Abstract
Tetrazine (Tz)–trans‐cyclooctene (TCO) ligation is an ultra‐fast and highly selective reaction and it is particularly suited to label biomolecules under physiological conditions. As such, a 3H‐Tz based synthon would have wide applications for in vitro/ex vivo assays. In this study, we developed a 3H‐labeled Tz and characterized its potential for application to pretargeted autoradiography. Several strategies were explored to synthesize such a Tz. However, classical approaches such as reductive halogenation failed. For this reason, we designed a Tz containing an aldehyde and explored the possibility of reducing this group with NaBT4. This approach was successful and resulted in [3H]‐(4‐(6‐(pyridin‐2‐yl)‐1,2,4,5‐tetrazin‐3‐yl)phenyl)methan‐t‐ol with a radiochemical yield of 22 %, a radiochemical purity of 96 % and a molar activity of 0.437 GBq/μmol (11.8 Ci/mmol). The compound was successfully applied to pretargeted autoradiography. Thus, we report the synthesis of the first 3H‐labeled Tz and its successful application as a labeling building block.
Keywords: autoradiography, bioorthogonal chemistry, pre-targeting, tetrazine ligation, tritiation
A tritiated tetrazine based on a phenyl/pyridyl core structure was successfully radiolabeled with NaBT4 by reducing an aldehyde moiety. The synthon was effectively employed to label an antibody in vitro with pretargeted autoradiography.
1. Introduction
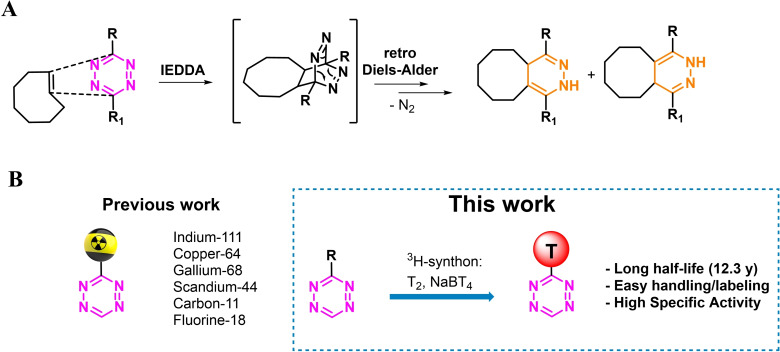
Recently, the interest in tetrazines (Tz) has exponentially increased due to the emergence of click‐chemistry as an important tool in drug development. These molecules are usually employed as bioorthogonal reagents with strained alkenes (e. g. trans‐cyclooctenes (TCOs)) in a reaction called tetrazine ligation. The Tz ligation occurs by means of the inverse electron‐demand Diels‐Alder (iEDDA) cycloaddition, followed by the retro‐Diels Alder resulting in N2 elimination (Figure 1A). [1] The wide range of applications of this transformation includes but are not limited to: live cell imaging, bioconjugation, nanoscience and nuclear medicine, the latter both for therapeutic and diagnostic (even theranostic) applications.[ 1c , 2 ] Moreover, the TCO‐Tz ligation holds favorable properties such as high selectivity and superior reaction kinetics rate constants (up to 106−7 M−1 s−1) compared to other (bio)orthogonal tools. [3] Over the years, several Tzs radiolabeling strategies emerged employing different nuclides such as fluorine‐18, carbon‐11, iodine‐123 or indium‐111 (Figure 1B). [4] However, to the best of our knowledge, the synthesis of a tritiated Tz has never been reported. Tritium labeled compounds are essential to drug discovery due to their relatively ease and safety to handle. [5] In this study, we report the first synthesis of a 3H‐labeled Tz as well as its application for pretargeted autoradiography (Figure 1B). [6]
Figure 1.
A) The tetrazine‐TCO ligation. B) Previously reported tetrazines were labeled with fluorine‐18, carbon‐11, copper‐64, gallium‐68, scandium‐44 (pet) and indium‐111 (SPECT) radionuclides. This study reports the first synthesis of a 3H‐labeled Tz.
