Abstract
Background
The vascular endothelium is markedly disrupted in sickle cell disease (SCD) and is the converging cascade of the complex pathophysiologic processes linked to sickle cell vasculopathy. Circulating endothelial activation and/or apoptotic markers may reflect this endothelial activation/damage that contributes to the pathophysiology of the SCD vascular complications.
Methods
Plasmatic levels of circulating endothelial cells (CECs), E‐selectin, progenitor's endothelial cells (EPCs), and circulating extracellular vesicles (EVs) were evaluated in 50 SCD patients, 16 with vasculopathy. The association between these markers and the occurrence of disease‐related microvascular injuries of the eye (retinopathy), kidney (nephropathy), and skin (chronic active ulcers) was explored.
Results
Among the endothelial activation markers studied, only higher plasma levels of E‐selectin were found in SCD patients with vasculopathy (p = .015). Increased E‐selectin levels were associated with retinopathy (p < .001) but not with nephropathy or leg ulcers. All patients, at steady state, with or without vasculopathy, did not display a high count of CEC and EPC, markers of endothelial injury and repair. We did not show any significant differences in EVs levels between vasculopathy and not vasculopathy SCD patients.
Conclusions
Further studies will be required to determine whether the E‐selectin could be used as an early biomarker of retinopathy sickle cell development.
Keywords: endothelium markers, E‐selectin, sickle cell disease, sickle cell vasculopathy
Novelty statement.
What is the new aspect of your work?
Plasmatic levels of circulating endothelial cells (CECs), E‐selectin, progenitor's endothelial cells (EPCs), and circulating extracellular vesicles (EVs) were evaluated in 50 SCD patients, 16 with vasculopathy. The association between these markers and the occurrence of disease‐related microvascular injuries of the eye (retinopathy), kidney (nephropathy), and skin (chronic active ulcers) was explored.
What is the central finding of your work?
Among the endothelial activation markers studied, only higher plasma levels of E‐selectin were found in SCD patients with vasculopathy (p = .015). Increased E‐selectin levels were associated with retinopathy (p < .001) but not with nephropathy or leg ulcers.
What is (or could be) the specific clinical relevance of your work?
Despite the fact that the elevation of E‐selectin levels in sickle cell retinopathy and its possible pathophysiological implications need to be confirmed by larger studies and might prompt us to evaluate this new molecule in the treatment of SCD retinopathy.
1. INTRODUCTION
Sickle cell disease (SCD), an autosomal recessive disorder, is the most common hereditary hemoglobinopathy affecting more than 300 000 newborns worldwide. 1 Despite the improvement of medical care leading to a longer life span of SCD patients during the last decades, the long‐term complications of the disease are still substantial and include silent cerebral infarct, stroke, end‐organ damage as well as early death. 2 , 3 , 4 , 5 , 6 SCD is caused by homozygosity for a Glu6Val mutation in HBB (sickle cell anemia; hemoglobin SS) or compound heterozygosity forms like hemoglobin SC and hemoglobin S‐β thalassemia. 1 The resulting abnormal Hb, named hemoglobin S (HbS), is prone to polymerization at low oxygen levels. Polymerized HbS interferes with red blood cells (RBCs) biconcave architecture and flexibility, resulting in crescent‐shaped cells with enhanced adherence to the vascular endothelium that obstructs the blood flow as well as increased RBC fragility leading to hemolysis. 7 , 8 The single sickle mutation is not sufficient to explain the heterogeneity of the disease phenotype observed clinically. Increased expression of adhesion molecules on erythrocytes and endothelial cells, interactions with leukocytes, increased levels of circulating inflammatory cytokines, enhanced microvascular thrombosis, and endothelial damage are all thought to contribute to obstruction of the capillaries and post‐capillary venules by sickled erythrocytes. 9 , 10 , 11 , 12 , 13 , 14
The clinical manifestations of SCD are thought to be not only related to “viscosity‐vaso‐occlusive” sub‐phenotype with vaso‐occlusive pain crisis (VOC), acute chest syndrome (ACS) and osteonecrosis, but also to “hemolysis‐endothelial dysfunction” with pulmonary arterial hypertension (PAH), priapism, leg ulcers, and stroke. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
Endothelium is a dynamic tissue in equilibrium with a circulating compartment reflecting both lesion and regeneration of the vascular tree and playing a key role in the control of vascular homeostasis such as vasomotion, permeability, hemostasis, inflammation, or angiogenesis. 16 , 17 In SCD, the vascular endothelium is markedly disrupted and dysregulated and is the converging cascade of the complex pathophysiologic processes linked to SCD vasculopathy. 8 Circulating endothelial cells (CECs) detached from injured vessels, 18 and extracellular vesicles (EVs) released from activated or apoptotic endothelial cells 19 constitute a hallmark of these deleterious responses affecting the vessel wall. In response to injury, regenerative mechanisms are triggered to restore endothelium integrity. Progenitor's endothelial cells (EPCs) are involved in vascular regeneration. 20 Dysregulated expression of pro‐adhesion molecules such as vascular adhesion molecule‐1 (VCAM‐1), intracellular adhesion molecule‐1 (ICAM‐1), or selectins (P‐selectin and E‐selectin) have been linked to SCD vascular pathophysiology. 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31
In this study, we hypothesized that plasma levels of endothelial activation markers: CEC, E‐selectin, EPC, and circulating EVs are associated with chronic vascular complications imputed to endothelial dysfunction in SCD patients. Thus, we explored the association of these markers with the occurrence of disease‐related microvascular injury of the eye (retinopathy), kidney (nephropathy), and skin (chronic active ulcers).
