ABSTRACT
The ability of a foodborne pathogen to tolerate environmental stress critically affects food safety by increasing the risk of pathogen survival and transmission in the food supply chain. Campylobacter jejuni, a leading bacterial cause of foodborne illnesses, is an obligate microaerophile and is sensitive to atmospheric levels of oxygen. Currently, the molecular mechanisms of how C. jejuni withstands oxygen toxicity under aerobic conditions have not yet been fully elucidated. Here, we show that when exposed to aerobic conditions, C. jejuni develops a thick layer of bacterial capsules, which in turn protect C. jejuni under aerobic conditions. The presence of both capsular polysaccharides and lipooligosaccharides is required to protect C. jejuni from excess oxygen in oxygen-rich environments by alleviating oxidative stress. Under aerobic conditions, C. jejuni undergoes substantial transcriptomic changes, particularly in the genes of carbon metabolisms involved in amino acid uptake, the tricarboxylic acid (TCA) cycle, and the Embden-Meyerhof-Parnas (EMP) pathway despite the inability of C. jejuni to grow aerobically. Moreover, the stimulation of carbon metabolism by aerobiosis increases the level of glucose-6-phosphate, the EMP pathway intermediate required for the synthesis of surface polysaccharides. The disruption of the TCA cycle eliminates aerobiosis-mediated stimulation of surface polysaccharide production and markedly compromises aerotolerance in C. jejuni. These results in this study provide novel insights into how an oxygen-sensitive microaerophilic pathogen survives in oxygen-rich environments by adapting its metabolism and physiology.
IMPORTANCE Oxygen-sensitive foodborne pathogens must withstand oxygen toxicity in aerobic environments during transmission to humans. C. jejuni is a major cause of gastroenteritis, accounting for 400 million to 500 million infection cases worldwide per year. As an obligate microaerophile, C. jejuni is sensitive to air-level oxygen. However, it has not been fully explained how this oxygen-sensitive zoonotic pathogen survives in aerobic environments and is transmitted to humans. Here, we show that under aerobic conditions, C. jejuni boosts its carbon metabolism to produce a thick layer of bacterial capsules, which in turn act as a protective barrier conferring aerotolerance. The new findings in this study improve our understanding of how oxygen-sensitive C. jejuni can survive in aerobic environments.
KEYWORDS: Campylobacter, oxygen sensitivity, aerotolerance, surface polysaccharides
INTRODUCTION
Campylobacter jejuni is a leading bacterial cause of foodborne illnesses worldwide (1, 2) and is the primary cause of Guillain-Barré syndrome, an acute and progressive neuromuscular paralysis (3, 4). Although C. jejuni is transmitted to humans mainly through the consumption of contaminated poultry meat (5), the wide distribution of C. jejuni in a range of animal species, including livestock, pets, and wildlife, and the environment makes human campylobacteriosis possible by direct contact with infected animals or exposure to contaminated environmental sources (5–7). As an obligate microaerophile, C. jejuni is sensitive to atmospheric levels of oxygen but still requires low (3 to 10%) levels of oxygen for growth (8, 9).
C. jejuni contains oxygen-sensitive metabolic proteins essential for survival, such as flavodoxin-dependent pyruvate:acceptor oxidoreductase (Por) and 2-oxoglutarate:acceptor oxidoreductase (Oor) (10). These oxygen-sensitive enzymes crucial for carbon metabolism contain iron-sulfur (Fe-S) clusters that are vulnerable to inactivation by reactive oxygen species (ROS) (10). Also, C. jejuni contains oxygen-labile l-serine dehydratase (SdaA), which plays a critical role in carbon catabolism and colonization of the gastrointestinal tract of chickens (10). Moreover, C. jejuni is unable to grow under strict anaerobic conditions (11), because C. jejuni possesses a single class I ribonucleotide reductase, which requires oxygen for the conversion of nucleotides to deoxynucleotides; thus, C. jejuni can not synthesize DNA without oxygen (8). Currently, it is not fully understood how C. jejuni survives during zoonotic transmission generally involving aerobic environments and causes approximately 400 million to 500 million diarrheal cases worldwide per year (1).
Bacterial tolerance to environmental stress significantly increases the risk of human infection by enabling foodborne pathogens to survive during transmission (12, 13). Through a comparative genomic fingerprinting analysis, we have demonstrated that C. jejuni strains tolerant to stress conditions present in food processing are more frequently implicated in human infections than stress-sensitive strains (14). Among various environmental stresses, oxygen toxicity under aerobic conditions is a common stressor that C. jejuni unavoidably encounters during the course of transmission from its animal hosts to humans, whether or not involving food contamination (15). For oxygen-sensitive pathogens, survival under aerobic conditions is a prerequisite to the initiation of infection (16–18). In our previous studies, we have shown that the levels of aerotolerance of C. jejuni isolates from clinical cases and food significantly vary and that C. jejuni strains with increased aerotolerance are highly prevalent in retail raw poultry products (14, 19–22). Moreover, aerotolerant C. jejuni can survive on poultry meat for an extended period compared to oxygen-sensitive C. jejuni (23). Whereas oxygen-sensitive C. jejuni strains lose viability on poultry meat rapidly within a few days, aerotolerant C. jejuni strains can survive ~2 to 4 times longer than oxygen-sensitive strains (23). Furthermore, aerotolerance has also been reported in Campylobacter coli, another important pathogenic species of Campylobacter (24, 25). Aerotolerant C. coli strain OR12 has increased peroxide stress tolerance, is motile under aerobic conditions, and can colonize chicken intestines (24). These data suggest that aerotolerance can directly affect food safety at both pre- and postharvest levels by increasing the survival of Campylobacter in food and the environment and by facilitating horizontal transmission among animal hosts.
Despite the importance of aerotolerance in food safety risks associated with Campylobacter, the molecular mechanisms of aerotolerance are largely unknown, particularly regarding the physiological features underpinning the survival of Campylobacter in aerobic environments. In this study, we demonstrate that under aerobic conditions, C. jejuni stimulates central carbon metabolism to develop a thick layer of bacterial capsules, which can act possibly as a permeability barrier protecting C. jejuni from excess oxygen under aerobic conditions.
RESULTS
Aerobiosis stimulates surface polysaccharide production in C. jejuni.
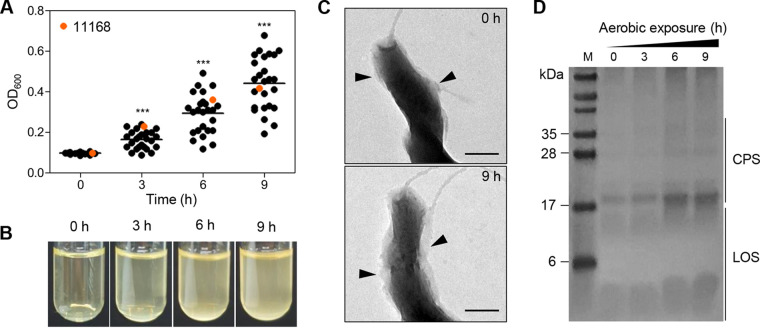
We assessed the aerobic survival of C. jejuni NCTC 11168 and 25 C. jejuni chicken isolates, which were collected from retail poultry in our previous study (21). Interestingly, the optical density at 600 nm (OD600) of aerobic cultures of all the tested strains was markedly increased (Fig. 1A and B) despite the inability of C. jejuni to grow aerobically (see Fig. S1 in the supplemental material). Since the increases in OD600 were not corelated with the CFU in aerobic cultures, we hypothesized that C. jejuni might undergo alterations in bacterial morphology and/or surface structures under aerobic conditions, which may result in OD changes. To examine our hypothesis, transmission electron microscopy (TEM) was conducted to observe the morphology of C. jejuni cells from aerobic cultures in combination with alcian blue staining to visualize surface polysaccharides (26). C. jejuni NCTC 11168, the first whole-genome-sequenced strain of Campylobacter (27), was used to investigate the molecular mechanisms of aerotolerance in the rest of the study. Remarkably, aerobiosis led to the formation of a thick layer of bacterial capsules in C. jejuni (Fig. 1C). Alcian blue staining analyses confirmed that the production of capsular polysaccharide (CPS) and lipooligosaccharide (LOS) was increased over time after aerobiosis (Fig. 1D). These results suggest that under aerobic conditions, C. jejuni increases the production of surface polysaccharides, which can affect the measurement of OD600 under aerobic conditions.
FIG 1.
