Abstract
Knowledge of life histories is crucial for understanding ecological and evolutionary processes, but for many hydrozoan species only incomplete life cycles have been described due to challenges in linking hydromedusae with their polyp stages. Using a combination of DNA barcoding, morphology, and ecological information, we describe for the first time the polyp stage of Halopsis ocellata Agassiz, 1865 and re-describe that of Mitrocomella polydiademata (Romanes, 1876). Campanulinid hydroids referable to Lafoeina tenuis Sars, 1874 and collected in the same biogeographic region as the type locality of this species are shown to be the polyp stage of these two mitrocomid hydromedusae. The nominal species L. tenuis thus is a species complex that includes the polyp stage of medusae belonging to at least two genera currently placed in a different family. Consistent morphological and ecological differences were found between the polyps linked to each of these two hydromedusae, but molecular results suggest that yet other species may have morphologically similar hydroids. Polyps morphologically identified to L. tenuis are therefore better referred to as Lafoeina tenuis-type until further associations are resolved, particularly when occurring outside of the area of distribution of H. ocellata and M. polydiademata. Molecular identification integrated with traditional taxonomy is confirmed as an effective approach to link inconspicuous stages of marine invertebrates with hitherto unknown life cycles, especially in often-overlooked taxa. Disentangling the relationships between L. tenuis, H. ocellata, and M. polydiademata lays the ground for future research aimed at resolving the taxonomy and systematics of the enigmatic families Mitrocomidae and Campanulinidae.
Keywords: Hydrozoa, Hydroids, Jellyfish, Life history, DNA barcoding, Integrative taxonomy, Life cycle
Introduction
Hydrozoans are widespread but often overlooked components of marine environments (Gili et al., 1998; Bouillon et al., 2006). They occur both in the benthos and the plankton of all oceans, where they act as predators (Purcell, 1989; Nicholas & Frid, 1999; Orejas et al., 2001), prey (Arai, 2005; Ayala et al., 2018), symbionts (Fernandez-Leborans, 2013; Montano et al., 2015), habitat-formers (Di Camillo et al., 2017; Gomes-Pereira et al., 2017; Puente-Tapia et al., 2021), and significantly contribute to bentho-pelagic coupling (Gili et al., 1998). As a group, hydrozoans are well-known for their wide array of life cycle strategies, including the classic textbook example of substrate-bound hydroid polyps that produce free-swimming hydromedusae (Boero et al., 1997; Bouillon et al., 2006). Because benthic hydroids and their corresponding medusae have different ecological niches, complete knowledge of their life cycle is crucial to understand the ecological and evolutionary processes underlying species diversity and diversification (Boero et al., 1997).
Documenting the entire life cycle of hydrozoans is a challenging task, complicated by hydroids and hydromedusae having been traditionally studied by different groups of researchers (Boero, Bouillon & Piraino, 1992). Hydrozoan species have often been described based on a single life stage, which has led to the development of parallel taxonomies with separate names for polyps and their respective medusae (Brooks, 1886; Cornelius, 1977). Many of these names are still in use, and the process of unifying these classification systems is far from finished (Schuchert, Hosia & Leclére, 2017; Maggioni et al., 2021). Even when the relationships between polyps and medusae are known, some stages have never been observed in nature and their morphology is described solely based on animals reared in laboratory and/or for juvenile or immature specimens (Russell, 1953; Bouillon et al., 2006). Hydrozoans have a high degree of phenotypic plasticity and lab-reared animals may be strikingly different from those collected in the field, depending on the conditions they have been exposed to (Dudgeon & Buss, 1996; Meroz-Fine et al., 2003; Griffith & Newberry, 2008). In the past, rearing experiments were the only way to link different life stages in Hydrozoa (e.g., Rees & Russell, 1937; Edwards, 1973a, 1973b; Widmer, 2004; Migotto & Cabral, 2005); however, culture procedures are time-consuming and not always successful (e.g., obtaining only immature specimens) (Martin, Chia & Koss, 1983; Freeman & Ridgway, 1990). Molecular species identification methods such as DNA barcoding offer an alternative solution to this problem by providing a rapid approach to correlate separate life stages in siphonophore, anthoathecate and leptothecate hydrozoans (Schuchert, Hosia & Leclére, 2017; Schuchert, 2016; Pyataeva et al., 2016; Grossmann, Lindsay & Collins, 2013; Grossmann, Collins & Lindsay, 2014). Despite recent advancements, our knowledge of hydrozoan life stages is still one of the least complete in all Cnidaria (Boero et al., 1997).
Among the Hydrozoa, Mitrocomidae Haeckel, 1879 and Campanulinidae Hincks, 1868 are two of the taxa with long-lasting taxonomic confusion due to poorly-known life cycles (Cornelius, 1995a). Mitrocomidae is a medusa-based taxon, i.e., it is defined based on morphological characters of the hydromedusa, a stage absent or unknown for most of the campanulinids (Bouillon et al., 2006). Campanulinidae, on the other hand, is defined based on characters present in the polyp stage, which is unknown for the majority of the mitrocomids (Bouillon et al., 2006). When known, most mitrocomid polyps are indistinguishable between species and—with the exception of three species in genera Cyclocanna and Earleria—they are described as “Cuspidella-type” (Cornelius, 1995a; Widmer, Cailliet & Geller, 2010; Schuchert, Hosia & Leclére, 2017), a morphological facies referable to Campanulinidae (Cornelius, 1995a). These polyps are also impossible to differentiate from similar Cuspidella-type polyps belonging to other hydrozoan families such as Laodiceidae and Tiaropsidae (Cornelius, 1995a; Bouillon et al., 2006). Campanulinid hydroids thus pose numerous taxonomic problems and inconsistencies, as the family has traditionally been used as a catch-all taxon for hydroids that release medusae referable to other hydrozoan taxa (Cornelius, 1995a; Bouillon et al., 2006). The complicated relationship between Mitrocomidae and Campanulinidae is only partially understood, as several—but not all—campanulinid hydroids produce mitrocomid hydromedusae (e.g., Schuchert, Hosia & Leclére, 2017), and many medusa-based and polyp-based species in these two families are in need of a description of their complete life cycle (Cornelius, 1995a).
In this contribution, we employ an integrative approach combining morphological, molecular, and ecological information to uncover the connection between the campanulinid polyps of Lafoeina tenuis Sars, 1874 and the mitrocomid hydromedusae of Halopsis ocellata Agassiz, 1865 and Mitrocomella polydiademata (Romanes, 1876). We redescribe the polyp stage of M. polydiademata and present a re-evaluation of the life cycle and taxonomy of these three leptothecate species to resolve part of the incongruences in these hydrozoan taxa.
Materials and Methods
Sampling and DNA work
Individual hydromedusae (Mitrocomella polydiademata and Halopsis ocellata) and hydroid colonies (Lafoeina tenuis) were collected during several sampling events at multiple locations in Norway as part of the Norwegian Taxonomy Initiative projects “Hydrozoan pelagic diversity in Norway (HYPNO)” and “Norwegian marine benthic Hydrozoa (NorHydro)” (Table 1, Fig. 1). The benthic hydroids were either collected with an Agassiz trawl, beam trawl, RP-sledge, Van Veen grab, triangular dredge, or by hand while scuba diving. The plankton samples were collected with either a modified WP3 (750 or 1,000 μm mesh size, non-filtering cod-end), MOCNESS (180 μm), or MIK plankton net. Live hydromedusae were carefully picked from the samples using a light table immediately after collection, and the morphology of each individual was documented with photographs prior to fixation in 96% EtOH. For each hydromedusa and polyp colony, photographs and associated metadata were assembled into electronic vouchers (e-vouchers) connected to the physical specimens deposited in the Invertebrate Collections of the University Museum of Bergen (UMB).
Table 1. List of specimens included in the analysis.
List of specimens of Mitrocomella polydiademata and Halopsis ocellata from NorHydro/HYPNO, and additional sequences used in the analysis from BOLD, GenBank and the University Museum Bergen (UMB). When available, the accession number for 16S and COI are listed. The specimen life stage (LS) is indicated as M (Medusa) or P (polyp).
| Catalogue number (ZMBN) | Definitive ID | Initial ID | LS | Location | Lat/Long | Depth (m) | Substrate | Source | 16S | COI |
|---|---|---|---|---|---|---|---|---|---|---|
| 150925 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.813 N 8.970 E | 190 | Polychaete | This study | OP951085 | OP945752 |
| 150926 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.430 N 8.826 E | 201 | Porifera | This study | OP951086 | OP945753 |
| 150927 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.430 N 8.826 E | 201 | Polychaete | This study | OP951087 | OP945754 |
| 150928 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.589 N 8.559 E | 187 | Polychaete | This study | OP951088 | OP945755 |
| 150929 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.971 N 8.354 E | 210 | Polychaete | This study | OP951089 | OP945756 |
| 150930 | Halopsis ocellata | Lafoeina tenuis | P | Haltenbanken | 64.430 N 8.826 E | 201 | Polychaete | This study | OP951090 | – |
| 150931 | Halopsis ocellata | Lafoeina tenuis | P | Korsfjord | 60.151 N 5.113 E | 680 | Polychaete | This study | OQ031447 | – |
| 150932 | Halopsis ocellata | Lafoeina tenuis | P | Fedje | 60.749 N 4.480 E | 381 | Hydroid | This study | OP951091 | – |
| 150933 | Halopsis ocellata | Halopsis ocellata | M | Raunefjord | 60.257 N 5.139 E | 250–0 | NA | This study | OQ031439 | OQ031460 |
| 150934 | Halopsis ocellata | Halopsis ocellata | M | Korsfjord | 60.184 N 5.195 E | 670–0 | NA | This study | OQ031441 | – |
