Abstract
Popliteal artery entrapment syndrome is an important albeit infrequent cause of serious disability among young adults and athletes with anomalous anatomic relationships between the popliteal artery and surrounding musculotendinous structures. We report our experience with 3 patients, in whom we used duplex ultrasonography, computed tomography, digital subtraction angiography, and conventional arteriography to diagnose popliteal artery entrapment and to grade the severity of dynamic circulatory insufficiency and arterial damage.
We used a posterior surgical approach to give the best view of the anatomic structures compressing the popliteal artery. In 2 patients, in whom compression had not yet damaged the arterial wall, operative decompression of the artery by resection of the aberrant muscle was sufficient. In the 3rd patient, operative reconstruction of an occluded segment with autologous vein graft was necessary, in addition to decompression of the vessel and resection of aberrant muscle. The result in each case was complete recovery, with absence of symptoms and with patency verified by Doppler examination. We conclude that clinicians who encounter young patients with progressive lower-limb arterial insufficiency should be aware of the possibility of popliteal artery entrapment. Early diagnosis through a combined approach (careful physical examination and history-taking, duplex ultrasonography, computerized tomography, and angiography) is necessary for exact diagnosis. The treatment of choice is the surgical creation of normal anatomy within the popliteal fossa.
Key words: Angiography, digital subtraction; popliteal artery/surgery; popliteal artery entrapment syndrome; tomography, x-ray computed; ultrasonography, Doppler, duplex
Despite technical advances in arterial repair, trauma to the popliteal artery continues to be associated with a relatively high amputation rate in most civilian and military experiences. 1–4
One rare popliteal arterial lesion is popliteal artery entrapment syndrome (PAES). This anomaly usually affects young men (aged 20 to 40 years) as the most common of several unusual entities that cause intermittent claudication in young adults. The other causes of acute vascular insufficiency of the limb in young persons are premature accelerated atherosclerosis, thromboangiitis obliterans, adventitial cystic disease, adductor canal outlet syndrome, microemboli, collagen vascular disease, Takayasu's arteritis, and coagulopathy. 5–7
Popliteal artery entrapment syndrome is a consequence of abnormal positioning of the popliteal artery in relation to its surrounding structures. In type I entrapment (Heidelberg classification system), the popliteal artery has an atypical course; in type II, the muscular insertion is atypical; and in type III, both conditions are present. 8 These abnormal anatomic relationships can produce extrinsic compression of the popliteal artery and cause vascular damage. 9–12 Eventually, an irreversible lesion of the popliteal artery can manifest itself as aneurysmal dilatation, thrombosis, or embolism and can result in ischemia, threatening limb viability. Diagnostic delay is common because this problem usually occurs in young, athletic patients, who lack the vascular conditions that would predispose them to atherosclerosis and limit their normal social and professional activities in the presence of even mild symptoms. Most commonly, PAES is found in young sportsmen or soldiers with well-developed muscles, 13–15 because the exercise and enlargement of muscles adjacent to the popliteal artery exacerbates the consequences of the anomalous relationship between muscle and artery. Therefore, military surgeons have taken a special interest in this disorder, which has increased the diagnostic rate of PAES in military personnel. 16–18
Patients and Methods
Three patients with popliteal artery stenosis or occlusion secondary to entrapment are described in this study. Before surgery, all patients underwent assessment that included Doppler ultrasonography, computerized tomography, and conventional arteriography or digital subtraction arteriography.
Duplex ultrasonographic scans were performed using an ATL Ultramark 9 duplex scanner (Advanced Technology Laboratories; Bothell, Wash). Popliteal arteries were examined by means of a 5-MHz transducer, with the patient in a prone position while the knee was fully extended, and in neutral position. Diagnostic maneuvers consisted of actions causing gastrocnemius muscle contraction. Such maneuvers included active ankle extension and passive dorsiflexion of the foot, and knee hyperextension. Arterial flow signals were identified using continuous-wave Doppler at the popliteal artery. After identification of the artery, the Doppler system was switched to the pulsed-wave mode and was guided by the flow profile; midstream signals were recorded on a Technics RS-B48R tape recorder. As a basis of comparison, we used Doppler waveforms with the least dampened signals from the contralateral popliteal artery.
Contrast-enhanced computed tomographic (CT) scanning has proved valuable in the diagnosis of PAES. The first 2 patients were scanned with the Somatom DR G (Siemens AG; Erlangen, Germany); the 3rd patient was scanned with the SCT-2800 TF Intelect (Shimadzu; Osaka, Japan). All scans were performed using continuous 10-mm slices (3-second scan time, 6-second intersection delay). Scanning was performed immediately after a manual bolus injection of nonionic contrast medium (iohexol 350, 40 mL) and during continuous infusion (at a rate of 20 mL/min) of an additional 60 mL of the same contrast agent. Scanning was limited to a 12- to 15-cm region of the popliteal area, from 8 cm above to 7 cm below the femoral junction. Helical parameters included 5-mm collimation, 5-mm/s table speed (1.0 pitch), and 5-mm-thick image reconstruction. The reduction in diameter of the artery was used for grading the stenosis, and the diameter of the area of the most severe arterial reduction in any plane was compared with the diameter of the most-normal-looking segment proximal or distal to the stenosis, or with the diameter of a normal segment from the other leg. Multiplane reformations were routinely performed to analyze the popliteal arteries in more detail. Final interpretation was based upon the data provided by analysis of the combination of axial transverse sections and multiplane reformation images.
