ABSTRACT
Candida auris and other Candida species (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei) are important causes of bloodstream infection. Early or prolonged treatment with antifungal agents is often required. The inhibitory effect of antifungal agents in the patients’ bloodstream may compromise the sensitivity of blood culture (BC) to diagnose and/or monitor patients with candidemia. Using a clinical BC simulation model, we compared antimicrobial drug-neutralizing BC media in BacT/Alert FA PLUS (FAP) or Bactec Plus Aerobic/F (PAF) bottles with non-neutralizing BC media in Bactec Mycosis IC/F (MICF) bottles to allow Candida growth in the presence of 100%, 50%, or 25% peak serum level (PSL) antifungal concentrations. In total, 117 organism/antifungal combinations were studied, and Candida growth was detected after incubating bottles into BacT/Alert VIRTUO or Bactec FX BC systems. Compared to control (without antifungal) bottles, both FAP and PAF bottles with 100% PSL antifungal concentrations allowed 100% recovery for C. auris, C. glabrata, and C. parapsilosis, whereas recovery was below 100% for C. albicans, C. krusei, and C. tropicalis. MICF bottles were less efficient at 100%, 50%, or 25% PSL antifungal concentrations, for all Candida species, except for C. auris. While azoles and amphotericin B did not hinder Candida growth in FAP or PAF bottles, echinocandins allowed C. auris, C. glabrata, and C. parapsilosis to grow in FAP, PAF, or MICF bottles. Overall, the maximum time to detection was 4.6 days. Taken together, our findings emphasize the reliability of BCs in patients undergoing antifungal treatment for candidemia.
IMPORTANCE While echinocandins remain the preferred antifungal therapy for candidemia, bloodstream infections caused by C. auris, C. glabrata, or, at a lesser extent, C. parapsilosis may be difficult to treat with these antifungal agents. This is in view of the high propensity of the above-mentioned species to develop antifungal resistance or tolerance during treatment. Azoles and amphotericin B are possible alternatives. Thus, optimizing the recovery of Candida from BCs is important to exclude the likelihood of negative BCs for Candida species, owing to the inhibitory effect of antifungal agents present in the blood sample with which BCs are inoculated. Consistently, our results about the recovery of medically important Candida species (including C. auris) from simulated BCs in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles containing clinically relevant antifungal concentrations add support to this research topic, as well as to the use of BCs for monitoring the clinical and therapeutic course of candidemia.
KEYWORDS: antifungal agents, blood cultures, candidemia, Candida species, BacT/Alert bottles, Bactec bottles
INTRODUCTION
Bloodstream infections (BSIs) caused by Candida species, including the emerging Candida auris (1), are associated with high mortality rates (2), especially in the case of fungal resistance, i.e., when the invading fungus can grow in the presence of antifungal agents that would otherwise kill (fungicidal agents) or inhibit the growth of (fungistatic agents) the fungus in vitro (3). Unlike other pathogenic Candida species, such as C. albicans, C. glabrata (Nakaseomyces glabrata), C. parapsilosis, C. tropicalis, and C. krusei (Pichia kudriavzevii), which represent (in the order) the most frequent cause of BSI worldwide (2), C. auris has the uniqueness of exhibiting multidrug resistance (4, 5). Consistently, C. auris isolates (most from BSI cases) studied by Lockhart et al. (6) in 2017 showed resistance to 2 classes (azoles and polyenes) and, less frequently, to 3 classes (azoles, polyenes, and echinocandins) of antifungal agents. While a rare cause of candidemia in most hospitals in Europe and the United States exists, C. auris continues to spread across the world, thus posing a global health threat (7).
In the intensive care unit (ICU) setting, which provides the paradigm of Candida BSIs associated with poor outcome (8, 9), patients suspected of having candidemia or other forms of invasive Candida infection usually undergo a blood culture (BC) before starting antifungals, and then a reevaluation of antifungal therapy at day 5 (10). This is the time the patient’s BC incubation in a BacT/Alert VIRTUO (bioMérieux) or Bactec FX (Becton, Dickinson) BC automated system to detect Candida growth is completed. If no Candida growth has been detected, antifungal therapy is usually stopped. However, a substantial number of critically ill patients (e.g., ICU patients with septic shock) are receiving early (e.g., before a BC is drawn) or prolonged (e.g., because of diagnostic uncertainties) treatment with antifungal agents (11). In both cases, optimizing the recovery of Candida from the patient’s BC should be essential to increase the likelihood that a negative (or delayed positive) BC result is due to the inherent low sensitivity of BC systems (12), rather than the effect of antifungal agents present in the patient sample with which the BC is inoculated (13).
Here, we used spiked BCs with C. auris, or 5 other medically important Candida species to test antimicrobial drug-neutralizing BC media in BacT/Alert FA PLUS (FAP) or FN PLUS (FNP) bottles (bioMérieux) for the ability to allow Candida growth in the presence of clinically relevant concentrations of antifungal agents. The recovery rate from, and in the mean time to detection (TTD) with these media, were compared to those with neutralizing BC media in Bactec Plus Aerobic/F (PAF), Plus Anaerobic/F (PNF) bottles (Becton, Dickinson), or with non-neutralizing BC media in Bactec Mycosis IC/F (MICF) bottles (Becton, Dickinson), respectively.
RESULTS
Our clinical Candida BC simulation approach in BacT/Alert or Bactec bottles used 6 Candida species (C. auris, C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis), each in the presence of antifungal agents. Three concentrations (expressed as μg/mL) each of anidulafungin (7.2, 3.6, and 1.8), caspofungin (9.9, 5.0, and 2.5), micafungin (16.4, 8.2, and 4.1), fluconazole (14.0, 7.0, and 3.5; except for C. auris and C. krusei), posaconazole (3.3, 1.7, and 0.8), voriconazole (3.0, 1.5, and 0.8), or amphotericin B (3.5, 1.8, and 0.9; except for C. auris) were used to mimic, respectively, 100%, 50%, or 25% peak serum level (PSL) concentrations, which are achievable in patients who receive standard intravenous doses of antifungal agents (14). Therefore, 117 Candida organism/antifungal drug combinations (each tested in duplicate), corresponding to 234 simulated BCs (each replicated in 5 bottles), were studied (Table S1).
Initially, to mirror the routine clinical BC practice (15), we included 1,170 test (with antifungal) bottles, of which 468 (234 FAP and 234 FNP) were BacT/Alert bottles, and 702 (234 PAF, 234 PNF, and 234 MICF) were Bactec bottles. We also included 390 control (without antifungal) bottles, of which 156 (78 FAP and 78 FNP) were BacT/Alert bottles, and 234 (78 PAF, 78 PNF, and 78 MICF) were Bactec bottles. In keeping with Candida studies, which have a priori included aerobic (BacT/Alert or Bactec) BC bottles only (13, 16–19), no Candida growth was detected in 100% (312/312; 234 test and 78 control) BacT/Alert FNP bottles and in 97.4% (304/312; 234 test and 70 control) Bactec PNF bottles. The 8 Candida positive Bactec PNF (control) bottles grew C. glabrata, which is the only Candida species found to grow in anaerobic BC bottles until recently (20). After excluding 312 (234 test and 78 control) anaerobic bottles for each (BacT/Alert or Bactec) bottle type, our BacT/Alert versus Bactec bottles’ comparison analysis included results for 312 BacT/Alert (FAP) bottles and 624 Bactec (PAF and MICF) bottles.
