Abstract
Chimeric antigen receptors (CAR) are engineered fusion proteins designed to target T cells to antigens expressed on cancer cells. CAR T cells are now an established treatment for patients with relapsed and/or refractory B cell lymphomas, B cell acute lymphoblastic leukaemia and multiple myeloma. At the time of this writing, over a decade of follow-up data are available from the initial patients who received CD19-targeted CAR T cells for B cell malignancies. Data on the outcomes of patients who received B cell maturation antigen (BCMA)-targeted CAR T cells for multiple myeloma are more limited owing to the more recent development of these constructs. In this Review, we summarize long-term follow-up data on efficacy and toxicities from patients treated with CAR T cells targeting CD19 or BCMA. Overall, the data demonstrate that CD19-targeted CAR T cells can induce prolonged remissions in patients with B cell malignancies, often with minimal long-term toxicities, and are probably curative for a subset of patients. By contrast, remissions induced by BCMA-targeted CAR T cells are typically more short-lived but also generally have only limited long-term toxicities. We discuss factors associated with long-term remissions, including the depth of initial response, malignancy characteristics predictive of response, peak circulating CAR levels and the role of lymphodepleting chemotherapy. We also discuss ongoing investigational strategies designed to improve the length of remission following CAR T cell therapy.
Subject terms: Cancer immunotherapy, B-cell lymphoma, Acute lymphocytic leukaemia, Myeloma, Immunotherapy
Chimeric antigen receptor (CAR) T cells have dramatically improved the outcomes of patients with certain relapsed and/or refractory haematological malignancies. Owing to the promising short-term survival outcomes achieved, long-term data on both safety and survival are becoming increasingly relevant. In this Review, the authors describe the available long-term follow-up data from early studies testing the safety and efficacy of receiving CAR T cells targeting CD19 as well as more recent data on BCMA-targeted CAR T cells in patients with relapsed and/or refractory multiple myeloma.
Key points
Among haematological malignancies, the indications for use of chimeric antigen receptor (CAR) T cells are rapidly expanding. CD19-targeted CAR T cells are now approved for relapsed and/or refractory B cell lymphoma and B cell acute lymphoblastic leukaemia, and B cell maturation antigen-targeted CAR T cells are approved for relapsed and/or refractory multiple myeloma.
Long-term follow-up data indicate that CD19-targeted CAR T cells are likely to be curative for a subset of patients with B cell lymphomas. These CAR T cells might need to be combined with consolidative allogeneic haematopoietic stem cell transplantation to enable long-term remissions for patients with B cell acute lymphoblastic leukaemia.
B cell maturation antigen-targeted CAR T cells can induce prolonged remissions in patients with relapsed and/or refractory multiple myeloma, although whether any of these responses are curative remains unclear.
Factors associated with durable remission after CAR T cell therapy include a deep initial response, lower baseline tumour volume, an absence of extramedullary disease, higher peak circulating CAR T cell levels and receipt of lymphodepleting chemotherapy.
The most prominent long-term toxicities after CAR T cell therapy include cytopenias and hypogammaglobulinaemia. The incidence of severe infections >1 month after CAR T cell therapy is low compared to infections seen in the acute period immediately after cell infusion.
Ongoing research efforts are attempting to improve the durability of responses after CAR T cell therapy, for example, through improved patient selection, novel CAR designs, including those targeting multiple antigens, and modifications to the manufacturing process.
Introduction
Chimeric antigen receptor (CAR) T cells are engineered fusion proteins that target T cells to a specific antigen present on tumour cells to generate an antitumour immune response1–3. CAR T cells targeting CD19, which is expressed on malignant B cells, were found to have potent activity in early phase clinical trials involving patients with relapsed and/or refractory (R/R) B cell malignancies over a decade ago4–8. In the multicentre trials that followed, complete response (CR) rates of 40–54%, 67% and 69–74% were observed in patients with R/R aggressive B cell lymphomas9–11, in patients with mantle cell lymphoma12 and in those with indolent B cell lymphomas13,14, respectively. These outstanding CR rates heralded a paradigm shift in the treatment of patients with R/R B cell lymphomas, who historically had dismal outcomes following salvage chemoimmunotherapy15. As discussed later in this Review, some of the responses were highly durable, suggesting that CD19-targeted CAR T cells are able to cure certain patients with B cell lymphomas16. CD19-targeted CAR T cells are now approved by the FDA for the treatment of R/R aggressive B cell lymphomas9–11, mantle cell lymphomas12 and indolent B cell lymphomas13,14. These CAR T cells have also generated CR rates of 71–81% in multicentre clinical trials involving patients with R/R B cell acute lymphoblastic leukaemia (B-ALL), who have limited treatment options17,18. Hence, CD19-targeted CAR T cells are now also approved by the FDA for patients with R/R B-ALL and provide an important standalone treatment or bridge to allogeneic haematopoietic stem cell transplantation (HSCT)19. More recently, CAR T cells targeting B cell maturation antigen (BCMA) have had remarkable successes in patients with R/R multiple myeloma (RRMM), with trials showing overall response rates (ORRs) of 73–98%20–22. BCMA-targeted CAR T cells are now approved by the FDA for patients with RRMM and are effective in those with disease progression on many other targeted agents. Overall, CAR T cell therapies have become an important component of the treatment landscape for a range of haematological malignancies, and the indications for these therapies continue to expand to earlier lines of treatment.
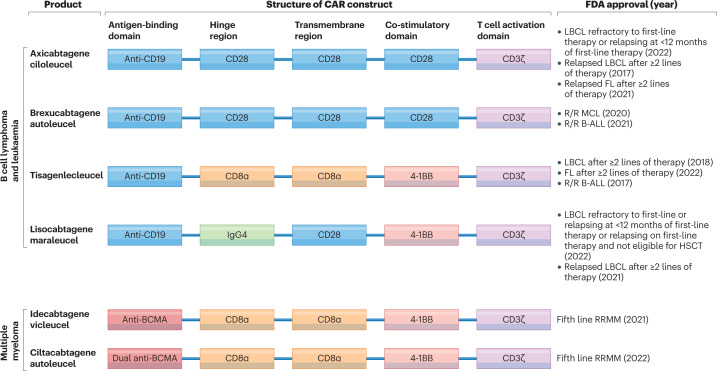
The CD19-targeted CAR T cell products currently approved by the FDA include axicabtagene ciloleucel9,14,23, tisagenlecleucel11,13,17, lisocabtagene maraleucel10,24 and brexucabtagene autoleucel12,18. The BCMA-targeted CAR T cell products idecabtagene vicleucel22 and ciltacabtagene autoleucel21 are also approved by the FDA (Fig. 1). All these products are generated by viral transduction of autologous (patient-derived) T cells to express the CAR construct2. The currently approved CAR T cell products use second-generation constructs that include an antigen-binding domain, hinge and transmembrane domains, a co-stimulatory domain (derived from either CD28 or 4-1BB), and a T cell activation domain derived from CD3ζ25,26. The products differ in some of the specific domains included in the CAR, the viral vector used for CAR transgene delivery and certain aspects of the manufacturing process25–27. Lymphodepleting chemotherapy is given in the week prior to CAR T cell infusion to support CAR T cell proliferation and possibly activation following infusion28. Thus far, all approved CAR T cell products share, to varying degrees, the class-specific adverse effects of cytokine-release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome, hypogammaglobulinaemia and cytopenias, particularly B cell aplasia29.
Fig. 1. FDA-approved CAR T cell therapies.
A total of six chimeric antigen receptor (CAR) products are currently available commercially, including four for patients with B cell lymphomas, two for patients with B cell acute lymphoblastic leukaemia (B-ALL) and two for those with multiple myeloma (MM). All approved products have a second-generation CAR construct, consisting of an antigen-binding domain, a hinge region, a transmembrane region, a co-stimulatory domain and a T cell activation domain. All CD19-targeted CARs contain the same antigen-binding domain, which is a single-chain variable fragment derived from the mouse FMC63 monoclonal antibody. Axicabtagene ciloleucel and brexucabtagene autoleucel use the same CAR but differ in their manufacturing processes, with production of brexucabtagene autoleucel including an additional step designed to remove malignant cells from the leukapheresis product. Tisagenlecleucel differs from these products in that it contains different hinge and transmembrane domains and includes a 4-1BB domain instead of a CD28 domain for co-stimulation. Lisocabtagene maraleucel is delivered at a defined CD4+:CD8+ T cell composition. The CAR gene for axicabtagene ciloleucel and brexucabtagene autoleucel is delivered using a gammaretrovirus, whereas those for tisagenlecleucel and lisocabtagene maraleucel are delivered using lentiviruses. Idecabtagene vicleucel includes a mouse 11D5-3 single-chain variable fragment targeting B cell maturation antigen (BCMA). Ciltacabtagene autoleucel has a binding domain consisting of two linked camelid heavy-chain-only variable (VHH) antigen-binding domains targeting BCMA. In both products, the CAR gene is delivered using a lentivirus. FL, follicular lymphoma; HSCT, haematopoietic stem cell transplantation; LBCL, large B cell lymphoma; MCL, mantle cell lymphoma; R/R, relapsed and/or refractory.