| 150935 | Halopsis ocellata | Halopsis ocellata | M | Ny Ålesund | 78.920 N 12.18 E | 94–0 | NA | This study | OQ031443 | OQ031464 |
| 150936 | Halopsis ocellata | Halopsis ocellata | M | Korsfjord | 60.151 N 5.099 E | 610–0 | NA | This study | OQ031442 | OQ031463 |
| Halopsis ocellata | Halopsis ocellata | M | Raunefjord | 60.274 N 5.202 E | 20–0 | NA | Schuchert, Hosia & Leclére (2017) | KY363947 | MF000506 | |
| Mitrocomella polydiademata | Lafoeina tenuis | P | Flatevossen | 60.268 N 5.208 E | 30 | Hard substrate | This study | OQ031446 | OQ031467 | |
| 150937 | Mitrocomella polydiademata | Lafoeina tenuis | P | Tvibyrge | 61.338 N 4.853 E | 38 | Halecium sp. | This study | OQ031431 | OQ031467 |
| 150938 | Mitrocomella polydiademata | Lafoeina tenuis | P | Gavlodden | 67.225 N 14.70 E | 33 | Abietinaria sp. | This study | OQ031435 | OQ031451 |
| 150939 | Mitrocomella polydiademata | Lafoeina tenuis | P | Tvibyrge | 61.338 N 4.853 E | 38 | Sertularella sp. | This study | OQ031434 | OQ031455 |
| Mitrocomella polydiademata | M. polydiademata | M | Flatevossen | 60.268 N 5.208 E | 30–0 | NA | This study | OQ031450 | OQ031454 | |
| 150940 | Mitrocomella polydiademata | M. polydiademata | M | North Sea | 57 N 3.65 E | 53–0 | NA | This study | OP951092 | – |
| 150941 | Mitrocomella polydiademata | M. polydiademata | M | Fanafjord | 60.247 N 5.286 E | 150–0 | NA | This study | OP951093 | – |
| 150942 | Mitrocomella polydiademata | M. polydiademata | M | North Sea | 57 N 3.65 E | 53–0 | NA | This study | OQ031437 | OQ031457 |
| 150943 | Mitrocomella polydiademata | M. polydiademata | M | Skagerrak | 58.882 N 9.685 E | 38–0 | NA | This study | OQ031432 | OQ031452 |
| Mitrocomella polydiademata | M. polydiademata | M | Skagerrak | 58.634 N 10.26 E | 292–274 | NA | This study | OQ031445 | OQ031466 | |
| 150944 | Mitrocomella polydiademata | M. polydiademata | M | Skagerrak | 58.882 N 9.685 E | 38–0 | NA | This study | OQ031448 | OQ031468 |
| Mitrocomella polydiademata | M. polydiademata | M | ND | ND | ND | NA | Kayal et al. (2015) | KU710349 | – | |
| Mitrocomella polydiademata | M. polydiademata | M | Fanafjord | 60.240 N 5.229 E | 20–0 | NA | Schuchert, Hosia & Leclére (2017) | KY363949 | MF000508 | |
| Mitrocomella polydiademata | M. polydiademata | M | Scotland | 56.455 N 5.434 W | 0 | NA | Schuchert, Hosia & Leclére (2017) | KY363939 | MF000501 | |
| Mitrocomella polydiademata | M. polydiademata | M | Canada | 58.856 N 94.23 W | ND | NA | GenBank | – | MG423333 | |
| Lafoeina sp. | Lafoeina tenuis | P | Azores | ND | 400–340 | ND | Moura et al. (2012) | JN714673 | – | |
| Earleria panicula | Lafoeina tenuis | P | Sweden | 58.693 N 11.04 E | 130 | ND | BOLD | – | SWEMA1022-15 | |
| Earleria panicula | Campanulina panicula | P | Norway | ND | ND | ND | Leclère et al. (2009) | FJ550511 | – | |
| Cosmetira pilosella | C. pilosella | M | Norway | 60.184 N 5.196 E | 600–0 | NA | Schuchert, Hosia & Leclére (2017) | KY363955 | – | |
| 150945 | Cosmetira pilosella | C. pilosella | M | Norway | 60.184 N 5.196 E | 670–0 | NA | This study | OQ031444 | OQ031465 |
| 150946 | Cosmetira pilosella | C. pilosella | M | Norway | 60.184 N 5.196 E | 670–0 | NA | This study | OQ031436 | OQ031456 |
| Cyclocanna producta | C. producta | P | Norway | 58.755 N 9.656 E | 240 | Hydroid | This study | OQ031433 | OQ031453 | |
| Cyclocanna producta | C. producta | M | Norway | 60.184 N 5.195 E | 670–0 | NA | Schuchert, Hosia & Leclére (2017) | KY570308 | KY570317 | |
| 150947 | Cyclocanna producta | C. producta | M | Norway | 60.257 N 5.139 E | 250–0 | NA | This study | OQ031449 | OQ031469 |
| 150948 | Cyclocanna producta | C. producta | M | Norway | 60.184 N 5.195 E | 670–0 | NA | This study | OQ031438 | OQ031459 |
| 150949 | Earleria panicula | Earleria panicula | M | Norway | 60.184 N 5.195 E | 670–0 | NA | Schuchert, Hosia & Leclére (2017) | KY570303 | KY570312 |
| 150950 | Earleria panicula | Earleria panicula | M | Norway | 60.184 N 5.195 E | 670–0 | NA | Schuchert, Hosia & Leclére (2017) | KY570306 | KY570315 |
| 150951 | Earleria panicula | Earleria panicula | M | Norway | 60.257 N 5.139 E | 250–0 | NA | Schuchert, Hosia & Leclére (2017) | KY570307 | KY570316 |
| 150952 | Earleria panicula | Earleria panicula | P | Norway | 60.162 N 5.176 E | 90 | Porifera | This study | OQ031440 | OQ031461 |
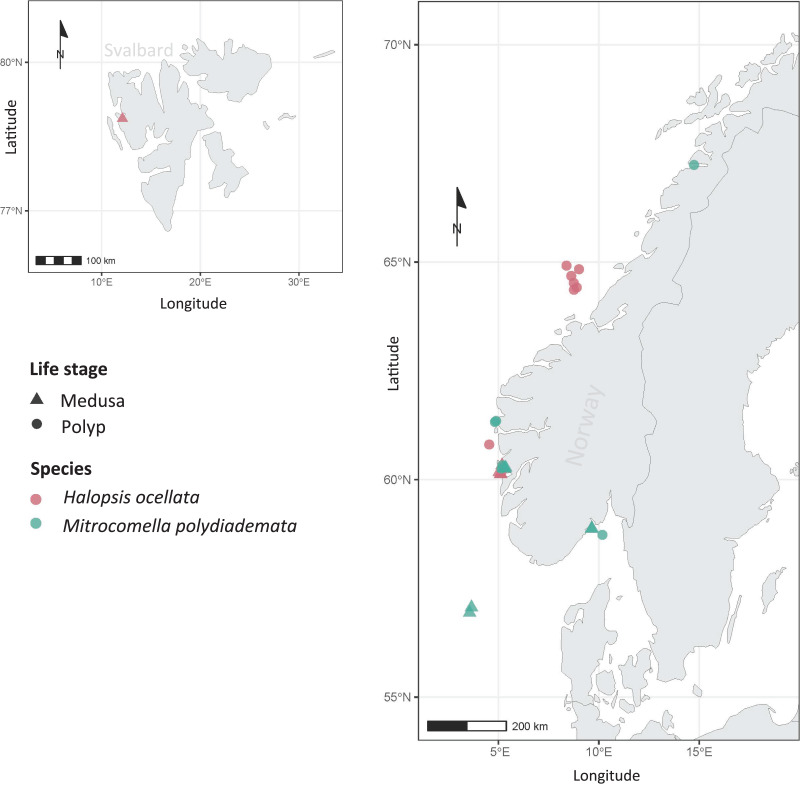
Figure 1. Sampling localities.
Sampling localities for Mitrocomella polydiademata (green) and Halopsis ocellata (red), including both medusa (triangles) and polyp stages (circles). All polyp colonies were initially identified as Lafoeina tenuis based on morphology, but were later re-allocated to their corresponding medusa-based species through the phylogenetic and species delimitation analyses.
DNA was extracted from 2–3 mm3 of soft tissue, selecting either a section of the umbrella margin (for hydromedusae) or 3–6 polyps (for benthic colonies). The samples were either further processed at the DNA lab of the University of Bergen (UiB) or sent to the sequencing facilities of the Canadian Centre for DNA Barcoding (CCDB—Centre for Biodiversity Genomics, University of Guelph). All samples sent to CCDB were processed according to the protocols described by Ratnasingham & Hebert (2007). At UiB, DNA was extracted using the QuickExtract™ DNA Extraction Solution Kit following the protocol described by Nygren et al. (2018). The mitochondrial molecular markers COI and 16S were subsequently amplified for each specimen following the specifications in Table 2. All PCR products were checked by electrophoresis on 1% agarose gels and those that yielded positive bands were then purified with ExoSAP-IT (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Table 2. The PCR specifications used for DNA barcoding.
PCR specifications for COI and 16S. S = number of sites in bp.
| Region | Forward primer | Reverse primer | S | Source | PCR settings |
|---|---|---|---|---|---|
| COI | LCO-1490 5′-GGTCAACAAATCATAAAGATATTGG-3′ |
HCO-2198 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ |
650 | Folmer et al. (1994) | a. 94 °C for 5 min. b. 94 °C for 45 s. c. 45 °C for 30 s. d. 72 °C for 1 min. e. Go to b. and repeat four times f. 94 °C for 45 s. g. 50 °C for 30 s. h. 72 °C for 1 min. i. Go to f. and repeat 30 times j. 72 °C for 10 min |
| 16S | SHA 5′ACGGAATGAACTCAAATCATGT-3′ |
SHB 5′-TCGACTGTTTACCAAAAACAT-3′ |
600 | Cunningham & Buss (1993) | a. 94 °C for 5 min. b. 94 °C for 30 s. c. 50 °C for 30 s. d. 72 °C for 1 min. e. Go to b. and repeat 39 times f. 72 °C for 7 min |
The resulting sequences were blasted against the nucleotide database of the National Centre for Biotechnology Information (NCBI, Bethesda, MD, USA) to check for apparent contaminations, and their chromatograms were visually checked in FinchTV (Geospiza, Inc., Denver, CO, USA) for weak or erroneous bases, which were then replaced using the nucleotide ambiguity code. The software Geneious v.11.1.5 (https://www.geneious.com) was used for contig assembly and generation of consensus sequences.
Phylogenetic analyses
All available COI and 16S sequences labelled as Lafoeina tenuis, Mitrocomella polydiademata, and Halopsis ocellata were mined from the DNA Barcode of Life Database (BOLD, www.boldsystems.org; Ratnasingham & Hebert, 2007) and NCBI GenBank as well as from the UMB hydrozoan database (see Table 1 for a complete overview of the included sequences). This resulted in a final dataset of 32 COI sequences with 658 bases, and 40 16S sequences with 599 bases. As putative and potential outgroups, the following leptothecate taxa were used: for the 16S dataset Hebella venusta (Allman, 1877) and Halisiphonia arctica Kramp, 1932; for the COI dataset Modeeria rotunda (Quoy & Gaimard, 1827); and for both the 16S and COI datasets Ptychogena crocea Kramp & Damas, 1925, Ptychogena lactea Agassiz, 1865 and Staurostoma mertensii (Brandt, 1834). Additionally, several novel sequences for Norwegian specimens of the mitrocomid species Cyclocanna producta (Sars, 1874), Earleria panicula (Sars, 1874), and Cosmetira pilosella Forbes, 1848 were included in the analysis. These potential outgroups and additional taxa were selected based on their phylogenetic position close to Mitrocomidae and Campanulinidae as shown by Maronna et al. (2016).
For each marker, the selected sequences were used to construct a multiple alignment with the program MUSCLE (Edgar, 2004) as implemented in AliView v.1.26 (Larsson, 2014). Both alignments were then checked for obvious errors and their ends were trimmed by a few bases to eliminate poorly aligned flanking regions and achieve similar lengths. For the COI dataset, sequences were additionally translated using the minimally derived genetic code (Mold, Protozoan, and Coelenterate Mitochondrial Code) to check for the presence of stop codons (TAA and TAG for cnidarians). Each alignment was processed separately during phylogenetic reconstruction with a maximum likelihood (ML) approach. All ML analyses were performed using the web server W-IQ-TREE (Trifinopoulos et al., 2016, http://iqtree.cibiv.univie.ac.at/). The substitution models were estimated with the implemented modeltest option and a set FreeRate heterogeneity. The best score models were TIM3+F+G4 and TIM2+F+I for 16S and COI, respectively. The analyses were subsequently run with 5,000 repetitions and the ultrafast bootstrap option (Hoang et al., 2017).
Molecular species delimitation
Molecular species delimitation was performed using two different methods: the Automatic Barcode Gap Discovery (ABGD; Puillandre et al., 2011) and the Bayesian Poisson tree process model (bPTP; Zhang et al., 2013). For both analyses the respective online servers were used with default settings. Reduced alignments (i.e., including all sequences with the exception of the outgroups) for both 16S and COI were used as input for ABGD. The ML trees were used as input for bPTP. In addition, pairwise distances were calculated for both alignments using the K80 (or Kimura’s two-parameter model) with 500 bootstrap replicates in MEGA version 10.2.5 (Kumar et al., 2018) in order to estimate inter- and intra-specific genetic distances. The distances were calculated based on the species hypothesis recovered in the phylogenetic and species delimitation analyses.