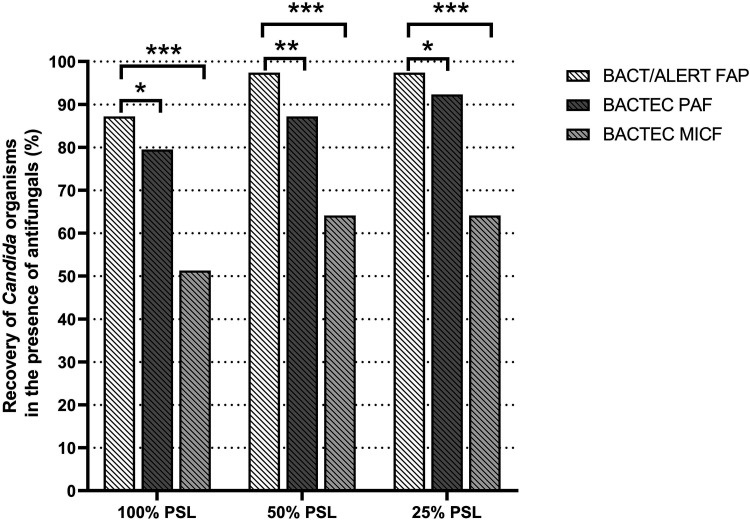
Table 1 and Fig. 1 show the overall recovery results for Candida species from BacT/Alert (FAP) or Bactec (PAF and MICF) bottles, respectively, according to the type (echinocandin, azole, or amphotericin B), or the PSL concentration of antifungal agents present in the bottles. As shown in Table 1, BacT/Alert FAP, Bactec PAF, and Bactec MICF control bottles allowed 100% recovery (78 bottles detected as positive/78 bottles tested) for all 6 Candida species included in the study. Regarding test bottles (Table 1), recovery was 100% in both BacT/Alert FAP (96/96) and Bactec PAF (30/30) bottles for Candida species grown in the presence of azole or amphotericin B antifungal agents. Conversely, recovery was 87.0% in BacT/Alert FAP (94/108), and 70.4% in Bactec PAF (76/108) bottles for Candida species grown in the presence of echinocandin antifungal agents. As expected, Bactec MICF bottles (which do not contain antimicrobial drug-neutralizing resins) allowed 50.0% (54/108), 72.9% (70/96), or 53.3% (16/30) recovery for Candida species grown in the presence of echinocandins, azoles, or amphotericin B, respectively. No recovery was noticed for C. albicans, C. krusei, or C. tropicalis in Bactec MICF bottles with echinocandins or for C. glabrata in Bactec MICF bottles with amphotericin B. As shown in Fig. 1, rates of Candida recovery in BacT/Alert FAP (68/78, 76/78, and 76/78), Bactec PAF (62/78, 68/78, and 72/78), and Bactec MICF (40/78, 50/78, and 50/78) bottles differed, depending on the antifungal concentrations (100% PSL versus 50% PSL or 25% PSL) present in the bottles. Using the McNemar’s test, we found statistically significant differences between BacT/Alert FAP and Bactec PAF bottles (P < 0.05, for both 100% and 25% PLS comparisons; P < 0.01, for 50% PLS comparison), and between BacT/Alert FAP and Bactec MICF bottles (P < 0.001, for all PLS comparisons).
TABLE 1.
Performances of BacT/Alert or Bactec blood culture bottles for the Candida species detection in the absence or presence of antifungal agentsa
| Organism and condition used | BacT/Alert FAP bottles |
Bactec PAF bottles |
Bactec MICF bottles |
|||
|---|---|---|---|---|---|---|
| Recovery, % (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery, % (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery, % (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | |
| Candida species grown without antifungal agents | ||||||
| C. auris | 100 (10/10) | 21.4 | 100 (10/10) | 21.2 | 100 (10/10) | 20.9 |
| C. albicans | 100 (14/14) | 21.5 | 100 (14/14) | 23.3 | 100 (14/14) | 19.9 |
| C. glabrata | 100 (14/14) | 17.8 | 100 (14/14) | 31.5 | 100 (14/14) | 17.5 |
| C. krusei | 100 (12/12) | 21.5 | 100 (12/12) | 27.1 | 100 (12/12) | 22.2 |
| C. parapsilosis | 100 (14/14) | 36.9 | 100 (14/14) | 31.5 | 100 (14/14) | 32.8 |
| C. tropicalis | 100 (14/14) | 20.5 | 100 (14/14) | 19.8 | 100 (14/14) | 19.3 |
| All species | 100 (78/78) | 23.4 | 100 (78/78) | 25.9 | 100 (78/78) | 22.1 |
| Candida species grown with echinocandins | ||||||
| C. auris | 100 (18/18) | 23.1 | 100 (18/18) | 34.1 | 100 (18/18) | 70.1 |
| C. albicans | 55.6 (10/18) | 40.0 | 33.3 (6/18) | 74.5 | 0 (0/18) | – |
| C. glabrata | 100 (18/18) | 18.0 | 100 (18/18) | 41.6 | 100 (18/18) | 20.5 |
| C. krusei | 88.9 (16/18) | 22.8 | 44.5 (8/18) | 42.6 | 0 (0/18) | – |
| C. parapsilosis | 100 (18/18) | 40.1 | 100 (18/18) | 34.8 | 100 (18/18) | 39.8 |
| C. tropicalis | 77.8 (14/18) | 26.8 | 44.5 (8/18) | 46.8 | 0 (0/18) | – |
| All species | 87.0 (94/108) | 27.7 | 70.4 (76/108) | 41.5 | 50.0 (54/108) | 43.5 |
| Candida species grown with azoles | ||||||
| C. auris | 100 (12/12) | 21.9 | 100 (12/12) | 22.7 | 100 (12/12) | 36.7 |
| C. albicans | 100 (18/18) | 22.1 | 100 (18/18) | 28.8 | 33.3 (6/18) | 97.8 |
| C. glabrata | 100 (18/18) | 19.1 | 100 (18/18) | 34.0 | 100 (18/18) | 20.4 |
| C. krusei | 100 (12/12) | 22.7 | 100 (12/12) | 29.8 | 83.3 (10/12) | 51.6 |
| C. parapsilosis | 100 (18/18) | 38.4 | 100 (18/18) | 33.1 | 33.3 (6/18) | 39.1 |
| C. tropicalis | 100 (18/18) | 21.9 | 100 (18/18) | 21.2 | 100 (18/18) | 41.9 |
| All species | 100 (96/96) | 24.6 | 100 (96/96) | 28.7 | 72.9 (70/96) | 41.4 |
| Candida species grown with amphotericin Bb | ||||||
| C. albicans | 100 (6/6) | 22.9 | 100 (6/6) | 39.4 | 66.6 (4/6) | 51.0 |
| C. glabrata | 100 (6/6) | 18.6 | 100 (6/6) | 44.9 | 0 (0/6) | – |
| C. krusei | 100 (6/6) | 24.3 | 100 (6/6) | 31.7 | 66.6 (4/6) | 35.2 |
| C. parapsilosis | 100 (6/6) | 38.3 | 100 (6/6) | 42.9 | 66.6 (4/6) | 50.1 |
| C. tropicalis | 100 (6/6) | 24.5 | 100 (6/6) | 30.0 | 66.6 (4/6) | 49.7 |
| All species | 100 (30/30) | 25.7 | 100 (30/30) | 35.8 | 53.3 (16/30) | 46.5 |
Growth of Candida species without or with antifungal agents (i.e., echinocandins, azoles, or amphotericin B) was detected after incubating the indicated blood culture bottles in the BacT/Alert VIRTUO or the Bactec FX blood culture automated system, respectively. In the case of recovery, the times to detection were recorded for each of Candida species allowed to grow in the absence or presence of antifungals agents. The symbol – indicates no recovery.
The list of Candida species allowed to grow in the presence of amphotericin B does not include C. auris because of a MIC value of 4 μg/mL to the polyene antifungal drug (see Table S1) that led us to consider the organism resistant to amphotericin B, as already noted (3).
FIG 1.
Percentages of overall recovery for Candida species in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles by the antifungal concentrations present in each type of bottle. Comparisons between groups of bottles were assessed using the McNemar’s test, which resulted in statistically significant differences (***, P < 0.001; **, P < 0.01; and *, P < 0.05). FAP, FA Plus; MICF, Mycosis IC/F; PAF, Plus Aerobic/F; PSL, peak serum level.
