Abstract
Background
While the positive effect of Trikafta on cystic fibrosis (CF) pulmonary disease is well established, there is limited data about its effect on bone mineral density (BMD), body composition and exercise capacity.
Methods
A pilot single center study. BMD and body composition were measured three months after the initiation of Trikafta (study group) and compared to values obtained 2 years earlier. CF patients not treated with Trikafta, for whom BMD was measured 2 years apart, served as controls. Spirometry, lung clearance index (LCI), sweat test, six‐min walk test (6MWT) and cardio‐pulmonary exercise test (CPET) were performed before and three months after the initiation of Trikafta.
Results
Nine study patients, aged 18.6 ± 4.7 years, and nine controls. For the study group, BMI and hip and spine BMD increased significantly (19.4 ± 2.6 to 20.3 ± 2.19 BMI, p = 0.05; 0.73 ± 0.098 to 0.81 ± 0.12 gr/cm2 hip, p = 0.017; 0.76 ± 0.14 to 0.82 ± 0.14 gr/cm2 spine, p = 0.025). For the control group, there was no difference in hip or spine BMD. Lean body mass, %fat z‐score and fat mass/height2 z‐score increased significantly (34770.23 ± 10521.21 to 37430.16 ± 10330.09gr, p = 0.017; –0.8 ± 0.75 to 0.46 ± 0.58, p = 0.012; and −0.98 ± 0.66 to −0.04 ± 0.51, p = 0.025, respectively). 6MWT improved from 541.1 ± 48.9 to 592.9 ± 54.5 m (p = 0.046). As expected, FEV1%pred increased (p = 0.008) and sweat chloride decreased significantly (p = 0.017). In CPET, VE/VCO2 improved, indicating better ventilatory efficiency.
Conclusions
To the best of our knowledge, this is the first study evaluating the metabolic effects of Trikafta. The results are encouraging and offer hope beyond the well‐established effect on pulmonary disease. Larger long‐term studies are warranted to unpin the underlying physiological mechanisms.
Keywords: body composition, bone mineral density, cardio‐pulmonary exercise testing, cystic fibrosis, six‐min walk test, Trikafta
1. INTRODUCTION
Cystic fibrosis (CF) is a severe progressive genetic disease, caused by diminished quantity or function of the CF transmembrane conductance regulator (CFTR) protein, an epithelial chloride channel. 1 In recent years, advances in CF research and care have led to the development of mutation‐specific therapies. Two major types of modifiers have been developed—“potentiators,” pharmacologic agents that increase chloride channel gating of CFTR; and “correctors,” defined as small molecules that “rescue” the misfolded protein and permit trafficking of the CFTR to the cell surface. 2
The most prevalent mutation is F508del, a deletion of three‐base pairs, accounting for 70% of all CFTR alleles. F508del CFTR causes misfolding of the protein, resulting in minimal protein expression at the plasma membrane. 2
At the end of 2019, the FDA approved Trikafta (elexacaftor/ivacaftor/tezacaftor, a combination of two correctors with a potentiator) for CF patients aged 12 years and older who carry at least one F508del mutation. 3 One year later, Trikafta was also approved for several other responsive mutations, based on in vitro data. 4
The effect of CFTR modulators on pulmonary disease is well established and includes improved lung function, as well as a decrease in sweat test values and improved quality of life; there is also evidence of improved nutritional status, but the pathogenesis is less clear. 5 Contributing factors include reduced resting energy expenditure, decreased gut inflammation, and decreased fat malabsorption. 6 In 245 patients with advanced lung disease, treatment with Trikafta led to an average weight gain of 4.2 kg. 7
Recently, there has been increased interest in more extra‐pulmonary effects of CFTR modulators; small studies found a positive effect of ivacaftor and ivacaftor/lumacaftor on bone density, body composition and exercise capacity. 6 , 8 , 9 While treatment with Trikafta holds promise for CF pulmonary disease, even in advanced stages, there is a paucity of data about its effect on other organs. Thus, our aim was to evaluate the effect of Trikafta on bone mineral density (BMD), body composition, pulmonary functions, and exercise capacity (evaluated by six‐min walk test [6MWT] and cardio‐pulmonary exercise test [CPET]).
2. METHODS
This was a pilot, single center, prospective‐retrospective study. The institutional board reviewed and approved the study, and informed consent was obtained from subjects or their legal guardians before recruitment. The study population included patients with CF who are being followed at our tertiary center. Patients eligible for mutation‐specific therapy with Trikafta (defined as the study group) underwent evaluations before and after the initiation of Trikafta.
The study group underwent dual energy X‐ray absorptiometry (DEXA) scans three months after the initiation of Trikafta. BMD and body composition were measured and compared to retrospective values obtained 2 years earlier. The control group consisted of CF patients not treated with Trikafta, for whom BMD was measured 2 years apart.
2.1. DEXA scans
Were performed with a Hologic densitometer (Discovery A; Hologic, Inc). The parameters retrieved included hip & spine BMD (gr/cm2). Body composition parameters included bone mineral content (BMC) (gr); lean body mass (LBM, gr); % fat; fat mass index (FMI, fat mass [FM] divided by height‐squared (kg/m2)).
