ABSTRACT
Nematode-trapping (NT) fungi are a unique group of carnivorous microorganisms that can capture and digest nematodes by producing ingenious trapping devices (traps). Arthrobotrys oligospora, a representative NT fungus, can develop adhesive three-dimensional networks for nematode predation. Hyphal fusion is indispensable for the trap formation of A. oligospora. Here, we characterized an orthologous Ste12 protein (AoSte12) in A. oligospora via gene disruption, DNA affinity purification sequencing (DAP-Seq), and multi-omics approaches. The disruption of the Aoste12 gene caused an increase in hyphal fusion and resulted in defects in mycelial growth, conidiation, trap morphology, and stress resistance, as well as reducing the number of nuclei and lipid droplet accumulation. Moreover, transcriptome and DAP-Seq analysis revealed that AoSte12 was involved in cellular processes associated with growth, cell fusion, the tricarboxylic acid cycle, vesicles, actin filaments, and lipid metabolism. In addition, combining metabolome with transcriptome and DAP-Seq analysis indicated that AoSte12 was involved in the mitogen-activated protein kinase signaling pathway, lipid metabolism, and secondary metabolites. A yeast two-hybrid assay revealed that AoSte12 can interact with diverse proteins, such as the MAK-2 orthologue protein Fus3, the vacuolar sorting protein Pep3, and UDP-glycosyltransferase. Our results suggest that AoSte12 plays an indispensable role in hyphal fusion and thus regulates sporulation and trap morphogenesis. These results provide deep insights into the connection between hyphal fusion and trap formation in NT fungi.
IMPORTANCE Nematode-trapping (NT) fungi are an important natural enemy of nematodes and can capture their prey by producing traps. Hyphal anastomosis and fusion are important for mycelial growth and the colony morphological development of filamentous fungi and are also crucial for the trap morphogenesis of NT fungi. Arthrobotrys oligospora can form complex three-dimensional networks (traps) when sensing the presence of nematodes. This study revealed that AoSte12 is indispensable for hyphal fusion and that it regulates mycelial growth, conidiation, trap morphogenesis, stress resistance, the number of nuclei, and lipid droplet accumulation in A. oligospora. In addition, DNA affinity purification sequencing, transcriptome, and metabolome analyses further revealed that AoSte12 is involved in the mitogen-activated protein kinase pathway, lipid metabolism, and secondary metabolism. Overall, these findings expand the important role of AoSte12 in NT fungus A. oligospora and provide a broad foundation for elucidating the regulatory mechanism of trap development and the lifestyle transitions of pathogenic fungi.
KEYWORDS: transcription factor Ste12, hyphal fusion, trap formation, conidiation, secondary metabolism, stress response
INTRODUCTION
Nematode-trapping (NT) fungi are a special ecological group that can use their own specialized structures (traps) to capture nematodes that move freely in the soil. Most NT fungi can live both saprophytically on organic matter and, as predators, by capturing tiny animals (1–3). NT fungi change their saprophytic lifestyle to a predacious lifestyle by producing traps, so traps are not only the weapons that NT fungi use to attack nematodes but are also an important indicator of their lifestyle transition (4, 5). As potential biocontrol agents, the origin of NT fungi and the regulation mechanism of trap formation and secondary metabolites have attracted extensive interest (6, 7). Arthrobotrys oligospora is a typical NT fungus that produces an adhesive three-dimensional network, and it is often used as a potential model for studying the interactions between fungi and nematodes (8, 9). In recent years, multiple signaling pathways and cellular processes, including the mitogen-activated protein kinase (MAPK) (7, 10), G protein signaling (11–13), small GTPases (14, 15), autophagy (2, 16), and peroxisome (17) have been shown to involve in mycelial development and trap formation of NT fungus in A. oligospora.
The formation of traps in A. oligospora initially involves a ring structure; on this basis, multiple ring structures are formed, and they are finally combined to form a three-dimensional network; the combination of these ring structures is closely related to the fusion between the hyphae (18). Hyphal fusion is manifested in conidial anastomosis tubes formed between conidia; it is also reflected in the stage of hyphal growth and development (19). Moreover, in the pathogenic stage of NT fungi, the initial stages of trap formation also require hyphal fusion (18). Similar to cell fusion in other organisms, the process of hyphal fusion requires cell recognition, adhesion, and membrane merging (20). Hyphal fusion is regulated by many aspects, including MAPK cascades, reactive-oxygen-species (ROS)-generating systems, cell polarity factors, Ca2+ signaling factors, and the so-called STRIPAK (striatin interacting phosphatase and kinase) complex (19). The MAK-1/ADV-1 pathway and the MAK-2/PP-1 pathway engage in cross talk, and both pathways form a regulatory network that mediates growth, communication, and fusion (20). Recently, an orthologue of the Neurospora crassa hyphal anastomosis gene sofT, which is involved in hyphal anastomosis and cell-to-cell communication, was identified in the NT fungus Arthrobotrys flagrans (Duddingtonia flagrans); the absence of sofT caused a reduction in aerial mycelia, incomplete trap closure, and the absence of hyphal anastomoses (21). Therefore, hyphal fusion plays an important role in the formation of three-dimensional networks of Arthrobotrys species.
Ste12 is a C2H2 zinc finger protein transcription factor, which is homologous with the pheromone response pathway transcription factor Ste12 in Saccharomyces cerevisiae (22). Ste12 transcription factors, including PP-1, contain two C2H2 zinc finger protein motifs and an STE domain involved in binding DNA. In N. crassa, the STE domain is crucial for the function of transcription factors (23). Ste12 transcription factors are necessary for the cell fusion of Neurospora crassa and Trichoderma atroviride (24); however, for Aspergillus oryzae (25), Fusarium oxysporum (26), and the plant endophytic fungus Epichloë festucae (27), the absence of the ste12 gene has no effect on cell fusion. Ste12 transcription factors are also involved in the regulation of fungal growth, virulence, dimorphic morphological transformation, and sexual and asexual development (28). For example, Ste12 regulates the dimorphic morphological transformation of yeast cells in response to starvation (29). In plant- and animal-pathogenic fungi such as Magnaporthe oryzae (30), Metarhizium acridum (31), and Fusarium oxysporum (26), Ste12 plays an important regulatory role in appressorium formation and pathogenicity. Recently, an orthologous Ste12 was identified in A. oligospora (strain TWF154); the disruption of ste12 resulted in a defect in the formation of aerial hyphae and traps (32). However, the function and regulatory mechanism of Ste12 in the growth and development of A. oligospora and other NT fungi remain largely unknown.
In this study, we investigated the homologous protein Ste12 (AoSte12) in A. oligospora via multiphenotypic comparison. AoSte12 plays a crucial role in hyphal fusion and is involved in conidiation, trap morphogenesis, and lipid droplet (LD) accumulation. We also carried out transcriptome, DNA affinity purification sequencing (DAP-Seq), and yeast two-hybrid (Y2H) analyses to further excavate the downstream target genes of Aoste12. In addition, metabolome analysis was carried out for probing the role of AoSte12 in secondary metabolism.
RESULTS
Analysis of protein sequence and conserved domains of AoSte12.
The homologous protein AoSte12 (AOL_s00079g294) in A. oligospora was obtained by comparing the amino acid sequence of N. crassa Ste12 (XP_957811). AoSte12 contains 686 amino acids, and the predicted molecular weight and isoelectric point were 77 kDa and 6.33, respectively. The orthologs of Ste12 in the NT fungi and other filamentous fungi were downloaded and analyzed through BLAST comparison. The orthologs of Ste12 from NT fungi form an independent evolutionary branch (see Fig. S1A in the supplemental material). All Ste12 homologous proteins contain the Ste12 domain (IPR003120), and they also contain two C2H2 domains (IPR013087), except for Purpureocillium lilacinum and S. cerevisiae (see Fig. S1B). These orthologs of Ste12 in different fungi share a high degree of sequence similarity, among which the sequence similarity between AoSte12 and homologous proteins from five NT fungi is 89.2 to 96.4%, and the sequence similarity between AoSte12 and homologous proteins from other filamentous fungi is 51.3 to 54.8% (see Fig. S1C).
Disruption of Aoste12.
The gene Aoste12 was disrupted using the homologous recombination method (see Fig. S2A); the positive transformants were screened on PDAS medium (potato dextrose agar [PDA] supplemented with 0.6 M sucrose) containing 200 μg/mL hygromycin and verified via PCR with the primers Ste12F and Ste12R (see Fig. S2B and Table S1 in the supplemental material). Southern blotting was used for further verification. The genomic DNA of the wild-type (WT) and mutant strains was digested using the restriction enzyme MfeI, and a North2South chemiluminescence hybridization and detection kit was used to verify the WT and mutant strains (see Fig. S2C). Finally, three independent transformants (ΔAoste12-8, ΔAoste12-9, and ΔAoste12-13) were confirmed, and one of them (ΔAoste12-8) was selected for subsequent analysis due to their phenotypes being consistent.
AoSte12 regulates mycelial growth and stress resistance.
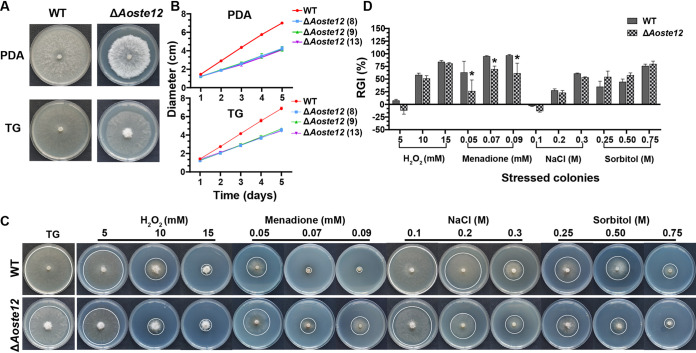
WT and mutant strains were inoculated on PDA and tryptone-glucose (TG) media at 28°C for 5 days, and the growth rates of the ΔAoste12 mutants were remarkably lower than WT strains on the two media (Fig. 1A and B). Furthermore, when cultured on a medium containing osmotic and oxidant agents, the sensitivity of the ΔAoste12 mutants was impaired. Notably, under the oxidative stress reagent containing menadione, the relative growth inhibition (RGI) values of the ΔAoste12 mutants were decreased by 27.05 to 36.81% compared to the WT strain, while under the condition of osmotic agents containing sorbitol, the RGI values were increased by 3.96 to 19.48%. At the same time, it was found that, under the conditions of 5 mM H2O2 and 0.1 M NaCl, the mycelial growth rate of the ΔAoste12 mutant was promoted (Fig. 1C and D).
