ABSTRACT
Colonization with nontoxigenic Clostridioides difficile strain M3 (NTCD-M3) has been demonstrated in susceptible hamsters and humans when administered after vancomycin treatment. NTCD-M3 has also been shown to decrease risk of recurrent C. difficile infection (CDI) in patients following vancomycin treatment for CDI. As there are no data for NTCD-M3 colonization after fidaxomicin treatment, we studied the efficacy of NTCD-M3 colonization and determined fecal antibiotic levels in a well-studied hamster model of CDI. Ten of 10 hamsters became colonized with NTCD-M3 after 5 days of treatment with fidaxomicin when NTCD-M3 was administered daily for 7 days after treatment discontinuation. The findings were nearly identical to 10 vancomycin-treated hamsters also given NTCD-M3. High fecal levels of OP-1118, the major fidaxomicin metabolite, and vancomycin were noted during treatment with the respective agents and modest levels noted 3 days after treatment discontinuation at the time when most of the hamsters became colonized. These findings support the ongoing development of NTCD-M3 for the prevention of recurrent CDI.
IMPORTANCE NTCD-M3 is a novel live biotherapeutic, that has been shown in a Phase 2 clinical trial to prevent recurrence of C. difficile infection (CDI) when administered shortly after antibiotic treatment of the initial CDI episode. Fidaxomicin was not, however, in widespread use at the time this study was conducted. A large multi-center Phase 3 clinical trial is now currently in the planning stage, and it is anticipated that many patients eligible for this study will be treated with fidaxomicin. Since efficacy in the hamster model of CDI has predicted success in patients with CDI, we studied the ability of NTCD-M3 to colonize hamsters after treatment with either fidaxomicin or vancomycin.
KEYWORDS: Clostridioides difficile, fidaxomicin, intestinal colonization, nontoxigenic, vancomycin
INTRODUCTION
Intentional colonization of volunteers with spores of nontoxigenic Clostridioides difficile restriction endonuclease analysis (REA) strain M3 (NTCD-M3) has been demonstrated to be safe in subjects receiving vancomycin (1). NTCD-M3 has also been shown to be effective in the prevention of recurrent C. difficile infection (CDI) in patients following treatment with metronidazole or vancomycin for CDI (2). Based on these data, NTCD-M3 is currently being developed as a novel live biotherapeutic to reduce recurrent CDI. However, since the clinical trials were not conducted on patients receiving fidaxomicin, and since fidaxomicin is now a preferred treatment of CDI (3), more data are needed to support use of NTCD-M3 in this scenario.
The pivotal preclinical studies supporting NTCD-M3 as a potential therapeutic agent have been conducted in hamsters and efficacy in the hamster model has predicted success in clinical trials (4). Administration of NTCD spores, particularly spores of the REA type strain, M3 effectively colonize hamsters given continuous administration of clindamycin, ampicillin, ceftriaxone, and fluoroquinolones (5–7). Administration of NTCD spores is also effective in preventing disease in hamsters challenged with highly virulent C. difficile strains, including the BI/NAP1/027 strain (8), but efficacy is dependent on establishing colonization (4).
Fidaxomicin has been reported to have an extended period of detection to day 5 in stool, following the stopping of the drug in humans (9, 10) and longer in a human gut model (11). Similar stool detection of vancomycin in human stool to day 5 has been reported in CDI-treated patients (12). Successful colonization by NTCD-M3 following vancomycin treatment has been demonstrated in volunteers and CDI patients (1, 2), however, similar data for fidaxomicin treatment are not available. There is a potential difference in NTCD-M3 colonization efficacy after fidaxomicin treatment due to carryover of drug in stool which could inhibit colonization. Therefore, we conducted a study of NTCD-M3 colonization in susceptible hamsters after fidaxomicin or vancomycin treatment, documenting time to colonization, duration of colonization, and fecal drug concentrations.
