Abstract
The coronavirus disease 19 (COVID-19), due to coronavirus 2 (SARS-CoV-2) infection, presents with an extremely heterogeneous spectrum of symptoms and signs. COVID-19 susceptibility and mortality show a significant sex imbalance, with men being more prone to infection and showing a higher rate of hospitalization and mortality than women. In particular, cardiovascular diseases (preexistent or arising upon infection) play a central role in COVID-19 outcomes, differently in men and women. This review will discuss the potential mechanisms accounting for sex/gender influence in vulnerability to COVID-19. Such variability can be ascribed to both sex-related biological factors and sex-related behavioural traits. Sex differences in cardiovascular disease and COVID-19 involve the endothelial dysfunction, the innate immune system and the renin-angiotensin system (RAS). Furthermore, the angiotensin-converting enzyme 2 (ACE2) is involved in disease pathogenesis in cardiovascular disease and COVID-19 and it shows hormone-dependent actions. The incidence of myocardial injury during COVID-19 is sex-dependent, predominantly in association with a greater degree of inflammation and coagulation disorders among men. Its pathogenesis is not fully elucidated, but the main theories foresee a direct role for the ACE2 receptor, the hyperimmune response and the RAS imbalance, which may also lead to isolated presentation of COVID-19-mediated myopericarditis. Moreover, the latest evidence on cardiovascular diseases and their relationship with COVID-19 during pregnancy will be discussed. Finally, authors will analyse the prevalence of the long-covid syndrome between the two sexes and its impact on the quality of life and cardiovascular health.
Keywords: cardiovascular health, COVID-19, pandemic, women
Introduction
Since March 2020, the coronavirus disease (COVID-19) pandemic has been continuing to spread across the globe and, although specific therapies and vaccines are now available, the onset of more contagious variants still presents today a persistent challenge for health systems because of the high number of infections, hospitalizations and deaths. During the past 2 years, population sub-groups with higher vulnerability and comorbidities have been identified. Some studies have already highlighted that advanced age and preexisting cardiovascular and metabolic conditions are associated with poor outcome.1–4 Moreover, some potential sex differences in the COVID-19 outcomes have been discussed.
In COVID-19, mortality is strongly associated with the presence of myocardial injury (7–40%)5,6 and cardiovascular diseases play a crucial role in determining the outcome, as preexistent or intercurrent. Thus, whether the incidence of COVID-19 related myocardial injury is sex-dependent remains not fully elucidated.7–9
Since the onset of the pandemic, men have appeared to suffer from a respiratory and cardiovascular poorer prognosis, exhibiting more severe and long-lasting disease and symptoms.10,11
The exact mechanisms of these differences are not completely understood and likely represent a complex mosaic of multiple factors. In this review, we discuss these sex and gender impacts on women's cardiovascular health of the COVID-19 pandemic, highlighting the recent studies reporting sex/gender-specific comorbidity profiles and outcomes.
Cardiovascular risk factors and outcomes of COVID-19 in women
Globally, cardiovascular disease (CVD) is the leading cause of death among women in high-income countries. In Europe, more women than men die from CVD, as they are less likely to be diagnosed and to be treated appropriately.12
Cardiovascular injury is associated with a substantial proportion of COVID-19 deaths and a preexisting CVD is one of the most common risk factors for hospitalization and death in COVID-19 patients.13
Furthermore, cardiovascular risk itself has been significantly affected by the health policy preventive measures to the COVID-19 pandemic, as the significant limitation of economic and social activities has led to unemployment and increased sedentary time and increased incidence of mental health issues, all of which are well recognized risk factors for CVD and associated with worsening cardiovascular outcomes.14
Despite women and men sharing similar ‘traditional’ cardiovascular risk factors, their relative weight and impact on CVD seems to be modulated by gender, as shown in the INTERHEART study, while traditional algorithms for cardiovascular risk underestimate the female risk of CAD.15 Among nontraditional risk factors for CVD, depressive disorders have been considered a relevant emergent risk factor for CVD in women, as they seem to be more prone to depressive disorders, with a doubled incidence compared with men. Moreover, CVD risk factors and risk markers that are unique to women exist.16,17
Several worldwide studies highlighted that many modifiable and nonmodifiable cardiovascular factors, such as older age, male sex, diabetes, overweight, hypertension, and depression, are strictly related to adverse outcomes in COVID-19 patients. Patients with old age (≥60 years), male sex, hypertension and diabetes mellitus have more than a 100 times higher risk for severe COVID-19 than those without these risk factors and a greater risk of in-hospital death.18,19 The role of both age and sex regarding COVID-19 outcomes is still a matter of debate. Collard et al.20 showed that in a cohort of 1604 Dutch patients with a mean age of 66 ± 15 years (60.5% men), the association of hypertension, dyslipidaemia and diabetes leads to a stepwise increased risk of short-term mortality in hospitalized COVID-19 patients, independently of age and sex. A meta-analysis by Matsushita et al.21 suggested that despite the potential for confounding, hypertension, diabetes and CVD are independently associated with severe COVID-19 and, together with age and male sex, can be informative for predicting the risk of severe COVID-19.