Stimulation of CPS and LOS synthesis by aerobiosis in C. jejuni. (A) The OD600 of C. jejuni NCTC 11168 and 25 C. jejuni isolates from retail chicken under aerobic conditions. C. jejuni strains were incubated with shaking at 200 rpm under aerobic conditions for 9 h. Solid horizontal lines indicate average OD600 values. Statistical significance was determined with Student’s t test compared to the OD600 of the previous time point, ***, P < 0.001. (B) Bacterial culture images of C. jejuni NCTC 11168 over the time of exposure to aerobic conditions. (C) Transmission electron microscopic (TEM) images of C. jejuni NCTC 11168 before and after exposure to aerobic conditions for 9 h. Black triangles indicate surface polysaccharides stained with alcian blue. Scale bars = 200 nm. (D) Alcian blue staining of CPS and LOS in C. jejuni NCTC 11168 after exposure to aerobic conditions. M, marker.
Disruption of surface polysaccharide integrity with EDTA decreases the optical density and compromises aerotolerance in C. jejuni.
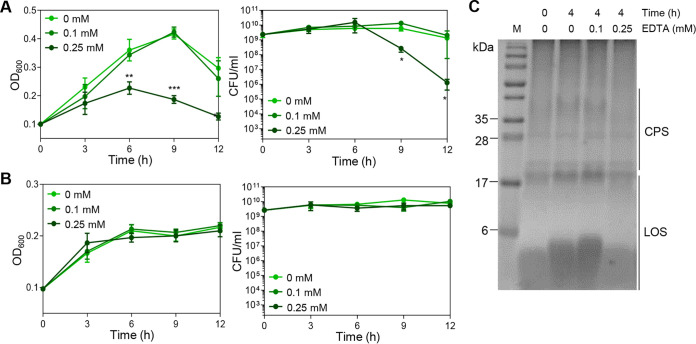
To evaluate whether OD600 increases in aerobic cultures are associated with surface polysaccharide synthesis, C. jejuni NCTC 11168 was subjected to aerobiosis in the presence of EDTA to disrupt the integrity of surface polysaccharides by chelating divalent ions. The bacterial surface of C. jejuni is decorated with CPS and LOS, a truncated version of lipopolysaccharide (LPS) lacking O-antigen (28). In Gram-negative bacteria, divalent ions are involved in maintaining the structural integrity of bacterial surfaces by cross-linking phosphate groups in the lipid anchor of CPS and the inner core of LPS (29–31). Notably, the supplementation of C. jejuni cultures with 0.25 mM EDTA significantly reduced the OD600 and CFU under aerobic conditions compared to a non-treated control (Fig. 2A). Consistent with a previous report (19), C. jejuni NCTC 11168 lost viability after exposure to aerobic stress for 24 h (data not shown). Under microaerobic conditions, 0.25 mM EDTA did not alter the OD600 and CFU (Fig. 2B), indicating that EDTA decreases the OD600 and CFU only in aerobic cultures. Furthermore, EDTA treatment depleted surface polysaccharides (Fig. S2) and decreased the abundance of CPS and LOS under aerobic conditions (Fig. 2C). Together, these results suggest that the stimulated production of surface polysaccharides by aerobiosis increases the OD600 and affects aerotolerance in C. jejuni.
FIG 2.
Compromised aerotolerance by disrupting the integrity of surface polysaccharides with EDTA. (A and B) The OD600 and CFU of C. jejuni NCTC 11168 under aerobic (A) and microaerobic (B) conditions in MH broth supplemented with different concentrations of EDTA. The data present the means and the standard errors of the mean (SEM) of the results of three independent experiments. Statistical significance was determined with Student’s t test compared to a nontreated sample at the same time point; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Alcian blue staining images of CPS and LOS in C. jejuni NCTC 11168 according to exposure time under aerobic conditions in the presence and absence of EDTA. M, marker.
Both CPS and LOS are required for aerotolerance in C. jejuni.
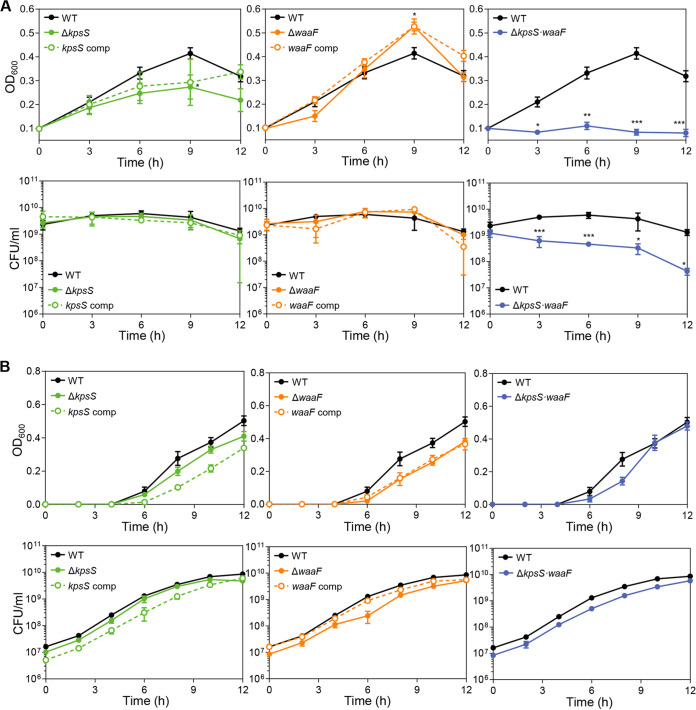
After examining the involvement of surface polysaccharides in aerotolerance with EDTA (Fig. 2), we validated this finding using the mutants defective in the production of surface polysaccharides. The synthesis of CPS and LOS is a complicated process involving a number of genes. A knockout mutation of a gene associated with the early step of CPS and LOS biosynthesis can lead to defects in CPS and LOS production. For this, we disrupted the synthesis of CPS and LOS by deleting kpsS and waaF, respectively (32–34). The kpsS gene encodes an enzyme responsible for the production of the 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) linker on the terminal lipid of CPS (35). A knockout mutation of waaF, which encodes the heptosyltransferase II enzyme, results in a truncation of the core oligosaccharide (32, 36). We measured the OD600 and viability of these mutants under aerobic conditions. The OD600 increase in the aerobic cultures of a CPS (ΔkpsS) mutant was less than that of the wild type (WT; Fig. 3A and Fig. S3). Aerobic exposure did not diminish the OD600 of the cultures of an LOS (ΔwaaF) mutant but, rather, increased it (Fig. 3A and Fig. S3), presumably because the lack of LOS resulted in auto-agglutination of bacterial cells exposing hydrophobic lipid membranes (34, 37). A double knockout mutation of kpsS and waaF eliminated the OD600 increases under aerobic conditions (Fig. 3A and Fig. S3). However, the OD600 of the ΔkpsS/ΔwaaF double mutant was comparable to that of the WT under microaerobic conditions (Fig. 3B). Remarkably, the ΔkpsS/ΔwaaF double mutant showed a substantial defect in aerotolerance (Fig. 3A).
FIG 3.
Compromised aerotolerance in mutants defective in CPS and LOS synthesis. The (A and B) OD600 and CFU of C. jejuni mutants defective in the synthesis of surface polysaccharides after aerobic (A) and microaerobic (B) cultivation with shaking at 200 rpm. Statistical significance was determined with Student’s t test compared to the values of the wild type (WT; C. jejuni NCTC 11168) at the same time point (*, P < 0.05; **, P < 0.01; ***; P < 0.001). A ΔkpsS mutant (ΔkpsS), a kpsS-complemented strain (kpsS comp), a ΔwaaF mutant (ΔwaaF), a waaF-complemented strain (waaF comp), and a ΔkpsS/ΔwaaF double mutant (ΔkpsS·waaF).
Genetic complementation in the ΔkpsS mutant and ΔwaaF mutant resulted in the recovery of aerotolerance. The phenotype recovery in these mutants by complementation was partial under some conditions (Fig. 3A), presumably because the transcription of the complemented genes was driven by different promoters. For instance, kpsS exists in an operon, and its transcription is modulated by its operon promoter; however, kpsS transcription in the complementation strain is controlled by the constitutively expressing promoter of the antibiotic resistance cassette (38). Additionally, a strain complemented with both kpsS and waaF could not be constructed in the ΔkpsS/ΔwaaF mutant because of the limited availability of antibiotic resistance markers in Campylobacter research. The two antibiotic-selective markers (i.e., kanamycin and tetracycline resistance markers) were already used to construct the ΔkpsS/ΔwaaF double mutant, leaving only the chloramphenicol resistance marker available. Due to this technical issue, we could test phenotype recovery by complementing the ΔkpsS/ΔwaaF double mutant only with a single gene, either kpsS or waaF. The complementation of the double mutant with a single gene partially restored the OD600 increases and CFU under aerobic conditions (Fig. S4). Nevertheless, these results from these knockout mutants and their complementation strains collectively confirm that the OD600 increase in the aerobic cultures of C. jejuni is associated with aerobiosis-mediated stimulation of surface polysaccharide production, which contributes to aerotolerance in C. jejuni.