Morphological analysis
To characterize the putative species recovered in the phylogenetic and species delimitation analyses, the following eight morphological characters were measured from each hydroid specimen: hydrothecal length, hydrothecal width, nematothecal length, nematothecal width, length and width of undischarged isorhiza capsules, and length and width of undischarged mastigophore capsules. Five replicate measurements of each character were performed per specimen.
Statistical analyses were carried out in RStudio version 1.4.1106 (R Core Team, 2017) to compare the measurements of morphological characters between putative species and to test for significant differences. Since the data were non-parametric and non-homogeneous, a series of Wilcoxon rank sum tests was performed. To explore the variation within specimens, all five measurements per specimen were plotted using the ggplot2 package (Wickham, 2016). A principal component analysis (PCA) was then used to characterize the distribution of the measurements along axes of major variation. For this PCA, all missing data were removed from the morphological dataset and the mean values of the measurements were taken for each specimen. The PCA was generated with the packages factoextra and FactoMineR (Sebastien, Josse & Husson, 2008), using the PCA() function which enables automatic scaling of the units. A Scree plot (Fig. S1) was used to check that the first two dimensions explain >75% of the variation. For visualization, a biplot was created using the fviz_pca_biplot() function (Fig. S2).
Results
A total of 23 specimens belonging to the target taxa were examined in this work. Four of them were morphologically identified as hydromedusae of Halopsis ocellata, seven as hydromedusae of Mitrocomella polydiademata, and twelve were polyp colonies morphologically referable to Lafoeina tenuis. The literature used for morphological identification included original species descriptions and later taxonomic studies (Agassiz, 1865; Sars, 1874; Romanes, 1876; Russell, 1953; Edwards, 1973a; Cornelius, 1995a). The sampling localities for all specimens are shown in Fig. 1.
Phylogenetic analysis and species delimitation
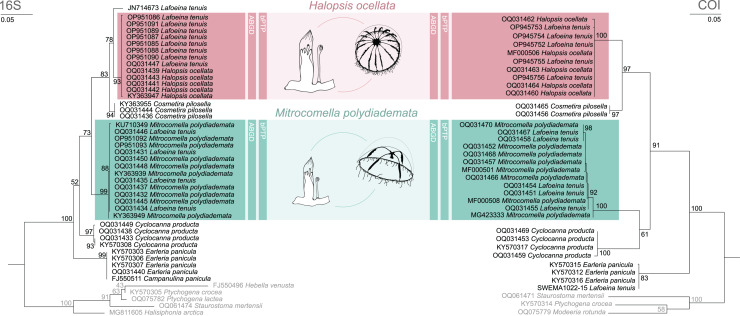
The phylogenetic and species delimitation analyses revealed a mismatch between the genetic identity of the specimens and their morphological species identification. Both 16S and COI trees resulted in one distinct clade with high support values for each of the mitrocomid species Halopsis ocellata and Mitrocomella polydiademata, but each of these clades also included sequences of Lafoeina tenuis which were genetically identical to those obtained from the respective hydromedusae (Fig. 2). Moreover, two additional L. tenuis sequences were recovered outside of these clades. COI sequence SWEMA1022-15 from the BOLD database grouped together with several sequences of Earleria panicula. A closer inspection of the associated voucher image in the BOLD database revealed that the specimen belongs to E. panicula as suggested by the phylogenetic placement of its COI sequence. The sequence is thus considered misidentified and is excluded from further discussion. The 16S sequence JN714673 from GenBank is placed separately from both H. ocellata and M. polydiademata and likely represents a distinct independent clade in the 16S tree. This sequence is closely related to but genetically distinct from the Halopsis ocellata clade. All clades identified in the phylogenetic analyses were recovered as putative species by the molecular species delimitation methods, confirming that H. ocellata forms a cohesive unit with some L. tenuis polyps, while other L. tenuis polyps correspond to the polyp stage of M. polydiademata (Fig. 2).
Figure 2. Maximum likelihood phylogenies for 16S and COI.
Maximum likelihood phylogenies of 16S (left) and COI (right) for the analysed specimens. Bars indicate delimitation results based on ABGD and bPTP. In grey the outgroup taxa. Support values are bootstrap values.
Genetic distances
Genetic distances between identified clades (between species) and intra-clade (within species) are presented in Table 3 (16S) and Table 4 (COI) for the target taxa plus additional mitrocomid species. For 16S (Table 3), mean intraspecific distances (±SE) ranged from 0.00 ± 0.00% in E. panicula and C. pilosella to 0.2 ± 0.13% in C. producta, with an overall mean of 0.06 ± 0.04%. Conversely, mean interspecific distances ranged from 2.5 ± 0.7% (between H. ocellata, L. tenuis JN714673, and C. pilosella) to 8.4 ± 1.3% (between H. ocellata and E. panicula), with an overall mean of 5.8 ± 1.0%. There is a distinct gap in interspecific distances of ca. 5.5% difference between H. ocellata and M. polydiademata, indicating that they are separate species. Within both species there is less than 0.1% genetic difference between the sequences originated from hydromedusae and polyps.
Table 3. Genetic distances for 16S.
Mean K80 pairwise genetic distances within clades (in bold, % ± standard error) and between clades (% ± standard error) for 16S.
| H. ocellata | M. polydiademata | E. panicula | L. tenuis (JN714673) | C. pilosella | C. producta | |
|---|---|---|---|---|---|---|
| Halopsis ocellata | 0.05 ± 0.04 | |||||
| Mitrocomella polydiademata | 5.5 ± 0.9 | 0.05 ± 0.03 | ||||
| Earleria panicula | 8.4 ± 1.3 | 8.1 ± 1.3 | 0 | |||
| Lafoeina tenuis (JN714673) | 2.5 ± 0.7 | 5.5 ± 1.1 | 8.1 ± 1.3 | – | ||
| Cosmetira pilosella | 2.5 ± 0.7 | 5.1 ± 1 | 7.1 ± 1.2 | 2.5 ± 0.7 | 0 | |
| Cyclocanna producta | 6.5 ± 1.1 | 5.3 ± 1 | 6.1 ± 1 | 6.7 ± 1.2 | 6.3 ± 1.1 | 0.2 ± 0.13 |
Table 4. Genetic distances for COI.
Mean K80 pairwise genetic distances within clades (in bold, % ± standard error) and between clades (% ± standard error) for COI.
| H. ocellata | M. polydiademata | E. panicula | C. pilosella | C. producta | |
|---|---|---|---|---|---|
| Halopsis ocellata | 0.1 ± 0.05 | ||||
| Mitrocomella polydiademata | 13.2 ± 1.5 | 0.37 ± 0.13 | |||
| Earleria panicula | 13.4 ± 1.4 | 15.2 ± 1.7 | 0.09 ± 0.09 | ||
| Cosmetira pilosella | 5.5 ± 0.9 | 11.7 ± 1.4 | 13.8 ± 1.6 | 0 | |
| Cyclocanna producta | 10.8 ± 1.3 | 10.2 ± 1.3 | 14 ± 1.6 | 12 ± 1.3 | 0.23 ± 0.13 |
For COI (Table 4) mean intra-species distances (±SE) ranged from 0.00 ± 0.00% in C. pilosella to 0.37 ± 0.13% in M. polydiademata, with an overall mean of 0.15 ± 0.08%. Mean interspecific distances ranged from 5.5 ± 0.9% (H. ocellata and C. pilosella) to 15.2 ± 1.7% (between M. polydiademata and E. panicula), with an overall mean of 11.9 ± 1.42%. There is a distinct gap in interspecific distances of ca. 13.2% difference between H. ocellata and M. polydiademata, indicating that they are separate species. The respective intraspecific distances are less than 0.4%.
Morphological analyses and ecological preferences
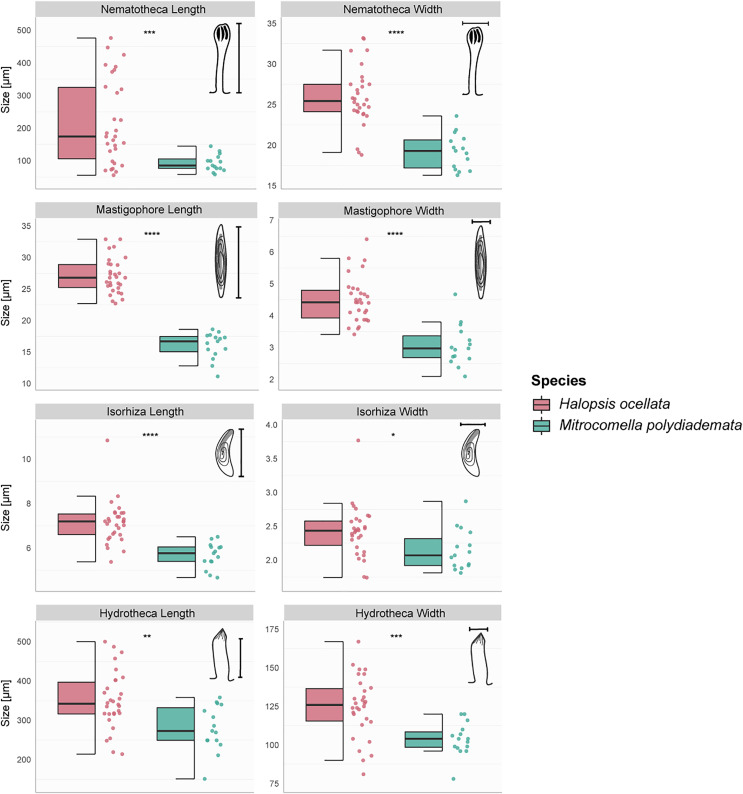
The hydroid colonies in the H. ocellata and M. polydiademata clades differed from each other in hydrothecal, nematothecal, and nematocyst size, as well as substrate preference and depth distribution. The morphometric analyses suggest that the different morphological characters analyzed from the polyp stage of M. polydiademata are, on average, consistently smaller than those of H. ocellata (Table 5, Figs. 3–6). All statistical comparisons showed significant differences between M. polydiademata and H. ocellata polyps, but the range of most characters had at least some overlap between the two groups, preventing characterization of the polyps based solely on their morphological traits (Fig. 3). The analyses identified the length of undischarged mastigophore capsules as the most promising diagnostic morphological character, as the differences observed between colonies of the two species were highly significant and the measurements obtained did not show any degree of overlap.
Table 5. Statistical results for the morphological measurements for polyp characters of Halopis ocellata and Mitrocomella polydiademata.
Mean (±SD) values and Wilcoxon rank sum test W and p values of all evaluated morphological polyp characters for Halopsis ocellata (n = 6) and Mitrocomella polydiademata (n = 3). Five replicate measurements of each character were performed per specimen.
| Character | Mean ± SD (in µm) | W | p value | |
|---|---|---|---|---|
| H. ocellata | M. polydiademata | |||
| Nematotheca length | 217 ± 131 | 93 ± 25 | 361 | 0.001105 |
| Nematotheca width | 26 ± 4 | 19 ± 2 | 424.5 | 1.653e−06 |
| Mastigophore length | 27 ± 3 | 16 ± 2 | 450 | 6.45e−08 |
| Mastigophore width | 4.5 ± 0.7 | 3 ± 0.6 | 421.5 | 2.351e−06 |
| Isorhiza length | 7 ± 1 | 5.7 ± 0.6 | 418.5 | 3.355e−06 |
| Isorhiza width | 2.4 ± 0.4 | 2.2 ± 0.3 | 323.5 | 0.01827 |
| Hydrotheca length | 350 ± 93 | 280 ± 58 | 344 | 0.004317 |
| Hydrotheca width | 125 ± 20 | 104 ± 11 | 370.5 | 0.0004772 |
Figure 3. Boxplots showing the morphological measurements from Halopsis ocellata and Mitrocomella polydiademata polyps.