2. Results and Discussion
2.1. Initial attempts
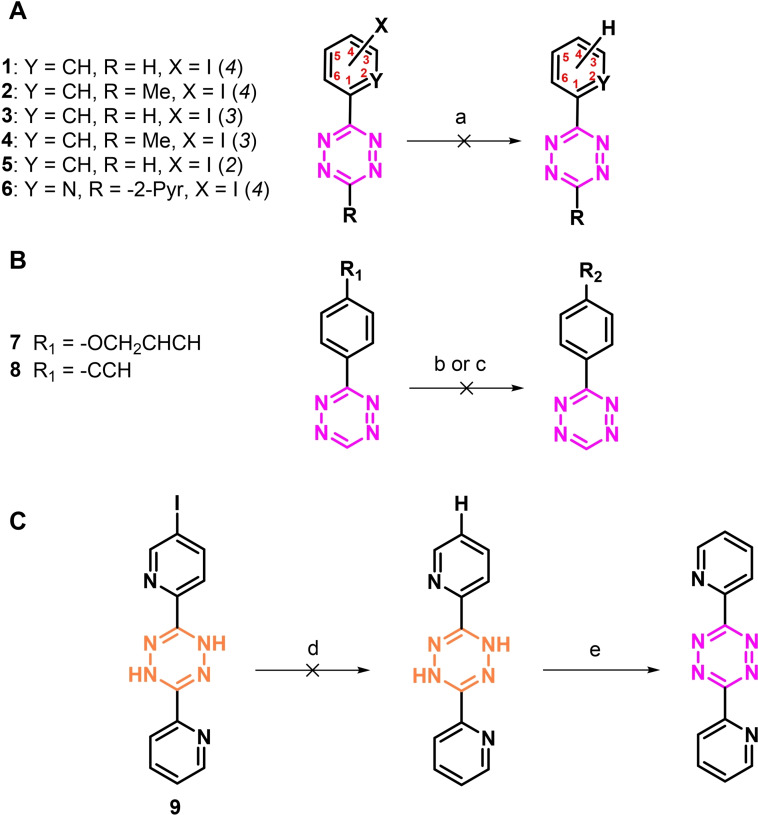
The feasibility to use tritium gas to radiolabel Tzs was evaluated using H2 (g) before attempting radioactive procedures. Reaction conditions were carefully screened with respect to catalyst, temperature, hydrogen source, solvents, and time. Various Tz precursors (e. g., methyl‐ Tzs, H‐Tzs or bispyridyl Tzs) substituted with an iodine atom at different positions were additional investigated. None of the applied procedures resulted in the desired product (Scheme 1A, SI Table S1). In a next step, alkene and alkyne bearing Tzs were unsuccessfully attempted to be reduced (Scheme 1B). Our results suggest that the tetrazine moiety is inhibiting the applied Pd‐catalyst. As Tzs are widely employed moieties to coordinate metals, the observed behavior is likely. [7] To confirm this hypothesis the stable dihydro tetrazine derivative 9 was synthesized. However, again no reduction was observed suggesting that the Tz core even in the dihydro form can inhibit Pd‐catalyzed reduction.
Scheme 1.
A) Reduction of different iodine‐substituted tetrazines with H2. Reagents and conditions: a) For further details please check Table S1 in the SI. B) Reduction of alkene group in 3‐(4‐(allyloxy)phenyl)‐1,2,4,5‐tetrazine 7 and alkyne group in 3‐(4‐ethynylphenyl)‐1,2,4,5‐tetrazine 8. Reaction conditions: b) Pd/C, H2, R.T. to 100 °C, EtOH, 72 h; or Pd/C, H2, R.T. to 100 °C, TEA, EtOH, 72 h; or Pd/C, H2, HCO2NH4, R.T. to 100 °C, MeOH/THF, 72 h; c) Lindlars catalyst, quinoline, H2 (g), R.T. 72 h. C) Alternative reduction of bispyridyl tetrazine. Reagents and conditions: d) Pd/C, H2, R.T., EtOH, 72 h; 0 % or Pd/C, H2, R.T. to 100 °C, TEA, EtOH, 72 h; or Pd/C, H2, HCO2NH4, R.T. to 100 °C, MeOH/THF, 72 h; e) NaNO2, AcOH, H2O, rt, 10 min.
2.2. Reduction with sodium borotritide (NaBT4)
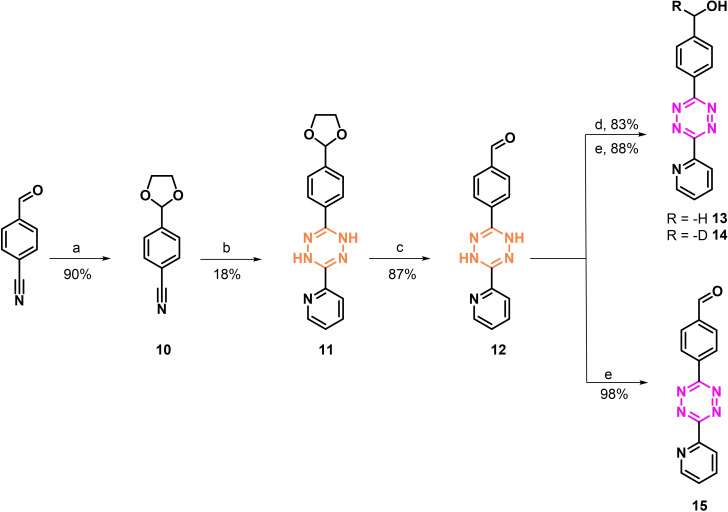
Since all attempts failed to synthesize a 3H‐labeled tetrazine using Pd‐chemistry, we explored if reduction of an aldehyde with NaBT4 could be employed. We investigate this strategy first non‐radioactively using NaBH4. As the Tz core is known to be reduced to its corresponding dihydro analogues with NaBH4, we decided to start the reaction from the dihydro‐Tz, sequentially reduced the aldehyde group and oxidized then selectively the dihydro‐Tz to the Tz core. [8] We selected a phenyl‐pyridyl Tz analogue in order to avoid autooxidation that is typically observed for diphenyl Tzs. Bispyridyl Tzs are known to be more reactive but less stable than phenyl‐pyridyl Tz and as such, we believed that phenyl‐pyridyl Tz display the best choice to develop a 3H‐labeled Tz derivative. [9] It is known that the limited stability of tetrazines in biological media strongly correlates with the electron‐withdrawing effect of the substituents and for this reason bispyridyl Tzs undergo faster decomposition compared to phenyl‐pyridyl Tz. [9] The synthesis of the precursor and the reference compounds are shown in Scheme 2. Briefly, 4‐formylbenzonitrile was converted to the corresponding acetal 10 under acidic catalysis in quantitative yields. The latter was reacted with an excess of 2‐carbonitrile and hydrazine hydrate to afford the dihydrotetrazine 11. [10] The dioxolane moiety was then hydrolyzed to the corresponding formyl compound 12 under microwave irradiation. Reduction with NaBH4 or NaBD4 followed by oxidation with NaNO2 afforded respectively Tz 13 and 14. Differently direct oxidation of compound 12 gave aldehyde 15.