Diagnostic arteriography was performed by the Seldinger technique, via the transfemoral approach. The arteriograms were examined to determine the presence or absence of popliteal artery stenosis (>50% reduction in diameter), to determine if there were signs of extrinsic compression of the popliteal artery by adjacent structures, and to determine if the course of the artery was abnormal. Arteriography with the foot in a neutral position was compared with arteriography during active plantar extension of the foot for assessment of compression of the artery in the popliteal fossa. Intraarterial digital subtraction angiography (DSA) or conventional angiography was used routinely. Arteriography was performed with a single-plane Siemens imaging system (Erlangen, Germany).
Case Presentations and Follow-Up
Case 1
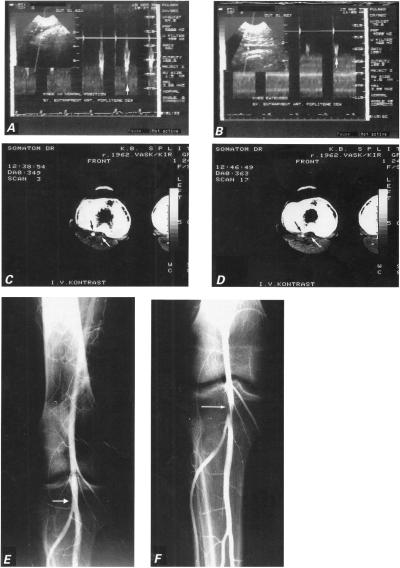
In September of 1995, a 31-year-old bus driver presented with a complaint of right calf pain, which he had experienced occasionally during the preceding 2 months and which intensified during ambulation or other exertion. He smoked 1 pack of cigarettes daily. Physical examination revealed an athletic young man in sinus rhythm, with a pulse rate of 65/min and a blood-pressure reading of 120/80 mmHg. Right pedal pulses were reduced with the knee in the neutral position and disappeared completely with the knee hyperextended. Doppler sonography showed normal right popliteal artery morphology and good flow with the knee in the neutral position (Fig. 1A). Active plantar extension against resistance and passive dorsiflexion of the foot compressed the popliteal artery and reduced blood flow (Fig. 1B). A CT scan on the level of the femoral condyles and the proximal 3rd of the calf showed localized stenosis of the right popliteal artery between the femoral condyles. Scans at the level of the intercondylar fossa showed a 2.5-cm segment of the right popliteal artery to be flattened like a sword sheath (Fig. 1C), while at the level of the upper margin of the joint surface of the tibia (that is, in the fossa poplitea) a ca. 2-cm segment of the right popliteal artery was compressed by the hypertrophic medial head of the gastrocnemius muscle (Fig. 1D). The axial transverse and the multiplane reformation images demonstrated extrinsic compression of the right popliteal due to an anomalous insertion of the aberrant head of the gastrocnemius. A functional angiogram of the right popliteal artery in active flexion did not reveal pathomorphologic changes (Fig. 1E), but a ca. 2-cm segment of the artery was seen to be narrowed in active extension, and a 1-cm-wide, beam-like extrinsic compression was seen before the trifurcation (Fig. 1F).
Fig. 1 Case 1: A) Doppler sonogram shows normal popliteal artery flow (vertical arrow) with knee in neutral position. B) Doppler tracing of popliteal artery flow with knee hyperextended during plantar extension of the foot shows monophasic configuration of the velocity waveform, with a blunt and rounded peak (vertical arrow) that suggests minor arterial stenosis. C) Computed tomographic scan performed after intravenous injection of contrast material shows enlarged medial head of gastrocnemius muscle joined with the accessory head (white arrow), and the normal lumen of the artery (black arrow). D) Computed tomographic scan with knee extended shows compression of artery (black arrow) by the accessory head of gastrocnemius muscle (white arrow). E) Arteriogram shows normal right popliteal artery (white arrow) with knee in the neutral position. F) Arteriogram of the hyperextended right knee shows constriction of the popliteal artery at the site of entrapment (white arrow).
During surgical intervention, we resected the accessory head of the gastrocnemius muscle, liberating the right popliteal artery. Postoperative necrosis of the skin on the margins of the operative wound necessitated a free-skin transplant graft. The subsequent postoperative course was uneventful and the patient was discharged in 3 weeks. A follow-up examination twelve months later showed normal popliteal artery patency.
Case 2
In June of 1994, a 33-year-old skilled mechanic was referred because of intermittent right-calf claudication, accompanied by coldness, numbness, and blanching of the foot. Symptoms were precipitated by walking 400 meters, were relieved by a short rest, and disappeared completely 1 hour after ambulation. Similar symptoms occurred while driving a car for 1 hour or more. These difficulties had 1st appeared 4 years before presentation, had been continual during the preceding 2 years, and had markedly worsened during the preceding week. The patient was a non-smoker and non-drinker who had kept active for 15 years by boxing, running, and weight-lifting.
Physical examination revealed an athletic young man, 85 kg in weight, 180 cm in height, in sinus rhythm, with a pulse rate of 75/min and a blood-pressure reading of 125/80 mmHg. In the lower limbs, there were no skin or temperature changes. All pulses were palpable in the neutral position. With the knee extended, no pulse could be detected in the popliteal artery or in the dorsalis pedis and posterior tibial arteries of the right foot.