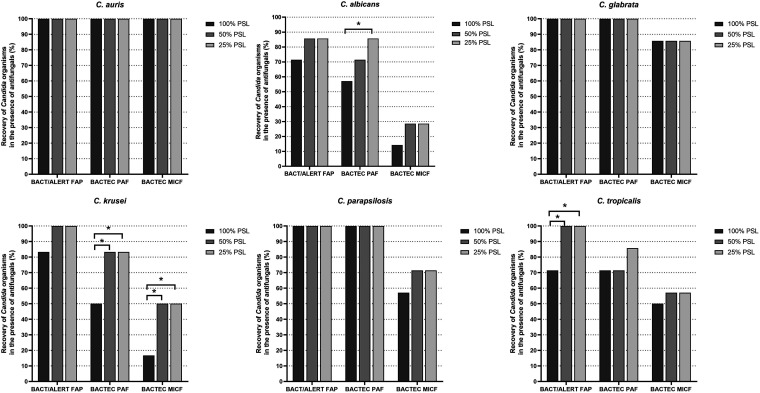
Tables 2, 3, and 4 show the recovery results for each Candida species from BacT/Alert (FAP) or Bactec (PAF and MICF) bottles according to the single antifungal agent (at the 100%, 50%, or 25% PLS concentration) present in the bottles. As shown in Table 2 (for echinocandins), BacT/Alert FAP and Bactec PAF bottles with anidulafungin did not allow growth for C. albicans (at all PSL concentrations), C. krusei (at the 100% PSL concentration), and C. tropicalis (at the 100% PSL concentration [FAP] or at all PSL concentrations [PAF]). Bactec PAF bottles with caspofungin did not allow growth for C. albicans (at both 100% and 50% PSL concentrations), C. krusei (at the 100% PSL concentration), and C. tropicalis (at both 100% and 50% PSL concentrations). BacT/Alert FAP bottles with micafungin (at the 100% PSL concentration) did not allow growth for C. albicans and C. tropicalis, whereas Bactec PAF bottles with micafungin did not allow growth for C. albicans (at the 100% PSL concentration) and C. krusei (at all PSL concentrations). In contrast, Bactec MICF bottles with anidulafungin, caspofungin, or micafungin (at all PSL concentrations) did not allow growth for C. albicans, C. krusei, and C. tropicalis. As shown in Table 3 (for azoles), Bactec MICF bottles did not allow growth for C. albicans with fluconazole or posaconazole (both at all PSL concentrations), C. krusei with posaconazole (at the 100% PSL concentration), C. parapsilosis with posaconazole (at all PSL concentrations), and C. parapsilosis with voriconazole (at all PSL concentrations). As shown in Table 4 (for amphotericin B), Bactec MICF bottles did not allow growth for all Candida species tested (C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis) at the 100% PLS concentration, and for C. glabrata at both 50% and 25% PSL concentrations of antifungal agent. Fig. 2 shows the recovery rates in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles with antifungal (100% PSL, 50% PSL, or 25% PSL) concentrations for each of the 6 Candida species included in the study. Using the McNemar’s test, we found statistically significant increases of recovery for C. albicans in Bactec PAF bottles (P = 0.021, for the 100% versus 25% PSL comparison), C. krusei in Bactec PAF or Bactec MICF bottles (P = 0.045 and P = 0.014, respectively, for all PSL comparisons), and C. tropicalis in BacT/Alert FAP bottles (P = 0.045, for all PSL comparisons).
TABLE 2.
Recovery and time to detection of Candida species from BacT/Alert or Bactec blood culture bottles according to clinically relevant echinocandin antifungal concentrationsa
| Antifungal drug and peak serum-level concn (%) usedb | Organism used | BacT/Alert FAP bottles |
Bactec PAF bottles |
Bactec MICF bottles |
||||
|---|---|---|---|---|---|---|---|---|
| Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | |||
| Anidulafungin | 100 | C. auris | 2/2 | 24.3 | 2/2 | 47.2 | 2/2 | 79.9 |
| 50 | 2/2 | 23.7 | 2/2 | 34.4 | 2/2 | 59.9 | ||
| 25 | 2/2 | 21.3 | 2/2 | 29.7 | 2/2 | 43.9 | ||
| No drug | 2/2 | 21.0 | 2/2 | 21.1 | 2/2 | 21.4 | ||
| 100 | C. albicans | 0/2 | – | 0/2 | – | 0/2 | – | |
| 50 | 0/2 | – | 0/2 | – | 0/2 | – | ||
| 25 | 0/2 | – | 0/2 | – | 0/2 | – | ||
| No drug | 2/2 | 21.2 | 2/2 | 23.3 | 2/2 | 20.3 | ||
| 100 | C. glabrata | 2/2 | 17.8 | 2/2 | 35.6 | 2/2 | 21.9 | |
| 50 | 2/2 | 17.6 | 2/2 | 33.1 | 2/2 | 20.7 | ||
| 25 | 2/2 | 17.6 | 2/2 | 32.9 | 2/2 | 20.2 | ||
| No drug | 2/2 | 17.8 | 2/2 | 32.1 | 2/2 | 18.1 | ||
| 100 | C. krusei | 0/2 | – | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 24.2 | 2/2 | 44.7 | 0/2 | – | ||
| 25 | 2/2 | 22.2 | 2/2 | 44.1 | 0/2 | – | ||
| No drug | 2/2 | 21.2 | 2/2 | 27.6 | 2/2 | 22.4 | ||
| 100 | C. parapsilosis | 2/2 | 40.2 | 2/2 | 33.4 | 2/2 | 35.0 | |
| 50 | 2/2 | 39.8 | 2/2 | 32.7 | 2/2 | 34.6 | ||
| 25 | 2/2 | 39.2 | 2/2 | 33.8 | 2/2 | 34.7 | ||
| No drug | 2/2 | 36.2 | 2/2 | 33.5 | 2/2 | 34.2 | ||
| 100 | C. tropicalis | 0/2 | – | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 29.7 | 0/2 | – | 0/2 | – | ||
| 25 | 2/2 | 22.8 | 0/2 | – | 0/2 | – | ||
| No drug | 2/2 | 20.8 | 2/2 | 18.6 | 2/2 | 18.8 | ||
| Caspofungin | 100 | C. auris | 2/2 | 26.8 | 2/2 | 47.2 | 2/2 | 68.2 |
| 50 | 2/2 | 22.9 | 2/2 | 34.4 | 2/2 | 63.3 | ||
| 25 | 2/2 | 22.3 | 2/2 | 29.7 | 2/2 | 57.0 | ||
| No drug | 2/2 | 21.2 | 2/2 | 21.1 | 2/2 | 20.4 | ||
| 100 | C. albicans | 2/2 | 39.1 | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 39.0 | 0/2 | – | 0/2 | – | ||
| 25 | 2/2 | 37.5 | 2/2 | 56.8 | 0/2 | – | ||
| No drug | 2/2 | 22.1 | 2/2 | 22.3 | 2/2 | 20.5 | ||
| 100 | C. glabrata | 2/2 | 18.5 | 2/2 | 41.6 | 2/2 | 22.0 | |
| 50 | 2/2 | 18.4 | 2/2 | 36.9 | 2/2 | 18.2 | ||
| 25 | 2/2 | 18.2 | 2/2 | 35.8 | 2/2 | 18.3 | ||
| No drug | 2/2 | 18.1 | 2/2 | 31.6 | 2/2 | 17.9 | ||
| 100 | C. krusei | 2/2 | 24.3 | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 19.8 | 2/2 | 50.5 | 0/2 | – | ||
| 25 | 2/2 | 19.7 | 2/2 | 31.2 | 0/2 | – | ||
| No drug | 2/2 | 20.5 | 2/2 | 27.4 | 2/2 | 21.9 | ||
| 100 | C. parapsilosis | 2/2 | 38.8 | 2/2 | 35.4 | 2/2 | 54.3 | |
| 50 | 2/2 | 38.8 | 2/2 | 34.5 | 2/2 | 45.0 | ||
| 25 | 2/2 | 37.1 | 2/2 | 32.2 | 2/2 | 42.4 | ||
| No drug | 2/2 | 36.5 | 2/2 | 32.1 | 2/2 | 33.1 | ||
| 100 | C. tropicalis | 2/2 | 27.7 | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 26.2 | 0/2 | – | 0/2 | – | ||
| 25 | 2/2 | 22.1 | 2/2 | 38.3 | 0/2 | – | ||
| No drug | 2/2 | 20.2 | 2/2 | 19.0 | 2/2 | 18.7 | ||
| Micafungin | 100 | C. auris | 2/2 | 22.9 | 2/2 | 35.9 | 2/2 | 110.5 |
| 50 | 2/2 | 22.0 | 2/2 | 30.7 | 2/2 | 92.2 | ||
| 25 | 2/2 | 21.5 | 2/2 | 27.8 | 2/2 | 55.8 | ||
| No drug | 2/2 | 21.0 | 2/2 | 21.1 | 2/2 | 21.3 | ||
| 100 | C. albicans | 0/2 | – | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 46.0 | 2/2 | 89.6 | 0/2 | – | ||