In addition, spirometry, lung clearance index (LCI), sweat test, 6MWT and CPET were performed before and 3 months after the initiation of Trikafta.
2.2. Spirometry
Was performed in accordance with the American Thoracic Society (ATS)/ERS (American Thoracic Society/European Respiratory Society) Task Force, using a KoKo spirometer (n‐Spire Health care, Inc.). 10 Results are expressed as absolute values and percent predicted (mean ± standard deviation [SD]) derived from Polgar and Quanjer et al. 11
2.3. LCI
Multiple breath washout (MBW) measurements were performed using the Easy‐One Pro, MBW Module (NDD Medical Technologies), as first described by Fuchs et al. in 2008. 12 LCI is the number of functional residual capacity (FRC) turnovers required to washout the nitrogen, and was calculated as the total expired volume during the washout phase divided by the FRC. 13 An increased LCI (>7) indicates more FRC turnovers required for the washout, reflecting inhomogeneous ventilation. 14 , 15
2.4. Sweat test
Was performed by the conductivity method. Sweat was stimulated by pilocarpine iontophoresis, collected by the Wescor Macroduct® tube, and analysed by the Wescor SWEAT·CHECK™ conductivity device. 16 Values are presented as mean ± SD.
2.5. 6MWT
Was performed according to the ATS guidelines. 17 Oxygen saturation (SpO2), blood pressure, heart rate (HR) and respiratory rate (RR) were evaluated pre‐ and post‐test. As recommended, patients were instructed to walk as far as possible along a 30‐m‐long flat corridor for six min. Six‐min walking distance (expressed in meters (m), 6MWD) was calculated from the total number of laps performed in six min.
2.6. CPET
Was carried out using a Quark CPET metabolic cart (Cosmed) according to ATS guidelines. 18 Cycle ergometer progressive exercise testing was performed to the limit of the participant's tolerance, with an incrementing resistance (10−25 W/min) adapted to the patient's functional capacities (ramp protocol) up to exhaustion. Subjects were asked to maintain a pedal speed at the desired protocol level, 60–70 rpm. Gas exchange variables through a designated face mask (V2 mask; Cosmed), 12‐lead ECG, blood pressure and oxygen saturation (SpO2) were recorded at rest, during the test and during the recovery period. Patients who were unable to perform the test on a cycle ergometer were tested on a treadmill with an equivalent incremental protocol (Bruce ramp).
2.7. Statistical methods
The primary outcome was BMD before and after Trikafta. Secondary outcome measures included anthropometric parameters (weight, height, and body mass index—BMI), body composition, spirometry, LCI, sweat test, 6MWT, and CPET results before and after Trikafta.
Statistical analysis was performed using SPSS version 21.
Results are expressed as absolute values, mean ± SD, median (range) and Z‐scores, as appropriate. Paired tests were used for differences between pre‐ and post‐Trikafta.
A value of p < 0.05 was considered as statistically significant.
3. RESULTS
Nine CF patients, aged 18.59 ± 4.67 years and treated with Trikafta, comprised the study group, and another nine CF patients served as controls. Two patients were unable to perform CPET before the initiation of Trikafta, and one patient had body composition and BMD measurements only 3 months after the initiation of Trikafta.
The anthropometric parameters, BMD, and body composition results before and after Trikafta are presented in Table 1. As can be seen, patients gained an average of 2.5 kg, with an increase in BMI from 19.4 ± 2.6 to 20.3 ± 2.19, p = 0.05. There was a significant increase in hip and spine BMD (0.73 ± 0.098 to 0.81 ± 0.12 gr/cm2 hip, p = 0.017; 0.76 ± 0.14 to 0.82 ± 0.14 gr/cm2 spine, p = 0.025). For the control patients, there was no difference in hip or spine BMD. Hip and spine BMD before and after Trikafta are presented in Figure 1. As can be seen, BMD improved in all patients except patient number 4. Notably, this patient was treated with repeated prolonged courses of corticosteroids due to allergic broncho‐pulmonary aspergillosis. The analysis of body composition found a significant increase in LBM, %fat z‐score and FM/height2 (FMI) z‐score (34770.23 ± 10521.21 to 37430.16 ± 10330.09, p = 0.017; −0.8 ± 0.75 to 0.46 ± 0.58, p = 0.012; and −0.98 ± 0.66 to −0.04 ± 0.51, p = 0.025, respectively). Table 1 supplement presents the anthropometric data and DEXA results divided by gender. The small number (five males and three females) may have resulted in a type II error. The only significant change that remained was %fat z‐score for males.
Table 1.
Anthropometric parameters, BMD, and body composition before and after Trikafta
| Before | After | p Value | |
|---|---|---|---|
| Weight (kg) | 51.63 ± 10.47 | 54 ± 10.82 | 0.05 |
| Height | 162.6 ± 13.23 | 162.5 ± 13.21 | 0.46 |
| BMI (kg/m2) | 19.4 ± 2.6 | 20.3 ± 2.19 | 0.05 |
| Hip BMD (gr/cm2) | 0.73 ± 0.098 | 0.81 ± 0.12 | 0.017 |
| Spine BMD (gr/cm2) | 0.76 ± 0.14 | 0.82 ± 0.14 | 0.025 |
| Total BMC (gr) | 1795.7 ± 457.6 | 1820.6 ± 411.1 | 0.58 |
| LBM (gr) | 34770.23 ± 10521.21 | 37430.16 ± 10330.09 | 0.017 |
| % Fat | 23.13 ± 6.08 | 27.55 ± 6.54 | 0.069 |
| % Fat z‐score | −0.8 ± 0.75 | 0.46 ± 0.58 | 0.012 |
| Fat mass/height2 z‐score | −0.98 ± 0.66 | −0.04 ± 0.51 | 0.025 |
Note: Values are presented as mean ± SD; n = 8 (body composition and BMD).