2. METHODS
2.1. Clinical data
This study recruited, over a period of 12 month, patients with SCD (homozygous hemoglobin S [HbSS], sickle hemoglobin C disease [HbSC], sickle β 0‐thalassemia [HbSβ 0‐thalassemia], sickle β +‐thalassemia [HbSβ +‐thalassemia]) at least 18 years old and at steady state. These patients were enrolled at the Internal medicine department, Timone Hospital, Marseille, France. Patients were considered at steady state if they had not undergone red blood‐cell transfusion or red‐cell exchange therapy within the 12 weeks before enrollment in the trial or not experienced VOC or ACS, or any other acute SCD‐related complication within the 4 weeks before inclusion, during the trial, and until 4 weeks after the study end. The included patients were screened for the presence of chronic micro‐vascular complications related to endothelial dysfunction. From chart review, the following related complications were recorded. Nephropathy: urinary protein to creatinine ratio of >50 mg/mmol (442 mg/g) for at least ≥2 annual measurements. 32 Renal failure: estimated glomerular estimation rate calculated by CKD epidemiology collaboration in adults <60 ml/min/1.73 m2. 33 Retinopathy: the presence of at least mild proliferative retinopathy >stage 2. 34 The patients followed in our center all received an annual slit lamp examination and fundus examination; proliferative retinopathy was defined by the presence of neovascularization in posterior pole or retinal periphery, and it was confirmed by fluorescein angiography. Leg ulcers: chronic and active ulcers of the ankle not otherwise explained. Patients were also screened for the occurrence of thrombo‐embolism events (deep venous, peripheral, or pulmonary thromboembolic disease).
Patients with macrovascular organ disease (PAH, stroke, and heart disease) were excluded because of a chronic blood transfusion or red cell exchange program. Postmenopausal women were excluded since previous studies reported abnormal changes in endothelial activation markers in these populations. 35 , 36 Patients with diabetes, 37 cancer or autoimmune disease or with hemochromatosis disease were also excluded. Pregnant and breastfeeding women were not included.
The study was conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki and has been approved by the local ethics committee (CPP Sud Mediterranée N° 12–037 and IDRCB Number 2012‐A00498‐35). All the patients provided written informed consent before enrolling in the trial.
2.2. Sample collection and laboratory analysis
Blood samples from each patient were collected twice at intervals of 2 weeks. Using samples taken at various times allows limitation of biases linked to intra‐individual variability of the biological parameters studied.
Total and fetal hemoglobin (HbF), platelets, and white blood cells (WBC) counts were evaluated. Biological markers of hemolysis (lactate dehydrogenase [LDH]) and of kidney dysfunction (creatinine) were also recorded. CEC, EPC, and E‐selectin analyses were performed on whole blood collected in 5‐ml vacutainer tubes containing K2EDTA. Patient's samples for EV analysis were collected into 5‐ml vacutainer tubes containing 0.129 mol/L sodium citrate (BD Diagnostics, Franklin Lakes, NJ).
In this study, we chose to investigate endothelial injury and repair biomarkers: CECs, EVs, and EPCs. VCAM‐1, ICAM‐1, and P‐selectin have not been analyzed because, unlike E‐selectin, these biomarkers are not specific to endothelial cells.
2.3. CEC and EPC analysis
CECs were counted according to a previously published, standardized protocol. 38 Briefly, CECs were isolated by immunomagnetic separation with beads (Dynabeads M‐450, Thermo Fisher, Carlsbad, CA) coated with CD146 (clone S‐endo1, Biocytex, Marseille, France) and enumerated using a fluorescence microscope (Eclipse TE2000‐S, Nikon, Tokyo, Japan) after acridine orange labeling. CECs were identified according to the following consensus criteria: stained rosette cells, size over 15 μm, bearing more than five beads. CEC normal values were defined in our laboratory from a cohort of 10 healthy donors and are below 20 CEC/ml.
Analysis of CD34+/KDR+ EPCs was performed with a three‐color flow cytometry protocol after direct peripheral blood mononuclear cell immunolabeling. The percentage of KDR+ cells among CD34+ cells was determined, and absolute values were calculated by multiplying the percentage of KDR+ cells by the absolute values of CD34+ cells. EPC normal values were defined in our laboratory from a cohort of 10 healthy volunteers matched for age and sex: 48 cells/ml (range: 17–202 cells/ml).
2.4. E‐selectin dosage
Plasma (EDTA) levels of E‐selectin were quantified by the enzyme‐linked immunosorbent assay (ELISA) method following the recommendations of the manufacturer (R&D Systems, Minneapolis, MN). The intra‐assay and inter‐assay CVs of the ELISA method used were 5.9% and 8.6%, respectively. The mean of the minimum detectable dose was 0.009 ng/ml.
2.5. EV enumeration and characterization by flow cytometry
Patient samples for EV analysis were collected and processed according to the current international guidelines. 39 Briefly, the samples were subjected to two successive centrifugations (2500 g for 15 min at room temperature), and platelet‐free plasma (PFP) was prepared. The PFP was homogenized before being aliquoted and stored at −80°C until use.
