ABSTRACT
Colistin is a bactericidal antibiotic identified decades ago which is active against a number of Gram-negative pathogens. After early elimination from clinical use due to toxicity issues, colistin has been reintroduced as a last-resort treatment for antibiotic-resistant Gram-negative infections lacking other therapeutic options. Inevitably, colistin resistance has emerged among clinical isolates, making the development of colistin adjuvants extremely beneficial. Clofoctol is a synthetic antibiotic active against Gram-positive bacteria, with low toxicity and high tropism for the airways. Interestingly, clofoctol has been found to have multiple biological activities and has been proposed for the treatment of several obstructive lung diseases, including asthma, lung cancer, and SARS-CoV-2 infection. In this study, the activity of clofoctol as a colistin adjuvant was investigated in Gram-negative lung pathogens that are critical for the high prevalence of multidrug-resistant isolates, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii. Clofoctol potentiated the bactericidal effect of colistin in all tested strains and reduced colistin MICs below the susceptibility breakpoint in nearly all colistin-resistant strains. Overall, this observation supports the development of inhaled clofoctol-colistin formulations for the treatment of difficult-to-treat airway infections caused by Gram-negative pathogens.
IMPORTANCE Colistin is used as a last-resort antibiotic against extensively drug-resistant Gram-negative pathogens. However, colistin resistance is on the rise. Clofoctol is an antibiotic used against Gram-positive bacteria, with low toxicity and high penetration and storage in the airways. Here, a strong synergistic activity of the colistin-clofoctol combination against colistin-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii isolates is reported, supporting the development of clofoctol-colistin formulations for the therapy of difficult-to-treat airways infections caused by these Gram-negative pathogens.
KEYWORDS: antibiotic resistance, adjuvants, colistin, clofoctol, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Gram-negative pathogens, antibiotic adjuvants, pulmonary infection
OBSERVATION
Colistin is a polymyxin antibiotic with bactericidal activity against Gram-negative pathogens. It was dismissed from clinical use in the late 1960s due to the concomitant development of less toxic antibiotics (1). However, the rise of infections caused by extensively drug-resistant (XDR) Gram-negative bacteria forced colistin reintroduction as a last-resort drug for the treatment of infections lacking other therapeutic options (1, 2). Consequently, colistin resistance is emerging among pathogens in clinical contexts such as ventilator-associated pneumonia and cystic fibrosis (3–5). Hence, the development of adjuvants lowering colistin therapeutic dosage and restoring susceptibility in XDR strains would be highly beneficial (6).
Clofoctol (octofene) is a synthetic antibiotic mainly active against Gram-positive bacteria. Thanks to its high tropism for the airways and low toxicity in humans (7), this drug is ordinarily prescribed in Italy and France to treat common pediatric airway infections caused by Gram-positive bacteria (8). Additional to antibacterial activity, clofoctol also regulates the unfolded protein response in eukaryotic cells, a process triggered in the airways of patients with respiratory diseases and contributing to inflammation. On these bases, clofoctol has been proposed for the treatment of different obstructive lung diseases, including asthma, lung cancer, and SARS-CoV-2 infection (8). Clofoctol also inhibits the quorum sensing (QS) cell-cell communication system of Pseudomonas aeruginosa, causing virulence attenuation (9, 10). Interestingly, a colistin-adjuvant activity has recently been discovered in two inhibitors of the P. aeruginosa QS, i.e., niclosamide and furanone C-30 (11, 12), raising the question of whether clofoctol could also display this activity.
Considering the high penetration and storage of clofoctol in airway tissues, the activity of this drug as colistin adjuvant has been investigated in three Gram-negative pulmonary pathogens for which new therapeutic options are urgently needed, namely, P. aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii (3, 13). One colistin-susceptible (CS) and three colistin-resistant (CR) strains have been analyzed for each species (Table S1 in the supplemental material).
MIC assays (14) revealed that clofoctol has no inhibitory activity against the selected strains (MIC > 1,170 μg/mL), while colistin MIC values ranged from 0.25 to 256 μg/mL (Table 1). Checkerboard assays (14) were performed to determine possible synergism between colistin and clofoctol, combined at different concentrations. A combination showing a fractional inhibitory concentration index (FICI) of ≤0.5 was considered synergistic (15). Synergism was slightly below the threshold in CS P. aeruginosa PAO1 (FICI = 0.502), while a synergistic effect was demonstrated against CS K. pneumoniae KP-MO-27 (FICI = 0.313) and A. baumannii ATCC 19606 (FICI = 0.125) (Table 1).