On the contrary, a meta-analysis published in 2021 about the impact of CVD and risk factors on fatal outcomes in patients with COVID-19 according to age (48 317 patients with COVID-19, median age 56 years, 58.1% men) suggested that CVD and its risk factors (hypertension and diabetes) are closely related to fatal outcomes in COVID-19 for patients across all ages. Although young patients had lower prevalence rates of cardiovascular comorbidities than elderly patients, the relative risk of fatal outcome in young patients with hypertension, diabetes and CVD was higher than in elderly patients.22
Although young patients had lower prevalence rates of cardiovascular comorbidities than elderly ones, the relative risk of fatal outcomes in young patients with hypertension, diabetes and CVD was higher than in elderly patients. Among young adults, hypertension, diabetes and CVD are prevalent in male participants, while women are substantially protected until menopause.23,24 This explains the tendency for worse COVID-19 outcomes in young male adults affected by CVD.
Furthermore, there is still a paucity of studies specifically focused on women. Among them, Tsai et al.25 analysed data from 77 364 U.S. women veterans (mean age 50.69 ± 12.80 years), confirming that older age, obesity, prior CVD and chronic obstructive pulmonary disease (COPD) represent relevant risk factors for mortality in COVID-19 disease.
Sex differences in COVID-19 pathogenesis
The higher COVID-19 case fatality rate and increased severity of disease in men compared with women is likely due to a combination of behavioural/lifestyle risk factors, prevalence of comorbidities, ageing and underlying biological sex differences.26
SARS-CoV-2 affects first tissues expressing the angiotensin-converting enzyme 2 (ACE2) receptor, namely the upper airway, lungs, heart, vascular endothelium, kidney, testis and gastrointestinal tract. ACE2 cleaves angiotensin-II into Ang 1–7, which induces vasodilatation; has antiarrhythmic, antihypertensive, anti-inflammatory and antithrombotic effects; and inhibits pathologic cardiac remodelling and insulin resistance.27,28 The SARS-CoV-2 spike protein interacts with the ACE2 receptor; then, a protein called type 2 transmembrane serine protease (TMPRSS2) induces membrane fusion.29,30 Viral invasion reduces ACE2 expression, causing the loss of the protective regulatory effect of ACE2.28
The ACE2 gene lies on the X chromosome and escapes X-chromosome inactivation.31 Furthermore, ACE2 expression might be regulated by estrogens.32 Nonetheless, ACE2 does not seem to be more expressed in alveolar cells in women compared with men.33 Furthermore, smoking status and COPD have been associated with a higher ACE2 expression,34,35 and both smoking and COPD36 are more prevalent among men.
Androgens are the only known stimulus to TMPRSS2 expression. Men with prostate cancer on androgen deprivation therapy may have a significantly lower risk of SARS-CoV-2 infection than the other male patients.37 The risk of severe infection mediated by androgen levels may, in part, explain why preadolescents are usually not severely affected by infection with SARS-CoV2.26
Morbidity and mortality associated with COVID-19 are mediated through intense viral stimulated inflammation and increasing levels of inflammatory biomarkers and cytokines, often referred to as a ‘cytokine storm’. Older and male patients have the highest risk of developing a cytokine storm during SARS-CoV-2 infection.38–40 Young and old men with COVID-19 exhibit significantly higher levels of the pro-inflammatory cytokines interleukin-2 and tumour necrosis factor alpha, regardless of comorbidities.41 Furthermore, men have a higher C-reactive protein concentration than women, independently of age and comorbidities.41
The neutrophil-to-lymphocyte ratio (NLR) is a well known marker of inflammation and appears to reflect the severity of COVID-19, particularly among patients older than 50 years.42,43 A single-centre retrospective study observed that more men had an NLR above 11.75, which was associated with a lower survival rate.44
Several genes involved in the innate and adaptive immune responses to infection are located on the X chromosome.44 Although X-chromosome inactivation is a mechanism of equalizing gene expression in women and men, some genes may escape silencing, thereby conferring on women an immune advantage over men.43 For example, toll-like receptors (TLRs), which upregulate type 1 interferon (IFN), an important protective mechanism against viral infections,45 may be up to 10-fold higher in women than in men.46–48 Furthermore, IFN levels after TLR7 stimulation were lower in men compared with women. TLR7-mediated IFN expression may be decreased in men due to the known negative effects of testosterone on IFN expression.49 Finally, a single-centre study showed that concentrations of SARS-CoV-2 immunoglobulin G were significantly higher in women compared with men, and remained higher until 4 weeks after hospital admission.39
Overall, sex-related differences in the pathogenetic mechanisms of COVID-19, the risk of infection, disease severity and its outcomes seem to exist. Nonetheless, the interplay between sex and COVID-19 features is complex. For example, although women have an overall stronger immune response, and appear to mount a stronger response to SARS-CoV-2, men are more likely to develop the cytokine storm associated with poor COVID-19 outcomes. Future studies are warranted to elucidate these points.