Carbon metabolism genes are up-regulated in C. jejuni under aerobic conditions.
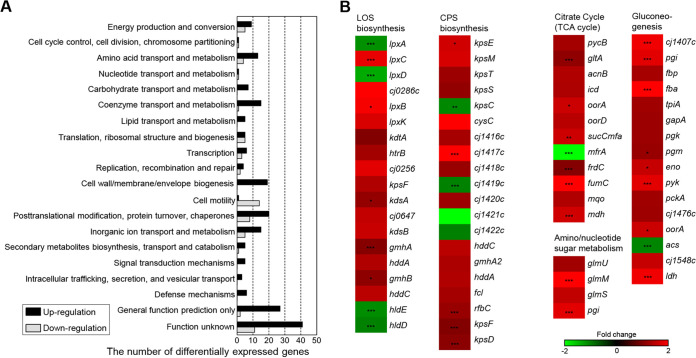
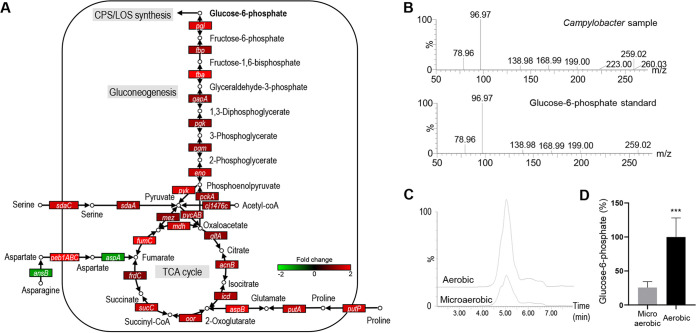
Next, we wondered whether aerobiosis may alter the transcription of genes of CPS and LOS synthesis. To answer this question, we performed transcriptome sequencing (RNA-Seq) to measure transcriptomic changes under aerobic conditions. The analysis revealed that the transcription of 16.5% (271/1,643) of the total genes of C. jejuni NCTC 11,168 was altered by aerobiosis compared to the transcriptomic profile of microaerobic cultures (Fig. 4A and Table S1). Among the differentially expressed genes under aerobic conditions, 208 of them were up-regulated, while 63 were down-regulated (Fig. 4A and Table S1). The genes associated with energy production and conversion, nutrient transport and metabolism, cell envelope biogenesis, posttranslational modification, protein turnover, chaperones, and signal transduction mechanisms were up-regulated under aerobic conditions, whereas the genes involved in cell motility were generally down-regulated (Fig. 4A and Table S1). Several oxidative stress defense genes, including ahpC (alkyl hydroperoxide reductase), sodB (superoxide dismutase), cosR (Campylobacter oxidative stress regulator), and perR (peroxide response regulator), were up-regulated in response to exposure to aerobic conditions (Table S1). Consistent with increases in the production of surface polysaccharides by aerobiosis, the genes related to CPS and LOS synthesis were up-regulated in aerobic cultures (Fig. 4B). Additionally, aerobiosis up-regulated genes encoding the enzymes of the TCA cycle and gluconeogenesis (Fig. 4B). These results show that aerobiosis up-regulates the genes of oxidative stress, CPS and LOS synthesis, and carbon metabolism in C. jejuni.
FIG 4.
Transcriptomic changes in C. jejuni under aerobic conditions. (A) Differentially expressed genes in C. jejuni under aerobic conditions based on RNA-Seq. The fold change was determined by comparing the transcriptional levels between aerobic and microaerobic conditions. The black and gray bars indicate genes up-regulation and down-regulation, respectively, after aerobic exposure. (B) Heat maps of the genes associated with CPS and LOS biosynthesis and carbon metabolism under aerobic conditions. The heat maps were constructed with Gitools.
Production of precursors for surface polysaccharides was increased under aerobic conditions.
Aerobiosis upregulates the genes of central carbon metabolism (Fig. 4B). Given the inability of aerobic growth of C. jejuni, the purpose of boosting carbon metabolism under aerobic conditions is not likely to promote bacterial growth but possibly to produce precursors for the synthesis of surface polysaccharides. Due to the lack of the genes encoding glucokinase (Glk) and phosphofructokinase (Pfk) of the Embden-Meyerhof-Parnas (EMP) pathway (27), C. jejuni is unable to catabolize glucose and primarily relies on amino acids and the TCA cycle intermediates as carbon sources (39–42). Using the remaining EMP pathway enzymes, C. jejuni synthesizes glucose and EMP pathway intermediates through gluconeogenesis, which is the only mechanism for C. jejuni to produce precursors for surface polysaccharides (43). In particular, glucose-6-phosphate generated by gluconeogenesis is the ultimate EMP pathway intermediate required to produce UDP-N-acetylglucosamine (UDP-GlcNAc) and sedoheptulose-7-phosphate (Fig. S5) for the synthesis of surface polysaccharides (Fig. 5A). We compared the intracellular levels of glucose-6-phosphate between the aerobic and microaerobic cultures of C. jejuni and found that aerobiosis significantly increased the level of glucose-6-phosphate by 3.9-fold more than microaerobiosis (Fig. 5B to D). Taken together, these results suggest that alterations in carbohydrate metabolism by aerobiosis increase the level of glucose-6-phosphate to supply precursors for surface polysaccharide synthesis under aerobic conditions.
FIG 5.
Increased production of precursors for surface polysaccharide synthesis under aerobic conditions. (A) Upregulation of the genes of the tricarboxylic acid (TCA) cycle and the Embden-Meyerhof-Parnas (EMP) pathway in C. jejuni NCTC 11168 under aerobic conditions. Fold change was determined by comparing the transcriptional levels between aerobic and microaerobic conditions. (B) Identification of glucose-6-phosphate in C. jejuni NCTC 11168 extract through comparison with the fragmentogram of its authentic standard. (C) Overlaid LC-MS chromatograph showing increased production of glucose-6-phosphate under aerobic conditions compared to microaerobic conditions. (D) Comparison of the levels of glucose-6-phosphate between aerobic and microaerobic cultures. The MS signals of glucose-6-phosphate in aerobic samples were arbitrarily set as 100%. Statistical significance was assessed with Student’s t test (***, P < 0.001).
The TCA cycle is involved in aerobiosis-mediated induction of surface polysaccharide synthesis and aerotolerance in C. jejuni.
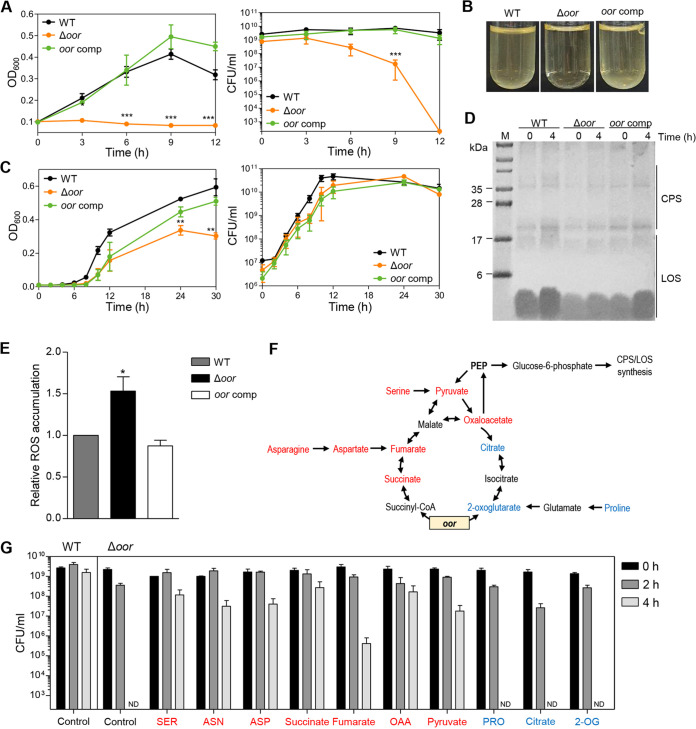
Several genes encoding the enzymes of the TCA cycle were up-regulated under aerobic conditions (Fig. 5A). Amino acids taken up by C. jejuni are integrated into carbohydrate metabolism after enzymatic conversion to the TCA cycle intermediates, which is the major metabolic pathway to absorb carbon sources in C. jejuni (43). The TCA cycle is the essential metabolism that provides precursors to the EMP pathway for gluconeogenesis, which produces glucose-6-phosphate for the synthesis of surface polysaccharides (Fig. 5A). We wondered about the role of the TCA cycle in the stimulation of surface polysaccharide synthesis under aerobic conditions and aerotolerance in C. jejuni. Thus, we evaluated the association of the TCA cycle with aerobiosis-mediated induction of surface polysaccharide synthesis. As reported in previous studies (44, 45), we observed that aerobiosis up-regulated oorDABC (Fig. 5A) encoding 2-oxoglutarate: acceptor oxidoreductase (Oor) that decarboxylates 2-oxoglutarate to succinyl-CoA (46, 47). The increased transcription level of oorDABC under aerobic conditions was confirmed with quantitative real-time PCR (qRT-PCR) (Fig. S6). A knockout mutation of oor eliminated the aerobiosis-mediated induction of the OD600 increase and significantly reduced the viability of C. jejuni under aerobic conditions (Fig. 6A and B). As opposed to obvious changes in the OD600 under aerobic conditions (Fig. 6A), the disruption of oor slightly reduced the OD600 of C. jejuni cultures under microaerobic conditions (Fig. 6C). The complementation of the Δoor mutant with an intact copy of oor did not restore the OD600 until 12 h but increased it close to the WT level after extended incubation under microaerobic conditions (Fig. 6C). Presumably, oor expression and/or regulation may differ between aerobic and microaerobic conditions. Consistently, the production of surface polysaccharides was not stimulated by aerobiosis in the Δoor mutant (Fig. 6D and Fig. S7). Furthermore, the accumulation of total reactive oxygen species (ROS) was significantly increased in the Δoor mutant under aerobic conditions compared to that in the WT (Fig. 6E), which accounts for compromised aerotolerance in the Δoor mutant.
