Steinberg, E. , Mez, J. , Alosco, M. L. , Cervantes‐Arslanian, A. , Stern, R. , & Killiany, R. (2020). Hippocampal resting‐state functional connectivity patterns are more closely associated with severity of subjective memory decline than whole hippocampal and subfield volumes. Cerebral Cortex Communications, 1(1), tgaa019. 10.1093/texcom/tgaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The demographic and neuropsychological data of the robust controls.
Table S2. The demographic, neuropsychological and CSF data.
Data Availability Statement
Data used in preparation of this article were obtained from the Alzheimer's disease Neuroimaging Initiative (ADNI) database (http://www.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.