FIG 1.
Comparison of mycelial growth and stress tolerance between wild-type (WT) and ΔAoste12 mutant strains. (A) Colonial morphologies of WT and ΔAoste12 mutant incubated on PDA and TG medium at 28°C for 5 days. (B) Mycelial growth rate of WT and ΔAoste12 mutants incubated on PDA and TG media. The numbers in parentheses after the strain names indicate the three independent ΔAoste12 mutants. (C) Colonial morphologies of WT and ΔAoste12 mutant in TG medium and medium supplemented with chemical agents (H2O2, menadione, NaCl, and sorbitol). The edge of colony was marked with a white circle. (D) Relative growth inhibition (RGI) of WT and ΔAoste12 mutant in the medium described above. Error bars represent the standard deviations. An asterisk indicates a significant difference between the mutant and the WT strain (Tukey’s HSD, P < 0.05).
AoSte12 regulates the morphology of conidiophores and conidia, the conidial yield, and the transcription of sporulation-related genes.
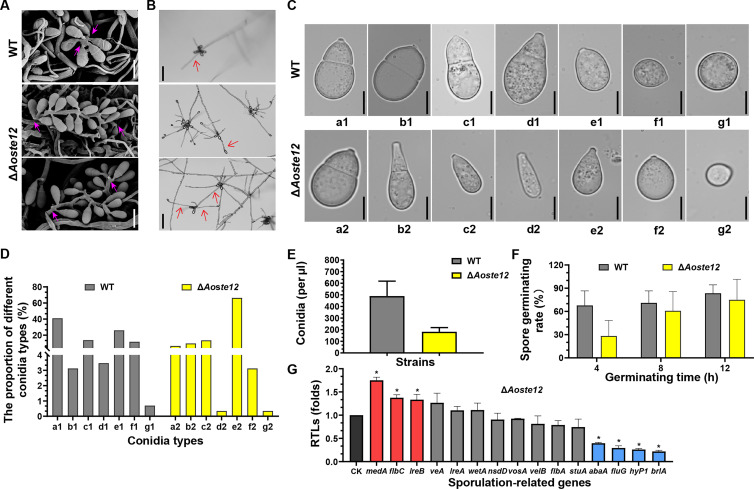
The deletion of Aoste12 caused remarkable defects in the morphologies of conidiophores and conidia. When the WT and ΔAoste12 mutant strains were cultured on cornmeal-molasses-yeast (CMY) medium for 7 days, the conidia of the ΔAoste12 mutant were not attached to the top of the conidiophores as in the WT strains, but conidia were attached to the conidiophores in a string shape (Fig. 2A). In addition, after 14 days of incubation, the WT conidia had germinated on the conidiophore, whereas the conidia of the ΔAoste12 mutant germinated and then formed conidiophores and conidia again (Fig. 2B). Furthermore, multiple morphologies (a to g) of conidia were observed in the WT and mutant strains; 66.3% of the conidia of the ΔAoste12 mutants were of type e2, which denotes the immature spore without a septum, whereas most of the WT conidia were of type a1 (40%), and only 6.6% of conidia were observed in the ΔAoste12 mutants to be in the form of type a1 (Fig. 2C and D). At the same time, the number of conidia of the ΔAoste12 mutant was significantly reduced (P < 0.05) by 62.8% compared to the WT strains, and the spore germination rate was decreased by 58.2% at 4 h, 14.3% at 8 h, and 10% at 12 h (Fig. 2E and F). In addition, the transcription of 15 genes associated with sporulation was detected via real-time quantitative PCR (RT-qPCR) analysis. The disruption of the Aoste12 gene led to upregulated expression of medA, flbC, and lreB, whereas the expression levels of abaA, fluG, hyp1, and brlA were significantly downregulated (P < 0.05) (Fig. 2G).
FIG 2.
Comparison of conidiophore and conidiation in WT and ΔAoste12 mutant. (A) Conidiophore morphologies observed via SEM. Scale bar, 20 μm. The pink arrows indicate conidia attached on conidiophore. (B) Morphologies of spore germination on the conidiophores of WT and ΔAoste12 mutant. Scale bar, 100 μm. The red arrow indicates the germinating hyphae on the conidiophore. (C) Different spore morphologies of WT (a1 to g1) and ΔAoste12 mutant (a2 to g2). Scale bar, 10 μm. (D) Statistical analysis of the proportion of different conidia types of WT and ΔAoste12 mutant. (E) The conidial yields of WT and ΔAoste12 mutant strains. (F) Comparison of the spore germination of WT and ΔAoste12 mutant at 4, 8, and 12 h. (G) The relative transcription levels (RTLs) of sporulation-related genes between WT and ΔAoste12 mutant at 5 days. CK is the standard (which has an RTL of 1) for statistical analysis of the RTL of each gene in the deletion mutant compared to that in the WT strain under a given condition. Error bars represent the standard deviations. An asterisk indicates a significant difference between the mutant and the WT strain (Tukey’s HSD, P < 0.05).
AoSte12 regulates hyphal fusion, mycelial networks, and trap morphogenesis.
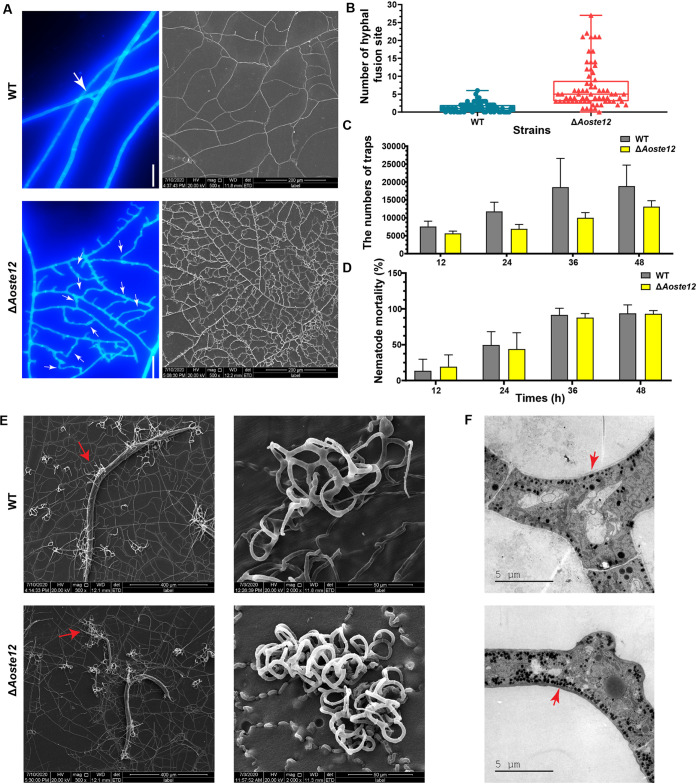
The WT and ΔAoste12 mutant strains were cultured on CMY medium at 28°C for 5 days, and the mycelia were stained using calcofluor white. The mycelial networks of the ΔAoste12 mutant became denser due to a remarkable increase in hyphal fusion (Fig. 3A). The average number of sites where hyphal fusion occurred was 7 in the ΔAoste12 mutants, whereas only 1.4 was observed in the WT strain (Fig. 3B). Similarly, the mycelial branching of the ΔAoste12 mutant was remarkably increased compared to the WT strain (Fig. 3A). To observe trap formation, the spores of the WT and mutant strains were incubated on water agar (WA) medium and cultured for 4 days; nematodes were then added for trap induction. The number of traps produced by the ΔAoste12 mutant were decreased compared to the WT strains, but the nematode predation efficiency was not correspondingly reduced (Fig. 3C and D). Moreover, the trap morphology was observed using scanning electron microscopy (SEM); the traps of the ΔAoste12 mutant contained more hyphal rings compared to the WT strain, which indicated that hyphal fusion was also increased in the traps of the ΔAoste12 mutant (Fig. 3E). Transmission electron microscope (TEM) observation of the traps showed that electron-dense bodies were increased in the ΔAoste12 mutant (Fig. 3F).
FIG 3.
Comparison of hyphal fusion, mycelial networks, trap formation and morphologies, electron-dense bodies, and nematicidal activity of wild-type (WT) and ΔAoste12 mutant strains. (A) Comparison of hyphal fusion and mycelial networks of WT and ΔAoste12 mutant. Hyphae were stained with calcofluor white and observed via fluorescent electron microscopy; mycelial networks were observed using SEM. White arrows: hyphal fusion sites. Scale bar, 10 μm. (B) Comparison of the number of hyphal fusion sites in WT and ΔAoste12 mutant. Hyphal fusion sites were counted under 68 fields viewed under a microscope. (C) Comparison of traps produced by WT and ΔAoste12 mutant. (D) Comparison of nematode mortality caused by WT and ΔAoste12 mutant. Error bars in panels B to D represent the standard deviations. (E) The traps and captured nematodes of the WT and ΔAoste12 mutant were observed via SEM. Red arrow, the captured nematodes. (F) Comparison of electron-dense bodies in traps of WT and ΔAoste12 mutant through TEM. Red arrow, electron-dense bodies.
AoSte12 regulates hyphal polar growth, the number of nuclei, and LD accumulation.
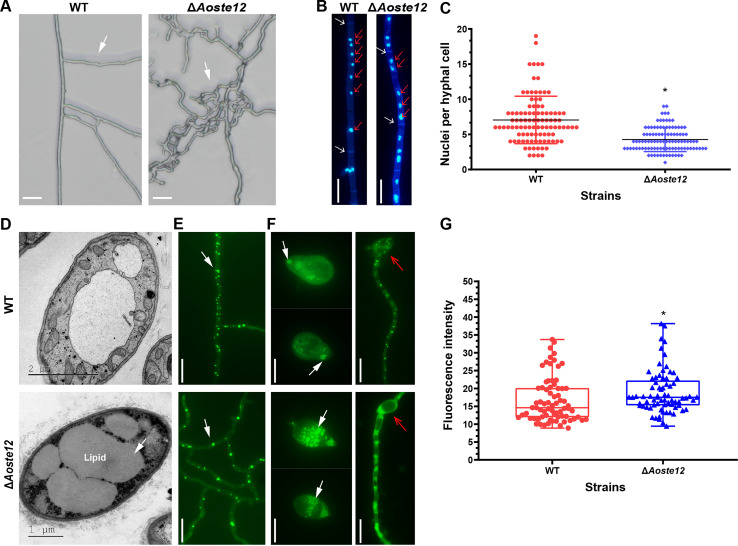
As mentioned above, the mycelial branch of the ΔAoste12 mutant was increased compared to the WT strain. When the WT and ΔAoste12 mutant strains were inoculated on WA at 28°C for 3 days, the polar growth of the ΔAoste12 mutant was altered, and the growth direction of its mycelium became confused (Fig. 4A). A further comparison of the number of nuclei in the mycelia showed that the average number of nuclei of the WT strains was seven, and that of the ΔAoste12 mutant was four (Fig. 4B and C). In addition, the lipid droplets (LDs) in the mycelia of the WT and mutants were observed using a BODIPY staining assay. More LDs were observed in the mycelia of the ΔAoste12 mutant, and the volume of the LDs became larger than that of the WT strain (Fig. 4D, E, and G). Similarly, LD accumulation in the conidia of the ΔAoste12 mutant was also increased, the vacuoles in the mycelia that germinated from conidia were larger in the ΔAoste12 mutant, and there was no accumulation of LDs when the conidia germinated for 12 h (Fig. 4F).
