Abstract
Background
Cardiogenic shock (CS) includes several phenotypes with heterogenous hemodynamic features. Timely prognostication is warranted to identify patients requiring treatment escalation. We explored the association of the updated Society for Cardiovascular Angiography and Interventions (SCAI) stages classification with in‐hospital mortality using a prospective national registry.
Methods
Between March 2020 and February 2022 the Altshock‐2 Registry has included 237 patients with CS of all etiologies at 11 Italian Centers. Patients were classified according to their admission SCAI stage (assigned prospectively and independently updated according to the recently released version). In‐hospital mortality was evaluated for association with both admission and 24‐h SCAI stages.
Results
The overall in‐hospital mortality was 38%. Of the 237 patients included and staged according to the updated SCAI classification, 20 (8%) had SCAI shock stage B, 131 (55%) SCAI stage C, 61 (26%) SCAI stage D and 25 (11%) SCAI stage E. In‐hospital mortality stratified according to the SCAI classification at 24 h was 18% for patients in SCAI stage B, 27% for SCAI stage C, 63% for SCAI stage D and 100% for SCAI stage E. Both the revised SCAI stages on admission and at 24 h were associated with in‐hospital mortality, but the classification potential slightly increased at 24‐h. After adjusting for age, sex, lactate level, eGFR, CVP, inotropic score and mechanical circulatory support [MCS], SCAI classification at 24 h was an independent predictor of in‐hospital mortality.
Conclusions
In the Altshock‐2 registry the utility of SCAI shock stages to identify risk of in‐hospital mortality increased at 24 h after admission. Escalation of treatment (either pharmacological or with MCS) should be tailored to achieve prompt clinical improvement within the first 24 h after admission. Registration: http://www.clinicaltrials.gov; Unique identifier: NCT04295252.
Keywords: cardiogenic shock, heart failure, SCAI stages
1. INTRODUCTION
Cardiogenic shock (CS) is the most severe form of acute heart failure, characterized by life‐threatening end‐organ hypoperfusion resulting from low cardiac output. 1 Given the high short‐term mortality of these patients and the need for developing dedicated CS teams and systems of care to provide timely interventions, 2 , 3 , 4 , 5 , 6 , 7 it is of utmost importance to optimize clinical phenotyping and implement dedicated outcome research. Since its original definition, the Society for Cardiovascular Angiography and Interventions (SCAI) stages classification has been widely used for risk stratification upon patient's admission across the whole spectrum of CS. 8 A clinical expert consensus group has recently redefined the SCAI classification using a 3‐axis model which focuses on a real time assignment of the SCAI shock stage and includes an analysis of serial changes over time. 9
The Altshock‐2 registry has been designed to include the full spectrum of consecutive “real world” CS patients and to collect data on etiology, clinical presentation, pharmacological treatments, use of mechanical circulatory support (MCS) and outcome. Accordingly, all patients were prospectively staged according to SCAI shock stages upon admission and at 24 h. We aimed to assess whether the updated SCAI shock stage classification can more accurately identify patients at risk for in‐hospital mortality.
2. MATERIALS AND METHODS
The Altshock‐2 Registry is a multicenter prospective data collection (ClinicalTrials. gov Identifier: NCT04295252), part of the Italian Altshock‐2 program. Recruitment started on 2 March 2020 with 11 Italian Centers contributing to patients' enrolment (Supporting Information: Appendix Table 1). Five of the 11 have active heart transplant and LVAD programs, whereas 3 have only an LVAD program. This study was approved by the Local Ethics Committee of Milano Area 3 of the ASST Grande Ospedale Metropolitano Niguarda (Piazza Ospedale Maggiore 3, 20162). In accordance to the EU Regulation 536/2014, all competent patients provided written informed consent, whereas consent was waived for patients who were not competent on admission. The study was conducted in accordance with ethical principles based on Helsinki's Declaration, 10 International Conference on Harmonization for Good Clinical Practice, and the current ethical rules. The Strengthening the Reporting of Observational Studies in Epidemiology Guidelines (STROBE) were followed for reporting the findings. 11
The Society for SCAI shock stages were prospectively assigned upon admission and at 24 h by physician assessment according to the original definition released in 2019, 8 without study‐specific criteria. A further refinement of the patients' shock stage was independently performed by two researchers (N. M. and D. B.) during data analysis using the updated SCAI shock stages classification. 9
Clinical, laboratory, procedural, pharmacological and follow‐up data of all consecutive patients enrolled were collected and registered in an electronic case report form provided through the RedCap platform. The primary outcome was in‐hospital mortality.
2.1. Data analysis
No formal sample size calculation was used to plan enrolment in the registry since the goal was to obtain participation of all eligible patients. Quantitative variables are summarized using medians and quartiles (Q1 and Q3) whereas categorical variables are presented as counts and percentages. Comparisons of patients' characteristics across SCAI shock stages were carried out using the χ 2 test—or the Fisher exact test when expected frequencies were less than 5—for categorical variables and the one‐way analysis of variance for continuous variables. Bar charts were used to graphically synthetize SCAI shock stage distribution and SCAI‐stratified in hospital‐mortality.
The paired t‐test was used to assess any difference in selected variables (lactates, estimated Glomerular Filtration Rate and central venous pressure) between measurements on admission and at 24 h. The association between SCAI shock stages on admission, by prospective physician assessment as well as for further refinement according to the updated criteria, and at 24 h with in‐hospital mortality was quantified in terms of Odd Ratio (OR) and its 95% confidence interval (CI). The latter was also assessed in a multivariable fashion adjusting for selected variables.
All analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing).