Abbreviations: BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; gr, grams; LBM, lean body mass; kg, kilograms; SD, standard deviation.
Figure 1.
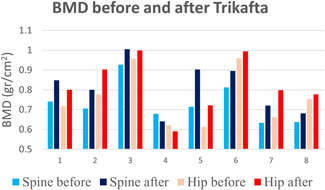
Bone mineral density of spine and hip before and after Trikafta. BMD, bone mineral density. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2 presents the results of spirometry, LCI, 6MWT and sweat test. 6MWD improved from 541.1 ± 48.93 to 592.867 ± 54.47 m (p = 0.046). As expected, FEV1% increased an average of 12% (p = 0.008) and sweat chloride decreased by 44 mmol/L (p = 0.017). There was a slight decrease in LCI that was not statistically significant. Before the initiation of Trikafta, FEV1 values of our patients had a wide range (31‐111%). Five patients had significant lung disease with moderate‐severe reduction in pulmonary function tests (FEV1 ≤ 50%). Of them, one had advanced lung disease (FEV1 31%) with restrictive impairment (FVC 39%). As can be seen in the Table, FEV1/FVC was in the lower limit of normal before Trikafta and did not change under treatment.
Table 2.
Lung functions and sweat chloride before and after Trikafta
| Before | After | p Value | |
|---|---|---|---|
| FVC (%) | 68.56 ± 22.85 | 80.78 ± 23.86 | 0.013 |
| FEV1 (%) | 58.44 ± 26.9 | 70.22 ± 27.054 | 0.008 |
| FEV1/FVC | 0.76 ± 0.12 | 0.75 ± 0.12 | 0.49 |
| FEF 25−75 (%) | 48.0 ± 34.99 | 54.11 ± 37.2 | 0.16 |
| LCIa | 11.2(6.6‐14.3) | 8.77(7.7‐13.27) | 0.6 |
| 6MWD (m) | 541.1 ± 48.93 | 592.87 ± 54.47 | 0.046 |
| Sweat chloride (mmol/L) | 85.13 ± 19.38 | 41.13 ± 20.28 | 0.017 |
Note: Values are presented as mean ± SD.
Abbreviations: FEF 25−75, forced expiratory flow between 25% and 75% of FVC; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; LCI, lung clearance index; m, meters; 6MWD, six‐min walk distance.
Median (25th‐75th).
Table 3 presents the CPET results before and after Trikafta. There was a significant decrease of VE/VCO2 at VT (ventilatory threshold), from 38.3 ± 7.28 to 33.7 ± 5.04, p = 0.018. As can be seen, there was an increase in BR and BR%, but it did not reach statistical significance (24.69 ± 20.32 to 36.64 ± 28.46 L/min, p = 0.091; and 21.71 ± 11.01 to 28.57 ± 14.28%, p = 0.089, respectively). Notably, before the initiation of Trikafta, two patients had peak exercise oxygen desaturations (from 97% at rest to 93% and from 98% to 92%) compared to none of the patients post Trikafta; this is consistent with the improvement in their pulmonary functions.
Table 3.
CPET results before and after Trikafta
| Before | After | p Value | |
|---|---|---|---|
| Peak VO2 (ml/min) | 1864.4 ± 603.0 | 1861.4 ± 501.2 | 1 |
| Peak VO2 (ml/min/kg) | 32.6 ± 9.0 | 32.4 ± 7.1 | 0.87 |
| Peak VO2%pred | 76.7 ± 19.9 | 78.4 ± 16.3 | 0.79 |
| Ventilatory threshold | 38.6 ± 9.3 | 41.3 ± 13.0 | 0.35 |
| Peak VO2/HR | 10.17 ± 3.68 | 10.49 ± 2.95 | 0.49 |
| Peak VO2/HR% | 85.00 ± 21.48 | 86.14 ± 18.85 | 0.87 |
| Peak VE/VO2 | 44.6 ± 12.25 | 40.7 ± 6.27 | 0.61 |
| VE/VCO2 at VT | 38.3 ± 7.28 | 33.7 ± 5.04 | 0.018 |
| VE/VCO2 slope | 32.97 ± 11.65 | 28.84 ± 3.32 | 0.49 |
| BR (L/min) | 24.69 ± 20.32 | 36.64 ± 28.46 | 0.091 |
| BR% | 21.71 ± 11.01 | 28.57 ± 14.28 | 0.089 |
| Low BR | 2 (28.6%) | 2 (28.6%) | 1.00 |
Note: n = 7, three on a cycle ergometer and four on a treadmill. Values are presented as mean ± SD.
