Abstract
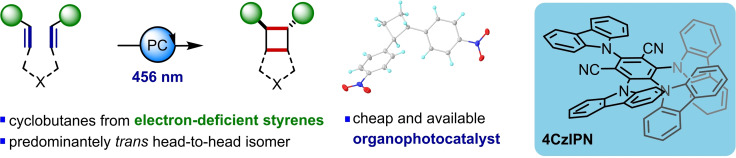
A visible‐light organophotocatalytic [2+2] cycloaddition of electron‐deficient styrenes is described. Photocatalytic [2+2] cycloadditions are typically performed with electron‐rich styrene derivatives or α,β‐unsaturated carbonyl compounds, and with transition‐metal‐based catalysts. We have discovered that an organic cyanoarene photocatalyst is able to deliver high‐value cyclobutane products bearing electron‐deficient aryl substituents in good yields. A range of electron‐deficient substituents are tolerated, and both homodimerisations and intramolecular [2+2] cycloadditions to fused bicyclic systems are available by using this methodology.
Keywords: cycloaddition, cyclobutane, electron-deficient styrenes, energy transfer, organophotocatalysis
The [2+2] cycloaddition of alkenes is a powerful method of preparing cyclobutanes, but the use of electron‐deficient styrenes has been largely neglected so far. By using the organophotocatalyst 4CzIPN, a visible‐light‐mediated procedure to cyclobutane motifs bearing electron‐deficient aryl substituents has been developed. Homodimerisations as well as intramolecular reactions to fused bicyclic motifs can be performed in good to excellent yields.
Introduction
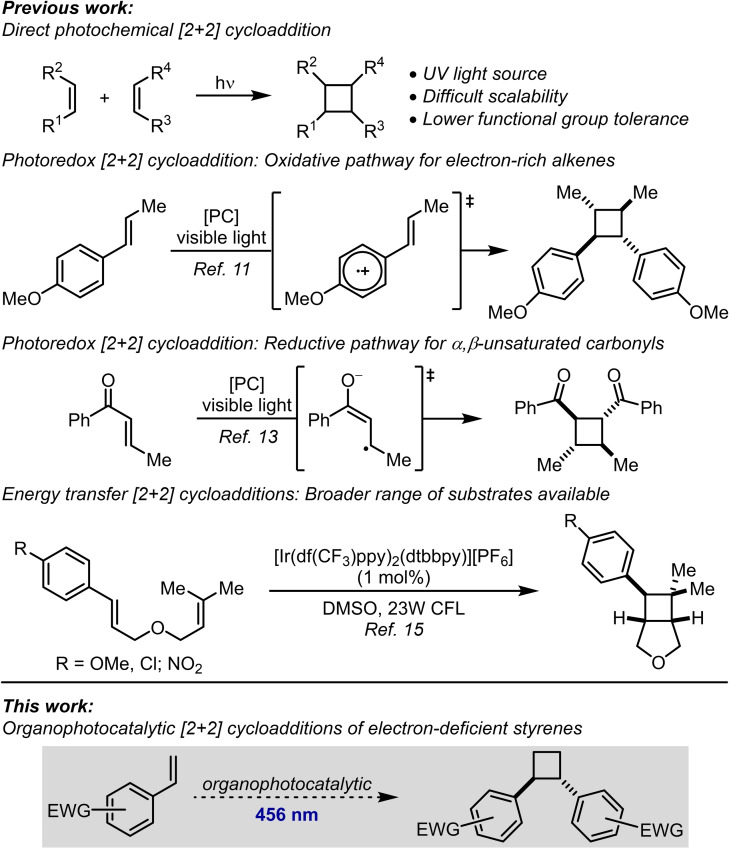
Cyclobutanes are strained four‐membered rings that are frequently encountered in natural products,[ 1 , 2 ] as synthetic intermediates [3] and within pharmaceutical and agrochemical compounds. [4] They are highly valuable building blocks in the pharmaceutical industry, where interest in nonplanar compound cores has recently increased.[ 4 , 5 ] Their high strain energy (ca. 26–27 kcal mol−1) [6] makes them highly reactive but also results in a high energetic cost associated with their synthesis. The direct photochemical excitation of alkenes and their subsequent participation in [2+2] cycloaddition reactions was first reported by Liebermann in 1877 [7] and is conceptually the simplest route to cyclobutanes (Scheme 1, top). [8] However, direct alkene excitation usually requires UV light, and the drawbacks associated with this include limited scalability and poor functional group tolerance. This has led to the development of alternative photochemical protocols. Visible‐light‐mediated energy transfer [9] and photoredox [10] catalysis methods have become extremely useful alternatives which avoid some of these problems. However, photoredox‐catalysed [2+2] cycloadditions operating under oxidative pathways are typically limited to electron‐rich substrates such as anisole derivatives, proceeding via a radical cation,[ 11 , 12 ] and reductive pathways are typically limited to α,β‐unsaturated carbonyl compounds, proceeding via the enolate‐based radical anion. [13] A more general approach has been found with visible light energy transfer catalysis which typically tolerates both styrene and α,β‐unsaturated carbonyl compounds, among