| 25 | 2/2 | 38.3 | 2/2 | 77.2 | 0/2 | – | ||
| No drug | 2/2 | 21.8 | 2/2 | 24.5 | 2/2 | 19.5 | ||
| 100 | C. glabrata | 2/2 | 18.1 | 2/2 | 54.7 | 2/2 | 21.2 | |
| 50 | 2/2 | 17.9 | 2/2 | 54.3 | 2/2 | 21.2 | ||
| 25 | 2/2 | 18.2 | 2/2 | 49.8 | 2/2 | 20.9 | ||
| No drug | 2/2 | 17.9 | 2/2 | 31.1 | 2/2 | 17.7 | ||
| 100 | C. krusei | 2/2 | 24.3 | 0/2 | – | 0/2 | – | |
| 50 | 2/2 | 24.2 | 0/2 | – | 0/2 | – | ||
| 25 | 2/2 | 24.1 | 0/2 | – | 0/2 | – | ||
| No drug | 2/2 | 21.3 | 2/2 | 26.9 | 2/2 | 21.2 | ||
| 100 | C. parapsilosis | 2/2 | 42.9 | 2/2 | 38.8 | 2/2 | 40.5 | |
| 50 | 2/2 | 42.5 | 2/2 | 36.3 | 2/2 | 35.9 | ||
| 25 | 2/2 | 42.0 | 2/2 | 36.2 | 2/2 | 35.8 | ||
| No drug | 2/2 | 37.0 | 2/2 | 31.8 | 2/2 | 33.3 | ||
| 100 | C. tropicalis | 0/2 | – | 2/2 | 53.8 | 0/2 | – | |
| 50 | 2/2 | 29.9 | 2/2 | 48.3 | 0/2 | – | ||
| 25 | 2/2 | 29.0 | 2/2 | 46.9 | 0/2 | – | ||
| No drug | 2/2 | 20.8 | 2/2 | 19.5 | 2/2 | 20.7 | ||
Candida species were allowed to grow in the presence of echinocandin antifungal agents in resin containing (BacT/Alert FAP and Bactec PAF) or non-containing (Bactec MICF) blood culture bottles, following bottles’ incubation in the BacT/Alert VIRTUO or the Bactec FX blood culture automated system, respectively. In the case of recovery, the times to detection were recorded for each of the Candida species used in the study. The symbol – indicates no recovery.
See text and Table S1 for details.
TABLE 3.
Recovery and time to detection of Candida species from BacT/Alert or Bactec blood culture bottles according to clinically relevant azole antifungal concentrationsa
| Antifungal drug and peak serum-level concn (%) usedb | Organism used | BacT/Alert FAP bottles |
Bactec PAF bottles |
Bactec MICF bottles |
||||
|---|---|---|---|---|---|---|---|---|
| Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | |||
| Fluconazole | 100 | C. albicans | 2/2 | 23.4 | 2/2 | 28.2 | 0/2 | – |
| 50 | 2/2 | 21.8 | 2/2 | 24.8 | 0/2 | – | ||
| 25 | 2/2 | 21.1 | 2/2 | 24.7 | 0/2 | – | ||
| No drug | 2/2 | 20.9 | 2/2 | 22.7 | 2/2 | 20.1 | ||
| 100 | C. glabrata | 2/2 | 19.7 | 2/2 | 36.7 | 2/2 | 21.4 | |
| 50 | 2/2 | 18.6 | 2/2 | 34.9 | 2/2 | 18.5 | ||
| 25 | 2/2 | 19.0 | 2/2 | 34.0 | 2/2 | 18.1 | ||
| No drug | 2/2 | 17.9 | 2/2 | 31.7 | 2/2 | 17.2 | ||
| 100 | C. parapsilosis | 2/2 | 40.3 | 2/2 | 32.3 | 2/2 | 50.8 | |
| 50 | 2/2 | 39.3 | 2/2 | 30.3 | 2/2 | 33.5 | ||
| 25 | 2/2 | 38.2 | 2/2 | 30.1 | 2/2 | 33.2 | ||
| No drug | 2/2 | 38.2 | 2/2 | 30.1 | 2/2 | 32.9 | ||
| 100 | C. tropicalis | 2/2 | 21.0 | 2/2 | 20.4 | 2/2 | 40.2 | |
| 50 | 2/2 | 20.3 | 2/2 | 20.7 | 2/2 | 29.4 | ||
| 25 | 2/2 | 20.2 | 2/2 | 20.5 | 2/2 | 28.7 | ||
| No drug | 2/2 | 19.7 | 2/2 | 20.1 | 2/2 | 18.2 | ||
| Posaconazole | 100 | C. auris | 2/2 | 22.4 | 2/2 | 25.6 | 2/2 | 74.3 |
| 50 | 2/2 | 22.8 | 2/2 | 24.7 | 2/2 | 50.0 | ||
| 25 | 2/2 | 21.7 | 2/2 | 23.2 | 2/2 | 26.4 | ||
| No drug | 2/2 | 22.4 | 2/2 | 22.1 | 2/2 | 21.1 | ||
| 100 | C. albicans | 2/2 | 23.4 | 2/2 | 41.8 | 0/2 | – | |
| 50 | 2/2 | 21.2 | 2/2 | 36.7 | 0/2 | – | ||
| 25 | 2/2 | 21.0 | 2/2 | 28.2 | 0/2 | – | ||
| No drug | 2/2 | 21.2 | 2/2 | 22.6 | 2/2 | 20.2 | ||
| 100 | C. glabrata | 2/2 | 20.0 | 2/2 | 33.0 | 2/2 | 21.4 | |
| 50 | 2/2 | 19.5 | 2/2 | 32.8 | 2/2 | 19.4 | ||
| 25 | 2/2 | 19.4 | 2/2 | 31.6 | 2/2 | 19.2 | ||
| No drug | 2/2 | 17.2 | 2/2 | 30.3 | 2/2 | 18.1 | ||
| 100 | C. krusei | 2/2 | 22.5 | 2/2 | 32.4 | 0/2 | – | |
| 50 | 2/2 | 22.2 | 2/2 | 32.1 | 2/2 | 69.1 | ||
| 25 | 2/2 | 22.1 | 2/2 | 31.3 | 2/2 | 58.2 | ||
| No drug | 2/2 | 21.8 | 2/2 | 28.5 | 2/2 | 22.9 | ||
| 100 | C. parapsilosis | 2/2 | 39.4 | 2/2 | 36.6 | 0/2 | – | |
| 50 | 2/2 | 38.3 | 2/2 | 36.2 | 0/2 | – | ||
| 25 | 2/2 | 38.2 | 2/2 | 32.2 | 0/2 | – | ||
| No drug | 2/2 | 37.9 | 2/2 | 30.7 | 2/2 | 31.8 | ||
| 100 | C. tropicalis | 2/2 | 23.2 | 2/2 | 24.6 | 2/2 | 54.8 | |
| 50 | 2/2 | 22.8 | 2/2 | 24.2 | 2/2 | 44.8 | ||
| 25 | 2/2 | 22.5 | 2/2 | 24.1 | 2/2 | 39.5 | ||
| No drug | 2/2 | 21.2 | 2/2 | 21.0 | 2/2 | 19.7 | ||
| Voriconazole | 100 | C. auris | 2/2 | 21.4 | 2/2 | 21.1 | 2/2 | 26.8 |
| 50 | 2/2 | 21.3 | 2/2 | 21.0 | 2/2 | 21.8 | ||
| 25 | 2/2 | 21.2 | 2/2 | 20.7 | 2/2 | 21.0 | ||
| No drug | 2/2 | 21.2 | 2/2 | 20.4 | 2/2 | 20.3 | ||
| 100 | C. albicans | 2/2 | 25.4 | 2/2 | 28.1 | 2/2 | 108.4 | |
| 50 | 2/2 | 20.9 | 2/2 | 23.8 | 2/2 | 99.2 | ||
| 25 | 2/2 | 20.8 | 2/2 | 23.4 | 2/2 | 85.7 | ||
| No drug | 2/2 | 20.6 | 2/2 | 23.4 | 2/2 | 20.1 | ||
| 100 | C. glabrata | 2/2 | 19.9 | 2/2 | 37.1 | 2/2 | 22.7 | |
| 50 | 2/2 | 18.0 | 2/2 | 33.3 | 2/2 | 21.9 | ||
| 25 | 2/2 | 17.9 | 2/2 | 32.5 | 2/2 | 20.9 | ||
| No drug | 2/2 | 17.9 | 2/2 | 31.7 | 2/2 | 17.2 | ||
| 100 | C. krusei | 2/2 | 24.3 | 2/2 | 28.6 | 2/2 | 61.8 | |
| 50 | 2/2 | 22.6 | 2/2 | 27.9 | 2/2 | 34.8 | ||
| 25 | 2/2 | 22.5 | 2/2 | 26.6 | 2/2 | 34.3 | ||
| No drug | 2/2 | 22.2 | 2/2 | 26.5 | 2/2 | 22.9 | ||
| 100 | C. parapsilosis | 2/2 | 37.5 | 2/2 | 37.3 | 0/2 | – | |
| 50 | 2/2 | 37.3 | 2/2 | 32.5 | 0/2 | – | ||
| 25 | 2/2 | 37.3 | 2/2 | 30.2 | 0/2 | – | ||
| No drug | 2/2 | 36.1 | 2/2 | 31.6 | 2/2 | 32.7 | ||
| 100 | C. tropicalis | 2/2 | 22.4 | 2/2 | 21.7 | 2/2 | 55.8 | |
| 50 | 2/2 | 22.3 | 2/2 | 21.7 | 2/2 | 42.8 | ||
| 25 | 2/2 | 22.2 | 2/2 | 21.7 | 2/2 | 41.1 | ||
| No drug | 2/2 | 19.6 | 2/2 | 19.9 | 2/2 | 19.2 | ||
Candida species were allowed to grow in the presence of azole antifungal agents in resin containing (BacT/Alert FAP and Bactec PAF) or non-containing (Bactec MICF) blood culture bottles, following bottles’ incubation in the BacT/Alert VIRTUO or the Bactec FX blood culture automated system, respectively. In the case of recovery, the times to detection were recorded for each of the Candida species used in the study. The symbol – indicates no recovery.