Scheme 2.
Reagents and conditions. a) Ethylene glycol, PTSA, toluene, reflux, 18 h; b) 2‐pyridinecarbonitrile, NH2NH2 ⋅ H2O, S8, EtOH, 90 °C, 12 h; c) H2O, DMSO, MW 110 °C, 1 h; d) NaBH4 or NaBD4, MeOH/CH2Cl2, rt, 5 min; e) NaNO2, AcOH, H2O, rt, 10 min.
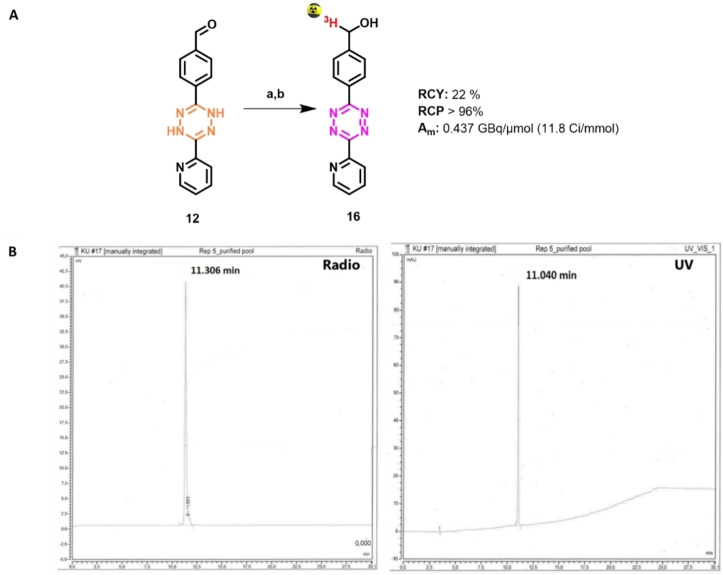
Encouraged by these results, the tritiation of dihydro‐Tz 12 to Tz 16 was performed using the same reduction‐oxidation sequence (Figure 2). The product 3H‐Tz 16 was obtained with a radiochemical yield (RCY) of 22 %, a radiochemical purity (RCP) of 96 % and a molar activity (Am) of 0.437 GBq/μmol (11.8 Ci/mmol). [11]
Figure 2.
A) Tritiation of 12: a) NaBT4, MeOH, CH2Cl2, rt, 5 min. b) NaNO2, AcOH, H2O, rt, 10 min. B) HPLC chromatograms of the final product [3H]16.
2.3. Pretargeted autoradiography
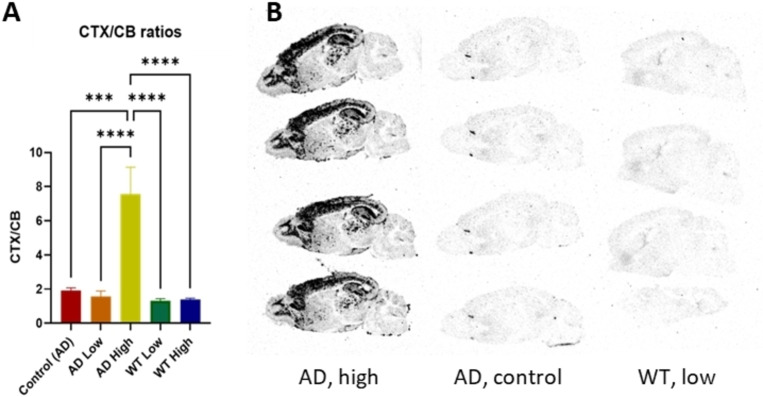
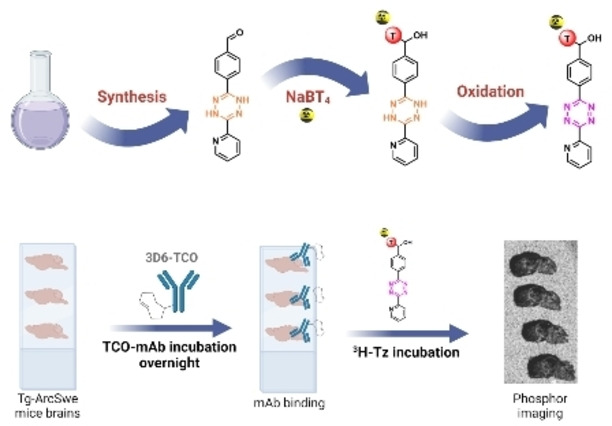
To determine the possibility of [3H]16 to be used as a synthon, we performed pretargeted imaging. TCO‐mAb 3D6 (anti‐Abeta) was applied to brain of an amyloid‐beta (Aβ) pathology mouse model (Tg‐ArcSwe). After incubation of the Tg‐ArcSwe mice brain slices with TCO‐modified 3D6 overnight, excess antibody was washed away followed by incubation of the [3H]16. Significant amount of cortical (CTX) binding was observed in comparison to the cerebellum (CB), which is in agreement with the Aβ deposition pattern (Figure 3). Wild type and control animals showed a 4‐fold lower CTX/CB ratio.