3. RESULTS
3.1. Study population characteristics
A total of 237 patients were hospitalized with confirmed diagnosis of CS between March 2020 and February 2022. The baseline characteristics of the study population are described in Table 1. The median age was 65 years (Q1−Q3, 55−74 years) and 77% were male. One‐hundred‐and‐one patients (43%) were admitted for CS related to acute myocardial infarction (AMI), 88 patients (37%) had acutely decompensated heart failure (ADHF) and 48 patients (20%) presented with other diagnoses.
Table 1.
Baseline characteristics of patients according to the Society for Cardiovascular Angiography and Interventions (SCAI) classification
| Characteristic | Admission updated SCAI shock stages | |||||
|---|---|---|---|---|---|---|
| Demographics | Overall N = 237a | B N = 20a | C N = 131a | D N = 61a | E N = 25a | p Valueb |
| Age, years | 65.0 (55.0−74.0) | 56.0 (49.2−65.0) | 66.0 (56.0−74.5) | 66.0 (58.0−76.0) | 63.0 (48.0−72.0) | 0.031 |
| BMI | 25.9 (23.0‐28.5) | 26.1 (23.1−29.2) | 25.7 (23.1−27.7) | 27.0 (23.1−29.4) | 25.7 (22.8–27.8) | 0.6 |
| Sex | 0.2 | |||||
| F | 54 (23%) | 4 (20%) | 24 (18%) | 17 (28%) | 9 (36%) | |
| M | 183 (77%) | 16 (80%) | 107 (82%) | 44 (72%) | 16 (64%) | |
| Medical History | ||||||
| COVID‐19 | 14 (6.3%) | 2 (11%) | 7 (5.9%) | 4 (6.7%) | 1 (4.2%) | 0.8 |
| Hypertension | 142 (60%) | 8 (40%) | 80 (61%) | 38 (62%) | 16 (64%) | 0.3 |
| Diabetes | 73 (31%) | 6 (30%) | 41 (31%) | 24 (39%) | 2 (8.0%) | 0.029 |
| Smoking | 63 (27%) | 7 (35%) | 35 (27%) | 15 (25%) | 6 (24%) | 0.8 |
| Dyslipidemia | 100 (42%) | 11 (55%) | 49 (37%) | 31 (51%) | 9 (36%) | 0.2 |
| Prior PCI | 51 (22%) | 2 (10%) | 28 (21%) | 13 (22%) | 8 (32%) | 0.4 |
| Prior CABG | 20 (8.4%) | 0 (0%) | 11 (8.4%) | 7 (11%) | 2 (8.0%) | 0.5 |
| Stroke or TIA | 13 (5.5%) | 0 (0%) | 9 (6.9%) | 4 (6.6%) | 0 (0%) | 0.5 |
| Peripheral artery disease | 40 (17%) | 0 (0%) | 23 (18%) | 12 (20%) | 5 (20%) | 0.14 |
| Atrial fibrillation | 66 (28%) | 6 (30%) | 36 (27%) | 21 (34%) | 3 (12%) | 0.2 |
| CKD | 59 (25%) | 5 (25%) | 35 (27%) | 17 (28%) | 2 (8.0%) | 0.2 |
| Anemia | 32 (14%) | 1 (5.0%) | 17 (13%) | 12 (20%) | 2 (8.0%) | 0.3 |
| Liver disease | 12 (5.1%) | 0 (0%) | 5 (3.8%) | 5 (8.2%) | 2 (8.0%) | 0.4 |
| Thyroid disease | 37 (16%) | 2 (10%) | 18 (14%) | 12 (20%) | 5 (20%) | 0.6 |
| Cancer | 25 (11%) | 0 (0%) | 16 (12%) | 7 (11%) | 2 (8.0%) | 0.4 |
| Prior EF | 39.0 (22.0,55.0) | 32.5 (23.2,51.2) | 35.0 (21.2,55.0) | 40.0 (25.0,55.0) | 40.0 (32.0,55.0) | 0.6 |
| Waiting list for HT | 10 (4.2%) | 0 (0%) | 9 (6.9%) | 0 (0%) | 1 (4.0%) | 0.11 |
| Etiology | 0.13 | |||||
| AMI | 101 (43%) | 6 (30%) | 51 (39%) | 29 (48%) | 15 (60%) | |
| ADHF | 88 (37%) | 11 (55%) | 54 (41%) | 19 (31%) | 4 (16%) | |
| Other | 48 (20%) | 3 (15%) | 26 (20%) | 13 (21%) | 6 (24%) | |
| Drug history | ||||||
| Betablocker | 106 (46%) | 7 (37%) | 65 (50%) | 24 (41%) | 10 (42%) | 0.5 |
| ACE‐I | 42 (18%) | 2 (11%) | 25 (19%) | 11 (19%) | 4 (17%) | 0.9 |
| ARB | 24 (10%) | 1 (5.0%) | 15 (12%) | 4 (6.8%) | 4 (17%) | 0.5 |
| Loop diuretics | 94 (40%) | 10 (50%) | 57 (44%) | 21 (36%) | 6 (25%) | 0.2 |
| MRA | 59 (25%) | 7 (35%) | 35 (27%) | 13 (22%) | 4 (17%) | 0.5 |
| Calcium antagonist | 27 (12%) | 0 (0%) | 16 (12%) | 7 (12%) | 4 (17%) | 0.3 |
| Oral anticoagulant | 60 (26%) | 7 (35%) | 34 (26%) | 18 (31%) | 1 (4.2%) | 0.034 |
| Ivabradine | 6 (2.6%) | 0 (0%) | 4 (3.1%) | 2 (3.4%) | 0 (0%) | >0.9 |
| Sacubitril/Valsartan | 29 (12%) | 2 (10%) | 19 (15%) | 5 (8.5%) | 3 (12%) | 0.7 |
| SAPT | 78 (34%) | 3 (15%) | 46 (36%) | 20 (34%) | 9 (38%) | 0.3 |
| DAPT | 22 (9.5%) | 2 (10%) | 11 (8.6%) | 6 (10%) | 3 (12%) | 0.8 |
| Oral antidiabetics | 40 (17%) | 6 (30%) | 24 (19%) | 8 (14%) | 2 (8.3%) | 0.2 |
| Insulin therapy | 25 (11%) | 4 (20%) | 16 (13%) | 5 (8.6%) | 0 (0%) | 0.12 |
Abbreviations: ACE‐I, Angiotensin‐convertin enzyme inhibitor; ADHF, acutely decompensated heart failure; AMI, acute myocardial infarction cardiogenic shock; ARB, Angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; DAPT, Dual antiplatelet therapy; EF, ejection fraction; HT, heart transplant; MRA, mineralcorticoid receptor antagonist; PCI, percutaneous coronary intervention; SAPT, Single antiplatelet therapy; TIA, transient ischemic attack.