The remarkable successes and rapid FDA approvals of new CAR T cell products are exciting and reflect an expanding role for such agents in the treatment of patients with haematological malignancies. However, as the manufacturing and administering CAR T cells is labour intensive and expensive, an examination of the long-term outcomes of patients who received these novel therapies is essential30. In this Review, we provide a summary of the long-term outcomes of patients with B cell malignancies or RRMM following CAR T cell therapy.
Long-term outcomes
CD19-targeted CAR T cell therapy for B cell lymphoma and CLL
Most data on long-term outcomes following infusion with CAR T cell therapies are from patients with R/R B cell lymphoma or chronic lymphocytic leukaemia (CLL) who received CD19-targeted CAR T cell therapies in the early trials (Table 1). A total of 10 studies have provided ≥24 months of follow-up data (range 24–123 months)16,23,31–38. These data indicate ORRs of 44–91% and CR rates of 28–68%16,23,31–38. All studies reported the existence of a subset of patients with ongoing responses at ≥2 years after infusion without any consolidative treatment16,23,31–38. These long-term durable remissions were reported for all malignancies treated, including aggressive B cell lymphomas, follicular lymphomas, mantle cell lymphomas and CLL, and with all currently approved CD19-targeted CAR T cell products16,23,31–38.
Table 1.
Long-term outcomes of patients with B cell lymphoma and/or CLL/SLL receiving CD19-targeted CAR T cells
| Study (year of publication) | CAR product and trial phase | Cancer types (n) | Median follow-up (range) | ORR and CRR | PFS or EFS | DOR in responding patients |
|---|---|---|---|---|---|---|
| Chong et al. (2021)31 | Tisagenlecleucel, single-centre case series | DLBCL (24); FL (14) | 61 months | ORR: 66%; CRR: 55% | 31% PFS at 5 years for DLBCL; 43% PFS at 5 years for FL | 60% remained in response at 5 years |
| Jacobson et al. (2021)32 | Axicabtagene ciloleucel, multicentre phase I/II | DLBCL (77); PMBCL (8); tFL (16)9 | 51 months | ORR: 74%; CRR: 54%67 | Median EFS: 5.7 months, with 24-month EFS of 38% | NR |
| Cappell et al. (2020)16 | FMC63-28Za, single-centre phase I | DLBCL/PMBCL (28); indL (8); CLL/SLL (7) | 42 months (1–123 months) | ORR: 81%; CRR: 58% | Median EFS: 55 months | 76% of patients with a CR remained in response at last follow-up with a DOR ranging from 43 to 113 months |
| Schuster et al. (2021)33 |
Tisagenlecleucel, multicentre phase II |
DLBCL, HGBCL or tFL (115) | 40 months (IQR 38–44 months) | ORR: 53%; CRR: 39% | Median PFS: 2.9 months; median EFS: 2.8 months | Median DOR: not estimable |
| Hirayama et al. (2019)34 | Lisocabtagene maraleucel, single-centre phase I/II | tFL (13) and FL (8) | 38 months for patients with tFL and 24 months for those with FL | ORR: NR for FL and 46% for tFL; CRR: 88% for FL and 46% for tFL | Median PFS: 1.4 months in tFL cohort; NR for FL cohort | All patients with FL with a CR remained in remission at a median follow-up duration of 24 months (range 5–37 months) |
| Wang et al. (2023)35 | Brexucabtagene autoleucel, multicentre phase II | MCL (68) | 36 months (26–56 months) | ORR: 91%; CRR: 68% | Median PFS: 26 months | Median DOR: 47 months in patients with a CR |
| Frey et al. (2020)36 | CART-19, single-centre phase II | CLL (38) | 32 months (2–75 months) | ORR: 44%; CRR: 28% | Median PFS: 1 month in all patients; 40 months in those with CR | 4/9 (44%) of patients with a CR had disease relapse |
| Abramson et al. (2021)37 | Lisocabtagene maraleucel, multicentre phase I | LBCL (270) | All patients had ≥24 months of follow-up data; median NR | ORR: 73%; CRR: 53% | Median PFS: 6.8 months | Median DOR: 26 months in those with a CR |
| Locke et al. (2022)23 b | Axicabtagene ciloleucel, multicentre phase III | DLBCL (126); HGBCL (31); NR (18); other (5) | 25 months | ORR: 83%; CRR: 65% | Median PFS: 15 months | Median DOR: 27 months |
| Siddiqi et al. (2022)38 | Lisocabtagene maraleucel, multicentre phase I | CLL/SLL (23) | 24 months | ORR: 82%; CRR: 45% | Median PFS: 18 months | Median DOR: not reached |
Studies meeting the following criteria were included in this table: ≥2 years of follow-up and >20 patients on study plus availability of the majority of the above data. Studies are listed in order of longest duration of follow-up. Studies of chimeric antigen receptor (CAR) T cells targeting CD19 in addition to other antigens and retrospective or real-world studies were not included. All studies involved adult patients. CLL, chronic lymphocytic leukaemia; CR, complete response; CRR, complete response rate; DLBCL, diffuse large B cell lymphoma; DOR, duration of response; EFS, event-free survival; FL, follicular lymphoma; HGBCL, high-grade B cell lymphoma; IQR, interquartile range; indL, indolent lymphoma; LBCL, large B cell lymphoma; MCL, mantle cell lymphoma; NR, not reported; ORR, overall response rate; PFS, progression-free survival; PMBCL, primary mediastinal B cell lymphoma; SLL, small lymphocytic lymphoma; tFL, transformed follicular lymphoma. aThe CAR used in this trial was later commercially developed into axicabtagene ciloleucel. bThe trial included randomization of patients to axicabtagene ciloleucel versus standard of care. Data presented are from patients who received axicabtagene ciloleucel only.
We conducted one of the longest follow-up studies to provide data on CD19-targeted CAR T cells; this study was designed to assess the outcomes of patients receiving a CAR T cell therapy that was developed at the National Cancer Institute and later commercialized as axicabtagene ciloleucel16. In our study involving 43 patients with R/R B cell lymphoma or CLL, 58% of treated patients had a CR and 76% of those with a CR remained in long-term remission16. At the latest follow-up, the duration of these CRs ranged from 43 to 113 months16. Long-term remissions were also observed in a study involving patients who received tisagenlecleucel in the initial single-centre clinical trial of tisagenlecleucel for lymphoma31. This study demonstrated a CR rate of 55%, and 60% of these patients remained in remission at 5 years31. Overall, these results demonstrate that some patients with R/R B cell lymphoma who receive CD19-targeted CAR T cell therapy are probably cured of their disease without a need for further intervention. This finding stands in contrast to the available chemoimmunotherapy approaches for R/R large B cell lymphoma (LBCL), which are usually used as a bridge to potentially curative autologous HSCT39. Moreover, the long-term remission rates of patients receiving salvage chemoimmunotherapy plus autologous HSCT have decreased since the adoption of rituximab as part of first-line therapy40,41.
CD19-targeted CAR T cell therapy for B-ALL
Data are now available from multiple long-term follow-up studies of the efficacy of CD19-targeted CAR T cell therapy in patients with B-ALL18,42–53. A total of 12 studies provide data on the outcomes of patients with a minimum median follow-up duration of 1 year (range 1–4.8 years)18,42–53 (Table 2). Data from these studies confirm the excellent initial CR rates ranging from 62% to 86%, with the majority of these being deep minimal residual disease (MRD)-negative remissions18,42–53. Median event-free survival (EFS) durations varied between studies, which probably reflects the fact that most studies included a substantial and variable fraction of patients (13–88%) who received consolidative allogeneic HSCT while in remission18,42–53. This approach obscures the interpretation of the ability of CAR T cells alone to elicit a curative response. Long-term data are available for both commercially available CAR T cell products used in adults with B-ALL, namely tisagenlecleucel and brexucabtagene autoleucel. Data from the initial study of tisagenlecleucel indicate a CR rate of 69% with a median EFS of 5.6 months at a median follow-up duration of 13 months48. Similarly, follow-up data from the initial study of brexucabtagene autoleucel also show a CR rate of 69%, with a median relapse-free survival duration of 7 months at a median follow-up duration of 22 months45. These data suggest similar efficacy of both products, albeit with short durations of remission in most adults with B-ALL relative to those with other B cell malignancies. By contrast, only tisagenlecleucel is currently approved for paediatric patients (defined as those ≤25 years of age) with B-ALL. Long-term follow-up of such patients (median 11 years of age, range 3–24) who received tisagenlecleucel in the ELIANA study indicate a complete remission rate of 82% and a median EFS duration of 24 months51. Comparably high EFS rates have also been noted in retrospective studies involving patients of a similar age54,55. These data clearly demonstrate that younger (paediatric and young adult) patients receiving CD19-targeted CAR T cell therapies have markedly superior survival outcomes compared with those of the adult population.