others. [14] An early example by Lu and Yoon demonstrated the utility of this approach in the intramolecular [2+2] cycloaddition of various styrenes of different electron character and an electronically unbiased alkene. [15] Despite these developments, an approach aimed at the intermolecular cycloaddition of simple electron‐deficient styrene derivatives has, to the best of our knowledge, not been described. [16] The use of organic dyes rather than transition‐metal complexes as photocatalysts is also underreported for these reactions. The possibility to use organic dyes provides benefits in terms of cost, toxicity, and sustainability for these useful processes.[ 17 , 18 ] In this report, we disclose a visible light‐mediated organophotocatalytic [2+2] cycloaddition reaction that allows the cycloaddition of electron‐deficient styrenes (Scheme 1, bottom).
Scheme 1.
Photochemical [2+2] cycloadditions of alkenes; [PC]=photocatalyst.
Results and Discussion
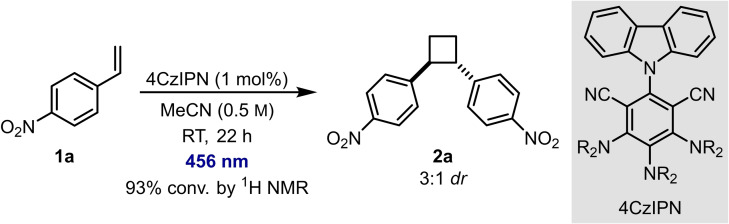
In the course of another project, we observed the [2+2] cycloaddition of para‐nitrostyrene (1 a) using the cyanoarene organophotocatalyst 4CzIPN. 93 % conversion of 1 a was estimated by 1H NMR, and cyclobutane 2 a was formed in a 3 : 1 trans:cis ratio (Scheme 2). Recognising the unusual reactivity of the electron‐deficient substrate with the organophotocatalyst, we decided to explore how general these reaction conditions were. We now report our findings.
Scheme 2.
Preliminary result. The diastereoselectivity was determined by 1H NMR spectroscopy of the crude reaction mixture; NR2=carbazole.
Initial investigations indicated that simple and commercially available styrene (1 b) could also undergo 4CzIPN‐catalysed [2+2] cycloaddition to cyclobutane 2 b. Full details of the optimisation of this reaction, including catalysts and solvents screened, are provided in the Supporting Information. Key control experiments are highlighted here. The previously used transition‐metal photocatalyst [Ir{dF(CF3)ppy}2(dtbbpy)]PF6 was less effective under our reaction conditions (entry 2). [19] DMF, previously used for the Iridium‐catalysed [2+2] cycloadditions, led only to very low levels of product (entry 3). [16a] Using freshly opened HPLC grade THF led to a small decrease in yield compared to using rigorously dry solvent, but was nonetheless still effective (entry 4). Degassing the solvent to remove oxygen was more critical; performing the reaction under air without freeze–pump–thaw degassing led to a much‐reduced yield of 23 % (entry 5). Sparging the solvent with a stream of argon restored some of the reactivity but was still not as effective as freeze‐pump‐thaw (entry 6). Both photocatalyst (entry 7) and irradiation (entry 8) were critical for the reaction. Scaling up the reaction to 1.00 mmol required a longer reaction time and a more concentrated reaction mixture, but homodimer 2 b could still be isolated in 56 % yield (entry 9).