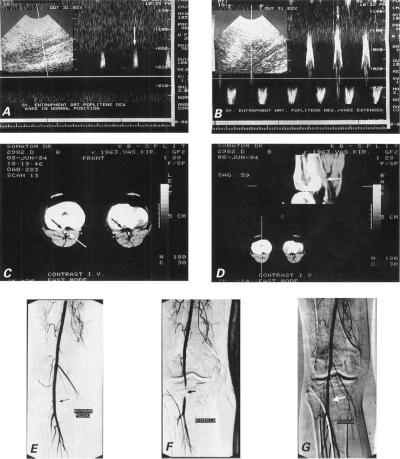
Electromyography showed a minor loss of motoneurons in the small muscles of the right foot and in the anterior muscle group of the right lower leg. Doppler sonography did not show any abnormality of the popliteal artery while the knee was in neutral position (Fig. 2A), but with the knee extended it revealed highly phasic, staccato waveforms (Fig. 2B), suggesting stenosis.
Fig. 2 Case 2: A) Doppler ultrasonographic image of right popliteal artery flow (vertical arrow) with knee in normal position shows no abnormality. B) Doppler tracing of popliteal artery flow (vertical arrow) with knee hyperextended during plantar extension of the foot reveals highly phasic, staccato waveforms, which suggest high-grade distal arterial stenosis. C) Axial dynamic computed tomographic scan with intravenous contrast enhancement during a submaximal calf muscle contraction demonstrates accessory head of the gastrocnemius muscle (white arrow) compressing small-caliber right popliteal artery (black arrow), in comparison with the opposite normal-caliber left popliteal artery (arrow). D) Phase-contrast images in sagittal plane depict flow in the popliteal artery during a submaximal calf muscle contraction. The vascular lumen is narrowed from side to side and flow signal intensity is declined in the sagittal aspect over a 3-cm length below the knee (white arrow) during plantar extension of the foot. Note the relationship between the popliteal artery and the abnormally inserted accessory head of the gastrocnemius muscle. E) Right transfemoral digital subtraction angiogram shows normal popliteal artery flow (black arrow) with knee in neutral position. F) Severe stenosis of the popliteal artery (black arrow) with full extension at the knee and active plantar extension at the ankle. G) Normal popliteal artery flow (white arrow) with foot in passive dorsiflexion.
Dynamic CT scanning of the popliteal fossa, performed in a 4-mm scan with intravenous bolus administration of contrast medium during a submaximal calf muscle contraction and plantar extension of the foot, showed an accessory medial head of the gastrocnemius muscle compressing the right popliteal artery (right leg is visible on the left side in Fig. 2C); images in the sagittal plane depicted the narrowed vascular lumen of the right popliteal artery (Fig. 2D).
Digital subtraction angiography performed with the knee in the neutral position yielded a normal radiographic finding (Fig. 2E), but during extension at the knee and active full plantar extension at the ankle, the popliteal artery appeared to discontinue near the joint fissure, and flow through the vessel was markedly slow (Fig. 2F). In flexion, the flow was normal (Fig. 2G).
Upon surgery, we used the posterior approach and found that the accessory head of the gastrocnemius muscle was inserted abnormally into the medial condyle. The popliteal artery was angulated and trapped behind the muscle. Below and above the site of compression, the wall of the popliteal artery and its internal diameter were normal. Myotomy was then performed. Postoperatively, the right dorsalis pedis and posterior tibial pulses become palpable in all positions. The patient had an uneventful postoperative course and was discharged home on the 6th postoperative day. At follow-up examinations after discharge, the patient complained of numbness on the medial aspect of his right leg.
Case 3
In October of 1997, a 21-year-old lorry driver complained of progressively increasing right-leg calf claudication. The pain and numbness in the right lower leg had started suddenly after swimming, 3 months before his referral. His pain-free walking distance was 100-200 m. He was smoking about 20 cigarettes daily. He had been playing amateur soccer as a defensive player for 4 years.
Physical examination revealed a muscular young man, 78 kg in weight and 175 cm in height, with a pulse rate of 70 per min and a blood-pressure reading of 120/80 mmHg. No pulses were palpable in the right leg below the popliteal fossa. The time required for restoration of color after application of finger-pressure to the skin was normal in both legs, and there were no morphologic changes.
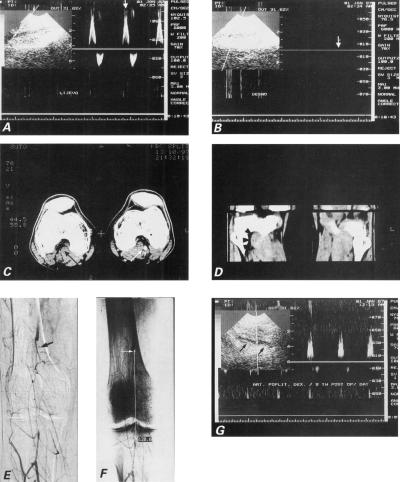
Two-dimensional and Doppler sonographic images of the left popliteal artery showed normal flow (Vmax 0.7 m/s) and diameter (Fig. 3A), while 2-D images on the right showed the proximal popliteal lumen but lost the distal lumen. No Doppler signal of arterial flow could be obtained on the right, which suggested complete interruption of arterial circulation (Fig. 3B).