RESULTS
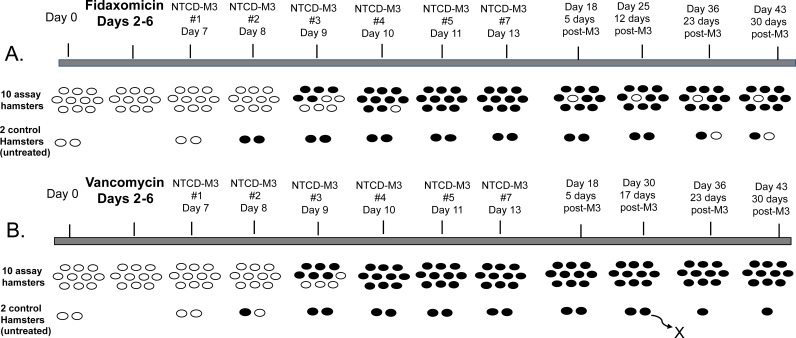
NTCD-M3 readily colonized all hamsters rendered susceptible by clindamycin conditioning at the start of the experiment (Fig. 1). Following 5 days of treatment (experimental days 2 to 6) with fidaxomicin and subsequent daily administration of NTCD-M3 for 7 days (experimental days 7 to 13), 10 out of 10 hamsters became colonized. Likewise, 10 out of 10 vancomycin-treated hamsters became colonized with NTCD-M3. The onset of NTCD-M3 colonization was nearly identical for both fidaxomicin and vancomycin groups; 48 to 96 h after the first day of NTCD-M3 administration for the fidaxomicin-treated hamsters and 48 to 72 h after the first day on NTCD-M3 administration for the vancomycin-treated hamsters. The onset of NTCD-M3 colonization was ~24 h earlier in the untreated control hamsters (24 to 48 h after the first day on NTCD-M3 administration). Onset of colonization occurred at high levels by semi-quantitative culture (3+ or 4+) for all hamsters, and the hamsters remained colonized at high levels to the end of the study (experimental day 43) with the exception of 1 fidaxomicin-treated hamster and 1 of the untreated control hamsters, which showed decreasing levels of colonization (1+ to rare) until finally becoming negative (Table S1 and S2). One control hamster expired on day 30.
FIG 1.
Colonization results for groups of 10 hamsters treated with fidaxomicin (A) and vancomycin (B) and groups of 2 untreated control hamsters. Open ovals indicated no C. difficile growth in the fecal pellets. Solid color ovals indicated positive fecal cultures for C. difficile. One control hamster in the second control group died on day 30 during an environmental problem in the animal facility.
To better understand the interaction of NTCD-M3 and antibiotic treatment given to the hamsters, we determined in vitro susceptibility of NTCD-M3 to the antibiotics as well as antibiotic levels in the hamster fecal pellets. The MIC of fidaxomicin was 0.125 μg/mL, the MIC of OP-1118 was 4 μg/mL. The MIC of vancomycin was 2 μg/mL for NTCD-M3. These MIC results are similar to MIC results for these antibiotics reported previously for other C. difficile strains (13–15).
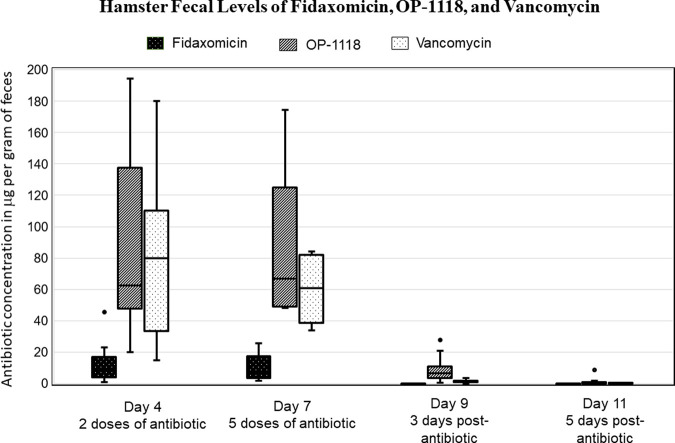
The fidaxomicin metabolite OP-1118, as well as vancomycin, remained at very high levels in hamster fecal pellets during antibiotic administration (experimental days 4 and 7, after 2 and 5 days of antibiotic treatment, respectively) (Fig. 2). The median (IQR) fecal OP-1118 levels on day 4 and 7 were 62.6 (52.6 to 128.4) and 66.7 (49.2 to 123.7) μg/gm feces, respectively, while the fecal vancomycin levels for the same time points were 80.0 (36.7 to 96.5) and 60.6 (39.8 to 81.3) μg/gm feces. The level of the fidaxomicin parent compound was much lower during treatment, but still above the MIC of the drug for NTCD-M3. The median (IQR) fecal fidaxomicin levels on day 4 and 7 were 8.3 (4.5 to 14.8) and 7.5 (3.6 to 17.1) μg/gm feces. Low fecal antibiotic levels of OP-1118 6.4 (3.9 to 7.3) μg/gm feces (P = 0.002, median change from day 4 to 10), and to a lesser extent, vancomycin 1.39 (0.93 to 1.69) μg/gm feces (P = 0.002, median change from day 4 to 10) were detectable 72 h after discontinuation of the antibiotics (experimental day 9). The median fecal concentration of OP-1118 for the hamsters who were colonized on day 9 was 5.32 (IQR: 2.43 to 6.54) compared to 7.33 (IQR: 6.16 to 20.98) μg/gm feces for those hamsters not yet colonized on day 9 (P = 0.14).