Abbreviations: BR, breathing reserve; CPET, cardio‐pulmonary exercise testing; HR, heart rate; ml, milliliters; min, minute; kg, kilograms; L, liters; SD, standard deviation; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake; VT, Ventilatory threshold.
Table 4 presents the CFTR mutations and VO2 values of the patients that performed CPET (n = 7). As can be seen, five patients carried at least one F508del mutation. The other two carried G85E, a missense mutation (one homozygous and one with a stop mutation). Two additional patients did not perform CPET before the initiation of Trikafta; both carried F508del (F508del/3849 + 10 kb C > T and F508del/R1066C). As can be seen in the Table, the response to Trikafta in terms of VO2 values was very heterogenous. While patients 1, 2, 4, 7 improved considerably, the other remained stable (patients 3, 5) or even had worse VO2 values results after Trikafta (patient 6). Interestingly, patient 6 had very poor compliance to other CF therapies; nonetheless, his FEV1 improved from 33% to 47% under Trikafta.
Table 4.
Individual patient data on CFTR mutations and VO2 values
| Patient | CFTR Mutations | Peak VO2 (ml/min)a | Peak VO2/kga | Peak VO2 (% pred)a |
|---|---|---|---|---|
| 1/M | F508del/W1282X | 2658/2798 | 41.86/43.05 | 85/90 |
| 2/F | F508del/G542X | 1379/1652 | 25.16/29.5 | 71/86 |
| 3/F | F508del/W1282X | 1822/1762 | 31.41/30.91 | 87/92 |
| 4/M | F508del/F508del | 1066/1574 | 17.77/24.29 | 37/52 |
| 5/M | F508del/Y1014C | 2350/2226 | 42.96/41.38 | 91/86 |
| 6/M | R1158X/G85E | 2133/1746 | 36.78/27.28 | 73/58 |
| 7/F | G85E/G85E | 1090/1272 | 32.06/30.5 | 87/86 |
Abbreviations: CFTR, Cystic fibrosis transmembrane regulator; F, female; kg, kilograms; M, male; min, minute; ml, milliliters; pred, predicted; VO2, oxygen uptake.
Before/after Trikafta.
Figure 1 supplement presents the CPET parameters before and after Trikafta—peak VO2 specific (ml/min/kg), BR (%) and VE/VCO2 at VT in Supporting Information: Figure 1a, 1b, and 1c, respectively. As can be seen, the response to Trikafta is variable; patient 4 had the worse peak VO2 specific before the initiation of Trikafta, and the greatest improvement after Trikafta. The small sample size and short time interval to exercise testing likely influenced the results.
4. DISCUSSION
In this pilot single‐center study, we investigated the effects of Trikafta on BMD, body composition and exercise capacity in CF patients. We found a significant increase in weight and BMI, as well as lean mass and FM, accompanied by increases in BMD parameters. In addition, there was a significant increase in 6MWT results; in CPET, peak VO2 did not improve, but VE/VCO2 decreased significantly, indicating better ventilatory efficiency. As expected, FEV1 improved and sweat test decreased in patients treated with Trikafta.
CF bone disease is characterized by low BMD (osteopenia and osteoporosis). The etiology is multifactorial and includes: decreased bone formation and increased resorption due to dysfunction of the CFTR; pancreatic insufficiency with malabsorption of vitamins D and K which are important for bone mineralization; and systemic inflammation leading to increased osteoclastic bone resorption. 6 Additional contributing factors are malnutrition, physical inactivity and use of glucocorticoids. 19 Our group previously examined 40 CF patients and found that 15 (37.5%) and 11 (27.5%) had osteopenia and osteoporosis, respectively. 20
In our study, there was a significant increase in hip and spine BMD in the study group, while BMD remained stable in the control group. We found an increase of 0.08 gr/cm2 in hip BMD and 0.06 gr/cm2 in spine BMD. Maghraou et al. suggested that a change of 0.02 gr/cm2 and 0.04 gr/cm2 in hip and spine BMD respectively, should be considered significant. 21 Thus, we believe that the increase of BMD in our patients is genuine, beyond the expected reproducibility. In addition, as we found that BMD improved in the study patients and not in the controls—we feel that the improvement may be attributed to Trikafta therapy.
There is a paucity of studies evaluating the effect of CFTR modulators on bone metabolism, and we are not aware of studies performed with Trikafta. In a small study, Ivacaftor led to a significant improvement in lumbar spine BMD z‐score in patients with the G551D gating mutation. 22 In another study, ivacaftor led to a significant increase in cortical volume, area, and porosity at the radius and tibia in adults, with no significant changes in BMD or estimated bone strength. 19 Suggested mechanisms for improvement include better nutritional status, increase in physical activity and/or reduced systemic inflammation. 6 Additionally, in vitro studies suggest increased osteoblastic CFTR activity, resulting in reduced receptor activator of nuclear factor k‐B ligand (RANK‐L) production and less osteoclast formation. 22
Notably, the increase in weight occurs almost immediately after the initiation of Trikafta. There is also evidence in the literature that CFTR modulators improve anthropometric parameters after a few weeks of treatment. 5 Although interventions to increase BMD usually take 6−12 months, we aimed to evaluate the short‐term effect of Trikafta on BMD.