Fig. 3 Case 3: A) Duplex sonogram waveform from a normal left popliteal artery demonstrates a rapid early rise in systole. B) Duplex sonogram from right popliteal artery shows complete occlusion (vertical arrow) and no Doppler signal. C) Dynamic computed tomographic scan with intravenous contrast enhancement demonstrates thrombosis of the right popliteal artery (black arrow), in contrast with patent left popliteal artery (white arrow). Accessory head of the right gastrocnemius muscle (white arrowhead) is clearly demonstrated, in contrast with normal anatomic relationships seen in left limb. D) Phase contrast computed tomographic images in sagittal plane depict increased mass of the 3rd head of the gastrocnemius muscle in the right eg (arrowheads). E) Digital subtraction arteriogram demonstrates a complete segmental occlusion of the right popliteal artery (arrow). Note the absence of atherosclerotic signs in the collateral arteries. F) The length of the occluded segment was 120.6 mm (white line). G) Control postoperative Doppler ultrasonogram demonstrates patent saphenous vein in situ bypass (arrowheads) between the proximal and distal portions of the popliteal artery.
A dynamic CT scan with intravenous bolus contrast enhancement (Fig. 3C) at the level of the popliteal fossa demonstrated occlusion of the right popliteal artery due to muscle entrapment by the 3rd head of the gastrocnemius muscle (Fig. 3D).
Digital subtraction angiography of the right leg showed a long occlusion (12 cm) of the popliteal artery and many unobstructed collateral vessels (Fig. 3E). Above the trifurcation, the circulation was established by well-developed collateral flow (Fig. 3F), which suggested that the occlusion was old.
At operation, the popliteal artery was seen to be completely occluded and to course below the 3rd head of the gastrocnemius muscle. The compromised arterial segment was replaced by an autologous venous graft, and the 3rd head of the gastrocnemius muscle was resected. The patient no longer experienced any impairment upon walking, and repeat Doppler ultrasonography showed unimpeded flow through the graft, even on plantar extension (Fig. 3G).
Discussion
In 1879, while still in medical school at Edinburgh, Anderson Stuart became the 1st to describe the anatomical basis of popliteal entrapment. 19 In 1925, Chambardel-Dubreuil described a case in which the popliteal artery was separated from the popliteal vein by an accessory gastrocnemius muscle. 20 Hamming and Vink, in 1959, performed the 1st operative decompression of an entrapped popliteal artery, at Leyden University in the Netherlands. 21 The 1st case diagnosed before surgical intervention was reported by Servello in 1962, at the University of Padua in Italy. 22 Love and Whelan, of Walter Reed General Hospital in the United States, introduced the term “popliteal artery entrapment syndrome” (PAES) in 1965. 16 Popliteal artery entrapment syndrome is an uncommon cause of lower-extremity claudication, which usually occurs in younger patients who lack the risk factors for atherosclerosis and who are healthier and more active than average for their age group. Most of them are sportsmen, and some play professionally. The most frequently involved activities are team sports (such as soccer, rugby, and basketball) and martial arts. All of these activities require repeated sudden and forceful contraction of the calf, which results in hypertrophy of the calf muscles. Similar contraction can cause PAES in heavy-vehicle drivers, such as military personnel who drive armored vehicles. 15
Popliteal artery entrapment syndrome occurs when these conditions exist: 1) an abnormal anatomical relationship between the popliteal artery and the surrounding musculotendinous structures; 2) hypertrophy of the musculotendinous structures; and 3) repeated arterial compression upon exercise. These anatomic variants of PAES have been proposed: atypical course of the popliteal artery from the medial or lateral head of the gastrocnemius muscle; compression of a normally running artery by anomalous musculotendinous formations lying between the 2 gastrocnemius heads (usually compression by the aberrant head of the gastrocnemius, or compression by the excessively hypertrophied soleus, plantaris, or popliteus muscle); or a combination of a dislocated artery and muscular abnormalities. 23–34 As of 1999, over 450 cases of PAES had been reported in the medical literature. 35,36 Uncommonly, the popliteal vein, rather than the popliteal artery, is involved. 11,37–46
Changes in the popliteal artery associated with external compression have ranged from poststenotic dilatation to true aneurysm formation. Thrombosis has also occurred at the site of entrapment. This localized area of thrombosis is usually found in the mid-popliteal artery, and extensive collateral development is frequently present. 47–49
Because PAES is both uncommon and difficult to diagnose, it poses a diagnostic pitfall. Awareness of the entity is of course a prerequisite for correct diagnosis. Of utmost importance are a careful history and a careful physical examination. The characteristic signs and symptoms are a history of leg swelling, aching pain, pain at rest, and tiredness or cramping of the calf; but symptoms can vary and, until complications develop, physical signs are absent at rest. In the early stages, when the artery is patent except during calf-muscle contraction, symptoms in young persons are usually limited to transitory cramps or a feeling of coldness. Patients may report numbness, blanching, coldness, or cramps of the limb in a variety of postures, which usually resolve with a change of position. The onset of the symptoms is often sudden, during intense physical exercise. Early in the course of entrapment syndrome, a provocative test is needed for diagnosis: the patient is asked to hyperextend his leg and to contract the gastrocnemius muscle by means of active plantar extension or maximal passive dorsal flexion, which should lead to a decrease or disappearance of pulses of the foot. 50
In the later stages of undiagnosed PAES, when the artery is affected by stable lesions (local stenosis or occlusion, local thrombotic interruption, or poststenotic aneurysm) typical symptoms are severe acute ischemia and intermittent calf claudication, usually monolateral. Such symptoms are surprising when they occur in healthy-looking young subjects who lack atherogenic risk factors. Acute ischemia occurs as a result of thrombosis in situ and is common in young patients who have not developed sufficient collateral circulation. 51,52 The high proportion of patients who are drivers of lorries, buses, or military vehicles may be a consequence of their sitting with an acutely flexed knee and of their repeatedly alternating forced plantar extension with forced plantar flexion, which can result in calf-muscle hypertrophy.