Data are presented as n (%) for categorical variables and median (25%, 75% percentiles) for continuous variables.
Derived through χ 2 or Fisher's exact test (frequencies < 5) for categorical variables and one‐way analysis of variance for continuous variables.
Patients with SCAI shock stage B were younger (median age 56 years, Q1−Q3, 49−65 years) than those with higher stages, and were mainly admitted with ADHF‐CS etiology (55%).
Table 2 reports clinical presentation, laboratory parameters, invasive monitoring, hemodynamic, and echocardiographic findings on admission. Forty‐seven patients (31%) suffered cardiac arrest, the proportion being significantly different (p < 0.001) among SCAI shock stages (21% vs. 33% vs. 79% in SCAI stage C, D, and E, respectively). Hypoperfusion and multiorgan failure in more advanced SCAI shock stages are reflected also by significantly higher values of SAPS (simplified acute physiology score) and SOFA (sequential organ failure assessment) in stages D and E.
Table 2.
Clinical presentation, blood examinations, and monitoring stratified according to the SCAI classification
| Admission updated SCAI shock stages | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall N = 237 a | B N = 20 a | C N = 131 a | D N = 61 a | E N = 25 a | p Value b |
| Clinical presentation | ||||||
| Cardiac arrest | 47 (31%) | 0 (0%) | 16 (21%) | 16 (33%) | 15 (79%) | <0.001 |
| Laboratory profile on admission | ||||||
| Lactate (nmol/L) | 2.8 (1.8−5.9) | 1.2 (0.9−1.6) | 2.7 (1.8−4.7) | 3.3 (2.2−7.1) | 11.7 (4.9−16.0) | <0.001 |
| Bilirubin (mg/dl) | 0.9 (0.5−1.5) | 1.0 (0.6−1.2) | 1.0 (0.6−1.7) | 0.8 (0.5−1.9) | 0.5 (0.3−1.0) | 0.36 |
| AST (U/L) | 113.0 (31.5−426.5) | 37.5 (23.8−126.8) | 116.5 (27.0−336.2) | 88.0 (34.8−435.5) | 509.0 (142.0−1394.0) | 0.001 |
| ALT (U/L) | 73.0 (27.0−220.0) | 40.0 (24.0−66.0) | 64.0 (25.0−176.0) | 79.0 (35.0−203.0) | 400.0 (157.0–1314.0) | <0.001 |
| INR | 1.3 (1.2−1.9) | 1.2 (1.1−1.7) | 1.3 (1.2−1.8) | 1.3 (1.2−1.9) | 1.5 (1.2–2.2) | 0.39 |
| eGFR (ml/min) | 55.0 (34.0−79.0) | 82.0 (54.5−96.5) | 55.0 (33.5−79.5) | 47.0 (29.5−64.8) | 62.5 (42.5–81.8) | 0.003 |
| NT‐proBNP (ng/l) | 8690.5 (4491.5−21,365.2) | 8,423.0 (5823.5−10,021.5) | 8,674.0 (4223.0−19,546.0) | 9,178.0 (3112.0−22,276.0) | 26,823.0 (26,823.0−26,823.0) | 0.65 |
| BNP (ng/l) | 530.0 (196.0−1651.0) | 334.0 (169.5−697.5) | 1,089.0 (491.0−2015.0) | 412.0 (175.8−571.0) | 38.0 (16.0−429.5) | 0.058 |
| Glycemia (mg/dl) | 164.5 (131.2,234.8) | 154.5 (130.0−200.5) | 160.0 (133.0−204.5) | 186.0 (135.2−263.8) | 208.0 (118.0−284.0) | 0.15 |
| Hemoglobin (g/dl) | 126.0 (106.5−144.0) | 130.0 (112.5−144.5) | 126.5 (108.0−144.0) | 126.0 (106.0−144.0) | 109.0 (101.0−135.0) | 0.20 |
| Platelet count (103/mm3) | 223.0 (166.0−284.5) | 236.0 (180.0−296.5) | 233.0 (174.2−292.5) | 212.0 (176.0−263.0) | 176.0 (132.0−216.0) | 0.042 |
| WBC count (103/mm3) | 12.9 (9.0−17.2) | 8.9 (8.2−15.4) | 12.4 (9.3−16.2) | 14.2 (9.0−19.4) | 14.7 (10.4,17.1) | 0.31 |
| CRP (mg/dl) | 4.0 (1.1−11.2) | 3.4 (1.1−6.2) | 4.9 (1.5−11.4) | 4.5 (1.4−17.3) | 0.6 (0.2−2.1) | 0.77 |
| SOFA score | 7.0 (4.0−9.0) | 4.0 (3.0−4.0) | 6.0 (4.0−8.8) | 8.5 (7.0−10.0) | 8.0 (7.0−11.2) | <0.001 |