FIG 4.
Comparison of hyphal polar growth, and nucleus and lipid droplet accumulation of wild-type (WT) and ΔAoste12 mutant strains. (A) Comparison of hyphal polar growth of WT and ΔAoste12 mutant. (B) Hyphae were stained with DAPI, and the nuclei of the WT and the ΔAoste12 mutant were observed. White arrow, hyphal septum; red arrow, nucleus. (C) Comparison of cell nuclei in hyphae of the WT and ΔAoste12 mutant. (D) Observation of cellular ultrastructure in hyphae of WT and ΔAoste12 mutant via TEM. (E) Observation of lipid droplets in hyphae of WT and ΔAoste12 mutant by staining with boron dipyrromethene dye. (F) Observation of lipid droplets in conidia and germinated hyphae of spores in WT and ΔAoste12 mutant. White arrow (D to F), lipid droplets. Red arrow, germinated conidium. (G) Comparison of lipid droplet content of WT and ΔAoste12 mutant, which were detected as fluorescence intensity, and determined for at least 70 fields observed under fluorescence electron microscopy. An asterisk indicates a significant difference between the mutant and the WT strain (Tukey’s HSD, P < 0.05). White arrow (D, E, and F), lipid droplet. Scale bar (A, B, E, and F), 10 μm.
Transcriptomic insight into the regulatory role of AoSte12.
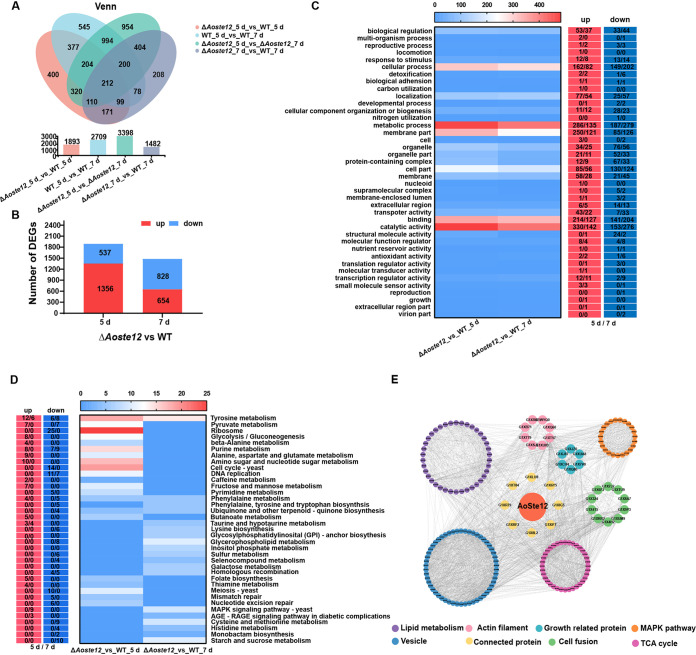
For further exploration into the regulatory role of AoSte12, the mycelial samples of WT and ΔAoste12 mutant strains were collected at 5 and 7 days postincubation (dpi) for transcriptome sequencing (RNA-Seq) analysis (see Table S2). Compared to the WT strain, the ΔAoste12 mutant shared 1,893 and 1,482 differentially expressed genes (DEGs) at 5 and 7 dpi, respectively (Fig. 5A). At 5 dpi, the ΔAoste12 mutant had 537 downregulated DEGs and 1,356 upregulated DEGs. At 7 dpi, there were 828 downregulated DEGs and 654 upregulated DEGs. Gene ontology (GO) enrichment analysis was carried out for these DEGs at 5 and 7 dpi (Fig. 5B). The upregulated and downregulated DEGs at 5 and 7 dpi displayed strong consistency, and they were mainly involved in biological regulation, cellular processes, localization, metabolic processes, membranes, membrane parts, cell parts, transport activity, binding, catalytic activity, organelles, and organelle parts (Fig. 5C).
FIG 5.
Comparison of DEGs between wild-type (WT) and ΔAoste12 mutant strains. (A) Venn diagram analysis of DEGs at two time points of ΔAoste12 mutant versus WT. (B) Numbers of upregulated and downregulated DEGs in the ΔAoste12 mutant versus the WT strain at different time points. (C) Gene ontology (GO) enrichment analysis of the ΔAoste12 mutant versus the WT strain at different time points. The red column indicates upregulated DEGs, the blue column indicates downregulated DEGs, and number/number indicates the DEGs of the ΔAoste12 mutant versus the WT strain at 5 and 7 days. (D) KEGG enrichment analysis of DEGs in the ΔAoste12 mutant versus the WT strain at different time points. (E) Proteins interact with enriched AoSte12 to correlate with the phenotypes and were analyzed through protein-protein interaction networks (STRING) and further visualized and analyzed with Cytoscape.
Similarly, a Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was carried out; the upregulated and downregulated DEGs at 5 and 7 dpi were enriched in different pathways. At 5 dpi, the upregulated DEGs were enriched in glycolysis/gluconeogenesis and multiple metabolic pathways, such as tyrosine metabolism; taurine and hypotaurine metabolism, pyruvate metabolism; purine metabolism; alanine, aspartate, and glutamate metabolism; amino sugar and nucleotide sugar metabolism; and fructose and mannose metabolism. The downregulated DEGs were remarkably enriched in ribosomes, the cell cycle, DNA replication, meiosis, mismatch repair, and nucleotide excision repair (Fig. 5D). At 7 dpi, the upregulated DEGs were enriched in tyrosine metabolism, taurine and hypotaurine metabolism, and the MAPK signaling pathway, while the downregulated DEGs were enriched in multiple metabolism and biosynthesis, such as pyruvate metabolism, phenylalanine metabolism, cysteine and methionine metabolism, glycerophospholipid metabolism, lysine biosynthesis, and ubiquinone and other terpenoid-quinone biosynthesis (Fig. 5D).
Combining the phenotypic and transcriptome analyses of the WT and ΔAoste12 mutant strains, proteins interact with AoSte12 were analyzed through protein-protein interaction networks (STRING) (see Table S3), we found that AoSte12 regulates multiple cellular processes, including lipid metabolism, the tricarboxylic acid (TCA) cycle, growth, vesicle transport, actin filament, the MAPK pathway, and cell fusion (Fig. 5E). Remarkably, phospholipases PldA (AOL_s00215g30) and PlaA (AOL_s00210g100), and UBX domain protein Ubx5 (AOL_s00215g489) related to lipid metabolism and fusion were enriched. SNARE domain protein (AOL_s00188g92) and Sec22 (AOL_s00076g350) associated with vesicle transport and membrane fusion were also enriched. In addition, several signaling proteins involved in mycelia growth, conidiation, polarized growth, and trap formation were enriched, including G protein complex alpha subunit GpaB (AOL_s00109g19), MAPK kinase kinase Ssk2 (AOL_s00006g209), cell division control protein Cdc42 (AOL_s00043g439), and protein kinase regulator Ste50 (AOL_s00004g530).
DAP-Seq analysis of AoSte12.
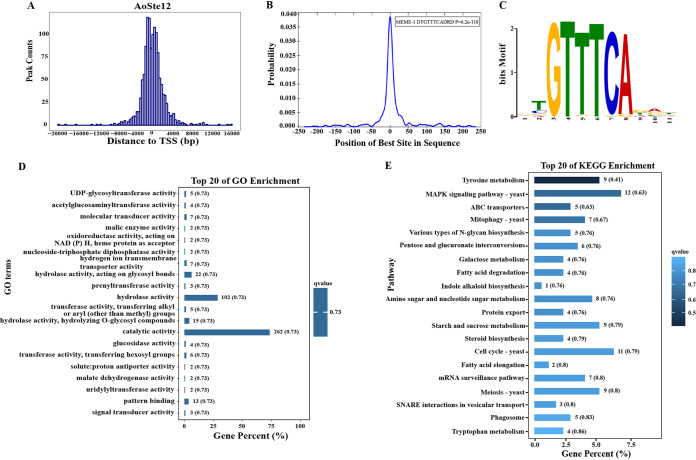
The downstream target genes of Aoste12 were analyzed using DAP-Seq; this method can be used as a partial substitute for the ChIP (chromatin immunoprecipitation) method to study transcription factor binding sites for nonmodel species (20). The distance between the peak summit and the transcriptional start site (TSS) was analyzed (Fig. 6A; see also Table S4); the distance from peak summit to the TSS was within 3 kb, accounting for about 85% of the total number of peaks (see Fig. S3). The consensus DNA binding motif of AoSte12 is “DTGTTTCADRD” (E value = 3.2E–154), and this was significantly enriched in the central region of the analyzed peak (P = 6.2E–118) (Fig. 6B and C). GO and KEGG enrichment analyses were further carried out on the target genes predicted by DAP-Seq. In the GO enrichment, the top 20 terms were significantly enriched in catalytic activity, hydrolase activity, and pattern binding (Fig. 6D). The top 20 KEGG pathways were enriched for the MAPK signaling pathway and autophagy processes, including mitophagy, phagosome, and SNARE interactions in vesicular transport, the cell cycle, meiosis, and fatty acid degradation and elongation (Fig. 6E).
FIG 6.
DAP-Seq analysis of AoSte12 in A. oligospora. (A) Genomic regional distribution of AoSte12 binding sites. TSS, transcriptional start site. (B) Positional distribution of motifs enriched significantly in the AoSte12 target peaks. (C) Motif base sequence specifically bound by AoSte12. (D) Top 20 GO enrichment terms of AoSte12 target genes. (E) Top 20 KEGG enrichment pathways of AoSte12 target genes.