FIG 2.
Fecal antibiotic levels during treatment on experimental days 4 and 7 (collected 24 h after the last treatment dose on day 6) and after treatment on days 9 and 11. Levels in μg/gm feces are presented in a box-and-whisker plot with the median (horizontal line), interquartile range (box), and the lower/upper fences (whiskers).
DISCUSSION
NTCD-M3 effectively colonized hamsters following treatment with fidaxomicin. Colonization after fidaxomicin treatment was complete, timely, and sustained in a nearly identical manner as that demonstrated following vancomycin treatment. These results support the rationale for using NTCD-M3 to colonize patients after treatment for CDI and prevent recurrence after antibiotic treatment discontinuation. NTCD-M3 has been demonstrated effective for prevention of recurrent CDI in a Phase 2 clinical trial, where vancomycin and metronidazole were the treatment antibiotics (2). A Phase 3 clinical trial is currently being developed to confirm this effect after treatment with current standard of care antibiotics that will likely include fidaxomicin, as well as vancomycin.
Fidaxomicin and vancomycin are similar in the respect that both antibiotics are minimally absorbed and achieve high concentrations in the stool of treated subjects (9, 10). Our findings in the hamster model confirmed high fecal concentrations of vancomycin during treatment but less so for fidaxomicin. However, the concentration of the main metabolite of fidaxomicin, OP-1118 which is active, was much higher than the parent compound (similar to a single dose human volunteer study where 82% of fecal recovery of fidaxomicin was OP-1118) (9) and was similar to that of vancomycin. The rapid colonization of NTCD-M3 in the hamsters following fidaxomicin and vancomycin appears to be associated with the decrease in fecal concentrations of both antibiotics and the active metabolite of fidaxomicin, where the concentrations reduced markedly between Day 7 and Day 9 which corresponds to the emergence of colonization of NTCD-M3 in both arms at Day 8/9. The relatively higher concentrations of OP-1118 on day 9 in the hamsters not yet colonized also support this association.
The timing of NTCD-M3 colonization was delayed approximately 24 h after stopping both fidaxomicin and vancomycin in comparison to the untreated control hamsters. This delay appeared to be associated with the residual fecal antibiotic levels for both vancomycin and OP-1118 after stopping treatment. Nevertheless, NTCD-M3 effectively colonized hamsters after fidaxomicin treatment and there was no delay in comparison to the vancomycin-treated hamsters.
As the hamster model has predicted clinical efficacy of NTCD-M3 in colonization of CDI patients after vancomycin treatment (1), the findings here support the rationale for ongoing trials of NTCD-M3 for prevention of recurrent CDI in current clinical arena where fidaxomicin treatment has taken an increasing role.
MATERIALS AND METHODS
Hamster husbandry and care.
As previously described, male Syrian Golden hamsters (age, 6 to 8 weeks; weight, approximately 90 to 100 g) were individually housed in polycarbonate cages with filter tops and disposable air filters to prevent cross-contamination (4). Food, bedding, water and water bottles, cages, wire tops, filters, and filter tops were autoclaved before usage. During the course of the experiment, bedding was changed once a day; the cage and water were changed once a week. Hamster fecal pellets were collected and cultured on selective taurocholate-cefoxitin-cycloserine-fructose-agar (TCCFA) prior to initiation of the protocol to assure they are free of the C. difficile. During the study protocol, 3 fecal pellets were collected for culture in 0.75 mL of sterile PBS, while 10 to 12 pellets were collected dry for subsequent antibiotic assays and frozen at −70°C until use. The experimental protocol was approved by the Hines VA Institutional Animal Care and Use Committee.
Antimicrobial susceptibility.
