Abstract
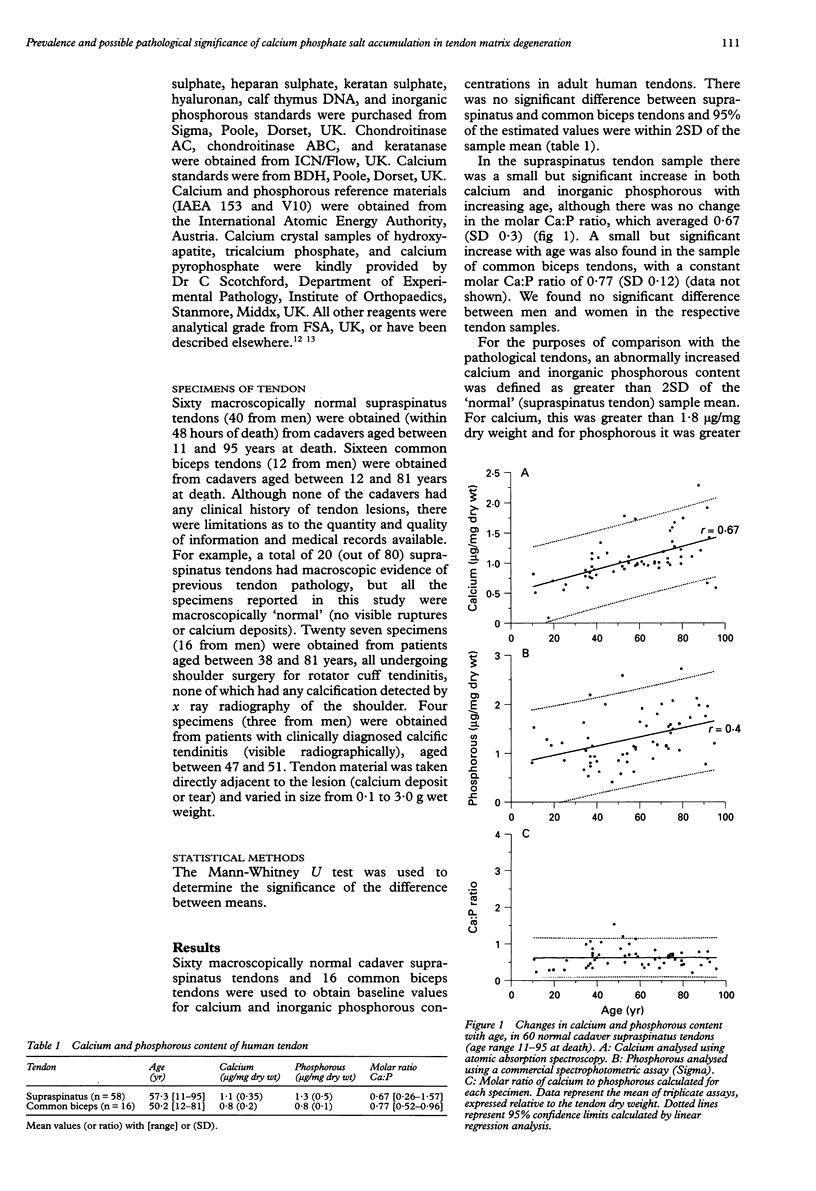
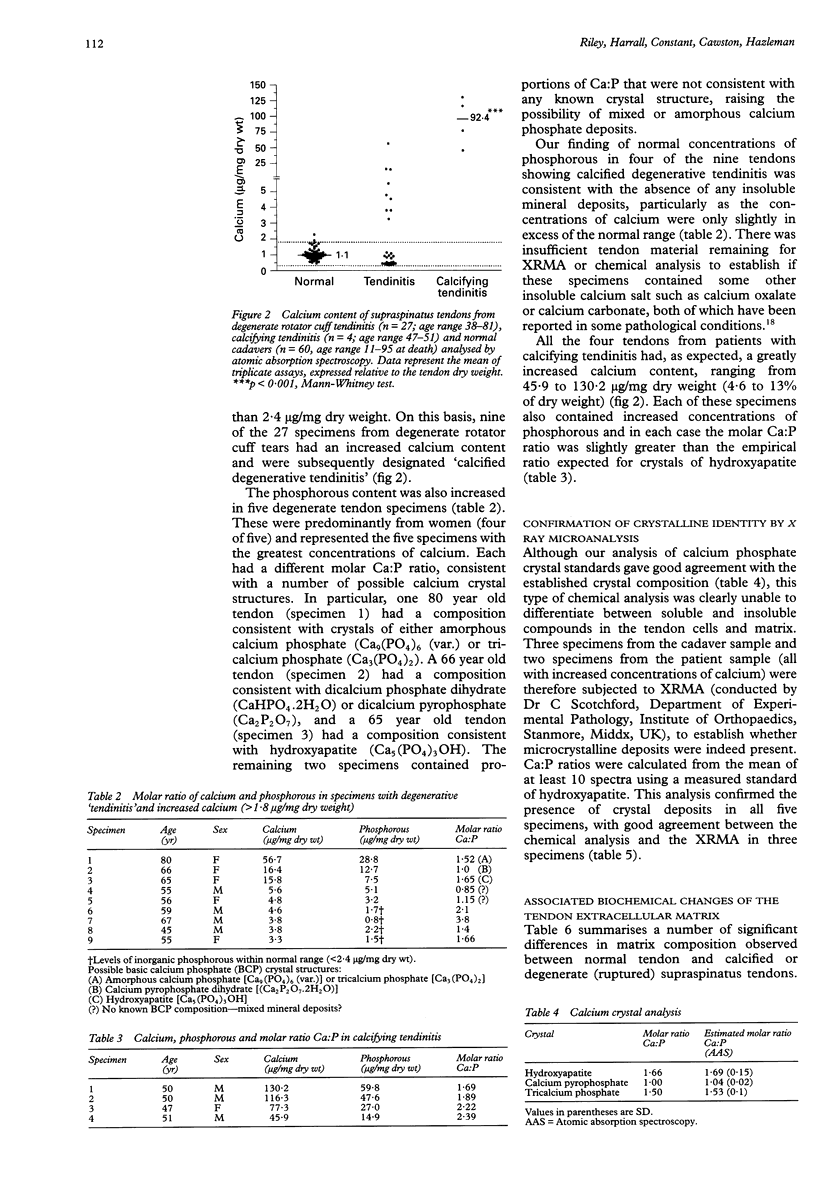
OBJECTIVES: To investigate the prevalence of calcium phosphate mineral salt accumulation in degenerative supraspinatus 'tendinitis' compared with a normal sample of human tendons, and to determine whether there is an association of calcium salt deposition with pathological changes in the tendon extracellular matrix. METHODS: Cadaver tendons (supraspinatus and common biceps tendons, n = 96) and fragments of supraspinatus tendons obtained during shoulder surgery (n = 31) were analysed for calcium content by atomic absorption spectroscopy, phosphorous content using a spectrophotometric assay, and matrix composition (collagen, glycosaminoglycans and DNA) using standard biochemical techniques. RESULTS: We established baseline values of calcium concentration in macroscopically normal cadaver tendons (mean 1.1 (SD 0.35) micrograms/mg dry wt, n = 60) and found that 33% (nine of 27) of ruptured tendons from patients with 'degenerative tendinitis' contained an excess of calcium (more than 2SD greater than the normal sample mean). Five of these specimens had increased concentrations of phosphorous and calcium:phosphorous (molar) ratios consistent with a variety of possible calcium crystals, including calcium pyrophosphate, hydroxyapatite, and tricalcium phosphate, in addition to mixed or amorphous calcium phosphate deposits. Four of these specimens contained normal concentrations of phosphorous, consistent with deposits of calcium oxalate or calcium carbonate, although this was not confirmed biochemically. In contrast, surgical specimens (n = 4) from patients with 'calcifying tendinitis' (radiographically detected calcium deposits) all contained salts with a mineral composition consistent with hydroxyapatite. The presence and identity of crystal deposits was subsequently confirmed in five specimens by radiographic microanalysis. Analysis of the tendon matrix demonstrated a number of significant differences between normal and degenerate (ruptured) tendons, including a reduction in collagen content, an increase in sulphated glycosaminoglycans (predominantly dermatan sulphate) and an increase in DNA (cellular) content. However, there were no significant differences between degenerate tendons that were 'calcified' and those degenerate specimens that contained normal concentrations of calcium. CONCLUSIONS: Although there was a relatively high prevalence of calcium salts in degenerate tendons, which might contribute to the pathological process (such as increased matrix collagen degradation), these data are consistent with the hypothesis that 'dystrophic calcification' of degenerate tendon matrix is a pathological entity distinct from cell mediated 'calcifying tendinitis'. Calcification is probably one possible outcome (or end point) of chronic tendon injury, although the possibility exists that in many cases, the presence of calcium salts may contribute to the tendon matrix degeneration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. Apatite-type crystal deposition in arthritic cartilage. Scan Electron Microsc. 1985;(Pt 4):1555–1566. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Boskey A. L., Maresca M., Armstrong A. L., Ehrlich M. G. Treatment of proteoglycan aggregates with physeal enzymes reduces their ability to inhibit hydroxyapatite proliferation in a gelatin gel. J Orthop Res. 1992 May;10(3):313–319. doi: 10.1002/jor.1100100302. [DOI] [PubMed] [Google Scholar]
- Boskey A. L. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 1989 May;6(2):111–123. doi: 10.1016/0169-6009(89)90044-5. [DOI] [PubMed] [Google Scholar]
- COTTON R. E., RIDEOUT D. F. TEARS OF THE HUMERAL ROTATOR CUFF; A RADIOLOGICAL AND PATHOLOGICAL NECROPSY SURVEY. J Bone Joint Surg Br. 1964 May;46:314–328. [PubMed] [Google Scholar]
- Chard M. D., Cawston T. E., Riley G. P., Gresham G. A., Hazleman B. L. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994 Jan;53(1):30–34. doi: 10.1136/ard.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. S., Halverson P. B., McCarty D. J. Release of collagenase, neutral protease, and prostaglandins from cultured mammalian synovial cells by hydroxyapatite and calcium pyrophosphate dihydrate crystals. Arthritis Rheum. 1981 Nov;24(11):1338–1344. doi: 10.1002/art.1780241102. [DOI] [PubMed] [Google Scholar]
- Cheung H. S., Story M. T., McCarty D. J. Mitogenic effects of hydroxyapatite and calcium pyrophosphate dihydrate crystals on cultured mammalian cells. Arthritis Rheum. 1984 Jun;27(6):668–674. doi: 10.1002/art.1780270610. [DOI] [PubMed] [Google Scholar]
- Cofield R. H. Rotator cuff disease of the shoulder. J Bone Joint Surg Am. 1985 Jul;67(6):974–979. [PubMed] [Google Scholar]
- Dalton S., Cawston T. E., Riley G. P., Bayley I. J., Hazleman B. L. Human shoulder tendon biopsy samples in organ culture produce procollagenase and tissue inhibitor of metalloproteinases. Ann Rheum Dis. 1995 Jul;54(7):571–577. doi: 10.1136/ard.54.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E. H., Spadaro J. A., Becker R. O. Trace elements in tendon collagen. Clin Orthop Relat Res. 1969 Jul-Aug;65:195–198. [PubMed] [Google Scholar]