Variation in eight morphological characters from the polyp stage of Halopsis ocellata (red) and Mitrocomella polydiademata (green). Significance for the Wilcoxon test: ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
The sampling events covered various locations in Norwegian waters for both polyps and medusae (Fig. 1). In this study, H. ocellata was collected from the Arctic Ocean (Svalbard) in the north to the North Sea (Bergen) in the south, while M. polydiademata was collected from the Norwegian Sea (Bodø), the North Sea, and the Skagerrak. Interestingly, the depth of collection for polyps differed between the two species. All polyp specimens assigned to M. polydiademata were collected at relatively shallow waters (≤40 m), whereas all H. ocellata polyps were collected from deeper sites between 187–680 m (Table 1). For M. polydiademata polyps, the substrate was other thecate hydroids (“macrocolonial” colonies such as Sertularella polyzonias, Halecium sp., Abietinaria sp.), algae and inorganic hard substrates, while H. ocellata was found growing mostly on polychaete tubes, but also sponges, and other unidentified thecate hydroids.
The analysis of morphological characters resulted in the following integrative descriptions for the polyp stages of H. ocellata and M. polydiademata:
Halopsis ocellata Agassiz, 1865
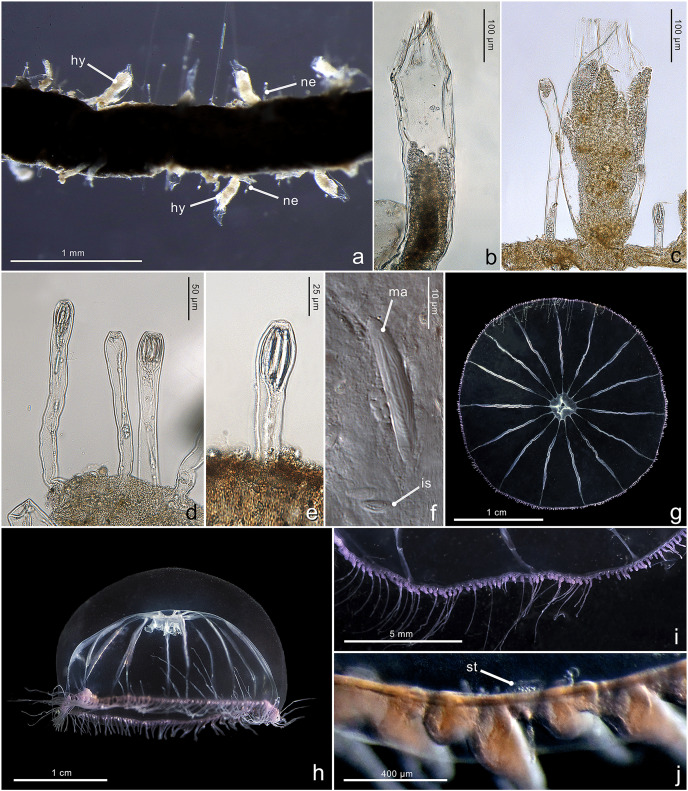
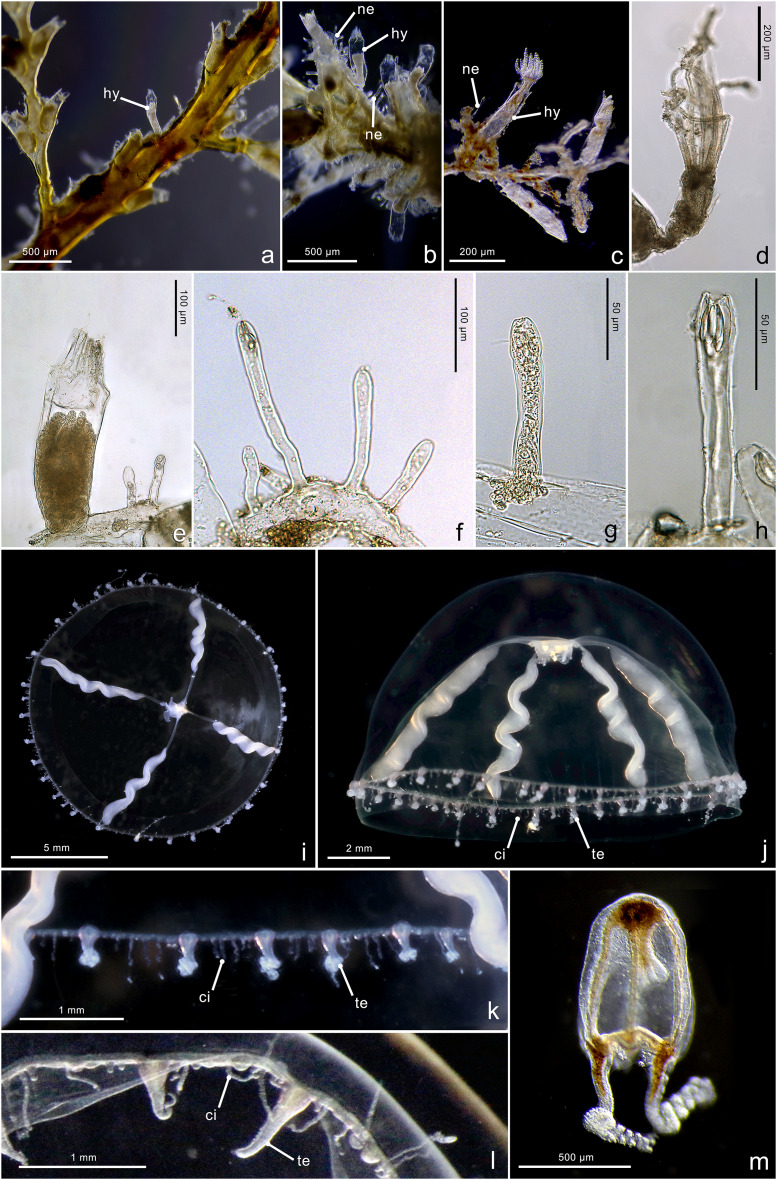
Figure 4. Morphological characters of Halopsis ocellata.
Halopsis ocellata. (A) Colony growing on a polychaete tube. (B, C) Hydrothecae (hy) with polyps. (D, E) Nematothecae (ne). (F) Mastigophore (ma) and Isorhiza (is). (G, H) Adult hydromedusa stage. (I, J) Details of the umbrella margin of the adult hydromedusa including a statocyst (st). Image Credits: Lara M. Beckmann, Fredrik Broms (G–I), Joan J. Soto-Angel.
Material examined: ZMBN150926, ZMBN150927, ZMBN150928, ZMBN150929, ZMBN150930, ZMBN150931 (Table 1).
Description. Colony minute, ‘Lafoeina tenuis’-type, stolonal. Density and distribution of hydrothecae and nematothecae variable within one colony and between different colonies, at times both structures concentrated and numerous, other times relatively separated from each other and with few nematothecae; hydrothecae and nematothecae arising directly from hydrorhiza. Hydrorhiza branching, anastomosing or not, with smooth perisarc, without septae. Hydrotheca 215–500 µm high, 81–167 µm wide, tubular but slightly flared at origin of operculum, straight or slightly curving, sessile, separated from hydrorhiza by basal constriction or less frequently having no evident constriction. Operculum steep, consisting of a folded continuation of the hydrothecal walls, giving the appearance of 9–11 triangular pleats meeting centrally, without basal crease-line in preserved material. Hydranth with approx. 12 tentacles, without intertentacular web, with conical hypostome. Nematotheca 56–476 µm high, 19–33 µm wide, always pedicellate and mostly long and narrow, 1/3 to 1/1 as long as the hydrotheca, often slightly bulbous distally, arising individually throughout the colony, with distal opening. Gonotheca unknown, but gives rise to medusae conforming to the species Halopsis ocellata. Two types of nematocysts identified: mastigophores (length 23–33 µm, width 3.4–6.4 µm) and isorhizas (length 5.4–10.8 µm, width 1.7–3.8 µm).
Substrate. Growing on various substrates, mostly on polychaete tubes, but also on the hydrocaulus of unidentified thecate hydroid colonies, and sponges.
Depth range. Waters below 180 m (polyps in this study were collected from 180–680 m)
Mitrocomella polydiademata (Romanes, 1876).
Figure 5. Morphological characters of Mitrocomella polydiademata.
Mitrocomella polydiademata. (A–C) Colony growing on a large hydroid. (D) Polyp with extended tentacles. (E) Hydrotheca (hy) with polyp and two nematothecae (ne). (F–H) Nematothecae. (I, J) Adult hydromedusa stage. (K, L) Margin details of the adult hydromedusa with cirri (ci) and tentacles (te). (M) Newly released hydromedusa. Image Credits: Lara M. Beckmann, Joan J. Soto-Angel.
Material examined: ZMBN150937, ZMBN150938, ZMBN150939 (Table 1).
Description. Colony minute, ‘Lafoeina tenuis’-type, stolonal. Hydrothecae and nematothecae variable in number and distribution, both inside each colony and between different colonies, always arising directly from hydrorhiza. Hydrorhiza branching, sometimes anastomosing, with smooth perisarc, lacking internal septa. Hydrotheca 151–358 µm high, 78–120 µm wide, tubular but slightly flared at origin of operculum, straight or slightly curving, sessile, most often separated from hydrorhiza by basal constriction but this not always evident. Operculum steep, a continuation of the hydrothecal wall which folds upon itself forming 8–12 pleats meeting centrally, without basal crease-line in either live or preserved material. Hydranth extensile, at least two times the length of the hydrotheca when extended, with 10–12 amphicoronate tentacles, without intertentacular web, with conical hypostome. Nematotheca 58–144 µm high, 16–24 µm wide, always pedicellate, 1/3 as long as the hydrotheca, often slightly bulbous distally, arising individually throughout the colony, with distal opening. Gonotheca unknown, but gives rise to medusae conforming to the species Mitrocomella polydiademata. Two types of nematocysts identified: mastigophores (length 11.1–18.6 µm, width 2.1–4.7 µm) and isorhizas (length 4.7–6.5 µm, width 1.8–2.9 µm).
Substrate. Growing on various substrates, including the hydrocaulus of other thecate hydroids (Sertularella polyzonias, Halcium sp., Abietinaria sp.), red algae, and inorganic hard substrates.
Depth range. Waters above 50 m (polyps in this study were collected from 30–38 m).
Discussion
Hydroid colonies morphologically referable to Lafoeina tenuis constitute the polyp stage of at least two different species of hydromedusae: Halopsis ocellata and Mitrocomella polydiademata. This finding, confirmed by the phylogenetic and molecular delimitation analyses of both COI and 16S markers, results in a series of taxonomic and biogeographic implications that challenge our current understanding of hydrozoan diversity in the North Atlantic.