Comprehensive transcriptomic and DAP-Seq analysis.
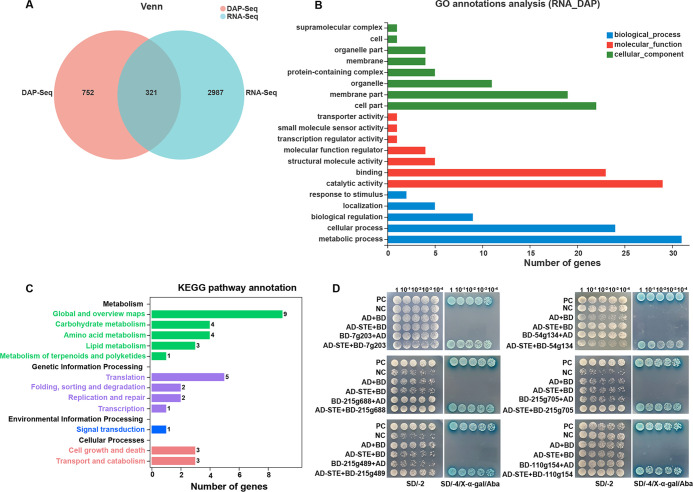
In order to further explore the function of AoSte12, transcriptomic and DAP-Seq data were comprehensively analyzed. First, the transcriptomic and DAP-Seq data were screened, 3,308 DEGs of the transcriptome were obtained, and 1,073 genes were selected due to the promoter of the target gene being located in the 2-kb sequence range upstream of the transcription start site. Venn analysis showed that there were 321 genes in both data sets (Fig. 7A). Then, GO and KEGG enrichment analyses showed that these genes were involved in multiple biological processes and metabolic pathways. In the GO analysis, cell parts, membrane parts, and organelles were enriched in the cellular component; catalytic activity and binding were enriched in molecular function; and metabolism and cellular processes were enriched in biological processes. In the KEGG pathway annotations, global and overview maps, carbohydrate metabolism, amino acid metabolism, lipid metabolism, translation, cell growth and death, and transport and catabolism were enriched (Fig. 7B and C).
FIG 7.
Function analysis of AoSte12 by combining transcriptome and DAP-Seq. (A) Venn diagram analysis of RNA-Seq and DAP-Seq data. RNA-Seq indicates transcriptomic data. (B) GO annotation of RNA-Seq and DAP-Seq data. (C) KEGG pathway annotation of RNA-Seq and DAP-Seq data. (D) Yeast two-hybrid (Y2H) assay of AoSte12 and six putative target proteins (AOL_s00007g203, AOL_s00054g134, AOL_s00215g688, AOL_s00215g705, AOL_s00215g489, and AOL_s00110g154) in A. oligospora. Plasmids pGBKT7-53 and pGADT7-T served as positive controls (PCs), whereas pGBKT7-Lam, pGADT7-T, pGBKT7, and pGADT7 served as negative controls (NCs). Yeast transformants were diluted in 0.9% NaCl, and on this basis, diluted four times with equal volume for 100, 10−1, 10−2, 10−3, 10−4. Growth was determined on SD/–Trp/–Leu (SD/-2), SD/–Trp/–Leu/–His/–Ade (SD/-4), and SD/-4/X-α-Gal/Aba media with serially diluted yeast cells.
Y2H analysis.
According to the phenotypic differences between the WT and the ΔAoste12 mutant strains, several genes were screened and verified using Y2H, and a total of six proteins interacting with AoSte12 were verified, including UDP-glycosyltransferase (AOL_s00007g203), malate dehydrogenase (AOL_s00054g134), vacuolar sorting protein Pep3 (AOL_s00215g688), ABC-type transporter (AOL_s00215g705), UBX domain-containing protein Ubx5 (AOL_s00215g489), and MAK-2 orthologue protein Fus3 (AOL_s00110g154) (Fig. 7D).
AoSte12 regulates secondary metabolites.
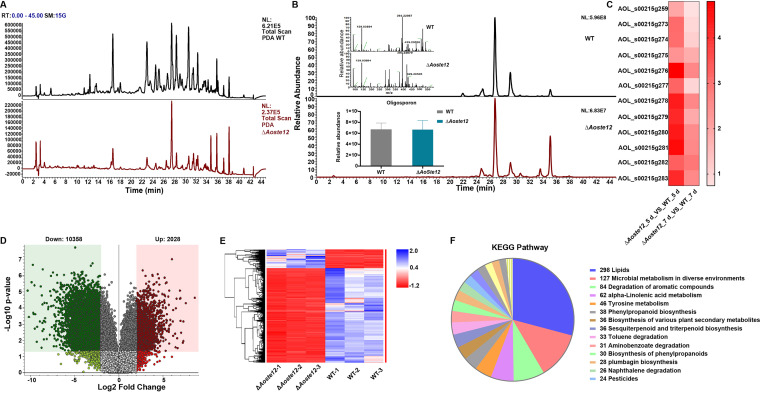
The extracts of the WT and ΔAoste12 mutant strains were determined via liquid chromatography-mass spectrometry (LC-MS) (see Table S5). The kurtosis values of the WT and ΔAoste12 mutant strains were compared through the chromatogram. Compared to the WT strain, the kurtosis of the ΔAoste12 mutant showed a downward trend over a 10- to 38-min retention period (Fig. 8A). Furthermore, the specific compound arthrobotrisins identified from A. oligospora and other NT fungi were analyzed, and their contents showed no differences between the WT and ΔAoste12 mutant strains (Fig. 8B), whereas the transcriptional levels of genes associated with the biosynthesis of arthrobotrisins were remarkably upregulated in the ΔAoste12 mutant compared to the WT strain (Fig. 8C).
FIG 8.
Comparison of metabolic profiling between wild-type (WT) and ΔAoste12 mutant strains. (A) Comparison of high-performance liquid chromatography (HPLC) profiles of the WT and ΔAoste12 mutant strains. PDA, photo-diode array. (B) Comparison of chromatogram of arthrobotrisin anion peaks of WT and ΔAoste12 mutant strains. Arthrobotrisins were verified via diagnostic ion fragments at m/z 139, 393, and 429 through a mass spectrogram. The histogram showed that the arthrobotrisin content was comparable to the peak area of the WT and ΔAoste12 mutant strains. (C) A heatmap shows the relative transcription levels of genes associated with the biosynthesis of arthrobotrisins in the ΔAoste12 mutant versus the WT strain at different time points. (D) Volcano plot of differential metabolites between WT and ΔAoste12 mutants. (E) Heatmap of upregulated and downregulated metabolic pathways between ΔAoste12 mutant and WT strain determined via KEGG enrichment. (F) Numbers of KEGG pathways in the ΔAoste12 mutant versus the WT strain.
An analysis of the metabolic data revealed that 10,358 compounds were downregulated and 2,028 compounds were upregulated in the ΔAoste12 mutant compared to the WT strain (Fig. 8D). Similarly, most of the enriched metabolic pathways were downregulated in the ΔAoste12 mutant (Fig. 8E). In addition, KEGG enrichment analysis of these metabolic pathways revealed that several pathways were highly enriched, such as the LD accumulation-related metabolic pathway, microbial metabolism in diverse environments, the degradation of aromatic compounds, alpha-linolenic acid metabolism, the biosynthesis of various plant secondary metabolites, sesquiterpenoid and triterpenoid biosynthesis, and toluene degradation (Fig. 8F).
DISCUSSION
Ste12 is a conserved transcription factor in fungi and plays an important regulatory role in mating, hyphal growth, conidiation, sexual development, hyphal fusion, and pathogenicity (26–31). In this study, we characterized an orthologue, Ste12, in the NT fungus A. oligospora using a combined analysis of multiple phenotypes and multi-omics. Our results show that AoSte12 plays an important role in mycelial growth, stress resistance, conidiation, hyphal fusion, trap morphogenesis, nucleus, LD accumulation, and secondary metabolism. Meanwhile, transcriptomic and DAP-Seq analyses also confirmed that AoSte12 is involved in the regulation of diverse biological processes.
The disruption of Aoste12 affects mycelial growth and stress resistance in A. oligospora. Similarly, the deletion of the ste12 gene results in the slow growth rate of mycelia in N. crassa (33), the NT fungus Drechslerella dactyloides (34), and the plant-pathogenic fungus Setosphaeria turcica (35). However, the absence of orthologs of ste12 has no influence on mycelial growth in several filamentous fungi, including Fusarium graminearum (36), Aspergillus nidulans (37), and M. grisea (30). Moreover, Δste12 mutant had the same sensitivity to H2O2 with WT strain in Alternaria alternata (38), while the ΔAoste12 mutant had lower sensitivity to oxidation reagents (H2O2 and menadione) than WT strain in A. oligospora. Accordingly, the responses to stimuli and antioxidant activity were enriched in GO terms. Therefore, Ste12 plays a varied role in the mycelial growth of different fungal species, and AoSte12 also plays a minor role in stress resistance for A. oligospora.
Conidia are special reproductive propagules with which filamentous fungi can expand their reproduction when their living conditions are no longer fit for apical extension growth (39). The deletion of ste12 in F. graminearum and M. grisea had no effect on the formation and germination of conidia (30, 36). In D. dactyloides, the ΔDdste12 mutant had defects for conidiation, and the number of conidia decreased by only 19.7% compared to the WT strains (34). The absence of Ste12 caused conidia to be produced only under the condition of 0.4 M KCl in the growth medium, in Metarhizium rileyi (40). Similarly, conidia yield was decreased in the Δste12 mutant of S. turcica (35), and A. oryzae (25). In this study, the conidia yield of the ΔAoste12 mutant was significantly reduced compared to the WT strain, and the morphology of the conidiophore of the ΔAoste12 mutant was also remarkably changed. Moreover, the morphology of the conidia of the ΔAoste12 mutant was mostly immature with no septum, and the spore germination rate was remarkably reduced. In addition, the transcription of genes related to conidiation was also altered, especially the genes encoding regulators abaA, brlA, and fluG, which coordinate conidiation-specific gene expression (39, 41), the expressions of which were remarkably downregulated in the ΔAoste12 mutant. Therefore, Ste12 plays a conserved role in sporulation in many fungi, and AoSte12 also plays an important role in regulating the development of conidiophores.