Myocardial damage/myopericarditis in COVID-19
Myocardial damage in patients with COVID-19 is difficult to acknowledge due to the different assessment methods, different clinical presentations and different studied populations. Myocardial damage has been demonstrated in 7–40% of COVID-19 patients depending on geographic area, with a higher prevalence among those patients admitted to intensive care.5,6,49,50 Mortality is approximately 22% among patients with cardiac troponin (cTn) above reference limits and 61.5% for those with cTn levels more than 10 times reference limits.
The mechanisms of myocardial damage in COVID-19 are multifactorial, ranging from coronary thrombotic events and atherosclerotic plaque rupture to sepsis-related cardiomyopathy triggered by systemic hyper-inflammatory state.51 The extensive systemic inflammatory reaction associated with severe pneumonia in COVID-19 may also lead to an increased propensity for plaque rupture,51 and thrombus formation,52 leading to type 1 myocardial infarction.
In this context, several research groups have highlighted a sex-specific propensity for the inflammatory response, outlining a different risk between the two sexes. In fact, as stated before, men are more likely to have a less powerful immune response and therefore a greater susceptibility or vulnerability to infections.53 Moreover, abdominal fat, which has been linked to more severe COVID-19 clinical presentations, is more prevalent in men and, containing more macrophages, inflammatory cells with higher cytokine concentrations, may play a central role in cytokine storm-related myocardial damage. Histopathological examination of autopsy specimens revealed that patients dying from COVID 19 infection have consistently higher macrophage and T cell content in coronary adventitia and peri-adventitial fat. In women, the immune response is mediated by estradiol and progesterone leading to less severe COVID-19 infections with lower mortality rates than in men.26,54,55
Furthermore, myocardial infarction patients with COVID-19 showed significantly higher rates of no coronary reflow (blush flow grade 0 or 1) with worse left ventricular function after revascularization, despite similar ischemic times. Nitric oxide is an important physiological vasodilator that exerts vasodilation through the activation of the cyclic guanosine monophosphate cyclase (cGMP) pathway.56 Several studies have shown that nitric oxide production is enhanced by oestrogen via oestrogen receptor mediated transcriptional upregulation of endothelial nitric oxide synthase (eNOS).57,58 Therefore, the oestrogen-dependent eNOS-NO-cGMP vasodilation pathway could be an advantage for female patients with COVID-19 by promoting improved coronary vasodilation and perfusion.
Several studies also report that patients with COVID-19 often present with type 2 myocardial damage for a variety of reasons. First, systemic inflammation is associated with marked haemodynamic changes, including sympathetic activation-mediated tachycardia, which results in increased myocardial oxygen requirements.59 Furthermore, mitochondrial dysfunction occurs with mitochondrial decoupling, which leads to an increase in mitochondrial oxygen uptake and thus to an increase in myocardial oxygen demand.60
The adrenal glands are a potential target for SARS-CoV-2 infection, predisposing patients with COVID-19 to adrenergic overdrive secondary to infection.61 The hypothalamus-pituitary-adrenal axis, responsible for the integration and management of the organism's internal and external stress stimuli, has significant sex-related differences in the neuroendocrine response. A more robust and enhanced release of stress hormones by the adrenal glands, including glucocorticoids, in response to acute stressors could not only contribute to greater protection against severe COVID-19 but also to less myocardial damage in patients with high cardiovascular risk.
Acute nonischemic myocardial injury is probably the main reason for the troponin increases. Common cardiac causes include myocarditis, Tako-Tsubo syndrome and acute heart failure due to systolic and diastolic dysfunction.62 Primary noncardiac conditions, such as pulmonary embolism and sepsis, also cause myocardial damage.62,63
However, myocarditis and myopericarditis are often causes of acute nonischemic myocardial injury that deserve special attention in COVID-19.
Pericarditis in COVID 19 has been described as one of the possible manifestations; however, data on true prevalence are not available. The clinical data available therefore derive mainly from clinical cases that attest a higher prevalence in men and an evolution towards myopericarditis in more than half of the cases.64 However, in most the studies, the definition of myopericarditis was based on troponin levels and left ventricular function, while there were other possible mechanisms not related to myocardial inflammation such as acute coronary thrombosis and Takotsubo cardiomyopathy, which could also result in elevation of troponins in COVID-19 patients.
