Abstract
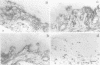
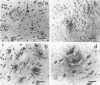
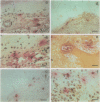
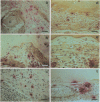
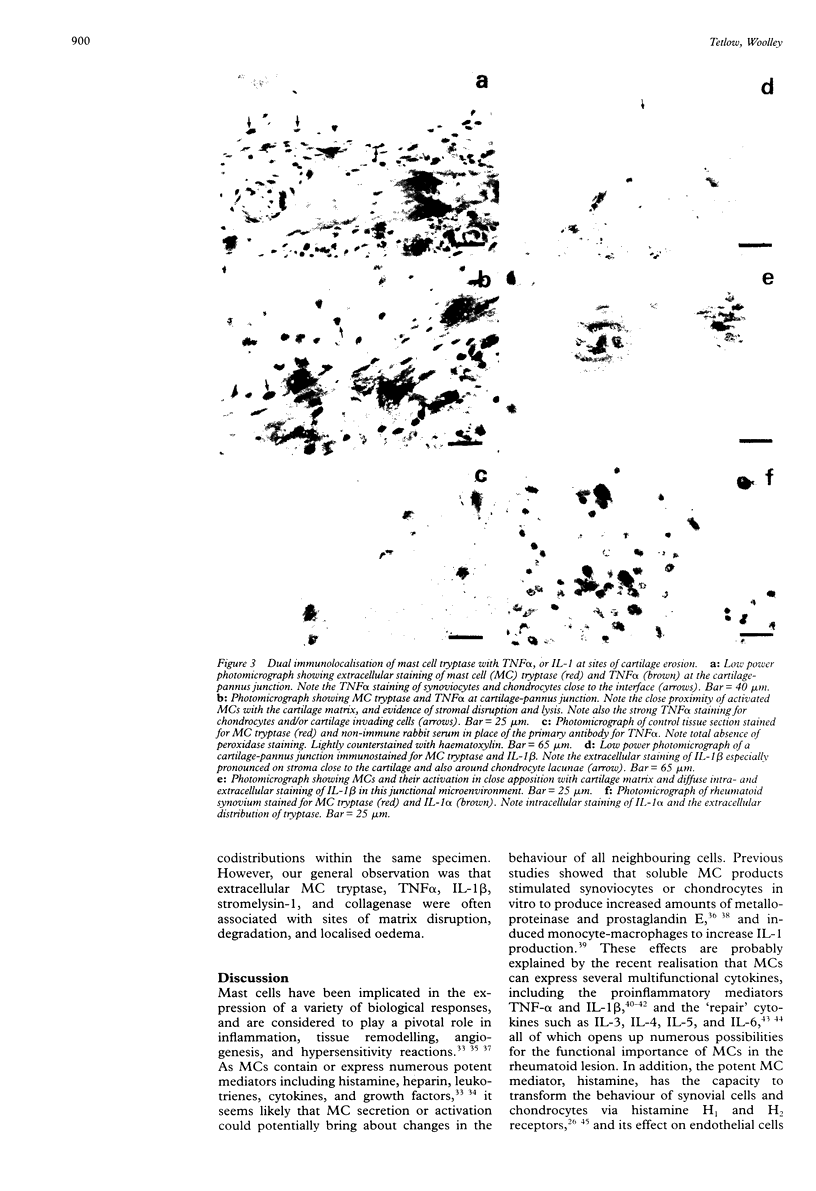
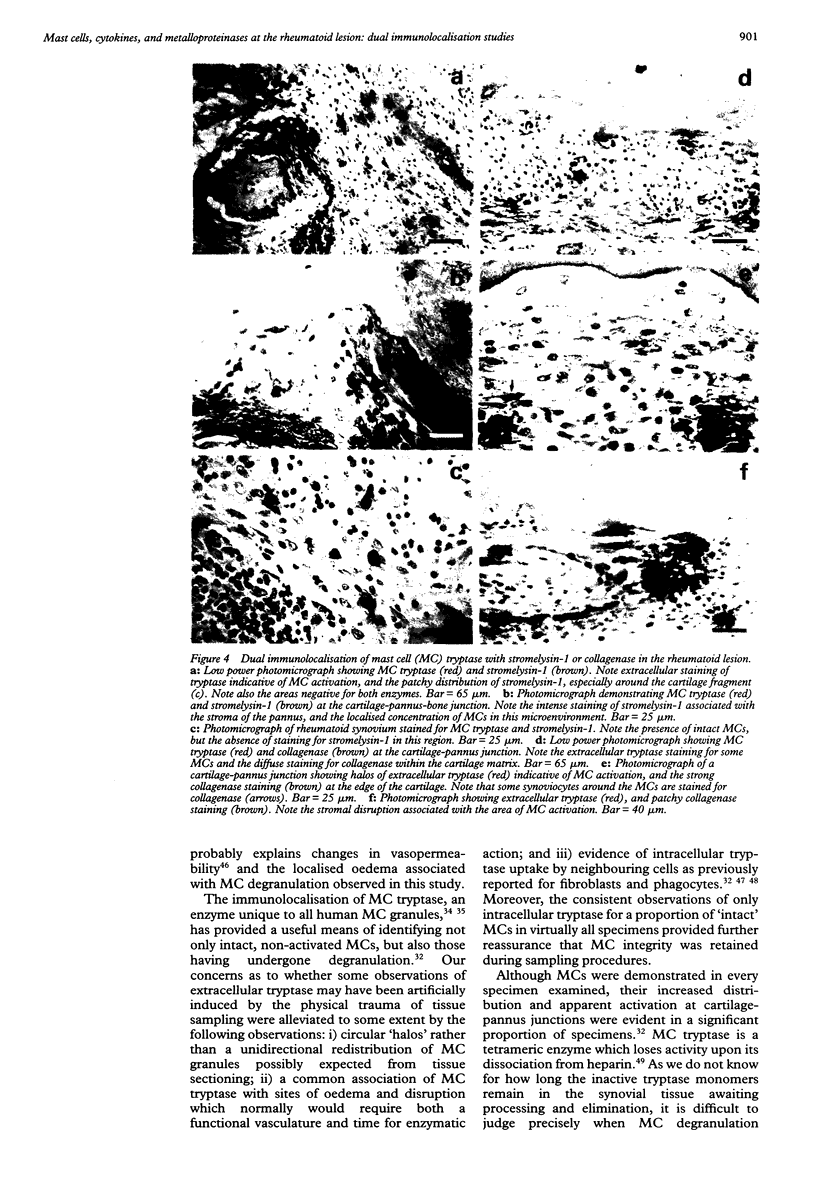
OBJECTIVES--To examine the distribution and activation of mast cells (MCs) in the rheumatoid lesion (cartilage-pannus junctions demonstrating cartilage erosion), and to determine whether or not their tissue distribution is related to that for tumour necrosis factor alpha (TNF alpha), interleukin-1 (IL-1), stromelysin-1, and collagenase. METHODS--Immunolocalisation of MC-tryptase was used to identify MCs and their states of degranulation in 35 specimens of cartilage-pannus junctions. Dual immunolocalisation techniques using alkaline phosphatase and peroxidase conjugated antibodies were used to compare the distributions of MCs with the proinflammatory cytokines TNF alpha and IL-1, and the cartilage or matrix degrading enzymes stromelysin-1 and collagenase. RESULTS--Stromelysin-1, TNF alpha, and IL-1 beta were especially prominent at the cartilage-pannus junctions, albeit with patchy distributions. Extracellular MC tryptase, indicative of MC activation/degranulation, was commonly observed at sites of cartilage erosion, and was often associated with the microenvironmental expression of TNF alpha, IL-1 beta, stromelysin-1, and collagenase. Such observations were often associated with localised sites of tissue oedema and stromal disruption. CONCLUSION--MC activation was frequently associated with proinflammatory cytokine and metalloproteinase expression by neighbouring cells, thereby suggesting an important contributory role for the MC in mediating matrix degradation and oedematous changes within microfoci of the rheumatoid lesion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter S. C., Metcalfe D. D., Bradford T. R., Schwartz L. B. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987 Dec 15;248(3):821–827. doi: 10.1042/bj2480821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Horisberger U., Martin U. Phagocytosis of mast cell granules by mononuclear phagocytes, neutrophils and eosinophils during anaphylaxis. Int Arch Allergy Appl Immunol. 1982;67(3):219–226. doi: 10.1159/000233022. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Wilson S., Bardin P. G., Heusser C. H., Holgate S. T., Howarth P. H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993 Oct 1;151(7):3853–3865. [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. TNF alpha--a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992 May;31(5):293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Campion G. V. The prospect for cytokine based therapeutic strategies in rheumatoid arthritis. Ann Rheum Dis. 1994 Aug;53(8):485–487. doi: 10.1136/ard.53.8.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Allard S., Abney E., Feldmann M., Maini R. N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992 Oct;31(10):653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Cooper A. L., Snowden N., Woolley D. E. IgE antibodies specific for cartilage collagens type II, IX and XI in rheumatic diseases. Scand J Rheumatol. 1993;22(5):207–214. doi: 10.3109/03009749309095124. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Mitchell T., Feldmann M., Maini R. N. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992 Oct;35(10):1170–1178. doi: 10.1002/art.1780351009. [DOI] [PubMed] [Google Scholar]