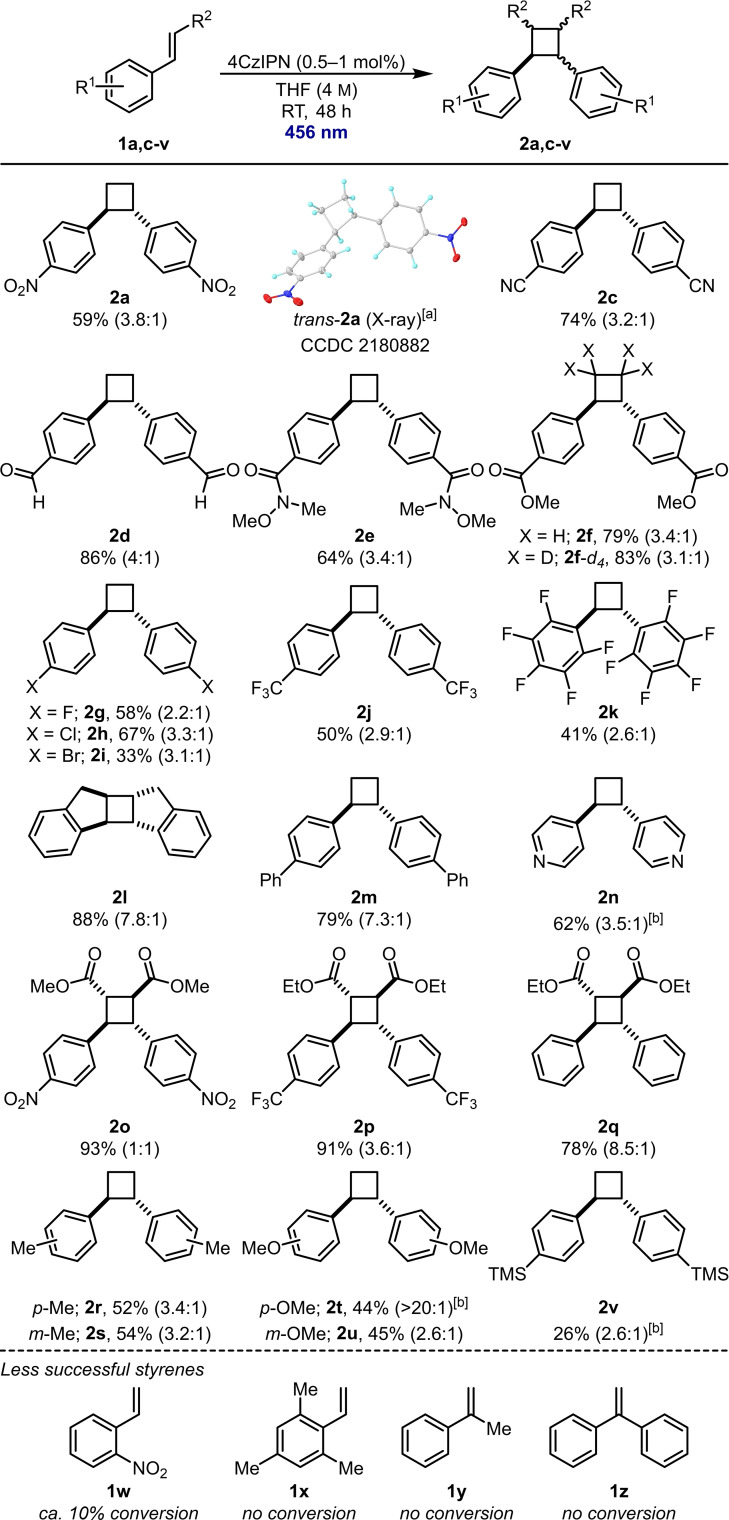
With optimised conditions in hand, we began to explore the scope of the reaction, with a focus on more electron‐deficient styrenes (Scheme 3). All reactions were performed on 1.00 mmol scale. Typical electron‐withdrawing groups such as nitro (in 2 a) and nitrile (in 2 c) were well tolerated and carbonyl‐based functionality including aldehydes (in 2 d), Weinreb amides (in 2 e), and esters (in 2 f) could also be incorporated. Using deuterated styrene [D2]‐1 f, tetradeuterated cyclobutane [D4]‐2 f could also be prepared in 83 % yield. A variety of halogen‐based substituents were also investigated. Fluoride (in 2 g) and chloride (in 2 h) substituted cyclobutanes could be produced in good yields. Bromide 2 i was prepared in slightly lower yield with some decomposition observed in the 1H NMR of the crude reaction mixture. para‐Trifluoromethylstyrene (1 j) and pentafluorostyrene (1 k) were less reactive, but the corresponding cyclobutanes could still be isolated in useful yields. Indene (1 l) performed well under the reaction conditions and afforded pentacycle 2 l in 88 % yield. Biphenyl‐substituted cyclobutane 2 m and pyridine‐substituted cyclobutane 2 n were also isolated in 79 % and 62 % yield respectively. Cinnamates have previously been shown to be excellent [2+2]‐cycloaddition precursors and proved to be so under our conditions too. [16] Cinnamate esters bearing electron‐deficient nitro and trifluoromethyl groups could be converted to cyclobutanes 2 o and 2 p in 93 and 91 % yield, respectively, and cyclobutane 2 q, from unsubstituted cinnamate ester 1 q, could also be prepared in 78 % yield. Groups with more electron‐donating character were also tolerated by our procedure, with methyl substituted cyclobutanes 2 r and 2 s isolated in 52 and 54 % yield, respectively. para‐Methoxyphenyl substituted cyclobutane 2 t was isolated in 44 % yield and meta‐methoxyphenyl‐substituted cyclobutane 2 u in 45 % yield. Finally, silyl‐substituted cyclobutane 2 v was obtained in 26 % yield. Some styrenes were less successful, including those with ortho substitution such as ortho‐nitrostyrene (1 w) and mesitylene 1 x. α‐Substituted styrenes 1 y and 1 z were also unreactive. Additionally, heterodimerisation could not be achieved with useful selectivity (see the Supporting Information for further details).