NTCD-M3 previously designated VP20621 (1, 2) was tested for susceptibility to fidaxomicin and the major fidaxomicin metabolite, OP-1118, and vancomycin by CLSI-recommended agar dilution methodology (16). Fidaxomicin (Sigma) and OP-1118 (Toronto Research Chemicals) were dissolved and diluted in dimethylsulfoxide (DMSO) to achieve final study concentrations that ranged from 0.125 to 8 μg/mL. Vancomycin (Sigma) was diluted in water to achieve concentrations from 0.125 to 16 μg/mL. C. difficile ATCC 700057 and Staphylococcus aureus ATCC 29213 were included as quality control strains for fidaxomicin and vancomycin (16). There are no established quality control break points for OP-1118, and only C. difficile ATCC 700057 was used per Goldstein, et al. (13). The MIC of OP-1118 for ATCC 700057 was 4 μg/mL which was 32-fold higher than the MIC of fidaxomicin for ATCC 700057 (0.125 μg/mL) and identical to the 32-fold higher MIC ratio previously reported for these drugs (17). Agar dilution assays were performed in triplicate.
Hamster colonization protocol.
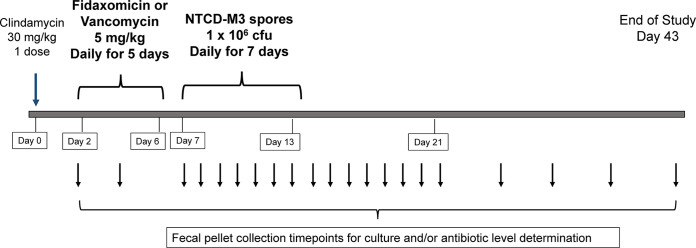
The experimental design and timeline for the protocol is shown in Fig. 3. Groups of 10 hamsters were given 30 mg/kg clindamycin orally (by gastric gavage needle) on day 0 to render the hamsters susceptible. On day 2 through day 6, antibiotics were given by once-daily oral gavage, either fidaxomicin (5 mg/kg) or vancomycin (5 mg/kg). On day 7 through day 13, 106 CFU of spores of NTCD-M3 were administered once-daily again, by oral gavage. Hamster fecal pellets were collected daily and cultured beginning the first day of NTCD-M3 administration and repeated daily until day 21 and then twice weekly thereafter until 30 days after the end of spore administration (day 43). Two additional hamsters in each treated cohort were given clindamycin on day 0 but no subsequent antibiotics before receiving NTCD-M3 on day 7 through day 13. These hamsters served as controls to confirm continued susceptibility to colonization by NTCD-M3 in the absence of study drug-mediated microbiota perturbations. Semi-quantitative cultures were performed on all fecal specimens by homogenization of the hamster pellets, inoculation onto TCCFA, then streaked for isolation. Colonization was recorded as 1+ to 4+ (growth in first to fourth quadrant of the agar plate) or ‘rare’ (less than 5 colonies in the first quadrant). Colonization was defined by positive fecal cultures on two or more consecutive days. Confirmation of NTCD-M3 was performed by REA typing of recovered C. difficile colonies from selected cultures for each colonized hamster (18).
FIG 3.
Experimental design of hamster experiments. Oral clindamycin was given on experimental day 0 to render the animals susceptible to C. difficile. Daily oral treatment with study drug (fidaxomicin or vancomycin) was given on days 2 through 6. Oral administration of NTCD-M3 was given on days 7 through 13. Fecal pellets were collected for culture and/or antibiotic level determination at the time points indicated by the arrows below the timeline.
Fecal antimicrobial assays.
Fecal pellets were also collected at intervals before, during and after antibiotic treatment, and during and after NTCD-M3 administration for determination of vancomycin or fidaxomicin and OP-1118 (the major metabolite of fidaxomicin) concentrations. The pellets were stored at −70°C until processing. Pellets were subsequently thawed, and 50 mg suspended in dH2O and 1% formic acid in methanol, homogenized, then spiked with an internal standard (methadone for fidaxomicin and OP-1118; codeine for vancomycin). Following sample vortexing and centrifugation at 13,000g for 10 min, 100 μL of supernatant was transferred into a well plate for analysis. Samples were prepared and ran in duplicate by liquid chromatography/tandem mass spectrometry (LC/MS/MS) on a Shimadzu UHPLC consisting of LC-30AD pumps, SIL-30ACMP autosampler and CBO-20A column oven connected to a SCIEX 6500+ Qtrap QQQ LC/MS/MS detector. A Phenomenex Kinetex 2.6 μm Biphenyl (50 × 2.1 mm) column was used for analyte separation via a gradient consisting of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B) according to the following program: 0.5 min 10% B, 2.0 min 25% B, 4.5 min 100% B, hold for 1 min, 5.51 min return to 10% B and hold for 2 min at this starting condition. A 3 stage wash of 0.1% formic acid in water, 50% methanol in water, and 40:40:20 methanol:acetonitrile:IPA was used with the autosampler needle and seat to avoid any compound carryover. MS source conditions were fully optimized for maximum analyte sensitivity for all compounds with the transitions (2 per analyte, T1 and T2) given in Table 1. Samples from both assays were quantified on matrix matched curves for fidaxomicin, OP-1118, and vancomycin. The curves were generated by spiking fidaxomicin, OP-1118, and vancomycin purified standards in quantities ranging from 0.1 μg/g to 100 μg/g into hamster fecal material that was collected prior to assay antibiotic treatment. Pooled high, mid, and low QC standards were created at 0.8 μg/g, 8 μg/g, and 80 μg/g concentrations, respectively, for the analytes of interest to confirm assay performance and accuracy.