The mycelium network structure formed by hyphal fusion in filamentous fungi is crucial for the organization and function of colonies (42). N. crassa is a model fungus for studying hyphal fusion, and the ste12 homologous protein gene pp-1 is essential for conidial anastomosis tubes and hyphal fusion (22). The Δste12 mutant in T. atroviride has the same phenotype as the Δpp-1 mutant in N. crassa, whereas in A. oryzae, Ste12 plays a negative regulatory role in hyphal fusion (24, 25). However, in E. festucae and F. oxysporum, the deletion of ste12 has little effect on hyphal fusion (27, 43). In this study, the deletion of Aoste12 remarkably promoted the frequency of hyphal fusion, and the ΔAoste12 mutant showed more dense mycelial networks. Therefore, orthologous Ste12 plays a divergent role in hyphal fusion in diverse fungi, and plays a role in negative regulation for hyphal fusion and the formation of mycelial networks in A. oligospora.
In addition, Ste12 is involved in virulence and is necessary for Candida glabrata to produce pseudomycelia in nitrogen-deficient cultures (44). Similarly, Ste12 is essential for pathogenicity in several pathogenic fungi, such as F. graminearum (36), F. oxysporum (26), M. grisea (30), M. rileyi (40), and S. turcica (35). Recently, orthologous Ste12 was identified in the two NT fungi D. dactyloides and A. oligospora (strain TWF154); disruption of the Ddaste12 gene in D. dactyloides disabled the cell inflation of constricting rings and led to an inability to capture nematodes (34). In A. oligospora (strain TWF154), the Δste12 mutant did not form traps when exposed to nematodes for 24 h; after prolonged incubation in the presence of nematodes, the Δste12 mutant developed a few traps with abnormal morphology (32). In this study, the deletion of Aoste12 resulted in a reduction in trap formation, whereas the traps of the ΔAoste12 mutant consisted of more hyphal loops, which may remedy the reduced trap formation. Thus, there is no obvious difference between nematode predation ability in the WT and ΔAoste12 mutant strains. Overall, these results show that Ste12 has a conserved role in fungal pathogenicity, and it plays a crucial role in the regulation of trap morphogenesis for NT fungi.
Filamentous fungi are a kind of fungi that show continuous growth at the tip of the growing hypha. Polar growth requires the continuous supply of proteins and LDs to the top of the hypha. This transportation is achieved by vesicles through the actin and microtubule cytoskeleton (45). In A. oligospora, the disruption of Aoste12 impaired the polar growth of hypha and affected the formation of hyphal networks, which caused the ΔAoste12 mutant to grow in a confused direction and increased the density of mycelial networks. Furthermore, BODIPY fluorescent staining showed that there were more LDs in the ΔAoste12 mutant, and the number of nuclei of the ΔAoste12 mutant was reduced. These results show that AoSte12 has a negative effect on the polar growth of hyphae, and it also plays an important role in regulating the accumulation of LDs and the number of nuclei.
In recent years, transcriptomic analysis has been broadly applied to elucidate the mechanism of fungal growth development (46–48). In this study, the absence of Aoste12 caused 16.5 and 12.9% of genes to be differentially expressed at 5 and 7 dpi, respectively. GO enrichment analysis showed that these DEGs were mainly enriched for cellular processes, localization, metabolic processes, membranes, cell parts, binding, catalytic activity, and organelles, indicating that AoSte12 plays a multifunctional regulatory role in the mycelial growth and development process. KEGG enrichment analysis showed that the upregulated genes were remarkably focused on glycolysis/gluconeogenesis, multiple metabolic pathways, and the MAPK signaling pathway, whereas the downregulated genes were remarkably focused on ribosome, cell cycle, DNA replication and repair, meiosis, multiple metabolism, and biosynthesis processes. These pathways are closely associated with phenotypic alteration, such as mycelial growth, conidiation, the cell nucleus, and trap formation. Moreover, several genes associated with lipids, actin, growth, the MAPK pathway, vesicles, cell fusion, and TCA cycles were significantly enriched and consistent with phenotypic traits. For example, G protein complex alpha subunit and cell division control protein Cdc42 have been proven to play a crucial role in mycelial growth, conidiation, trap formation, and stress response in A. oligospora (11, 49). Meanwhile, Ssk2 and Ste50 are indispensable components of the MAPK signaling cascade and have been reported to be involved in growth, conidiation, stress response, and virulence in A. oligospora and other pathogenic fungi (32, 50). In addition, phospholipase PldA is required for polarized growth and cell fusion in E. festucae (51), and PlaA regulates conidiation, appressorial turgor pressure, and pathogenicity in M. oryzae (52). The ortholog of SNARE domain protein (AOL_s00188g92) mediates vesicle membrane fusion with target compartments (53), and vesicle transport protein Sec22 is involved in regulating cell wall integrity, growth, reproduction, pathogenicity, the regulation of ROS, and the expression of extracellular enzymes in filamentous fungi (54).
DAP-Seq data showed that the consensus DNA binding motif of AoSte12 was consistent with PP-1 in N. crassa (20). In the GO enrichment, catalytic activity, hydrolase activity, and hydrolase activity acting on glycolytic bonds were significantly enriched. Furthermore, tyrosine metabolism, the MAPK signaling pathway, ABC transport, mitophagy, and fatty acid degradation were enriched in the KEGG enrichment, which was consistent with the transcriptomic analysis. Combining the transcriptome with DAP-Seq for a comprehensive analysis, the GO enrichment result is consistent with the transcriptomic analysis, and the KEGG enrichment analysis indicates that AoSte12 regulates diverse metabolic pathways such as global and overview maps, carbohydrate metabolism, amino acid metabolism, lipid metabolism, translation, cell growth and death, and transport and catabolism. Based on these results, AoSte12 is involved in cellular growth and metabolic processes. Several genes involved in these processes were further verified through Y2H. In N. crassa, Ste12 is regulated by the MAPK pathway, and its ortholog of PP-1 is regulated by MAK-2 (55). Moreover, there are no potential Adv-1 binding motifs upstream of ste12 (pp-1), but there are several potential Ste12 binding motifs upstream of adv-1, which suggested that Ste12 regulates adv-1 and Adv-1 directly regulates downstream gene expression in N. crassa (20). However, our DAP-Seq data failed to identify AoSte12 binding to the promoter of Aoadv-1, and no interaction between the AoSte12 and AoAdv-1 was verified by Y2H analysis (data not shown); therefore, the interaction between the AoSte12 and AoAdv-1 remains to be further explored. In our analysis, the MAK-2 homologous protein Fus3 can interact with AoSte12, which further proves the regulatory relationship between Fus3 and AoSte12. At the same time, AoSte12 can interact with vacuolar sorting protein Pep3, UDP-glycosyltransferase, UBX domain-containing protein Ubx5, ABC-type transporter, and malate dehydrogenase. Of these, UDP glycosyltransferases are major phase II enzymes involved in the metabolism and detoxification of exogenous organisms (56), Ubx5 is related to lipid accumulation and the stress response (57), malate dehydrogenase is involved in the regulation of the TCA cycle (58), Pep3 is a low-abundance peripheral vacuolar membrane protein (59), and ABC-type transporter is involved in membrane transporters (60); they might regulate membrane transport and hyphal fusion. These results suggest that AoSte12 plays a pleiotropic role in diverse biological processes by regulating diverse targets.
Fungi are rich sources of secondary metabolites with important medicinal value, with potential uses as antibiotics, antitumor medications, and immunosuppressants (61, 62). Secondary metabolites play an important role in the development and pathogenesis of fungi, for example, the cycloundecapeptide cyclosporine was isolated from the insect-pathogenic fungus Tolypocladium inflatum for its antifungal activity and later developed as an immunosuppressant drug (63). The interaction between nematodes and NT fungi requires sophisticated inter-organismic communication with low-molecular-weight signaling molecules (64). The Mak-2/PP-1 regulation pathway also regulates the synthesis of secondary metabolites in N. crassa (33). In this study, compound kurtosis was significantly downregulated at multiple retention times, indicating that AoSte12 is involved in the regulation of the biosynthesis of secondary metabolites in A. oligospora.
Combing the analysis of phenotypic traits, transcriptomics, DAP-Seq, and metabolic analyses between the WT and the ΔAoste12 mutant strains, our results show that AoSte12 plays an important regulatory role in mycelial growth, hyphal fusion, conidiation, pathogenicity, secondary metabolism, and so on. Similar to the homologous protein PP-1 in N. crassa, AoSte12 is regulated by Fus3 (ortholog of MAK-2 in N. crassa) and then regulates multiple biological processes such as the TCA cycle, lipid metabolism, actin filament, and vesicle transport (Fig. 9). However, the physiology of the ΔAoste12 mutant was different from that of the Δpp-1 mutant in N. crassa, whose frequency of hyphal fusion was reduced. For the ΔAoste12 mutant, the frequency of hyphal fusion in hyphal networks and traps was significantly increased. In addition, our results show obvious differences from the phenotypic alteration (trap morphology and conidiation) of another species of A. oligospora (strain TWF154). These divergences may be caused by a difference between fungal species, whereas the detailed mechanism is still unknown. In summary, our results provide deep insights for revealing the function and regulatory mechanisms of AoSte12, and our results show that AoSte12 has pleiotropic roles especially in hyphal fusion, conidiation, and trap development. These results lay a foundation for further study into the function and regulatory mechanism of orthologs of Ste12 in hyphal fusion and trap formation in A. oligospora and other NT fungi.
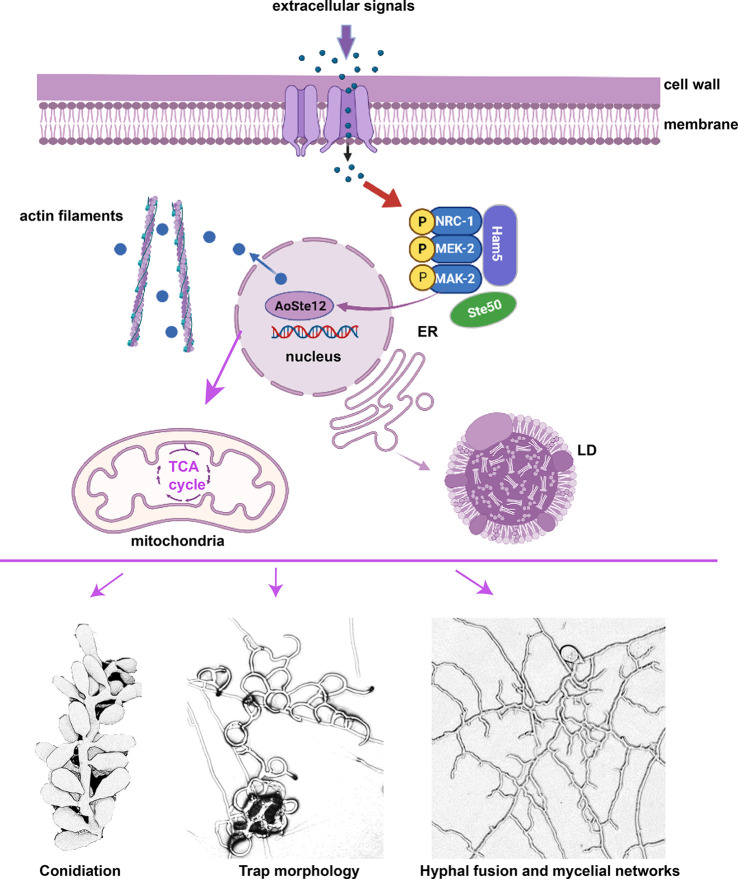
FIG 9.