We present the first description of the previously unknown polyp stage of H. ocellata, as well as the only hitherto known hydroid in genus Halopsis. A large and distinctive mitrocomid hydromedusa, H. ocellata has a mostly disjunct distribution in temperate-cold regions, except for one record from the tropical Pacific (Cely & Chiquillo, 1993) that requires confirmation. It occurs in the North Atlantic and Arctic Oceans, as well as in subantarctic waters in the southwestern Atlantic and southeastern Pacific Oceans (Kramp, 1961; Zelickman, 1972; Genzano, Mianzan & Bouillon, 2008; Oliveira et al., 2016). Many of its records come from moderately deep or oceanic waters, particularly when these occur near the coast as in the Norwegian and Chilean fjords (Russell, 1953; Cornelius, 1995a; Palma et al., 2014). While it has long been assumed that there is a polyp stage in the life cycle of H. ocellata (e.g., Russell, 1953), few attempts have been made to discover its identity. This is probably due to difficulties in rearing the jellyfish in laboratory conditions and the fact that, although relatively common, the medusae are never caught in large enough numbers to allow for extensive experimentation (Russell, 1953; Cornelius, 1995a). Hadzi (1917) speculated that Campanulina hincksii Hartlaub, 1897 could be the polyp of H. ocellata, but this hydroid has since been shown to produce hydromedusae referable to Eucheilota maculata Hartlaub, 1894 (Werner, 1968), and no other candidate polyp species has been seriously considered as a potential match for H. ocellata until now. By describing the polyp stage of a Halopsis species, we reduce the number of genera in Mitrocomidae for which no information exists regarding benthic stages to only one (genus Cosmetirella Browne, 1910).
The uncovered link between M. polydiademata and L. tenuis reveals an incorrect or incomplete description of the polyp stage in the past. The polyp stage of this species was previously described by Edwards (1973a) as Cuspidella-like based on laboratory-reared cultures obtained from medusae collected in the wild. The hydroids of ‘Cuspidella’-type and ‘L. tenuis’-type are morphologically similar, as they differ only in the presence of nematothecae in the latter, and since these structures are sometimes minute and inconspicuous the two nominal taxa can easily be confused (Bouillon, 1971; Migotto & Cabral, 2005). Edwards, however, was a careful observer and an experienced hydrozoologist (Edwards, 1964, 1965, 1973a, 1973b, 1978, 1983), and it is unlikely he would have missed the presence of nematothecae had these occurred in his specimens. Subsequent attempts to culture M. polydiademata seemed to partially confirm his results by obtaining young Cuspidella-type primary polyps (Martin, Chia & Koss, 1983; Freeman & Ridgway, 1990), but a degree of uncertainty persists because fully grown colonies have not been observed so far under laboratory conditions. Edwards collected his medusae on the west coast of Scotland, not far from the type locality of M. polydiademata. The morphology of those medusae matches the original description for the species as well as that of the specimens analyzed here. Consequently, it is unlikely that the different morphologies of the polyp stages for M. polydiademata could have stemmed from a mistaken identity. All known sequences obtained from Scottish and Canadian specimens form a monophyletic clade with the Norwegian specimens, and the species delimitation analyses also support a single species occurring in the entire North-Atlantic Ocean, further suggesting that the differences observed are not likely due to misidentification or the presence of cryptic species.
The lack of nematothecae in laboratory-reared colonies of M. polydiademata could instead be explained as a result of normal ontogenetic development and/or environmentally-induced variation. The colonies of M. polydiademata reared by Edwards remained immature and never developed gonophores (Edwards, 1973a), and all subsequent attempts at rearing polyps from individual medusae have also failed at obtaining mature colonies (Martin, Chia & Koss, 1983; Freeman & Ridgway, 1990). As the development of L. tenuis from planula to reproductive colonies has never been observed, young L. tenuis colonies could go through a Cuspidella-type stage in which nematothecae are not yet developed. Non-reproductive L. tenuis colonies with fully-developed nematothecae are nonetheless commonly encountered in the field (Sars, 1874; Vervoort, 2006; Moura, 2015), suggesting no correlation between the presence of nematothecae and gonothecae. Environmental variability, on the other hand, impacts hydroid morphology and results in polymorphism within colonies (e.g., Dudgeon & Buss, 1996; Griffith & Newberry, 2008; Prudkovsky, Ekimova & Neretina, 2019). Some characters in the polyp may not be expressed when the colonies are grown in the laboratory (Brinckmann-Voss, 1970; Migotto, 1996; Migotto & Cabral, 2005). Nematothecae, for example, did not arise in cultured colonies of Cirrholovenia tetranema Kramp, 1959 obtained from planuloids, and they were scarce or absent in some sections of the hydrorhiza in colonies tied in glass slides (Migotto & Cabral, 2005). Other structures, such as the filiform tentacles of Stauridiosarsia ophiogaster (Haeckel, 1879) and Stauridiosarsia producta (Wright, 1858), are absent from field-collected animals but develop in polyps kept in the laboratory (Brinckmann-Voss, 1970; Migotto, 1996).
Unexpected as it is, the link between L. tenuis polyps and H. ocellata and M. polydiademata medusae is not entirely surprising, as all mitrocomid jellyfish for which the polyp stage is known are produced by campanulinid hydroids. The first reference to a campanulinid polyp stage in Mitrocomidae was made as early as 1886, when the polyps of Mitrocoma annae Haeckel, 1864 were described and morphologically attributed to genus Cuspidella Hincks, 1866 (Metchnikoff, 1886), a taxon of questionable validity since it encompasses a collection of polyps belonging to various leptothecate hydromedusae referable to different unrelated families (Cornelius, 1995a). Metchnikoff further suggested that other taxa within Mitrocomidae might develop similar campanulinid hydroids, which was proven true when the Cuspidella-type polyps of Cosmetira pilosella Forbes, 1848, Mitrocomella brownei (Kramp, 1930), Earleria purpurea (Foerster, 1923), and Mitrocoma cellularia (Agassiz, 1862) were described (Rees & Russell, 1937; Rees, 1941; Widmer, 2004; Widmer, 2011). The polyp stage of the rest of the mitrocomid species with known life cycle is also a campanulinid, albeit not a Cuspidella: Earleria corachloeae Widmer, Cailliet & Geller, 2010, Earleria panicula (Sars, 1874), and Cyclocanna producta (Sars, 1874) possess a Campanulina-type, Racemoramus-type, and Egmundella-type polyp stage, respectively (Widmer, Cailliet & Geller, 2010; Schuchert, Hosia & Leclére, 2017).
Although morphological identification of L. tenuis-type polyps is currently unattainable, at least for the populations in Norwegian waters we have identified several morphological and ecological characters that consistently separate the polyps of H. ocellata from those of M. polydiademata. Too little is known of the variability of these characters in other campanulinid polyps to use them for reliable identification outside of our study area (Calder, 1991; Cornelius, 1995a; Migotto & Cabral, 2005), but our results provide a starting set of hypotheses that will allow researchers to test for morphological differences in small, inconspicuous L. tenuis-type hydroids from other regions. Besides genetic identification, the polyp stages of H. ocellata and M. polydiademata were distinguished by a combination of their preferred habitat and the size of their hydrothecae, nematothecae, and mastigophore capsules, even if the analyzed characters (except for mastigophore length) overlap between the two taxa. In addition, vertical distribution was a good predictor of the species in our dataset, as the polyp stages of these two species apparently occupy different ecological niches in the region. Taken together, these characters allowed us to characterize M. polydiademata as a smaller and shallower species with shorter mastigophore capsules and comparatively small hydrothecae and nematothecae, and predominantly found in shallow waters <50 m in depth. In contrast, H. ocellata is larger, has longer mastigophores capsules, comparatively larger hydrothecae and nematothecae, and occurs in waters deeper than 150 m. Finally, while not as straightforward as the other characters, substrate preference offers an alternative clue for species identity in Norwegian waters, as H. ocellata polyps were more frequently encountered in association with polychaete tubes whereas M. polydiademata polyps were mostly found growing on other thecate hydroids. The size of hydrothecae, nematothecae, and nematocysts are all characters used in the diagnosis of other taxa within Hydrozoa (Cornelius, 1995a, 1995b; Schuchert, 2012), but a thorough evaluation of their usefulness in distinguishing campanulinid and mitrocomid taxa is still lacking.
The existence of at least one 16S sequence from a L. tenuis specimen that does not cluster with either of the two analyzed hydromedusan species suggests that, besides H. ocellata and M. polydiademata, other hydromedusa-based taxa likely possess hydroids morphologically referable to L. tenuis in their life cycle. The geographic distribution of L. tenuis also does not match that of H. ocellata, M. polydiademata, or the combined distribution of these two hydromedusan taxa, supporting the status of the former as a species complex. Lafoeina tenuis is a widespread taxon with confirmed records from the northeastern and northwestern Atlantic Ocean (Sars, 1874; Calder, 2003; Vervoort, 2006; Moura, 2015), as well as the Arctic Ocean (Ronowicz, Kukliński & Mapstone, 2015); but the species has not been reliably observed in subantarctic waters, where H. ocellata occurs (Kramp, 1961; Genzano, Mianzan & Bouillon, 2008), or in the northwestern Pacific Ocean, where M. polydiademata occurs (Arai & Brinkmann-Voss, 1980; Larson, 1987; Mills, 1993). Conversely, L. tenuis is present in tropical and subtropical waters of the Gulf of Mexico and southern Atlantic (Bouzon, Brandini & Rocha, 2012; Mendoza-Becerril, Simões & Genzano, 2018), as well as in the Mediterranean Sea—the latter in the form of the synonymized name Lafoeina vilaevelebiti Hadzi, 1917—(Isinibilir et al., 2015; Topcu et al., 2018; Yilmaz et al., 2020), where no confirmed records of the two hydromedusan species exist. Disentangling the complicated taxonomic history of L. tenuis and testing the potential for cryptic diversity in H. ocellata and M. polydiademata requires a thorough taxonomic revision, an aim that is outside the scope of the present contribution. Until such a revision is made, we suggest that the nominal species Lafoeina tenuis Sars, 1874 be considered a partial synonym of both H. ocellata and M. polydiademata and that polyps morphologically identified to L. tenuis be referred to as ‘Lafoeina tenuis’-type until further associations are resolved. This temporary solution, while not optimal, is analogous to the one currently adopted for genus Cuspidella (Cornelius, 1995a), and has the benefit of incorporating the new findings in the status of these three species while minimizing the potential for a confusing situation in which the name of either hydromedusa is applied to unidentifiable polyps.
Conclusions
Complete descriptions of life cycles are essential to correctly estimate the diversity of cnidarian species in an area, but for most campanulinid and mitrocomid hydrozoans only incomplete accounts exist based on the presence of either the polyp or the medusa stage. In the Atlantic and Arctic Oceans, our results linking L. tenuis polyps to two different hydromedusae call into question the validity of all records of M. polydiademata based solely on polyp morphology (Ramil & Vervoort, 1992; Lundsteen, Hauksson & Gunnarson, 2020), and cast further doubts on the previously suggested synonymy between M. polydiademata and the nominal species Cuspidella grandis Hincks, 1868 (Schuchert, 2022). Our solution to the conundrum posed by H. ocellata, L. tenuis, and M. polydiademata serves as a confirmation that combining DNA barcoding, morphology and ecological information is an effective approach to link inconspicuous stages of marine invertebrates with hitherto unknown life cycles, especially in often-overlooked taxa. Furthermore, disentangling the relationships between these three taxa lays the ground for more robust analyses aimed at resolving the taxonomy and systematics of the enigmatic families Mitrocomidae and Campanulinidae.
Supplemental Information
A line plot of the eigenvalues of factors or principal components used to determine the number of principal components to keep in the principal component analysis (PCA).