Lower BMI in CF patients has been known to affect clinical outcomes, including pulmonary function, frequency of hospital admission, and quality of life. 23 The beneficial effect of Trikafta on nutritional status is well established. In our study, Trikafta led to a mean of 2.5 kg weight gain and one point in BMI, which were statistically significant. In previous studies, Trikafta led to increased weight and BMI, compared to placebo or to dual combination therapy. 5 Suggested mechanisms include reduced resting energy expenditure, increased fat absorption and decreased gut inflammation. 24 However, there is an increased body of evidence for the need to evaluate body composition in addition to BMI. Recently, body composition emerged as a key determinant of clinical outcomes in children and adults with CF. 25
In adults, fat‐free mass (FFM) was found to correlate negatively with CF disease severity, inflammatory state, and pulmonary function. FFM was also found to correlate with better BMD, while greater FM was associated with greater loss of spine BMD; in youth, LBM was associated with pulmonary function. 26 Additionally, patients with CFRD were found to have a lower FMI z‐score, weight z‐score and leptin levels compared to the control group. 27
Hence, we evaluated the effect of Trikafta on body composition. We found that weight gain was associated with significant increases both in parameters of lean mass and FM; LBM, %fat z‐score and FM/height 2 z‐score increased significantly. A few studies examined the effect of CFTR modulators on body composition, with varying results. In a double‐blind, placebo‐controlled study, 28 days of treatment with Ivacaftor led to a small, nonsignificant increase in FFM. 28 An open‐label extension of the study led to a mean weight increase of 2.5 kg and 0.8 BMI units after 6 months, comprised predominantly of FM, with small gains in FFM; after 2 years, weight and FM gain attenuated, with stability of FFM. 8 In contrast, Stallings et al found that Ivacaftor treatment for 3 months led to an increase of 2.5 ± 2.2 kg in weight, with significant increases in both FFM and FM. 24 The increase in FM may raise concerns. Normal weight adiposity, defined by high body fat with normal BMI, may have adverse effects on patients' health. Thus, nutritional intake should be assessed and monitored, especially in patients experiencing weight gain on CFTR modulator therapy. 8
Exercise capacity is a biomarker of whole body metabolic performance, and is associated with lung function trajectories and quality of life in CF. 29 , 30 Oxygen uptake, exercise duration and workload measured by CPET have been found as useful prognostic indicators for survival in CF. 28 We assessed 6MWT and CPET, which reflect everyday functional capacity (submaximal) and maximal exercise capacity, respectively. In our study, 6MWT improved significantly after treatment with Trikafta, with a 51.8 m increase in the distance walked. In healthy children, an increase of at least 79.69 m 6MWD was considered necessary to attribute the improvement to an intervention and not to the individual's growth. 31 However, in adults with chronic lung disease, 30 m was suggested as the minimal important difference in 6MWD. 32 Moreover, in a pilot study assessing exercise capacity and quality of life over 1 year, the MCID (minimal clinically important difference) in 6MWD was found to be 33 m. 33 In the current study, five adult patients were included; thus, we cannot draw a firm conclusion if the change in 6MWD was clinically significant.
In our study, there was no change in peak VO2 after Trikafta; however, there was a significant decrease in VE/VCO2 at VT. Although there was an improvement in BR, it did not reach statistical significance.
Patients with chronic lung disease often have impaired ventilatory efficiency and increased dead space, which contribute to respiratory morbidity. VE/VCO2 is a submaximal marker for ventilatory drive, that is related to the amount and sensitivity of central chemoreceptors and the ventilatory dead space. A previous study in our institute found a correlation between computed tomography (CT) scores and VE/VCO2. 29 In a study evaluating longitudinal changes in exercise capacity in adult CF patients, VE/VCO2 at anaerobic threshold was higher (p = 0.047) and the ventilatory reserve by the end of the exercise was lower (p = 0.019) during follow‐up. 34 Another study found correlations between peripheral muscles (biceps and quadriceps) strength and ventilatory equivalents (VE/VO2 and VE/VCO2). 35 Thus, the improved VE/VCO2 may potentially improve respiratory physiology and may result in an apparent clinical benefit. Notably, our patient had a wide range of their baseline condition. The small sample size and short time interval to exercise testing may have influenced the results. Further significant changes in exercise are likely to be seen in those who were markedly limited from the onset.
In concordance with the improvement in lung functions, two patients who experienced desaturations at the end of CPET before treatment had normal saturations at the beginning and end of the test under Trikafta. A study in 10 CF patients treated with Lumacaftor/ivacaftor found an increase of 78 m in 6MWT by four weeks of treatment, which increased to 118 m after 52 weeks. Similar to our findings, significant improvements were also found in oxygen saturation after 6MWT. 9 In a retrospective study of severe CF patients on compassionate therapy with Trikafta, 6MWT increased by a mean of 42 m. 36 A pilot study in three patients found that treatment with Trikafta led to significant improvement in 6MWT. 37 These results could correlate to the significant improvement in breathing indices post Trikafta.