Moreover, according to the current European Society of Cardiology guidelines, the diagnosis of viral myocarditis is a diagnosis of exclusion made with certainty only in the event that a viral genome within the cardiomyocytes is demonstrated together with the histological results of active myocarditis.65
The SARS-CoV-2 genome has been identified in endomyocardial biopsies of patients with suspected myocarditis.66 However, there is no direct evidence of SARS-CoV-2 within cardiomyocytes, as the presence of the virus has been documented in interstitial cells within heart tissue but not in cardiomyocytes, suggesting that the presence of the viral genome was due to the migration of infected macrophages.67
Recently, rare and self-limiting cases of myocarditis with temporal association with immunization with an mRNA-based COVID-19 vaccine have been reported, mainly in young men (mean age 25 years).68 Data from the Israeli Ministry of Health suggest a crude incidence rate of around 24 cases per million after a second dose.68 However, the true incidence of this adverse event is unknown at this time. Although the specific mechanisms are unclear, an immune-mediated mechanism is likely for which the female sex appears to be protected.69
Pregnancy outcomes and COVID-19 infection
Physiological changes during pregnancy in the pulmonary system, such as increased oxygen consumption, increased minute ventilation and decreased lung capacity, can make pregnant women more susceptible to severe respiratory infections. Moreover, some adaptive immune responses are downregulated in pregnant women (e.g. the decrease in the number of T cells and B cells). Therefore, pregnant women were identified as a vulnerable group.
The majority of studies assessing the impact of COVID-19 on pregnant women were conducted before the vaccination campaign. According to reviews and meta-analyses, almost half of pregnant women were asymptomatic, while the most commonly reported symptoms were those typically related to the virus (fever, cough, fatigue, anosmia, ageusia, headache).70,71 Increased risk of severe COVID-19 has been associated with different factors such as increasing age, high BMI, chronic hypertension, past smoking history, preexisting diabetes, CVDs, gestational diabetes mellitus and preeclampsia, although no link has been reported with race/ethnicity and parity.70,71
Although not confirmed by all studies, COVID-19 infection in pregnant women was shown to significantly increase the risk of preeclampsia/eclampsia, preterm birth, stillbirth, ICU admission, maternal mortality, need for mechanical ventilation, vasopressor support, venous thromboembolism, lower birth weight, neonatal ICU admission, severe perinatal morbidity and mortality.72–74 It has been proposed that COVID-19 infection might lead to adverse pregnancy outcomes through the deregulation of otherwise tightly regulated Treg/Th17 ratios and to subsequent uncontrolled systemic inflammation.75 Inflammation can also adversely affect the placenta and higher rates of placenta vascular dys-perfusion, thrombosis and inflammation have been reported among infected women. COVID-19 was not related with caesarean delivery or postpartum haemorrhage compared with no COVID-19.72–75 There are conflicting results about the effect of first-trimester COVID-19 infection on miscarriage rates.
As regards the stratification of the outcomes by maternal COVID-19 severity, a systematic review and meta-analysis has found that, compared with mild COVID-19, severe COVID-19 (presence of dyspnoea, respiratory rate at 30 breaths per minute or more and oxygen saturation <93% or on room air, or findings consistent with pneumonia) was strongly associated with preeclampsia, gestational diabetes, ICU admission, mechanical ventilation, preterm birth, caesarean delivery, low birth weight and neonatal ICU admission.72
Although there is a surge in studies demonstrating the harmful effects of COVID-19 on the cardiovascular system, only a few cases of COVID-19 related cardiomyopathy have been reported in pregnancy. Mercedes et al.76 described a series of 154 pregnant women with COVID 19 infection, of whom 15 developed myocardial injury. All these women needed to be transferred to the ICU and presented with left ventricular dysfunction at echocardiography and highly elevated levels of troponin and B-type natriuretic peptide. Two patients died from arrhythmias.
Mounting evidence supports the safety of COVID-19 vaccination in pregnancy. No difference in rates of adverse pregnancy outcomes or newborn complications has been detected between women who received at least one dose of the COVID-19 vaccine during the second or third trimesters of pregnancy and unvaccinated pregnant women.77 Moreover, the vaccination in the first pregnancy trimester does not increase the risk of early pregnancy loss.
The risk of vertical mother-to-baby transmission of SARS-CoV-2 is low, likely because the placental cells co-expressing ACE2 and TMPRSS2 proteins, required for SARS-CoV-2 viral cell entry, are rare.78 Moreover, according to the current evidence, breast milk is unlikely to be a source of transmission of SARS-COV-2. Conversely, antibodies of SARS-CoV-2 may be transferred from infected or healthy vaccinated lactating mothers to newborns through breastfeeding, with a potential protective effect.79
Long-COVID-19 syndrome: differences between men and women
As above mentioned, the SARS-CoV-2 pandemic has clearly demonstrated that sex and gender-related factors play role in COVID-19 vulnerability, both in the acute phase and in the postinfection period.