- Farahat M. N., Yanni G., Poston R., Panayi G. S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993 Dec;52(12):870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M. Stromelysin and tissue inhibitor of metalloproteinases gene expression in rheumatoid arthritis synovium. Am J Pathol. 1992 Jun;140(6):1309–1314. [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Gordon J. R., Wershil B. K. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991 Dec;3(6):865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Ilardi C., Engber W., Graziano F. M. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984 Aug;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Suzuki K., Schwartz L. B., Okada Y., Nagase H., Ramamurthy N. S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989 Nov;84(5):1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber B. Activation of rheumatoid synovial mast cells. Role of IgE-associated antiglobulins. Monogr Allergy. 1989;26:120–134. [PubMed] [Google Scholar]
- Hembry R. M., Bagga M. R., Reynolds J. J., Hamblen D. L. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995 Jan;54(1):25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasser M. Z., Mitchell P. G., Cheung H. S. Induction of stromelysin-1 and collagenase synthesis in fibrochondrocytes by tumor necrosis factor-alpha. Matrix Biol. 1994 Apr;14(3):241–249. doi: 10.1016/0945-053x(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Lacraz S., Isler P., Vey E., Welgus H. G., Dayer J. M. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem. 1994 Sep 2;269(35):22027–22033. [PubMed] [Google Scholar]
- Lees M., Taylor D. J., Woolley D. E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994 Jul 1;223(1):171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Irani A. M., Schwartz L. B., Barrett K. E., Metcalfe D. D. Mast cell numbers and histamine levels in synovial fluids from patients with diverse arthritides. Arthritis Rheum. 1986 Aug;29(8):956–963. doi: 10.1002/art.1780290803. [DOI] [PubMed] [Google Scholar]
- McCachren S. S., Haynes B. F., Niedel J. E. Localization of collagenase mRNA in rheumatoid arthritis synovium by in situ hybridization histochemistry. J Clin Immunol. 1990 Jan;10(1):19–27. doi: 10.1007/BF00917494. [DOI] [PubMed] [Google Scholar]
- Mican J. M., Metcalfe D. D. Arthritis and mast cell activation. J Allergy Clin Immunol. 1990 Oct;86(4 Pt 2):677–683. doi: 10.1016/s0091-6749(05)80240-4. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Dodge G. R., Roughley P. J., Liu J., Finch S. J., DiPasquale G., Poole A. R. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix. 1993 Mar;13(2):95–102. doi: 10.1016/s0934-8832(11)80068-5. [DOI] [PubMed] [Google Scholar]