Proposed model for AoSte12 regulation in A. oligospora. Our findings suggest that AoSte12 is regulated by Fus3 and then regulates multiple cellular processes such as the TCA cycle, lipid metabolism, actin filament, and vesicle transport. It also plays an important regulatory role in conidiation, hyphal fusion, trap formation, and pathogenicity. ER, endoplasmic reticulum; LD, lipid droplet. This image was created with BioRender.com.
MATERIALS AND METHODS
Strains and culture conditions.
Arthrobotrys oligospora (ATCC 24927) and derived mutant strains were incubated on PDA medium. PDA and TG media were used for phenotypic analyses of the WT and mutant strains, as previously described (14). S. cerevisiae (FY834) was cultured on yeast extract peptone dextrose medium for the recombinational cloning procedure, and yeast strains with the correct knockout vector were selected on SC-Ura defective medium (65). pCSN44 and pRS426 plasmids were carried by Escherichia coli strain DH5α (TaKaRa, Shiga, Japan) for hygromycin B resistance gene hph cloning and construction of the knockout vector, respectively. The regeneration of A. oligospora protoplasts was carried out on PDAS supplemented with 200 μg/mL hygromycin. Caenorhabditis elegans (strain N2) worms were cultured on an oatmeal medium at 26°C and used for bioassays.
Sequence and conserved domain analysis of AoSte12 in A. oligospora.
The protein sequences of Ste12 from S. cerevisiae and N. crassa were used as a query to seek out the ortholog of Ste12 in A. oligospora. The homologous sequences of Ste12 from different fungi were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/); the sequence similarity was analyzed using the DNAman software package (v5.2.2; Lynnon Biosoft, St. Louis, Canada), and the structural domains of the homologs of Ste12 were analyzed on the Pfam website (https://www.ebi.ac.uk/interpro). The amino acid sequences of Ste12 proteins were aligned with ClustalX, and the MEGA 7 software package with default parameter settings was used to construct a neighbor-joining tree (66).
Aoste12 deletion and verification.
The disruption of the Aoste12 gene was performed using a modified yeast cloning procedure, as previously described (67, 68). The 5′ and 3′ flanking sequences of the Aoste12 gene and the hph cassette were amplified from A. oligospora genome DNA and pCSN44, respectively, using the paired primers listed in Table S1. The amplified fragments and the linearized pRS426 plasmid were cotransformed into S. cerevisiae (FY834) to obtain the correct knockout vector pRS426-AoSte12-hph, and the disruption sequences were amplified from it using Ste12LF1 and Ste12RR1 and then transformed into A. oligospora protoplasts, as previously described (67, 68). The positive transformants were confirmed via PCR and Southern blotting, as described previously (45, 69).
Analysis of vegetative growth and stress tolerance.
The WT and mutant strains were cultured on PDA and TG medium for observing mycelial growth. The growth rate of mycelia was recorded in 24 intervals. In order to observe the cell morphology, calcofluor white (Sigma-Aldrich, St. Louis, MO), 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and boron dipyrromethene (BODIPY) dye (Sigma-Aldrich) were used to stain the mycelia, as described previously (50). The mycelia were examined using transmission electron microscopy (TEM; Hitachi, Tokyo, Japan).
In addition, the TG medium was used as the base medium supplemented with different chemical reagents to test the stress tolerance; two osmotic agents (0.1, 0.2, and 0.3 M NaCl and 0.25, 0.5, and 0.75 M sorbitol) and two oxidant agents (5, 10, and 15 mM H2O2 and 0.05, 0.07, and 0.09 mM menadione) were used. The relative growth inhibition (RGI) values of the strains were calculated as previously described (10, 14).
Observation of conidiospores, colony morphology, trap formation, and morphology.
The fungal strains were inoculated on CMY medium at 28°C for 7 days, and the conidiophores and conidia were observed using SEM (FEI Quanta-200, Hillsboro, OR) and TEM (Hitachi, Tokyo, Japan). The spores were washed from the medium, as described previously (50). A total of 2 × 104 spores were spread on water agar medium. The spore germination rate was counted at 4, 8, and 12 h. Similarly, 2 × 104 spores were spread on water agar medium at 28°C for 4 days. After spore germination, approximately 200 nematodes were added to each plate for the induction of trap formation. The mycelial morphology, traps, and the captured nematodes were imaged using SEM, and the intracellular structures of the traps were observed using TEM.
Transcriptome sequencing and analysis.
The WT and mutant strains were cultured on CMY medium with cellophane at 28°C, and the mycelia were collected at 5 and 7 days postinoculation. Three independent biological replicates were used for each sample, and the samples were sent to Shanghai Meiji Biological Company (Shanghai, China) for RNA sequencing and data analysis. High-quality RNA samples were used to construct a sequencing library that was sequenced on an Illumina HiSeq 4000 platform (Illumina, San Diego, CA). An average of 43.5 million clean reads was obtained per sample. The percentage of Q30 was 93.49 to 94.21%, and the GC content was 47.82 to 48.43% (see Table S2). Principal-component analysis showed that the three repeats of the WT and mutant strains at each time stage had high similarity and correlation (see Fig. S4). Furthermore, the transcriptional levels of 12 genes were determined using RT-qPCR analysis, and the results show that all the selected genes had the same expression pattern, thus verifying the accuracy of the transcriptome data (see Fig. S5). DEGs were identified based on the thresholds of | log2 ratio | ≥ 1 and adjusted P < 0.05. The RNA-Seq data were analyzed through the OmicShare online platform.
In addition, proteins interact with AoSte12 were analyzed through protein-protein interaction networks STRING (https://cn.string-db.org/) and further visualized and analyzed with Cytoscape.
DAP-Seq and data analysis.
The cDNA sequence encoding protein AoSte12 was constructed in the Halo Tag plasmid. The open reading frame of Aoste12 was amplified from the cDNA of the WT strain. This was inserted into the DB3 vector with a Halo-tag, the construct vector pAoSte12-halo was identified via sequencing, and then the vector and the genome DNA of A. oligospora were sent to Genedenovo Biotechnology (Guangzhou, China) for DAP-Seq analysis. The AoSte12 protein binds to the target genome fragment, then the DNA fragment of the genome bound by the target protein can be enriched and the input group used as the control. In combination with high-throughput sequencing technology, DNA products after DAP were sequenced and analyzed, DNA binding sites of target proteins were searched from the whole genome, and high-throughput data results were obtained using efficient sequencing methods. The DNA libraries were sequenced on the Illumina sequencing platform by Genedenovo Biotechnology Co. The average total number of effective reads after IP group (experimental group) and input filtering was about 80 million (see Table S4). The number of bases whose quality value was above Q30 and the percentage of them in the total number of filtered effective bases was 94.12%, on average (see Table S4). The distribution of peaks on gene functional elements is provided in Table S4 in the supplemental material. MEME-ChIP (https://meme-suite.org/meme/doc/meme-chip-output-format.html) was used to detect significant motif sequences in the peak sequence.
RT-qPCR analysis.
WT and mutant strains were cultured on CMY at 28°C for 5 day, and mycelial samples were collected for total RNA extraction according to the Axygen kit procedure (Axygen Biotech Company, Hangzhou, China) and then reverse transcribed into cDNA using a PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). They were then used as the template for qPCR. Specific primers (see Table S1) to detect the transcript levels of sporulation-related genes, and the A. oligospora β-tubulin gene (AOL_s00076g640) was used as an internal standard using a Roche LightCycler 480 relative quantitative method (Roche Center, China). The relative transcription level (RTL) of each gene was calculated as the ratio of the transcription level between the mutant and the WT strain according to the 2–ΔΔCT method (70).
Y2H analysis.
The cDNA of A. oligospora was obtained as mentioned, and then the cDNA sequences of Aoste12, AOL_s00215g688, AOL_s00007g203, AOL_s00215g489, AOL_s00215g705, AOL_s00054g134, and Fus3 were amplified using the primers listed in Table S1. The cDNA sequence of Aoste12 was cloned into the prey plasmid pGADT-7, and other cDNA sequences were cloned into the bait plasmid pGBKT-7. The correctly constructed vectors were cotransformed in S. cerevisiae strain Y2H Gold according to the LiAc/SS-DNA/PEG transformation protocol (TaKaRa, Dalian, China). The transformants were spread on SD/–Trp/–Leu and SD/–Trp/–Leu/–His/–Ade medium and verified using SD/–Trp/–Leu/–His/–Ade+X-α-Gal/Aba medium at 30°C for 3 days.
Metabolomic comparison via UPLC-MS.
The WT and mutant strains were inoculated into PD broth at 28°C and 180 rpm for 5 days, and then the fermentation broth was extracted using ethyl acetate and dried under a vacuum. The dried crude extract samples were dissolved into methanol, and the volume was fixed to 1 mL according to the dry weight of mycelia of each sample and the mass volume ratio. The experiment was repeated three times independently and analyzed by LC-MS using a Thermo Scientific Dionex Ultimate 3000 UHPLC system with a Thermo high-resolution Q Exactive focus mass spectrometer (Thermo, Bremen, Germany). Untargeted metabolomics analysis was performed using Compounds Discoverer 3.0 software (Thermo Fisher Scientific, Carlsbad, CA).
Statistical analysis.
All experiments were repeated three times, and the data are expressed as the means ± the standard deviations (SD). Prism 8.0 (GraphPad Software, San Diego, CA) was used as the tool for statistical analysis, with one-way analysis of variance, followed by Tukey’s honestly significant difference (HSD) test performed with a set of P < 0.05.
Data availability.
All data generated or analyzed during this study are included here or in the associated supplemental material. RNA-Seq and DAP-Seq data were deposited in the NCBI Gene Expression Omnibus (GEO) database and are accessible under GEO series accession numbers GSE213447 (RNA-Seq) and GSE213449 (DAP-Seq).