TABLE 1.
Effect of the clofoctol-colistin combination on the MIC of the indicated strains
| Strain | Colistin MIC (μg/mL) at clofoctol concn (μg/mL) of: |
Colclofa MIC | Clofcolb MIC | Maximum fold changec | FICId | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.14 | 2.28 | 4.56 | 9.13 | 18.25 | 36.5 | 73 | |||||
| P. aeruginosa | ||||||||||||
| PAO1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 2.28 | 2 | 0.502 |
| PAO1 colr1 | 64 | 64 | 16e | 4e | 2e | 2e | 2e | 2e | 2 | 9.13 | 32 | 0.039 |
| BG98 | 32 | 4e | 1e | 1e | 1e | 1e | 1e | 1e | 1 | 2.28 | 32 | 0.033 |
| KK27 colr7 | 256 | 256 | 64e | 16e | 8e | 4e | 4e | 4e | 4 | 18.25 | 64 | 0.031 |
| PAO1 ΔpqsR | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 4.56 | 2 | 0.504 |
| K. pneumoniae | ||||||||||||
| KP-MO-27 | 0.25 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.0625e | 0.0625 | 73 | 4 | 0.313 |
| KP-MO-5 | 64 | 32 | 8e | 8e | 4e | 4e | 4e | 4e | 4 | 9.13 | 16 | 0.070 |
| KP-MO-6 | 32 | 16 | 4e | 2e | 2e | 1e | 1e | 1e | 1 | 18.25 | 32 | 0.047 |
| KP-MO-25 | 128 | 8e | 4e | 4e | 1e | 1e | 1e | 1e | 1 | 9.13 | 128 | 0.016 |
| A. baumannii | ||||||||||||
| ATCC 19606 | 1 | 0.5 | 0.5 | 0.25e | 0.125e | 0.125e | 0.125e | 0.0625e | 0.0625 | 73 | 16 | 0.125 |
| Ab249 | 128 | 1e | 0.5e | 0.5e | 0.5e | 0.5e | 0.5e | 0.5e | 0.5 | 2.28 | 256 | 0.006 |
| Ab347 | 16 | 2e | 0.5e | 0.5e | 0.25e | 0.25e | 0.25e | 0.25e | 0.25 | 9.13 | 64 | 0.023 |
| Ab4452 | 32 | 8e | 2e | 0.5e | 0.5e | 0.5e | 0.5e | 0.5e | 0.5 | 4.56 | 64 | 0.020 |
Lowest MIC of colistin in combination with clofoctol (μg/mL).
Lowest MIC of clofoctol in combination with colistin (μg/mL).
Ratio between colistin MIC and the MIC of Colclof.
Fractional inhibitory concentration index. Clofoctol had no activity against any of the strains; therefore, the MIC of clofoctol was considered 1,170 μg/mL for calculation of the FICI [FICI = (MIC Colclof/MIC Col) + (MIC Clofcol/MIC Clof)] (15).
MIC values in which colistin/clofoctol combinations showed a synergistic activity (FICI ≤ 0.5).
Interestingly, the combination showed a strong synergistic effect in all CR strains, with MIC reductions ranging from 16- to 256-fold and FICI values of ≤0.070. In particular, clofoctol reduced colistin MICs to values below the susceptibility breakpoint (16) in all CR strains except KP-MO-5 (Table 1).
The minimal bactericidal concentration (MBC) of the combination was tested by checkerboard assays to evaluate the effect of clofoctol on colistin bactericidal activity (Table 2). In the presence of clofoctol, the MBC of colistin decreased almost in parallel with MIC reduction in all strains (MBC/MIC ratio ≤ 4), indicating that clofoctol promotes colistin-mediated bacterial killing (Table 2). Time-kill assays were performed to confirm the above-described result. One CR strain for each species was selected among the less susceptible to the colistin-clofoctol combination, namely, P. aeruginosa KK27 colr7, K. pneumoniae KP-MO-5, and A. baumannii Ab4452 (Table 1). Colistin and clofoctol concentrations reflecting those reached in patients’ lungs during treatment were used for this assay (17–19), i.e., colistin was tested at the minimum concentration maintained for at least 12 h in the sputum of most cystic fibrosis patients after inhaled colistin therapy (4 μg/mL) (18, 19), while clofoctol was tested at a concentration measured in the human lung 4 h after rectal administration (18 μg/mL) (17). In agreement with MBC results, the time-kill assays confirmed the bactericidal activity of the combination at colistin and clofoctol concentrations that are ineffective as monotherapy (Fig. 1), i.e., reduction of ≥3 log10 of the initial bacterial inoculum after 4 h of incubation. However, all cultures were able to regrow after 24 h of treatment, suggesting the emergence of resistant mutants and/or loss of activity of the combination. Further studies will be required to understand the nature of this phenomenon. Similar results were obtained with time-kill experiments carried out with the CS strains (Fig. S1).