Therefore, it is imperative to collect sex-disaggregated data in order to better understand the differences in the pathophysiologic mechanisms of COVID-19 and to implement effective treatment and prevention.
Many studies showed gender differences in outcomes and symptoms between the two sexes in the acute phase of infection. As already stated, men have higher mortality and women have more symptoms; nevertheless, few data are available on the role of sex in long-COVID-19 syndrome80 even if women tend to be at a higher risk for long-term manifestations.81,82
Long-COVID-19 syndrome is defined as persistent symptomatology extending beyond 12 weeks after the initial symptoms of acute infection. The most common symptoms are fatigue, shortness of breath, palpitations, chest pain and sleep disturbances. Other symptoms include neuro-psychiatric involvement such as depression, anxiety, attention disorders and headache.83,84
In a prospective multicentre cohort study by ISARIC (the International Severe Acute Respiratory and emerging Infections Consortium), enrolling 327 hospitalized participants who were evaluated at least 3 months postdischarge, Sigfrid et al.85 showed that 93% of participants reported persistent symptoms, with fatigue being the most common (83%), followed by breathlessness (54%). In addition, women younger than 50 years were five times less likely to report feeling recovered, twice as likely to report worse fatigue and seven times more likely to report breathlessness and more likely to have greater disability than men of the same age.
Female gender is associated with long-COVID syndrome in the prospective cohort study that included 377 hospitalized patients by Bai et al.86 In this study, 69% of patients had a diagnosis of long-COVID syndrome. Interestingly, women were characterized by a higher proportion of most physical symptoms (fatigue, dyspnoea, brain fog, anosmia, dysgeusia, gastrointestinal symptoms) and psychological symptoms (depression, anxiety, posttraumatic stress disorder) than men. At multivariable logistic regression analysis, female gender was as strong a predictor of long-COVID as advanced age and active smoking.86
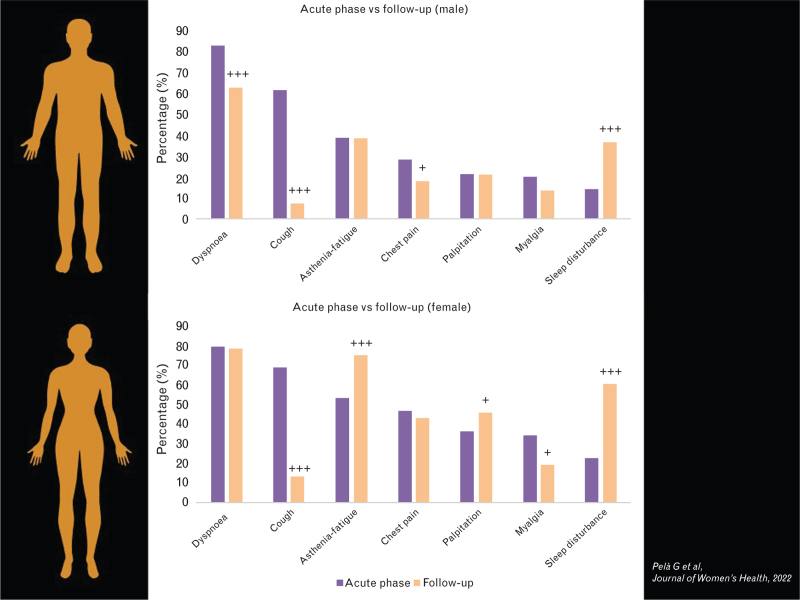
A retrospective/prospective study that included 223 patients with a history of SARS-CoV-2 infection (72% hospitalized, 28% outpatients) has recently demonstrated that women were more symptomatic than men not only in the acute phase but also at follow-up. The persistence of each symptom reported in male and female groups from baseline to follow-up is depicted in Fig. 1 and clearly demonstrate that there are significant differences in long-COVID-19 syndrome between the two groups: men improved significantly in dyspnoea and thoracic pain without change in fatigue and palpitations; by contrast, women did not improve in dyspnoea and thoracic pain and worsened in fatigue and palpitations. At multivariable analysis, female sex was confirmed as an independent predictor of long-lasting COVID-19 symptoms.87
Fig. 1.
Chance in symptoms from the acute phase (purple column) to follow-up (orange column) (160+80 days) in men (on the top) and women (on the bottom) with COVID-19. Adapted from 87.