See text and Table S1 for details.
TABLE 4.
Recovery and time to detection of Candida species from BacT/Alert or Bactec blood culture bottles according to clinically relevant amphotericin B antifungal concentrationsa
| Antifungal drug and peak serum-level concn (%) usedb | Organism used | BacT/Alert FAP bottles |
Bactec PAF bottles |
Bactec MICF bottles |
||||
|---|---|---|---|---|---|---|---|---|
| Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | Recovery (no. of bottles detected as positive/no. of bottles tested) | Mean time (h) to detection | |||
| Amphotericin B | 100 | C. albicans | 2/2 | 23.5 | 2/2 | 33.5 | 0/2 | – |
| 50 | 2/2 | 22.7 | 2/2 | 27.5 | 2/2 | 51.9 | ||
| 25 | 2/2 | 22.5 | 2/2 | 27.1 | 2/2 | 50.2 | ||
| No drug | 2/2 | 22.2 | 2/2 | 24.6 | 2/2 | 18.7 | ||
| 100 | C. glabrata | 2/2 | 18.7 | 2/2 | 49.4 | 0/2 | – | |
| 50 | 2/2 | 18.7 | 2/2 | 43.2 | 0/2 | – | ||
| 25 | 2/2 | 18.5 | 2/2 | 42.1 | 0/2 | – | ||
| No drug | 2/2 | 17.4 | 2/2 | 32.1 | 2/2 | 16.2 | ||
| 100 | C. krusei | 2/2 | 25.7 | 2/2 | 35.4 | 0/2 | – | |
| 50 | 2/2 | 23.7 | 2/2 | 30.6 | 2/2 | 37.2 | ||
| 25 | 2/2 | 23.6 | 2/2 | 29.1 | 2/2 | 33.2 | ||
| No drug | 2/2 | 22.1 | 2/2 | 26.0 | 2/2 | 21.7 | ||
| 100 | C. parapsilosis | 2/2 | 39.8 | 2/2 | 46.9 | 0/2 | – | |
| 50 | 2/2 | 38.6 | 2/2 | 41.6 | 2/2 | 52.1 | ||
| 25 | 2/2 | 36.5 | 2/2 | 40.3 | 2/2 | 48.2 | ||
| No drug | 2/2 | 36.4 | 2/2 | 30.9 | 2/2 | 31.7 | ||
| 100 | C. tropicalis | 2/2 | 27.0 | 2/2 | 31.2 | 0/2 | – | |
| 50 | 2/2 | 24.3 | 2/2 | 30.6 | 2/2 | 50.7 | ||
| 25 | 2/2 | 22.1 | 2/2 | 28.1 | 2/2 | 48.8 | ||
| No drug | 2/2 | 21.0 | 2/2 | 20.4 | 2/2 | 19.4 | ||
Candida species were allowed to grow in the presence of amphotericin B antifungal agent in resin containing (BacT/Alert FAP and Bactec PAF) or non-containing (Bactec MICF) blood culture bottles, following bottles’ incubation in the BacT/Alert VIRTUO or the Bactec FX blood culture automated system, respectively. In the case of recovery, the times to detection were recorded for each of the Candida species used in the study. The symbol – indicates no recovery.
See text and Table S1 for details.
FIG 2.
Percentages of recovery for single Candida species in the presence of antifungal concentrations by BacT/Alert FAP, Bactec PAF, or Bactec MICF types of bottles. Comparisons between groups of bottles were assessed using the McNemar’s test, which resulted in statistically significant differences (*, P < 0.05). FAP, FA Plus; MICF, Mycosis IC/F; PAF, Plus Aerobic/F; PSL, peak serum level.
As also shown in Table 1, mean TTD (h) values for Candida species grown with echinocandins, azoles, or amphotericin B were, respectively, 27.7, 24.6, and 25.7 in BacT/Alert FAP bottles, 41.5, 28.7, and 35.8 in Bactec PAF bottles, and 43.5, 41.4, and 46.5 in Bactec MICF bottles. Based on ΔTTD (h) values (i.e., derived from the difference between the mean TTD values of test and control bottles for each antifungal class), these times were arbitrated to be slightly (ΔTTD, < 2 h), moderately (ΔTTD, 2 to 5 h), or highly (ΔTTD, > 5 h) delayed compared to those in bottles without antifungals (P < 0.001, for all comparisons; paired t test). Tables 2, 3, and 4 detail the mean TTD (h) values for each of 117 Candida organism/antifungal drug combinations in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles. For echinocandins (Table 2), the highest values noticed for C. albicans/micafungin were in BacT/Alert FAP or Bactec PAF bottles with the 50% PSL antifungal concentration (46.0 h and 89.6 h, respectively), or for C. auris/micafungin in Bactec MICF bottles with the 100% PSL antifungal concentration (110.5 h). For azoles (Table 3), the highest values (in bottles with the 100% PSL antifungal concentration) were noticed for C. parapsilosis/fluconazole in BacT/Alert FAP bottles (40.3 h), for C. albicans/posaconazole in Bactec PAF bottles (41.8 h), and for C. albicans/voriconazole in Bactec MICF bottles (108.4 h). For amphotericin B (Table 4), the highest values were noticed for C. parapsilosis in BacT/Alert FAP bottles with the 100% PSL antifungal concentration (39.8 h), for C. glabrata in Bactec PAF bottles with the 100% PSL antifungal concentration (49.4 h), and for C. parapsilosis in Bactec MICF bottles with the 50% PSL antifungal concentration (52.1 h).