Scheme 3.
Scope of photocatalytic [2+2] cycloaddition. Reactions were performed on a 1.00 mmol scale. The diastereoselectivity was determined by 1H NMR spectroscopy of the crude reaction mixture. [a] Ellipsoids drawn at 50 % probability level. [b] 144 h reaction time.
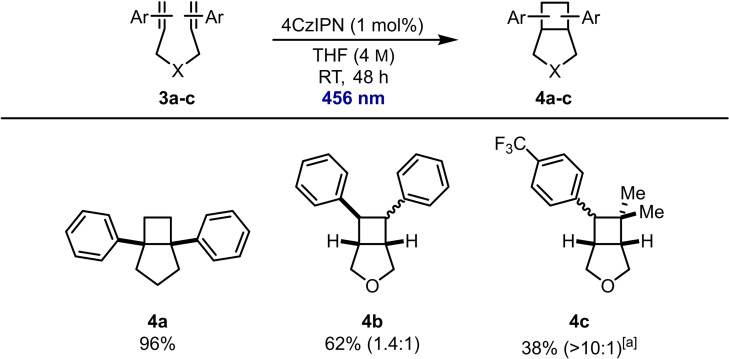
We were then interested to see whether these conditions could be applied to intramolecular cycloaddition reactions to provide valuable fused bicyclo[3.2.0]heptane frameworks (Scheme 4). [20] The necessary hepta‐1,6‐dienes 3 were readily prepared (see the Supporting Information) and subjection of α‐substituted styrene 3 a to the reaction conditions successfully afforded fused bicycle 4 a in 96 % yield. Interestingly, α‐substituted styrene 1 y had failed to react in an intermolecular reaction (Scheme 3). β‐Substituted styrene 3 b could also be converted to fused bicycle 4 b in 63 % yield. Finally, trifluoromethyl‐substituted bicycle 4 c was obtained in 38 % isolated yield.
Scheme 4.
Scope of intramolecular photocatalytic [2+2] cycloaddition to fused bicyclic compounds. The diastereoselectivity was determined by 1H NMR spectroscopy of the crude reaction mixture. [a] 90 h reaction time.