Table 2.
Long-term outcomes of patients with B-ALL receiving CD19-targeted CAR T cells
| Study (year of publication) | CAR design, namea and trial phase | Trial population (n) | Median follow-up (range) | CR/CRi | Survival outcomes | Proportion of patients in CR receiving consolidative alloHSCT | Outcomes of patients in CR not receiving consolidative alloHSCT |
|---|---|---|---|---|---|---|---|
| Shah et al. (2021)42 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domainsb,153; CD19.28ζ; single-centre phase I | Children and adults aged 4–30 years (50) | 4.8 years (3.5–7.2 years) | 31/50 (62%) | Median EFS: 3.1 months | 21/28 (75%) of those with MRD-negative CR | 7/7 (100%) of patients with MRD-negative CR relapsed |
| Laetsch et al. (2023)51 | Mouse scFv, CD8α H/T, 4-1BB co-stimulatory domains; tisagenlecleucel; multicentre phase II | Children and young adults aged 3–24 years (79) | 39 months | 65/79 (82%) | Median EFS: 24 months | 11/66 (17%) | NR |
| Jacoby et al. (2022)50 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domains; CD19 CAR T cells; single-centre phase II | Children and adults aged 1–36 years (37) | 3 years | 30/35 (86%) | Median EFS: 17 months (not censored for consolidative alloHSCT) | 25/30 (83%) | 4/5 (80%) of patients with CR relapsed; the remaining patient was on maintenance treatment |
| Wayne et al. (2022)52 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domains; KTE-X19; multicentre phase I/II | Children and young adults aged 3–20 years (24) | 36 months (24–54 months) | 16/24 (67%) | Median RFS: 5.2 months | 14/16 (88%) | One patient died of progressive disease and the other was lost to follow-up |
| Hay et al. (2019)43 | Mouse scFv, IgG4 hinge, CD28 transmembrane, 4-1BB co-stimulatory domains, delivered in a defined CD4+-to-CD8+ cell ratioc,79; CD19 CAR T cells; single-centre phase I/II | Adults aged 20–76 years (53) | 31 months | 45/53 (85%) | Median EFS: 7.6 months (in those achieving MRD-negative CR) | 18/45 (40%) of those with MRD-negative CR | NR |
| Park et al. (2018)44 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domainsb,154; 19-28z; single-centre phase I | Adults aged 23–74 years (53) | 29 months (1–65 months) | 44/53 (83%) | Median EFS: 6.1 months | 17/44 (39%) | 17/26 (65%) relapsed or died |
| Shah et al. (2021)45 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domainsb,25; brexucabtagene autoleucel; multicentre phase I | Adults aged 18–77 years (45) | 22 months (7–36 months) | 31/45 (69%) | Median RFS: 7 months | 6/45 (13%) of all patients received alloHSCT | 8/31 (26%) of CRs were ongoing at data cut-off, with 5/8 (63%) not having received alloHSCT |
| Roddie et al. (2021)46 | Mouse scFv from the CAT131E10 hybridoma, CD8α H/T domain, with a 4-1BB co-stimulatory domainsc,46; AUTO1; multicentre phase I | Adults aged 18–62 years (20) | 22 months (1–34 months) | 17/20 (85%), MRD-negative CR | 24-month EFS 44% by MRD relapse criteria | 3/17 (18%) | NR |
| Shah et al. (2021)18 | Mouse scFv, CD28 H/T, CD28 co-stimulatory domainsb,25; brexucabtagene autoleucel; multicentre phase II | Adults aged 28–52 years (55) | 16 months (IQR 14–20 months) | 39/55 (71%) | Median RFS: 11.6 months | 9/39 (23%) | 18/30 (60%) relapsed, died or proceeded to other therapies |
| Wang et al. (2021)47 | Humanized scFv, CD8α H/T, 4-1BB co-stimulatory domainsc,47; hCART19s; single-centre phase I | Children aged 3–17 years (24) | 16 months (3–45 months) | 20/24 (83%) | EFS: 37% at 3 years | 8/20 (40%) | 9/12 (75%) relapsed |
| Frey et al. (2020)48 | Mouse scFv, CD8α H/T, 4-1BB co-stimulatory domainsc,25; tisagenlecleucel; single-centre phase I/II | Adults aged 20–70 years (35) | 13 months (0.2–53 months) | 24/35 (69%) | Median EFS: 5.6 months | 9/24 (38%) | NR |
| An et al. (2020)49 | Mouse scFv, IgG4 hinge, CD28 TM, 4-1BB co-stimulatory domainsb,49; Sino19; multicentre phase II | Children and adults aged 3–72 years (47) | NR (survival data are from 1 year) | 38/47 (81%) | Median RFS: 10.5 months | 10/38 (26%) | 19/28 (68%) relapsed |
Studies meeting the following criteria were included in this table: median follow-up duration ≥1 year and had ≥20 patients on study in addition to including most of the above data in the manuscript. Studies are listed in order of longest duration of follow-up. Studies testing CARs targeting CD19 in addition to other antigens and retrospective or real-world studies were not included. alloHSCT, allogeneic haematopoietic stem cell transplantation; B-ALL, B cell acute lymphoblastic leukaemia; CAR, chimeric antigen receptor; CR, complete response; CRi, CR with incomplete blood count recovery; EFS, event-free survival; H/T, hinge and transmembrane; IQR, interquartile range; MRD, minimal residual disease; NR, not reported; RFS, relapse-free survival; scFv, single-chain variable fragment; TM, transmembrane. aCAR name is included if the CAR construct was developed into a commercial product. bThese studies used a gammaretroviral vector for construct delivery. cThese studies used a lentiviral vector for construct delivery. If a commercial product is not yet approved by the FDA, the construct name is listed.
The need for consolidative allogeneic HSCT for durable remissions after CAR T cell therapy in patients with B-ALL is currently debatable19. The markedly different long-term outcomes in paediatric and adult patients warrant separate discussions of the role of consolidative HSCT in these populations. In the paediatric population, a substantial proportion of patients have long-term remissions after tisagenlecleucel alone without consolidative allogeneic HSCT51. For example, in the ELIANA study, 17/79 (22%) patients underwent allogeneic HSCT, including 11 who were in tisagenlecleucel-mediated remission at the time of transplantation51; the 3-year relapse-free survival was 52% with censoring for allogeneic HSCT or other therapies and 48% without censoring51. Overall, these data suggest that a cure is possible without consolidative allogeneic HSCT in some paediatric patients treated with tisagenlecleucel. Data from four studies have detailed the outcomes of paediatric patients who received other CD19-targeted CAR T cell products, achieved a CR and did not proceed to consolidative allogeneic HSCT42,47,49,50. These studies showed a rate of relapse from CR in non-consolidated patients of 68–100%42,47,49,50. Therefore, a higher percentage of paediatric patients might have long-term remissions without consolidative allogeneic HSCT after treatment with tisagenlecleucel as compared with other CD19-targeted CAR T cell products. In contrast to data from the paediatric population, consolidative allogeneic HSCT is routinely recommended for adults with B-ALL with a CR after CD19-targeted CAR T cell therapy56. This recommendation reflects a markedly lower median EFS duration, regardless of the CAR construct used, in this population18,43–46,48. The importance of consolidative allogeneic HSCT is highlighted by data from a long-term follow-up study involving adult patients who received tisagenlecleucel, which showed markedly improved EFS in those who received consolidative allogeneic HSCT48. Factors that might have an important role in determining which patients require consolidative allogeneic HSCT are discussed in detail in an excellent recent review and include receipt of previous HSCT, loss of B cell aplasia, previous treatments received, cytogenetics, detection of MRD and disease burden prior to CAR T cell infusion19.