TABLE 1.
Mass spectrometry conditionsa for determination of fecal antibiotic concentrations
| Analyte | Q1 (m/z) | Q3 (m/z) |
|---|---|---|
| Vancomycin (T1) | 724.900 | 144.000 |
| Vancomycin (T2) | 724.900 | 1143.500 |
| Fidaxomicin (T1) | 1079.500 | 847.600 |
| Fidaxomicin (T2) | 1079.500 | 617.200 |
| Op1118 (T1) | 1009.500 | 777.500 |
| Op1118-T2 | 1009.500 | 505.000 |
| Codeine ISb (T1) | 300.100 | 152.000 |
| Methadone ISb (T1) | 310.200 | 265.200 |
Mass transitions (in m/z) are shown for each compound run. Mass transitions are tandem MS (MS/MS) gas-phase dissociation reactions wherein the ionized parent molecule is selected in the first quadrupole (Q1), subjected to collisions with gas to affect molecule fragmentation and fragmentation products are chosen in the final quadrupole (Q3). Two mass transitions are chosen: (1) Quantifier (T1) for quantitation via peak area and (2) Qualifier (T2) for confirmation of the molecule and MS/MS experiment by comparing height ratios of T1 and T2.
Internal standard.
Statistical analysis.
Groups of 10 hamsters were used to allow for detection of a significant decline in colonization of 50% from that of the expected colonization rate which is 90% to 100% based on historical experience (P = 0.03) (4–8).
Normality of fecal pellet drug concentration and drug concentration difference between days 4, 7, and 10 were assessed using Shapiro-Wilk (19). Fecal drug concentrations were reported as median and interquartile range. Fecal drug concentrations were compared between days by Wilcoxon Sign-Rank Test and fecal OP-1118 concentrations between colonized and non-colonized hamsters were compared by Wilcoxon Rank-Sum Test. All statistical analysis was completed using SAS statistical software v9.4 (SAS Institute).
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel Hynes, William Rhys-Williams and William Love of Destiny Pharma, PLC, Brighton, UK for their support.
Funding for this study and the NTCD-M3 spores were supplied by Destiny Pharma PLC, Brighton, UK.
D.N.G. and S.J. are supported by the US Department of Veterans Affairs Research Service.
We thank Arsen Gaisin, PhD, for facilitating the fecal antibiotic assays which were performed at the Integrated Molecular Structure Education and Research Center MS Facility at Northwestern University, Evanston, IL.
We also thank Adam Cheknis and Laurica Petrella-Zitko for their help with the REA typing and the hamster experiments.
Footnotes
Supplemental material is available online only.
Contributor Information
Stuart Johnson, Email: stuart.johnson2@va.gov.
Karen C. Carroll, Johns Hopkins Hospital
Kevin Garey, University of Houston.
Matthew Schnizlein, University of Michigan-Ann Arbor.