TABLE 2.
Effect of the clofoctol-colistin combination on the MBC of the indicated strains
| Strain | Colistin MBC (μg/mL) at clofoctol concn (μg/mL) |
Colclofa MBC | Clofcolb MBC | MBC/MICc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1.14 | 2.28 | 4.56 | 9.13 | 18.25 | 36.5 | 73 | ||||
| P. aeruginosa | |||||||||||
| PAO1 | 4 | 4 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 9.13 | 2 |
| PAO1 colr1 | 256 | 128 | 64 | 16 | 8 | 8 | 8 | 8 | 8 | 9.13 | 4 |
| BG98 | 128 | 16 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 4.56 | 1 |
| KK27 colr7 | 512 | 256 | 256 | 64 | 32 | 16 | 16 | 16 | 16 | 18.25 | 4 |
| K. pneumoniae | |||||||||||
| KP-MO-27 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.125 | 0.0625 | 0.0625 | 73 | 1 |
| KP-MO-5 | 128 | 32 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 9.13 | 1 |
| KP-MO-6 | 64 | 16 | 4 | 4 | 2 | 1 | 1 | 1 | 1 | 18.25 | 1 |
| KP-MO-25 | 128 | 16 | 8 | 8 | 1 | 1 | 1 | 1 | 1 | 9.13 | 1 |
| A. baumannii | |||||||||||
| ATCC 19606 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.0625 | 0.0625 | 73 | 1 |
| Ab249 | 128 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 4.56 | 2 |
| Ab347 | 32 | 8 | 2 | 2 | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 | 36.5 | 1 |
| Ab4452 | 64 | 16 | 4 | 2 | 2 | 2 | 1 | 1 | 1 | 36.5 | 2 |
Lowest MBC of colistin in combination with clofoctol (μg/mL).
Lowest MBC of clofoctol in combination with colistin (μg/mL).
Ratio between Colclof MBC and Colclof MIC. According to Pankey and Sabath (25), a ratio of ≤4 indicates bactericidal activity.
FIG 1.
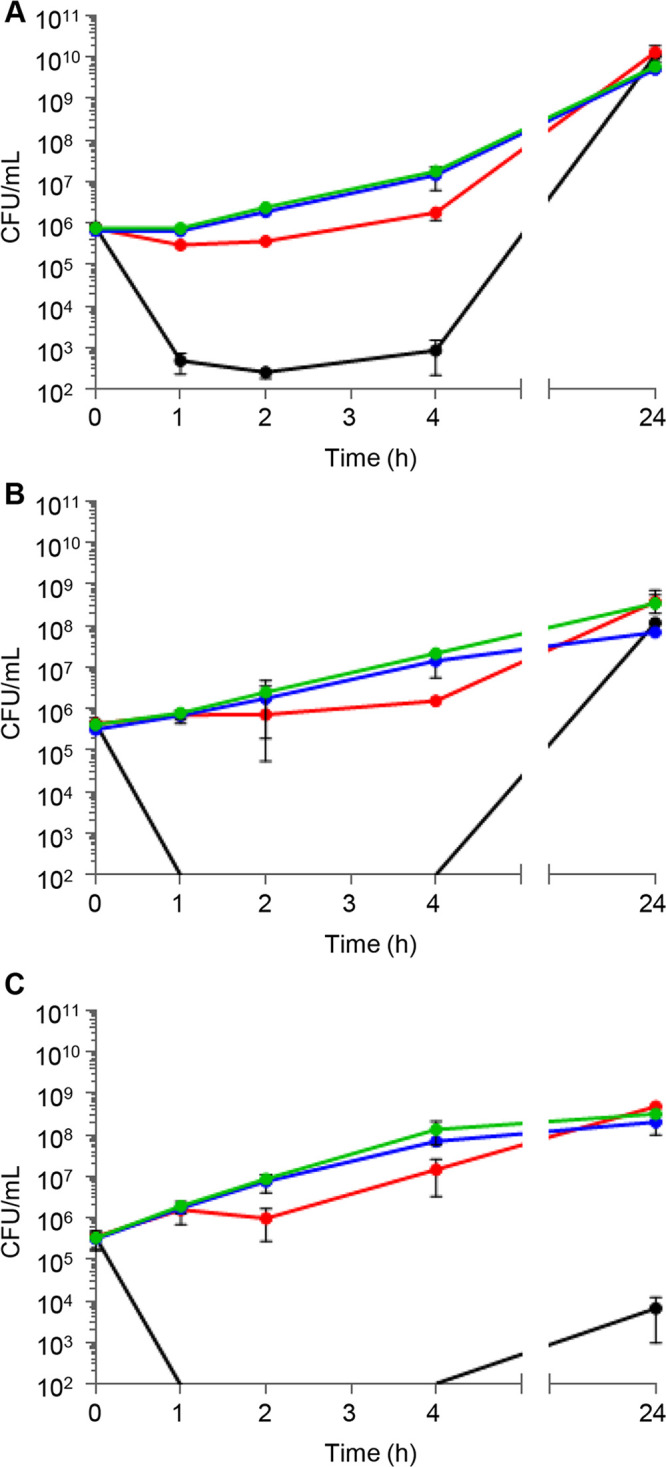
Time-kill curves of P. aeruginosa KK27 colr7 (A), K. pneumoniae KP-MO-5 (B), and A. baumannii Ab4452 (C) treated with 4 μg/mL colistin (red lines), 18 μg/mL clofoctol (blue lines), or the same concentrations of colistin and clofoctol in combination (black lines). The untreated controls are shown with green lines. Data are mean values from three independent experiments, and the error bars represent standard deviations. The detection limit of the assay was 102 CFU/mL.
In P. aeruginosa, clofoctol inhibits QS by targeting the PqsR signal receptor (9). However, K. pneumoniae and A. baumannii lack the pqs QS system, suggesting that the clofoctol adjuvant effect could not be related to its anti-QS activity. Accordingly, P. aeruginosa PAO1 and its isogenic ΔpqsR mutant showed the same MIC for colistin, either alone or in combination with clofoctol (Table 1).