Extensive efforts have been made to diagnose PAES by noninvasive means. Duplex sonography of the compressed popliteal artery is a noninvasive, quick, and relatively inexpensive test. The popliteal artery is ideally situated for ultrasonographic examination, and the effect of dynamic maneuvers can be assessed with Doppler examination. Popliteal arterial stenosis can be quantified by measuring the peak systolic velocity (PSV) ratio across a lesion: the PSV within a stenosis is compared with that in a disease-free popliteal segment in the opposite limb, and a ratio is created that is independent of individual variations in blood pressure, vascular compliance, and cardiac function. We defined a significant stenosis as one with a PSV ratio greater than 2. Turbulence with aliasing of the signal is required to diagnose hemodynamically significant stenosis. Vessels were considered to be occluded if no flow could be detected by such means as DSA and no pulsatile flow could be detected by pulsed Doppler. The opposite limb is always screened as well, because the syndrome is bilateral in up to two-thirds of all cases. However, mapping both legs of a patient requires about 60 to 90 minutes.
One purpose of our study was to adopt duplex imaging as the primary diagnostic technique for popliteal artery entrapment, because the superficial location of the popliteal artery renders it easily accessible for ultrasound examination. The present results show that duplex imaging may be useful as the primary investigational tool in the early diagnosis of PAES. Duplex ultrasonographic scanning is the 1st diagnostic tool that has the ability, through intensive graft surveillance, to provide clear anatomical and functional data about the quality of peripheral bypass grafts. Follow-up monitoring of bypass grafts with duplex scanning, after decompression and resection of an occluded segment of an artery, is an almost ideal screening procedure, because of its simplicity and noninvasive character. 53–56 Ultrasonography, however, lacks the image clarity for accurate analysis of soft-tissue structures.
Computed tomography of PAES is a new diagnostic approach that has been made possible by improvements in CT-scanner technology. The main advantage of the CT scan arises from its capacity to view the 3-dimensional model from any angle after data acquistion in order to best visualize soft-tissue anatomy—in our case, the position of the artery in relation to that of the surrounding muscles. Careful analysis of axial scans on the monitor enables accurate grading of popliteal arterial stenoses and evaluation of surrounding muscular anomalies. Once images are loaded at the workstation, axial scans can be viewed rapidly by scrolling up and down the vascular tree. Interpretation of axial scans on the monitor also enables electronic enlargement of each affected leg segment and rapid changing of window parameters. When axial CT scans are interpreted, diagnostic errors caused by the superimposition of overlying structures such as bone are avoided by increased versatility of image postprocessing. Lateral and oblique views of CT images are useful for detection of arterial deviation and aberrant muscle, but axial slices provide the most accurate view. Computed tomography may be more accurate than digital subtraction angiography, due to its multiplane reconstruction capabilities. The speed of current CT scanners enables dynamic imaging during peak bolus contrast enhancement. The inherent high contrast available with CT—along with its ability to reconstruct sans the superimposition of overlying structures—yields excellent visualization of normal, stenosed, and thrombosed vascular lumen. For acutely ischemic limbs, it is of major importance to know the site and origin of vascular occlusion and the degree to which distal vessels are being refilled. Therefore, the accuracy of CT in imaging acute disease should be at least as high as that in imaging chronic disease. For the acutely ischemic leg, CT may provide important information not provided by DSA, such as the presence of aberrant muscle, the relationship between the popliteal artery and surrounding structures, and information about other conditions affecting the popliteal artery, such as cystic adventitial disease and thrombosed popliteal artery aneurysm. Computed tomography can detect occlusion, deviation, and stenosis of the popliteal arteries. It can be diagnostic when arteriography is not helpful (for example, in a case of late-stage PAES with thrombosed artery), and it can confirm normal contralateral anatomy, thereby eliminating suspicion of bilateral entrapment. 57–63 The major limitations of current CT technology are attributable to limited tube capacity.
Traditionally, contrast arteriography, preferably bilateral, has been considered the definitive test. The following angiographic signs can be observed: internal deviation, flatness or a clinched appearance of the popliteal artery, narrowing or slight angulation, and sometimes poststenotic dilatation. Transient partial lesions of the popliteal artery wall, with absence of lesions at other levels, are certainly reason for suspicion of PAES. When suspected, PAES can be established by dynamic arteriography with runoff during stress, performed with the patient's leg in hyperextension. Deviation (medial or lateral) and occlusion of the proximal popliteal artery with extended knee confirms the diagnosis of popliteal entrapment. When entrapment has produced popliteal artery occlusion, positional maneuvers will not, of course, aid diagnosis. Complete obstruction of the popliteal artery in the presence of large collateral arteries and normal proximal and distal arteries is usually seen in later stages of PAES.
It is well known that conventional plain film arteriography often leads to overestimation of the length of occluded vessel segments, a phenomenon that may be caused by dilution of the bolus of contrast material as it traverses segmental occlusions or stenosis between the site of injection and the distal target.
When DSA is used, overestimation should not occur, because the acquisition series are not stopped until there is no further filling of arteries. Digital subtraction angiography is well suited for the detection of slow flow and retrograde flow. The length of the popliteal occlusion and the degree of refilling via collateral vessels are important in planning therapy for patients with late-phase PAES. In the preoperative work-up of patients with severe occlusive PAES, the identification of patent runoff vessels is crucial. The use of the digital subtraction technique has overcome the problem of bone superimposition. At our institution, it is standard practice to obtain multiple comparative series of popliteal artery studies for functional diagnosis of entrapment. 64–68 In doing this, we perform intraarterial digital subtraction angiography of the lower extremities in combination with Doppler ultrasonographic examination and computed tomographic angiography.