Several small studies examined the effect of CFTR modulators on CPET results. In the study mentioned earlier, 28 days of Ivacaftor did not change VO2max or min ventilation (VE) compared to placebo; however, exercise time increased significantly. 28 In seven adults treated with Lumacaftor/Ivacaftor, there was no change in exertional dyspnea and leg discomfort at submaximal exercise; six participants improved their endurance time, but this did not achieve statistical significance. 38 In eight adolescents, Tezacaftor/Ivacaftor led to improved VO2 peak and ventilatory anaerobic threshold. 39 The lack of improvement in peak VO2 may reflect the small sample size. However, previous studies also found that exercise parameters did not mirror the effect of Ivacaftor on FEV1, BMI and sweat chloride. The authors postulated that non‐respiratory factors, such as cardiovascular and muscular systems, play a greater role in exercise limitation in CF than previously suspected. 28 In addition, we repeated CPET three months after the initiation of Trikafta; we may postulate that whole body metabolic response to low intensity exercise (e.g., 6MWT) occurs in a short period of time, while a longer period of treatment is needed for the response to high intensity exercise (CPET). Interestingly, a large multicenter study did not find an association between CFTR mutation class and exercise capacity; however, those carrying F508del and a class V mutation had lower maximal exercise capacity. 40 We had one patient carrying F508del and 3849 + 10 kb C > T (class V). This patient did not respond to Trikafta, but further conclusions cannot be made based on a single case.
As expected, we found significant improvement in spirometry and sweat chloride. Previous studies found a significant improvement in FEV1 and sweat test following the initiation of Trikafta. Even in severe CF patients eligible for compassionate treatment, FEV1 increased by a mean of 10.7% after one month and 14.2% by 6 months, accompanied by a mean decrease of 45 mmol/L in sweat chloride. 36 Interestingly, we found a 2.5‐point decrease in median LCI that did not reach statistical significance. In a postapproval study of Lumacaftor/Ivacaftor, despite no improvement in spirometry, there was a significant decrease in LCI—0.81 units at 1 month, 0.77 units at 3 months, and 0.55 units at 12 months. 41 Recently, Trikafta was found to improve LCI by 1.4−2.04 points in patients with at least one F508del mutation. 42 As stated earlier with other parameters, the lack of statistical significance in our study may reflect the small sample size.
The main limitation of our study is the small number of patients. Not all assessments were available for all patients, resulting in even smaller sub‐groups. The lack of improvement in some parameters (e.g., peak VO2 in CPET) may reflect a type II error due to the small sample size. Some patients did the study on treadmill and some on cycle ergometer which makes comparison difficult; however, each patient served as his own control. The control group performed only BMD measurements. We did not account for possible changes in dietary habits or daily physical activity after the initiation of Trikafta, which may have affected our results. Long‐term studies can assess the possible effect of Trikafta on CPET parameters.
However, to the best of our knowledge, this is the first study evaluating the metabolic effects of Trikafta, and the initial results are encouraging. Understanding the extra‐pulmonary effects of Trikafta may aid in managing other organs involved in CF, in the prevention of long‐term complications, and may enable dietary and exercise guidance for people on CFTR modulators. Larger multi‐center, long term studies are warranted to confirm our results.
AUTHOR CONTRIBUTIONS
Michal Gur: Conceptualization; writing − original draft; methodology; data curation; investigation. Ronen Bar–Yoseph: Visualization; validation; methodology; supervision. Moneera Hanna: Data curation; formal analysis; validation. Dana Abboud: Data curation; visualization. Zohar Keidar: Writing − review and editing; formal analysis; data curation. Tala Palchan: Data curation; formal analysis; writing − review and editing. Yazeed Toukan: Data curation; visualization. Kamal Masarweh: Visualization; data curation. Irit Alisha: Data curation; investigation. Nehama Zuckerman‐Levin: Formal analysis; writing − review and editing; validation. Lea Bentur: Writing − review and editing; conceptualization; investigation; methodology; supervision; project administration.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure 1 supplement – CPET parameters before and after Trikafta. 1a – Peak VO2/kg (specific) before and after Trikafta.
1b – BR (%) before and after Trikaft.
1c – VE/VCO2 at VT before and after Trikafta. VO2 = oxygen uptake; ml=milliliters; min=minute; kg=kilograms; BR = breathing reserve; VE = minute ventilation; VCO2 = carbon dioxide output; VT = Ventilatory threshold.
Supplementary information.