Figure 3.
Results of the pretargeted autoradiography. A) CTX/CB ratio of [3H]16 binding. Control=AD Brain, 0 μg/mL mAb‐TCO. AD low=AD brain, 0.006 μg/mL mAb‐TCO. AD High=AD brain, 0.06 μg/mL mAb‐TCO. WT low=WT brain, 0.006 μg/mL mAb‐TCO. WT High=WT brain, 0.06 μg/mL mAb‐TCO. Standard deviation calculated ANOVA. B) Autoradiographic image, showing the image contrast between AD High, AD control and WT low.
3. Conclusion
In conclusion, a tritiation strategy was successfully developed for the labeling of Tz 16 using sodium borotritide. The compound was obtained in satisfactory RCY of 22 % and high RCP of >96 %. [3H]16 was thereafter used for pretargeted autoradiography. Preincubation of TCO‐3D6 followed by application of [3H]16 confirmed the ability of the [3H]16 to be used for evaluation experiments. This study might pave the road to develop other tritiated Tzs which could even be used for ex vivo applications.
Experimental Section
Chemistry
General
All reagents and solvents were dried prior to use according to standard methods. Commercial reagents were used without further purification. Analytical TLC was performed using silica gel 60 F254 (Merck) with detection by UV absorption and/or by charring following immersion in a 7 % ethanolic solution of sulfuric acid or KMnO4‐solution (1.5 g of KMnO4, 10 g K2CO3, and 1.25 mL 10 % NaOH in 200 mL water). Purification of compounds was carried out by column chromatography on silica gel (40–60 μm, 60 Å) or employing a CombiFlash NextGen 300+ (Teledyne ISCO). 1H and 13C NMR spectra were recorded on Brucker (400 and 600 MHz instruments), using chloroform‐d, methanol‐d 4 or DMSO‐d 6 as deuterated solvent and with the residual solvent as the internal reference. For all NMR experiments the deuterated solvent signal was used as the internal lock. Chemical shifts are reported in δ parts per million (ppm). Coupling constants (J values) are given in Hertz (Hz). Multiplicities of 1H NMR signals are reported as follows: s, singlet; d, doublet; dd, doublet of doublets; ddd, doublet of doublets of doublets; dt, doublet of triplets; t, triplet; q, quartet; m, multiplet; br, broad signal. NMR spectra of all compounds are reprocessed in MestReNova software (version 12.0.22023) from original FID's files. Mass spectra analysis was performed using MS‐Acquity‐A: Waters Acquity UPLC with QDa‐detector. Purification by preparative HPLC was performed on Agilent 1260 infinity system, column SymmetryPrep‐C18, 17 mL/min H2O‐MeCN gradient 50–100 % 15 min with 0.1 % trifluoroacetic acid. All final compounds were >95 % pure as determined by analytical HPLC. Analytical HPLC method: (Thermo Fisher® UltiMate 3000) with a C‐18 column (Luna® 5u C18(2) 100 Å, 150×4.6 mm), eluents: A: H2O with 0.1 % TFA, B: MeCN with 0.1 % TFA. Gradient from 100 % A ‐>100 % B over 15minutes, back to 100 % A over 4 minutes, flow rate 1.5 mL/min. Detection by UV‐absorption at λ=254 nm on a UVD 170 U detector.
The modified antibody used in this study was kindly provided by Tagworks Pharmaceuticals. NaBT4 (specific activity: 2.96 TBq/mmol, 3.7 GBq, 1.25 μmol) was obtained from RC TRITEC AG (Teufen, Switzerland), stored as a solid in a sealed ampoule under argon gas at 750 mbar at room temperature. HPLC purification was carried out on a semi‐preparative C18 Luna Column (5 μm, 100 Å, 150×10 mm) and monitored with a 254 nm UV detector and a Raytest Gabi NaI detector. The solvent systems used were water (0.1 % TFA, solvent A) and acetonitrile (0.1 % TFA, solvent B) with a flow rate of 3 mL min−1. The RCY was determined by comparison of the starting activity which was present in the reaction mixture vial and the radioactivity present in the purified and formulated product. Radioactivity was measured by liquid scintillation counting. Molar activity was quantified by standard curve.