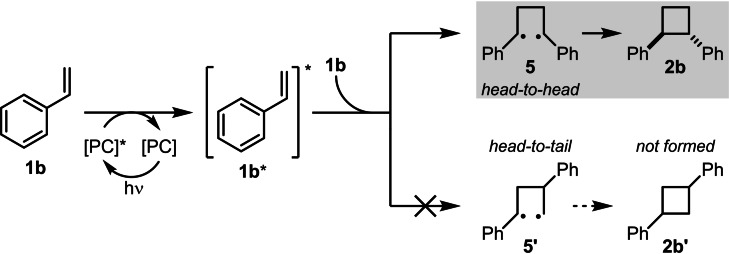
Mechanistically, the reaction is likely to proceed through an energy‐transfer pathway (Scheme 5). [9] 4CzIPN (E(PC*/PC⋅−)=+1.43 V vs. SCE; E(PC⋅+/PC*)=−1.18 V vs. SCE) [21] is unlikely to be able to oxidise the styrenes (styrene (1 b): E(1 b +/1 b)=+1.97 V vs. SCE [22] ; para‐nitrostyrene (1 a): E(1 a +/1 a)=+1.68 V vs. SCE [15] ), or reduce them (styrene (1 b) has a reduction potential of E(1 b/1 b− )=−2.65 V vs. SCE) [23] but has a high enough excited‐state energy (2.67 eV) [21] to allow energy transfer with a styrene (excited‐state energy∼2.6 eV). [24] Experimental support for this mechanism was obtained by performing the cycloaddition of styrene (1 b) in the presence of the triplet quencher isoprene. A reduced yield of 23 % was determined by 1H NMR, compared to the yield of 83 % obtained in its absence (Table 1, entry 1). For further details, see the Supporting Information. The mechanism begins by excitation of the photocatalyst at 456 nm. Energy transfer to styrene 1 b gives excited state 1 b* from which dimerisation likely occurs stepwise. First, diradical 5 is formed and free rotation about the C−C single bonds then allows ring‐closure to both trans and cis‐2 b. trans‐2 b is favoured, likely on steric grounds. The preference for head‐to‐head regioselectivity (i. e., 2 b rather than 2 b′) likely arises, at least in part, due to the favoured formation of more the stable benzylic radicals in 5 compared to 5′. [16b] Previous work by Wu and co‐workers also suggested a beneficial pre‐associative π‐stacking interaction between two styrene molecules prior to energy‐transfer. [16b] Our observations of improved reactivity and appearance of a new lower energy absorption band in the UV‐Vis spectrum (see the Supporting Information) at high concentrations of styrene would be consistent with this hypothesis.
Scheme 5.
Proposed energy‐transfer reaction mechanism; [PC]=photocatalyst.
Table 1.
Optimisation of photocatalytic [2+2] cycloaddition.[a]
|
| |||
|---|---|---|---|
|
|
Deviation from standard conditions |
dr (trans:cis)[b] |
Yield [%][c] |
|
1 |
none |
3.6 : 1 |
83 |
|
2 |
[Ir{dF(CF3)ppy}2(dtbbpy)]PF6 (1 mol %) |
3.3 : 1 |
41 |
|
3 |
DMF |
1.6 : 1 |
6 |
|
4 |
HPLC grade THF |
3.3 : 1 |
71 |
|
5 |
under air |
3.2 : 1 |
23 |
|
6 |
argon sparged THF |
3.5 : 1 |
63 |
|
7 |
no 4CzIPN |
n.d. |
<5 |
|
8 |
Reaction in the dark |
n.d. |
<5 |
|
9 |
0.5 mol % 4CzIPN, 1.00 mmol 1 b, 4 M, 48 h |
3.4 : 1 |
56[d] |
[a] The reaction mixture was degassed by three freeze–pump–thaw cycles prior to irradiation. For further details see the Supporting Information. [b] Diastereoselectivity was determined by 1H NMR spectroscopy of the crude reaction mixture. [c] Yield estimated from the 1H NMR of the reaction mixture relative to 1,3,5‐trimethoxybenzene as internal standard. [d] Isolated yield.
Conclusion
In summary, we have developed a visible‐light‐mediated organophotocatalytic [2+2] cycloaddition reaction that operates well with electron‐deficient styrene derivatives to deliver cyclobutanes bearing electron‐deficient aromatic substituents. The cyanoarene photocatalyst enables a departure from the current literature, which has generally focused on electron‐rich aryl and α,β‐unsaturated carbonyl compounds, and allows the incorporation of typical electron‐withdrawing functionality such as esters, nitriles and nitro groups. We hope this method proves useful to synthetic practitioners.
Experimental Section
General procedure for photochemical dimerisation: A 10 mL Schlenk tube was charged with alkene (1 equiv.), 4CzIPN (0.5–1 mol %) and anhydrous THF (4 M with respect to the alkene) and the reaction mixture was subsequently deoxygenated by three freeze–pump–thaw cycles. The reaction mixture was irradiated with 456 nm LEDs for 48 h (or as indicated). See the Supporting Information for details of the photochemical setup. The reaction was concentrated in vacuo and directly purified by flash column chromatography (SiO2) to afford the title compound(s).
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
We wish to thank Martin Simon, University of Göttingen, for his assistance with HPLC chromatography. M.G. and J.C.L.W. thank the Georg‐August‐Universität Göttingen for financial support. A.G. thanks the ERASMUS+ programme of the European Union for financial support. J.C.L.W. wishes to thank Prof. Manuel Alcarazo for his continuous generous support and guidance. Open Access funding enabled and organized by Projekt DEAL.