Biplot of the principal component analysis based on measurements of eight morphological characters of the newly identified polyp stage of Mitrocomella polydiademata (green) and Halopsis ocellata (red).
Measurement data for polyps of Halopsis ocellata and Mitrocomella polydiademata. Five replicate polyps were measured within one individual for the following characters: Nematocyst length (NTL), nematocyst width (NTW), hydrothecate length (HTL), hydrothecate width (HTW), mastigophore length (MML), mastigophore length (MML), isorhiza length (IL) and isorhiza width (IW).
Acknowledgments
The authors express their thanks to the crew of RV ‘Hans Brattström’, Peter Schuchert, Bernard Picton, Marta Gil, the student diving club at UiB, and project MAREANO for help collecting the samples used in this study. Thanks are also due to Louise Lindblom for her help in the DNA laboratory and to Fredrik Broms for granting permission to use his images of Halopsis ocellata.
Funding Statement
The present study was funded by the Norwegian Biodiversity Information Centre through the Norwegian Taxonomy Initiative projects 70184240/NORHYDRO (LM) and 70184233/HYPNO (AH, LM), in collaboration with the Norwegian Barcode of Life (NorBOL). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Lara M. Beckmann conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Joan J. Soto-Angel conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Aino Hosia conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Luis Martell conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The PCA scree plot, the Biplot, and measurement data of morphological characters, and sequences for 16S and COI are available in the Supplemental Files.
The 16S sequences are available at GenBank: OP951085–OP951093 and OQ031431–OQ031450.
The COI sequences are available at GenBank: OP945752–OP945756 and OQ031451–OQ031470.
The outgroup sequences are available at GenBank: OQ075779–OQ075782; OQ061474 and OQ061471.
Some sequences are available at BOLD: NOHYD168-21; HYPNO121-16; HYPNO144-16; HYPNO241-17; HYPNO327-18, GBCI9686-19; NOHYD024-20; NOHYD022-20; NOHYD127-21; NBCNI182-20; NOHYD125-21; NOHYD023-20; HYPNO256-17; HYPNO001-15; HYPNO296-18; HYPNO006-15; GBCI9688-19; HYPNO332-18; HYPNO180-16; HYPNO292-18; HYPNO179-16; HYPNO304-18; HYPNO339-18; HYPNO136-16; HYPNO150-16; HYPNO156-16; NOHYD020-20.
https://www.boldsystems.org/index.php/Public_RecordView?processid=NOHYD168-21.
References
- Agassiz (1862).Agassiz L. Contributions to the natural history of the United States of America. Vol. 4. Boston: Little Brown; 1862. pp. 1–380. page 350. [Google Scholar]
- Agassiz (1865).Agassiz A. Proceedings of the Boston society of natural history. Vol. 9. Boston: Boston Society of Natural History; 1865. pp. 219–220. [Google Scholar]
- Allman (1877).Allman GJ. Memoirs of the Museum of Comparative Zoology. Vol. 5. Cambridge: Welch, Bigelow, and Company, University Press; 1877. Report on the Hydroida collected during the Exploration of the Gulf Stream by L. F. de Pourtalès, Assistant United States Coast Survey; pp. 1–66. plate 6, Figure. 3–4. [Google Scholar]
- Arai (2005).Arai MN. Predation on pelagic coelenterates: a review. Journal of the Marine Biological Association of the United Kingdom. 2005;85(3):523–536. doi: 10.1017/s0025315405011458. [DOI] [Google Scholar]
- Arai & Brinkmann-Voss (1980).Arai MN, Brinkmann-Voss A. Hydromedusae of British Columbia and Puget Sound. Canadian Bulletin of Fisheries and Aquatic Sciences. 1980;204:1–192. [Google Scholar]
- Ayala et al. (2018).Ayala DJ, Munk P, Lundgreen RBC, Traving SJ, Jaspers C, Jørgensen TS, Hansen LH, Riemann L. Gelatinous plankton is important in the diet of European eel (Anguilla anguilla) larvae in the Sargasso Sea. Scientific Reports. 2018;8(1):6156. doi: 10.1038/s41598-018-24388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boero, Bouillon & Piraino (1992).Boero F, Bouillon J, Piraino S. On the origins and evolution of hydromedusan life cycles (Cnidaria, Hydrozoa) In: Dallai R, editor. Sex Origin and Evolution. Selected Symposia and Monographs U.Z.I., Vol. 6. 1992. pp. 59–68. [Google Scholar]
- Boero et al. (1997).Boero F, Bouillon J, Piraino S, Schmid V. Diversity of hydroidomedusan life cycles: ecological implications and evolutionary patterns. Proceedings of the 6th International Conference on the Coelenterate Biology.1997. [Google Scholar]
- Bouillon (1971).Bouillon J. Sur quelques hydroïdes de Roscoff. Cahiers de Biologie Marine. 1971;12:323–364. [Google Scholar]
- Bouillon et al. (2006).Bouillon J, Gravili C, Pages F, Gili JM, Boreo F. An introduction to Hydrozoa. Mémoires du Muséum national d’Histoire naturelle. 2006;194:1–591. [Google Scholar]
- Bouzon, Brandini & Rocha (2012).Bouzon JL, Brandini FP, Rocha RM. Biodiversity of sessile fauna on rocky shores of coastal islands in Santa Catarina, Southern Brazil. Marine Science. 2012;2(5):39–47. doi: 10.5923/j.ms.20120205.01. [DOI] [Google Scholar]
- Brandt (1834).Brandt JF. Prodromus descriptionis animalium ab H. Mertensio in orbis terrarum circumnavigatione observatorum. Fascic. I., Polypos, Acalephas Discophoras et Siphonophoras, nec non Echinodermata continens. Recueil Actes des séances publiques de l’Acadadémie impériale des Science de St. Pétersbourg. 1834;1834:201–275. doi: 10.5962/bhl.title.10196. [DOI] [Google Scholar]
- Brinckmann-Voss (1970).Brinckmann-Voss A. Anthomedusae/Athecatae (Hydrozoa, Cnidaria) of the Mediterranean. Part I. Capitata. Fauna e Flora del Golfo di Napoli. 1970;39:1–96. [Google Scholar]
- Brooks (1886).Brooks WK. The life-history of the hydromedusae: a discussion of the origin of the medusae and of the significance of metagenesis. Memoirs of the Boston Society of Natural History. 1886;3:359–430. [Google Scholar]
- Browne (1910).Browne ET. Coelenterata V. Medusae. National Antarctic expedition, 1901–1904. Natural History. 1910;5:1–62. doi: 10.5962/bhl.title.14923. plates 1–7, page 32. [DOI] [Google Scholar]
- Calder (1991).Calder DR. Shallow-water hydroids of Bermuda. The Thecatae, exclusive of Plumularioidea. Life Sciences Contributions. 1991;154:1–140. [Google Scholar]
- Calder (2003).Calder DR. Subtidal hydroids (Cnidaria) of Northumberland Strait, Atlantic Canada, with observations on their life cycles and distributions. Canadian Field-Naturalist. 2003;117(4):555–564. doi: 10.22621/cfn.v117i4.824. [DOI] [Google Scholar]
- Cely & Chiquillo (1993).Cely HA, Chiquillo JE. Quetognatos, sifonóforos e hidromedusas de la región costera del Pacífico colombiano. 1993. p. 120. Undergraduate Thesis, Universidad Jorge Tadeo Lozano, Bogotá.
- Cornelius (1977).Cornelius PFS. The linking of polyp and medusa stages in Obelia and other coelenterates. Biological Journal of the Linnean Society. 1977;9(1):45–57. doi: 10.1111/j.1095-8312.1977.tb00258.x. [DOI] [Google Scholar]
- Cornelius (1995a).Cornelius PFS. North-West European thecate hydroids and their medusae—part 1: introduction, laodiceidae and haleciidae. London: Linnean Society of London; 1995a. [Google Scholar]
- Cornelius (1995b).Cornelius PFS. North-West European thecate hydroids and their medusae—part 2: sertulariidae and campanulariidae. London: Linnean Society of London; 1995b. [Google Scholar]
- Cunningham & Buss (1993).Cunningham CW, Buss LW. Molecular evidence for multiple episodes of paedomorphosis in the family hydractiniidae. Biochemical Systematics and Ecology. 1993;21(1):57–69. doi: 10.1016/0305-1978(93)90009-G. [DOI] [Google Scholar]
- Di Camillo et al. (2017).Di Camillo CGD, Bavestrello G, Cerrano C, Gravili C, Piraino S, Puce S, Boero F. Hydroids (Cnidaria, Hydrozoa): a neglected component of animal forests. In: Rossi S, Bramanti L, Gori A, Orejas C, editors. Marine Animal Forests. Berlin: Springer; 2017. [Google Scholar]
- Dudgeon & Buss (1996).Dudgeon SR, Buss LW. Growing with the flow: on the maintenance and malleability of colony form in the hydroid Hydractinia. The American Naturalist. 1996;147(5):667–691. doi: 10.1086/285874. [DOI] [Google Scholar]
- Edgar (2004).Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards (1964).Edwards C. On the hydroids and medusae Bougainvillia pyramidata and B. muscoides. Journal of the Marine Biological Association of the United Kingdom. 1964;44(3):725–752. doi: 10.1017/S0025315400027892. [DOI] [Google Scholar]
- Edwards (1965).Edwards C. The hydroid and the medusa Neoturris pileata. Journal of the Marine Biological Association of the United Kingdom. 1965;45(2):443–468. doi: 10.1017/S0025315400054941. [DOI] [Google Scholar]
- Edwards (1973a).Edwards C. The medusa Mitrocomella polydiademata and its hydroid. Journal of the Marine Biological Association of the United Kingdom. 1973a;53(3):601–607. doi: 10.1017/s0025315400058793. [DOI] [Google Scholar]
- Edwards (1973b).Edwards C. The medusa Modeeria rotunda and its hydroid Stegopoma fastigiatum, with a review of Stegopoma and Stegolaria. Journal of the Marine Biological Association of the United Kingdom. 1973b;53(3):573–600. doi: 10.1017/S0025315400058781. [DOI] [Google Scholar]
- Edwards (1978).Edwards C. The hydroids and medusae Sarsia occulta sp. nov., Sarsia tubulosa and Sarsia loveni. Journal of the Marine Biological Association of the United Kingdom. 1978;58(2):291–311. doi: 10.1017/S0025315400027995. [DOI] [Google Scholar]
- Edwards (1983).Edwards C. The hydroids and medusae Sarsia piriforma sp. nov. and Sarsia striata sp. nov. from the west coast of Scotland, with observations on other species. Journal of the Marine Biological Association of the United Kingdom. 1983;63(1):49–60. doi: 10.1017/S0025315400049791. [DOI] [Google Scholar]
- Fernandez-Leborans (2013).Fernandez-Leborans G. A review of cnidarian epibionts on marine crustacea. Oceanological and Hydrobiological Studies. 2013;42(3):347–357. doi: 10.2478/s13545-013-0092-9. [DOI] [Google Scholar]
- Foerster (1923).Foerster RE. The Hydromedusae of the west coast of North America, with special reference to those of the Vancouver Island Region. Contributions to Canadian Biology and Fisheries. 1923;1a(2):219–277. doi: 10.1139/f22-012. plate 4, page 250, Figures 2–5. [DOI] [Google Scholar]