In symptomatic cases of PAES, surgery is definitely indicated in order to establish normal anatomy within the popliteal space and restore normal arterial flow to the extremity. The best surgical approach is a posterior S-shaped incision in the popliteal fossa, which enables complete exposure of the popliteal artery and its surrounding structures. Although the medial approach yields better exposure of the superficial femoral and tibial arteries, it does not enable identification of the structures around the popliteal artery and confers, therefore, a high risk of incomplete section of the musculotendinous structures causing the entrapment. When the diagnosis of entrapment is made at an early stage and the popliteal artery is intact, the treatment of choice is the release of the popliteal artery by division of the anomalous musculotendinous tissue. If the diagnosis is made later, when the popliteal artery is occluded, stenotic, or aneurysmal, the treatment of choice is vascular reconstruction, in addition to division of the anomalous musculotendinous structure.
Thromboendarterectomy is difficult to perform in the entrapped segment of popliteal artery, because no plane of cleavage exists in the wall of the fibrous, cord-like artery. If decompression of the popliteal artery by division of the anomalous muscle is not in itself adequate, the interposition of autologous vein graft is also necessary. This is usually a popliteo-popliteal bypass, kept as short as possible. Our material of choice is usually the contralateral long saphenous vein. 25,69,70 The ipsilateral long and short saphenous veins are usually spared, because of their importance in this area, in which the circulation already has been compromised.
Footnotes
Address for reprints: Vedran Radonić, MD, Department of Surgery, University Hospital, Split, Spinc̆ićeva 1, 21 000 Split, Croatia
References
- 1.Rich NM, Baugh JH, Hughes CW. Popliteal artery injuries in Vietnam. Am J Surg 1969;118:531–4. [DOI] [PubMed]
- 2.McCabe CJ, Ferguson CM, Ottinger LW. Improved limb salvage in popliteal artery injuries. J Trauma 1983;23:982–5. [DOI] [PubMed]
- 3.Krige JE, Spence RA. Popliteal artery trauma: a high risk injury. Br J Surg 1987;74:91–4. [DOI] [PubMed]
- 4.Radonic V, Baric D, Petricevic A, Andric D, Radonic S. Military injuries to the popliteal vessels in Croatia. J Cardiovasc Surg (Torino)1994;35:27–32. [PubMed]
- 5.Darling RC, Buckley CJ, Abbott WM, Raines JK. Intermittent claudication in young athletes: popliteal artery entrapment syndrome. J Trauma 1974;14:543–52. [DOI] [PubMed]
- 6.Girvan RJ, Stone PA, McGarry JJ. Acute dysvascular limb in a young adult. A case study. J Am Podiatr Med Assoc 1984;84:591–7. [DOI] [PubMed]
- 7.Cohn SL, Taylor WC. Vascular problems of the lower extremity in athletes. Clin Sports Med 1990;9:449–70. [PubMed]
- 8.Hoelting T, Schuermann G, Allenberg JR. Entrapment of the popliteal artery and its surgical management in a 20-year period. Br J Surg 1997;84:338–41. [PubMed]
- 9.Inada K, Hirose M, Iwashima Y, Matsumoto K. Popliteal artery entrapment syndrome: a case report. Br J Surg 1978; 65:613–5. [DOI] [PubMed]
- 10.Ikeda M, Iwase T, Ashida K, Tankawa H. Popliteal artery entrapment syndrome. Report of a case and study of 18 cases in Japan. Am J Surg 1981;141:726–30. [DOI] [PubMed]
- 11.Koplic S, Maskovic J, Radonic V. Musculotendinous pressure on the arteries of the knee observed in a patient with obstructive entrapment syndrome of the popliteal artery and vein [in Serbo-Croatian (Roman)]. Acta Chir Iugosl 1982;29(Suppl 2):189–93. [PubMed]
- 12.Iwai T, Konno S, Soga K, Hatano R, Sato S, Yamada T, et al. Diagnostic and pathological considerations in the popliteal artery entrapment syndrome. J Cardiovasc Surg (Torino) 1983;24:243–9. [PubMed]
- 13.Taunton JE, Maxwell TM. Intermittent claudication in an athlete—popliteal artery entrapment: a case report. Can J Appl Sport Sci 1982;7:161–3. [PubMed]
- 14.Bouhoutsos I, Goulios A. Popliteal artery entrapment. Report of a case. J Cardiovasc Surg (Torino) 1977;18:481–4. [PubMed]
- 15.Al Shahri AMG, Al Haddad MC. Popliteal artery entrapment syndrome: report of two cases. Saudi Medical Journal 1993;14:557–61.
- 16.Love JW, Whelan TJ. Popliteal artery entrapment syndrome. Am J Surg 1965;109:620–4. [DOI] [PubMed]
- 17.Rich NM, Collins GJ Jr, McDonald PT, Kozloff L, Clagett GP, Collins JT. Popliteal vascular entrapment. Its increasing interest. Arch Surg 1979;114:1377–84. [DOI] [PubMed]
- 18.Rich NM. Popliteal entrapment and adventitial cystic disease. Surg Clin North Am 1982;62:449–65. [DOI] [PubMed]
- 19.Stuart TPA. Note on a variation in the course of the popliteal artery. J Anat Physiol 1879;13:162–5. [PMC free article] [PubMed]
- 20.Chambardel-Dubreuil LL. Variations des artères du pelvis et du membre inferieur. Paris: Masson and Cie, 1925.