- Murphy G., Docherty A. J., Hembry R. M., Reynolds J. J. Metalloproteinases and tissue damage. Br J Rheumatol. 1991;30 (Suppl 1):25–31. [PubMed] [Google Scholar]
- Murphy M., Friend D. S., Pike-Nobile L., Epstein L. B. Tumor necrosis factor-alpha and IFN-gamma expression in human thymus. Localization and overexpression in Down syndrome (trisomy 21). J Immunol. 1992 Oct 1;149(7):2506–2512. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Ruoss S. J., Hartmann T., Caughey G. H. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991 Aug;88(2):493–499. doi: 10.1172/JCI115330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen J., Kalkkinen N., Welgus H. G., Kovanen P. T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994 Jul 8;269(27):18134–18140. [PubMed] [Google Scholar]
- Schwartz L. B. Mast cells: function and contents. Curr Opin Immunol. 1994 Feb;6(1):91–97. doi: 10.1016/0952-7915(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Sillaber C., Bevec D., Butterfield J. H., Heppner C., Valenta R., Scheiner O., Kraft D., Lechner K., Bettelheim P., Valent P. Tumor necrosis factor alpha and interleukin-1 beta mRNA expression in HMC-1 cells: differential regulation of gene product expression by recombinant interleukin-4. Exp Hematol. 1993 Aug;21(9):1271–1275. [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Suzuki K., Lees M., Newlands G. F., Nagase H., Woolley D. E. Activation of precursors for matrix metalloproteinases 1 (interstitial collagenase) and 3 (stromelysin) by rat mast-cell proteinases I and II. Biochem J. 1995 Jan 1;305(Pt 1):301–306. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Yoffe J. R., Brown D. M., Woolley D. E. Histamine stimulates prostaglandin E production by rheumatoid synovial cells and human articular chondrocytes in culture. Arthritis Rheum. 1986 Feb;29(2):160–165. doi: 10.1002/art.1780290202. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Lees M., Ogata Y., Nagase H., Woolley D. E. Differential expression of gelatinase B (MMP-9) and stromelysin-1 (MMP-3) by rheumatoid synovial cells in vitro and in vivo. Rheumatol Int. 1993;13(2):53–59. doi: 10.1007/BF00307734. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann Rheum Dis. 1995 Jul;54(7):549–555. doi: 10.1136/ard.54.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. C., Dickens E., Symons J. A., Duff G. W. In situ hybridization of interleukin-1 in CD14-positive cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1992 Mar;62(3):295–300. doi: 10.1016/0090-1229(92)90106-x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lark M. W., Chun L. E., Eyre D. R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991 Mar 25;266(9):5625–5628. [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Wooley D. E. Mast cell products stimulate collagenase and prostaglandin E production by cultures of adherent rheumatoid synovial cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):270–276. doi: 10.1016/0006-291x(84)90470-4. [DOI] [PubMed] [Google Scholar]