ACKNOWLEDGMENTS
We are grateful to Microbial Library of the Germplasm Bank of Wild Species from Southwest China for preserving and providing experimental strains and to Guo Ying-qi (Kunming Institute of Zoology, Chinese Academy of Sciences) for help with taking and analyzing TEM images.
Funding for this study was provided by the National Natural Science Foundation of China (grant 31960556) and Applied Basic Research Foundation of Yunnan Province (grant 202001BB050004).
We declare that we have no conflicts of interest.
J.Y. conceived and designed the study. N.B. performed the experiments. M.X., Q.L., W.W., and Y.L. analyzed the data. N.B. prepared figures and tables. N.B. and J.Y. contributed to manuscript preparation and revision. All authors read and approved the final manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Jinkui Yang, Email: jinkui960@ynu.edu.cn.
Christina A. Cuomo, Broad Institute
REFERENCES
- 1.Zhu MC, Zhao N, Liu YK, Li XM, Zhen ZY, Zheng YQ, Zhang KQ, Yang JK. 2022. The cAMP-PKA signalling pathway regulates hyphal growth, conidiation, trap morphogenesis, stress tolerance, and autophagy in Arthrobotrys oligospora. Environ Microbiol 24:6524–6538. doi: 10.1111/1462-2920.16253. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Zhu M, Liu Y, Yang L, Yang J. 2023. Aoatg11 and Aoatg33 are indispensable for mitophagy, and contribute to conidiation, the stress response, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Microbiol Res 266:127252. doi: 10.1016/j.micres.2022.127252. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Liu J, Fan Y, Xiang M, Kang S, Wei D, Liu X. 2022. SNARE protein DdVam7 of the nematode-trapping fungus Drechslerella dactyloides regulates vegetative growth, conidiation, and the predatory process via vacuole assembly. Microbiol Spectr 10:e0187222. doi: 10.1128/spectrum.01872-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su H, Zhao Y, Zhou J, Feng HH, Jiang DW, Zhang KQ, Yang JK. 2017. Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biol Rev Camb Philos Soc 92:357–368. doi: 10.1111/brv.12233. [DOI] [PubMed] [Google Scholar]
- 5.Zhu MC, Li XM, Zhao N, Yang L, Zhang KQ, Yang JK. 2022. Regulatory mechanism of trap formation in the nematode-trapping fungi. J Fungi 8:406. doi: 10.3390/jof8040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji XL, Li H, Zhang WH, Wang JA, Liang LM, Zou CG, Yu ZF, Liu SQ, Zhang KQ. 2020a. The lifestyle transition of Arthrobotrys oligospora is mediated by microRNA-like RNAs. Sci China Life Sci 63:543–551. doi: 10.1007/s11427-018-9437-7. [DOI] [PubMed] [Google Scholar]
- 7.Kuo CY, Chen SA, Hsueh YP. 2020. The high osmolarity glycerol (HOG) pathway functions in osmosensing, trap morphogenesis and conidiation of the nematode-trapping fungus Arthrobotrys oligospora. J Fungi 6:191. doi: 10.3390/jof6040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JK, Wang L, Ji XL, Feng Y, Li XM, Zou CG, Xu JP, Ren Y, Mi QL, Wu JL, Liu SQ, Liu Y, Huang XW, Wang HY, Niu XM, Li J, Liang LM, Luo YL, Ji KF, Zhou W, Yu ZF, Li GH, Liu YJ, Li L, Qiao M, Feng L, Zhang KQ. 2011. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7:e1002179. doi: 10.1371/journal.ppat.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji XL, Yu ZF, Yang JK, Xu JP, Zhang Y, Liu SQ, Zou CG, Li J, Liang LM, Zhang KQ. 2020b. Expansion of adhesion genes drives pathogenic adaptation of nematode-trapping fungi. iScience 23:101057. doi: 10.1016/j.isci.2020.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen ZY, Xing XJ, Xie MH, Yang L, Yang XW, Zheng YQ, Chen YL, Ma N, Li Q, Zhang KQ, Yang JK. 2018. MAP kinase Slt2 orthologs play similar roles in conidiation, trap formation, and pathogenicity in two nematode-trapping fungi. Fungal Genet Biol 116:42–50. doi: 10.1016/j.fgb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Bai N, Zhang GS, Wang WJ, Feng HH, Yang XW, Zheng YQ, Yang L, Xie MH, Zhang KQ, Yang JK. 2022. Ric8 acts as a regulator of G-protein signalling required for nematode-trapping lifecycle of Arthrobotrys oligospora. Environ Microbiol 24:1714–1730. doi: 10.1111/1462-2920.15735. [DOI] [PubMed] [Google Scholar]
- 12.Ma N, Zhao YN, Wang YC, Yang L, Li DN, Yang JL, Jiang KX, Zhang KQ, Yang JK. 2021. Functional analysis of seven regulators of G protein signaling (RGSs) in the nematode-trapping fungus Arthrobotrys oligospora. Virulence 12:1825–1840. doi: 10.1080/21505594.2021.1948667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie MH, Yang JL, Jiang KX, Bai N, Zhu MC, Zhu YM, Zhang KQ, Yang JK. 2021. AoBck1 and AoMkk1 are necessary to maintain cell wall integrity, vegetative growth, conidiation, stress resistance, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front Microbiol 12:649582. doi: 10.3389/fmicb.2021.649582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XW, Ma N, Yang L, Zheng YQ, Zhen ZY, Li Q, Xie MH, Li J, Zhang KQ, Yang JK. 2018. Two Rab GTPases play different roles in conidiation, trap formation, stress resistance, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Appl Microbiol Biotechnol 102:4601–4613. doi: 10.1007/s00253-018-8929-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Li XM, Xie MH, Bai N, Yang JL, Jiang KX, Zhang KQ, Yang JK. 2021. Pleiotropic roles of Ras GTPases in the nematode-trapping fungus Arthrobotrys oligospora identified through multi-omics analyses. iScience 24:102820. doi: 10.1016/j.isci.2021.102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou DX, Zhu Y, Bai N, Yang L, Xie M, Yang J, Zhu M, Zhang KQ, Yang J. 2022. AoATG5 plays pleiotropic roles in vegetative growth, cell nucleus development, conidiation, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Sci China Life Sci 65:412–425. doi: 10.1007/s11427-020-1913-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu QQ, Li DN, Jiang KX, Zhang KQ, Yang JK. 2022. AoPEX1 and AoPEX6 are required for mycelial growth, conidiation, stress response, fatty acid utilization, and trap formation in Arthrobotrys oligospora. Microbiol Spectr 10:e0027522. doi: 10.1128/spectrum.00275-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordbring-Hertz B, Friman E, Veenhuis M. 1989. Hyphal fusion during initial stages of trap formation in Arthrobotrys oligospora. Antonie Van Leeuwenhoek 55:237–244. doi: 10.1007/BF00393852. [DOI] [PubMed] [Google Scholar]
- 19.Herzog S, Schumann MR, Fleissner A. 2015. Cell fusion in Neurospora crassa. Curr Opin Microbiol 28:53–59. doi: 10.1016/j.mib.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Fischer MS, Wu VW, Lee JE, O’Malley RC, Glass NL. 2018. Regulation of cell-to-cell communication and cell wall integrity by a network of MAP kinase pathways and transcription factors in Neurospora crassa. Genetics 209:489–506. doi: 10.1534/genetics.118.300904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youssar L, Wernet V, Hensel N, Yu X, Hildebrand HG, Schreckenberger B, Kriegler M, Hetzer B, Frankino P, Dillin A, Fischer R. 2019. Intercellular communication is required for trap formation in the nematode-trapping fungus Duddingtonia flagrans. PLoS Genet 15:e1008029. doi: 10.1371/journal.pgen.1008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeder AC, Jonkers W, Li J, Glass NL. 2013. Early colony establishment in Neurospora crassa requires a MAP kinase regulatory network. Genetics 195:883–898. doi: 10.1534/genetics.113.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell E, Kim HJ, Stone DE. 2007. The pheromone-induced nuclear accumulation of the Fus3 MAPK in yeast depends on its phosphorylation state and on Dig1 and Dig2. BMC Cell Biol 8:44. doi: 10.1186/1471-2121-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruber S, Zeilinger S. 2014. The transcription factor Ste12 mediates the regulatory role of the Tmk1 MAP kinase in mycoparasitism and vegetative hyphal fusion in the filamentous fungus Trichoderma atroviride. PLoS One 9:e111636. doi: 10.1371/journal.pone.0111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katayama T, Bayram Ö, Mo T, Karahoda B, Valerius O, Takemoto D, Braus GH, Kitamoto K, Maruyama JI. 2021. Novel Fus3- and Ste12-interacting protein FsiA activates cell fusion-related genes in both Ste12-dependent and -independent manners in Ascomycete filamentous fungi. Mol Microbiol 115:723–738. doi: 10.1111/mmi.14639. [DOI] [PubMed] [Google Scholar]
- 26.Asunción García-Sánchez M, Martín-Rodrigues N, Ramos B, de Vega-Bartol JJ, Perlin MH, Díaz-Mínguez JM. 2010. Fost12, the Fusarium oxysporum homolog of the transcription factor Ste12, is upregulated during plant infection and required for virulence. Fungal Genet Biol 47:216–225. doi: 10.1016/j.fgb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Kamiya S, Ozaki Y, Kameoka S, Kayano Y, Saikia S, Akano F, Uemura A, Takagi H, Terauchi R, Maruyama JI, Hammadeh HH, Fleissner A, Scott B, Takemoto D. 2020. A nuclear protein NsiA from Epichloë festucae interacts with a MAP kinase MpkB and regulates the expression of genes required for symbiotic infection and hyphal cell fusion. Mol Microbiol 114:626–640. doi: 10.1111/mmi.14568. [DOI] [PubMed] [Google Scholar]
- 28.Hoi JWS, Dumas B. 2010. Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot Cell 9:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlini L, Dudin O, Martin SG. 2013. Mate and fuse: how yeast cells do it. Open Biol 3:130008. doi: 10.1098/rsob.130008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park G, Xue C, Zheng L, Lam S, Xu JR. 2002. MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol Plant Microbe Interact 15:183–192. doi: 10.1094/MPMI.2002.15.3.183. [DOI] [PubMed] [Google Scholar]
- 31.Wei QL, Du YR, Jin K, Xia YX. 2017. The Ste12-like transcription factor MaSte12 is involved in pathogenicity by regulating the appressorium formation in the entomopathogenic fungus, Metarhizium acridum. Appl Microbiol Biotechnol 101:8571–8584. doi: 10.1007/s00253-017-8569-x. [DOI] [PubMed] [Google Scholar]