| SAPS | 45.0 (34.0−58.0) | 32.0 (24.0−41.2) | 42.0 (31.0−53.0) | 54.5 (40.8−62.2) | 62.0 (45.0−71.5) | 0.014 |
| Invasive monitoring | ||||||
| Arterial pressure | 227 (97%) | 19 (95%) | 125 (96%) | 58 (98%) | 25 (100%) | 0.64 |
| Central venous line | 210 (90%) | 19 (95%) | 116 (89%) | 52 (88%) | 23 (92%) | 0.90 |
| Pulmonary artery catheter | 42 (18%) | 7 (35%) | 26 (20%) | 8 (14%) | 1 (4.0%) | 0.038 |
| Hemodynamic findings on admission | ||||||
| MAP (mmHg) | 70.0 (60.5−78.0) | 70.0 (62.0−77.8) | 70.0 (63.0−78.0) | 70.5 (63.0−83.0) | 57.5 (47.8,70.0) | 0.003 |
| SAP (mmHg) | 93.0 (82.0−110.0) | 89.0 (83.8−105.5) | 95.0 (85.0−110.0) | 98.0 (84.2−117.5) | 78.0 (60.0−100.0) | 0.006 |
| HR (bpm) | 90.0 (75.0−110.0) | 85.0 (74.5−94.5) | 90.0 (75.0,110.0) | 96.0 (75.0,114.5) | 96.0 (83.0,110.0) | 0.43 |
| CVP (mmHg) | 12.0 (8.0−17.0) | 11.0 (9.0−15.0) | 12.0 (7.0−16.5) | 14.0 (10.0−17.0) | 14.0 (11.0−19.0) | 0.16 |
| ScvO2 (%) | 61.0 (47.0−71.0) | 58.6 (44.5−69.3) | 60.1 (45.0−70.0) | 68.0 (47.3−72.0) | 59.0 (54.2−71.4) | 0.56 |
| CI (L/min/m2) | 2.0 (1.6−2.3) | 2.1 (1.5−2.5) | 2.0 (1.7−2.4) | 1.9 (1.7−2.1) | 2.3 (2.3−2.3) | 0.95 |
| PAPm (mmHg) | 28.0 (25.0−35.0) | 34.0 (30.5−38.0) | 26.5 (22.8−31.2) | 29.0 (28.0−35.0) | 25.0 (25.0−25.0) | 0.91 |
| PCWP (mmHg) | 21.0 (14.0−25.0) | 20.0 (15.0−28.0) | 21.0 (11.0−25.0) | 22.0 (18.8−24.5) | 18.0 (18.0−18.0) | 0.89 |
| Echocardiographic findings on admission | ||||||
| LVEF (%) | 20.0 (16.0−30.0) | 20.0 (15.0−33.0) | 20.0 (18.0−28.0) | 22.5 (15.0−35.2) | 26.5 (19.2−33.0) | 0.71 |
| LVEDV (ml) | 163.5 (122.0−223.8) | 190.0 (134.8−245.0) | 167.5 (130.2−226.5) | 121.0 (88.5−208.0) | 145.0 (142.5−182.5) | 0.87 |
| MR | 0.25 | |||||
| None | 15 (8.4%) | 1 (5.6%) | 7 (6.4%) | 6 (15%) | 1 (10%) | |
| 1+/4+ | 52 (29%) | 3 (17%) | 33 (30%) | 11 (27%) | 5 (50%) | |
| 2+/4+ | 36 (20%) | 2 (11%) | 23 (21%) | 11 (27%) | 0 (0%) | |
| 3+/4+ | 35 (20%) | 5 (28%) | 21 (19%) | 8 (20%) | 1 (10%) | |
| 4+/4+ | 40 (22%) | 7 (39%) | 25 (23%) | 5 (12%) | 3 (30%) | |
| AR | 0.89 | |||||
| None | 114 (67%) | 14 (78%) | 67 (63%) | 26 (67%) | 7 (88%) | |
| 1+/4+ | 32 (19%) | 3 (17%) | 23 (22%) | 5 (13%) | 1 (12%) | |
| 2+/4+ | 18 (11%) | 1 (5.6%) | 10 (9.4%) | 7 (18%) | 0 (0%) | |
| 3+/4+ | 5 (2.9%) | 0 (0%) | 4 (3.8%) | 1 (2.6%) | 0 (0%) | |
| 4+/4+ | 2 (1.2%) | 0 (0%) | 2 (1.9%) | 0 (0%) | 0 (0%) | |
| TAPSE (mm) | 15.0 (12.0−18.0) | 15.0 (12.0−20.0) | 16.0 (13.0−18.0) | 14.0 (11.0−17.0) | 15.0 (11.5−19.0) | 0.16 |
| Systolic PAP (mmHg) | 45.0 (36.0−54.0) | 45.5 (33.5−49.8) | 45.0 (35.5−55.0) | 47.5 (40.0−50.0) | 49.5 (40.0−60.5) | 0.69 |
Abbreviations: ALT, alanine aminotransferase; AR, aortic regurgitation; AST, aspartate aminotransferase; BNP, B‐type natriuretic peptide; CI, cardiac index; CRP, C‐reactive protein; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; ICU, intensive care unit; INR, international normalized ratio; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MR, mitral regurgitation; NT‐proBNP, N‐terminal proB‐type natriuretic peptide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; SAP: systolic arterial pressure; SAPS: simplified acute physiology score; ScvO2, central venous oxygen saturation; SOFA, sequential organ failure assessment; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell.