EVs were enumerated by high‐sensitivity flow cytometry following standardization, as previously described. 40 Briefly, 30 μl PFP were incubated with the appropriate amount of specific antibody and 10 μl of AnnV‐FITC (fluorescein, Beckman‐Coulter, Miami, FL). Each stained sample was analyzed on a NAVIOS 3‐lasers instrument (Beckman‐Coulter, Miami, FL), following a protocol standardized with Megamix‐Plus FSC beads (BioCytex, Marseille, France). Platelet‐(PEVs), erythrocyte‐(Ery‐EVs), leucocyte‐(LEVs), and endothelial‐(EEVs)derived EVs were defined as AnnV+/CD41+, AnnV+/CD235a+, AnnV+/CD11b+, or AnnV+/CD31+/CD41− events, respectively. The absolute EV counts (events per μl) were determined using ad hoc counting beads (CytoCount®, Dako, Copenhagen, Denmark). EV's normal values were defined in our laboratory from a cohort of 10 healthy volunteers.
3. STATISTICAL ANALYSIS
Continuous variables were reported as mean ± SD or as median and interquartile range if non‐normally distributed. Categorical data were expressed as percentages. According to the distribution of the variables, Student's t‐test or Wilcoxon signed‐rank test was used to compare the sample mean or median. The relationship between two categorical variables was evaluated using chi‐squared test or Fisher's exact test, as appropriate. As repeated measurements were made on the same statistical units (two samples for each patient), a univariate and multivariate mixed‐effect model, and a generalized estimating equation (GEE) were used. Variables significantly associated with the outcome and those that were marginally significant (p < .10) in univariate analysis were included in multivariate analysis. For all analyses, a two‐tailed test was used, and p < .05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics version 20 (IBM SPSS Inc., Chicago, IL).
4. RESULTS
4.1. Characteristics of the study population
Hundred SCD patients, over the age of 18, were followed in our center. Thirty‐five patients were excluded: 20 because of red blood cell transfusion or exchange therapy, four because of VOC during the trial, five for autoimmune disease, two for diabetes, and one woman for postmenopause. Fifteen patients refused to participate in this trial. Fifty SCD patients, at steady state, could be included in this study (35 females and 15 males). The median age was 28 years (range, 18–54 years). Twenty‐five patients were homozygous HbSS, eight have sickle hemoglobin C disease (Hb SC), and 17 have sickle β‐thalassemia (11 HbS β0‐thal and 6 HbS β+‐thal). Ten patients were treated with hydroxyurea (9 HbSS and 1 HbS β0‐thal) for at least 2 years. Thirteen patients have experienced thromboembolic complications.
Sixteen patients (32%) had SCD‐related retinal, renal, and/or cutaneous vasculopathy. Retinopathy and nephropathy were found in 9 out of 50 and 6 out of 50 patients, respectively. Three patients had leg ulcers. Retinopathy was found to be associated with other organ damage in 77% of cases. Three patients have a nephropathy–retinopathy combination, and one HbSC female patient, 47 years old, has three organ disorders (nephropathy, retinopathy, and leg ulcers). Patients' characteristics are reported in Table 1.
TABLE 1.
Clinical characteristics of SCD patients with and without vasculopathy
| Total SCD patients | SCD patients with no vasculopathy | SCD patients with vasculopathy | p‐value | |
|---|---|---|---|---|
| n (%) | 50 | 34 (68) | 16 (32) | ‐ |
| Age a (year) | 28 (22–39) | 27.5 (21–33) | 37.5 (26. 5–41) | .039 |
| Sex F/M | 35/15 | 24/10 | 10/6 | .514 |
| SCD genotype | .48 | |||
| SS patients n, (%) | 25 (50) | 18 (52.9) | 7 (43.8) | ‐ |
| Sβ patients n, (%) | 17 (34) | 12 (35) | 5 (31) | ‐ |
| SC patients n, (%) | 8 (16) | 4 (11.8) | 4 (25) | ‐ |
| Hydroxyurea n, (%) | 10 (20) | 7 (20.5) | 3 (18.75) | 1.0 |
| Thromboembolic complications n, (%) | 13 (26) | 9 (26) | 4 (25) | .746 |
Note: Bold values indicate the statistically significant with p‐value < 0.05 (p < .05).
Abbreviation: SCD, sickle cell disease.
Data are shown as medians and corresponding interquartile ranges.
SCD patients with vasculopathy were older than SCD patients without vasculopathy [37.5 (26.5–41) vs. 27.5 (21–33) years, p = .039]. No differences were found between groups with or without vasculopathy for sex ratio, sickle cell genotype or hydroxyurea treatment (Table 1). Levels of Hb, HbF, platelets, WBC, LDH, and creatinine were not statistically different between the two groups (Table 2).
TABLE 2.
Biological markers, circulating biomarkers of endothelium activity, and extracellular microvesicles in SCD patients with and without vasculopathy
| Total SCD patients | SCD patients with no vasculopathy | SCD patients with vasculopathy | p‐value | |
|---|---|---|---|---|
| Hb (g/dl) | 9,7 ± 1.4 | 9,47 ± 1.3 | 10,19 ± 1.6 | .437 |
| HbF (%) | 9.3 ± 5.8 | 10.17 ± 6.5 | 7.8 ± 3.8 | .364 |
| WBC (×109/L) | 8,91 ± 2.5 | 8,50 ± 2.4 | 9,85 ± 2.5 | .314 |
| Platelet count (109/L) | 356 ± 189 | 363 ± 193 | 342 ± 174 | .801 |
| LDH (IU/L) | 342 ± 119 | 325 ± 124 | 377 ± 104 | .684 |
| Creatinine (μmol/L) | 64,5 ± 15 | 64,1 ± 13.8 | 65,7 ± 17.9 | .175 |
| CEC (cel/μl) | 2.5 [0.5–7] | 2 [0.5–5.5] | 6 [1–10.5] | .390 |
| EPC (cel/μl) | 0.13 [0.07–0.24] | 0.165 [0.07–0.27] | 0.1 [0.02–0.15] | .230 |
| E‐selectin (ng/ml) | 41 [32–56] | 33 [25–48] | 49 [38–67] | .015 * |
| Ery‐EVs (events/μl) | 2815 [1007–4750] | 2969 [1018–4750] | 2767 [970–5589] | .887 |
| PEVs (events/μl) | 6532 [4693–11 183] | 7374 [5025–13 127] | 5768 [3431–8061] | .365 |
| EEV (events/μl) | 118 [57–156] | 122 [68–158] | 86 [56–167] | .116 |
| LEV (events/μl) | 40 [17–126] | 68 [22–242] | 25 [12–47] | .937 |
Note: Data expressed as median [25th–75th percentile] or as mean ± SD of the two samples mean of each patient. Bold values indicate the statistically significant with p‐value < 0.05 (p < .05).