Overall, data from patients with B-ALL indicate a very high CR rate with CD19-targeted CAR T cells, exceeding 80% in many studies18,43,44,46,47,49–51. However, even in paediatric patients who received tisagenlecleucel, ≤50% had long-term EFS51. Thus, in comparison to those with B cell lymphoma, patients with B-ALL are more likely to have a CR, although a lower proportion of patients with a CR are likely to be cured without subsequent therapy.
BCMA-targeted CAR T cell therapy for RRMM
In comparison with CD19-targeted CAR T cells, fewer data on the long-term outcomes of patients with RRMM who received BCMA-targeted CAR T cells are available owing to the later development of these constructs57,58. Outcomes are currently available from a total of six studies with a median follow-up duration of ≥1 year (range 13–48 months)21,22,59–62 (Table 3). These studies involved patients with RRMM and reported ORRs of 73–100% and CR or stringent CR rates ranging from 33% to 83%21,22,59–62. MRD-negative remissions were frequently observed in many of these studies21,22,59–61. Furthermore, a subset of patients with prolonged remissions lasting several years was observed in all studies without the need for consolidative or maintenance therapies21,22,59–62. The frequency of prolonged remissions, defined as those with progression-free survival (PFS) of >1 year, varied markedly between studies with median PFS durations ranging from 5.2 to 27 months21,22,59–62. In our trial of a fully human BCMA-targeted CAR with a heavy-chain-only antigen-recognition domain, patients had a median PFS duration of 18 months without any maintenance therapy at the time of a report presented in November 2021 (ref. 62). This single treatment contrasts with monoclonal antibody-based approaches targeting BCMA in patients with RRMM, which require ongoing treatment63,64.
Table 3.
Long-term outcomes of patients with relapsed and/or refractory multiple myeloma receiving BCMA-targeted CAR T cells
| Study and year of publication (n) | CAR design, name and trial phase | Median follow-up (range) | ORR and ≥CRR | PFS | DOR |
|---|---|---|---|---|---|
| Zhao et al. 2022 (74)59 | Two BCMA-binding domains, CD8α H/T domain, 4-1BB co-stimulatory domain; LCAR-B38Ma,b; multicentre phase I | 48 months | ORR: 65/74 (88%); ≥CRR: 54/74 (73%) | Median PFS: 18 months | Median DOR: 23 months |
| Martin et al. 2023 (97)21 | Two BCMA-binding domains, a CD8α H/T domain, 4-1BB co-stimulatory domainb,117; ciltacabtagene autoleucel; multicentre phase Ib/II | 28 months | ORR: 95/97 (98%); ≥CRR: 80/97 (83%) | PFS: 55% at 27 months | Median DOR: NE (range 23 months to NE) |
| Mikkilineni et al. 2021 (25)62 | Fully human scFv, CD8α H/T domain, 4-1BB co-stimulatory domainc; FHVH33-CD8BBZ | NR (median PFS of 78 weeks) | ORR: 23/25 (92%); ≥CRR: 12/25 (48%) | Median PFS: 18 months | Median DOR: NR |
| Munshi et al. 2021 (128)22 | Mouse scFv, CD8α H/T domain, 4-1BB co-stimulatory domainb,20,116; idecabtagene vicleucel; multicentre phase II | 13 months (0.2–21 months) | ORR: 94/128 (73%); ≥CRR: 42/128 (33%) | Median PFS: 8.8 months | Median DOR: 11 months in all patients and 19 months in those with CR or better |
| Wang et al. 2021 (18)60 | Fully human scFv, CD8α H/T domain, 4-1BB co-stimulatory domainb,60; CT103A; single-centre phase I | 13 months | ORR: 18/18 (100%); ≥CRR: 13/18 (72%) | 1-year PFS: 58% | Median DOR: 325 days for all patients and 412 days for patients with CR or better |
| Li et al. 2021 (30)61 | Mouse scFv, CD8α hinge, CD28 co-stimulatory domainb,61; anti-BCMA CAR T cells; phase I | 13 months | ORR: 27/30 (90%); ≥CRR: 13/30 (43%) | Median PFS: 5.2 months | Median DOR: 148 days for responding patients (range 16–625 days) |
Studies meeting the following criteria were included in this table: median follow-up duration ≥1 year and >15 patients on study in addition to including most of the above data in the manuscript. Studies are listed in order of longest duration of follow-up. Studies targeting B cell maturation antigen (BCMA) in addition to other antigens and retrospective or real-world studies were not included. Chimeric antigen receptor (CAR) name is included if the CAR construct was developed into a commercial product; otherwise, the construct name is listed. ≥CRR, complete response rate or better; CR, complete response; DOR, duration of response; H/T, hinge and transmembrane; NE, not estimable; NR, not reported; ORR, overall response rate; PFS, progression-free survival; scFv, single-chain variable fragment. aLCAR-B38M is the same CAR as used in ciltacabtagene autoleucel. bThese studies used a lentiviral vector for construct delivery. cThis study used a gammaretroviral vector for construct delivery.
Long-term follow-up data are available for both commercially available BCMA-targeted CAR T cell products, idecabtagene vicleucel22 and ciltacabtagene autoleucel21,59. The longest reported follow-up data for idecabtagene vicleucel (median 13 months) demonstrate a median PFS duration of 8.8 months for all patients, increasing to 12.1 months for patients who received the highest dose level22. A CR or better was achieved in 33% of patients, and patients obtaining a CR had a median duration of response of 19 months22. Data are available from two important long-term follow-up studies testing the CAR construct used in ciltacabtagene autoleucel21,59. The first study was a multicentre trial conducted in the USA and Japan with a median follow-up duration of 28 months21. This study demonstrated a CR or better in 83% of patients, a PFS of 55% at 27 months of follow-up, and a median duration of response that was not estimable at the reported data analysis cut-off21. The PFS curve trended downwards over time, although the median PFS duration was still not reached at the time of the report. The second important long-term study of the CAR used in ciltacabtagene autoleucel was conducted in China, where this construct was originally developed59. This long-term follow-up study of 74 patients with RRMM had a median follow-up duration of 48 months59. The data indicate a median PFS duration of 18 months, and the PFS curves demonstrated that increasing numbers of patients develop disease progression with longer follow-up monitoring59. These data suggest that patients with RRMM can have prolonged maintenance-free remissions after BCMA-target CAR T cell therapy, albeit with a continued risk of disease progression over time.
Factors associated with long-term remissions
The factor most consistently associated with durable long-term remissions following CAR T cell therapy is the depth of initial response to treatment, which is usually quantifiable within the first few months after cell infusion and often in the first month10,12,21,22,33,65,66 (Box 1). The importance of the depth of initial response in predicting duration of remission has been demonstrated in studies involving patients with various haematological malignancies, including B cell lymphomas9,16,34,35,67, CLL36,38, B-ALL18,43,44,47 and MM21,22,59; in each of these malignancies, patients obtaining a deeper response remained in remission for longer than those who did not. The depth of response can be assessed by determining MRD negativity or by measuring circulating-tumour DNA: both approaches can predict the durability of remission68,69. Patients with B cell lymphoma, with a best response constituting a partial response, are unlikely to have subsequent long-term curative remissions16. By contrast, those with a CR can have curative remissions16. Importantly, patients with MM and those with B-ALL often have very deep MRD-negative CRs yet later have disease relapse18,42,44,47,49. Therefore, a deep initial response seems to be necessary, yet not sufficient, for long-term remissions after CAR T cell therapy.
Both the type and characteristics of the malignancy are also clearly predictive of response durability. Patients with B cell lymphomas are less likely to have a CR compared to those with B-ALL or MM; however, CRs of patients with B cell lymphoma are more likely to be durable once they are achieved. Baseline tumour burden is another factor that predicts response to CAR T cell therapy across malignancies. Patients with higher tumour burdens at the start of treatment are less likely to both attain and maintain a deep response compared to those with a lower tumour burden in all malignancies, including B cell lymphomas70,71, B-ALL42,44,47,50,54 and MM21. Another shared factor across malignancies is that the presence of extra-nodal B cell lymphoma70,72 is predictive of inferior outcomes, as is the presence of extramedullary B-ALL43,49 or extramedullary MM21,59,60,73.
Receipt of lymphocyte-depleting chemotherapy is another factor consistently associated with response. Lymphocyte-depleting chemotherapy is given in the week prior to CAR T cell infusion and is usually a combination of fludarabine and cyclophosphamide, although other regimens can be used28. Studies involving patients with B cell lymphomas74–77 and B-ALL42,76,78,79 have shown improved responses when lymphocyte-depleting chemotherapy is given before CAR T cell infusion. Therefore, CAR T cell studies involving patients with MM included lymphocyte-depleting chemotherapy27. Lymphocyte depletion creates a favourable immune environment that enables optimal CAR T cell proliferation and function; the underlying mechanism of this effect that is currently best supported by human and mouse data involves enhancement of T cell proliferation and function owing to induction of an increase in certain serum cytokines such as IL-7 and IL-15 (refs. 74,80,81). The ideal lymphocyte-depleting regimen remains an active area of investigation28.