Previously described compounds
Tzs 1–6, 8 and 9 were synthesized as previously described by Garcia et al. and Steen et al.[ 4b , 12 ]
Synthesis of 3‐(4‐(allyloxy)phenyl)‐1,2,4,5‐tetrazine (7)
The title compound was obtained from 4‐(allyloxy)benzonitrile (637 mg, 4.00 mmol) following the procedure from Garcia et al.[ 4b , 12 ] The tetrazine was purified via flash chromatography using n‐heptane: EtOAc (ethyl acetate) (95/5) as eluent, yielded as a pink solid (50 mg, 4 %). Rf=0.41 (n‐heptane: EtOAc: 9/1). 1H NMR (400 MHz, CDCl3) δ 10.12 (s, 1H), 8.64–8.43 (m, 2H), 7.16–7.00 (m, 2H), 6.17–5.91 (m, 1H), 5.55–5.39 (m, 1H), 5.39–5.27 (m, 1H), 4.66 (s, 2H).
4‐(6‐(Pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (12)
4‐(1,3‐dioxolan‐2‐yl)benzonitrile (10): The compound was synthesized accordingly to the previously published procedure Boyer et al. [13] Ethylene glycol (3.07 mL, 54.90 mmol) and p‐toluene sulfonic acid (0.46 g, 2.44 mmol) were added into a solution of 4‐cyanobenzaldehyde (4 g, 30.50 mmol) in 150 mL of toluene. The reaction mixture was refluxed for 12 h and a Dean‐Stark trap was used to remove generated water during the reaction. After cooled to room temperature, 40 mL of a 5 % NaHCO3 aqueous solution was added. The organic layer was extracted with CH2Cl2, washed with water three times, and dried over Na2SO4. The solvent was removed under reduced pressure to yield 5.2 g (97 %) of 4‐(1,3‐dioxolan‐2‐yl)benzonitrile 10 as a white crystalline solid. Spectral data were identical to what was previously reported. Rf=0.35 (n‐heptane/EtOAc 70/30); 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J=8.4 Hz, 2H), 7.59 (d, J=8.2 Hz, 2H), 5.84 (s, 1H), 4.86–2.69 (m, 4H).
3‐(4‐(1,3‐Dioxolan‐2‐yl)phenyl)‐6‐(pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazine (11): The compound was synthesized accordingly to the previously published procedure. [10] 4‐(1,3‐Dioxolan‐2‐yl)benzonitrile (1.12 g, 6.39 mmol) 2‐cyanopyridine (5.32 g, 51.14 mmol) were dissolved in 100 mL of ethanol with hydrazine hydrate (6.67 mL, 95.89 mmol) and warmed to 60 °C. After 15 minutes, sulfur (410 mg, 1.59 mmol) was added, and the solution refluxed for 12 h. The mixture was cooled to room temperature and the ethanol was removed under reduced pressure. Purification by flash chromatography (CH2Cl2/MeOH 95/5) afforded 0.35 g (18 %) of the desired product as a yellow solid. Rf=0.48 (n‐Heptane/EtOAc 50/50); 1H NMR (400 MHz, CDCl3) δ 8.78–8.46 (m, 2H), 8.04 (dt, J=8.0, 1.1 Hz, 1H), 7.78 (td, J=7.8, 1.7 Hz, 1H), 7.57 (d, J=8.3 Hz, 2H), 7.38 (ddd, J=7.6, 4.9, 1.2 Hz, 2H), 7.18 (s, 1H), 5.87 (s, 1H), 4.28–4.00 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 148.43, 147.35, 147.25, 147.13, 140.44, 136.76, 130.94, 126.94, 125.95, 124.99, 121.22, 103.06, 65.35; HPLC‐MS [M+H]+ m/z: calcd for [C16H16N5O2]+, 310.1; found, 310.1.
4‐(6‐(Pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (12): 3‐(4‐(1,3‐Dioxolan‐2‐yl)phenyl)‐6‐(pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazine (0.12 g, 0.39 mmol) was added to a microwave vial equipped with a stir bar which was then sealed and purged with N2. Water (6 mL) and DMSO (1 mL) were added via a syringe and the reaction allowed to stir at 110 °C in a microwave for 1 hour. The reaction was allowed to cool to room temperature and unsealed. The suspension was extracted with CH2Cl2 washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (n‐Heptane/EtOAc 60/40) afforded 0.09 g (87 %) of the desired compound as a yellow solid. Rf=0.38 (n‐Heptane/EtOAc 50/50); 1H NMR (400 MHz, DMSO) δ 10.06 (s, 1H), 9.44 (s, 1H), 8.91 (s, 1H), 8.65 (dd, J=4.8, 1.5 Hz, 1H), 8.04 (d, J=8.2 Hz, 2H), 8.00–7.89 (m, 4H), 7.54 (ddd, J=6.5, 5.0, 1.5 Hz, 1H); 13C NMR (101 MHz, DMSO) δ 193.10, 149.08, 147.62, 147.36, 146.78, 137.88, 137.50, 135.68, 130.06, 127.15, 125.82, 121.47; HPLC‐MS [M+H]+ m/z: calcd for [C14H12N5O]+, 266.1; found, 266.1.