FIG 6.
Defects in surface polysaccharide synthesis and aerotolerance in an Δoor mutant. (A) The OD600 and CFU of the wild type (WT; C. jejuni NCTC 11168), an Δoor mutant, and an oor-complemented strain in MH broth under aerobic conditions are shown. The data present the means and the standard errors of the mean (SEM) of the results of three experiments. Statistical significance was determined with Student’s t test compared to WT at the same time point (**, P < 0.01; ***, P < 0.001). (B) Bacterial culture images of the WT, an Δoor mutant, and an oor-complemented strain under aerobic conditions for 4 h. (C) The OD600 and CFU of microaerobic cultures of WT, an Δoor mutant, and an oor-complemented strain in MH broth. The data present the means and SEM of the results of three experiments. Statistical significance was determined with Student’s t test compared to the WT at the same time point (**, P < 0.01). (D) Surface polysaccharides under aerobic conditions in the WT, an Δoor mutant, and an oor-complemented strain. The image shows alcian blue staining of CPS and LOS in the WT, an Δoor mutant, and an oor-complemented strain before/after aerobiosis. M, marker. (E) Relative levels of the total ROS in the WT, an Δoor mutant, and an oor-complemented strain after aerobiosis for 4 h. The level of the WT was set as 1. The data present the means and SEM of the results of six experiments. Statistical significance was determined by Student’s t test compared to the WT (*, P < 0.05). (F) A simplified model of the TCA cycle and gluconeogenesis in C. jejuni. Carbon sources increasing aerotolerance in the Δoor mutant are indicated in red, and those that did not are in blue. (G) Aerotolerance of WT (control) and an Δoor mutant in the presence of amino acids and TCA cycle intermediates. The CFU levels of the WT and the Δoor mutant were determined under aerobic conditions for 4 h with shaking (200 rpm) in MEMα with or without (control) supplemental carbon sources. SER, serine; ASN, asparagine; ASP, aspartate; OAA, oxaloacetate; PRO, proline; 2-OG, 2-oxoglutarate; ND, not detected. The data present the means and SEM of the results of at least three experiments. For all data except for F, an Δoor mutant (Δoor) and an oor-complemented strain (oor comp) were used.
The association of the TCA cycle with aerotolerance was evaluated in an alternative way by chemically complementing the Δoor mutant with TCA cycle intermediates and amino acids. Notably, the aerotolerance of the Δoor mutant was restored when the Δoor mutant was cultured on the minimum essential medium (MEM) α supplemented with various amino acids and TCA cycle intermediates, which can be converted to phosphoenolpyruvate (PEP; Fig. 6F). Some carbon sources, such as serine, asparagine, aspartate, succinate, oxaloacetate, and pyruvate, restored aerotolerance to levels close to that of the WT (Fig. 6G). These compounds are commonly involved in steps following the process mediated by Oor (Fig. 6F and G). However, aerotolerance was not restored by supplementing with proline, citrate, and 2-oxoglutarate, which are associated with the TCA cycle steps preceding the Oor step (Fig. 6F and G). These data suggest that the stimulated TCA cycle under aerobic conditions is required to facilitate surface polysaccharide synthesis and aerotolerance in C. jejuni.
Oxidative stress is increased in mutants defective in surface polysaccharide production under aerobic conditions.
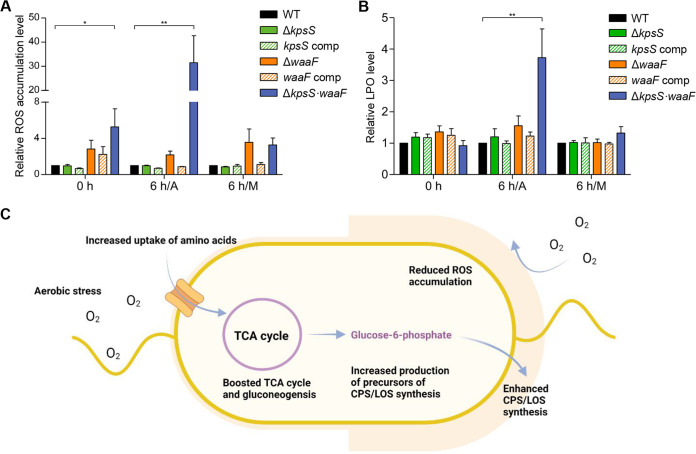
The disruption of the TCA cycle reduces surface polysaccharide synthesis and increases oxidative stress (Fig. 6D and E). This indicates that the presence of surface polysaccharides can increase aerotolerance by alleviating oxidative stress. To examine this possibility, we measured the levels of total ROS and lipoperoxide (LPO) in mutants defective in the biosynthesis of CPS (ΔkpsS), LOS (ΔwaaF), and both (ΔkpsS and ΔwaaF). Compared to a CPS (ΔkpsS) mutant, an LOS (ΔwaaF) mutant showed increased levels of total ROS under aerobic and microaerobic conditions (Fig. 7A). Notably, ROS and LPO were significantly accumulated in the ΔkpsS/ΔwaaF double mutant under aerobic conditions (Fig. 7A and B). Complementation of the ΔkpsS/ΔwaaF double mutant with either kpsS or waaF partially decreased the level of ROS accumulation under aerobic conditions and reduced it to the WT level under microaerobic conditions (Fig. S8), indicating that CPS and LOS are required to alleviate oxidative stress mainly under aerobic conditions. These results suggest that the presence of CPS and LOS confers aerotolerance by protecting C. jejuni from oxidative damage under aerobic conditions.
FIG 7.
Contribution of CPS and LOS to aerotolerance by alleviating oxidative stress. (A and B) Relative levels of the total ROS accumulation (A) and lipoperoxide (LPO) (B) of C. jejuni strains before/after exposure to aerobic and microaerobic conditions for 6 h. The levels of ROS and LPO in the WT were set as 1. A, aerobic conditions; M, microaerobic conditions. Data for a ΔkpsS mutant (ΔkpsS), a kpsS-complemented strain (kpsS comp), a ΔwaaF mutant (ΔwaaF), a waaF-complemented strain (waaF comp), and a ΔkpsS/ΔwaaF double mutant (ΔkpsS waaF) are shown. The data present the means and the standard errors of the mean (SEM) of the results of three experiments. Significance was assessed using one-way ANOVA (*, P < 0.05; **, P < 0.01; ns; not significant). (C) Stimulation of carbon metabolism and surface polysaccharide synthesis under aerobic conditions protects C. jejuni from excess oxygen in aerobic environments.
DISCUSSION
Aerotolerance is the critical mechanism for sustaining the viability of oxygen-sensitive C. jejuni in aerobic environments (48, 49). The aerobic survival of C. jejuni can be mediated by other mechanisms, such as biofilm development and the viable-but-non-culturable state; however, these survival mechanisms generally necessitate extensive physiological changes in C. jejuni, such as the physiological transition from planktonic to sessile lifestyle and from a culturable to nonculturable state (50–52). Regarding the molecular mechanism of aerotolerance, oxidative stress responses involving the ROS-detoxification enzymes, particularly alkyl hydroperoxide reductase (AhpC), are the key determinant of aerotolerance in C. jejuni (48, 49). In line with this, C. jejuni responds to oxidative stress differentially depending on the strain, which appears to determine whether a C. jejuni strain is oxygen sensitive or aerotolerant (22, 24, 44). Previously, we measured the activities of catalase and superoxide dismutase in 70 C. jejuni isolates from retail chicken and discovered that aerotolerant C. jejuni strains were more tolerant to peroxide and superoxide stress than oxygen-sensitive strains (22). C. jejuni strain Bf, an aerotolerant strain isolated from a clinical case, exhibits increased tolerance to oxidative stress and can better survive in the presence of oxidants compared to a reference strain (44). In addition, aerotolerant C. coli strains are more frequently isolated from retail food products than aerotolerant C. jejuni (25). Interestingly, the prevalence of aerotolerant C. coli is associated with the presence of catalase-like protein in C. coli (25). Other than the classic aerotolerance mechanism driven by oxidative stress responses, little information is available about the physiological features contributing to aerotolerance in C. jejuni. The data in this study demonstrate that under aerobic conditions, C. jejuni boosts carbon metabolism to increase amino acid uptake, stimulates the production of surface polysaccharides, and develops a thick layer of surface polysaccharides, which possibly acts as a permeability barrier to protect C. jejuni from excess oxygen in aerobic environments (Fig. 7C).