Data are presented as n (%) for categorical variables and median (25%, 75% percentiles) for continuous variables.
Derived through χ 2 or Fisher's exact test (frequencies < 5) for categorical variables and one‐way analysis of variance for continuous variables.
Only 42 patients (18%) were monitored using a Swan‐Ganz catheter. No significant differences emerged across SCAI shock stages for left ventricular ejection fraction (LVEF) and end diastolic volume (LVEDV), aortic (AR) and mitral regurgitation (MR), tricuspid annular plane systolic excursion (TAPSE) and systolic pulmonary artery pressure.
3.2. Pharmacological and mechanical support
Table 3 describes pharmacological and mechanical treatments stratified according to the updated SCAI classification on admission. Mechanical ventilation before intensive care unit admission was more often used in patients with SCAI shock stages D and E. The inotropic score significantly differs (p < 0.001) across SCAI shock stages, being 19 (Q1−Q3, 9.0−30.5) in SCAI stage B and 40 (Q1−Q3, 26.5−57.0) in SCAI stage E. Conversely, sodium nitroprusside was used in 47% of patients with SCAI shock stage B as compared to one patient only (4.2%) with SCAI stage E. Overall, 144 (62%) patients received a temporary MCS device.
Table 3.
Pharmacological and mechanical treatment stratified according to the SCAI classification
| Admission updated SCAI shock stages | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall N = 237 a | B N = 20 a | C N = 131 a | D N = 61 a | E N = 25 a | p Value b |
| Treatments before ICU admission | ||||||
| Inotropic/vasopressor support | 119 (52%) | 7 (39%) | 67 (52%) | 29 (49%) | 16 (67%) | 0.3 |
| Noninvasive ventilation | 17 (7.4%) | 3 (16%) | 11 (8.6%) | 2 (3.4%) | 1 (4.2%) | 0.3 |
| Mechanical ventilation | 73 (32%) | 3 (16%) | 28 (22%) | 23 (40%) | 19 (79%) | <0.001 |
| ICU procedures | ||||||
| Inotropic/vasopressor support | 219 (95%) | 19 (95%) | 122 (95%) | 54 (93%) | 24 (96%) | >0.9 |
| Epinephrine | 124 (58%) | 12 (63%) | 62 (52%) | 37 (70%) | 13 (54%) | 0.2 |
| Norepinephrine | 138 (64%) | 8 (42%) | 70 (58%) | 41 (77%) | 19 (79%) | 0.006 |
| Levosimendan | 81 (38%) | 9 (47%) | 50 (42%) | 16 (30%) | 6 (25%) | 0.2 |
| Milrinone | 13 (6.4%) | 3 (16%) | 5 (4.5%) | 5 (10%) | 0 (0%) | 0.10 |
| Dobutamine | 88 (40%) | 9 (47%) | 53 (44%) | 21 (39%) | 5 (21%) | 0.2 |
| Dopamine | 33 (15%) | 4 (21%) | 22 (18%) | 7 (13%) | 0 (0%) | 0.070 |
| Sodium nitroprusside | 80 (37%) | 9 (47%) | 54 (45%) | 16 (30%) | 1 (4.2%) | <0.001 |
| Inotropic score | 16.0 (10.0,36.5) | 19.0 (9.0,30.5) | 13.0 (7.0,25.0) | 24.0 (13.0,43.5) | 40.0 (26.5,57.0) | <0.001 |
| Mechanical circulatory support | ||||||
| Mechanical circulatory support | 144 (62%) | 12 (60%) | 77 (59%) | 37 (63%) | 18 (75%) | 0.5 |
| IABP | 117 (82%) | 12 (100%) | 69 (90%) | 27 (75%) | 9 (50%) | <0.001 |
| Time from index admission to IABP (days) | 1.0 (0.0−3.0) | 2.0 (0.5−6.0) | 1.0 (0.0−3.8) | 0.0 (0.0−1.2) | 0.0 (0.0−0.0) | 0.3 |
| IABP duration (days) | 4.0 (2.3−7.0) | 4.0 (1.5−6.2) | 4.2 (2.8−8.0) | 4.0 (2.4−5.0) | 4.0 (0.4−6.5) | 0.6 |
| Impella | 22 (15%) | 2 (17%) | 8 (10%) | 10 (27%) | 2 (11%) | 0.12 |
| Impella duration (days) | 4.9 (2.1−8.0) | 7.5 (5.2−9.8) | 4.2 (1.9−7.2) | 4.9 (2.7−8.7) | 2.6 (1.4−3.8) | 0.6 |
| ECMO | 39 (27%) | 3 (25%) | 8 (10%) | 14 (38%) | 14 (78%) | <0.001 |
| ECMO duration (days) | 4.0 (1.9−10.1) | 13.0 (7.4−22.0) | 8.5 (5.8−11.6) | 6.5 (4.0−12.0) | 1.8 (0.5−2.5) | 0.004 |
| Respiratory support | 158 (68%) | 12 (60%) | 81 (62%) | 45 (78%) | 20 (83%) | 0.055 |
| Noninvasive ventilation | 62 (43%) | 5 (56%) | 40 (53%) | 15 (36%) | 2 (11%) | 0.005 |
| Mechanical ventilation | 118 (76%) | 10 (83%) | 54 (69%) | 35 (78%) | 19 (95%) | 0.085 |
| Noninvasive ventilation duration (days) | 2.0 (1.0−4.0) | 1.0 (0.4−1.0) | 2.0 (0.9−5.0) | 2.0 (1.1−3.6) | 1.5 (1.2,1.8) | 0.6 |
| Mechanical ventilation duration (days) | 5.0 (2.0−10.6) | 5.9 (3.0−10.0) | 4.5 (2.0−10.0) | 8.2 (2.0−18.6) | 2.7 (1.1−5.4) | 0.067 |
| CRRT | 59 (25%) | 3 (15%) | 27 (21%) | 24 (41%) | 5 (22%) | 0.025 |
| CRRT duration (days) | 4.2 (3.0−8.5) | 5.0 (4.0−5.4) | 6.0 (3.0−9.5) | 3.7 (2.0−6.2) | 2.4 (0.6−8.7) | 0.4 |
Abbreviations: CRRT, continuous renal replacement therapy; ECMO, ExtraCorporeal Membrane Oxygenation; IABP, intraortic balloon pump.