- 21.Hamming JJ. Intermittent claudication at an early age, due to an anomalous course of the popliteal artery. Angiology 1959;10:369–71. [DOI] [PubMed]
- 22.Servello M. Clinical syndrome of anomalous position of the popliteal artery. Differentiation from juvenile arteriopathy. Circulation 1962;26:885–90. [DOI] [PubMed]
- 23.Delaney TA, Gonzalez LL. Occlusion of popliteal artery due to muscular entrapment. Surgery 1971;69:97–101. [PubMed]
- 24.Laubach K, Trede M, Perera R, Saggau W. Compression syndrome of the popliteal artery [in German]. Chirurg 1973;44:74–7. [PubMed]
- 25.Schuermann G, Mattfeldt T, Hofmann W, Hohenberger P, Allenberg JR. The popliteal artery entrapment syndrome: presentation, morphology and surgical treatment of 13 cases. Eur J Vasc Surg 1990;4:223–31. [DOI] [PubMed]
- 26.Kogel H, Vollmar JF, Hutschenreiter S. A new variant of the popliteal artery compression syndrome [in German]. Langenbecks Arch Chir 1990;375:171–4. [DOI] [PubMed]
- 27.Ohta M, Kusaba A, Shrestha DR, Koja K, Kina M, Shiroma H, et al. Popliteal artery entrapment syndrome. Report of two cases. J Cardiovasc Surg (Torino) 1991;32:697–701. [PubMed]
- 28.Simon JJ, Soppelsa A. The trapped popliteal artery syndrome. About one bilateral case [in French]. J Chir (Paris) 1981;118:179–83. [PubMed]
- 29.Ferrero R, Barile C, Bretto P, Buzzacchino A, Ponzio F. Popliteal artery entrapment syndrome. Report on seven cases. J Cardiovasc Surg (Torino) 1980;21:45–52. [PubMed]
- 30.Bouhoutsos J, Daskalakis E. Muscular abnormalities affecting the popliteal vessels. Br J Surg 1981;68:501–6. [DOI] [PubMed]
- 31.Represa JA, De Diego JA, Molina LM, Delgado I, Calvo MG, Canizo JF, et al. Popliteal artery entrapment syndrome. J Cardiovasc Surg (Torino)1986;27:426–30. [PubMed]
- 32.Choghari C, Bosschaerts T, Locufier JL, Barthel J, Barroy JP. Variant form of popliteal artery entrapment syndrome. Acta Chir Belg 1993;93:34–7. [PubMed]
- 33.Erdoes LS, Devine JJ, Bernhard VM, Baker MR, Berman SS, Hunter GC. Popliteal vascular compression in a normal population. J Vasc Surg 1994;20:978–86. [DOI] [PubMed]
- 34.Turnipseed WD, Pozniak M. Popliteal entrapment as a result of neurovascular compression by the soleus and plantaris muscles. J Vasc Surg 1992;15:285–94. [PubMed]
- 35.di Marzo L, Cavallaro A, Sciacca V, Mingoli A, Stipa S. Natural history of entrapment of the popliteal artery. J Am Coll Surg 1994;178:553–6. [PubMed]
- 36.di Marzo L, Cavallaro A, Sciacca V, Mingoli A, Tamburelli A. Surgical treatment of popliteal artery entrapment syndrome: a ten-year experience. Eur J Vasc Surg 1991;5:59–64. [DOI] [PubMed]
- 37.Lambert AW, Wilkins DC. Popliteal artery entrapment syndrome. Br J Surg 1999;86:1365–70. [DOI] [PubMed]
- 38.Andrikopoulos V, Papacharalambous G, Antoniou I, Panoussis P. The popliteal artery entrapment syndrome. Vasc Surg 1999;33:357–65.
- 39.Ring DH Jr, Haines GA, Miller DL. Popliteal artery entrapment syndrome: arteriographic findings and thrombolytic therapy. J Vasc Interv Radiol 1999;10:713–21. [DOI] [PubMed]
- 40.Schäfer K, Sell G, Schäfer B, Rumpelt HJ. Die cystische adventitia-degeneration der arteria poplitea als mogliche folge eines entrapment-syndroms. Der Chirurg 1995;66:154–7. [PubMed]
- 41.Rich NM, Hughes CW. Popliteal artery and vein entrapment. Am J Surg 1967;113:696–8. [DOI] [PubMed]
- 42.Connell J. Popliteal vein entrapment. Br J Surg 1978;65:351. [DOI] [PubMed]
- 43.Mas̆kovic J, Koplic S, Gacina M, Cambj Lj, Radonic V, Gjakun K. Entrapment syndrome of popliteal artery and vein. Radiol Iugosl 1981;15:125–8.