CAR T cell levels following infusion are a final and very important factor in predicting response durability. CAR T cells expand rapidly after cell infusion, reach a peak level and can then persist at a much lower level for years after treatment. Higher peak CAR-expressing cell levels and higher levels of CAR-expressing cells during the first month of infusion, quantified by area under the curve, are consistently associated with improved responses in the majority of9,10,16,23,35,74,82 but not in all11,83 studies involving patients with B cell lymphoma. Higher peak CAR-expressing cell levels and CAR-expressing cell area under the curve within the first month of treatment have also been associated with response in patients with CLL36,84, B-ALL18,42–44,66,82,85 and RRMM22,57,58,61. The available data therefore clearly support the importance of robust early in vivo CAR T cell levels for durable responses. Long-term low-level persistence lasting many months to years after infusion has been documented in multiple studies with a range of different CAR construct designs25,35,83,84,86. Data from several studies indicate durable responses without a need for long-term persistence of CAR T cells in patients with B cell lymphomas, and evidence of durable responses without detectable persisting T cells also comes from patients with MM12,16,21,35,67,87. However, the evidence does indicate a role of long-term CAR T cell persistence for a durable response in patients with B-ALL17,51. Nonetheless, the length and degree of persistence necessary for a durable response are unclear from the available data and could conceivably vary with different CAR constructs and malignancies.
Box 1 Factors associated with durable remissions after CAR T cell therapy.
- Malignancy type
Long-term adverse effects
CAR T cells are associated with a substantial risk of acute adverse events, including, most notably, CRS and immune effector cell-associated neurotoxicity syndrome29,88. The majority of clinical studies have focused on these acute toxicities, which generally occur within the first month of treatment29,88. Data from studies designed to investigate longer-term adverse events, especially in patients who are in long-term remission after CAR T cell infusion, are much more limited. The most common long-term adverse effects observed thus far are B cell depletion (aplasia), hypogammaglobulinaemia, cytopenias and infections (Table 4).
Table 4.
Long-term adverse effects of CAR T cells
| Study (year of publication) | CAR name and patient population (n) | Median follow-up (range) | Prevalence of persistent B cell/IgG depletion in patients with a CRa | Prevalence of late severe cytopeniasb | Incidence of late infections | Incidence of second malignancy |
|---|---|---|---|---|---|---|
| Chong et al. (2021)31 | Tisagenlecleucel; adults with B cell lymphomas (38) | 61 months | B cell: 4/12 (33%); IgG: 2/11 (18%) | 1/38 (3%), ongoing at 57 months | NR | 6/38 (16%) |
| Zhao et al. (2022)59 | LCAR-B38Mc; adults with multiple myeloma (74) | 48 months (0.4–61 months) | NR | NR | NR | 4/74 (5%) |
| Cappell et al. (2020)16 | FMC63-28Zd; adults with B cell lymphoma or CLL (43) | 42 months (1–123 months) | B cell: 9/24 (38%); IgG: 5/24 (21%) | NR | 4/43 (9%) developed an infection requiring hospitalization ≥6 months after CAR T cell infusion | 7/43 (16%) |
| Cordeiro et al. (2020)89 | Lisocabtagene maraleucel; adults with ALL, NHL or CLL (86) | 28 months (13–63 months) | B cell: NR; IgG: 14/19 (74%) | 3/19 (16%) of patients in CR | 33/54 (61%) developed an infection and 80% of these were non-severe infections (mostly URIs); 20% of infections required hospitalization at ≥3 months after infusion | 13/86 (15%) |
| Locke et al. (2019)67 | Axicabtagene ciloleucel; adults with B cell lymphomas (108) | 27 months (IQR 26–29 months) | B cell: 8/32 (25%); IgG: NR | 18/108 (17%) of all patients | 2 grade 3 infections occurred ≥12 months in patients in ongoing remission | 1 case of MDS |
| Locke et al. (2022)23,e | Axicabtagene ciloleucel; adults with B cell lymphomas (170) | 25 months | B cell: 55/160 (34%) of all patients; IgG: NR | NR | NR | NR |
Included studies must have documented long-term adverse events occurring at least 90 days after CAR T cell infusion and include data for most of the above categories. Studies are listed in order of longest duration of follow-up. Studies targeting multiple antigens or antigens other than CD19 and B cell maturation antigen are not listed. Chimeric antigen receptor (CAR) name is included if the CAR construct was developed into a commercial product; otherwise, the construct name is listed. ALL, acute lymphoblastic leukaemia; CLL, chronic lymphocytic leukaemia; CR, complete remission; IQR, interquartile range; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; NR, not reported; URI, upper respiratory tract infection. aPatients with persistent B cell/IgG depletion, defined as those with ongoing depletion at time of last follow-up. bSevere cytopenias were defined as cytopenias requiring either transfusion or growth factor support; grade ≥3 cytopenias were included in this category. All cytopenias must have occurred ≥3 months after CAR infusion. cThe CAR used in this trial was later commercially developed into ciltacabtagene autoleucel. dThe CAR used in this trial was later commercially developed into axicabtagene ciloleucel. eTrial included randomization of patients to axicabtagene ciloleucel versus standard of care. Data only from patients who received axicabtagene ciloleucel are presented here.
CAR T cell therapy clearly has long-lasting effects on the immune system. In addition to being expressed on malignant cells, CD19 is expressed on non-malignant B cells and BCMA is expressed on non-malignant plasma cells5,27. Long-lasting B cell depletion following CD19-targeted CAR T cell therapy is a common occurrence, with data from long-term follow-up studies indicating persistent B cell depletion in 25–38% of patients even several years after CAR T cell infusion16,23,31,67. B cell depletion can persist for years in such patients, sometimes despite loss of detectable CAR-expressing T cells16. Immunoglobulin depletion is a consequence of both impaired B cell and plasma cell activity. Long-term follow-up data indicate IgG depletion persisting several years after cell infusion in 18–74% of patients who received CD19-targeted CAR T cells16,31,89. Prolonged immunoglobulin depletion has also been observed in patients who received BCMA-targeted CAR T cells59. Patients with persistent hypogammaglobulinaemia after CAR T cell therapy often receive immunoglobulin infusions, although data on whether this is necessary for all patients are unclear90,91. An impaired response to vaccines is an important effect of B cell depletion and hypogammaglobulinaemia in patients receiving CAR T cells92,93.
Cytopenias, including anaemia, thrombocytopenia and neutropenia, are all common acute toxicities associated with CAR T cell therapy29,88. Data from several studies also indicate the occurrence of chronic cytopenias lasting ≥3 months after CAR T cell infusion. The incidence of grade 3–4 cytopenias at ≥3 months after CAR T cell infusion is approximately 15% in patients with B cell lymphoma67,94. In a long-term follow-up study, clinically significant cytopenias occurred in 3/19 (16%) of patients with B cell malignancies in CR following CD19-targeted CAR T cell therapy and lasted for 15–22 months after cell infusion89. Similarly, ongoing grade ≥3 neutropenia (in 20%) and thrombocytopenia (in 47%) can be observed in patients with MM at 100 days after infusion of idecabtagene vicleucel22. Chronic cytopenias are also common after ciltacabtagene autoleucel21. The mechanisms of prolonged cytopenias after CAR infusion are poorly understood, although these events generally occur in patients who are in ongoing remission and with no evidence of myelodysplastic syndrome (MDS)21,22,67,89,94,95. The risk of cytopenias is associated with higher-grade CRS, multiple previous lines of therapy, receipt of allogeneic HSCT ≤1 year prior to CAR T cell infusion, baseline cytopenia and the presence of bone marrow malignancy95,96.