- Folmer et al. (1994).Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- Forbes (1848).Forbes E. A monograph of the British naked-eyed Medusae: with figures of all the species. London: Ray Society; 1848. plate 8, page 48, Figure 1a–e. [Google Scholar]
- Freeman & Ridgway (1990).Freeman G, Ridgway EB. Cellular and intracellular pathways mediating the metamorphic stimulus in hydrozoan planulae. Roux’s Archives of Developmental Biology. 1990;199(2):63–79. doi: 10.1007/bf02029553. [DOI] [PubMed] [Google Scholar]
- Genzano, Mianzan & Bouillon (2008).Genzano G, Mianzan H, Bouillon J. Hydromedusae (Cnidaria: Hydrozoa) from the temperate southwestern Atlantic Ocean: a review. Zootaxa. 2008;1750(1):1–18. doi: 10.11646/zootaxa.1750.1.1. [DOI] [Google Scholar]
- Gili et al. (1998).Gili AV, Coma R, Orejas C, Pages F, Ribes M, Zabala M, Arntz W, Bouillon J, Boero F, Hughes RG. The impact of small benthic passive suspension feeders in shallow marine ecosystems: the hydroids as an example. Zoologische Verhandelingen. 1998;323:99–105. [Google Scholar]
- Gomes-Pereira et al. (2017).Gomes-Pereira JN, Carmo V, Catarino D, Jakobsen J, Alvarez H, Aguilar R, Hart J, Giacomello E, Menezes G, Stefanni S, Colaço A, Morato T, Santos RS, Tempera F, Porteiro F. Cold-water corals and large hydrozoans provide essential fish habitat for Lappanella fasciata and Benthocometes robustus. Deep Sea Research Part II: Topical Studies in Oceanography. 2017;145(Suppl. 3):33–48. doi: 10.1016/j.dsr2.2017.09.015. [DOI] [Google Scholar]
- Griffith & Newberry (2008).Griffith KA, Newberry AT. Effect of flow regime on the morphology of a colonial cnidarian. Invertebrate Biology. 2008;127(3):259–264. doi: 10.1111/j.1744-7410.2008.00127.x. [DOI] [Google Scholar]
- Grossmann, Collins & Lindsay (2014).Grossmann MM, Collins AG, Lindsay DJ. Description of the eudoxid stages of Lensia havock and Lensia leloupi (Cnidaria: Siphonophora: Calycophorae), with a review of all known Lensia eudoxid bracts. Systematics and Biodiversity. 2014;12(2):163–180. doi: 10.1080/14772000.2014.902867. [DOI] [Google Scholar]
- Grossmann, Lindsay & Collins (2013).Grossmann MM, Lindsay DJ, Collins AG. The end of an enigmatic taxon: Eudoxia macra is the eudoxid stage of Lensia cossack (Siphonophora, Cnidaria) Systematics and Biodiversity. 2013;11(3):381–387. doi: 10.1080/14772000.2013.825658. [DOI] [Google Scholar]
- Hadzi (1917).Hadzi J. Rezultati biolokih istraivanja Jadranskoga mora. Hidroidi II. Halocoryne epizoica g. n. sp. n.; Lafoeina vilae-velebiti sp. n. Prirodoslovna istrazivanja hrvatske i Slavonije. Potaknuta matematicko-prirodoslovnim razredom Jugoslavenske Akademije Znanosti Umjetnosti. 1917;12:1–61. [Google Scholar]
- Haeckel (1864).Haeckel E. Beschreibung neuer Craspedoter Medusen aus dem Golfe von Nizza. Jenaer Zeitschrift für Medizin und Naturwissenschaften. 1864;1:326–342. page 332. [Google Scholar]
- Haeckel (1879).Haeckel E. Das System der Medusen. Erster Teil einer Monographie der Medusen. Denkschriften der Medizinisch-Naturwissenschaftlichen Gesellschaft zu Jena. 1879;1:163. doi: 10.5962/bhl.title.46856. [DOI] [Google Scholar]
- Hartlaub (1894).Hartlaub C. Die Coelenteraten Helgolands. Vorläufiger Bericht. Wissenschaftliche Meeresuntersuchungen. 1894;1(1):161–206. page 193. [Google Scholar]
- Hincks (1866).Hincks T. On new British Hydroida. Annals and Magazine of Natural History. 1866;18(3):296–299. page 298. [Google Scholar]
- Hincks (1868).Hincks T. A history of the British hydroid zoophytes. London: John van Voorst; 1868. [Google Scholar]
- Hoang et al. (2017).Hoang DT, Chernomor O, Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution. 2017;35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isinibilir et al. (2015).Isinibilir M, Martell L, Topçu EN, Yilmaz IN, Piraino S. First inventory of the shallow-water benthic hydrozoan assemblages of Gökçeada Island (northern Aegean Sea) Italian Journal of Zoology. 2015;82(2):281–290. doi: 10.1080/11250003.2014.977970. [DOI] [Google Scholar]
- Kayal et al. (2015).Kayal E, Bentlage B, Cartwright P, Yanagihara AA, Lindsay DJ, Hopcroft RR, Collins AG. Phylogenetic analysis of higher-level relationships within Hydroidolina (Cnidaria: Hydrozoa) using mitochondrial genome data and insight into their mitochondrial transcription. PeerJ. 2015;19(3):e1403. doi: 10.7717/peerj.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramp (1930).Kramp PL. Hydromedusae collected in the South Western part of the North Sea and in the eastern part of the Channel in 1903–1914. Mémoires du Musée royal d’histoire naturelle de Belgique. 1930;45:1–55. page 23, figures 9–11. [Google Scholar]
- Kramp (1959).Kramp PL. Some new and little known Indo-Pacific medusae. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i København. 1959;121:223–259. page 253, figure 17a–b. [Google Scholar]
- Kramp (1961).Kramp PL. Synopsis of the medusae of the world. Journal of the Marine Biological Association of the United Kingdom. 1961;40:7–382. doi: 10.1017/S0025315400007347. [DOI] [Google Scholar]
- Kramp & Damas (1925).Kramp PL, Damas D. Les Méduses de la Norvège. Introduction et partie speciale. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i København. 1925;80:217–323. plate 1, page 290, Figures 1–7. [Google Scholar]
- Kumar et al. (2018).Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson (1987).Larson RJ. Trophic ecology of planktonic gelatinous predators in Saanich Inlet, British Columbia: diets and prey selection. Journal of Plankton Research. 1987;9(5):811–820. doi: 10.1093/plankt/9.5.811. [DOI] [Google Scholar]
- Larsson (2014).Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30(22):3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclère et al. (2009).Leclère L, Schuchert P, Cruaud C, Couloux A, Manuel M. Molecular phylogenetics of thecata (Hydrozoa, Cnidaria) Reveals long-term maintenance of life history traits despite high frequency of recent character changes. Systematic Biology. 2009;58(5):509–526. doi: 10.1093/sysbio/syp044. [DOI] [PubMed] [Google Scholar]
- Lundsteen, Hauksson & Gunnarson (2020).Lundsteen S, Hauksson E, Gunnarson K. Hydrozoan colonization and succession in the tidal and subtidal zones in Surtsey during the period 1967 to 1984. Surtsey Research. 2020;14:131–139. doi: 10.33112/surtsey.14.11. [DOI] [Google Scholar]
- Maggioni et al. (2021).Maggioni D, Schuchert P, Arrigoni R, Hoeksema BW, Huang D, Strona G, Seveso D, Berumen ML, Montalbetti E, Collins R, Galli P, Montano S. Integrative systematics illuminates the relationships in two sponge-associated hydrozoan families (Capitata: Sphaerocorynidae and Zancleopsidae) Contributions to Zoology. 2021;90(4–5):487–525. doi: 10.1163/18759866-bja10023. [DOI] [Google Scholar]
- Maronna et al. (2016).Maronna MM, Miranda TP, Cantero Á.LP, Barbeitos MS, Marques AC. Towards a phylogenetic classification of Leptothecata (Cnidaria, Hydrozoa) Scientific Reports. 2016;6(1):18075. doi: 10.1038/srep18075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, Chia & Koss (1983).Martin V, Chia F, Koss R. A fine structural study of metamorphosis of the hydrozoan Mitrocomella polydiademata. Journal of Morphology. 1983;176(3):261–287. doi: 10.1002/jmor.1051760303. [DOI] [PubMed] [Google Scholar]
- Mendoza-Becerril, Simões & Genzano (2018).Mendoza-Becerril MA, Simões N, Genzano G. Benthic hydroids (Cnidaria, Hydrozoa) from Alacranes Reef, Gulf of Mexico, Mexico. Bulletin of Marine Science. 2018;94(1):125–142. doi: 10.5343/bms.2017.1072. [DOI] [Google Scholar]
- Meroz-Fine et al. (2003).Meroz-Fine E, Brickner I, Loya Y, Ilan M. The hydrozoan coral Millepora dichotoma: speciation or phenotypic plasticity? Marine Biology. 2003;143(6):1175–1183. doi: 10.1007/s00227-003-1135-3. [DOI] [Google Scholar]
- Metchnikoff (1886).Metchnikoff E. Embryologische Studien an Medusen: Ein Beitrag zur Genealogie der Primitivorgane. Vienna, Austria: A. Holder; 1886. [Google Scholar]
- Migotto (1996).Migotto AE. Benthic shallow-water hydroids (Cnidaria, Hydrozoa) of the coast of São Sebastião, Brazil, including a checklist of Brazilian hydroids. Zoologische Verhandelingen, Zootaxa. 1996;306:1–125. [Google Scholar]
- Migotto & Cabral (2005).Migotto AE, Cabral AS. Lafoeina amirantensis (Cnidaria: Hydrozoa, Campanulinoidea), the hydroid stage of the medusa Cirrholovenia tetranema (Cnidaria: Hydrozoa, Lovenelloidea) Zootaxa. 2005;919(1):1–16. doi: 10.11646/zootaxa.919.1.1. [DOI] [Google Scholar]
- Mills (1993).Mills CE. Natural mortality in NE Pacific coastal hydromedusae: grazing predation, wound healing and senescence. Bulletin of Marine Science. 1993;53(1):194–203. [Google Scholar]
- Montano et al. (2015).Montano S, Arrigoni R, Pica D, Maggioni D, Puce S. New insights into the symbiosis between Zanclea (Cnidaria, Hydrozoa) and scleractinians. Zoologica Scripta. 2015;44(1):92–105. doi: 10.1111/zsc.12081. [DOI] [Google Scholar]
- Moura (2015).Moura CJ. The hydrozoan fauna (Cnidaria: Hydrozoa) from the peaks of the Ormonde and Gettysburg seamounts (Gorringe Bank, NE Atlantic) Zootaxa. 2015;3972(2):148–180. doi: 10.11646/zootaxa.3972.2.2. [DOI] [PubMed] [Google Scholar]
- Moura et al. (2012).Moura C, Cunha MR, Porteiro F, Yesson C, Rogers A. Evolution of Nemertesia hydroids (Cnidaria: Hydrozoa, Plumulariidae) from the shallow and deep waters of the NE Atlantic and western Mediterranean. Zoologica Scripta. 2012;41(1):79–96. doi: 10.1111/j.1463-6409.2011.00503.x. [DOI] [Google Scholar]
- Nicholas & Frid (1999).Nicholas KR, Frid CLJ. Occurrence of hydromedusae in the plankton off Northumberland (western central North Sea) and the role of planktonic predators. Journal of the Marine Biological Association of the United Kingdom. 1999;79(6):979–992. doi: 10.1017/s0025315499001216. [DOI] [Google Scholar]