Synthesis of (4‐(6‐(pyridin‐2‐yl)‐1,2,4,5‐tetrazin‐3‐yl)phenyl)methanol (13)
To a solution of 4‐(6‐(pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (0.03 g, 0.11 mmol) in MeOH/CH2Cl2 (6 mL, 50/50 v/v) was added NaBH4 (0.005 g, 0.13 mmol). The reaction was stirred at room temperature for 10 minutes. A solution of NaNO2 (0.08 g, 1.13 mmol) in water (5 mL) was added to the mixture. Glacial acetic acid (3 mL) was added, and the reaction turned bright red. The mixture was stirred for 30 minutes and subsequently extracted with CH2Cl2 washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (CH2Cl2/MeOH 95/5) afforded 0.025 g (83 %) of the desired compound as a red solid. Rf=0.33 (CH2Cl2/MeOH 98/2); 1H NMR (600 MHz, MeOD) δ 8.76 (d, J=4.9 Hz, 1H), 8.64 (dd, J=8.0, 1.3 Hz, 1H), 8.55 (d, J=8.3 Hz, 1H), 8.05 (td, J=7.8, 1.7 Hz, 1H), 7.61 (ddd, J=7.5, 4.8, 1.2 Hz, 1H), 7.56 (d, J=8.0 Hz, 1H), 4.66 (s, 2H); 13C NMR (151 MHz, MeOD) δ 164.46, 163.04, 150.08, 149.99, 147.15, 138.05, 130.61, 127.97, 127.13, 126.59, 123.82, 63.22; HPLC‐MS [M+H]+ m/z: calcd for [C14H12N5O]+, 266.1; found, 266.1.
Synthesis of (4‐(6‐(pyridin‐2‐yl)‐1,2,4,5‐tetrazin‐3‐yl)phenyl)methan‐d–ol (14)
To a solution of 4‐(6‐(Pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (0.017 g, 0.06 mmol) in MeOH/CH2Cl2 (6 mL, 50/50) was added NaBD4 (0.003 g, 0.07 mmol). The reaction was stirred at room temperature for 10 minutes. A solution of NaNO2 (0.044 g, 0.64 mmol) in water (5 mL) was added to the mixture. Glacial acetic acid (3 mL) was added, and the reaction turned bright red. The mixture was stirred for 30 minutes and subsequently extracted with CH2Cl2 washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (CH2Cl2/MeOH 95/5) afforded 0.015 g (88 %) of the desired compound as a red solid. Rf=0.35 (CH2Cl2/MeOH 98/2); 1H NMR (600 MHz, CDCl3) δ 9.07–8.92 (m, 1H), 8.74–8.64 (m, 3H), 8.00 (td, J=7.7, 1.8 Hz, 1H), 7.61 (d, J=8.2 Hz, 2H), 7.57 (ddd, J=7.6, 4.7, 1.2 Hz, 1H), 4.84 (t, J=1.9 Hz, 1H); 13C NMR (151 MHz, CDCl3) δ 164.20, 163.38, 150.90, 150.31, 146.28, 137.47, 130.67, 128.61, 127.47, 126.34, 123.92, 64.45 (t, J=21.9 Hz); HPLC‐MS [M+H]+ m/z: calcd for [C14H11DN5O]+, 267.1; found, 267.1.
Synthesis of 4‐(6‐(pyridin‐2‐yl)‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (15)
To a solution of 4‐(6‐(Pyridin‐2‐yl)‐1,4‐dihydro‐1,2,4,5‐tetrazin‐3‐yl)benzaldehyde (0.05 g, 0.18 mmol) in MeOH/CH2Cl2 (6 mL, 50/50) was added a solution of NaNO2 (0.13 g, 1.88 mmol) in water (5 mL). Glacial acetic acid (3 mL) was added, and the reaction turned bright red. The mixture was stirred for 30 minutes and subsequently extracted with CH2Cl2 washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. Purification by flash chromatography (CH2Cl2/MeOH 95/5) afforded 0.045 g (91 %) of the desired compound as a red solid. Rf=0.27 (CH2Cl2/MeOH 99/1); 1H NMR (400 MHz, CDCl3) δ 10.18 (s, 1H), 9.04–8.97 (m, 1H), 8.89 (d, J=8.3 Hz, 2H), 8.74 (dd, J=8.0, 1.1 Hz, 1H), 8.14 (d, J=8.4 Hz, 2H), 8.03 (td, J=7.8, 1.8 Hz, 1H), 7.60 (ddd, J=7.7, 4.8, 1.2 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 191.57, 163.84, 163.58, 151.11, 150.01, 139.18, 137.53, 136.73, 130.36, 128.95, 126.65, 124.28; HPLC‐MS [M+H]+ m/z: calcd for [C14H10N5O]+, 264.1; found, 264.1.