ACKNOWLEDGMENT
The authors acknowledge the help of Mrs. Faten Daud from the Nuclear Medicine Institute, Rambam Health Care Campus; Mrs. Myrna Perlmutter for English – language editing; and the statistical help of Mrs. Ronit Leiba from the Medical Statistics Unit, Rambam Health Care Campus. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Gur M, Bar–Yoseph R, Hanna M, et al. Effect of Trikafta on bone density, body composition and exercise capacity in CF: a pilot study. Pediatric Pulmonology. 2023;58:577‐584. 10.1002/ppul.26243
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Keating D, Marigowda G, Burr L, et al. VX‐445–Tezacaftor–Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379(17):1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors: cystic fibrosis potentiators and correctors. Pediatr Pulmonol. 2015;50(0 40):S3‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FDA . FDA approves new breakthrough therapy for cystic fibrosis. Accessed May 2, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis
- 4. Vertex Pharmaceuticals . Vertex Announces FDA Approvals of TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor), SYMDEKO® (tezacaftor/ivacaftor and ivacaftor) and KALYDECO® (ivacaftor) for Use in People With CF With Certain Rare Mutations. Accessed May 2, 2021. https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-fda-approvals-trikaftar
- 5. Bailey J, Rozga M, McDonald CM, et al. Effect of CFTR modulators on anthropometric parameters in individuals with cystic fibrosis: an evidence analysis center systematic review. J Acad Nutr Diet. 2021;121(7):1364‐1378. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/32532673/ [DOI] [PubMed] [Google Scholar]
- 6. Sergeev V, Chou FY, Lam GY, Hamilton CM, Wilcox PG, Quon BS. The extrapulmonary effects of cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Ann Am Thorac Soc. 2020;17(2):147‐154. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/31661636/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgel P‐R, Durieu I, Chiron R, et al. Rapid improvement after starting elexacaftor‐tezacaftor‐ivacaftor in patients with cystic fibrosis and advanced pulmonary disease. Am J Respir Crit Care Med. 2021;204(1):64‐73. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/33600738/ [DOI] [PubMed] [Google Scholar]
- 8. King SJ, Tierney AC, Edgeworth D, et al. Body composition and weight changes after ivacaftor treatment in adults with cystic fibrosis carrying the G551 D cystic fibrosis transmembrane conductance regulator mutation: a double‐blind, placebo‐controlled, randomized, crossover study with open‐label. Nutrition. 2021;85:111124. [DOI] [PubMed] [Google Scholar]
- 9. Wark PAB, Cookson K, Thiruchelvam T, Brannan J, Dorahy DJ. Lumacaftor/Ivacaftor improves exercise tolerance in patients with cystic fibrosis and severe airflow obstruction. BMC Pulm Med. 2019;19(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller MR, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 11. Quanjer PH, Borsboom GJJM, Brunekreef B, et al. Spirometric reference values for White european children and adolescents: polgar revisited. Pediatr Pulmonol. 1995;19(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 12. Fuchs SI, Sturz J, Junge S, Ballmann M, Gappa M. A novel sidestream ultrasonic flow sensor for multiple breath washout in children. Pediatr Pulmonol. 2008;43(8):731‐738. [DOI] [PubMed] [Google Scholar]
- 13. Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13(2):123‐138. [DOI] [PubMed] [Google Scholar]
- 14. Fuchs SI, Eder J, Ellemunter H, Gappa M. Lung clearance index: normal values, repeatability, and reproducibility in healthy children and adolescents. Pediatr Pulmonol. 2009;44(12):1180‐1185. [DOI] [PubMed] [Google Scholar]
- 15. Robinson PD, Latzin P, Verbanck S, et al. Consensus statement for inert gas washout measurement using multiple‐ and single‐ breath tests. Eur Respir J. 2013;41(3):507‐522. http://www.ncbi.nlm.nih.gov/pubmed/23397305 [DOI] [PubMed] [Google Scholar]
- 16. Greaves RF, Jolly L, Massie J, et al. Laboratory performance of sweat conductivity for the screening of cystic fibrosis. Clin Chem Lab Med. 2018;56(4):554‐559. https://www-degruyter-com.ezlibrary.technion.ac.il/document/doi/10.1515/cclm-2017-0530/html [DOI] [PubMed] [Google Scholar]
- 17. Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166(1):111‐117. [DOI] [PubMed] [Google Scholar]
- 18. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211‐277. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/12524257/ [DOI] [PubMed] [Google Scholar]
- 19. Putman MS, Greenblatt LB, Bruce M, et al. The effects of ivacaftor on bone density and microarchitecture in children and adults with cystic fibrosis. J Clin Endocrinol Metab. 2021;106(3):e1248‐e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gur M, Bar‐Yoseph R, Diab G, et al. Understanding the interplay between factors that influence bone mineral density in CF. Pediatr Pulmonol. 2020;55(10):2667‐2673. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/32584478/ [DOI] [PubMed] [Google Scholar]
- 21. El Maghraoui A, Do Santos Zounon AA, Jroundi I, et al. Reproducibility of bone mineral density measurements using dual X‐ray absorptiometry in daily clinical practice. Osteoporos Int. 2005;16(12):1742‐1748. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/15937633/ [DOI] [PubMed] [Google Scholar]
- 22. Sermet‐Gaudelus I, Delion M, Durieu I, Jacquot J, Hubert D. Bone demineralization is improved by ivacaftor in patients with cystic fibrosis carrying the p.Gly551Asp mutation. J Cyst Fibros. 2016;15(6):e67‐e69. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/27745802/ [DOI] [PubMed] [Google Scholar]