REFERENCES
- 1.Villano SA, Seiberling M, Tatarowicz W, Monnot-Chase E, Gerding DN. 2012. Evaluation of an oral suspension of VP20621, spores of nontoxigenic Clostridium difficile strain M3, in healthy subjects. Antimicrob Agents Chemother 56:5224–5229. doi: 10.1128/AAC.00913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, Van Schooneveld TC, Pardi DS, Ramos A, Barron MA, Chen H, Villano S. 2015. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA 313:1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. 2021. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 73:e1029–e1044. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 4.Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. 2002. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis 186:1781–1789. doi: 10.1086/345676. [DOI] [PubMed] [Google Scholar]
- 5.Merrigan MM, Sambol SP, Johnson S, Gerding DN. 2003. Prevention of fatal Clostridium difficile-associated disease during continuous administration of clindamycin in hamsters. J Infect Dis 188:1922–1927. doi: 10.1086/379836. [DOI] [PubMed] [Google Scholar]
- 6.Merrigan MM, Sambol SP, Johnson S, Gerding DN. 2009. New approach to the management of Clostridium difficile infection: colonisation with non-toxigenic C. difficile during daily ampicillin or ceftriaxone administration. Int J Antimicrob Agents 33 Suppl 1:S46–S50. doi: 10.1016/S0924-8579(09)70017-2. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ST, Nagaro K, Sambol SP, Johnson S, Gerding DN. 2011. Susceptibility of hamsters to infection by historic and epidemic BI Clostridium difficile strains during daily administration of three fluoroquinolones. Anaerobe 17:166–169. doi: 10.1016/j.anaerobe.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Nagaro KJ, Phillips ST, Cheknis AK, Sambol SP, Zukowski WE, Johnson S, Gerding DN. 2013. Nontoxigenic Clostridium difficile protects hamsters against challenge with historic and epidemic strains of toxigenic BI/NAP1/027 C. difficile. Antimicrob Agents Chemother 57:5266–5270. doi: 10.1128/AAC.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shue YK, Sears PS, Shangle S, Walsh RB, Lee C, Gorbach SL, Okumu F, Preston RA. 2008. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother 52:1391–1395. doi: 10.1128/AAC.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima H, Yamazaki T, Benner L, Miki T, Michon I, Wojtkowski T, Kaibara A, Mujais S. 2015. Comparison of the safety, tolerability, and pharmacokinetics of fidaxomicin in healthy Japanese and caucasian subjects. Clin Drug Invest 35:375–384. doi: 10.1007/s40261-015-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilton CH, Crowther GS, Freeman J, Todhunter SL, Nicholson S, Longshaw CM, Wilcox MH. 2014. Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. J Antimicrob Chemother 69:451–462. doi: 10.1093/jac/dkt347. [DOI] [PubMed] [Google Scholar]
- 12.Abujamel T, Cadnum JL, Jury LA, Sunkesula VC, Kundrapu S, Jump RL, Stintzi AC, Donskey CJ. 2013. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One 8:e76269. doi: 10.1371/journal.pone.0076269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein EJ, Babakhani F, Citron DM. 2012. Antimicrobial activities of fidaxomicin. Clin Infect Dis 55 Suppl 2:S143–S148. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorpe CM, McDermott LA, Tran MK, Chang J, Jenkins SG, Goldstein EJC, Patel R, Forbes BA, Johnson S, Gerding DN, Snydman DR. 2019. U.S.-based national surveillance for fidaxomicin susceptibility of Clostridioides difficile-associated diarrheal isolates from 2013 to 2016. Antimicrob Agents Chemother 63:e00391-19. doi: 10.1128/AAC.00391-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargis AS, Karlsson M, Paulick AL, Anderson KF, Adamczyk M, Vlachos N, Kent AG, McAllister GA, McKay SL, Halpin AL, Albrecht V, Campbell D, Korhonen L, Elkins CA, Rasheed JK, Guh AY, McDonald LC, Lutgring JD, Emerging Infections Program CdIWG . 2022. Reference susceptibility testing and genomic surveillance of Clostridioides difficile, United States, 2012–17. Clin Infect Dis 76:890–896. doi: 10.1093/cid/ciac817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. 2018. Methods for antimicrobial susceptibility testing of anaerobic bacteria 9th Edition: M11. [Google Scholar]
- 17.Anonymous . 2011. Dificlir (fidaxomicin) assessment report EMA/857570/2011. https://www.ema.europa.eu/en/documents/assessment-report/dificlir-epar-public-assessment-report_en.pdf. Accessed 21 March 2023.
- 18.Clabots CR, Johnson S, Bettin KM, Mathie PA, Mulligan ME, Schaberg DR, Peterson LR, Gerding DN. 1993. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J Clin Microbiol 31:1870–1875. doi: 10.1128/jcm.31.7.1870-1875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi A, Zahediasl S. 2012. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10:486–489. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00517-23-s0001.xlsx, XLSX file, 0.03 MB (29.4KB, xlsx)
Supplemental material. Download spectrum.00517-23-s0002.xlsx, XLSX file, 0.03 MB (32.2KB, xlsx)