Abbreviations: Ery‐EV, erythrocyte‐derived extracellular microvesicles; EEV, endothelial‐derived extracellular microvesicles; Hb, hemoglobin; HbF, fetal hemoglobin, LDH: lactate dehydrogenase; LEV, leucocytes‐derived extracellular microvesicles; PEV, platelet‐derived extracellular microvesicles.
Statistically significant p‐value (p < .05).
4.2. Plasma levels of circulating Ery‐EVs and PEVs are increased in SCD patients
Ery‐EV and PEV amounts were significantly increased in SCD patients compared to laboratory reference ranges defined in healthy volunteers 41 [2815 (1007–4750) vs. 240 (90–450) EVs/μl, p < .001 for Ery‐EVs and 6532 (4693–11 183) vs. 1180 (740–1670) EVs/μl, p < .001 for PEV]. In contrast, EVs that originated from leucocytes and endothelial cells were not significantly increased in SCD patients compared to in healthy volunteers 41 [40 (17–126) vs. 21 (0–55) EVs/μl; p = .13 for LEVs and 118 (57–156) vs. 71 (50–96) EVs/μl; p = .14 for EEVs] (Tables S1–S3).
No significant differences between vasculopathy and not vasculopathy SCD patients regarding Ery‐EVs, PEVs, LEVs, and EEVs levels were observed (Table 2).
4.3. Circulating cellular biomarkers of endothelial activation and injury
In univariate analysis, E‐selectin level was significantly higher in SCD vasculopathy patients than in non‐vasculopathy patients [49 (38–67) vs. 33 (25–48) ng/ml, p = .015] (Table 2). This increased level in patients with vasculopathy was confirmed after the adjustment for age, sex, SCD genotype, and hydroxyurea treatment, Hb level, HbF proportion, platelet and WBC count, LDH, and creatinine levels (p = .02).
CEC and PEC counts were detected at very low levels in our SCD patients [2.5 (0.5–7) and 0.13 (0.07–0.24) cell/μl, respectively] whether in those with or without vasculopathy (Table 2).
4.4. E‐selectin levels are significantly higher in SCD patients with retinopathy (Table 3)
TABLE 3.
Clinical characteristics, circulating biomarkers of endothelium activity and extracellular microvesicles in SCD patients with and without retinopathy
| Total SCD patients | SCD patients with No retinopathy | SCD patients with retinopathy | p‐value | |
|---|---|---|---|---|
| n (%) | 50 | 41(82) | 9 (18) | ‐ |
| Age (year) | 28 (22–39) | 28 (21.5–37) | 39 (27–44.5) | .034 * |
| Sex F/M | 35/15 | 27/14 | 8/1 | .25 |
| SCD genotype | .26 | |||
| SS patients n, (%) | 25 (50) | 19 (46.3) | 6 (66.6) | ‐ |
| Sβ patients n, (%) | 17 (34) | 16 (39) | 1 (11.11) | ‐ |
| SC patients n, (%) | 8 (16) | 6 (14.6) | 2 (22.2) | ‐ |
| Hydroxyurea n, (%) | 10 (20) | 8 (19.5) | 2 (22.2) | .99 |
| CEC (cel/μl) | 2.5 [0.5–7] | 2 [0.5–6.5] | 2.5 [0.75–23.8] | .35 |
| EPC (cel/μl) | 0.13 [0.07–0.24] | 0.13 [0.07–0.27] | 0.09 [0.03–0.13] | .12 |
| E‐selectin (ng/ml) | 41 [32–56] | 39.1 [28.9–51] | 58.9 [50.7–86.8] | <.001 * |
| Ery‐EVs (events/μl) | 2815 [1007–4750] | 2820 [1011–5559] | 2810 [989–4064] | .31 |
| PEVs (events/μl) | 6532 [4693–11 183] | 7000 [4792–13 044] | 5529 [3915–7508] | .22 |
| EEV (events/μl) | 118 [57–156] | 118 [52–165] | 105 [70–157] | .66 |
| LEV (events/μl) | 40 [17–126] | 40 [15–147] | 27 [17–157] | .82 |
Note: Data expressed as median [25th–75th percentile] or as mean ± SD of the two samples mean of each patient. Bold values indicate the statistically significant with p‐value < 0.05 (p < .05).
Abbreviations: Ery‐EV, erythrocyte‐derived extracellular microvesicles; EEV, endothelial‐derived extracellular microvesicles; Hb, hemoglobin; HbF, fetal hemoglobin, LDH: lactate dehydrogenase; LEV, leucocytes‐derived extracellular microvesicles; PEV, platelet‐derived extracellular microvesicles.
Statistically significant p‐value (p < .05).