More recently, a multicentre cohort study including 1969 hospitalized COVID-19 patients, interviewed by telephone questionnaire, found that women were more vulnerable to developing post-COVID-19 symptoms 8 months after discharge. Female sex was linked to at least three post-COVID-19 symptoms, including fatigue, dyspnoea, pain, hair loss, ocular issues, depressive levels, anxiety and poor sleep quality. The authors concluded that healthcare systems should consider sex differences in the management of the long haulers.88
The causes of long-COVID-19 syndrome are not still elucidated. Some hypotheses have been proposed, including a persistent inflammatory state, an autoimmune reaction, persistent circulating SARS-CoV-2 spike protein, postinfection organ damage and genetic predisposition involving innate and interferon-mediated immunity.89,90 In addition, we do not know why women are more likely to experience long-lasting COVID-19 symptoms. In this regard, recent work that demonstrated a persistent immune dysregulation in women is worth mentioning.91
In conclusion, COVID-19 affects women and men differently both in the acute and post-COVID phases. Sex-disaggregated longitudinal studies are necessary to understand the sex-related pathophysiology of the symptoms and their impact on quality of life and work activity to promote preventive and personalized therapeutic approaches and to prevent gender inequality.
Conclusion
As previously described, there is a clear impact of sex and gender on the cardiovascular systems of women and men, which not only implies different susceptibility, clinical course and outcomes of numerous chronic diseases, but also, as demonstrated, in the case of infections, even during COVID-19.7,8 In fact, some related aspects have been identified that explain the differences observed in the clinical evolution of COVID-19 that characterize the two sexes7,8,92; some features are related to gender (high-risk behaviours, nutrition and access to healthcare), others related to sex (influence of sex hormones, variability in the function of the immune system and in the regulation of the inflammatory response, expression of genes and receptors). As noted, significant changes in ACE2 gene expression, immune response and pathophysiology of cardiovascular risk factors and comorbidities help reduce susceptibility to an infection and better survival in women and, conversely, make men more at risk of hospitalization and mortality.16,27,28
Even before the onset of the pandemic, it was observed that the lifestyle of women tended to be less compatible with the nonpharmacological prevention of CVDs93,94; subsequently, a significant impact on the cardiovascular health of women was certainly imputable to those aspects related to mental overload, to family responsibility, stress, and to the further reduction of physical activity experienced during the lockdown.95,96
Unfortunately, confinement at home has also meant a significant increase in cases of domestic violence, which increased stress and depression.14,15,92
The cardiovascular health of the entire population has been severely threatened by the pandemic because of reduced and/or delayed access to treatments and lack of screening. Women have previously experienced this kind of bias, which intensified during the pandemic.
Moreover, the higher susceptibility of women to the long-term consequences of COVID-19 will also be further evaluated and framed in the future, to understand what impact on general health and, specifically, on cardiovascular health long-COVID-19 may have.80–82
More studies are needed to provide a better understanding of sex and gender influence in the reaction to various treatment options or in the long-term sequelae of COVID-19 infection, with the aim of setting up personalized, clinical prevention programmes evaluation and therapies for a more efficient resolution of the disease, minimizing its impact on cardiovascular health.
Acknowledgements
The authors thank the Italian Society of Cardiology as the promoter of this study for the present Special Issue.
All authors equally contributed to the writing process of this paper.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang H, Wu Y, He Y, et al. Age-related risk factors and complications of patients with COVID-19: a population-based retrospective study. Front Med (Lausanne) 2022; 8:757459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. Lancet Glob Health 2020; 8:e1003–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection: a matched cohort study in the UK. PLoS Med 2022; 19:e1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcari L, Luciani M, Cacciotti L, et al. Coronavirus disease 2019 in patients with cardiovascular disease: clinical features and implications on cardiac biomarkers assessment. J Cardiovasc Med (Hagerstown) 2021; 22:832–839. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Shen Y, Wu N, Sun X. J Myocardial injury in severe and critical coronavirus disease 2019 patients. Card Surg 2021; 36:82–88. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Hou K, Xu R, et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc 2020; 9:e016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Aidaoui K, Benhamou RA, Haoudar A, et al. Sex differences in COVID-19 outcomes. Cureus 2022; 14:e25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejpal A, Gianos E, Cerise J, et al. Sex-based differences in COVID-19 outcomes. J Womens Health (Larchmt) 2021; 30:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi CM, Specchia C, Conforti F, et al. Sex-related differences in patients with coronavirus disease 2019: results of the Cardio-COVID-Italy multicentre study. J Cardiovasc Med (Hagerstown) 2022; 23:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita Y, Yokoyama T, Hayakawa K, et al. We should pay more attention to sex differences to predict the risk of severe COVID-19: men have the same risk of worse. J Epidemiol 2022; doi: 10.2188/jea.JE20220056. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percivale I, Danna PSC, Falaschi Z, et al. Men and women affected by Sars-CoV-2 pneumonia: same CT features but different outcome. Clin Radiol 2021; 76:235e25–235e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend N, Kazakiewicz D, Lucy Wright F, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol 2022; 19:133–143. [DOI] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucciarelli V, Nasi M, Bianco F, et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc Med 2022; 32:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucciarelli V, Caterino AL, Bianco F, et al. Depression and cardiovascular disease: the deep blue sea of women's heart. Trends Cardiovasc Med 2020; 30:170–176. [DOI] [PubMed] [Google Scholar]