Data are presented as n (%) for categorical variables and median (25%, 75% percentiles) for continuous variables.
Derived through χ 2 or Fisher's exact test (frequencies < 5) for categorical variables and one‐way analysis of variance for continuous variables.
3.3. SCAI classification stages
Figure 1 describes the SCAI shock classification upon admission, by prospective physician assessment as well as for further refinement according to the updated criteria, and at 24‐h. For what regards the updated SCAI shock classification on admission, 20 (8%) patients had a SCAI stage B, while 131 (55%), 61 (26%) and 25 (11%) patients had SCAI stages C, D, and E, respectively. Such updated classification restaged 41 (17%) patients, 8 (3%) of whom with SCAI stage B, 20 (8%) with SCAI stage C, 7 (3%) with SCAI stage D and 6 (2.5%) with SCAI stage E. At 24 h, 202 out of 237 patients (85%) had a SCAI assessment: 15 (7%) SCAI stage A, 28 (19%) SCAI stage B, 102 (50%) SCAI stage C, 35 (17%) SCAI stage D and 12 (6%) SCAI stage E. Worth note, 42% of the SCAI B patients worsened their classification at 24 h compared to the admission evaluation, whereas only 8.3% improved (Figure 2, Figure 3). Although statistical significance is not achieved, a 100% and 50% increase in median values of lactates and central venous pressure (CPV) has been observed at 24 h for SCAI B patients who worsened their status (Supporting Information: Appendix Table 2).
Figure 1.
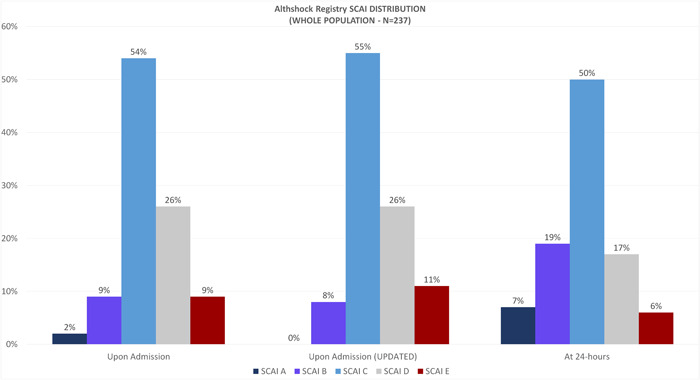
SCAI classification upon admission (prospective physician assessment and further refinement according to the updated criteria) and at the 24‐h evaluation in the whole population. SCAI, Society for Cardiovascular Angiography and Interventions.
Figure 2.
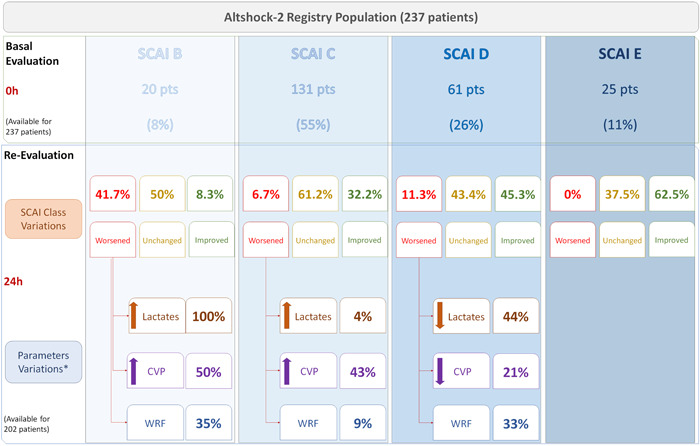
SCAI classification upon admission (prospective physician assessment and further refinement according to the updated criteria) and at the 24‐h evaluation, with SCAI class variations. CVP, central venous pressure; SCAI, Society for Cardiovascular Angiography and Interventions; WRF, worsening renal function.
Figure 3.
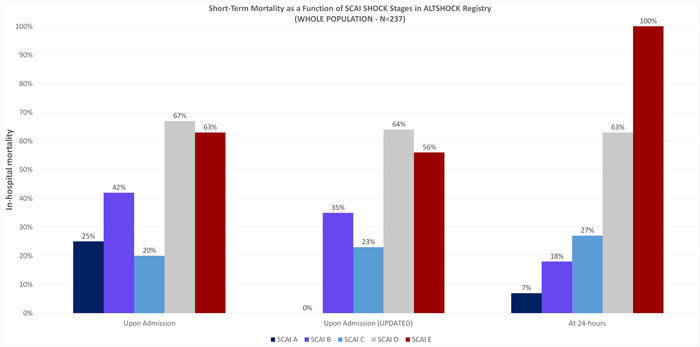
In‐hospital mortality for different SCAI stages upon admission (prospective assessment and updated classification) and at the 24‐h evaluation, in the whole study population. SCAI, Society for Cardiovascular Angiography and Interventions.