Despite these widespread changes to the immune system, the incidence of severe infections >1 month after CAR T cell therapy is relatively low compared to the incidence of severe infections in the first month after CAR T cell infusion, and the incidence of such infections decreases with time after infusion16,89,94,97. However, data in this area are sparse because most studies have not focused on infections occurring many months after CAR T cell infusion98. Furthermore, survivors of lymphoma are already known to have increased long-term risks of infection, which makes interpretation of the effects of CAR T cell therapy difficult in this population99. In our long-term follow-up study involving 43 patients with B cell malignancies who received CD19-targeted CAR T cells at the National Cancer Institute, 4/43 (9%) required hospital admission for infections >6 months after CAR T cell therapy with a median follow-up duration of 42 months16. Similarly, data from a long-term follow-up study involving patients who received lisocabtagene maraleucel show that only 5% of patients had severe infections >91 days after infusion37. Patients who received CAR T cells seem to have an increased risk of mortality from COVID-19 (refs. 100–102) and also have impaired antibody production following COVID-19 vaccination92. However, studies in this area are limited in several respects, including being conducted early in the pandemic when fewer therapeutic options existed, having retrospective designs, including many patients who were not in remission after CAR T cell therapy and including many patients who were diagnosed with COVID-19 within a year of receiving CAR T cells, when immunosuppression is most prominent100–102. Whether patients in durable long-term remissions after CAR T cell therapy share these poor outcomes is currently unclear. Overall, the available data demonstrate an increased risk of infection following CAR T cell infusion, albeit less than would be expected in the context of substantial ongoing changes to the immune system in patients previously exposed to multiple lines of chemotherapy.
The possibility of malignant transformation of transduced cells was previously a concern surrounding the use of gene therapy approaches103. In the context of CAR T cell therapy, in which peripheral blood mononuclear cells undergo transduction for expression of the CAR, haematological malignancies, such as MDS, could theoretically emerge from adverse gene integration events. Data from large-cohort follow-up studies indicate an incidence of secondary malignancies after CAR infusion of 4–16%16,31,59,89,104. These incidences are not higher than expected given that all patients had a history of substantial chemotherapy exposure, which itself increases the risk of secondary malignancies105,106. The incidences of haematological malignancies, and particularly MDS, were also not higher than those expected in all trials using standard viral transduction approaches16,23,31,59,67,89,104. Overall, the available data provide no evidence of an increased risk of secondary malignancies with approved gammaretroviral or lentiviral CAR-delivery systems.
Ongoing research efforts
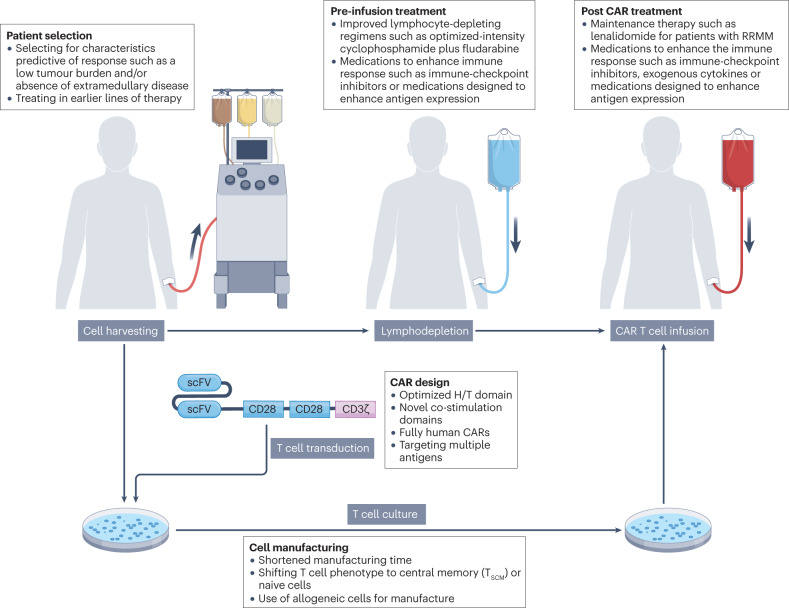
Investigational approaches designed to optimize nearly every part of the CAR T cell therapy process are currently being developed and/or tested (Fig. 2). A combination of changes at several different stages of this process will probably be needed to optimize the crucial parameters of CR rate and long-term PFS.
Fig. 2. Investigational strategies designed to improve remission duration following CAR T cell therapy.
The process of chimeric antigen receptor (CAR) T cell therapy involves harvesting peripheral blood mononuclear cells from a patient by apheresis, followed by transduction with viruses encoding the CAR, ex vivo T cell expansion, and re-infusion into the patient after completion of lymphocyte-depleting chemotherapy. Studies attempting to optimize each step of this process, and thus improve the durability of remissions following CAR T cell therapy, are currently ongoing. H/T, hinge and transmembrane; scFv, single-chain variable fragment; RRMM, relapsed and/or refractory multiple myeloma; TSCM, stem central memory T.
Antigen escape is a well-described mechanism of relapse after CAR T cell therapy, which occurs when malignant cells lose target antigen expression107. Antigen escape has been identified as the mechanism of disease relapse after CAR T cell therapy in 20–28% of patients with B cell lymphoma9,83,108, in 16–68% with B-ALL17,18 and at lower incidences in those with MM22,58,109,110. Antigen escape is therefore one of the most important factors affecting the durability of response to CAR T cell therapy. Dual antigen targeting is being actively investigated in several clinical trials in an attempt to overcome this problem. The most extensively studied additional antigens to target are CD20 and CD22, which are both expressed in malignant B cells. Thus far, long-term outcome data after CAR T cell therapy targeting both CD19 and CD20 are available from two studies involving patients with R/R B cell lymphoma111,112. One of these trials demonstrated no relapses owing to antigen loss111 and, in the other trial, 1/12 (8%) of patients with a biopsy sample available on disease relapse had antigen loss112. Dual targeting of CD19 and CD22 has been reported in two trials involving patients with R/R B-ALL113,114. These trials included post-relapse biopsy sampling and reported loss of one or both antigens in 25–33% of patients with disease relapse113,114. Another trial in which patients with R/R B cell malignancies received CD19/CD22-targeted CAR T cells again identified CD19 loss in 5/10 (50%) patients with B-ALL and in 4/14 (29%) patients with B cell lymphomas115. These data suggest that targeting more than one antigen does not always circumvent the problem of antigen escape and this approach might need to be combined with other improvements in CAR therapies.
The antigen-binding domains of the CAR can also be optimized to improve CAR T cell function. The antigen-binding domains of all approved CAR T cell products, except ciltacabtagene autoleucel, are currently derived from mouse antibodies20,25,116,117. The inclusion of a mouse-derived component might promote anti-CAR immune responses and thus limit CAR T cell levels following infusion. Several groups have therefore developed fully human CAR T cell products that have been tested in clinical trials27,60,118–120. Some of these studies have shown improved persistence of the fully human CAR, although, thus far, this has not translated into an improvement in efficacy58,60,120. Another potential area of improvement in the antigen-binding domain is the substitution of single-chain variable fragments with a heavy-chain-only variable domain. Antigen-binding domains comprised of only heavy chains, without an associated light chain, were originally described in antibodies derived from camelids and cartilaginous fish121,122. These heavy-chain-only binding domains have the advantage of a smaller size, easier genetic manipulation and potentially reduced immunogenicity given the absence of a linker region122,123. An example of the success of heavy-chain-only binding domains is provided by ciltacabtagene autoleucel, which incorporates two linked camelid heavy-chain-only variable domains targeting BCMA117. Although not compared prospectively, patients receiving this product have been shown to have higher ORRs with longer response durations compared to those receiving the single-chain variable fragment-containing CAR idecabtagene vicleucel21,22 (Table 3).
The trials leading to the approvals of all current CAR T cell therapies were conducted in patients with highly refractory malignancies who had previously received multiple lines of chemotherapy9–11,21,22. These patients often have baseline lymphopenia, and their previous treatments could impair T cell fitness124. Achieving a level of disease control that is sufficient to proceed to later-line CAR T cells might also not be feasible for patients with malignancies that are refractory to first-line chemoimmunotherapy given that the time from harvesting peripheral blood mononuclear cells to CAR T cell infusion can be as long as several weeks. Administering CAR T cells earlier in the course of malignancy might avoid these problems and improve response rates. Promising results with such an approach were described in a report from the ZUMA-12 trial. This trial involved patients with high-risk aggressive B cell lymphomas who had a positive interim PET scan after two cycles of first-line chemoimmunotherapy65. An impressive CR rate of 78% was observed, with 86% of these responses ongoing at a median follow-up duration of 16 months65. Circulating CAR T cell numbers were also higher in the ZUMA-12 study, in which CAR T cells were the second line of treatment, as compared to ZUMA-1, in which CAR T cells were the third line of treatment, despite the use of a similar methodology in both trials125. Multiple trials evaluating CAR T cell therapy in earlier lines of treatment are currently ongoing (such as NCT04923893 and NCT05605899).