Radiolabeling [3H]‐(4‐(6‐(pyridin‐2‐yl)‐1,2,4,5‐tetrazin‐3‐yl)phenyl)methan‐t–ol (16)
To 100 μL of a solution of the precursor 12 (3 mg/mL in DCM, 130 μL, 1.5 μmol, 1.2 eq.) was added 100 μL of NaBT4 (3.7 GBq, 1.25 μmol, 1 eq.) solution in MeOH was added. The reaction was stirred for 5 minutes at room temperature. The reaction was monitored with HPLC and after 5 minutes, a conversion was observed from precursor 12 (RT=12.9 min) to the reduced intermediate (RT=10.5 min). A saturated solution of NaNO2 was added in excess. The reaction was acidified with AcOH until the color changed to red (pH 2–3) to fully oxidize the compound to the Tz 16. The DCM layer was extracted twice, and the crude was injected on analytical HPLC, showing a full conversion to product 16 (RT=11.3 min). The crude was purified with semi‐prep HPLC. The product was [3H]16 with an RCY of 22 %, based on liquid scintillation counting, RCP of 96 %, based on radio‐HPLC and a molar activity of 11.8 Ci/mmol.
Pretargeted autoradiography
All animal procedures were performed in accordance with the European Commission's Directive 2010/63/EU, as well as the ARRIVE guidelines, and were approved by the Danish Council of Animal Ethics (Journal no. 2017‐15‐0201‐01375). Autoradiography was performed using 20 μm cryosections containing sagittal sections of Tg‐ArcSwe mice brains. Frozen brains were sectioned on a cryostat (Leica CM1800, Leica Biosystems, Buffalo Grove, IL, United States) and mounted on Superfrost Plus™ adhesion microscope slides (Thermo Fischer Scientific, MS, United States). Sections were stored at −80 °C for the remaining period of the study. The sections were thawed at 4 °C before the study, and consequently pre‐incubated with PBS containing 1 % BSA (700 mL/slide) for 30 minutes. Buffer was removed from the slides and PBS containing 0.05 % TWEEN‐20 was added to the slides (700 μL/slide) and incubated for 5 minutes. After 5 minutes two concentrations of TCO‐mAb 3D6 solution was added to the slides, the high concentration being 0.06 μg/mL mAb‐TCO and the low concentration=0.006 μg/mL mAb‐TCO, this was incubated for 24 h. After 24 h excess TCO‐mAb was removed by washing in cold PBS (3×15 min) and cold distilled water (1×30 s). Consequently, 20 nM of radiolabeled [3H]16 in PBS containing 0.05 % TWEEN‐20 was added to all slides (700 μL/slide) and incubated for 1 h at room temperature. Non‐specific binding was determined with two control slides of WT brain slices and one slide containing Tg‐ArcSwe mice brain with no mAb‐TCO incubated but PBS. Excess radioactive solution was washed with cold distilled water (1×30 s). The slides were subjected to a gentle airflow and fixated overnight in a paraformaldehyde vapor chamber in cold storage (4 °C). After 24 h the slides were moved to an exicator for 1 h to remove any excess moisture and then arranged for exposure to a tritium sensitive imaging plate (BAS‐IP TR2040, Science Imaging Scandinavia AB, Nacka, Sweden), together with tritium standards (RPA510, Amersham Bioscience, GE Healthcare, Chicago, IL, USA. After six days of exposure the plate was read out by a Fujifilm BAS 1000 scanner (RPA510, Amersham Bioscience, GE Healthcare, Chicago, IL, USA). Analysis and quantification of the autoradiography were performed using OptiQuant and Excel respectively. The four‐parameter general curve fit (David Rodbard, NIH) of decay corrected tritium standards was used to convert mean pixel density (grayscale) to nCi/mg tissue equivalent (TE).
Author Contributions
Conceptualization (lead): M. M. H., U. B. Chemistry: U. B. Labeling: N. B. and A. M. D. Autoradiography: N. B., V. S. and S. L. B. Original draft preparation, review and editing: N. B., U. B. Review and editing: N. B., U. B., M. M. H. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
4.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement No 813528. V. S. was supported by BRIDGE – Translational Excellence Program at the Faculty of Health and Medical Sciences, University of Copenhagen, funded by the Novo Nordisk Foundation (grant agreement no. NNF18SA0034956). TCO‐mAb 3D6 was obtained kindly from Tagworks Pharmaceuticals.
N. S. Radjani Bidesi, U. M. Battisti, S. Lopes van de Broek, V. Shalgunov, A.-M. Dall, J. Bøggild Kristensen, D. Sehlin, S. Syvänen, G. Moos Knudsen, M. M. Herth, ChemBioChem 2022, 23, e202200539.
Data Availability Statement
Data will be made available on request.
References
- 1.
- 1a. Kolb H. C., Sharpless K. B., Drug Discovery Today 2003, 8, 1128–1137; [DOI] [PubMed] [Google Scholar]
- 1b. Zeng D., Zeglis B. M., Lewis J. S., Anderson C. J., J. Nucl. Med. 2013, 54, 829–832; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1c. Devaraj N. K., Weissleder R., Hilderbrand S. A., Bioconjugate Chem. 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.