Supporting Information: Appendix Figure 1 shows the SCAI shock stages classification in the Altshock‐2 registry and in other recent international studies.
3.4. In‐hospital outcome
Ninety patients died (38%) in‐hospital, with a statistically significant (p < 0.001) higher rate in SCAI stages D and E. Mortality rates stratified according to SCAI classification upon admission and at 24 h are described in Figure 3, while Supporting Information: Appendix Figure 2 shows the mortality rate according to SCAI shock classification upon admission and at 24 h assessment in the Altshock‐2 registry and in other recent international studies.
The SCAI classification prospectively assessed by the physician taking care of the patient upon admission as well as the updated SCAI classification are significantly associated with in‐hospital mortality. According to the latter, a statistically significant increased risk of in‐hospital mortality was detected in patients with SCAI shock stage D (OR 3.29, 95% CI 1.17−9.94, p = 0.03) as compared to SCAI shock stage B. The risk magnitude increased at 24 h; compared to SCAI shock stage A, patients classified as SCAI shock stage D had an OR of 23.7 (95% CI 4.05−455, p = 0.004), while all patients classified with SCAI shock stage E died before discharge (Supporting Information: Appendix Table 3). After adjusting for age, sex, lactates, eGFR, PVC, inotropic score and MCS, SCAI classification at 24 h was still an independent predictor of in‐hospital mortality (Supporting Information: Appendix Table 4).
4. DISCUSSION
Our prospective multicenter Altshock‐2 registry provides new insight on a real‐world population of patients admitted for CS. We report that in a CS population with a relevant prevalence of ADHF‐CS patients, the SCAI shock stage classification, both in the original definition prospectively assigned and in the updated version refined in a retrospective fashion, was associated to in‐hospital mortality. Such classification potential slightly improved, although not significantly, at the 24 h evaluation, as shown by an increased in‐hospital mortality risk for all SCAI stages. This process of deterioration through the SCAI stages is tracked by increasing CVP and inotropic score.
This registry shows that SCAI staging is a dynamic process and suggests that its evaluation 24 h after shock onset is better suited to predict mortality, compared with the initial assessment.
Baran et al. 12 prospectively validated the SCAI shock staging in a series of 166 consecutive single center patients; several of them suffered from lung failure and required VV ECMO. They similarly showed that demographics, hemodynamics, LVEF and laboratory values do not correlate with the SCAI stage and that the 24 h reassessment improves mortality prediction. This was true regardless of intervention, either circulatory support or medical management alone, underlining the importance of timely treatment.
Our registry is in line with this report 12 in suggesting that the treatment of CS should be timely and aggressive, as it should be able to quickly restore the hemodynamic status of the sickest patients. This concept is consistent with the improved prognostication of the 24‐h classification, which takes into account the results of the early phase management. This finding is novel and further applies to SCAI B patients which deteriorate in half of cases within 24 h using contemporary therapeutic approaches.
The 100% mortality reported among patients classified as SCAI Stage E at the 24 h evaluation highlights the need of rapid and appropriate mechanical support in the sickest patients to provide any hemodynamic improvement and prompt shock reversal within 24 h. To act accordingly during this limited time‐window, it is essential to identify reliable markers of clinical deterioration. Although high lactates were associated with SCAI level E, only a minority of SCAI D patients deteriorating over the first 24 h showed an increase in lactates or a worsening renal function. On the contrary, CVP and inotropic score identified deterioration; however, they were not found to be independently associated with in‐hospital mortality at multivariable analysis.
The study found that inotropes are routinely used across all stages of CS with doses increasing with worsening SCAI stages; on the other hand, the use of MCS and respiratory support did not increase significantly with more advanced SCAI stages.
Our work shows some real‐life practices that should be considered in the clinical application of the SCAI classification and, interestingly, major differences between US and Italy. The use of the pulmonary artery catheter was very low and right heart catheterization was eventually performed after the initiation of mechanical support, markedly reducing the applicability of the hemodynamic criteria for the SCAI classification and promoting a wider use of the concepts of hemodynamic failure and hypoperfusion as well as a systematic use of echocardiography. From a pragmatic standpoint, this difference is favored by the distinct infrastructural approach to CS which is mostly based on input from critical care and cardiology in Italy as opposed to algorithms driven by interventional cardiology in the US.
As far as echocardiography, the data from the Alt Shock2 registry show that patients suffered from severe LV dysfunction (median LVEF 20%, Q1−Q3 16−30) without any statistically significant difference according to the SCAI stage; the same was true for other echocardiographic parameters, such as TAPSE (median 15 mm, Q1–Q3 12–18) or estimated systolic PAP (median 45 mmHg, Q1−Q3 36−54). Moderate‐severe MR affected almost half of the population and that warrants thorough consideration, especially when considering device selection for MCS. At baseline only mean arterial pressure, lactates and transaminases discriminate between SCAI stages.
Thayer et al. 13 showed that CVP is associated with increased mortality and higher SCAI stage and envisioned the need for future management protocols calling for early venous decongestion. Taken together with the data from Schrage, 14 which showed the association of lactates with mortality, the current data would suggest the opportunity to rapidly treat patients with CS with the aim of reducing lactates and venous congestion rapidly and ideally within 24 h. Future studies are warranted to identify the best strategies in this regard.