- Nygren et al. (2018).Nygren A, Parapar J, Pons J, Meißner K, Bakken T, Kongsrud JA, Oug E, Gaeva D, Sikorski A, Johansen RA, Hutchings PA, Lavesque N, Capa M. A mega-cryptic species complex hidden among one of the most common annelids in the North East Atlantic. PLOS ONE. 2018;13(6):e0198356. doi: 10.1371/journal.pone.0198356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira et al. (2016).Oliveira OM, Miranda TP, Araujo EM, Ayon P, Cedeno-Posso CM, Cepeda-Mercado AA, Cordova P, Cunha AF, Genzano GN, Haddad MA, Mianzan HW, Migotto AE, Miranda LS, Morandini AC, Nagata RM, Nascimento KB, Júnior MN, Palma S, Quinones J, Rodriguez CS, Scarabino F, Schiariti A, Stampar SN, Tronolone VB, Marques AC. Census of Cnidaria (Medusozoa) and Ctenophora from South American marine waters. Zootaxa. 2016;4194(1):1–256. doi: 10.11646/zootaxa.4194.1.1. [DOI] [PubMed] [Google Scholar]
- Orejas et al. (2001).Orejas C, Gili J, López-González PJ, Arntz W. Feeding strategies and diet composition of four Antarctic cnidarian species. Polar Biology. 2001;24(8):620–627. doi: 10.1007/s003000100272. [DOI] [Google Scholar]
- Palma et al. (2014).Palma S, Córdova P, Silva N, Silva C. Biodiversity and spatial distribution of medusae in the Magellan Region (Southern Patagonian Zone) Latin American Journal of Aquatic Research. 2014;42(5):1175–1188. doi: 10.3856/vol42-issue5-fulltext-21. [DOI] [Google Scholar]
- Prudkovsky, Ekimova & Neretina (2019).Prudkovsky AA, Ekimova IA, Neretina TV. A case of nascent speciation: unique polymorphism of gonophores within hydrozoan Sarsia lovenii. Scientific Reports. 2019;9(1):15567. doi: 10.1038/s41598-019-52026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente-Tapia et al. (2021).Puente-Tapia FA, Garese A, Delpiani SM, Acuña F, Genzano G. Association between the parasitic larvae of the sea-anemone Peachia sp. (Cnidaria: Haloclavidae) and hydromedusae (Cnidaria: Hydrozoa) in the temperate Southwestern Atlantic Ocean. Marine Biodiversity. 2021;51(5):72. doi: 10.1007/s12526-021-01209-5. [DOI] [Google Scholar]
- Puillandre et al. (2011).Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology. 2011;21(8):1864–1877. doi: 10.1111/j.1365-294x.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- Purcell (1989).Purcell JE. Predation on fish larvae and eggs by the hydromedusa Aequorea victoria at a herring spawning ground in British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46(8):1415–1427. doi: 10.1139/f89-181. [DOI] [Google Scholar]
- Pyataeva et al. (2016).Pyataeva SV, Hopcroft RR, Lindsay DJ, Collins AG. DNA barcodes unite two problematic taxa: the meiobenthic Boreohydra simplex is a life-cycle stage of Plotocnide borealis (Hydrozoa: Aplanulata) Zootaxa. 2016;4150(1):85–92. doi: 10.11646/zootaxa.4150.1.5. [DOI] [PubMed] [Google Scholar]
- Quoy & Gaimard (1827).Quoy JRC, Gaimard JP. Observations zoologiques faites à bord de l’Astrolabe, en mai 1826, dans le Détroit de Gibraltar. Annales des Sciences Naturelles. 1827;10:181. plate 6a, page 181, Figure 1–2. [Google Scholar]
- R Core Team (2017).R Core Team . R: a language and environment for statistical computing. Austria, Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Ramil & Vervoort (1992).Ramil F, Vervoort W. Report on the Hydroida collected by the “BALGIM” expedition in and around the Strait of Gibraltar. Nationaal Natuurhistorisch Museum. 1992.
- Ratnasingham & Hebert (2007).Ratnasingham S, Hebert PDN. BOLD: the barcode of life data system (http://www.barcodinglife.org) Molecular Ecology Notes. 2007;7(3):355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees (1941).Rees WJ. IV. The hydroid of the medusa cosmetira pilosella forbes. Proceedings of the Royal Society of Edinburgh, Section B: Biological Sciences. 1941;61(1):55–58. doi: 10.1017/S0080455X00011358. [DOI] [Google Scholar]
- Rees & Russell (1937).Rees WJ, Russell FS. On rearing the hydroids of certain medusae, with an account of the methods used. Journal of the Marine Biological Association of the United Kingdom. 1937;22(1):61–82. doi: 10.1017/s0025315400011875. [DOI] [Google Scholar]
- Romanes (1876).Romanes GJ. An account of some new species, varieties, and monstrous forms of medusae. Journal of the Linnean Society of London, Zoology. 1876;12(64):524–531. doi: 10.1111/j.1096-3642.1876.tb00228.x. [DOI] [Google Scholar]
- Ronowicz, Kukliński & Mapstone (2015).Ronowicz M, Kukliński P, Mapstone GM. Trends in the diversity, distribution and life history strategy of Arctic Hydrozoa (Cnidaria) PLOS ONE. 2015;10(3):e0120204. doi: 10.1371/journal.pone.0120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell (1953).Russell FS. The medusae of the British Isles vol. I: anthomedusae, leptomedusae, limnomedusae, trachymedusae, and narcomedusae. UK: Cambridge University Press; 1953. [Google Scholar]
- Sars (1874).Sars GO. Bidrag til Kundskaben om Norges Hydroider. Forhandlinger i Videnskabs-Selskabet i Kristiana 1873. 1874. https://www.biodiversitylibrary.org/page/43853964 https://www.biodiversitylibrary.org/page/43853964
- Schuchert (2012).Schuchert P. North-West European athecate hydroids and their medusae: keys and notes for the identification of the species. Synopsis of the British Fauna. 2012;59:364. [Google Scholar]
- Schuchert (2016).Schuchert P. The polyps of Oceania armata identified by DNA barcoding (Cnidaria, Hydrozoa) Zootaxa. 2016;4175(6):539–555. doi: 10.11646/zootaxa.4175.6.3. [DOI] [PubMed] [Google Scholar]
- Schuchert (2022).Schuchert P. World hydrozoa database. 2022. https://www.marinespecies.org/hydrozoa. [21 October 2022]. https://www.marinespecies.org/hydrozoa
- Schuchert, Hosia & Leclére (2017).Schuchert P, Hosia A, Leclére L. Identification of the polyp stage of three leptomedusa species using DNA barcoding. Revue suisse de Zoologie. 2017;124:167–182. doi: 10.5281/zenodo.322675. [DOI] [Google Scholar]
- Sebastien, Josse & Husson (2008).Sebastien L, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software. 2008;25(1):1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- Topcu et al. (2018).Topcu NE, Martell LF, Yilmaz IN, Isinibilir M. Benthic hydrozoans as potential indicators of water masses and anthropogenic impact in the Sea of Marmara. Mediterranean Marine Science. 2018;19(2):273–283. doi: 10.12681/mms.15117. [DOI] [Google Scholar]
- Trifinopoulos et al. (2016).Trifinopoulos J, Nguyen LT, Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research. 2016;44(W1):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort (2006).Vervoort W. Leptolida (Cnidaria: Hydrozoa) collected during the CANCAP and Mauritania-II expeditions of the National Museum of Natural History, Leiden, the Netherlands (Anthoathecata, various families of Leptothecata and addenda). CANCAP-project. Contributions, no. 128. Zoologische Mededelingen (Leiden) 2006;80(1):181–318. [Google Scholar]
- Werner (1968).Werner B. Polypengeneration und Entwicklungsgeschichte von Eucheilota maculata (Thecata-Leptomedusae) Helgoländer wissenschaftliche Meeresuntersuchungen. 1968;18:136–168. doi: 10.1007/BF01611672. [DOI] [Google Scholar]
- Wickham (2016).Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016. pp. 65–90. [Google Scholar]
- Widmer (2004).Widmer C. The hydroid and early medusa stages of Mitrocoma cellularia (Hydrozoa, Mitrocomidae) Marine Biology. 2004;145(2):315–321. doi: 10.1007/s00227-004-1322-x. [DOI] [Google Scholar]
- Widmer (2011).Widmer CL. The hydroid and early medusa stages of the deep sea jellyfish Earleria purpurea (Hydrozoa: Mitrocomidae) from the Monterey Bay Submarine Canyon, USA. Zootaxa. 2011;2902(1):59–68. doi: 10.11646/zootaxa.2902.1.3. [DOI] [Google Scholar]
- Widmer, Cailliet & Geller (2010).Widmer CL, Cailliet G, Geller J. The life cycle of Earleria corachloeae n. sp. (Cnidaria: Hydrozoa) with epibiotic hydroids on mid-water shrimp. Marine Biology. 2010;157(1):49–58. doi: 10.1007/s00227-009-1294-y. [DOI] [Google Scholar]
- Wright (1858).Wright TS. Observations on British Zoophytes. Edinburgh new Philosophical Journal (New Series) 1858;7:282–287. page 283. [Google Scholar]
- Yilmaz et al. (2020).Yilmaz IN, Martell L, Topcu NE, Isinibilir M. Βenthic hydrozoan assemblages as potential indicators of environmental health in a Mediterranean Marine protected area. Mediterranean Marine Science. 2020;21(1):36–46. doi: 10.12681/mms.20593. [DOI] [Google Scholar]
- Zelickman (1972).Zelickman EA. Distribution and ecology of the pelagic hydromedusae, siphonophores and ctenophores of the Barents Sea, based on perennial plankton collections. Marine Biology. 1972;17(3):256–264. doi: 10.1007/BF00366301. [DOI] [Google Scholar]
- Zhang et al. (2013).Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29(22):2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A line plot of the eigenvalues of factors or principal components used to determine the number of principal components to keep in the principal component analysis (PCA).
Biplot of the principal component analysis based on measurements of eight morphological characters of the newly identified polyp stage of Mitrocomella polydiademata (green) and Halopsis ocellata (red).
Measurement data for polyps of Halopsis ocellata and Mitrocomella polydiademata. Five replicate polyps were measured within one individual for the following characters: Nematocyst length (NTL), nematocyst width (NTW), hydrothecate length (HTL), hydrothecate width (HTW), mastigophore length (MML), mastigophore length (MML), isorhiza length (IL) and isorhiza width (IW).
Data Availability Statement
The following information was supplied regarding data availability:
The PCA scree plot, the Biplot, and measurement data of morphological characters, and sequences for 16S and COI are available in the Supplemental Files.
The 16S sequences are available at GenBank: OP951085–OP951093 and OQ031431–OQ031450.
The COI sequences are available at GenBank: OP945752–OP945756 and OQ031451–OQ031470.
The outgroup sequences are available at GenBank: OQ075779–OQ075782; OQ061474 and OQ061471.
Some sequences are available at BOLD: NOHYD168-21; HYPNO121-16; HYPNO144-16; HYPNO241-17; HYPNO327-18, GBCI9686-19; NOHYD024-20; NOHYD022-20; NOHYD127-21; NBCNI182-20; NOHYD125-21; NOHYD023-20; HYPNO256-17; HYPNO001-15; HYPNO296-18; HYPNO006-15; GBCI9688-19; HYPNO332-18; HYPNO180-16; HYPNO292-18; HYPNO179-16; HYPNO304-18; HYPNO339-18; HYPNO136-16; HYPNO150-16; HYPNO156-16; NOHYD020-20.
https://www.boldsystems.org/index.php/Public_RecordView?processid=NOHYD168-21.