The medications patients receive prior to apheresis, as bridging therapy between apheresis and CAR T cell infusion and as lymphocyte-depleting chemotherapy before infusion, is another area for optimization28. Limited data are available on how medications administered at any of these stages affect subsequent responses to CAR T cells126–129 and most current practices are driven by a combination of the protocols used in previous trials and expert guidance130. Lymphocyte-depleting chemotherapy is an important area of investigation that clearly improves response rates, although more intensive lymphodepletion also leads to increased toxicities28. Agents aside from lymphocyte-depleting chemotherapy could be given before or after infusion either to alter antigen expression on malignant cells or to directly alter CAR T cell function. Examples of these approaches include γ-secretase inhibitors given prior to BCMA-targeted CAR T cells to increase BCMA expression131, administration of ibrutinib concurrently with CD19-targeted CAR T cells in patients with CLL to alter the immune environment132,133, delivery of IL-15 or other cytokines to enhance CAR T cell expansion, and immune-checkpoint inhibitors given after CAR T cells to promote anticancer immunity134. Overall, we have much more to learn about the effects of specific medications at all steps of the CAR T cell process.
Cell manufacturing protocols are another area of potential improvement135. T cells can have a range of differentiation states with heterogenous functions136,137. Data from several studies suggest a link between the characteristics of T cells in the infusion product and subsequent CAR responses120,138–140. The ideal T cell composition is not yet known, although, in general, the presence of less-differentiated naive T cells or central memory T cells seems to be important for a response to adoptive cell therapies141–143. Shifting T cell phenotypes by growing the cells in the presence of specific cytokines144–146 or inhibiting specific cell signalling pathways is another possibility135,147,148. Longer durations of ex vivo culture have also been associated with increased T cell exhaustion and less favourable T cell phenotypes135,149. Multiple efforts to shorten T cell manufacturing times are therefore currently ongoing150,151. The advent of new manufacturing methods might enable the generation of products both with improved phenotypes and with faster production times to ameliorate the problem of disease progression during cell manufacture. Similarly, efforts to use allogeneic cells might eventually improve efficacy because more robust T cells derived from donors without cancer could be used as the source material152.
Conclusions
CAR T cells are a potent treatment option for patients with haematological malignancies, with long-term data demonstrating robust efficacy and overall low levels of toxicity. The highly durable remissions observed after CD19-targeted CAR T cell therapy in patients with B cell lymphoma demonstrate the potential of this therapeutic modality to induce curative remission in patients with chemotherapy-refractory malignancies. CAR T cells can also serve as an important bridge to allogeneic HSCT in patients with B-ALL and can provide prolonged treatment-free remission for patients with MM. Numerous promising areas of investigation have the potential to improve the durability of remission after this therapy. Overall, we are at an exciting time in the development of CAR T cells, with improving responses and additional treatment indications continuing to emerge.
Acknowledgements
The work of the authors is supported by National Cancer Institute intramural funding. K.M.C. thanks the staff at INOVA Schar Cancer Institute for support and S.D. Cappell for critically reviewing the manuscript.
Author contributions
Both authors made a substantial contribution to all aspects of the preparation of this manuscript.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks E. Jacoby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
J.N.K. has received research funding from Bristol Myers Squibb and Kite and receives royalties relating to patents from Kite and Kyverna Therapeutics. K.M.C. declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 11.Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler NH, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat. Med. 2022;28:325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson CA, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
- 15.Crump M, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cappell KM, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J. Clin. Oncol. 2020;38:3805–3815. doi: 10.1200/JCO.20.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. doi: 10.1016/S0140-6736(21)01222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qayed M, Bleakley M, Shah NN. Role of chimeric antigen receptor T-cell therapy: bridge to transplantation or stand-alone therapy in pediatric acute lymphoblastic leukemia. Curr. Opin. Hematol. 2021;28:373–379. doi: 10.1097/MOH.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raje N, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin T, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 2023;41:1265–1274. doi: 10.1200/JCO.22.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munshi NC, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2021;384:705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 23.Locke FL, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 2022;386:640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 24.Kamdar M, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 25.Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 2021;18:715–727. doi: 10.1038/s41571-021-00530-z. [DOI] [PubMed] [Google Scholar]
- 26.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2021;18:71–84. doi: 10.1038/s41571-020-0427-6. [DOI] [PubMed] [Google Scholar]
- 28.Amini L, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 2022;19:342–355. doi: 10.1038/s41571-022-00607-3. [DOI] [PubMed] [Google Scholar]
- 29.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JK, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J. Clin. Oncol. 2019;37:2105–2119. doi: 10.1200/JCO.18.02079. [DOI] [PubMed] [Google Scholar]
- 31.Chong EA, Ruella M, Schuster SJ, Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N. Engl. J. Med. 2021;384:673–674. doi: 10.1056/NEJMc2030164. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson C, et al. Long-term (≥4 year and ≥5 year) overall survival (OS) by 12- and 24-month event-free survival (EFS): an updated analysis of ZUMA-1, the pivotal study of axicabtagene ciloleucel (axi-cel) in patients (pts) with refractory large B-cell lymphoma (LBCL) Blood. 2021;138(Suppl. 1):1764. doi: 10.1182/blood-2021-148078. [DOI] [Google Scholar]
- 33.Schuster SJ, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403–1415. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 34.Hirayama AV, et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood. 2019;134:636–640. doi: 10.1182/blood.2019000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J. Clin. Oncol. 2023;41:555–567. doi: 10.1200/JCO.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey NV, et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T cells in relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 2020;38:2862–2871. doi: 10.1200/JCO.19.03237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abramson JS PM, et al. Two-year follow-up of transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed or refractory (R/R) large B-cell lymphomas (LBCL) Blood. 2021;138(Suppl. 1):2840. doi: 10.1182/blood-2021-148948. [DOI] [Google Scholar]
- 38.Siddiqi T, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139:1794–1806. doi: 10.1182/blood.2021011895. [DOI] [PubMed] [Google Scholar]
- 39.Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract. Res. Clin. Haematol. 2018;31:209–216. doi: 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Gisselbrecht C, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin A, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 42.Shah NN, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J. Clin. Oncol. 2021;39:1650–1659. doi: 10.1200/JCO.20.02262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hay KA, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–1663. doi: 10.1182/blood-2018-11-883710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah BD, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138:11–22. doi: 10.1182/blood.2020009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roddie C, et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J. Clin. Oncol. 2021;39:3352–3363. doi: 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, et al. Humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells for relapsed/refractory pediatric acute lymphoblastic leukemia. Am. J. Hematol. 2021;96:E162–E165. doi: 10.1002/ajh.26123. [DOI] [PubMed] [Google Scholar]
- 48.Frey NV, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J. Clin. Oncol. 2020;38:415–422. doi: 10.1200/JCO.19.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An F, et al. Influence of patient characteristics on chimeric antigen receptor T cell therapy in B-cell acute lymphoblastic leukemia. Nat. Commun. 2020;11:5928. doi: 10.1038/s41467-020-19774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacoby E, et al. Parameters of long-term response with CD28-based CD19 chimaeric antigen receptor-modified T cells in children and young adults with B-acute lymphoblastic leukaemia. Br. J. Haematol. 2022;197:475–481. doi: 10.1111/bjh.18105. [DOI] [PubMed] [Google Scholar]
- 51.Laetsch TW, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J. Clin. Oncol. 2023;41:1664–1669. doi: 10.1200/JCO.22.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wayne AS, et al. Three-year results from phase 1 of ZUMA-4: KTE-X19 in pediatric relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2022 doi: 10.3324/haematol.2022.280678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grupp SA, et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood. 2018;132:895–895. doi: 10.1182/blood-2018-99-112599. [DOI] [Google Scholar]
- 54.Myers RM, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J. Clin. Oncol. 2022;40:932–944. doi: 10.1200/JCO.21.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dourthe ME, et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia. 2021;35:3383–3393. doi: 10.1038/s41375-021-01281-7. [DOI] [PubMed] [Google Scholar]
- 56.Gauthier, J. in The EBMT/EHA CAR-T Cell Handbook (eds N. Kroger et al.) 165–168 (Springer, 2022).