Surface polysaccharides are a frontline barrier protecting Gram-negative bacteria from the surrounding environment, playing various roles in host colonization (43, 53), bacteriophage infection (54), biofilm formation (55), serum resistance (56, 57), and antibiotic resistance (34). In particular, LPS makes the bacterial outer membrane impermeable to hydrophobic compounds (58–60), and so does LOS in C. jejuni (34). Oxygen, as a small nonpolar molecule, can freely diffuse across biological membranes (61, 62). However, the hydrophilic polar headgroup regions of phospholipids are barriers to oxygen permeation through membranes (63, 64). Thus, hydrophilic surface polysaccharide layers can limit the access of oxygen to the membrane of C. jejuni. Notably, C. jejuni thickens surface capsules under aerobic conditions, not microaerobic conditions, suggesting that the stimulation of surface polysaccharide synthesis is a unique survival mechanism to sustain the viability of C. jejuni under aerobic conditions. Similar findings have been reported in other bacteria. The nitrogen-fixing bacterium Azotobacter vinelandii produces thick alginate capsules on the bacterial surface as a barrier for oxygen transfer into the cell, which helps protect the O2-sensitive nitrogenase from inactivation (65). The formation of thick surface capsules appears to be a novel survival mechanism protecting oxygen-sensitive bacteria from aerobic stress.
Since polysaccharides are a predominant component of bacterial biofilms (66), the enhancement of surface polysaccharide production by aerobiosis can be an environmental factor enhancing biofilm development in C. jejuni. In our previous study, we showed that oxidative stress promotes the biofilm formation of C. jejuni (15, 67). Increased production of polysaccharides under aerobic conditions can enhance biofilm formation, possibly facilitating transition from a planktonic to sessile state. However, the validation of this hypothesis requires further investigation.
Previous studies also demonstrate the association of carbon metabolism with aerotolerance in Campylobacter. Proteomics and transcriptomics analyses of C. jejuni have shown that aerobiosis increases the abundance of several amino acid transporters and TCA cycle enzymes, including Oor (45). Similarly, aerobiosis of the aerotolerant C. jejuni strain Bf overexpresses the proteins of the TCA cycle and amino acid uptake in addition to the enzymes of oxidative stress responses compared to the proteomics profile of microaerobic conditions (44). Whole-genome sequencing of C. coli strain OR12 shows that this aerotolerant strain has insertional or deletional mutations in genes related to carbohydrate metabolism, such as the b subunit of pyruvate carboxylase (24). These studies show that carbohydrate metabolism genes are up-regulated under aerobic conditions. However, little was known about why aerobiosis activates carbon metabolism in C. jejuni, although this obligate microaerophile is unable to grow aerobically. The findings in this study demonstrate that carbon metabolism through the TCA cycle plays a critical role in the aerotolerance of C. jejuni by producing precursors for surface polysaccharide synthesis.
Oxygen-sensitive bacteria are generally equipped with unique aerotolerance mechanisms unseen in aerobic bacteria to protect from oxygen toxicity in oxygen-rich environments (17). For instance, aerobic bacteria use superoxide dismutase, which catalyzes the dismutation of superoxide into molecular oxygen. To avoid the enzymatic formation of oxygen by superoxide dismutase, anaerobes use superoxide reductases that reduce superoxide to hydrogen peroxide, which is converted to water by peroxidases (68, 69). Although the presence of superoxide reductase has been reported only in some microaerophiles, such as Treponema pallidum (70), it is not a general aerotolerance mechanism in microaerophiles. Unlike aerobes, microaerophiles must maintain the physiological balance between oxygen sensitivity and the requirement to respire with low levels of oxygen (16, 18). Based on the distribution of high-affinity terminal oxidases active at low oxygen concentrations, bacteria with the capability of microaerobic respiration are widespread in nature (18). However, little attention has been paid to molecular mechanisms whereby microaerophiles sustain viability in aerobic environments. Our study first demonstrates that the development of a thick layer of surface polysaccharides induced by aerobiosis protects the obligate microaerophilic bacterium C. jejuni from excess oxygen in aerobic environments. Since aerotolerance involves various cellular functions, such as ROS detoxification, respiration, and carbon metabolisms, our findings may provide only one mechanism underlying aerotolerance. However, our work overall expands our understanding of how an oxygen-sensitive microaerophilic pathogen sustains viability in oxygen-rich environments by adapting bacterial metabolism and physiology.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni NCTC 11168 and 25 C. jejuni isolates from retail raw chicken in our previous study were used in this study (21). The strain was grown on Mueller-Hinton (MH) media (Oxoid, UK) at 42°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). When needed, MH media were supplemented with kanamycin (50 μg/mL), chloramphenicol (12.5 μg/mL), and tetracycline (5 μg/mL). Escherichia coli DH5α was grown at 37°C on Luria-Bertani (LB) media (Difco, USA), which were occasionally supplemented with carbenicillin (100 μg/mL), kanamycin (50 μg/mL), tetracycline (5 μg/mL), and chloramphenicol (12.5 μg/mL), where required.
Aerotolerance test.
The aerotolerance test was performed as described previously (19). Briefly, overnight cultures of C. jejuni on MH agar plates at 42°C were suspended in MH broth or MEMα medium to an OD600 of 0.1. The bacterial suspension (3 mL) in a 19-mL glass culture tube (catalog [cat.] no. T16-55-337; DWK Life Sciences, Germany) was incubated at 42°C with shaking (200 rpm) under aerobic conditions. Samples were taken at 2- or 3-h intervals for serial dilution and bacterial counting. To disrupt the cross-links of surface polysaccharides, EDTA was used at different concentrations (0.1 or 0.25 mM). MEMα was supplemented with serine, asparagine, aspartate, succinate, fumarate, oxaloacetate, pyruvate, proline, citrate, or 2-oxoglutarate to a final concentration of 20 mM to evaluate the effects of carbon sources on the restoration of aerotolerance in an Δoor mutant.
TEM analysis.
TEM using alcian blue staining was performed as described previously (71). Briefly, C. jejuni was fixed in ice-cold 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M cacodylate buffer, and left overnight at room temperature with gentle inversion. After centrifugation at 10,000 × g for 5 min, C. jejuni strains were positively stained with saturated alcian blue solution. Samples were loaded on Formvar/carbon copper grids (200 mesh), and morphology was examined with an energy-filtering transmission microscope (EF-TEM; Libra 120, Germany) at a voltage of 120 kV.
Detection of LOS and CPS by alcian blue staining.
CPS and LOS were detected with Alcian blue as described previously (26). Briefly, overnight cultures of C. jejuni strains on MH agar were suspended in MH broth or MEMα to an OD600 of 0.1. After exposure to either microaerobic or aerobic conditions, bacterial cultures were centrifuged at 10,000 × g for 5 min (MH broth) or 15,000 × g for 10 min (MEMα medium). Pellets were resuspended in lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.05% bromophenol blue) and boiled for 10 min. After centrifugation at 10,000 × g for 5 min, supernatants were mixed with proteinase K (final concentration of 1 mg/mL; Sigma, USA). The samples were incubated at 50°C for 1 h and then fractionated by SDS-PAGE. CPS and LOS were visualized with alcian blue staining (0.1% alcian blue dissolved in 40% ethanol/5% acetic acid).
Growth experiments.
Overnight cultures of C. jejuni strains were harvested from MH agar plates and suspended in MH broth to an OD600 of 0.001 or 0.1. Cultures (20 mL) in 100-mL flasks were incubated at 42°C with shaking under microaerobic conditions. Samples were taken at 2- or 3-h intervals, and the OD600 was measured in an Ultrospec 2000 spectrophotometer (Amersham Pharmacia Biotech, USA) or serially diluted in phosphate-buffered saline (PBS). The suspension was serially diluted and plated on MH agar to enumerate CFU. All experiments were repeated three times.
Construction of C. jejuni mutants and complemented strains.