- 32.Chen SA, Lin HC, Schroeder FC, Hsueh YP. 2021. Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway. Genetics 217:iyaa008. doi: 10.1093/genetics/iyaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104. doi: 10.1534/genetics.104.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y, Zhang WW, Chen Y, Xiang MC, Liu XZ. 2021. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl Microbiol Biotechnol 105:7379–7393. doi: 10.1007/s00253-021-11455-z. [DOI] [PubMed] [Google Scholar]
- 35.Gu SQ, Li P, Wu M, Hao ZM, Gong XD, Zhang XY, Tian L, Zhang P, Wang Y, Cao ZY, Fan YS, Han JM, Dong JG. 2014. StSTE12 is required for the pathogenicity of Setosphaeria turcica by regulating appressorium development and penetration. Microbiol Res 169:817–823. doi: 10.1016/j.micres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Gu Q, Zhang CQ, Liu X, Ma ZH. 2015. A transcription factor FgSte12 is required for pathogenicity in Fusarium graminearum. Mol Plant Pathol 16:1–13. doi: 10.1111/mpp.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallim MA, Miller KY, Miller BL. 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol Microbiol 36:290–301. doi: 10.1046/j.1365-2958.2000.01874.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma H, Zhang B, Gai Y, Sun X, Chung KR, Li H. 2019. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of citrus. Front Microbiol 10:2514. doi: 10.3389/fmicb.2019.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang AX, Mouhoumed AZ, Tong SM, Ying SH, Feng MG. 2019. BrlA and AbaA govern virulence-required dimorphic switch, conidiation, and pathogenicity in a fungal insect pathogen. mSystems 4:e00140-19. doi: 10.1128/mSystems.00140-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin YL, Wang J, Yang K, Fan L, Wang ZK, Yin YP. 2021. Regulation of conidiation, polarity growth, and pathogenicity by MrSte12 transcription factor in entomopathogenic fungus, Metarhizium rileyi. Fungal Genet Biol 155:103612. doi: 10.1016/j.fgb.2021.103612. [DOI] [PubMed] [Google Scholar]
- 41.Guo CT, Luo XC, Tong SM, Zhou Y, Ying SH, Feng MG. 2022. FluG and FluG-like FlrA coregulate manifold gene sets vital for fungal insect-pathogenic lifestyle but not involved in asexual development. mSystems 7:e00318-22. doi: 10.1128/msystems.00318-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Read ND, Lichius A, Shoji JY, Goryachev AB. 2009. Self-signalling and self-fusion in filamentous fungi. Curr Opin Microbiol 12:608–615. doi: 10.1016/j.mib.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Rispail N, Di Pietro A. 2009. Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol Plant Microbe Interact 22:830–839. doi: 10.1094/MPMI-22-7-0830. [DOI] [PubMed] [Google Scholar]
- 44.Calcagno AM, Bignell E, Warn P, Jones MD, Denning DW, Mühlschlegel FA, Rogers TR, Haynes K. 2003. Candida glabrata STE12 is required for wild-type levels of virulence and nitrogen starvation induced filamentation. Mol Microbiol 50:1309–1318. doi: 10.1046/j.1365-2958.2003.03755.x. [DOI] [PubMed] [Google Scholar]
- 45.Takeshita N. 2016. Coordinated process of polarized growth in filamentous fungi. Biosci Biotechnol Biochem 80:1693–1699. doi: 10.1080/09168451.2016.1179092. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Li XM, Bai N, Yang XW, Zhang KQ, Yang JK. 2022. Transcriptomic analysis reveals that Rho GTPases regulate trap development and lifestyle transition of the nematode-trapping fungus Arthrobotrys oligospora. Microbiol Spectr 10:e0175921. doi: 10.1128/spectrum.01759-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cea-Sánchez S, Corrochano-Luque M, Gutiérrez G, Glass NL, Cánovas D, Corrochano LM. 2022. Transcriptional regulation by the velvet protein VE-1 during asexual development in the fungus Neurospora crassa. mBio 13:e01505-22. doi: 10.1128/mbio.01505-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie M, Ma N, Bai N, Yang L, Yang X, Zhang KQ, Yang J. 2022. PKC-SWI6 signaling regulates asexual development, cell wall integrity, stress response, and lifestyle transition in the nematode-trapping fungus Arthrobotrys oligospora. Sci China Life Sci 65:2455–2471. doi: 10.1007/s11427-022-2118-0. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Li XM, Ma YX, Zhang KQ, Yang JK. 2022. The Arf-GAP proteins AoGcs1 and AoGts1 regulate mycelial development, endocytosis, and pathogenicity in Arthrobotrys oligospora. J Fungi 8:463. doi: 10.3390/jof8050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang KX, Liu QQ, Bai N, Zhu MC, Zhang KQ, Yang JK. 2022. AoSsk1, a response regulator required for mycelial growth and development, stress responses, trap formation, and the secondary metabolism in Arthrobotrys oligospora. J Fungi 8:260. doi: 10.3390/jof8030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassing B, Eaton CJ, Winter D, Green KA, Brandt U, Savoian MS, Mesarich CH, Fleissner A, Scott B. 2020. Phosphatidic acid produced by phospholipase D is required for hyphal cell-cell fusion and fungal-plant symbiosis. Mol Microbiol 113:1101–1121. doi: 10.1111/mmi.14480. [DOI] [PubMed] [Google Scholar]
- 52.Liu XH, Zhuang FL, Lu JP, Lin FC. 2011. Identification and molecular cloning Moplaa gene, a homologue of Homo sapiens PLAA, in Magnaporthe oryzae. Microbiol Res 167:8–13. doi: 10.1016/j.micres.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Yoon TY, Munson M. 2018. SNARE complex assembly and disassembly. Curr Biol 28:R397–R401. doi: 10.1016/j.cub.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Zhou D, Bai N, Liu Q, Zhao N, Yang J. 2023. SNARE protein AoSec22 orchestrates mycelial growth, vacuole assembly, trap formation, stress response, and secondary metabolism in Arthrobotrys oligospora. J Fungi 9:75. doi: 10.3390/jof9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer MS, Glass NL. 2019. Communicate and fuse: How filamentous fungi establish and maintain an interconnected mycelial network. Front Microbiol 10:619. doi: 10.3389/fmicb.2019.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Zhu JS, Cui L, Wang QQ, Huang WL, Yang QJ, Ji XJ, Rui CH. 2021. Overexpression of multiple UDP-glycosyltransferase genes involved in sulfoxaflor resistance in Aphis gossypii Glover. J Agric Food Chem 69:5198–5205. doi: 10.1021/acs.jafc.1c00638. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Yu QL, Liu Z, Liang C, Zhang B, Li MC. 2017. UBX domain-containing proteins are involved in lipid homeostasis and stress responses in Pichia pastoris. Int J Biochem Cell Biol 90:136–144. doi: 10.1016/j.biocel.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Sui YF, Schütze T, Ouyang LM, Lu H, Liu P, Xiao X, Qi J, Zhuang YP, Meyer V. 2020. Engineering cofactor metabolism for improved protein and glucoamylase production in Aspergillus niger. Microb Cell Fact 19:198. doi: 10.1186/s12934-020-01450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preston RA, Manolson MF, Becherer K, Weidenhammer E, Kirkpatrick D, Wright R, Jones EW. 1991. Isolation and characterization of PEP3, a gene required for vacuolar biogenesis in Saccharomyces cerevisiae. Mol Cell Biol 11:5801–5812. doi: 10.1128/mcb.11.12.5801-5812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martins MP, Franceschini ACC, Jacob TR, Rossi A, Martinez-Rossi NM. 2016. Compensatory expression of multidrug-resistance genes encoding ABC transporters in dermatophytes. J Med Microbiol 65:605–610. doi: 10.1099/jmm.0.000268. [DOI] [PubMed] [Google Scholar]
- 61.Saha P, Ghosh S, Roy-Barman S. 2020. MoLAEA regulates secondary metabolism in Magnaporthe oryzae. mSphere 5:e00936-19. doi: 10.1128/mSphere.00936-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson DM, Donzelli BG, Krasnoff SB, Keyhani NO. 2014. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat Prod Rep 31:1287–1305. doi: 10.1039/c4np00054d. [DOI] [PubMed] [Google Scholar]
- 63.Yang XQ, Feng P, Yin Y, Bushley K, Spatafora JW, Wang CS. 2018. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. mBio 9:e01211-18. doi: 10.1128/mBio.01211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu X, Hu XD, Pop M, Wernet N, Kirschhöfer F, Brenner-Weiss G, Keller J, Bunzel M, Fischer R. 2021. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat Commun 12:5462. doi: 10.1038/s41467-021-25535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci USA 103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie MH, Wang YC, Tang LY, Yang L, Zhou DX, Li Q, Niu XM, Zhang KQ, Yang JK. 2019. AoStuA, an APSES transcription factor, regulates the conidiation, trap formation, stress resistance and pathogenicity of the nematode-trapping fungus Arthrobotrys oligospora. Environ Microbiol 21:4648–4661. doi: 10.1111/1462-2920.14785. [DOI] [PubMed] [Google Scholar]
- 68.Wang WJ, Zhao YN, Bai N, Zhang KQ, Yang JK. 2022. AMPK is involved in regulating the utilization of carbon sources, conidiation, pathogenicity, and stress response of the nematode-trapping fungus Arthrobotrys oligospora. Microbiol Spectr 10:e0222522. doi: 10.1128/spectrum.02225-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding JL, Li XH, Lei JH, Feng MG, Ying SH. 2022. Succinate dehydrogenase subunit C contributes to mycelial growth and development, stress response, and virulence in the insect parasitic fungus Beauveria bassiana. Microbiol Spectr 10:e0289122. doi: 10.1128/spectrum.02891-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.03957-22-s0001.pdf, PDF file, 1.7 MB (1.7MB, pdf)
Supplemental material. Download spectrum.03957-22-s0002.xlsx, XLSX file, 4.1 MB (4.1MB, xlsx)
Data Availability Statement
All data generated or analyzed during this study are included here or in the associated supplemental material. RNA-Seq and DAP-Seq data were deposited in the NCBI Gene Expression Omnibus (GEO) database and are accessible under GEO series accession numbers GSE213447 (RNA-Seq) and GSE213449 (DAP-Seq).