- 2a. Blackman M. L., Royzen M., Fox J. M., J. Am. Chem. Soc. 2008, 130, 13518–13519; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Stéen E. J. L., Edem P. E., Nørregaard K., Jørgensen J. T., Shalgunov V., Kjaer A., Herth M. M., Biomaterials 2018, 179, 209–245; [DOI] [PubMed] [Google Scholar]
- 2c. Ros E., Prades A., Forson D., Smyth J., Verdaguer X., d. Pouplana L. R., Riera A., Chem. Commun. 2020, 56, 11086–11089; [DOI] [PubMed] [Google Scholar]
- 2d. Best M. D., Biochemistry 2009, 48, 6571–6584; [DOI] [PubMed] [Google Scholar]
- 2e. Billaud E. M. F., Belderbos S., Cleeren F., Maes W., Van de Wouwer M., Koole M., Verbruggen A., Himmelreich U., Geukens N., Bormans G., Bioconjugate Chem. 2017, 28, 2915–2920. [DOI] [PubMed] [Google Scholar]
- 3. Darko A., Wallace S., Dmitrenko O., Machovina M. M., Mehl R. A., Chin J. W., Fox J. M., Chem. Sci. 2014, 5, 3770–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Denk C., Svatunek D., Mairinger S., Stanek J., Filip T., Matscheko D., Kuntner C., Wanek T., Mikula H., Bioconjugate Chem. 2016, 27, 1707–1712; [DOI] [PubMed] [Google Scholar]
- 4b. García-Vázquez R., Battisti U. M., Jørgensen J. T., Shalgunov V., Hvass L., Stares D. L., Petersen I. N., Crestey F., Löffler A., Svatunek D., Kristensen J. L., Mikula H., Kjaer A., Herth M. M., Chem. Sci. 2021, 12, 11668–11675; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Price E. W., Orvig C., Chem. Soc. Rev. 2014, 43, 260–290; [DOI] [PubMed] [Google Scholar]
- 4d. Herth M. M., Andersen V. L., Lehel S., Madsen J., Knudsen G. M., Kristensen J. L., Chem. Commun. 2013, 49, 3805–3807; [DOI] [PubMed] [Google Scholar]
- 4e. Battisti U. M., Bratteby K., Jørgensen J. T., Hvass L., Shalgunov V., Mikula H., Kjær A., Herth M. M., J. Med. Chem. 2021, 64, 15297–15312; [DOI] [PubMed] [Google Scholar]
- 4f. Edem P. E., Sinnes J.-P., Pektor S., Bausbacher N., Rossin R., Yazdani A., Miederer M., Kjær A., Valliant J. F., Robillard M. S., Rösch F., Herth M. M., EJNMMI Res. 2019, 9, 49; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4g. Andersen I. V., García-Vázquez R., Battisti U. M., Herth M. M., Molecules 2022, 27, 4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Lockley W. J. S., McEwen A., Cooke R., J. Labelled Compd. Radiopharm. 2012, 55, 235–257; [Google Scholar]
- 5b. Atzrodt J., Derdau V., Kerr W. J., Reid M., Angew. Chem. Int. Ed. 2018, 57, 1758–1784; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 1774–1802. [Google Scholar]
- 6. Shalgunov V., Lopes van den Broek S., Vang Andersen I., García Vázquez R., Raval N., Palner M., Mori Y., Schäfer G., Mikula H., Beschorner N., Nedergaard M., Syvänen S., Barz M., Knudsen G. M., Battisti U. M., Herth M., ChemRxiv. 2022; 10.26434/chemrxiv-2022-gj597-v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Kaim W., Coord. Chem. Rev. 2002, 230, 127–139; [Google Scholar]
- 7b. Stetsiuk O., Abhervé A., Avarvari N., Dalton Trans. 2020, 49, 5759–5777. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Counotte-Potman A., Van der Plas H. C., Van Veldhuizen B., J. Org. Chem. 1981, 46, 2138–2141; [Google Scholar]
- 8b. Hu W.-X., Xu F., J. Chem. Res. 2006, 2006, 797–799. [Google Scholar]
- 9. Svatunek D., Wilkovitsch M., Hartmann L., Houk K. N., Mikula H., J. Am. Chem. Soc. 2022, 144, 8171–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Vázquez R., Jørgensen J. T., Bratteby K. E., Shalgunov V., Hvass L., Herth M. M., Kjær A., Battisti U. M., Pharmaceuticals 2022, 15, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herth M. M., Ametamey S., Antuganov D., Bauman A., Berndt M., Brooks A. F., Bormans G., Choe Y. S., Gillings N., Häfeli U. O., James M. L., Kopka K., Kramer V., Krasikova R., Madsen J., Mu L., Neumaier B., Piel M., Rösch F., Ross T., Schibli R., Scott P. J. H., Shalgunov V., Vasdev N., Wadsak W., Zeglis B. M., Nucl. Med. Biol. 2021, 93, 19–21. [DOI] [PubMed] [Google Scholar]
- 12. Stéen E. J. L., Jørgensen J. T., Denk C., Battisti U. M., Nørregaard K., Edem P. E., Bratteby K., Shalgunov V., Wilkovitsch M., Svatunek D., Poulie C. B. M., Hvass L., Simón M., Wanek T., Rossin R., Robillard M., Kristensen J. L., Mikula H., Kjaer A., Herth M. M., ACS Pharmacol. Transl. Sci. 2021, 4, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyer N., Gloanec P., De Nanteuil G., Jubault P., Quirion J.-C., Eur. J. Org. Chem. 2008, 4277–4295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
Data will be made available on request.