- 57.Ali SA, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brudno JN, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 2018;36:2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao WH, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in China (LEGEND-2) J. Hematol. Oncol. 2022;15:86. doi: 10.1186/s13045-022-01301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D, et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood. 2021;137:2890–2901. doi: 10.1182/blood.2020008936. [DOI] [PubMed] [Google Scholar]
- 61.Li C, et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin. Transl. Med. 2021;11:e346. doi: 10.1002/ctm2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikkilineni L, et al. Treatment of patients with T cells expressing a fully-human anti-BCMA CAR with a heavy-chain antigen-recognition domain caused high rates of sustained complete responses and relatively mild toxicity. Blood. 2021;138:3837–3837. doi: 10.1182/blood-2021-152688. [DOI] [Google Scholar]
- 63.Lonial S, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–221. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 64.Moreau P, et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2022;387:495–505. doi: 10.1056/NEJMoa2203478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neelapu SS, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat. Med. 2022;28:735–742. doi: 10.1038/s41591-022-01731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pulsipher MA, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3:66–81. doi: 10.1158/2643-3230.BCD-21-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank MJ, et al. Monitoring of circulating tumor DNA improves early relapse detection after axicabtagene ciloleucel infusion in large B-cell lymphoma: results of a prospective multi-institutional trial. J. Clin. Oncol. 2021;39:3034–3043. doi: 10.1200/JCO.21.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vercellino L, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4:5607–5615. doi: 10.1182/bloodadvances.2020003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iacoboni G, et al. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy. Ann. Hematol. 2021;100:2303–2310. doi: 10.1007/s00277-021-04560-6. [DOI] [PubMed] [Google Scholar]
- 72.Cherng HJ, et al. Risk assessment with low-pass whole-genome sequencing of cell-free DNA before CD19 CAR T-cell therapy for large B-cell lymphoma. Blood. 2022;140:504–515. doi: 10.1182/blood.2022015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl Acad. Sci. USA. 2019;116:9543–9551. doi: 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kochenderfer JN, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 Levels. J. Clin. Oncol. 2017;35:1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turtle CJ, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gauthier J, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137:323–335. doi: 10.1182/blood.2020006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirayama AV, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133:1876–1887. doi: 10.1182/blood-2018-11-887067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gardner RA, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turtle CJ, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wrzesinski C, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J. Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brudno JN, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porter DL, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melenhorst JJ, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602:503–509. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kochenderfer JN, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol. Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cordeiro A, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol. Blood Marrow Transpl. 2020;26:26–33. doi: 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T — and a side order of IgG, to go? — Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38:100596. doi: 10.1016/j.blre.2019.100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–935. doi: 10.1182/blood.2019004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Atanackovic D, et al. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell-depleted lymphoma after CART therapy. Blood. 2022;140:152–156. doi: 10.1182/blood.2022016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sesques P, et al. Immune response to three doses of mRNA SARS-CoV-2 vaccines in CD19-targeted chimeric antigen receptor T cell immunotherapy recipients. Cancer Cell. 2022;40:236–237. doi: 10.1016/j.ccell.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Logue JM, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106:978–986. doi: 10.3324/haematol.2019.238634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brudno JN, et al. Acute and delayed cytopenias following CAR T-cell therapy: an investigation of risk factors and mechanisms. Leuk. Lymphoma. 2022;63:1849–1860. doi: 10.1080/10428194.2022.2056172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fried S, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 2019;54:1643–1650. doi: 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
- 97.Little JS, et al. Low incidence of invasive fungal disease following CD19 chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Adv. 2022;6:4821–4830. doi: 10.1182/bloodadvances.2022007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gudiol C, Lewis RE, Strati P, Kontoyiannis DP. Chimeric antigen receptor T-cell therapy for the treatment of lymphoid malignancies: is there an excess risk for infection? Lancet Haematol. 2021;8:e216–e228. doi: 10.1016/S2352-3026(20)30376-8. [DOI] [PubMed] [Google Scholar]
- 99.Shree T, et al. Impaired immune health in survivors of diffuse large B-cell lymphoma. J. Clin. Oncol. 2020;38:1664–1675. doi: 10.1200/JCO.19.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheok KPL, et al. Severe presentations and high mortality from SARS-CoV-2 in patients undergoing chimeric antigen receptor (CAR-T) therapy: a UK NCCP analysis. Leuk. Lymphoma. 2022;63:1980–1984. doi: 10.1080/10428194.2022.2057487. [DOI] [PubMed] [Google Scholar]
- 101.Spanjaart AM, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–3588. doi: 10.1038/s41375-021-01466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Busca A, et al. COVID-19 and CAR T cells: a report on current challenges and future directions from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv. 2022;6:2427–2433. doi: 10.1182/bloodadvances.2021005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 104.Steffin DHM, et al. Long-term follow-up for the development of subsequent malignancies in patients treated with genetically modified IECs. Blood. 2022;140:16–24. doi: 10.1182/blood.2022015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Travis LB, et al. Second cancers among long-term survivors of non-Hodgkin’s lymphoma. J. Natl Cancer Inst. 1993;85:1932–1937. doi: 10.1093/jnci/85.23.1932. [DOI] [PubMed] [Google Scholar]
- 106.Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 107.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 108.Plaks V, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138:1081–1085. doi: 10.1182/blood.2021010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Da Via MC, et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 2021;27:616–619. doi: 10.1038/s41591-021-01245-5. [DOI] [PubMed] [Google Scholar]
- 110.Samur MK, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021;12:868. doi: 10.1038/s41467-021-21177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zurko JC, et al. Long-term outcomes and predictors of early response, late relapse, and survival for patients treated with bispecific LV20.19 CAR T-cells. Am. J. Hematol. 2022 doi: 10.1002/ajh.26718. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1-2 trial. Leukemia. 2022;36:189–196. doi: 10.1038/s41375-021-01345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cordoba S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shalabi H, et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140:451–463. doi: 10.1182/blood.2022015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spiegel JY, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Friedman KM, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum. Gene Ther. 2018;29:585–601. doi: 10.1089/hum.2018.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao WH, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brudno JN, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 2020;26:270–280. doi: 10.1038/s41591-019-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mikkilineni L, et al. Deep and durable remissions of relapsed multiple myeloma on a first-in-humans clinical trial of T cells expressing an anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) with a fully-human heavy-chain-only antigen recognition domain. Blood. 2020;136:50–51. doi: 10.1182/blood-2020-138839. [DOI] [Google Scholar]
- 120.Cohen AD, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamers-Casterman C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 122.Asaadi Y, Jouneghani FF, Janani S, Rahbarizadeh F. A comprehensive comparison between camelid nanobodies and single chain variable fragments. Biomark. Res. 2021;9:87. doi: 10.1186/s40364-021-00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lam N, et al. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat. Commun. 2020;11:283. doi: 10.1038/s41467-019-14119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qayed M, et al. Leukapheresis guidance and best practices for optimal chimeric antigen receptor T-cell manufacturing. Cytotherapy. 2022;24:869–878. doi: 10.1016/j.jcyt.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Neelapu SS, et al. Primary analysis of ZUMA-12: a phase 2 study of axicabtagene ciloleucel (axi-cel) as first-line therapy in patients with high-risk large B-cell lymphoma (LBCL) Blood. 2021;138:739–739. doi: 10.1182/blood-2021-148009. [DOI] [Google Scholar]
- 126.Liebers N, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv. 2021;5:2707–2716. doi: 10.1182/bloodadvances.2020004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pinnix CC, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871–2883. doi: 10.1182/bloodadvances.2020001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nastoupil LJ, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 2020;38:3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lutfi F, et al. The impact of bridging therapy prior to CD19-directed chimeric antigen receptor T-cell therapy in patients with large B-cell lymphoma. Br. J. Haematol. 2021;195:405–412. doi: 10.1111/bjh.17738. [DOI] [PubMed] [Google Scholar]
- 130.Jain T, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transpl. 2019;25:2305–2321. doi: 10.1016/j.bbmt.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 131.Pont MJ, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134:1585–1597. doi: 10.1182/blood.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gauthier J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–1660. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geyer MB, et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019 doi: 10.1172/jci.insight.122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chong EA, et al. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139:1026–1038. doi: 10.1182/blood.2021012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watanabe N, Mo F, McKenna MK. Impact of manufacturing procedures on CAR T cell functionality. Front. Immunol. 2022;13:876339. doi: 10.3389/fimmu.2022.876339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 137.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rossi J, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132:804–814. doi: 10.1182/blood-2018-01-828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deng Q, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat. Med. 2020;26:1878–1887. doi: 10.1038/s41591-020-1061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fraietta JA, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sommermeyer D, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu Y, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sabatino M, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128:519–528. doi: 10.1182/blood-2015-11-683847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van der Waart AB, et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood. 2014;124:3490–3500. doi: 10.1182/blood-2014-05-578583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghassemi S, et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 2018;6:1100–1109. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu TL, et al. A rapid cell expansion process for production of engineered autologous CAR-T cell therapies. Hum. Gene Ther. Methods. 2016;27:209–218. doi: 10.1089/hgtb.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ghassemi S, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 2022;6:118–128. doi: 10.1038/s41551-021-00842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mailankody S, et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 2023;29:422–429. doi: 10.1038/s41591-022-02182-7. [DOI] [PubMed] [Google Scholar]
- 153.Kochenderfer JN, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hollyman D, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J. Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]