- Hunter G. K., Szigety S. K. Effects of proteoglycan on hydroxyapatite formation under non-steady-state and pseudo-steady-state conditions. Matrix. 1992 Nov;12(5):362–368. doi: 10.1016/s0934-8832(11)80032-6. [DOI] [PubMed] [Google Scholar]
- Józsa L., Bálint B. J., Réffy A. Calcifying tendinopathy. Arch Orthop Trauma Surg. 1980;97(4):305–307. doi: 10.1007/BF00380713. [DOI] [PubMed] [Google Scholar]
- Józsa L., Réffy A., Bálint J. B. The pathogenesis of tendolipomatosis; an electron microscopical study. Int Orthop. 1984;7(4):251–255. doi: 10.1007/BF00266836. [DOI] [PubMed] [Google Scholar]
- Kannus P., Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991 Dec;73(10):1507–1525. [PubMed] [Google Scholar]
- Lipman J. M. Fluorophotometric quantitation of DNA in articular cartilage utilizing Hoechst 33258. Anal Biochem. 1989 Jan;176(1):128–131. doi: 10.1016/0003-2697(89)90282-0. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Alini M., Webber C., Poole A. R. Characterization of aggregating proteoglycans from the proliferative, maturing, hypertrophic, and calcifying zones of the cartilaginous physis. J Bone Joint Surg Am. 1991 Aug;73(7):1064–1074. [PubMed] [Google Scholar]
- McLAUGHLIN H. L., ASHERMAN E. G. Lesions of the musculotendinous cuff of the shoulder. IV. Some observations based upon the results of surgical repair. J Bone Joint Surg Am. 1951 Jan;33 A(1):76–86. [PubMed] [Google Scholar]
- Riley G. P., Harrall R. L., Constant C. R., Chard M. D., Cawston T. E., Hazleman B. L. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994 Jun;53(6):367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G. P., Harrall R. L., Constant C. R., Chard M. D., Cawston T. E., Hazleman B. L. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994 Jun;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney P., Walker D., Grant M. E., McClure J. Cartilage and bone formation in repairing Achilles tendons within diffusion chambers: evidence for tendon-cartilage and cartilage-bone conversion in vivo. J Pathol. 1993 Mar;169(3):375–381. doi: 10.1002/path.1711690315. [DOI] [PubMed] [Google Scholar]
- Uhthoff H. K. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch A Pathol Anat Histol. 1975;366(1):51–58. doi: 10.1007/BF00438677. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Williams I. F., McCullagh K. G., Silver I. A. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12(3-4):211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]