We analyzed the relationship between E‐selectin levels and SCD organ damages (retinopathy, nephropathy, and leg ulcers). In univariate analysis, increased E‐selectin levels were associated with retinopathy (Figure 1) but not with nephropathy or leg ulcers. Multivariate analysis using GEE and integrating age, sex, SCD genotype, and hydroxyurea treatment indicated that among SCD organ damages, only retinopathy could be identified as independently associated with E‐selectin counts (B‐coefficients of .09 and a p‐value of .0002).
FIGURE 1.
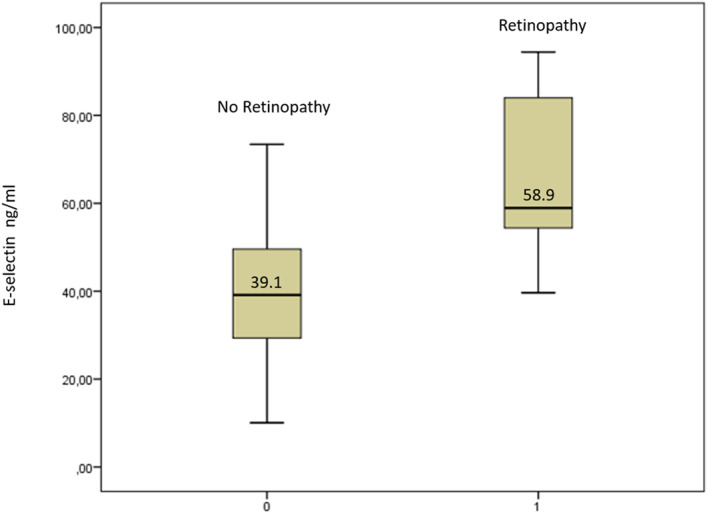
E‐selectin levels in sickle cell disease patients with and without retinopathy. Data shown are the average of the two samples mean of each patient.
Since patients with vasculopathy were older, and to exclude the risk that the elevation of the E‐selectin levels was the result of the vasculopathy and not the cause, the effect of age on E‐selectin levels was analyzed, and no interaction was found (Figure 2).
FIGURE 2.
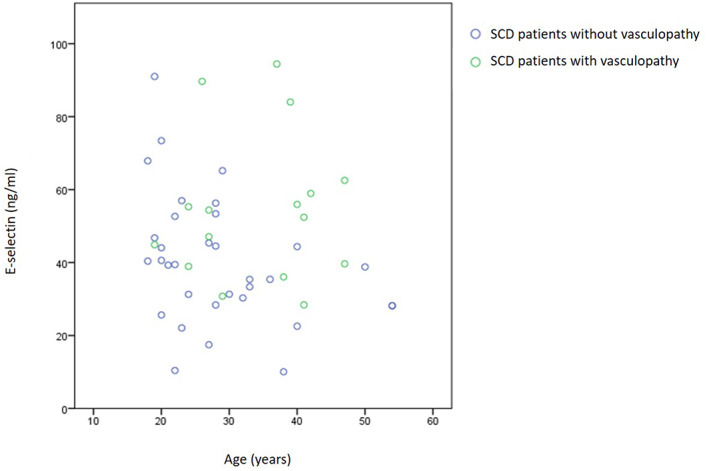
E‐selectin levels in sickle cell disease (SCD) patients with and without retinopathy according to patient's age. Data shown are the average of the two samples mean of each patient.
5. DISCUSSION
In this study, we investigated at steady state the relationship between plasma endothelial activation markers (CEC, E‐selectin, EPC, and circulating EVs) and SCD‐related microvascular complications in three different territories, retinopathy, nephropathy, and leg ulcers. It is important to note that patients with macrovascular organ disease (pulmonary hypertension, stroke, and heart disease) were excluded. Interestingly, our population with about 50% HbSS, exhibited a moderate hemolytic anemia with a median Hb level of 9.7 gr/dl, a median Hb F level of 9.3%, a mild median LDH level of 342 IU/L, and no inflammatory thrombocytosis or hyperleukocytosis (Table 2). Chronic microvascular complications were found in 32% of our SCD adult patients.
Patients with microvascular complications are significantly older in our cohort, but there is no other clinical or standard biological characteristic in this group. Among the markers of endothelial activation studied, only higher plasma levels of E‐selectin were found in SCD patients with microvasculopathy. However, despite our small sample size, which decreased the statistical power of this study, and even after the multiplicity correction test, we conserved a trend toward higher plasma levels of E‐selectin in SCD patients with microvasculopathy (p = .19). In subgroup analysis, an increased E‐selectin level was associated with retinopathy but not with nephropathy nor leg ulcers. In our study, only three patients with leg ulcers and six with nephropathy were included, thus we cannot exclude that the lack of association is due to the small sample size and low statistical power of this study. It is interesting to note that all patients, at steady state, did not display a high count of CEC and EPC, which are markers of endothelial injury and repair. High levels of circulating Ery‐EVs and PEVs were found in our SCD patients. In contrast, amounts of LEVs and EEVs, respectively, were most often within the normal range. Significant variability in EV levels was found between the two samples, even in steady state. We did not show any significant differences in EVs levels between vasculopathy and not vasculopathy SCD patients.