Dedicated to the memory of Prof. Dr. Ulf Diederichsen.
Golfmann M., Glagow L., Giakoumidakis A., Golz C., Walker J. C. L., Chem. Eur. J. 2023, 29, e202202373.
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv‐2022‐trn3c).
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1.
- 1a. Sergeiko A., Poroikov V. V., Hanuš L. O., Dembitsky V. M., Open Med. Chem. J. 2008, 2, 26–37; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Dembitsky V. M., J. Nat. Med. 2008, 62, 1–33. [DOI] [PubMed] [Google Scholar]
- 2.For reviews on the synthesis of cyclobutane natural products, see:
- 2a. Li J., Gao K., Bian M., Ding H., Org. Chem. Front. 2020, 7, 136–154; [Google Scholar]
- 2b. Bach T., Hehn J. P., Angew. Chem. Int. Ed. 2011, 50, 1000–1045; [DOI] [PubMed] [Google Scholar]
- 2c. Iriondo-Alberdi J., Greaney M. F., Eur. J. Org. Chem. 2007, 4701–4815. [Google Scholar]
- 3.For reviews on the use of cyclobutanes as synthetic intermediates, see:
- 3a. Nandy M., Das S., Nanda S., Org. Biomol. Chem. 2022, 20, 1582–1622; [DOI] [PubMed] [Google Scholar]
- 3b. Murakami M., Ishida N., Chem. Rev. 2021, 121, 264–299; [DOI] [PubMed] [Google Scholar]
- 3c. Fumagalli G., Stanton S., Bower J. F., Chem. Rev. 2017, 117, 9404–9432; [DOI] [PubMed] [Google Scholar]
- 3d. Seiser T., Saget T., Tran D. N., Cramer N., Angew. Chem. Int. Ed. 2011, 50, 7740–7752; [DOI] [PubMed] [Google Scholar]
- 3e. Namyslo J. C., Kaufmann D. E., Chem. Rev. 2003, 103, 1485–1538; [DOI] [PubMed] [Google Scholar]
- 3f. Lee-Ruff E., Mladenova G., Chem. Rev. 2003, 103, 1449–1483. [DOI] [PubMed] [Google Scholar]
- 4. van der Kolk M. R., Janssen M. A. C. H., Rutjes F. P. J. T., Blanco-Ania D., ChemMedChem. 2022, 17, e202200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Lovering F., Bikker J., Humblet C., J. Med. Chem. 2009, 52, 6752–6756; [DOI] [PubMed] [Google Scholar]
- 5b. Lovering F., MedChemComm 2013, 4, 515–519; [Google Scholar]
- 5c. Brown D. G., Boström J., J. Med. Chem. 2016, 59, 4443–4458; [DOI] [PubMed] [Google Scholar]
- 5d. Boström J., Brown D. G., Young R. J., Keserü G. M., Nat. Rev. Drug Discovery 2018, 17, 709–727; [DOI] [PubMed] [Google Scholar]
- 5e. Blakemore D. C., Castro L., Churcher I., Rees D. C., Thomas A. W., Wilson D. M., Wood A., Nat. Chem. 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- 6. Wiberg K. B., Angew. Chem. Int. Ed. Engl. 1986, 25, 312–322. [Google Scholar]
- 7. Liebermann C., Ber. Dtsch. Chem. Ges. 1877, 10, 2177–2179. [Google Scholar]
- 8.For reviews on the photochemical [2+2] cycloaddition and its uses in organic synthesis, see:
- 8a. Sarkar D., Bera N., Ghosh S., Eur. J. Org. Chem. 2020, 1310–1326; [Google Scholar]
- 8b. Poplata S., Tröster A., Zou Y.-Q., Bach T., Chem. Rev. 2016, 116, 9748–9815; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c. Xu Y., Connor M. L., Brown K. M., Angew. Chem. Int. Ed. 2015, 54, 11918–11928; [DOI] [PubMed] [Google Scholar]
- 8d. Bach T., Synthesis 1998, 5, 683–703; [Google Scholar]
- 8e. Crimmins M. T., Chem. Rev. 1988, 88, 1453–1473. [Google Scholar]
- 9.For general reviews on energy transfer catalysis, see:
- 9a. Strieth-Kalthoff F., James M. J., Teders M., Pitzer L., Glorius F., Chem. Soc. Rev. 2018, 47, 7190–7202; [DOI] [PubMed] [Google Scholar]
- 9b. Zhou Q.-Q., Zou Y.-Q., Lu L.-Q., Xioa W.-J., Angew. Chem. Int. Ed. 2019, 58, 1586–1604. [DOI] [PubMed] [Google Scholar]