- 16.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 2016; 118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women's cardiovascular risk. Circulation 2020; 141:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong KA, Jung S, Yu M, Park J, Kang IS. Association between cardiovascular risk factors and the severity of coronavirus disease 2019: nationwide epidemiological study in Korea. Front Cardiovasc Med 2021; 8:732518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma AK, Baig VN, Sharma S, et al. Cardiovascular risk factors and outcomes in COVID-19: a hospital-based study in India. PLoS Glob Public Health 2022; 2:e0000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collard D, Nurmohamed NS, Kaiser Y, et al. Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: a prospective cohort study. BMJ Open 2021; 11:e045482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita K, Ding N, Kou M, et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Global Heart 2020; 15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae S, Kim SR, Kim M, et al. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart 2021; 107:373–380. [DOI] [PubMed] [Google Scholar]
- 23.Sciomer S, De Carlo C, Moscucci F, Maffei S. Age at menopause: a fundamental data of interest to acquire in female patients’ anamnesis. Int J Cardiol 2016; 215:358–359. [DOI] [PubMed] [Google Scholar]
- 24.Mattioli AV, Sciomer S, Moscucci F, et al. Cardiovascular prevention in women: a narrative review from the Italian Society of Cardiology working groups on ‘Cardiovascular Prevention, Hypertension and peripheral circulation’ and on ‘Women Disease’. J Cardiovasc Med (Hagerstown) 2019; 20:575–583. [DOI] [PubMed] [Google Scholar]
- 25.Tsai S, Nguyen H, Ebrahimi R, et al. COVID-19 associated mortality and cardiovascular disease outcomes among US women veterans. Sci Rep 2021; 11:8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haitao T, Vermunt JV, Abeykoon J, et al. COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc 2020; 95:2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circulation research 2020; 126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aimo A, Vergaro G, Passino C, Clerico A. Evaluation of pathophysiological relationships between renin-angiotensin and ACE-ACE2 systems in cardiovascular disorders: from theory to routine clinical practice in patients with heart failure. Crit Rev Clin Lab Sci 2021; 58:530–545. [DOI] [PubMed] [Google Scholar]
- 29.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020; 176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181:281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tukiainen T, Villani AC, Yen A, et al. Landscape of X chromosome inactivation across human tissues. Nature 2017; 550:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukowska A, Spiller L, Wolke C, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 2017; 242:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020; 55:2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakladar J, Shende N, Li WT, Rajasekaran M, Chang EY, Ongkeko WM. Smoking-mediated upregulation of the androgen pathway leads to increased SARS-CoV-2 susceptibility. Int J Mol Sci 2020; 21:3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer discovery 2014; 4:1310–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020; 31:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 2020; 96:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19: a systematic review. Life Sci 2020; 254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J Med Virol 2020; 92:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F, Li L, Zeng J, et al. Can we predict the severity of coronavirus disease 2019 with a routine blood test? Pol Arch Intern Med 2020; 130:400–406. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 2020; 18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan X, Li F, Wang X, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J Med Virol 2020; 92:2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science 1994; 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 46.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathogens 2011; 7:e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 2011; 118:5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb K, Peckham H, Radziszewska A, et al. Sex and pubertal differences in the Type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol 2018; 9:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei JF, Huang FY, Xiong TY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart 2020; 106:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J 2007; 34:11–18. [PMC free article] [PubMed] [Google Scholar]
- 52.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–638. [DOI] [PubMed] [Google Scholar]
- 54.Wray S, Arrowsmith S. The physiological mechanisms of the sexbased difference in outcomes of COVID19 infection. Front Physiol 2021; 12:627260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology 2020; 161:bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Archer SL, Huang JM, Hampl V, et al. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A 1994; 91:7583–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacRitchie AN, Jun SS, Chen Z, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 1997; 81:355–362. [DOI] [PubMed] [Google Scholar]