For the construction of an Δoor mutant, the gene with a flanking region was amplified by PCR using oor-SalI-F and oor-BamHI-R primers (Table S2). After restriction digestion of PCR products and pUC19, the PCR product was ligated to pUC19. pUC19::oor was inverse PCR-amplified with oor-inverse-F and oor-inverse-R primers (Table S2) and ligated with a kanamycin resistance cassette which amplified from pMW10 using Kan-F and Kan-R primers (Table S2). The suicide plasmid was transferred to C. jejuni NCTC 11168 by electroporation. In addition, a chromosomal integration method was used to construct an oor-complemented strain (38). The oor gene was amplified by PCR using oor-compl-F and oor-compl-R primers (Table S2) and digested by XbaI. After ligation with pFMBcomCM (72), the complementation plasmid was introduced to the oor mutant.
The kpsS (CPS) and waaF (LOS) single and double mutants were constructed previously (34). To construct kpsS- and waaF-complemented strains, the genes were amplified with PCR using kpsS-compl-F, kpsS-compl-R primers or waaF-compl-F, waaF-compl-R primers, respectively (Table S2). The PCR product was digested by XbaI and ligated with pFMBcomCM (72). The complementation plasmid was introduced to a ΔkpsS mutant or a ΔwaaF mutant, and the transformants were selected by growing on MH agar plates supplemented with chloramphenicol (12.5 μg/mL). The single-gene (kpsS or waaF)-complemented double mutant was constructed by natural transformation with the genomic DNA of the kpsS- or waaF-complemented strains. The double mutant was grown overnight on MH agar, and the genomic DNA of the kpsS- or waaF-complemented strains was added to the culture. The C. jejuni strains were further grown for 5 h and plated on MH agar plates supplemented with kanamycin (50 μg/mL), tetracycline (5 μg/mL), and chloramphenicol (12.5 μg/mL).
The ΔahpC mutant, ahpC-complemented strain, ΔkatA mutant, katA-complemented strain, ΔsodB mutant, and sodB-complemented strain were constructed previously (72, 73).
Total RNA extraction, RNA sequencing, and analysis.
To prepare bacterial total RNA, overnight cultures of C. jejuni on MH agar were harvested and suspended in MH broth to an OD600 of 0.1. Bacterial suspension (6 mL) in a 19-mL glass culture tube was incubated for 3 h with shaking under microaerobic conditions, and cultures were equally divided for additional cultivation at 42°C for 3 h with shaking under microaerobic and aerobic conditions (3 mL culture in a 19-mL glass culture tube). Bacterial cultures (μL) were treated with 5% ice-cold phenol-ethanol solution, and total bacterial RNAs were isolated using the RNeasy minikit (Qiagen, Germany) according to the manufacturer’s instructions. The quantity and quality of total RNA samples were examined using a NanoPhotometer N60 (Implen, USA), and two biological replicate RNA samples were submitted to Macrogen (Seoul, Republic of Korea) for RNA sequencing. Before sequencing, the quality and quantity of total RNA were rechecked using an Agilent Technologies 2100 Bioanalyzer with an RNA integrity number (RIN) value larger than 7. After mRNA-Seq library construction using the Illumina TruSeq RNA sample preparation kit v.2 (Illumina, USA), RNA-Seq was performed by two runs with an Illumina NovaSeq 6000 instrument to generate paired-end reads of around 101 bp in length. The expression level of each gene was normalized by calculating the reads per kilobase per million mapped reads (RPKM) using CLC Workbench. Fold change was defined as RPKMaerobic conditions/RPKMmicroaerobic conditions. The differentially expressed genes (DEGs; fold change ≥2 or ≤−2; P < 0.05) were filtered and visualized using the Gitools.
Quantitative real-time PCR (qRT-PCR).
The extraction of total RNA is described above. Using extracted RNA samples, cDNA was synthesized with cDNA EcoDry premix (Clontech, USA). The synthesized cDNA was mixed with 2 × iQ SYBR green supermix (Bio-Rad, USA) and 0.3 μM each primer in a reaction volume of 20 μL. All qRT-PCR primer sets used in this study are listed in Table S2. qRT-PCRs were performed using the CFX Connect real-time PCR detection system (Bio-Rad, USA). The cycling parameters were as follows: 95°C for 5 min; 39 cycles at 95°C for 15 s, 55°C for 15 s, 72°C for 30 s; 72°C for 7 min.
Analysis of glucose-6-phosphate.
The extraction and the liquid chromatography-mass spectrometry (LC-MS) analysis of glucose-6-phosphate in C. jejuni were conducted according to a previously described protocol (74). C. jejuni NCTC 11168 was cultured on an MH agar plate overnight and resuspended in 200 mL of fresh MH broth to an OD600 of 0.08. The C. jejuni suspension was grown at 42°C microaerobically with shaking (200 rpm) for 4 h and was divided into two equal volumes. One volume was cultured at 42°C microaerobically with shaking (200 rpm) for 3 h, and the other was exposed to aerobic conditions with shaking (200 rpm) at 42°C for 3 h. C. jejuni was harvested with centrifugation at 3,000 × g at 4°C for 10 min and suspended in 0.5 mL of a methanol solution containing 1 μM sulfadimethoxine as the internal standard, sonicated for 15 s using an ultrasonic processor, and then mixed with 0.4 mL of water and 0.5 mL of chloroform for phase separation. After centrifugation at 13,000 × g at 4°C for 10 min, the aqueous phase was transferred to a fresh 1.5-mL tube and stored at −80°C prior to the analysis. For the LC-MS analysis, 5 μL of the extracted aqueous phase was injected into an Acquity ultraperformance liquid chromatography system (Waters, Milford, MA, USA). Separation was achieved in a 10-min run at a flow rate of 0.5 mL/min in a BEH amide column. The mobile phase used a gradient ranging from 99.5% aqueous Acetonitrile (ACN) containing 0.1% formic acid to 50% water. The LC eluent was then introduced into a Xevo-G2-S quadrupole time-of-flight mass spectrometer (Waters) for accurate mass measurement and ion counting. The accuracy of the MS was monitored by the intermittent injection of leucine encephalin ([M-H]– = m/z 554.2615). The capillary voltage and cone voltage for electrospray ionization (ESI) were maintained at −3 kV and −35 V for negative-mode detection, respectively. The source temperature and desolvation temperature were set at 120°C and 350°C, respectively. Nitrogen was used as both cone gas (50 L/h) and desolvation gas (600 L/h), and argon was used as collision gas. Structural information of glucose-6-phosphate was obtained by tandem MS (MS/MS) fragmentation with collision energies ranging from 15 to 50 eV, in comparison with an authentic standard.
Measurement of total ROS.
The level of total ROS accumulation in C. jejuni was measured using CM-H2DCFDA (Life Technologies, USA). Briefly, overnight cultures of C. jejuni strains on MH agar were suspended in MH broth to an OD600 of 0.1. Bacterial suspension (3 mL) in a 19-mL glass culture tube was incubated at 42°C with shaking (200 rpm) under aerobic and microaerobic conditions for 6 h. After exposure to each condition, bacterial cultures were centrifuged at 10,000 × g for 5 min and washed twice with PBS (pH 7.4). Then, the pellet was resuspended with PBS and treated CM-H2DCFDA at a final concentration of 10 μM for 30 min. Fluorescence was measured with a SpectraMax i3 platform (Molecular Devices, USA). To normalize the ROS level, protein concentrations of each sample were measured with a Bradford assay (Bio-Rad, USA).
LPO assay.
LPO levels were measured using a commercial kit (Cayman Chemical Co., USA) according to the manufacturer’s instructions. Briefly, overnight cultures of C. jejuni strains on MH agar were suspended in MH broth to an OD600 of 0.1. The bacterial suspension (3 mL) in a 19-mL glass culture tube was incubated at 42°C with shaking (200 rpm) under aerobic and microaerobic conditions for 6 h. After exposure to each condition, bacterial cultures were centrifuged at 10,000 × g for 5 min. LPOs were extracted with chloroform and methanol and mixed with the Chromogen reagent. After incubation at room temperature for 5 min, the OD at 500 nm was measured with a SpectraMax i3 platform (Molecular Devices, USA). A standard curve was generated with 13-hydroperoxy-octadecadienoic acid. The results were normalized with the protein concentration of each sample that was measured with the Bradford assay.
Statistical analysis.
The data present the means and the standard errors of the mean (SEM) of the results of independent experiments. Statistical significance was evaluated with Student’s t test or one-way analysis of variance (ANOVA) using Prism version 5.01 (GraphPad Software, Inc., San Diego, CA, USA). P values of <0.05 were considered statistically significant.
Data availability.
All RNA-Seq reads generated in this study were deposited in the Gene Expression Omnibus database (accession no. SRR17344685).