DISCUSSION
We showed that both BacT/Alert FAP and Bactec PAF bottles were highly (100%) efficient for detection of C. auris, C. glabrata, or C. parapsilosis in the presence of highest (100% PSL) concentrations of antifungal agents. The efficiency did not reach 100% with C. albicans, C. krusei, and C. tropicalis. In contrast, Bactec MICF bottles were considerably less efficient with all (excluding C. auris) the Candida species allowed to grow, and also with lowest (50% PSL or 25% PSL) concentrations of antifungal agents. Looking at the single antifungal agents used in the study, we noticed that azoles and amphotericin B did not hinder the growth of Candida species in both BacT/Alert FAP and Bactec PAF bottles. In contrast, we noticed that echinocandins did considerably affect the recovery of Candida species, which was apparent not only in Bactec MICF, but also in BacT/Alert FAP or Bactec PAF bottles. Overall, the maximum TTD (4.6 days) we recorded was nearly at the fixed upper limit (5 days) by the BacT/Alert or Bactec FX system’s manufacturer.
Unlike the BacT/Alert FAP or Bactec PAF medium (15), Bactec MICF medium contains supplements that specifically adapt for the growth of Candida or other fungal species in BC bottles (18). In this context, it should be recalled that bioMérieux has adjusted the micronutrients in the BacT/Alert FAP medium to improve Candida detection from BC bottles. Consistent with our results, 2 studies (17, 18), independently, showed that fluconazole delayed the growth (17), whereas echinocandins or amphotericin B (but not fluconazole or voriconazole) hindered it (18) for Candida species (6 tested overall) in Bactec MICF bottles. The Köck et al. study (18) also revealed that echinocandins (particularly anidulafungin and micafungin) at the therapeutic peak serum concentration (i.e., the Cmax, which is nearly equivalent to the 100% PSL antifungal concentration in our study) did not allow reliable detection of C. albicans and C. glabrata in Bactec PAF bottles, to which Bactec MICF bottles were compared. In that study (18), recovery of both Candida species improved in Bactec (PAF and MICF) bottles with concentrations of 6.25% to 50% of the Cmax for echinocandins. Unfortunately, these values corresponded to the minimum serum concentrations (range, 1 to 6 μg/mL) achieved with the standard echinocandin therapy (18).
In both BacT/Alert FAP and Bactec PAF bottles, antimicrobial drug-neutralizing resins are likely to have allowed higher Candida recovery rates, in the presence of azoles or amphotericin B, than those in Bactec MICF bottles (Table 1). Therefore, Bactec MICF bottles may not be useful to detect candidemia or monitor Candida clearance from the bloodstream in patients undergoing treatment with these antifungal agents (21). However, Candida recovery rates were much less than 100% (albeit to a different extent) in all 3 types of BC bottles in the presence of echinocandins, suggesting that the resins’ ability to neutralize antifungal agents in BacT/Alert FAP and Bactec PAF bottles may not be sufficient with these antifungal agents. Of the 6 Candida species used in our study, C. auris, C. glabrata, and C. parapsilosis grew undisturbed in the presence of anidulafungin, caspofungin, or micafungin. The remaining 3 species (C. albicans, C. krusei, and C. tropicalis) did not grow at 100% PSL concentrations, or grew slowly (i.e., had a longer TTD) at 50% or 25% PSL concentrations of echinocandins (particularly anidulafungin), and this occurred more in Bactec MICF bottles than in Bactec PAF or BacT/Alert FAP bottles. Echinocandins are cyclic lipopeptides with hydrophobic fatty acid chains, which act as a “hook” for anchoring the drug in the fungal cell membrane, where it exerts an inhibitory action against the transmembrane enzyme beta-(1,3)-d-glucan synthase. Due to their chemical structure, echinocandins bind strongly (97 to 99%) to plasma proteins (22). In our experimental BC setting, protein-bound echinocandins could be presented as altered molecules for binding to polymeric resins—which are capable of binding to the hydrophobic regions of virtually any antimicrobial agent—and could, therefore, be less adsorbed by resins in the bottles compared to azoles or amphotericin B. Factors related to Candida growth kinetics (23) could have further influenced the interaction between polymeric resins and echinocandins, and this would have been evident for Candida species that grow faster or are morphologically more flexible.
Detection of C. auris, C. glabrata, and C. parapsilosis in BC bottles may be particularly important, considering the high propensity of these species to develop antifungal drug resistance or tolerance (the latter often termed “trailing growth” in clinical studies), following short-term antifungal exposure (3, 24), or in the presence of inhibitory or subinhibitory antifungal drug concentrations (25). Unlike azoles, echinocandins exert fungicidal activity against most Candida species but, remarkably, can paradoxically promote the growth in vitro of C. parapsilosis or other Candida species (such as C. auris) at concentrations above the MIC (7, 26). In our study, echinocandin antifungal effects, coupled with the absence (in Bactec MICF bottles) or the suboptimal (we suppose) ability (in BacT/Alert FAP or Bactec PAF bottles) of antifungal drug-neutralizing resins, may have prompted some Candida species to thrive in the presence of echinocandins. This occurred regardless of MICs for the species that were below the CLSI-established resistant breakpoints for echinocandin antifungals. Thus, echinocandins remain the preferred treatment for candidemia (21), particularly for C. auris, which is the only species with isolates shown to be pan-resistant to all 3 classes of antifungal drugs (1). Unlike previous studies (16, 18), the range of azole antifungal agents in our study included posaconazole, which may be a treatment chance in the case of C. auris BSI (27), as well as, like previous studies (16, 18), voriconazole, which is an alternative to fluconazole for the treatment of C. glabrata or C. krusei BSIs (21).
Our study has both strengths and limitations. To the best of our knowledge, this is the first study to use BacT/Alert FAP bottles to simulate BCs of patients receiving antifungal therapy for candidemia. However, we used only antifungal drug-susceptible organisms for each Candida species included in the study to appreciate the abilities of BacT/Alert FAP or Bactec PAF bottles’ media to hinder or delay the fungistatic/fungicidal activity of antifungal agents in BC bottles. Additionally, our clinical BC simulation model was rigorous in terms of (i) volume of blood inoculated, (ii) antifungal drug concentrations mimicking the antifungal drug exposure level in patients, (iii) Candida inoculum, or (iv) incubation conditions in BacT/Alert or Bactec FX BC systems complying with the manufacturers’ recommendations. However, we acknowledge that the median number of Candida organisms present in a Candida BSI episode may be ≤1 cell/mL (range, 0.1 and >1,000 cells/mL), a concentration of 0.5 to 1.0-fold below the yeast cell number tested by us. Thus, detection rates in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles spiked with Candida species might not mirror those in clinical BC bottles.
In conclusion, our study extends and confirms previous results about the recovery of medically important Candida species from simulated BCs in BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles containing clinically relevant concentrations of antifungal agents. While both BacT/Alert FAP and Bactec PAF bottles showed excellent performances with azoles and amphotericin B, 3 species (C. auris, C. glabrata, and C. parapsilosis) were recovered from all the BacT/Alert FAP, Bactec PAF, or Bactec MICF bottles with echinocandins. Our results emphasize the importance of surveillance BCs for the clinical and therapeutic monitoring of patients with candidemia.
MATERIALS AND METHODS
Yeast organisms and antifungal agents.