4.1. Limitations
Our study has some limitations. First, at the time of writing the Altshock‐2 registry sample size is still limited to allow generalizable findings. The registry started to recruit patients in March 2020, during the first COVID‐19 pandemic wave, when several of the 11 North Italian centers who contributed to patients' enrollment were fully dedicated to manage COVID patients. In addition, our registry collects data from tertiary centers with large experience in CS and with active transplantation and VAD programs. Secondly, the updated SCAI stage refinement stratification has been performed by two investigators and is not externally validated. However, this refinement did not substantially change the prognostic role of the prospective assessment. Third, clinicians applied institutional protocols and best practices which might differ across centers. Finally, outcome differences across AMI‐CS and ADHF‐CS patients will be investigated in a separate investigation of the Altshock‐2 registry. Although at time of writing our limited sample did not powerfully allow to draw definitive conclusions, a preliminary analysis did not show a significant interaction effect of CS etiology and updated SCAI score upon admission on in‐hospital mortality. However, mixing the two groups of patients may skew the outcome as ADHF‐CS is a chronic disease with a different degree of end organ adaptation.
4.2. Strengths
This is the first registry in Italy to enroll and collect data from patients who are not able to consent, following the endorsement of the legislation and direction from the UE 536/2014/EC. This allowed the enrolment of every consecutive patient, thus markedly reducing selection bias and increasing the generalization of findings.
Participating centers are experienced in MCS and have all contemporary devices available for clinical use.
For the first time the updated SCAI classification is implemented in a large series of patients who were prospectively stratified according to the original version. Expending the SCAI stage classification to 24 h after admission gives an opportunity to have a trajectory of the impact of hospital treatments to identify the need for escalation.
5. CONCLUSIONS
Using the data of the prospective multicenter Altshock‐2 registry, we were able to provide new insights on a all‐comers real‐world population of patients admitted for CS in Italy. We report the following major findings: (1) the prevalence of ADHF‐CS appears to be increasing relative to AMICS; (2) the potential for SCAI shock stages to identify the risk of in‐hospital mortality increases at the 24‐h reassessment rather than upon admission; (3) the deterioration of the SCAI stage is tracked by CVP and inotropic score; (4) timely interventions might be deployed as deterioration is captured via the worsening of the SCAI stage.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix Figure 1: SCAI shock stages distribution among different international studies. Data are presented as %.
Appendix Figure 2: Short term mortality comparison between different studies related to SCAI classification. Data are presented as %. @= in‐hospital mortality. #= 30‐day mortality.
Supplementary information.
ACKNOWLEDGMENTS
We thank Dr Dario Brunelli for data management and graphical support.
We thank all the Investigators involved in the Altshock program. Open access funding provided by BIBLIOSAN.
Morici N, Frea S, Bertaina M, et al. SCAI stage reclassification at 24 h predicts outcome of cardiogenic shock: insights from the Altshock‐2 registry. Catheter Cardiovasc Interv. 2023;101:22‐32. 10.1002/ccd.30484
DATA AVAILABILITY STATEMENT
Data will become available to interested investigators, upon submission of a reasonable research request, approved by the chief investigators
REFERENCES
- 1. Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8:e011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldberg RJ, Makam RCP, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade‐long trends (2001‐2011) in the incidence and hospital death rates associated with the in‐hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock: clinical picture and outcome of cardiogenic shock. Eur J Heart Fail. 2015;17(5):501‐509. 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 5. Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12(3):e005618. 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valente S, Marini M, Battistoni I, et al. [Cardiogenic shock is a rare disease: the dedicated network]. G Ital Cardiol (Rome). 2017;18(10):719‐726. 10.1714/2790.28261 [DOI] [PubMed] [Google Scholar]
- 7. Rab T, Ratanapo S, Kern KB, et al. Cardiac shock care centers. J Am Coll Cardiol. 2018;72(16):1972‐1980. 10.1016/j.jacc.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 8. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American college of cardiology (ACC), the American heart association (AHA), the society of critical care Medicine (SCCM), and the society of thoracic surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29‐37. 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 9. Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies: this statement was endorsed by the American college of cardiology (ACC), American college of emergency physicians (ACEP), American heart association (AHA), european society of cardiology (ESC) association for acute cardiovascular care (ACVC), international society for heart and lung transplantation (ISHLT), society of critical care Medicine (SCCM), and society of thoracic surgeons (STS) in December 2021. J Am Coll Cardiol. 2022. 79(9):933‐946. 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 10. World Medical Association . World medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11. Vandenbroucke JP, von Elm E, Altman DG, et al. STROBE Initiative Strengthening the reporting of observational studies in Epidemiology(STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: single center analysis. Catheter Cardiovasc Interv. 2020;Dec 96(7):1339‐1347. 10.1002/ccd.29319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thayer KL, Zweck E, Ayouty M, et al. Invasive hemodynamic assessment and classification of In‐Hospital mortality risk among patients with cardiogenic shock. Circulation: Heart Failure. 2020;13(9):e007099. 10.1161/CIRCHEARTFAILURE.120.007099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96(3):E213‐E219. 10.1002/ccd.28707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1: SCAI shock stages distribution among different international studies. Data are presented as %.
Appendix Figure 2: Short term mortality comparison between different studies related to SCAI classification. Data are presented as %. @= in‐hospital mortality. #= 30‐day mortality.
Supplementary information.
Data Availability Statement
Data will become available to interested investigators, upon submission of a reasonable research request, approved by the chief investigators