ACKNOWLEDGMENTS
J.K. was supported by the BK21 Plus Program of the Department of Agricultural Biotechnology, Seoul National University, Seoul, Republic of Korea. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01050990 and 2022R1A6A1A03055869). B.J. was supported by MnDRIVE (Minnesota’s Discovery, Research, and InnoVation Economy).
J.K., S.R., and B.J. designed the study. J.K., M.P., E.A., and Q.M. performed the experiments. J.K., C.C., S.R., and B.J. analyzed the data. S.R. and B.J. supervised the study. J.K. and B.J. wrote the initial draft, and J.K., B.J., and S.R. reviewed and edited the manuscript.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Sangryeol Ryu, Email: sangryu@snu.ac.kr.
Byeonghwa Jeon, Email: bjeon@umn.edu.
Beile Gao, South China Sea Institute of Oceanology.
REFERENCES
- 1.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnham PM, Hendrixson DR. 2018. Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat Rev Microbiol 16:551–565. doi: 10.1038/s41579-018-0037-9. [DOI] [PubMed] [Google Scholar]
- 3.Poropatich KO, Walker CL, Black RE. 2010. Quantifying the association between Campylobacter infection and Guillain-Barre syndrome: a systematic review. J Health Popul Nutr 28:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimesaat MM, Backert S, Alter T, Bereswill S. 2021. Human campylobacteriosis-a serious infectious threat in a one health perspective. Curr Top Microbiol Immunol 431:1–23. doi: 10.1007/978-3-030-65481-8_1. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey T, O’Brien S, Madsen M. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Stuart TL, Sandhu J, Stirling R, Corder J, Ellis A, Misa P, Goh S, Wong B, Martiquet P, Hoang L, Galanis E. 2010. Campylobacteriosis outbreak associated with ingestion of mud during a mountain bike race. Epidemiol Infect 138:1695–1703. doi: 10.1017/S095026881000049X. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Brooks BW, Lowman R, Carrillo CD. 2015. Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can J Microbiol 61:701–721. doi: 10.1139/cjm-2014-0770. [DOI] [PubMed] [Google Scholar]
- 8.Sellars MJ, Hall SJ, Kelly DJ. 2002. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J Bacteriol 184:4187–4196. doi: 10.1128/JB.184.15.4187-4196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaakoush NO, Miller WG, De Reuse H, Mendz GL. 2007. Oxygen requirement and tolerance of Campylobacter jejuni. Res Microbiol 158:644–650. doi: 10.1016/j.resmic.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AJ, Kelly DJ. 2019. The function, biogenesis and regulation of the electron transport chains in Campylobacter jejuni: new insights into the bioenergetics of a major food-borne pathogen. Adv Microb Physiol 74:239–329. doi: 10.1016/bs.ampbs.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Véron M, Lenvoisé-Furet A, Beaune P. 1981. Anaerobic respiration of fumarate as a differential test between Campylobacter fetus and Campylobacter jejuni. Curr Microbiol 6:349–354. doi: 10.1007/bf01567010. [DOI] [Google Scholar]
- 12.Begley M, Hill C. 2015. Stress adaptation in foodborne pathogens. Annu Rev Food Sci Technol 6:191–210. doi: 10.1146/annurev-food-030713-092350. [DOI] [PubMed] [Google Scholar]
- 13.Humphrey T. 2004. Salmonella, stress responses and food safety. Nat Rev Microbiol 2:504–509. doi: 10.1038/nrmicro907. [DOI] [PubMed] [Google Scholar]
- 14.Oh E, Chui L, Bae J, Li V, Ma A, Mutschall SK, Taboada EN, McMullen LM, Jeon B. 2018. Frequent implication of multistress-tolerant Campylobacter jejuni in human infections. Emerg Infect Dis 24:1037–1044. doi: 10.3201/eid2406.171587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J-C, Oh E, Kim J, Jeon B. 2015. Regulation of oxidative stress resistance in Campylobacter jejuni, a microaerophilic foodborne pathogen. Front Microbiol 6:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg N, Hoffman P. 1986. Microaerophily and oxygen toxicity. Annu Rev Microbiol 40:107–130. doi: 10.1146/annurev.mi.40.100186.000543. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Imlay JA. 2021. When anaerobes encounter oxygen: mechanisms of oxygen toxicity, tolerance and defence. Nat Rev Microbiol 19:774–785. doi: 10.1038/s41579-021-00583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris RL, Schmidt TM. 2013. Shallow breathing: bacterial life at low O2. Nat Rev Microbiol 11:205–212. doi: 10.1038/nrmicro2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh E, McMullen L, Jeon B. 2015. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front Microbiol 6:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guk JH, Kim J, Song H, Kim J, An JU, Kim J, Ryu S, Jeon B, Cho S. 2019. Hyper-aerotolerant Campylobacter coli from duck sources and its potential threat to public health: virulence, antimicrobial resistance, and genetic relatedness. Microorganisms 7:579. doi: 10.3390/microorganisms7110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Park H, Kim J, Kim JH, Jung JI, Cho S, Ryu S, Jeon B. 2019. Comparative analysis of aerotolerance, antibiotic resistance, and virulence gene prevalence in Campylobacter jejuni isolates from retail raw chicken and duck meat in South Korea. Microorganisms 7:433. doi: 10.3390/microorganisms7100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh E, Andrews KJ, McMullen LM, Jeon B. 2019. Tolerance to stress conditions associated with food safety in Campylobacter jejuni strains isolated from retail raw chicken. Sci Rep 9:11915. doi: 10.1038/s41598-019-48373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh E, McMullen LM, Chui L, Jeon B. 2017. Differential survival of hyper-aerotolerant Campylobacter jejuni under different gas conditions. Front Microbiol 8:954. doi: 10.3389/fmicb.2017.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Kane PM, Connerton IF. 2017. Characterisation of aerotolerant forms of a robust chicken colonizing Campylobacter coli. Front Microbiol 8:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karki AB, Marasini D, Oakey CK, Mar K, Fakhr MK. 2018. Campylobacter coli from retail liver and meat products is more aerotolerant than Campylobacter jejuni. Front Microbiol 9:2951. doi: 10.3389/fmicb.2018.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlyshev AV, Wren BW. 2001. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using alcian blue dye. J Clin Microbiol 39:279–284. doi: 10.1128/JCM.39.1.279-284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 28.Karlyshev AV, Ketley JM, Wren BW. 2005. The Campylobacter jejuni glycome. FEMS Microbiol Rev 29:377–390. [DOI] [PubMed] [Google Scholar]
- 29.Bertani B, Ruiz N. 2018. Function and biogenesis of lipopolysaccharides. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corcoran AT, Annuk H, Moran AP. 2006. The structure of the lipid anchor of Campylobacter jejuni polysaccharide. FEMS Microbiol Lett 257:228–235. doi: 10.1111/j.1574-6968.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 31.Domenico P, Schwartz S, Cunha BA. 1989. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun 57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldfield NJ, Moran AP, Millar LA, Prendergast MM, Ketley JM. 2002. Characterization of the Campylobacter jejuni heptosyltransferase II gene, waaF, provides genetic evidence that extracellular polysaccharide is lipid A core independent. J Bacteriol 184:2100–2107. doi: 10.1128/JB.184.8.2100-2107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chisanga M, Linton D, Muhamadali H, Ellis DI, Kimber RL, Mironov A, Goodacre R. 2020. Rapid differentiation of Campylobacter jejuni cell wall mutants using Raman spectroscopy, SERS and mass spectrometry combined with chemometrics. Analyst 145:1236–1249. doi: 10.1039/C9AN02026H. [DOI] [PubMed] [Google Scholar]
- 34.Jeon B, Muraoka W, Scupham A, Zhang Q. 2009. Roles of lipooligosaccharide and capsular polysaccharide in antimicrobial resistance and natural transformation of Campylobacter jejuni. J Antimicrob Chemother 63:462–468. doi: 10.1093/jac/dkn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis LM, Whitfield C. 2013. KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci USA 110:20753–20758. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwata T, Chiku K, Amano K-i, Kusumoto M, Ohnishi-Kameyama M, Ono H, Akiba M. 2013. Effects of lipooligosaccharide inner core truncation on bile resistance and chick colonization by Campylobacter jejuni. PLoS One 8:e56900. doi: 10.1371/journal.pone.0056900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen VT, Barlow RS, Fegan N, Turner MS, Dykes GA. 2013. Role of capsular polysaccharides and lipooligosaccharides in Campylobacter surface properties, autoagglutination, and attachment to abiotic surfaces. Foodborne Pathog Dis 10:506–513. doi: 10.1089/fpd.2012.1365. [DOI] [PubMed] [Google Scholar]