Studies of the individual function of single selectins in a mouse model of SCD have revealed a key role for E‐selectin, but not P‐selectin, in sending activating signals leading to the upregulation of the β2 integrin, Mac‐1, specifically at the leading edge of crawling neutrophils in inflamed venules. 27 In this study, we chose to evaluate E‐selectin, an appropriate marker of endothelial activation. This cytokine is only triggered by endothelial cells; however, P‐selectin is both expressed by platelets and endothelial cells. Soluble circulating form of E‐selectin resulted from a proteolytic cleavage of the membrane protein or from the synthesis of a protein without a transmembrane domain. Significant elevation in plasma levels of soluble E‐selectin has been previously observed in sickle cell patients compared with controls. 42 In addition, the study conducted in 2005 by Kato et al. 43 showed increased E‐selectin levels in SCD patients with PAH. In this study, markers of endothelial activation, including E‐selectin, were associated with risk for early mortality in patients with SCD. 44 Although various systemic complications of SCD are known to be more frequent in patients with the Hb SS genotype, vision impairment secondary to proliferative sickle cell retinopathy is more common in patients with the Hb SC genotype. In our study, only eight Hb SC patients were included, which was insufficient to show the association between sickle cell genotypes and the occurrence of retinopathy. It's important to note that the nine SCD patients with related retinal vasculopathy had proliferative retinopathy.
In our study, increased E‐selectin levels were associated with retinopathy but not with nephropathy or leg ulcers in the subgroup of patients with microvasculopathy. This suggests that the various organ damages described in sickle cell patients implicate different pathophysiological mechanisms. However, only three patients with leg ulcers and six with nephropathy were included, thus we cannot exclude that the lack of association is due to the small sample size and then low statistical power of this study. This finding needs to be confirmed by larger studies. It is worthwhile to notice that unlike SCD retinopathy, SCD skin ulcers and nephropathy are now known to be associated with the hemolysis‐related macrovascular involvement of pulmonary hypertension or stroke in HbSS patients. 15 , 44 , 45
Kunz et al. reported that SCD retinas show prominent immunoreactivity against ICAM‐1 (intercellular cell adhesion molecule‐1), VCAM‐1 (vascular cell adhesion molecule‐1), and P‐selectin, compared to retinas from healthy controls. E‐selectin was not measured. 46 In contrast to our results, Cruz et al., who evaluated E‐selectin levels in 37 SCD patients with retinopathy and 34 without retinopathy, did not find any correlation between E‐selectin levels and retinopathy. However, only 14/36 SCD patients had proliferative retinopathy in their study. 47
High levels of soluble endothelial adhesion molecules, especially E‐selectin, were reported in diabetic patients with proliferative retinopathy. 37 , 48 , 49 Sickle cell and diabetic retinopathies appear to share common pathophysiological mechanisms. 50 , 51 Previous studies on a pan‐selectin inhibitor with strong activity against E‐selectin showed significantly reduced opioid requirements during VOC. 52 , 53 The elevation of E‐selectin levels in sickle cell retinopathy and its possible pathophysiological implications need to be confirmed by larger studies and might prompt us to evaluate this new molecule in the treatment of SCD retinopathy.
In our study, CEC and PEC counts were detected at very low levels. In contrast, previous preliminary data reported significantly higher CEC counts in SCD patients at steady state compared to controls. 54 CEC counts were higher in SCD patients with PAH; however, no significant differences were observed related to retinopathy, nephropathy, leg ulcers, and the number of VOC or ACS. 54 The authors suggest a possible relation between CEC counts and SCD macrovasculopathy. Our results confirm the absence of a significant correlation between CEC and EPC counts in the presence of microvasculopathy. In fact, at steady state, patients with proliferative retinopathy seem to have an endothelial activation reflected in the release of high levels of E‐selectin but no evidence of vascular endothelial death or repair. Our data suggest that the endothelial activation is not necessarily related to the endothelial damage and detachment in SCD.
High plasma concentrations of Ery‐EVs, PEVs, EEVs, and LEVs have been identified in sickle cell patients. 55 , 56 The roles of circulating EVs in the pathogenic mechanisms of SCD are poorly known, 57 , 58 some of these EVs express both anionic phospholipids and tissue factors and may exert procoagulant effects. 59 , 60 A recent study reported that EVs derived from RBCs and isolated from SCD patients' blood may increase ICAM‐1 endothelial expression and induce a pro‐adherent phenotype. 61 However, results are controversial as to whether EVs numbers and/or cellular origin are different in acute pain episodes compared to steady state. 62 Tantawy et al. showed that both PEV and Ery‐EV levels were positively correlated with VOC and pulmonary hypertension, suggesting that these EVs may be considered as a potential biological marker for macrovascular dysfunction and disease severity in SCD. 56 As previously reported, 59 , 60 Ery‐EV and PEV levels, in our study, remained high in steady state, but we do not show a correlation between Ery‐EVs and PEVs with the presence of microvasculopathy. Interestingly, far from any acute event, LEVs and EEVs remain low even in patients with high E‐selectin levels.
Our study provides evidence that circulating E‐selectin is associated with sickle cell retinopathy. These interesting results need further studies in large cohorts of SCD patients. Moreover, it would be interesting to investigate in SCD children and young adults, whether E‐selectin could be a biomarker for the development of retinopathy and if E‐selectin is able to predict the progression of retinopathy once it is established.
AUTHOR CONTRIBUTIONS
Emmanuelle Bernit, Romaric Lacroix, and Elodie Masson designed the research. Imane Agouti and Elodie Masson performed the research. Imane Agouti, Elodie Masson, Romaric Lacroix, and Emmanuelle Bernit analyzed and interpreted the data. Imane Agouti and Emmanuelle Bernit wrote the manuscript. Elodie Masson, Laurent Arnaud, Evelyne Abdili, and Patricia Berenger performed the laboratory work for this study. Anderson Loundou and Imane Agouti performed the statistical analysis. Elodie Masson, Estelle Jean, Julie Séguier, Virginie Lavoipierre, and Emmanuelle Bernit recruited patients. Françoise Dignat‐George supervised the laboratory work. Each author contributed important intellectual content during manuscript drafting. All authors read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
TABLE S1: Extracellular microvesicles in sickle cell disease patients
TABLE S2: Extracellular microvesicles in sickle cell disease patients without vasculopathy
TABLE S3: Extracellular microvesicles in sickle cell disease patients with vasculopathy
ACKNOWLEDGMENTS
This research was supported by AORC 2012‐13 (Appel d'Offres de Recherche Clinique). The authors would like to thank Dr. Marc Romana, Laboratoire d'Excellence du Globule Rouge (Labex GR‐Ex), PRES Sorbonne, Paris, France, for his critical review of the manuscript.