The yeast organisms included in the study consisted of 1 clinical isolate (C. auris Fondazione Policlinico Universitario A. Gemelli IRCCS [FPG]1), and 5 type/reference strains (C. albicans ATCC 90028, C. glabrata ATCC 2001, C. krusei ATCC 6258, C. parapsilosis ATCC 22019, and C. tropicalis ATCC 750) of Candida species. The C. auris FPG1 term means the “C. auris isolate one” at the FPG hospital of Rome (Italy), which is our study’s location. Before testing, yeast organisms were retrieved from their frozen stocks, subcultured on Sabouraud dextrose agar (SDA) plates, and colonies were analyzed by matrix-assisted laser desorption/ionization time-of flight mass spectrometry (7, 28) to confirm the organism’s identity to the species level. Antifungal agents (whose powders were provided by Toku-E) used in the study were as follows: anidulafungin (ANF), caspofungin (CSF), micafungin (MCF), fluconazole (FLZ), posaconazole (PSZ), voriconazole (VRZ), and amphotericin B (AMB). Prior to use in clinical BC simulation experiments (see below for details), each of the 6 Candida species was tested for susceptibility to ANF, CSF, MCF, FLZ, PSZ, VRZ, and AMB, which was performed according to the CLSI M27-A3 broth dilution reference method guidelines (29). Briefly, drug-free and yeast-free controls were included in 96-well microtiter plates, which were incubated at 35°C, and read visually after 24 h, whereas C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 served as quality control strains, as recommended by the CLSI. The MIC endpoints were defined as the lowest antifungal drug concentration that caused a prominent decrease in or (only for AMB) a full inhibition of the visual growth relative to the drug-free control wells. As detailed in Table S1, for each Candida species, MICs were interpreted according to antifungal clinical breakpoints (CBPs) or, in the absence of CBPs, epidemiological cutoff values (ECVs), which the CLSI has established and reported, respectively, in the M60 (30) and M57S (31) documents. In the case of C. auris, for which CLSI CBPs/ECVs to azoles or AMB are lacking, azole MIC of ≥4 μg/mL (3) and AMB MIC of ≥2 μg/mL (27) were considered resistant, respectively. These MIC values were consistent with the tentative MIC breakpoints proposed by the CDC for C. auris and antifungal agents: FLZ, ≥32 μg/mL; AMB, ≥2 μg/mL; ANF, ≥4 μg/mL; CSF, ≥2 μg/mL; and MCF, ≥4 μg/mL (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html). It should be recalled that the modal MIC to FLZ among all C. auris isolates tested at the CDC was ≥256 μg/mL (as for our isolate; see Table S1). Nonetheless, the CDC proposed a FLZ MIC of ≥32 μg/mL, with the goal of capturing only those C. auris isolates that had a Erg11 gene mutation-based associated azole resistance mechanism, and were, therefore, unlikely to respond to the FLZ antifungal agent. In parallel, ANF, CSF, MCF, FLZ, PSZ, VRZ, and AMB powders were dissolved in dimethyl sulfoxide (DMSO), as appropriate (28), to concentrations of 1,440 μg/mL, 1,980 μg/mL, 3,280 μg/mL, 2,800 μg/mL, 660 μg/mL, 600 μg/mL, or 700 μg/mL, respectively. The antifungal stock solutions were aliquoted and stored at −80°C prior to use in clinical BC simulation experiments, when a 1:10, 1:20, or 1:40 dilution series was prepared from each of 7 antifungal agents with 100%, 50%, or 25% PSL concentrations (14).
Clinical Candida BC simulation model.
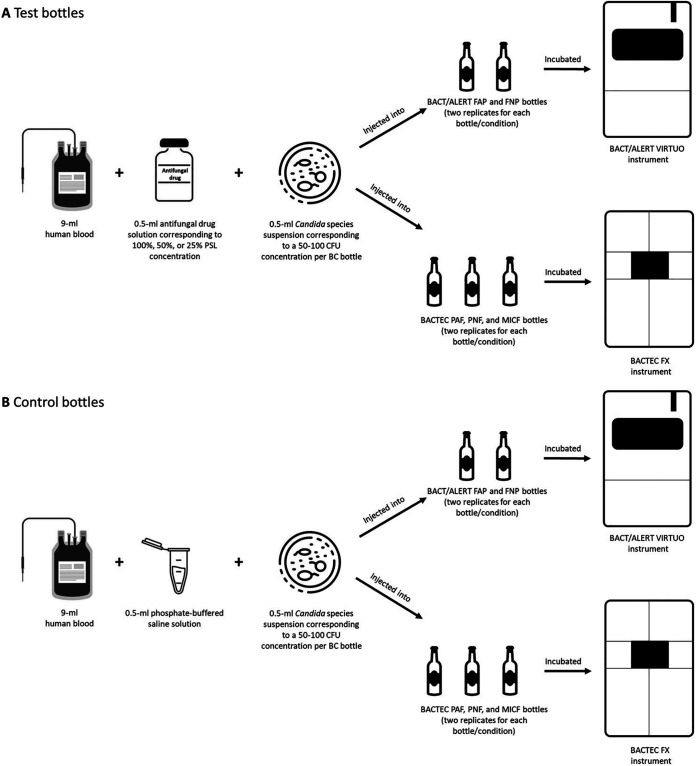
To make this model, we followed a previously developed protocol (19, 32) with some adaptations, as depicted in Fig. 3. After growth on SDA plates, fresh colonies of Candida (yeast) organisms grown on SDA were suspended in phosphate-buffered saline (PBS) to approximately 1 to 2 × 106 cells/mL (equivalent to a 0.5-McFarland standard density). The suspensions were diluted 10,000-fold in PBS, and aliquots (100 μL) of each suspension were plated on SDA to determine CFU counts after 48 h incubation of plates at 37°C. To simulate a candidemia level of 5 to 10 cells/mL, 0.5-mL Candida organism’s suspension (containing 50 to 100 cells/mL) was used together with 9 mL human whole blood (obtained from the Transfusion Medicine Division of the FPG hospital), and 0.5 mL antifungal solution/PBS (see below) to fill each BC bottle with a final 10 mL injection volume. This volume corresponds to the optimal blood fill volume (8 to 10 mL, as per BacT/Alert or Bactec FX system manufacturer’s instructions), to which the BC yield should be maximal (33). Unlike previous studies (13, 17) that used 1 to 5 (low inoculum) or 10 to 50 (high inoculum) Candida cells/mL in spiked BCs, we decided to use 1 inoculum, which represents a compromise between a lower and higher number of yeast cells that may be circulating in the blood during candidemia (33). As detailed in Fig. 3, simulated BC bottles were divided in 2 series, namely, test or control bottles, according to whether each antifungal drug concentration solution (0.5 mL; prepared as described above) was used in place of PBS (0.5 mL). We also included a negative (i.e., only blood-containing bottle) control for each simulated BC. In brief (Fig. 3), for any experimental condition (i.e., one of 117 Candida organism/antifungal drug combinations tested in total), 2 replicates of each bottle’s type were separately filled using sterile precautions with each component (added in sequence) of the injection volume mentioned above (Fig. 3). All BacT/Alert or Bactec BC bottles were incubated, respectively, in a BacT/Alert VIRTUO BC system’s (bioMérieux) or Bactec FX BC system’s (Becton, Dickinson) instrument at 37°C for up to 5 days. The incubation period (h) for each bottle was recorded, and was used to calculate the TTD, i.e., the time elapsed from when the BC bottle was entered into the BC system instrument to when the bottle was flagged positive by the instrument. Additionally, for each Candida organism-antifungal combination tested, we calculated a ΔTTD value, which was based on the difference between the test (with antifungal) bottle’s mean TTD, and the control (without antifungal) bottle’s mean TTD (Table 1). We arbitrarily categorized the mean TTD values of test bottles with echinocandins, azoles, or AMB as slightly (ΔTTD, < 2 h), moderately (ΔTTD, 2 to 5 h), or highly (ΔTTD, > 5 h) delayed.
FIG 3.
Schematic diagram illustrating the protocol to obtain simulated Candida blood cultures with (A) or without (B) antifungal agents. Together with a Candida species, antifungal drug solution, which mimicked a given PSL concentration, or phosphate-buffered saline solution, was injected in a test (A) or control (B) blood culture bottle, respectively. For each experimental condition, replicates of test or control bottles were incubated, respectively, in the BacT/Alert VIRTUO or the Bactec FX blood culture system’s instrument, until Candida growth was detected, or the manufacturer-recommended 5-day incubation period was completed. BC, blood culture; CFU, colony-forming unit; FAP, FA Plus; FNP, FN Plus; MICF, Mycosis IC/F; PAF, Plus Aerobic/F; PNF, Plus Anaerobic/F.
Statistical analysis.
Results were reported as numbers and percentages for Candida organism recovery, or as a mean for TTD in the BacT/Alert BC or the Bactec FX BC systems, and differences between results in BC bottle groups were assessed using the McNemar’s test or the paired t test, as appropriate. For all comparisons, the level of statistical significance was set at a P value of <0.05. The Intercooled Stata program version 11 and GraphPad Prism 7 were used to analyze data and/or to construct figures.
Supplementary Material
ACKNOWLEDGMENTS
This work was presented, in part, at the 32nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) held in Lisbon, Portugal (23 to 26 April 2022).
We are grateful to bioMérieux for providing reagents and funding this study, participating in the study design, and critically reviewing the manuscript before submission. In reviewing the manuscript, bioMérieux suggested only minor changes to the manuscript that had no impact on how the authors presented or interpreted the study results.