- 10.For a general review on photoredox catalysis, see: Shaw M. H., Twilton J., MacMillan D. W. C., J. Org. Chem. 2016, 81, 6898–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For examples of photoredox-catalysed [2+2] cycloadditions initiated by substrate oxidation, see:
- 11a. Ischay M. A., Lu Z., Yoon T. P., J. Am. Chem. Soc. 2010, 132, 8572–8574; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Ischay M. A., Ament M. S., Yoon T. P., Chem. Sci. 2012, 3, 2807–2811; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c. Riener M., Nicewicz D. A., Chem. Sci. 2013, 4, 2625–2629; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11d. Lin S., Lies S. D., Gravatt C. S., Yoon T. P., Org. Lett. 2017, 19, 368–371; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11e. Li R., Ma B. C., Huang W., Wang L., Wang D., Lu H., Landfester K., Zhang K. A. I., ACS Catal. 2017, 7, 3097–3101; [Google Scholar]
- 11f. Shin J. H., Seong E. Y., Mun H. J., Jang Y. J., Kang E. J., Org. Lett. 2018, 20, 5872–5876; [DOI] [PubMed] [Google Scholar]
- 11g. Horibe T., Katagiri K., Ishihara K., Adv. Synth. Catal. 2020, 362, 960–963; [Google Scholar]
- 11h. Tanaka K., Iwama Y., Kishimoto M., Ohtsuka N., Hoshino Y., Honda K., Org. Lett. 2020, 22, 5207–5211. [DOI] [PubMed] [Google Scholar]
- 12.For a review of radical cation-induced [2+2] cycloadditions, see: Horibe T., Ishihara K., Chem. Lett. 2020, 49, 107–113. [Google Scholar]
- 13.For examples of photoredox-catalysed [2+2] cycloadditions initiated by substrate reduction, see:
- 13a. Ischay M. A., Anzovino M. E., Du J., Yoon T. P., J. Am. Chem. Soc. 2008, 130, 12886–12887; [DOI] [PubMed] [Google Scholar]
- 13b. Du J., Yoon T. P., J. Am. Chem. Soc. 2009, 131, 14604–14605; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13c. Tyson E. L., Farney E. P., Yoon T. P., Org. Lett. 2012, 14, 1110–1113; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13d. Du J., Skubi K. L., Schultz D. M., Yoon T. P., Science 2014, 344, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For examples of photocatalysed [2+2] cycloadditions initiated by energy transfer, see:
- 14a. Zou Y.-Q., Duan S.-W., Meng X.-G., Hu X.-Q., Gao S., Chen J.-R., Xiao W.-J., Tetrahedron 2012, 68, 6914–6919; [Google Scholar]
- 14b. Alonso R., Bach T., Angew. Chem. Int. Ed. 2014, 53, 4368–4371; [DOI] [PubMed] [Google Scholar]
- 14c. Hurtley A. E., Lu Z., Yoon T. P., Angew. Chem. Int. Ed. 2014, 53, 8991–8994; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14d. Mojr V., Svobodová E., Straková K., Neveselý T., Chudoba J., Dvořáková H., Cibulka R., Chem. Commun. 2015, 51, 12036–12039; [DOI] [PubMed] [Google Scholar]
- 14e. Blum T. R., Miller Z. D., Bates D. M., Guzei I. A., Yoon T. P., Science 2016, 354, 1391–1395; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14f. Lei T., Zhou C., Huang M.-Y., Zhao L.-M., Yang B., Ye C., Xiao H., Meng Q.-Y., Ramamurthy V., Tung C.-H., Wu L.-Z., Angew. Chem. Int. Ed. 2017, 56, 15407–15410; [DOI] [PubMed] [Google Scholar]
- 14g. Zhou C., Lei T., Wei X.-Z., Liu Z., Chen B., Ramamurthy V., Tung C.-H., Wu L.-Z., Org. Lett. 2018, 20, 6808–6811; [DOI] [PubMed] [Google Scholar]
- 14h. Higgins R. F., Fatur S. M., Damrauer N. H., Ferreira E. M., Rappé A. K., Shores M. P., ACS Catal. 2018, 8, 9216–9225; [Google Scholar]