- 23. Papalexopoulou N, Dassios TG, Lunt A, et al. Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir Med. 2018;142(July):60‐65. [DOI] [PubMed] [Google Scholar]
- 24. Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy balance and mechanisms of weight gain with ivacaftor treatment of cystic fibrosis gating mutations. J Pediatr. 2018;201:229‐237.e4. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/30029855/ [DOI] [PubMed] [Google Scholar]
- 25. Calella P, Valerio G, Brodlie M, Donini LM, Siervo M. Cystic fibrosis, body composition, and health outcomes: a systematic review. Nutrition. 2018;55–56:131‐139. https://pubmed.ncbi.nlm.nih.gov/29981489/ [DOI] [PubMed] [Google Scholar]
- 26. Soltman S, Hicks RA, Naz Khan F, Kelly A. Body composition in individuals with cystic fibrosis. J Clin Transl Endocrinol. 2021;26:100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Granados A, Beach EA, Christiansen AJ, Patterson BW, Wallendorf M, Arbeláez AM. The association between body composition, leptin levels and glucose dysregulation in youth with cystic fibrosis. J Cyst Fibros. 2021;20(5):796‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edgeworth D, Keating D, Ellis M, et al. Improvement in exercise duration, lung function and well‐being in G551D‐cystic fibrosis patients: a double‐blind, placebo‐controlled, randomized, cross‐over study with ivacaftor treatment. Clin Sci. 2017;131(15):2037‐2045. [DOI] [PubMed] [Google Scholar]
- 29. Bar‐Yoseph R, Ilivitzki A, Gur M, et al. Exercise capacity in patients with cystic fibrosis vs non‐cystic fibrosis bronchiectasis. 2018:OA484. [DOI] [PMC free article] [PubMed]
- 30. Williams CA, Wedgwood KCA, Mohammadi H, Prouse K, Tomlinson OW, Tsaneva‐Atanasova K. Cardiopulmonary responses to maximal aerobic exercise in patients with cystic fibrosis. PLoS One. 2019;14(2):e0211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. del Corral T, Tapia‐Castañeda J, Ríos‐Pérez G, et al. Assessment of the determinants of changes and test‐retest reliability in the 6‐min walk test performance over a 4‐month period in healthy 6‐12‐year‐old children. Eur J Appl Physiol. 2022;122(4):935‐944. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/35044496/ [DOI] [PubMed] [Google Scholar]
- 32. Holland AE, Spruit MA, Troosters T, et al. An official european respiratory Society/American thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428‐1446. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/25359355/ [DOI] [PubMed] [Google Scholar]
- 33. Bhatia R, Kaye M, Roberti‐Miller A. Longitudinal assessment of exercise capacity and quality of life outcome measures in cystic fibrosis: a year‐long prospective pilot study. J Eval Clin Pract.26(1), 2020:236‐241 https://onlinelibrary.wiley.com/doi/full/10.1111/jep.13105 [DOI] [PubMed] [Google Scholar]
- 34. Boutou A, Manika K, Hajimitrova M, et al. Longitudinal changes in exercise capacity among adult cystic fibrosis patients. Adv Respir Med. 2020;88(5):420‐423. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/33169814/ [DOI] [PubMed] [Google Scholar]
- 35. Vendrusculo FM, Bueno GS, Gheller MF, et al. Peripheral muscle strength is associated with aerobic fitness and use of antibiotics in patients with cystic fibrosis. Int J Clin Pract. 2021;75(5)e14050. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/33497024/ [DOI] [PubMed] [Google Scholar]
- 36. Carnovale V, Iacotucci P, Terlizzi V, et al. Effectiveness and safety of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease with the Phe508del/minimal function genotype. Respir Med. 2021;189(May):106646. [DOI] [PubMed] [Google Scholar]
- 37. Terlizzi V, Colangelo C, Marsicovetere G, et al. Effectiveness of elexacaftor/tezacaftor/ivacaftor therapy in three subjects with the cystic fibrosis genotype phe508del/unknown and advanced lung disease. Genes. 2021;12(8):1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quon BS, Ramsook AH, Dhillon SS, et al. Short‐term effects of Lumacaftor/Ivacaftor (orkambi™) on exertional symptoms, exercise performance, and ventilatory responses in adults with cystic fibrosis. Respir Res. 2020;21(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed M, Dayman N, Madge J, Gaillard E. P91 Cardiopulmonary exercise testing in CF adolescents after starting Tezacaftor/Ivacaftor. in: monitoring and care delivery for children with respiratory disease. BMJ. 2021;76:A136.1. https://thorax.bmj.com/lookup/doi/10.1136/thorax-2020-BTSabstracts.236 [Google Scholar]
- 40. Radtke T, Hebestreit H, Gallati S, et al. CFTR genotype and maximal exercise capacity in cystic fibrosis: a cross‐sectional study. Ann Am Thorac Soc. 2018;15(2):209‐216. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/29140739/ [DOI] [PubMed] [Google Scholar]
- 41. Shaw M, Khan U, Clancy JP, et al. Changes in LCI in F508del/F508del patients treated with lumacaftor/ivacaftor: results from the prospect study. J Cyst Fibros. 2020;19(6):931‐933. https://pubmed-ncbi-nlm-nih-gov.ezlibrary.technion.ac.il/32513528/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graeber SY, Renz DM, Stahl M, et al. Effects of Elexacaftor/Tezacaftor/Ivacaftor therapy on lung clearance index and magnetic resonance imaging in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med. 2022;206(3):311‐320. https://pubmed.ncbi.nlm.nih.gov/35536314/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 supplement – CPET parameters before and after Trikafta. 1a – Peak VO2/kg (specific) before and after Trikafta.
1b – BR (%) before and after Trikaft.
1c – VE/VCO2 at VT before and after Trikafta. VO2 = oxygen uptake; ml=milliliters; min=minute; kg=kilograms; BR = breathing reserve; VE = minute ventilation; VCO2 = carbon dioxide output; VT = Ventilatory threshold.
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