Footnotes
Supplemental material is available online only.
Contributor Information
Maurizio Sanguinetti, Email: maurizio.sanguinetti@unicatt.it.
Alexandre Alanio, Institut Pasteur.
REFERENCES
- 1.Spivak ES, Hanson KE. 2018. Candida auris: an emerging fungal pathogen. J Clin Microbiol 56:e01588-17. doi: 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. 2018. Invasive candidiasis. Nat Rev Dis Primers 4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 3.Pristov KE, Ghannoum MA. 2019. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 25:792–798. doi: 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhary A, Voss A, Meis JF. 2016. Multidrug-resistant Candida auris: 'new kid on the block' in hospital-associated infections? J Hosp Infect 94:209–212. doi: 10.1016/j.jhin.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Arendrup MC, Patterson TF. 2017. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S445–S451. doi: 10.1093/infdis/jix131. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart SR, Lyman MM, Sexton DJ. 2022. Tools for detecting a “superbug”: updates on Candida auris testing. J Clin Microbiol 60:e0080821. doi: 10.1128/jcm.00808-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F, French Mycosis Study Group . 2014. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med 40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassetti M, Giacobbe DR, Vena A, Trucchi C, Ansaldi F, Antonelli M, Adamkova V, Alicino C, Almyroudi MP, Atchade E, Azzini AM, Carannante N, Carnelutti A, Corcione S, Cortegiani A, Dimopoulos G, Dubler S, García-Garmendia JL, Girardis M, Cornely OA, Ianniruberto S, Kullberg BJ, Lagrou K, Le Bihan C, Luzzati R, Malbrain MLNG, Merelli M, Marques AJ, Martin-Loeches I, Mesini A, Paiva JA, Peghin M, Raineri SM, Rautemaa-Richardson R, Schouten J, Brugnaro P, Spapen H, Tasioudis P, Timsit JF, Tisa V, Tumbarello M, van den Berg CHSB, Veber B, Venditti M, Voiriot G, Wauters J, Montravers P. 2019. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care 23:219. doi: 10.1186/s13054-019-2497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouzé A, Estella Á, Timsit JF. 2022. Is (1,3)-β-D-glucan useless to guide antifungal therapy in ICU? Intensive Care Med 48:930–932. doi: 10.1007/s00134-022-06766-2. [DOI] [PubMed] [Google Scholar]
- 11.Leroy O, Bailly S, Gangneux JP, Mira JP, Devos P, Dupont H, Montravers P, Perrigault PF, Constantin JM, Guillemot D, Azoulay E, Lortholary O, Bensoussan C, Timsit JF, AmarCAND2 study group . 2016. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care 6:2. doi: 10.1186/s13613-015-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clancy CJ, Nguyen MH. 2013. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 56:1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 13.Beyda ND, Amadio J, Rodriguez JR, Malinowski K, Garey KW, Wanger A, Ostrosky-Zeichner L. 2018. In vitro evaluation of BacT/Alert FA blood culture bottles and T2Candida assay for detection of Candida in the presence of antifungals. J Clin Microbiol 56:e00471-18. doi: 10.1128/JCM.00471-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pharmacological features of selected antimicrobial agents (Table 9.A), p 102–103. 2020. In Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia A (ed), The Sanford guide to antimicrobial therapy, 50th ed. Antimicrobial Therapy, Inc., Sperryville, VA. [Google Scholar]
- 15.Gonzalez MD, Chao T, Pettengill MA. 2020. Modern blood culture: management decisions and method options. Clin Lab Med 40:379–392. doi: 10.1016/j.cll.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riedel S, Eisinger SW, Dam L, Stamper PD, Carroll KC. 2011. Comparison of BD Bactec Plus Aerobic/F medium to VersaTREK Redox 1 blood culture medium for detection of Candida spp. in seeded blood culture specimens containing therapeutic levels of antifungal agents. J Clin Microbiol 49:1524–1529. doi: 10.1128/JCM.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jekarl DW, Lee SY, Lee S, Park YJ, Lee J, Baek SM, An YJ, Ock SM, Lee MK. 2012. Comparison of the Bactec Fx Plus, Mycosis IC/F, Mycosis/F Lytic blood culture media and the BacT/Alert 3D FA media for detection of Candida species in seeded blood culture specimens containing therapeutic peak levels of fluconazole. J Clin Lab Anal 26:412–419. doi: 10.1002/jcla.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köck R, Eißing LC, Boschin MG, Ellger B, Horn D, Idelevich EA, Becker K. 2013. Evaluation of Bactec Mycosis IC/F and Plus Aerobic/F blood culture bottles for detection of Candida in the presence of antifungal agents. J Clin Microbiol 51:3683–3687. doi: 10.1128/JCM.02048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menchinelli G, Liotti FM, Fiori B, De Angelis G, D'Inzeo T, Giordano L, Posteraro B, Sabbatucci M, Sanguinetti M, Spanu T. 2019. In vitro evaluation of BACT/ALERT VIRTUO, BACT/ALERT 3D, and BACTEC FX automated blood culture systems for detection of microbial pathogens using simulated human blood samples. Front Microbiol 10:221. doi: 10.3389/fmicb.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farfour E, Le Brun C, Mizrahi A, Bargain P, Durieux MF, Boquel F, Corvec S, Jeddi F, Muggeo A, Huguenin A, Barraud O, Amara M, Fihman V, Bailly E, Botterel F, Guillard T, Vasse M, GMC study group . 2022. Contribution of the anaerobic blood culture vial for the recovery of Candida glabrata: a retrospective multicentric study. Med Mycol 60:myac021. doi: 10.1093/mmy/myac021. [DOI] [PubMed] [Google Scholar]
- 21.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szymański M, Chmielewska S, Czyżewska U, Malinowska M, Tylicki A. 2022. Echinocandins — structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem 37:876–894. doi: 10.1080/14756366.2022.2050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bordallo-Cardona MÁ, Sánchez-Carrillo C, Muñoz P, Bouza E, Escribano P, Guinea J. 2019. Growth kinetics in Candida spp.: differences between species and potential impact on antifungal susceptibility testing as described by the EUCAST. Med Mycol 57:601–608. doi: 10.1093/mmy/myy097. [DOI] [PubMed] [Google Scholar]
- 24.Tóth R, Nosek J, Mora-Montes HM, Gabaldon T, Bliss JM, Nosanchuk JD, Turner SA, Butler G, Vágvölgyi C, Gácser A. 2019. Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev 32:e00111-18. doi: 10.1128/CMR.00111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gow NAR, Johnson C, Berman J, Coste AT, Cuomo CA, Perlin DS, Bicanic T, Harrison TS, Wiederhold N, Bromley M, Chiller T, Edgar K. 2022. The importance of antimicrobial resistance in medical mycology. Nat Commun 13:5352. doi: 10.1038/s41467-022-32249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagener J, Loiko V. 2017. Recent insights into the paradoxical effect of echinocandins. J Fungi 4:5. doi: 10.3390/jof4010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Carolis E, Vella A, Vaccaro L, Torelli R, Posteraro P, Ricciardi W, Sanguinetti M, Posteraro B. 2014. Development and validation of an in-house database for matrix-assisted laser desorption ionization-time of flight mass spectrometry-based yeast identification using a fast protein extraction procedure. J Clin Microbiol 52:1453–1458. doi: 10.1128/JCM.03355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CLSI. 2008. M27-A3: reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.CLSI. 2017. M60: performance standards for antifungal susceptibility testing of yeasts; supplement, 1st ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.CLSI. 2022. M57S: epidemiological cutoff values for antifungal susceptibility testing; supplement, 4th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Menchinelli G, Liotti FM, Giordano L, De Angelis G, Sanguinetti M, Spanu T, Posteraro B. 2019. Efficient inactivation of clinically relevant antimicrobial drug concentrations by BacT/Alert or Bactec resin-containing media in simulated adult blood cultures. Antimicrob Agents Chemother 63:e00420-19. doi: 10.1128/AAC.00420-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. 2016. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol 7:697. doi: 10.3389/fmicb.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download spectrum.04104-22-s0001.pdf, PDF file, 0.2 MB (175KB, pdf)