Since we showed that clofoctol potentiates colistin activity against bacteria characterized by different colistin resistance mechanisms (i.e., lipid A aminoarabinosylation for P. aeruginosa and K. pneumoniae, lipid A phosphoethanolamination for A. baumannii) (20–22), it is tempting to speculate that clofoctol does not interfere with specific resistance mechanisms. Unfortunately, the mechanisms underlying clofoctol antibiotic activity and bacterial resistance in Gram-positive pathogens have been poorly studied so far (8). Hence, further studies are required to confirm this hypothesis and investigate the mechanism of action of the colistin-clofoctol combination.
Overall, clofoctol synergizes with colistin against both CR and CS isolates, suggesting that it could be useful not only to treat infections caused by CR bacteria but also to reduce the colistin dosing regimen so as to minimize its toxic effects. Indeed, colistin toxicity is a concern due to its limited therapeutic range, close to the plasma nephrotoxic concentration (23). However, nebulized colistin treatment is less toxic than other administration routes, and clinical and microbiological efficacies are promising (24). Compared with other colistin adjuvants, the obvious benefit of clofoctol is that it is a commonly used antibacterial drug with known pharmacological properties, very low toxicity, and high tropism for the lungs (7, 17).
Overall, this study demonstrates that the colistin-clofoctol combination is active against relevant XDR Gram-negative pulmonary pathogens, supporting the development of inhalable clofoctol-colistin formulations.
ACKNOWLEDGMENTS
This work was supported by the Italian Ministry of Education, University and Research (MIUR) with the following grants: Excellence Departments (art. 1, commi 314-337 Legge 232/2016) to the Department of Science, Roma Tre University; PRIN 2017 (prot. 20177J5Y3P) to P.V. and F.I.; and PRIN 2020 to F.I. (prot. 20208LLXEJ) and to L.L. (Prot. 202089LLEH). This work was also supported by Regione Lazio (Gruppi di Ricerca 2020, POR A0375E0026) to F.I.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
Contributor Information
Livia Leoni, Email: livia.leoni@uniroma3.it.