- 44.Leconte D, Wagner JM, Maguin D, Hiebel G, Peter R. An uncommon syndrome: the trapped popliteal vein. Apropos of a case. J Chir 1986;123:358–61. [PubMed]
- 45.Iwai T, Sato S, Yamada T, Muraoka Y, Sakurazawa K, Kinoshita H, et al. Popliteal vein entrapment caused by the third head of the gastrocnemius muscle. Br J Surg 1987;74:1006–8. [DOI] [PubMed]
- 46.Gerkin TM, Beebe HG, Williams DM, Bloom JR, Wake-field TW. Popliteal vein entrapment presenting as deep venous thrombosis and chronic venous insufficiency. J Vasc Surg 1993;18:760–6. [DOI] [PubMed]
- 47.Lundell C, Kadir S. The jogger's aneurysm: unusual presentation of popliteal artery trauma. Cardiovasc Intervent Radiol 1981;4:239–41. [DOI] [PubMed]
- 48.Gyftokostas D, Koutsoumbelis C, Mattheou T, Bouhoutsos J. Post stenotic aneurysm in popliteal artery entrapment syndrome. J Cardiovasc Surg (Torino) 1991;32:350–2. [PubMed]
- 49.Fong H, Downs AR. Popliteal artery entrapment syndrome with distal embolization. A report of two cases. J Cardiovasc Surg (Torino) 1989;30:85–8. [PubMed]
- 50.Gedeon A, Puel P, Castany R, Boccardo JP, Barret A, Cerene A, et al. Non-atheromatous obliterations of the popliteal artery [in French]. Chirurgie 1975;101:355–60. [PubMed]
- 51.McDonald PT, Easterbrook JA, Rich NM, Collins GJ Jr, Kozloff L, Clagett GP, et al. Popliteal artery entrapment syndrome. Clinical, noninvasive and angiographic diagnosis. Am J Surg 1980;139:318–25. [DOI] [PubMed]
- 52.Cavallaro A, Di Marzo L, Gallo P, Cisternino S, Mingoli A. Popliteal artery entrapment. Analysis of the literature and report of personal experience. Vasc Surg 1986;20:404–23.
- 53.Miles S, Roediger W, Cooke P, Mieny CJ. Doppler ultrasound in the diagnosis of the popliteal artery entrapment syndrome. Br J Surg 1977;64:883–4. [DOI] [PubMed]
- 54.Collins PS, McDonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg 1989;10:484–90. [DOI] [PubMed]
- 55.di Marzo L, Cavallaro A, Sciacca V, Lepidi S, Marmorale T, Tamburelli A, et al. Diagnosis of popliteal artery entrapment syndrome: the role of duplex scanning. J Vasc Surg 1991;13:434–8. [DOI] [PubMed]
- 56.MacSweeney ST, Cuming R, Greenhalgh RM. Colour Doppler ultrasonographic imaging in the diagnosis of popliteal artery entrapment syndrome. Br J Surg 1994;81:822–3. [DOI] [PubMed]
- 57.Alder W, Zwicker H. Computer tomographic demonstration of the popliteal artery entrapment syndrome [in German]. ROFO Fortschr Geb Rontgenstr Nuklearmed 1979; 130:543–5. [DOI] [PubMed]
- 58.Muller N, Morris DC, Nichols DM. Popliteal artery entrapment demonstrated by CT. Radiology 1984;151:157–8. [DOI] [PubMed]
- 59.Goebel N, Brunner U, Bollinger A. CT aspects of the most common variant of the popliteal artery entrapment syndrome [in German]. ROFO Fortschr Geb Roentgenstr Nuklearmed 1985;142:698–700. [DOI] [PubMed]
- 60.Wehmann TW. Computed tomography in the diagnosis and management of popliteal artery entrapment syndrome. J Am Osteopath Assoc 1993;93:1039–42, 1047–50. [PubMed]
- 61.Rizzo RJ, Flinn WR, Yao JS, McCarthy WJ, Vogelzang RL, Pearce WH. Computed tomography for evaluation of arterial disease in the popliteal fossa. J Vasc Surg 1990;11:112–9. [DOI] [PubMed]
- 62.Takase K, Imakita S, Kuribayashi S, Onishi Y, Takamiya M. Popliteal artery entrapment syndrome: aberrant origin of gastrocnemius muscle shown by 3D CT. J Comput Assist Tomogr 1997;21:523–8. [DOI] [PubMed]
- 63.Williams LR, Flinn WR, McCarthy WJ, Yao JS, Bergan JJ. Popliteal artery entrapment: diagnosis by computed tomography. J Vasc Surg 1986;3:360–3. [PubMed]
- 64.Rieker O, Duber C, Schmiedt W, von Zitzewitz H, Schweden F, Thelen M. Prospective comparison of CT angiography of the legs with intraarterial digital subtraction angiography. AJR Am J Roentgenol 1996;166:269–76. [DOI] [PubMed]
- 65.Gost AL, Mestre M, Magrinya J, Llobera M. Entrapment syndrome of the popliteal artery. J Cardiovasc Surg (Torino) 1981;22:353–9. [PubMed]
- 66.Greenwood LH, Yrizarry JM, Hallett JW Jr. Popliteal artery entrapment: importance of the stress runoff for diagnosis. Cardiovasc Intervent Radiol 1986;9:93–9. [DOI] [PubMed]
- 67.Sanders RJ, Alston GK. Variations and anomalies of the popliteal and tibial arteries. Am J Surg 1986;152:531–4. [DOI] [PubMed]
- 68.Fontanetta P, Kirshbom I, Fisher MM, Katz M, Clauss RH. Popliteal artery entrapment: lateral deviation and compression of artery. Vasa 1974;3:399–403. [PubMed]
- 69.Barabas AP, Macfarlane R. Popliteal artery entrapment syndrome. Br J Hosp Med 1985;34:304–6. [PubMed]
- 70.Zund G, Roggo A, Etter C, Brunner U. Differential surgical therapy of popliteal entrapment syndrome 1967 to 1992 [in German]. Helv Chir Acta 1994;60:879–81. [PubMed]