Agouti I, Masson E, Loundou A, et al. Plasma levels of E‐selectin are associated with retinopathy in sickle cell disease. Eur J Haematol. 2023;110(3):271‐279. doi: 10.1111/ejh.13902
Funding information AORC 2012‐13 (Appel d'Offres de Recherche Clinique).
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary data. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Saunthararajah Y, Vichinsky PE, Embury HS. Sickle cell disease. In: Hoffman R, Benz JE, Shattil JS Jr, et al., eds. Hematology: Basic Principles and Practice. Elsevier; 2005:605‐644. [Google Scholar]
- 2. Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 3. Van Tuijn CF, van Beers EJ, Schnog JJ, Biemond BJ. Pain rate and social circumstances rather than cumulative organ damage determine the quality of life in adults with sickle cell disease. Am J Hematol. 2010;85(7):532‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease––life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639‐1644. [DOI] [PubMed] [Google Scholar]
- 5. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561‐1573. [DOI] [PubMed] [Google Scholar]
- 6. Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model‐based map and population estimates. Lancet. 2013;381(9861):142‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. [DOI] [PubMed] [Google Scholar]
- 8. Morris CR. Vascular risk assessment in patients with sickle cell disease. Haematologica. 2011;96(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008;177‐185. [DOI] [PubMed] [Google Scholar]
- 10. Rees DC, Williams TN, Gladwin MT. Sickle‐cell disease. Lancet. 2010;376(9757):2018‐2031. [DOI] [PubMed] [Google Scholar]
- 11. Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle‐cell anemia—a possible determinant of disease severity. N Engl J Med. 1980;302(18):992‐995. [DOI] [PubMed] [Google Scholar]
- 12. Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K, Cheung A. Platelet activation and platelet erythrocyte aggregates in patients with sickle cell anemia. J Lab Clin Med. 1997;129(5):507‐516. [DOI] [PubMed] [Google Scholar]
- 13. Zarbock A, Polanowska‐Grabowska RK, Ley K. Platelet‐neutrophil‐interactions: linking hemostasis and inflammation. Blood Rev. 2007;21(2):99‐111. [DOI] [PubMed] [Google Scholar]
- 14. Frenette PS. Sickle cell vasoocclusion: heterotypic, multicellular aggregations driven by leukocyte adhesion. Microcirculation. 2004;11(2):167‐177. [PubMed] [Google Scholar]
- 15. Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84(9):618‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gimbrone MA Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;99(8):1809‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801‐809. [DOI] [PubMed] [Google Scholar]
- 18. Erdbruegger U, Dhaygude A, Haubitz M, Woywodt A. Circulating endothelial cells: markers and mediators of vascular damage. Curr Stem Cell Res Ther. 2010;5(4):294‐302. [DOI] [PubMed] [Google Scholar]
- 19. Vítková V, Živný J, Janota J. Endothelial cell‐derived microvesicles: potential mediators and biomarkers of pathologic processes. Biomark Med. 2018;12(2):161‐175. [DOI] [PubMed] [Google Scholar]
- 20. Sabatier F, Camoin‐Jau L, Anfosso F, Sampol J, Dignat‐George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13(3):454‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manwani D, Frenette PS. Vaso‐occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood. 2013;122(24):3892‐3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Semin Thromb Hemost. 2011;37(3):226‐236. [DOI] [PubMed] [Google Scholar]
- 23. Vinchi F, De Franceschi L, Ghigo A, et al. Hemopexin therapy improves cardiovascular function by preventing heme‐induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 24. Kalish BT, Matte A, Andolfo I, et al. Dietary omega‐3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica. 2015;100(7):870‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Telen MJ. Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease. Blood. 2016;127(7):810‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thrombo‐inflammatory injury. Nat Med. 2009;15(4):384‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris CR, Morris SM Jr, Hagar W, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 29. Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107(2):271‐278. [DOI] [PubMed] [Google Scholar]
- 30. Sullivan KJ, Kissoon N, Gauger C, Sullivan KJ. Nitric oxide metabolism and the acute chest syndrome of sickle cell anemia. Pediatr Crit Care Med. 2008;9(2):159‐168. [DOI] [PubMed] [Google Scholar]
- 31. Nader E, Romana M, Connes P. The red blood cell‐inflammation vicious circle in sickle cell disease. Front Immunol. 2020;11:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niss O, Lane A, Asnani MR, et al. Progression of albuminuria in patients with sickle cell anemia: a multicenter, longitudinal study. Blood Adv. 2020;4(7):1501‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharpe CC, Thein SL. How I treat renal complications in sickle cell disease. Blood. 2014;123(24):3720‐3726. [DOI] [PubMed] [Google Scholar]
- 34. Goldberg MF. Classification and pathogenesis of proliferative sickle retinopathy. Am J Ophthalmol. 1971;71(3):649‐665. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy G, McLaren M, Belch JJ, Seed M. Elevated levels of sE‐selectin in post‐menopausal females are decreased by hormone replacement therapy to levels observed in pre‐menopausal females. Thromb Haemost. 1999;82(5):1433‐1436. [PubMed] [Google Scholar]