- 58.Salerni S, Di Francescomarino S, Cadeddu C, Acquistapace F, Maffei S, Gallina S. The different role of sex hormones on female cardiovascular physiology and function: not only oestrogens. Eur J Clin Invest 2015; 45:634–645. [DOI] [PubMed] [Google Scholar]
- 59.Liu PP, Blet A, Smyth D, Li H. The Science underlying COVID-19: implications for the cardiovascular system. Circulation 2020; 142:68–78. [DOI] [PubMed] [Google Scholar]
- 60.Pan P, Wang X, Liu D. The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. J Int Med Res 2018; 46:2157–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanczkowski W, Evert K, Stadtmüller M, et al. COVID-19 targets human adrenal glands. lancet diabetes endocrinol 2021. Lancet Diabetes Endocrinol 2022; 10:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thygesen K, Alpert JS, Jaffe AS, et al. ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur Heart J 2019; 40:237–269. [DOI] [PubMed] [Google Scholar]
- 63.Giustino G, Pinney SP, Lala A, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC Focus Seminar. J Am Coll Cardiol 2020; 76:2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diaz-Arocutipa C, Saucedo-Chinchay J, Imazio M. Pericarditis in patients with COVID-19: a systematic review. J Cardiovasc Med 2021; 22:693–700. [DOI] [PubMed] [Google Scholar]
- 65.Caforio AL, Pankuweit S, Arbustini E, et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34:2636–2648. 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 66.Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail 2020; 7:2440–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020; 5:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol 2021; 6:1115–1117. [DOI] [PubMed] [Google Scholar]
- 69.Petersen BW, Damon IK, Pertowski CA, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recomm Rep 2015; 64:1–26. [PubMed] [Google Scholar]
- 70.Lassi ZS, Ana A, Das JK, et al. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J Glob Health 2021; 11:05018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allotey J, Fernandez S, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020; 370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 2021; 193:E540–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatrics 2021; 175:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metz TD, Clifton RG, Hughes BL, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 2022; 327:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am J Reprod Immunol 2020; 84:e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mercedes BR, Serwat A, Naffaa L, et al. New-onset myocardial injury in pregnant patients with coronavirus disease 2019: a case series of 15 patients. Am J Obstet Gynecol 2021; 224:387.e381–387.e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol 2022; 226:236.e231–236.e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotlyar M, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 2021; 224:35–53.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Chen H, An M, et al. Recommendations for breastfeeding during Coronavirus Disease 2019 (COVID-19) pandemic. Int Breastfeed J 2022; 17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penna C, Mercurio V, Tocchetti CG, Pagliaro P. Sex-related differences in COVID-19 lethality. Br J Pharmacol 2020; 177:4375–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 2020; 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Stadio A, Ricci G, Greco A, de Vincentiis M, Ralli M. Mortality rate and gender differences in COVID-19 patients dying in Italy: a comparison with other countries. Eur Rev Med Pharmacol Sci 2020; 24:4066–4067. [DOI] [PubMed] [Google Scholar]
- 83.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crook MG. Post-COVID-19 symptom burden: what is Long-COVID and how should we manage it? Lung 2021; 199:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pela’ G, Goldoni M, Cavalli C, et al. Long-term cardiac sequelae in patients referred into a diagnostic post-COVID-19 pathway: the different impacts on the right and left ventricles. Diagnostics 2021; 11:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Heal Eur 2021; 8:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect 2022; 28:611e9–611e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pela’ G, Goldoni M, Solinas E, et al. Sex-related differences in long-COVID-19 syndrome. J Womens Health 2022; 31:620–630. [DOI] [PubMed] [Google Scholar]
- 88.Fernández-De-las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med 2022; 11:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Indolfi C, Barillà F, Ciccone MM, et al. Expert consensus document of the Italian Society of Cardiology (SIC): post acute Cardiovascular sequelae of SARS-CoV-2 infection. G Ital Cardiol 2022; 23:491–503. [DOI] [PubMed] [Google Scholar]
- 90.Novelli G, Michela Biancolella M, Novelli G, Michela Biancolella M. COVID-19 and molecular genetics. Genes 2022; 13:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ganesh R, Grach SL, Ghosh AK, et al. The female-predominant persistent immune dysregulation of the post-COVID syndrome. Mayo Clin Proc 2022; 97:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet 2021; 397:2385–2438. [DOI] [PubMed] [Google Scholar]
- 93.Sciomer S, Moscucci F, Maffei S, Gallina S, Mattioli AV. Prevention of cardiovascular risk factors in women: the lifestyle paradox and stereotypes we need to defeat. Eur J Prev Cardiol 2019; 26:609–610. [DOI] [PubMed] [Google Scholar]
- 94.Sciomer S, Gallina S, Mattioli AV, Agostoni PG, Moscucci F. Slow and steady wins the race: better walking than running: the turtle's lesson in the times of COVID-19. Heart Lung 2021; 50:587–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mattioli AV, Sciomer S, Maffei S, Gallina S. Lifestyle and stress management in women during COVID-19 pandemic: impact on cardiovascular risk burden. Am J Lifestyle Med 2020; 15:356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ricci F, Izzicupo P, Moscucci F, et al. Recommendations for physical inactivity and sedentary behavior during the Coronavirus Disease (COVID-19) pandemic. Front Public Health 2020; 8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]