Monika Kumaraswamy, University of California, San Diego.
REFERENCES
- 1.Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. 2021. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol Rev 73:679–728. doi: 10.1124/pharmrev.120.000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Rafailidis PI, Matthaiou DK. 2010. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat 13:132–138. doi: 10.1016/j.drup.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Nowak J, Zander E, Stefanik D, Higgins PG, Roca I, Vila J, McConnell MJ, Cisneros JM, Seifert H, MagicBullet Working Group WP4 . 2017. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother 72:3277–3282. doi: 10.1093/jac/dkx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worthington RJ, Melander C. 2013. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 31:177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danesi R, Del Tacca M. 1985. Clinical study on the efficacy of clofoctol in the treatment of infectious respiratory diseases. Int J Clin Pharmacol Res 5:175–179. [PubMed] [Google Scholar]
- 8.Bailly C, Vergoten G. 2021. A new horizon for the old antibacterial drug clofoctol. Drug Discov Today 26:1302–1310. doi: 10.1016/j.drudis.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 9.D’Angelo F, Baldelli V, Halliday N, Pantalone P, Polticelli F, Fiscarelli E, Williams P, Visca P, Leoni L, Rampioni G. 2018. Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob Agents Chemother 62:e1296-18. doi: 10.1128/AAC.01296-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collalto D, Giallonardi G, Fortuna A, Meneghini C, Fiscarelli E, Visca P, Imperi F, Rampioni G, Leoni L. 2022. In vitro activity of antivirulence drugs targeting the las or pqs quorum sensing against cystic fibrosis Pseudomonas aeruginosa isolates. Front Microbiol 13:845231. doi: 10.3389/fmicb.2022.845231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domalaon R, Malaka P, Silva D, Kumar A, Zhanel GG, Schweizer F. 2019. The anthelmintic drug niclosamide synergizes with colistin and reverses colistin resistance in Gram-negative bacilli. Antimicrob Agents Chemother 63:e02574-18. doi: 10.1128/AAC.02574-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Lin Y, Zhang X, Chen L, Xu C, Liu S, Cao J, Zheng X, Jia H, Chen L, Zhou T. 2021. Combining colistin with furanone C-30 rescues colistin resistance of Gram-negative bacteria in vitro and in vivo. Microbiol Spectr 9:e01231-21. doi: 10.1128/Spectrum.01231-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 8 November 2022.
- 14.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed. CLSI M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Doern CD. 2014. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EUCAST. 2022. Breakpoint tables for interpretation of MICs and zone diameters v.12.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf.
- 17.Del Tacca M, Danesi R, Senesi S, Gasperini M, Mussi A, Angeletti CA. 1987. Penetration of clofoctol into human lung. J Antimicrob Chemother 19:679–683. doi: 10.1093/jac/19.5.679. [DOI] [PubMed] [Google Scholar]
- 18.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 19.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeannot K, Bolard A, Plésiat P. 2017. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents 49:526–535. doi: 10.1016/j.ijantimicag.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Esposito EP, Cervoni M, Bernardo M, Crivaro V, Cuccurullo S, Imperi F, Zarrilli R. 2018. Molecular epidemiology and virulence profiles of colistin-resistant Klebsiella pneumoniae blood isolates from the hospital agency “Ospedale dei Colli,” Naples, Italy. Front Microbiol 9:1463. doi: 10.3389/fmicb.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo Sciuto A, Imperi F. 2018. Aminoarabinosylation of lipid A is critical for the development of colistin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 23:e01820-17. doi: 10.1128/AAC.01820-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrest A, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Li J, Silveira FP, Nation RL. 2017. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob Agents Chemother 61:e01367-17. doi: 10.1128/AAC.01367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vardakas KZ, Voulgaris GL, Samonis G, Falagas ME. 2018. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int J Antimicrob Agents 51:1–9. doi: 10.1016/j.ijantimicag.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Pankey GA, Sabath LD. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis 38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1, Table S1, and supplemental materials and methods. Download spectrum.04275-22-s0001.pdf, PDF file, 0.3 MB (272.5KB, pdf)