- 38.Karlyshev A, Wren B. 2005. Development and application of an insertional system for gene delivery and expression in Campylobacter jejuni. Appl Environ Microbiol 71:4004–4013. doi: 10.1128/AEM.71.7.4004-4013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leach S, Harvey P, Wali R. 1997. Changes with growth rate in the membrane lipid composition of and amino acid utilization by continuous cultures of Campylobacter jejuni. J Appl Microbiol 82:631–640. doi: 10.1111/j.1365-2672.1997.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 40.Guccione E, Del Rocio Leon-Kempis M, Pearson BM, Hitchin E, Mulholland F, Van Diemen PM, Stevens MP, Kelly DJ. 2008. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol 69:77–93. doi: 10.1111/j.1365-2958.2008.06263.x. [DOI] [PubMed] [Google Scholar]
- 41.Kelly D. 2001. The physiology and metabolism of Campylobacter jejuni and Helicobacter pylori. J Appl Microbiol 90:16S–24S. doi: 10.1046/j.1365-2672.2001.01350.x. [DOI] [PubMed] [Google Scholar]
- 42.Stahl M, Butcher J, Stintzi A. 2012. Nutrient acquisition and metabolism by Campylobacter jejuni. Front Cell Infect Microbiol 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao B, Vorwerk H, Huber C, Lara-Tejero M, Mohr J, Goodman AL, Eisenreich W, Galán JE, Hofreuter D. 2017. Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni. PLoS Biol 15:e2001390. doi: 10.1371/journal.pbio.2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues RC, Haddad N, Chevret D, Cappelier J-M, Tresse O. 2016. Comparison of proteomics profiles of Campylobacter jejuni strain Bf under microaerobic and aerobic conditions. Front Microbiol 7:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guccione EJ, Kendall JJ, Hitchcock A, Garg N, White MA, Mulholland F, Poole RK, Kelly DJ. 2017. Transcriptome and proteome dynamics in chemostat culture reveal how Campylobacter jejuni modulates metabolism, stress responses and virulence factors upon changes in oxygen availability. Environ Microbiol 19:4326–4348. doi: 10.1111/1462-2920.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weerakoon DR, Borden NJ, Goodson CM, Grimes J, Olson JW. 2009. The role of respiratory donor enzymes in Campylobacter jejuni host colonization and physiology. Microb Pathog 47:8–15. doi: 10.1016/j.micpath.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Kendall JJ, Barrero-Tobon AM, Hendrixson DR, Kelly DJ. 2014. Hemerythrins in the microaerophilic bacterium Campylobacter jejuni help protect key iron–sulphur cluster enzymes from oxidative damage. Environ Microbiol 16:1105–1121. doi: 10.1111/1462-2920.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh E, McMullen L, Jeon B. 2015. Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front Microbiol 6:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baillon ML, van Vliet AH, Ketley JM, Constantinidou C, Penn CW. 1999. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol 181:4798–4804. doi: 10.1128/JB.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reuter M, Mallett A, Pearson BM, van Vliet AH. 2010. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl Environ Microbiol 76:2122–2128. doi: 10.1128/AEM.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson DN, Davis B, Tirado SM, Duggal M, van Frankenhuyzen JK, Deaville D, Wijesinghe M, Tessaro M, Trevors J. 2009. Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Van Leeuwenhoek 96:377–394. doi: 10.1007/s10482-009-9378-8. [DOI] [PubMed] [Google Scholar]
- 52.Klančnik A, Botteldoorn N, Herman L, Možina SS. 2006. Survival and stress induced expression of groEL and rpoD of Campylobacter jejuni from different growth phases. Int J Food Microbiol 112:200–207. doi: 10.1016/j.ijfoodmicro.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect Immun 72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holst Sørensen MC, van Alphen LB, Fodor C, Crowley SM, Christensen BB, Szymanski CM, Brøndsted L. 2012. Phase variable expression of capsular polysaccharide modifications allows Campylobacter jejuni to avoid bacteriophage infection in chickens. Front Cell Infect Microbiol 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naito M, Frirdich E, Fields JA, Pryjma M, Li J, Cameron A, Gilbert M, Thompson SA, Gaynor EC. 2010. Effects of sequential Campylobacter jejuni 81-176 lipooligosaccharide core truncations on biofilm formation, stress survival, and pathogenesis. J Bacteriol 192:2182–2192. doi: 10.1128/JB.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, Ferderber JS, Porter CK, Trent MS, Guerry P. 2013. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 81:665–672. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keo T, Collins J, Kunwar P, Blaser MJ, Iovine NM. 2011. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence 2:30–40. doi: 10.4161/viru.2.1.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barua S, Yamashino T, Hasegawa T, Yokoyama K, Torii K, Ohta M. 2002. Involvement of surface polysaccharides in the organic acid resistance of Shiga Toxin-producing Escherichia coli O157:H7. Mol Microbiol 43:629–640. doi: 10.1046/j.1365-2958.2002.02768.x. [DOI] [PubMed] [Google Scholar]
- 59.Guo Y, Rowe-Magnus DA. 2010. Identification of a c-di-GMP-regulated polysaccharide locus governing stress resistance and biofilm and rugose colony formation in Vibrio vulnificus. Infect Immun 78:1390–1402. doi: 10.1128/IAI.01188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snyder DS, McIntosh TJ. 2000. The lipopolysaccharide barrier: correlation of antibiotic susceptibility with antibiotic permeability and fluorescent probe binding kinetics. Biochemistry 39:11777–11787. doi: 10.1021/bi000810n. [DOI] [PubMed] [Google Scholar]
- 61.Windrem DA, Plachy WZ. 1980. The diffusion-solubility of oxygen in lipid bilayers. Biochim Biophys Acta 600:655–665. doi: 10.1016/0005-2736(80)90469-1. [DOI] [PubMed] [Google Scholar]
- 62.Zuniga-Hertz JP, Patel HH. 2019. The evolution of cholesterol-rich membrane in oxygen adaption: the respiratory system as a model. Front Physiol 10:1340. doi: 10.3389/fphys.2019.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subczynski WK, Hyde JS, Kusumi A. 1989. Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proc Natl Acad Sci USA 86:4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widomska J, Raguz M, Subczynski WK. 2007. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta 1768:2635–2645. doi: 10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabra W, Zeng A-P, Lünsdorf H, Deckwer W-D. 2000. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol 66:4037–4044. doi: 10.1128/AEM.66.9.4037-4044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limoli DH, Jones CJ, Wozniak DJ. 2015. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3:3.3.29. doi: 10.1128/microbiolspec.MB-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh E, Kim J-C, Jeon B. 2016. Stimulation of biofilm formation by oxidative stress in Campylobacter jejuni under aerobic conditions. Virulence 7:846–851. doi: 10.1080/21505594.2016.1197471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenney FE, Jr, Verhagen MFJM, Cui X, Adams MWW. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306–309. doi: 10.1126/science.286.5438.306. [DOI] [PubMed] [Google Scholar]
- 69.Thorgersen MP, Stirrett K, Scott RA, Adams MWW. 2012. Mechanism of oxygen detoxification by the surprisingly oxygen-tolerant hyperthermophilic archaeon, Pyrococcus furiosus. Proc Natl Acad Sci USA 109:18547–18552. doi: 10.1073/pnas.1208605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lombard M, Touati D, Fontecave M, Nivière V. 2000. Superoxide reductase as a unique defense system against superoxide stress in the microaerophile Treponema pallidum. J Biol Chem 275:27021–27026. doi: 10.1016/S0021-9258(19)61474-2. [DOI] [PubMed] [Google Scholar]
- 71.Corcionivoschi N, Alvarez LA, Sharp TH, Strengert M, Alemka A, Mantell J, Verkade P, Knaus UG, Bourke B. 2012. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe 12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang S, Zhang Q, Ryu S, Jeon B. 2012. Transcriptional regulation of the CmeABC multidrug efflux pump and the KatA catalase by CosR in Campylobacter jejuni. J Bacteriol 194:6883–6891. doi: 10.1128/JB.01636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh E, Jeon B. 2014. Role of alkyl hydroperoxide reductase (AhpC) in the biofilm formation of Campylobacter jejuni. PLoS One 9:e87312. doi: 10.1371/journal.pone.0087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao Q, Liu J, Wiertzema JR, Chen D, Chen P, Baumler DJ, Ruan R, Chen C. 2021. Identification of quinone degradation as a triggering event for intense pulsed light-elicited metabolic changes in Escherichia coli by metabolomic fingerprinting. Metabolites 11:102. doi: 10.3390/metabo11020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.03761-22-s0001.xlsx, XLSX file, 0.08 MB (77.8KB, xlsx)
Supplemental material. Download spectrum.03761-22-s0002.pdf, PDF file, 0.5 MB (469.7KB, pdf)
Data Availability Statement
All RNA-Seq reads generated in this study were deposited in the Gene Expression Omnibus database (accession no. SRR17344685).