- 14i. Hörmann F. M., Chung T. S., Rodriguez E., Jakob M., Bach T., Angew. Chem. Int. Ed. 2018, 57, 827–831; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14j. James M. J., Schwarz J. L., Strieth-Kalthoff F., Wibbeling B., Glorius F., J. Am. Chem. Soc. 2018, 140, 8624–8628; [DOI] [PubMed] [Google Scholar]
- 14k. Jiang Y., Wang C., Rogers C. R., Kodaimati M. S., Weiss E. A., Nat. Chem. 2019, 11, 1034–1040; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14l. Zhao L.-M., Lei T., Liao R.-Z., Xiao H., Chen B., Ramamurthy V., Tung C.-H., Wu L.-Z., J. Org. Chem. 2019, 84, 9257–9269; [DOI] [PubMed] [Google Scholar]
- 14m. Daub M. E., Jung H., Lee B. J., Won J., Baik M.-H., Yoon T. P., J. Am. Chem. Soc. 2019, 141, 9543–9547; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14n. Kerres S., Plut E., Malcherek S., Rehbein J., Reiser O., Adv. Synth. Catal. 2019, 361, 1400–1407; [Google Scholar]
- 14o. de Souza W. C., Matsuo B. T., Matos P. M., Correia J. T. M., Santos M. S., König B., Paixão M. W., Chem. Eur. J. 2021, 27, 3722–3728; [DOI] [PubMed] [Google Scholar]
- 14p. Murray P. R. D., Bussink W. M. M., Davies G. H. M., van der Mei F. W., Antropow A. H., Edwards J. T., D'Agostino L. A., Ellis J. M., Hamann L. G., Romanov-Michailidis F., Knowles R. R., J. Am. Chem. Soc. 2021, 143, 4055–4063; [DOI] [PubMed] [Google Scholar]
- 14q. Shcherbakova V., Dibchak D., Snisarenko M., Skalenko Y., Denisenko A. V., Kuznetsova A. S., Mykhailiuk P. K., J. Org. Chem. 2021, 86, 2200–2209; [DOI] [PubMed] [Google Scholar]
- 14r. Pecho F., Sempere Y., Graumüller J., Hörmann F. M., Gschwind R. M., Bach T., J. Am. Chem. Soc. 2021, 143, 9350–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu Z., Yoon T. P., Angew. Chem. Int. Ed. 2012, 51, 10329–10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A small number of electron-deficient styrenes were used in the following reports:
- 16a. Pagire S. K., Hossain A., Traub L., Kerres S., Reiser O., Chem. Commun. 2017, 53, 12072–12075; [DOI] [PubMed] [Google Scholar]
- 16b. Liu Z., Zhou C., Lei T., Nan X.-L., Chen B., Tung C.-H., Wu L.-Z., CCS Chem. 2019, 1, 582–588. [Google Scholar]
- 17.For reviews on organophotoredox catalysis, see:
- 17a. Tlili A., Lakhdar S., Angew. Chem. Int. Ed. 2021, 60, 19526–19549; [DOI] [PubMed] [Google Scholar]
- 17b. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]
- 18.For a recent example of an organophotoredox catalysed [2+2] cycloaddition with electron-rich alkenes, see: Grantham H. F., Kimber M. C., ChemPhotoChem 2022, 6, e202100273. [Google Scholar]
- 19.The reasons for this are not clear as the triplet excited energy for [Ir{dF(CF3)ppy}2(dtbbpy)]PF6 (∼61 kcal mol−1) is very similar to that of 4CzIPN (∼61.6 kcal mol−1).
- 20.For previous examples of similar intramolecular photocatalytic [2+2] cycloaddition reactions, see: refs. [11a] and [14d,n].
- 21. Speckmeier E., Fischer T. G., Zeitler K., J. Am. Chem. Soc. 2018, 140, 15353–15365. [DOI] [PubMed] [Google Scholar]
- 22. Roth H. G., Romero N. A., Nicewicz D. A., Synlett 2016, 27, 714–723. [Google Scholar]
- 23. Ruoff R. S., Kadish K. M., Boulas P., Chen E. C. M., J. Phys. Chem. 1995, 99, 8843–8850. [Google Scholar]
- 24. Ni T., Caldwell R. A., Melton L. A., J. Am. Chem. Soc. 1989, 111, 457–464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.