- 36. Jayachandran M, Litwiller RD, Lahr BD, et al. Alterations in platelet function and cell‐derived microvesicles in recently menopausal women: relationship to metabolic syndrome and atherogenic risk. J Cardiovasc Transl Res. 2011;4(6):811‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soedamah‐Muthu SS, Chaturvedi N, Schalkwijk CG, Stehouwer CD, Ebeling P, Fuller JH. EURODIAB prospective complications study group. Soluble vascular cell adhesion molecule‐1 and soluble E‐selectin are associated with micro‐ and macrovascular complications in type 1 diabetic patients. J Diabetes Complications. 2006;20(3):188‐195. [DOI] [PubMed] [Google Scholar]
- 38. Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of Vascular Disease Thromb Haemost. 2005;93(2):228‐235. [DOI] [PubMed] [Google Scholar]
- 39. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lacroix R, Judicone C, Poncelet P, et al. Impact of pre‐analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10(3):437‐446. [DOI] [PubMed] [Google Scholar]
- 41. Agouti I, Cointe S, Robert S, et al. Platelet and not erythrocyte microparticles are procoagulant in transfused thalassaemia major patients. Br J Haematol. 2015;171(4):615‐624. [DOI] [PubMed] [Google Scholar]
- 42. Mohan JS, Lip GY, Wright J, Bareford D, Blann AD. Plasma levels of tissue factor and soluble E‐selectin in sickle cell disease: relationship to genotype and to inflammation. Blood Coagul Fibrinolysis. 2005;16(3):209‐214. [DOI] [PubMed] [Google Scholar]
- 43. Kato GJ, Martyr S, Blackwelder WC, et al. Levels of soluble endothelium‐derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130(6):943‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254‐2265. [DOI] [PubMed] [Google Scholar]
- 45. Hariri E, Mansour A, El Alam A, Daaboul Y, Korjian S, Aoun Bahous S. Sickle cell nephropathy: an update on pathophysiology, diagnosis, and treatment. Int Urol Nephrol. 2018;50(6):1075‐1083. [DOI] [PubMed] [Google Scholar]
- 46. Kunz Mathews M, McLeod DS, Merges C, Cao J, Lutty GA. Neutrophils and leucocyte adhesion molecules in sickle cell retinopathy. Br J Ophthalmol. 2002;86(6):684‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cruz PR, Lira RP, Pereira Filho SA, et al. Increased circulating PEDF and low sICAM‐1 are associated with sickle cell retinopathy. Blood Cells Mol Dis. 2015;54(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 48. Nowak M, Wielkoszyński T, Marek B, et al. Blood serum levels of vascular cell adhesion molecule (sVCAM‐1), intercellular adhesion molecule (sICAM‐1) and endothelial leucocyte adhesion molecule‐1 (ELAM‐1) in diabetic retinopathy. Clin Exp Med. 2008;8(3):159‐164. [DOI] [PubMed] [Google Scholar]
- 49. Kasza M, Meleg J, Vardai J, et al. Plasma E‐selectin levels can play a role in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 50. Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86(4):363‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124‐136. [DOI] [PubMed] [Google Scholar]
- 52. Telen MJ, Wun T, McCavit TL, et al. Randomized phase 2 study of GMI‐1070 in SCD: reduction in time to resolution of vaso‐occlusive events and decreased opioid use. Blood. 2015;125(17):2656‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Telen MJ, Malik P, Vercellotti GM. Therapeutic strategies for sickle cell disease: towards a multi‐agent approach. Nat Rev Drug Discov. 2019;18(2):139‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strijbos MH, Landburg PP, Nur E, et al. Circulating endothelial cells: a potential parameter of organ damage in sickle cell anemia? Blood Cells Mol Dis. 2009;43(1):63‐67. [DOI] [PubMed] [Google Scholar]
- 55. Hebbel RP, Key NS. Microparticles in sickle cell anaemia: promise and pitfalls. Br J Haematol. 2016;174(1):16‐29. [DOI] [PubMed] [Google Scholar]
- 56. Tantawy AA, Adly AA, Ismail EA, Habeeb NM, Farouk A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: relation to cardiovascular complications. Platelets. 2013;24(8):605‐614. [DOI] [PubMed] [Google Scholar]
- 57. Nebor D, Bowers A, Connes P, et al. Plasma concentration of platelet‐derived microparticles is related to painful vaso‐occlusive phenotype severity in sickle cell anemia. PLoS One. 2014;9(1):e87243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor‐positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678‐2683. [DOI] [PubMed] [Google Scholar]
- 60. Van Beers EJ, Schaap MC, Berckmans RJ, et al. Circulating erythrocyte‐derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94(11):1513‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garnier Y, Ferdinand S, Garnier M, et al. Plasma microparticles of sickle patients during crisis or taking hydroxyurea modify endothelium inflammatory properties. Blood. 2020;136(2):247‐256. [DOI] [PubMed] [Google Scholar]
- 62. Kasar M, Boğa C, Yeral M, Asma S, Kozanoglu I, Ozdogu H. Clinical significance of circulating blood and endothelial cell microparticles in sickle‐cell disease. J Thromb Thrombolysis. 2014;38(2):167‐175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1: Extracellular microvesicles in sickle cell disease patients
TABLE S2: Extracellular microvesicles in sickle cell disease patients without vasculopathy
TABLE S3: Extracellular microvesicles in sickle cell disease patients with vasculopathy
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary data. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.


